In a cohort of hospitalized febrile patients in India, clinicians prescribed fewer antibiotics when mosquito-borne disease was suspected and discontinued antibiotics faster when mosquito-borne disease was confirmed. Improved mosquito-borne disease identification has potential to reduce antibiotic use in India.
Keywords: antibiotic usage, acute febrile illness, India, diagnosis, vector-borne
Abstract
Background
Antibiotic resistance mechanisms originating in low- and middle- income countries are among the most common worldwide. Reducing unnecessary antibiotic use in India, the world’s largest antibiotic consumer, is crucial to control antimicrobial resistance globally. Limited data describing factors influencing Indian clinicians to start or stop antibiotics are available.
Methods
Febrile adults and children admitted to a public tertiary care hospital in Pune, India, were enrolled. Antibiotic usage and clinical history were recorded. Immunoassays for mosquito-borne disease and bacterial cultures were performed by protocol and clinician-directed testing. Clinical factors were assessed for association with empiric antibiotic initiation and discontinuation by day 5 using multivariable logistic regression and propensity score–matched Cox proportional hazard models.
Results
Among 1486 participants, 683 (82%) adults and 614 (94%) children received empiric antibiotics. Participants suspected of having mosquito-borne disease were less likely to receive empiric antibiotics (adjusted odds ratio [AOR], 0.5; 95% confidence interval [CI], .4–.8). Empiric antibiotics were discontinued in 450 (35%) participants by day 5. Dengue or malaria testing performed before day 4 was positive in 162 (12%) participants, and was associated with antibiotic discontinuation (AOR, 1.7; 95% CI, 1.2–2.4). In a propensity score–matched model accounting for admission suspicion of mosquito-borne disease, positive dengue or malaria tests increased hazard of antibiotic discontinuation (hazard ratio, 1.6; 95% CI, 1.2–2.0).
Conclusions
Most patients with acute febrile illness in an Indian public hospital setting receive empiric antibiotics. Mosquito-borne disease identification is associated with reduced empiric antibiotic use and faster antibiotic discontinuation.
India is the largest overall consumer of antibiotics worldwide, and has among the highest reported rates of antibiotic resistance [1–4]. A worrying trend toward increased use of broad-spectrum antibiotics as well as last-resort antibiotics, such as carbapenems, in India will drive resistance to these important classes of antibiotics higher both domestically and internationally [5–8]. Reducing the use of antibiotics is key to addressing the problem of resistance; however, there has been only limited work investigating drivers of antibiotic use in India.
As in other low- and middle-income countries, febrile illness in India is one of the most common reasons to seek healthcare [9, 10], and most patients in India with febrile illness receive antibiotics [5]. Early efforts to implement antibiotic stewardship in India have proceeded slowly, particularly in public healthcare institutions [11]. To devise effective strategies to reduce unnecessary antibiotic use in India, it is important to understand the factors that influence providers to start and stop antibiotics. Here we examine the role of both suspected and diagnosed mosquito-borne disease on antibacterial use in a prospective observational cohort of febrile adults and children admitted to a public tertiary care center in Pune, India.
METHODS
Setting and Participants
Adults and children admitted to Byramjee Jeejeebhoy Government Medical College–Sassoon General Hospital, a 1300-bed public teaching hospital in Pune, India, with acute febrile illness (AFI) were enrolled into a prospective observational cohort. Care on medicine and pediatric wards is performed primarily by general internists, pediatricians, and their trainees. From July 2013 through December 2015, a dedicated study physician reviewed the admission records, 5 days per week, for adult and pediatric general medicine wards and intensive care units (ICUs). Starting in December 2013, antibiotic administration data was recorded. Participants >6 months of age, admitted within 1 day of screening or within 3 days in the case of a weekend, with self-reported or measured fever ≥38.0°C of >1 day duration were approached for enrollment into a prospective cohort. Written informed consent was given by the participant, or in the case of children <18 years of age, by the guardian, and with assent from the child if between 12 and 18 years of age. Inpatient transfers from other hospitals, minor-age orphans, patients unable to consent, and cases that involved a police investigation were excluded. This study was approved by the Byramjee Jeejeebhoy Government Medical College–Sassoon General Hospital ethics committee and the Johns Hopkins School of Medicine Institutional Review Board.
Diagnostic Testing and Antibiotic Administration
A study physician and social worker obtained a standardized clinical and social history at the time of enrollment from the participant or accompanying family member. The medical record was reviewed for admission diagnosis as written by the admitting clinician. Peripheral blood was sent for malaria antigen rapid test (SD Bioline, Gyeonggi-do, Republic of Korea) [12], parasite smear, and chikungunya immunoglobulin M (IgM) combo rapid test (CTK Onsite, San Diego, California) [13], which were performed on the day of collection and reported to clinicians by the next day. As previously reported, bacterial blood cultures were systematically collected [14]. Positive results were provided to clinicians by a phone call from the study clinician. Samples were stored for dengue early enzyme-linked immunosorbent assay nonstructural protein 1 (NS1) antigen capture (PanBio, Brisbane, Australia) [15], which was batch-tested every 2 weeks; results were not available to clinicians in real time and are therefore not the focus of this study. In addition to results of laboratory testing performed as part of the study protocol, the study team abstracted results of laboratory tests ordered by treating clinicians from the medical record, which included malaria rapid antigen rapid test and smear, dengue NS1 antigen, dengue IgM, chikungunya, and culture of blood, urine, cerebrospinal fluid (CSF), and sputum with speciation. At either discharge or day 7 of enrollment, whichever came first, the study physician reviewed the medical record and recorded medication administration records for the admission.
Definitions and Analysis
Participants ≥12 years of age are admitted to adult wards at Sassoon General Hospital and were considered adults in our analyses. Age was divided into 5 brackets for analysis. Antibiotics were classified into 4 tiers according to prior work (Supplementary Table) [16]. Antibiotics started on the first calendar day of admission were defined as empiric antibiotics. Only inpatient antibiotic days were collected. Highest-tier antibiotic usage on calendar day 5 of admission was compared to the admission day to determine antibiotic use outcomes. Escalation was defined as use of a higher tier antibiotic on follow-up than admission. De-escalation was defined as use of a lower-tier antibiotic on follow-up than admission. Discontinuation was defined as cessation of antibiotic therapy at follow-up after having started empiric antibiotics. Dengue, malaria, or chikungunya laboratory testing results including study protocol driven and clinician-ordered tests were assessed if the tests were performed before day 4 of admission. Bacterial cultures collected within the first 2 days of admission were considered for analysis.
Clinical and demographic factors were assessed for association with antibiotic use outcomes using Fisher exact tests. Clinical factors demonstrating individual association with antibiotic use outcomes (P < .05) and demographic factors regardless of significance were included in multivariable logistic regression models. Propensity score–matched analysis was utilized to estimate the additional role of confirmed dengue and malaria diagnosis beyond initial clinical suspicion and basic laboratory testing by comparing participants who had similar clinical characteristics other than testing positive for dengue or malaria [17]. Participants were matched based on demographics, clinical symptoms, basic laboratory findings, illness severity, and admission clinical impression of mosquito-borne disease. Participants with a positive dengue or malaria diagnosis were matched in a 1:1 ratio to participants without positive dengue or malaria diagnosis using nearest neighbor matching with caliper specified to 0.5 times the propensity score standard deviation logit. A Cox proportional hazard model was used to compare antibiotic discontinuation between participants with positive malaria or dengue tests and propensity score–matched participants without malaria or dengue diagnosis. Antibiotic use was censored at the day of follow-up for participants who remained on antibiotics at the time. Statistical analyses were performed using R software version 3.2.1 (http://www.cran.r-project.org).
RESULTS
Study Participants and Admission Diagnosis
A total of 1598 participants were enrolled during the study period; follow-up yielded antibiotic prescription details for 1486 (93%) participants, including 831 (56%) adults and 655 (44%) children <12 years of age (Table 1). Three hundred nineteen (22%) participants reported taking antibiotics within the week prior to admission. The most common nonexclusive diagnoses given by clinicians at enrollment were pneumonia, dengue, meningitis, and malaria, which accounted for 65% of all participants (Table 1). Initial intensive care admission was required for 30 (4%) adults and 195 (30%) children.
Table 1.
Clinical Characteristics by Empiric Antibiotic Usage
| Clinical Characteristic | Entire Population
(n = 1486) |
Received Empiric Antibiotics
(n = 1297) |
No Empiric Antibiotics
(n = 189) |
P Value |
|---|---|---|---|---|
| Male | 855 (58) | 726 (56) | 129 (68) | <.01 |
| Age, y | ||||
| <5 | 431 (29) | 410 (32) | 21 (11) | <.01 |
| 5–11 | 224 (15) | 204 (16) | 20 (11) | <.01 |
| 12–23 | 270 (18) | 215 (17) | 55 (29) | Ref |
| 24–59 | 466 (31) | 385 (30) | 81 (43) | .31 |
| ≥60 | 95 (6) | 83 (6) | 12 (6) | .1 |
| Low income | 559 (38) | 496 (38) | 63 (33) | .2 |
| Received antibiotics in the past week | 319 (21) | 288 (22) | 31 (16) | .07 |
| HIV infection | 127 (16) | 111 (17) | 16 (12) | .2 |
| Diabetes | 49 (4) | 40 (4) | 9 (5) | .41 |
| Altered mental status | 213 (14) | 198 (15) | 15 (8) | <.01 |
| Bilirubin >1.8 mg/dL | 97 (7) | 84 (7) | 13 (7) | .87 |
| Creatinine >1.4 mg/dL | 124 (8) | 115 (9) | 9 (5) | .07 |
| Leukocytosis | 458 (31) | 430 (34) | 28 (15) | <.01 |
| Admitted to ICU | 225 (15) | 220 (17) | 5 (3) | <.01 |
| Admission diagnosis | ||||
| Mosquito-borne disease | 253 (17) | 187 (14) | 66 (35) | <.01 |
| Dengue | 129 (9) | 86 (7) | 43 (23) | <.01 |
| Malaria | 71 (5) | 55 (4) | 16 (8) | <.01 |
| Mixed mosquito-borne disease | 21 (1) | 18 (1) | 3 (2) | .42 |
| Pneumonia | 260 (17) | 253 (20) | 7 (4) | .01 |
| Meningitis | 109 (7) | 104 (8) | 5 (3) | .18 |
| Gastroenteritis/ enterocolitis | 81 (5) | 77 (6) | 4 (2) | .27 |
| No diagnosis | 487 (33) | 399 (31) | 88 (47) | <.01 |
| URTI | 71 (5) | 68 (5) | 3 (2) | .22 |
| Multiple | 53 (4) | 51 (4) | 2 (1) | .24 |
| Other | 204 (14) | 186 (14) | 18 (10) | Ref |
Data are presented as No. (%) of patients. P values were calculated using Fisher exact tests with the exception of age and admission diagnosis, which were assessed as categorical variables with logistic regression.
Abbreviations: HIV, human immunodeficiency virus; ICU, intensive care unit; URTI, upper respiratory tract infection.
Empiric Antimicrobial Therapy
On admission, 683 (82%) adults and 614 (94%) children received empiric antibiotics (Table 1). Prescribing rates differed by age, with participants <5 years of age having the highest rate (95%), followed by participants 5–11 years of age (91%), ≥60 years of age (87%), and 24–59 years of age (83%). Participants suspected of having pneumonia and meningitis were more likely to receive empiric antibiotics than participants not suspected of having pneumonia or meningitis (P < .01; Table 1). Participants suspected of having dengue or malaria were less likely to receive empiric antibiotics (P < .01 and P = .03, respectively). In a multivariable model adjusted for age, altered mental status, leukocytosis, and ICU admission, clinical impression of dengue or malaria was associated with decreased empiric antibiotic prescription (adjusted odds ratio [OR], 0.5; 95% confidence interval [CI], .4–.8; P < .01).
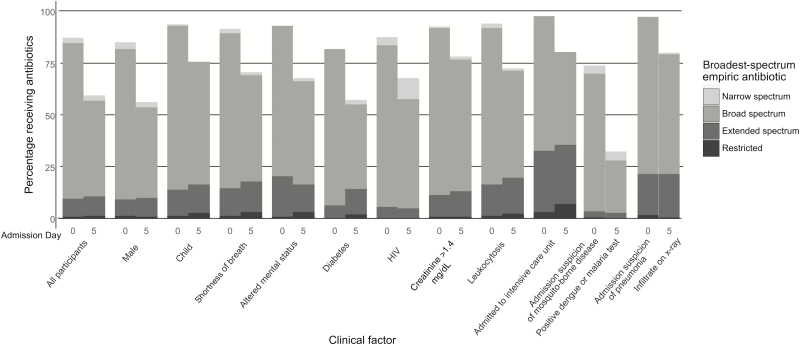
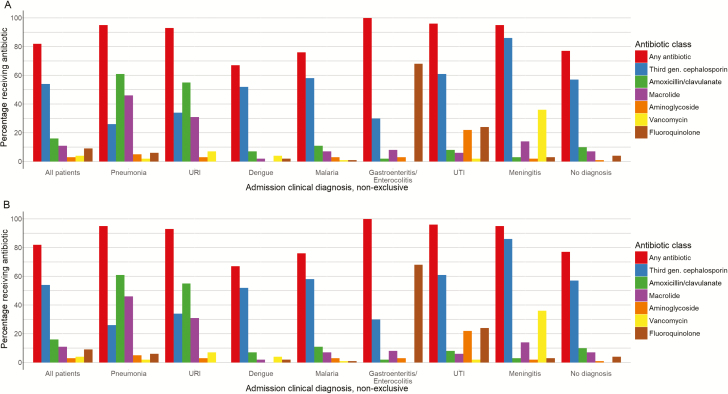
Narrow-spectrum antibiotics were started in 392 (26%) participants, broad-spectrum antibiotics were started in 1250 (84%) of participants, extended-spectrum antibiotics were started in 135 (9%) participants, and restricted antibiotics were started in 13 (1%) participants (Figure 1) including 460 (31%) participants who received antibiotics from multiple classes. Third-generation cephalosporins were the most commonly prescribed antibiotics overall, given to 774 (52%) participants, followed by amoxicillin/clavulanate (413 [28%]) and macrolides (302 [20%]) (Figure 2A and 2B).
Figure 1.
Empiric and day 5 antibiotic administration by spectrum and clinical factor, admission diagnosis, or test result. Abbreviation: HIV, human immunodeficiency virus.
Figure 2.
Empiric antibiotic administration by diagnosis for 831 adults (A) and 655 children (B) with acute febrile illness. Abbreviations: URI, upper respiratory infection; UTI, urinary tract infection.
Follow-up and Diagnostic Test Results
Median follow-up was performed on day 6 of admission (interquartile range [IQR], 4–7 days); median length of stay was 5 days (IQR, 3–8 days). Results of diagnostic tests have been previously reported [14, 18]. Mosquito-borne disease testing performed prior to admission day 5 was positive for 148 of 393 (38%) participants tested for dengue, 93 of 1448 (6%) participants tested for malaria, and 29 of 1292 (2%) participants tested for chikungunya. There were 17 participants with positive results for >1 mosquito-borne disease. Among participants without a positive dengue result, 92 (7%) were later found to have a positive dengue test either by clinician-directed testing later in the admission or via testing of stored specimens. Chest radiographs revealed an infiltrate in 179 of 381 (47%) participants. CSF analysis revealed pleocytosis in 64 of 226 (28%) participants. Bacterial cultures collected within the first 2 days of admission and reported within the first 5 days of admission included respiratory cultures (positive in 13 of 60 [22%] participants), blood cultures (positive in 46 of 1466 [3%] participants), and urine cultures (positive in 29 of 143 [20%] participants). Among the 251 patients with positive mosquito-borne testing before day 5, 6 (2%) had community-associated culture-positive bacterial infections.
Follow-up Antibiotic Administration
Most participants received >1 antibiotic during their hospital admission (Supplementary Figure 1). At day 5 of admission, 881 (59%) patients were receiving antibiotics, including 34 (2%) patients who started antibiotics, 744 (50%) patients who remained on the same level of antibiotic therapy, 66 (4%) who escalated therapy, and 37 (2%) who de-escalated therapy, but remained on antibiotics. There were 605 (41%) patients not receiving antibiotics on day 5, including 129 (9%) who had never received antibiotics, 26 (2%) who started and then stopped antibiotics, and 450 (30%) who discontinued antibiotics.
Positive bacterial culture results were associated with antibiotic therapy escalation (OR, 2.5; 95% CI, 1.2–4.8), which remained significant in a model adjusted for age, leukocytosis, and ICU admission (OR, 2.3; 95% CI, 1.1–4.5). Among the 37 participants whose antibiotic therapy was de-escalated, bacterial cultures were positive in 2 (5%) participants, similar to the 73 (6%) positive cultures among 1260 participants whose therapy was not de-escalated (P = .98).
Among the 1297 participants started on empiric antibiotics, 450 (35%) had been discontinued by day 5 of admission (Figure 1). Participants aged 12–23 years were most likely to discontinue antibiotics by day 5 (Table 2). Participants with leukocytosis and ICU admission were less likely to discontinue antibiotic therapy. Negative culture results were associated with decreased likelihood of antibiotic discontinuation. Absence of CSF pleocytosis or infiltrate on chest radiograph among participants in which these tests were performed was not associated with antibiotic discontinuation. Positive tests for dengue or malaria, but not chikungunya, were associated with antibiotic discontinuation. In a model adjusted for markers of disease severity including leukocytosis and ICU admission, positive test results for dengue or malaria were associated with increased odds of antibiotic discontinuation (OR, 1.7; 95% CI, 1.2–2.4).
Table 2.
Clinical Characteristics and Test Results Associated With Discontinuation of Antibiotic Therapy by Day 5 Among Patients Started on Empiric Therapy
| Clinical Characteristic | Received Empiric Antibiotics
(n = 1297) |
Discontinued
(n = 450) |
Continued
(n = 847) |
OR
(95% CI) |
P Value | AOR
(95% CI) |
P Value |
|---|---|---|---|---|---|---|---|
| Male sex | 726 (56) | 268 (60) | 458 (54) | 1.3 (1–1.6) | .06 | 1.1 (.9–1.5) | .28 |
| Age, y | |||||||
| <5 | 410 (32) | 86 (19) | 324 (38) | 0.2 (.2–.3) | <.01 | 0.3 (.2–.4) | <.01 |
| 5–11 | 204 (16) | 45 (10) | 159 (19) | 0.2 (.2–.4) | <.01 | 0.3 (.2–.4) | <.01 |
| 12–23 | 215 (17) | 118 (26) | 97 (11) | Ref | … | … | … |
| 24–59 | 385 (30) | 181 (40) | 204 (24) | 0.7 (.5–1) | .06 | 0.8 (.5–1.1) | .13 |
| ≥60 | 83 (6) | 20 (4) | 63 (7) | 0.3 (.1–.5) | <.01 | 0.3 (.2–.6) | <.01 |
| Altered mental status | 198 (15) | 59 (13) | 139 (16) | 0.8 (.5–1.1) | .12 | … | … |
| Cough | 626 (48) | 188 (42) | 438 (52) | 0.7 (.5–.8) | <.01 | 0.9 (.7–1.2) | .56 |
| Leukocytosis | 430 (34) | 109 (24) | 321 (38) | 0.5 (.4–.7) | <.01 | 0.7 (.6–1) | .04 |
| CSF pleocytosisa | 70 (33) | 13 (27) | 57 (35) | 0.7 (.3–1.5) | .38 | … | … |
| Infiltrate on chest radiographb | 173 (48) | 35 (47) | 138 (48) | 1 (.6–1.7) | .9 | … | … |
| Admitted to ICU | 288 (22) | 56 (12) | 232 (27) | 0.4 (.3–.5) | <.01 | 0.6 (.4–.9) | <.01 |
| Negative culture | |||||||
| Respiratory | 45 (3) | 15 (3) | 30 (4) | 0.9 (.5–1.8) | 1 | … | … |
| Urine | 110 (8) | 36 (8) | 74 (9) | 0.9 (.6–1.4) | .68 | … | … |
| CSF | 97 (7) | 21 (5) | 76 (9) | 0.5 (.3–.8) | <.01 | … | … |
| Negative respiratory, urine, or CSF culture | 240 (19) | 67 (15) | 173 (20) | 0.7 (.5–.9) | .02 | 0.7 (.5–.9) | .01 |
| Positive test, first 5 d of admission | |||||||
| Dengue or malaria | 162 (12) | 92 (20) | 70 (8) | 2.8 (2–4) | <.01 | 1.7 (1.2–2.4) | .01 |
| Chikungunya | 21 (2) | 6 (1) | 15 (2) | 0.7 (.2–2.1) | .65 | … | … |
| Dengue | 96 (7) | 59 (13) | 37 (4) | 3.3 (2.1–5.2) | <.01 | … | … |
| Malaria | 71 (5) | 35 (8) | 36 (4) | 1.9 (1.1–3.2) | .01 | … | … |
Data are presented as No. (%) of patients; unadjusted ORs and associated P values were calculated using Fisher exact tests, with the exception of age, which was assessed as a categorical variable with logistic regression; AORs and associated P values were calculated using a multivariable logistic regression model.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; CSF, cerebrospinal fluid; ICU, intensive care unit; OR, odds ratio; Ref, reference.
aLumbar puncture performed on 212 participants.
bChest radiography performed on 360 participants.
Propensity Score Matching to Compare Impact of a Confirmed Versus a Suspected Diagnosis of Malaria or Dengue
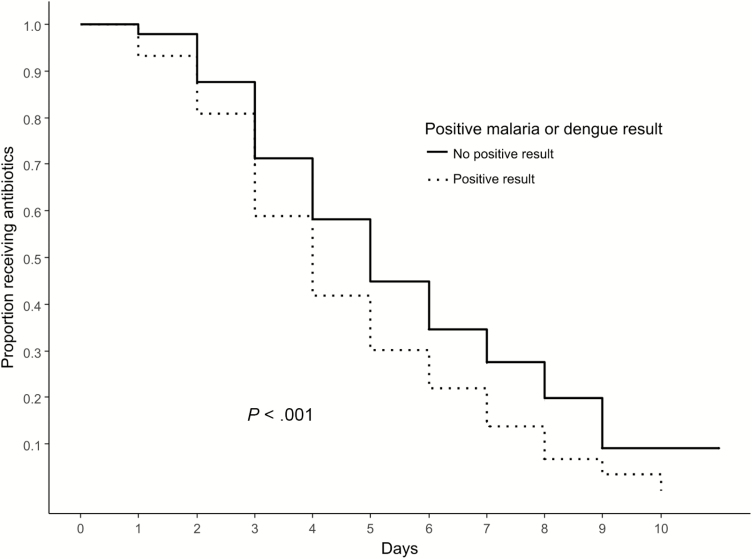
Among the 162 participants who were given empiric antibiotic therapy and had a positive malaria or dengue test prior to day 5 of admission, 146 (90%) participants without a positive malaria or dengue test result were matched using propensity scores which included initial clinical impression of dengue or malaria (Table 3). Clinicians were more likely to discontinue antibiotics among participants with a positive test for dengue or malaria in the propensity score–matched model (Cox proportional hazard ratio, 1.6; 95% CI, 1.2–2.0; P < .001; Figure 3).
Table 3.
Comparison of Clinical Characteristics Among Participants With Malaria or Dengue Diagnosis and Propensity Score–Matched Participants Without Dengue or Malaria Diagnosis
| Clinical Characteristic | All Patients
(N = 292) |
Positive Dengue or Malaria Test (n = 146) | No Positive Dengue or Malaria Test (n = 146) | P Value |
|---|---|---|---|---|
| Male sex | 193 (66) | 99 (68) | 94 (64) | .62 |
| Age, y | ||||
| <5 | 21 (7) | 11 (8) | 10 (7) | .59 |
| 5–11 | 44 (15) | 18 (12) | 26 (18) | .58 |
| 12–23 | 98 (34) | 45 (31) | 53 (36) | Ref |
| 24–59 | 117 (40) | 65 (45) | 52 (36) | .16 |
| ≥60 | 12 (4) | 7 (5) | 5 (3) | .42 |
| Low income | 78 (27) | 36 (25) | 42 (29) | .51 |
| Hospitalized within the past 3 months | 25 (9) | 12 (8) | 13 (9) | 1 |
| Received antibiotics in the past week | 67 (23) | 34 (23) | 33 (23) | 1 |
| HIV | 7 (2) | 4 (3) | 3 (2) | 1 |
| Symptoms | ||||
| Altered mental status | 34 (12) | 15 (10) | 19 (13) | .58 |
| Rigors | 252 (86) | 132 (90) | 120 (82) | .06 |
| Cough | 68 (23) | 37 (25) | 31 (21) | .49 |
| Diarrhea | 34 (12) | 17 (12) | 17 (12) | 1 |
| Headache | 123 (42) | 65 (45) | 58 (40) | .48 |
| White blood cell count, median (IQR) | 5.6 (3.7–9.1) | 5.5 (3.9–8.6) | 6 (3.6–9.9) | .36 |
| Platelets, median (IQR) | 90 (36–155) | 85.5 (36.2–149.8) | 90 (35.5–159.2) | .72 |
| Admitted to ICU | 24 (8) | 11 (8) | 13 (9) | .83 |
| Admission suspicion of mosquito-borne disease | 121 (41) | 66 (45) | 55 (38) | .23 |
Data are presented as No. (%) of patients unless otherwise indicated. P values were calculated using Fisher exact tests, with the exception of age, which was assessed as a categorical variable with logistic regression.
Abbreviations: HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range.
Figure 3.
Antibiotic discontinuation among propensity score–matched participants started on empiric antibiotics by malaria or dengue test result.
DISCUSSION
In this study, we analyzed the antibiotic utilization of 1486 patients admitted with AFI to a public tertiary care hospital in Pune, India, for patterns that could have important implications for the design of effective antibiotic stewardship. We found, first, that despite the preponderance of nonbacterial illness, in the context of diagnostic uncertainty, almost every patient hospitalized with AFI received antibiotics. Second, physicians were twice as likely to avoid empiric antibiotic therapy in patients they suspected to have a mosquito-borne disease. Third, positive bacteriology results were associated with antibiotic escalation, but negative bacteriology results were not associated with antibiotic discontinuation. Finally, clinicians discontinued antibiotics more rapidly in patients diagnosed with mosquito-borne disease, even after adjustment for initial clinical suspicion.
Nearly every patient admitted with AFI received antibiotics, which were mostly broad spectrum. Similar to other studies conducted in India, most patients received multiple antibiotics [5, 6]. In resource-rich settings such as the United States, 50%–60% of inpatients receive antibiotics [19]. Most febrile inpatients in resource-rich settings also receive antibiotics, including >78% of children [20]. Because only 6% of patients had culture-positive bacterial infection and nonbacterial mosquito-borne diseases were the most common cause of illness [18], it is likely that a large portion of antibiotic use was unnecessary. However, the majority of antibiotics were started empirically, on the day of admission, when few test results were available. Empiric antibiotic use that was ultimately deemed unnecessary was frequently appropriate. Most patients eventually confirmed to have mosquito-borne disease had admission platelet, creatinine, and bilirubin values that would fulfill criteria for sepsis [21]. When clinicians suspected a diagnosis of a mosquito-borne disease, they were twice as likely to avoid empiric antibiotic therapy, even when adjusted for measures of disease severity.
Implementation of more sensitive and rapid identification of bacterial infection and antibiotic susceptibility has been promoted as necessary to reduce and narrow antibiotic use [16, 22]. In US hospitals, performance of bacterial cultures was associated with narrowing or discontinuing empiric antibiotics, which occurred in 29% of patients by day 5 [16]. We found that although positive cultures were associated with antibiotic escalation, they were not associated with antibiotic de-escalation, and negative cultures were not associated with discontinuation. As previously reported [14], the majority of isolates in this study population were multidrug resistant, which may prohibit therapy narrowing for patients with positive cultures.
Participants who had a positive test for malaria or dengue before day 5 were more likely to discontinue antibiotics. Determining the additional impact of pathogen-specific testing for malaria or dengue on antibiotic prescribing practices beyond clinical intuition is complicated by the association of clinician suspicion of mosquito-borne disease and decreased antibiotic prescription. Using a propensity score–adjusted model, we were able to compare participants who, other than receiving a positive dengue or malaria test, were similar in their presenting symptoms, basic laboratory findings, and initial suspicion of mosquito-borne disease. The finding that propensity score–matched participants with a positive dengue or malaria test discontinued antibiotics more rapidly suggests that pathogen-specific testing for malaria and dengue has an impact on the decision to stop antibiotics beyond clinical suspicion and basic laboratory testing.
The result that testing for mosquito-borne illnesses reduced antibiotic use is qualitatively different than prior reports of the impact of rapid malaria diagnostic tests, which found that their introduction was associated with a 21% increase in antibiotic prescriptions [23]. Patients in these trials who tested negative for malaria were more likely to be prescribed antibiotics, driving an overall increase in antibiotic use. However, the included trials were conducted in sub-Saharan Africa and Afghanistan, where the overall antibiotic use of 56% is far lower than we report and what has previously been reported in India [5, 6]. Studies of antibiotic use in Africa may not be generalizable to Asian settings where antibiotic use is more common and arboviruses are more prevalent [4, 24]. In this study, identifying nonbacterial infection reduced antibiotic use, whereas negative culture results for bacterial infection did not. In settings where antibiotic use for AFI is near universal, and identifiable nonbacterial illnesses are more common, a counterintuitive approach of providing more rapid and sensitive diagnostics for nonbacterial illness may reduce unnecessary antibiotic use more effectively than bolstering bacteriology laboratory capacity.
Our study has several limitations. It was conducted at a single, public, academic medical center, where limited resources may restrict use of costly antibiotics and academic scrutiny may encourage more judicious antibiotic use compared to private hospitals. Additionally, our study design did not allow us to discern the date on which clinicians reviewed laboratory results, relying on the date when laboratory reports were generated. At the time of enrollment, many participants were suspected of having multiple diagnoses. In a setting where most adults and older children have some degree of malaria immunity, patients who require admission for malaria may have other complications. In other Asian settings, some hospitalized patients with malaria also have bacteremia, possibly due to gut microthrombi causing ischemia and bacterial translocation [25]. Therefore, even in a case of confirmed malaria, initial empiric antibiotics may not always be inappropriate, as the failure to institute timely antibiotics may result in preventable mortality.
We have shown that physicians in a public Indian hospital setting start empiric antibiotics less frequently when they suspect mosquito-borne disease and, when given results of a positive dengue or malaria test, they discontinue antibiotics more quickly. Because we found that initial clinical impression, largely formed without pathogen-specific testing, and pathogen-specific diagnostics have independent impacts on antibiotic prescribing behavior, rapid diagnostics identifying nonbacterial illness have the potential to reduce empiric antibiotic use. Future work is necessary to determine the impact of rapid diagnostics for diseases other than malaria on empiric antibiotic prescription. Informed with more data on the impact of diagnostic speed and sensitivity on antibiotic prescribing practices, the cost-effectiveness of competing diagnostic approaches must be evaluated. Since the release of the Chennai Declaration—“a roadmap to tackle the challenge of antimicrobial resistance”—and the completion of this study, the Indian National Center for Disease Control has released the first national treatment guidelines for antimicrobial use in infectious diseases [26, 27]. Using these guidelines, institutions in India can work toward limiting antibiotic use where possible and focusing therapy to curtail the emergence and spread of antibiotic resistance.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study participants and staff for their immense contribution. We thank Purva Ranchal for helping draft sections of the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the Ujala Foundation, Newton Square, PA; Wyncote Foundation; Sacharuna Foundation; Gilead Foundation; the NIH Baltimore-Washington-India HIV Clinical Trials Unit (grant number UM1AI069465); NIH Research Training Grants (grant numbers R25 TW009340 funded by the Fogarty International Center and the NIH Office of the Director and Office of AIDS Research and UM1AI104681 and T32 AI007291 funded by the National Institute of Allergy and Infectious Diseases); and NIH Fogarty International Center (grant number D43TW009574).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Antimicrobial resistance: global report on surveillance. 2014. [Google Scholar]
- 2. Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. Emergence of high levels of extended-spectrum-beta-lactamase-producing gram-negative bacilli in the Asia-Pacific region: data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrob Agents Chemother 2009; 53:3280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganguly NK, Arora NK, Chandy SJ et al. ; Global Antibiotic Resistance Partnership (GARP)–India Working Group Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res 2011; 134:281–94. [PMC free article] [PubMed] [Google Scholar]
- 4. Van Boeckel TP, Gandra S, Ashok A et al. . Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14:742–50. [DOI] [PubMed] [Google Scholar]
- 5. Indira Kumari KS, Chandy SJ, Jeyaseelan L, Kumar R, Suresh S. Antimicrobial prescription patterns for common acute infections in some rural and urban health facilities of India. Indian J Med Res 2008; 128:165–71. [PubMed] [Google Scholar]
- 6. Blomberg M, Blomberg Jensen M, Henry A et al. . Antimicrobial drug use in a small Indian community hospital. Trop Doct 2010; 40:194–8. [DOI] [PubMed] [Google Scholar]
- 7. Chandy SJ, Thomas K, Mathai E, Antonisamy B, Holloway KA, Stalsby Lundborg C. Patterns of antibiotic use in the community and challenges of antibiotic surveillance in a lower-middle-income country setting: a repeated cross-sectional study in Vellore, South India. J Antimicrob Chemother 2013; 68:229–36. [DOI] [PubMed] [Google Scholar]
- 8. Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: drivers and opportunities for action. PLoS Med 2016; 13:e1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Hemelrijck MJ, Lindblade KA, Kubaje A et al. . Trends observed during a decade of paediatric sick visits to peripheral health facilities in rural western Kenya, 1997–2006. Trop Med Int Health 2009; 14:62–9. [DOI] [PubMed] [Google Scholar]
- 10. Salvi S, Apte K, Madas S et al. . Symptoms and medical conditions in 204 912 patients visiting primary health-care practitioners in India: a 1-day point prevalence study (the POSEIDON study). Lancet Glob Health 2015; 3:e776–84. [DOI] [PubMed] [Google Scholar]
- 11. Walia K, Ohri VC, Mathai D; Antimicrobial Stewardship Programme of ICMR Antimicrobial stewardship programme (AMSP) practices in India. Indian J Med Res 2015; 142:130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ratsimbasoa A, Fanazava L, Radrianjafy R, Ramilijaona J, Rafanomezantsoa H, Ménard D. Evaluation of two new immunochromatographic assays for diagnosis of malaria. Am J Trop Med Hyg 2008; 79:670–2. [PubMed] [Google Scholar]
- 13. Yap G, Pok KY, Lai YL et al. . Evaluation of chikungunya diagnostic assays: differences in sensitivity of serology assays in two independent outbreaks. PLoS Negl Trop Dis 2010; 4:e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mave V, Chandanwale A, Kagal A et al. . High burden of antimicrobial resistance and mortality among adults and children with community-onset bacterial infections in India. J Infect Dis 2017; 215:1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aryati A, Trimarsanto H, Yohan B, Wardhani P, Fahri S, Sasmono RT. Performance of commercial dengue NS1 ELISA and molecular analysis of NS1 gene of dengue viruses obtained during surveillance in Indonesia. BMC Infect Dis 2013; 13:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Braykov NP, Morgan DJ, Schweizer ML et al. . Assessment of empirical antibiotic therapy optimisation in six hospitals: an observational cohort study. Lancet Infect Dis 2014; 14:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bai AD, Showler A, Burry L et al. . Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015; 60:1451–61. [DOI] [PubMed] [Google Scholar]
- 18. Robinson M, Balasubramanian U, Raichur P et al. . Etiology of acute febrile illness among hospitalized adults and children at a public tertiary-care center in Pune, India. In: IDWeek, New Orleans, LA, 2016. [Google Scholar]
- 19. Magill SS, Edwards JR, Beldavs ZG et al. . Prevalence of antimicrobial use in us acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Irwin AD, Grant A, Williams R et al. . Predicting risk of serious bacterial infections in febrile children in the emergency department. Pediatrics 2017; 140. [DOI] [PubMed] [Google Scholar]
- 21. Singer M, Deutschman CS, Seymour CW et al. . The Third International Consensus Definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caliendo AM, Gilbert DN, Ginocchio CC et al. ; Infectious Diseases Society of America (IDSA) Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 2013; 57(Suppl 3):S139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hopkins H, Bruxvoort KJ, Cairns ME et al. . Impact of introduction of rapid diagnostic tests for malaria on antibiotic prescribing: analysis of observational and randomised studies in public and private healthcare settings. BMJ 2017; 356:j1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhatt S, Gething PW, Brady OJ et al. . The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nyein PP, Aung NM, Kyi TT et al. . High frequency of clinically significant bacteremia in adults hospitalized with falciparum malaria. Open Forum Infect Dis 2016; 3:ofw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghafur A, Mathai D, Muruganathan A et al. . The Chennai Declaration: a roadmap to tackle the challenge of antimicrobial resistance. Indian J Cancer 2013; 50:71–3. [DOI] [PubMed] [Google Scholar]
- 27. National Centre for Disease Control. National treatment guidelines for antimicrobial use in infectious diseases. New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare Government of India, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.