One component of HPTN 065 examined the feasibility of universally offering HIV testing to emergency department and hospital admissions community-wide in the Bronx, New York and Washington, DC. All hospitals identified new HIV diagnoses, but none achieved universal coverage.
Keywords: routine HIV screening, HIV testing in emergency departments, HIV testing of hospital inpatients
Abstract
Background
Human immunodeficiency virus (HIV) testing is critical for both HIV treatment and prevention. Expanding testing in hospital settings can identify undiagnosed HIV infections.
Methods
To evaluate the feasibility of universally offering HIV testing during emergency department (ED) visits and inpatient admissions, 9 hospitals in the Bronx, New York and 7 in Washington, District of Columbia (DC) undertook efforts to offer HIV testing routinely. Outcomes included the percentage of encounters with an HIV test, the change from year 1 to year 3, and the percentages of tests that were HIV-positive and new diagnoses.
Results
From 1 February 2011 to 31 January 2014, HIV tests were conducted during 6.5% of 1621016 ED visits and 13.0% of 361745 inpatient admissions in Bronx hospitals and 13.8% of 729172 ED visits and 22.0% of 150655 inpatient admissions in DC. From year 1 to year 3, testing was stable in the Bronx (ED visits: 6.6% to 6.9%; inpatient admissions: 13.0% to 13.6%), but increased in DC (ED visits: 11.9% to 15.8%; inpatient admissions: 19.0% to 23.9%). In the Bronx, 0.4% (408) of ED HIV tests were positive and 0.3% (277) were new diagnoses; 1.8% (828) of inpatient tests were positive and 0.5% (244) were new diagnoses. In DC, 0.6% (618) of ED tests were positive and 0.4% (404) were new diagnoses; 4.9% (1349) of inpatient tests were positive and 0.7% (189) were new diagnoses.
Conclusions
Hospitals consistently identified previously undiagnosed HIV infections, but universal offer of HIV testing proved elusive.
Testing to identify human immunodeficiency virus (HIV) infection is an essential first step for both treatment and prevention [1]. With sustained viral suppression, persons living with HIV can have a nearly normal life expectancy and substantially reduce their risk of transmitting HIV [2, 3]. Public health authorities in the United States recommend routine HIV screening in healthcare settings and targeted testing for persons at increased risk to facilitate the diagnosis of unrecognized HIV infection [4, 5]. Numerous studies have demonstrated high levels of testing and the yield of new HIV diagnoses from emergency department (ED) screening programs [6–12], but few have examined routine HIV screening among hospital inpatients [13–15] and none have involved whole communities.
The HIV Prevention Trials Network (HPTN) 065 study was conceived to evaluate the feasibility of an enhanced community-level test and link to care plus treat strategy in the United States. The study included several components designed to expand HIV testing, to evaluate the effectiveness of financial incentives for linkage to care and viral suppression and a computer-based prevention intervention for HIV-infected persons in care, and surveys of providers and patients to assess attitudes about antiretroviral therapy for prevention [16–18]. We describe here the expanded hospital testing component, which sought to achieve the universal offer of HIV testing during ED visits and inpatient admissions.
METHODS
Study Facilities
Two communities, the Bronx, New York (Bronx) and Washington, District of Columbia (DC), were selected for participation in HPTN 065 because of their high estimated HIV prevalence (1.7% and 2.3%, respectively) and existence of initiatives instituted by the cities’ health departments to increase HIV testing and linkage to care [19–21]. Sixteen hospitals (9 in the Bronx and 7 in DC) agreed to participate. In DC, 1 hospital did not undertake ED testing and 1 did not conduct inpatient testing. All but 2 hospitals (1 Veterans Administration facility in each community) received some health department funding to support HIV testing before and during the 3-year study period.
Intervention
The study was an uncontrolled prospective observational evaluation of hospital-level interventions to encourage staff to offer HIV testing to all ED patients and inpatients as part of usual clinical practice, consistent with Centers for Disease Control and Prevention (CDC) recommendations [4]. The study supported a testing coordinator and part-time data manager at each hospital and provided some additional funding that could be used, at the hospital’s discretion, for activities to promote HIV screening including the purchase of testing reagents and supplies, support for staff, or other activities related to HIV testing. Testing coordinators and study leaders at each hospital identified approaches to increase testing relevant for their institution. These included fostering administrative and clinical staff support, streamlining informed consent procedures, modifying electronic medical records to identify patients who had not been tested and developing prompts to encourage the order of HIV tests, incorporating HIV testing as part of routine admission orders, and shifting from point-of-care rapid testing by dedicated staff to centralized, high volume, fast-turnaround testing in the hospital laboratory. An important part of the strategy was to identify “champions” in key leadership roles who could promote the testing effort. Hospitals established their own testing goals and reported aggregate testing data monthly to a centralized database, and each participated in periodic study-specific conference calls to monitor progress, discuss challenges, and provide feedback.
Data Completeness
The study relied on the hospitals’ usual reporting mechanisms for data collection. Most facilities provided a fairly complete record of HIV tests, some recorded manually. However, few existing systems could record the overall number of HIV tests offered or declined, and some facilities could not provide data for all months throughout the study period on the number of ED or inpatient admissions or the number of positive tests that represented new HIV diagnoses. Therefore, we excluded monthly observations from facilities for those months with incomplete data for the calculation of percentages of HIV tests, positive HIV tests, and new diagnoses.
Outcomes
Because information on unique patient visits could not be obtained, the primary endpoint—change in testing from year 1 to year 3—was defined in terms of the percentage of ED encounters and inpatient admissions during which an HIV test was conducted. We analyzed data for the number and percentage of annual HIV tests during ED visits and inpatient admissions, the percentage of positive HIV tests, and percentage of new HIV diagnoses, defined as those for which there was no previous HIV diagnosis in the medical record or by patient self-report. We also assessed the total number of point-of-care and laboratory-based HIV tests conducted during each year of the study at each facility.
Statistical Analysis
The absolute difference from year 1 to year 3 in the percentage of ED visits and inpatient admissions during which an HIV test was conducted was computed for each hospital and compared, using a z test, to an absolute change of 5 percentage points. Facilities with a significant increase in testing of at least 5 percentage points were considered to have meaningfully improved HIV testing, and facilities with a significant decrease of at least 5 percentage points were considered to have meaningfully worsened. Facilities with <5 percentage point change in testing (increase or decrease) were classified as no meaningful change. Analyses were performed using SAS software version 9.3 (SAS Institute, Cary, North Carolina).
ETHICAL REVIEW
Because hospital HIV testing was already being conducted as public health practice for the benefit of individuals in the 2 communities, no additional intervention with patients was planned, and no personally identifying or study-specific information was collected, this component of the HPTN 065 protocol was deemed to be nonresearch by each site’s affiliated institutional review board.
RESULTS
From February 2011 through January 2014, there were 1621016 ED visits and 361745 inpatient admissions in the Bronx and 729172 ED visits and 150655 inpatient admissions in DC (Table 1). Of 324 total study months in the Bronx (36 months at 9 hospitals), ED data were complete, but 16 months (4.9%) of inpatient data were missing for new diagnoses. Of 252 total study months in DC (36 months at 7 hospitals), 9 months (3.7%) of ED data were missing for percentages of HIV tests, positive tests, and new diagnoses; for inpatient admissions, 48 months (19.0%) of data were missing for percentages of HIV tests and positive tests, and 84 months (33.3%) of data were missing for new diagnoses.
Table 1.
Human Immunodeficiency Virus Tests Conducted During Emergency Department Visits and Inpatient Admissions, by Community and Study Year
| Bronx, NY | Washington DC | |||||
|---|---|---|---|---|---|---|
| Emergency Department HIV Tests | Emergency Department HIV Tests | |||||
| Year | Visits N |
HIV Tests N (% of visits) | Positive HIV Tests N (% of HIV Tests) | Visits N | HIV Tests N (% of visits) | Positive HIV Tests N (% of HIV Tests) |
| 2/2011-1/2012 | 525,137 | 34,620 (6.6) | 153 (0.4) | 233,767 | 27,860 (11.9) | 172 (0.6) |
| 2/2012-1/2013 | 555,113 | 34,166 (6.2) | 122 (0.4) | 264,337 | 36,449 (13.8) | 161 (0.4) |
| 2/2013-1/2014 | 540,766 | 37,390 (6.9) | 133 (0.4) | 231,068 | 36,496 (15.8) | 285 (0.8) |
| Overall | 1,621,016 | 106,176 (6.5) | 408 (0.4) | 729,172 | 100,805 (13.8) | 618 (0.6) |
| Inpatient HIV Tests | Inpatient HIV Tests | |||||
| Year | Admissions N | HIV Tests N (% of admissions) | Positive HIV Tests N (% of HIV Tests) | Admissions N | HIV Tests N (% of admissions) | Positive HIV Tests N (% of HIV Tests) |
| 2/2011-1/2012 | 122,021 | 15,811 (13.0) | 230 (1.5) | 42,758 | 8,133 (19.0) | 519 (6.4) |
| 2/2012-1/2013 | 120,900 | 15,112 (12.5) | 251 (1.7) | 54,668 | 12,294 (22.5) | 561 (4.6) |
| 2/2013-1/2014 | 118,824 | 16,163 (13.6) | 366 (2.3) | 53,229 | 12,702 (23.9) | 540 (4.3) |
| Overall | 361,745 | 47,086 (13.0) | 847 (1.8) | 150,655 | 33,129 (22.0) | 1,620 (4.9) |
Abbreviation: HIV, Human Immunodeficiency Virus.
HIV Testing in EDs
HIV tests were conducted during 6.5% (106176) of ED visits in the Bronx hospitals and 13.8% (100805) of ED visits in the DC hospitals (Table 1), but testing varied widely by hospital. In the 9 Bronx hospitals, the total number of HIV tests per hospital ED during the 3-year period ranged from 527 to 27743, and the proportion of ED visits with an HIV test ranged from 2.0% to 12.5% (Supplementary Table 1). Among the 7 DC hospitals, the total number of HIV tests per hospital ED ranged from 2916 to 27160 and the proportion tested ranged from 4.8% to 39.3%. (Supplementary Table 2).
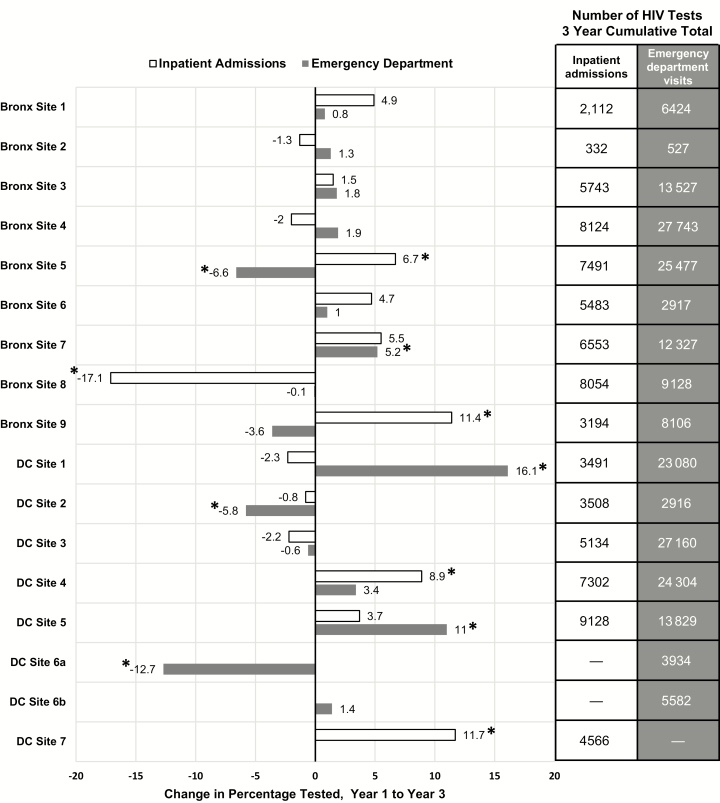
The percentage of ED visits with HIV testing showed little overall change from year 1 to year 3 in the Bronx hospitals (6.6% to 6.9%), but increased from 11.9% to 15.8% in the DC hospitals (Table 1). At individual hospitals in both the Bronx and DC, the change in the absolute percentage of ED visits with HIV testing over the 3 years showed a wide range (Figure 1). Over the study period, 3 EDs experienced a meaningful (>5%) increase in the absolute percentage of HIV tests, 3 experienced a meaningful decrease in the absolute percentage of tests, and 10 EDs showed little change (Figure 1).
Figure 1.
Absolute difference from year 1 to year 3 in the percentage of tests conducted during emergency department visits and inpatient admissions, by hospital, 2011–2014. *Denotes significant change in percentage tested of ≥5%. Abbreviations: Bronx, Bronx, New York; DC, Washington, District of Columbia; HIV, human immunodeficiency virus.
HIV Testing Among Inpatient Admissions
In Bronx hospitals overall, HIV tests were conducted during 13.0% (47086) of inpatient admissions, and in DC, during 22% (33129) (Table 1). The proportion of inpatient admission HIV tests ranged widely at individual hospitals, from 5.1% to 26.8% in the Bronx (Supplementary Table 1) and from 9.6% to 49.1% in DC (Supplementary Table 2). There was little change in the annual percentage of inpatient admissions with HIV testing in the Bronx hospitals (13.0% in year 1, 13.6% in year 3) but a small increase in DC, from 19.0% in year 1 to 23.9% in year 3 (Table 1). Similar to ED testing, the absolute change from year 1 to year 3 in the percentage of tests ranged widely (Figure 1). Four hospitals experienced meaningful increases in inpatient admission HIV testing from year 1 to year 3, and only 1 hospital experienced a meaningful decrease.
Positive HIV Tests
The overall percentage of HIV-positive tests was higher among inpatient admissions than among ED visits in both cities: 1.8% of inpatient tests vs 0.4% of ED tests in the Bronx, and 4.9% of inpatient tests vs 0.6% of ED tests in DC (Table 1), but many of these were from previously diagnosed patients (Table 2). The percentage of positive ED HIV tests remained stable at 0.4% over the 3 study years in the Bronx, but increased slightly from 0.6% in year 1 to 0.8% in year 3 in DC, while the percentage of positive inpatient admission HIV tests increased slightly in Bronx hospitals but decreased in DC (Table 1).
Table 2.
Percentage of Positive Human Immunodeficiency Virus (HIV) Tests and New HIV Diagnoses in Emergency Department Visits and Inpatient Admissions, by Community and Study Year
| Setting | Bronx, New York | Washington, District of Columbia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV Tests, No. | Positive HIV Tests, No. (% of HIV Tests) | New Diagnoses | HIV Tests, No. | Positive HIV Tests, No. (% of HIV Tests) | New Diagnoses | |||||
| No. | % of HIV Tests | % of Positive HIV Tests | No. | % of HIV Tests | % of Positive HIV Tests | |||||
| Emergency departments | ||||||||||
| Feb 2011–Jan 2012 | 34620 | 153 (0.4) | 119 | 0.34 | 77.8 | 27860 | 172 (0.6) | 133 | 0.48 | 77.3 |
| Feb 2012–Jan 2013 | 34166 | 122 (0.4) | 86 | 0.25 | 70.5 | 36449 | 161 (0.4) | 121 | 0.33 | 75.2 |
| Feb 2013–Jan 2014 | 37390 | 133 (0.4) | 72 | 0.19 | 54.1 | 36496 | 285 (0.8) | 150 | 0.41 | 52.6 |
| Overall | 106176 | 408 (0.4) | 277 | 0.26 | 67.9 | 100805 | 618 (0.6) | 404 | 0.40 | 65.4 |
| Inpatient admissions | ||||||||||
| Feb 2011–Jan 2012 | 14909 | 211 (1.4) | 78 | 0.53 | 37.0 | 6396 | 414 (6.5) | 91 | 1.50 | 22.0 |
| Feb 2012–Jan 2013 | 15112 | 251 (1.7) | 92 | 0.62 | 36.7 | 10659 | 467 (4.4) | 51 | 0.50 | 10.9 |
| Feb 2013–Jan 2014 | 16163 | 366 (2.3) | 71 | 0.45 | 19.4 | 10940 | 468 (4.3) | 47 | 0.45 | 10.0 |
| Overall | 46184 | 828 (1.8) | 241 | 0.53 | 29.1 | 27995 | 1349 (4.8) | 189 | 0.70 | 14.0 |
Excludes monthly observations from hospitals for months during which any data needed for calculation of percentage of new diagnoses are missing.
Abbreviation: HIV, human immunodeficiency virus.
New HIV Diagnoses
Analyses were restricted to the subset of HIV testing data for which information on new diagnoses was complete (Table 2). The overall prevalence of undiagnosed HIV among persons tested was lower in EDs (0.3% in the Bronx, 0.4% in DC) than among inpatients (0.5% in the Bronx and 0.7% in DC). However, more positive tests in the ED represented new HIV diagnoses in both communities (67.9% in the Bronx; 65.4% in DC) than positive tests among inpatient admissions (29.1% in the Bronx; 14.0% in DC). The percentages of positive tests that were new diagnoses decreased from year 1 to year 3 in both cities, for both ED tests and inpatient admission tests.
Laboratory-Based Testing
The percentage of all ED HIV tests that were conducted in the laboratory increased over the course of the study in both cities. In the Bronx, despite 4 hospitals that did not perform any laboratory-based tests on ED patients during the study, there was an overall 19.6 percentage point increase in laboratory-based testing, from 0.3% in year 1 to 19.9% in year 3 (Supplementary Table 1). In DC, the percentage of ED HIV tests that were performed in the laboratory increased from 0.3% in year 1 to 24.5% in year 3 (Supplementary Table 2). Among Bronx inpatient admissions, laboratory-based testing increased from 28.8% in year 1 to 55.8% in year 3 (Supplementary Table 1). The percentage of inpatient admission HIV tests conducted in the laboratory in DC was high at the start of the study (97.3%) but declined to 87.0% overall in year 3 due to large reductions in laboratory testing at 2 sites (Supplementary Table 2).
DISCUSSION
The HPTN 065 study sought to promote the universal offer of HIV testing during ED visits and inpatient admissions at participating hospitals in the Bronx, New York and Washington, District of Columbia. During 2350188 ED visits and 512400 inpatient admissions, >280000 HIV tests were conducted. Despite concerted efforts to increase testing, HIV tests were performed during <25% of all ED visits and inpatient admissions. However, the number of HIV tests performed and the percentage of visits and admissions with an HIV test varied widely among the hospitals. Over the 3-year study period, the percentage of encounters during which an HIV test was conducted showed only small increases in DC and little change in the Bronx. Consistent with numerous previous studies (summarized in [12]), hospitals in both communities successfully conducted HIV screening and consistently identified previously undiagnosed HIV infections, but were unable to perform testing during the majority of encounters. However, because many persons make repeated visits to the ED, consistently screening even a relatively modest number of patients for HIV over time can have important cumulative effects on increasing the overall proportion of persons who have been tested and newly diagnosed [22].
Participating hospitals attempted to implement different operational changes that have been shown to increase HIV testing, such as eliminating written consent, integrating the offer of HIV screening into the triage or intake process, automating identification of patients who should be tested, and real-time electronic reminders and clinical decision support tools [22–26]. Optimizing such processes can also reduce the cost of HIV screening programs [27]. However, the necessary modifications often required engagement and approval at several administrative levels, vied with other priorities for attention, and could be time-consuming and resource intensive. At several facilities, because of practical and logistical challenges, procedural changes proposed early in the study period were not adopted before the study ended, which might explain why only 7 of the 16 hospitals were able to meaningfully increase HIV testing during ED visits or inpatient admissions despite having identified champions and the funding, albeit limited, provided by the study.
Hospitals were encouraged to use routine laboratory-based HIV tests for screening because they facilitate testing larger numbers of patients and were better at identifying acute infections that would be missed by point-of-care antibody tests [10, 11, 28–30]. At the 2 hospitals in the Bronx and the 2 in DC that successfully adopted centralized laboratory HIV testing during the study, the annual number of tests conducted during ED visits more than doubled. However, in most participating hospitals, point-of-care rapid HIV tests constituted the majority of tests performed during the study period. In some hospitals, obstacles such as lead times for acquiring new laboratory equipment or existing purchasing contracts prevented changes to testing procedures. Hospitals also might have had less motivation to change from point-of-care testing because health departments provided support for testing personnel and free rapid HIV tests before and throughout the 3-year study period.
Recommendations and regulations surrounding HIV testing might also have affected hospital testing practices. CDC has recommended routine HIV screening in healthcare settings since 2006 [4], but the US Preventive Services Task Force did not endorse routine testing of all persons, not just those deemed at increased risk, until April 2013, during the last year of the study [5, 31]. New York State passed a statute in 2010 that required health care providers to offer HIV testing to persons aged 13–64 years in hospitals, EDs, and primary care settings, but the mandate had only a modest effect on the odds of HIV testing [32]. Until 2014, New York State required written informed consent for HIV tests, which providers frequently cite as a major impediment to routine HIV testing [33, 34]. This might explain why HIV testing showed small increases in DC, a municipality that did not require written consent, in contrast to the Bronx.
The study had several limitations. First, it relied on data collected during the delivery of clinical services, which proved to be incomplete for key outcomes. Testing could be offered by different staff members at different times, and the various information systems at participating hospitals could not collect information on the number of HIV tests that were offered or declined by diverse people in various settings.
Second, although the protocol sought to achieve universal offer of testing, staff were encouraged to follow current recommendations, with the more pragmatic goal of HIV testing persons who had not been tested previously and those at increased risk [4, 5]. Specific data are not available from the participating institutions, but the Behavioral Risk Factor Surveillance System suggests that it is likely many individuals who made ED visits or had inpatient admissions had already been tested: in 2011, 50% of persons aged 18–65 in New York State and 73% in DC reported that they had been tested for HIV at least once [35]. Third, the percentages tested for HIV were based on the numbers of visits or admissions, not unique patients. Specific utilization data are not available for the study hospitals, but other studies suggest that frequent users account for up to 28% of ED visits [36]. Thus, the percentage of ED visits during which an HIV test was conducted is likely to underestimate considerably the percentage of unique, eligible (previously untested) patients who were tested. A fourth limitation is the lack of access, at the time of offer of testing at these institutions, to historical testing information, which resulted in repeat testing of persons who had tested HIV-positive before. The study found that one-third of positive ED tests and 80% of positive inpatient tests were obtained from persons who had a previous HIV diagnosis. Notwithstanding, repeat testing for those with a prior HIV-positive test is not without benefit. Other routine HIV testing programs have demonstrated that when previously diagnosed patients undergo repeat testing in the context of a screening program, engagement in HIV care and viral suppression improve after retesting, regardless of the number of years since initial diagnosis [37, 38].
CONCLUSIONS
In the HPTN 065 study, during the 3-year effort to increase hospital testing in the Bronx and DC, the percentage of ED visits and inpatient admissions during which an HIV test was conducted showed only modest change. However, large-scale facility-based HIV testing was feasible, and focusing testing efforts on those with no record of HIV testing appears to be warranted. In both communities, the prevalence of new HIV diagnoses among those screened exceeded the 0.1% threshold at which, based on cost-effectiveness, CDC recommends routine HIV screening in healthcare settings [4]. Opportunities remain to expand HIV testing in both ED and inpatient settings, but will require additional strategies and resources to fully realize its potential.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the hospital lead investigators: Ralph Belloise, Sheldon Brown, MD, Kim Bullock, MD, Akinola Fisher, MD, Fred Gordin, MD, Princy Kumar, MD, Natella Rakhmanina, MD, and Gary Simon, MD. The authors also appreciate the appreciate the assistance during the study of David Burns, National Institute of Allergy and Infectious Diseases (NIAID) and Steven Ethridge, Centers for Disease Control and Prevention (CDC).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of the CDC, the NIAID, or the National Institutes of Health (NIH).
Financial support. The HIV Prevention Trials Network (HPTN) 065 study is sponsored by the NIAID, the National Institute of Mental Health, and the National Institute on Drug Abuse, of the US NIH (cooperative agreements UM1 AI 068619 and UM1 AI 068617), as well as the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC.
Potential conflicts of interest. B. M. B. reports personal fees from FHI 360 and Gilead Sciences, Inc, and has been on the speakers’ bureau for Siemens Healthcare Diagnostics. W. M. E.-S., T. G., E. G., B. H., and B. S. Z. report grants from NIAID and NIH during the conduct of the study. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 23–25 February 2015.
References
- 1. Bradley H, Hall HI, Wolitski RJ et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:1113–7. [PMC free article] [PubMed] [Google Scholar]
- 2. Samji H, Cescon A, Hogg RS et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen MS, Chen YQ, McCauley M et al. ; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Branson BM, Handsfield HH, Lampe MA et al. ; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 5. Moyer VA; US Preventive Services Task Force Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 159:51–60. [DOI] [PubMed] [Google Scholar]
- 6. Lyss SB, Branson BM, Kroc KA, Couture EF, Newman DR, Weinstein RA. Detecting unsuspected HIV infection with a rapid whole-blood HIV test in an urban emergency department. J Acquir Immune Defic Syndr 2007; 44:435–42. [DOI] [PubMed] [Google Scholar]
- 7. White DA, Scribner AN, Schulden JD, Branson BM, Heffelfinger JD. Results of a rapid HIV screening and diagnostic testing program in an urban emergency department. Ann Emerg Med 2009; 54:56–64. [DOI] [PubMed] [Google Scholar]
- 8. Sattin RW, Wilde JA, Freeman AE, Miller KM, Dias JK. Rapid HIV testing in a southeastern emergency department serving a semiurban-semirural adolescent and adult population. Ann Emerg Med 2011; 58:S60–4. [DOI] [PubMed] [Google Scholar]
- 9. Lyons MS, Lindsell CJ, Ruffner AH et al. Randomized comparison of universal and targeted HIV screening in the emergency department. J Acquir Immune Defic Syndr 2013; 64:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoxhaj S, Davila JA, Modi P et al. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Ann Emerg Med 2011; 58:S79–84. [DOI] [PubMed] [Google Scholar]
- 11. Geren KI, Lovecchio F, Knight J et al. Identification of acute HIV infection using fourth-generation testing in an opt-out emergency department screening program. Ann Emerg Med 2014; 64:537–46. [DOI] [PubMed] [Google Scholar]
- 12. Haukoos JS, Lyons MS, White DA, Hsieh YH, Rothman RE. Acute HIV infection and implications of fourth-generation HIV screening in emergency departments. Ann Emerg Med 2014; 64:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walensky RP, Losina E, Steger-Craven KA, Freedberg KA. Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary inpatient testing. Arch Intern Med 2002; 162:887–92. [DOI] [PubMed] [Google Scholar]
- 14. Greenwald JL, Rich CA, Bessega S, Posner MA, Maeda JL, Skolnik PR. Evaluation of the Centers for Disease Control and Prevention’s recommendations regarding routine testing for human immunodeficiency virus by an inpatient service: who are we missing?Mayo Clin Proc 2006; 81:452–8. [DOI] [PubMed] [Google Scholar]
- 15. Siegel M, Kennedy L, Rexroth K et al. Better but not ideal acceptance of routine inpatient HIV point-of-care testing among veterans in a high prevalence area. J Acquir Immune Defic Syndr 2010; 55:205–10. [DOI] [PubMed] [Google Scholar]
- 16. Gamble T, Branson B, Donnell D et al. Design of the HPTN 065 (TLC-Plus) study: a study to evaluate the feasibility of an enhanced test, link-to-care, plus treat approach for HIV prevention in the United States. Clin Trials 2017; 14:322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Sadr WM, Donnell D, Beauchamp G et al. ; HPTN 065 Study Team. Financial incentives for linkage to care and viral suppression among HIV-positive patients: a randomized clinical trial (HPTN 065). JAMA Intern Med 2017; 177:1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buchacz K, Farrior J, Beauchamp G et al. ; HPTN 065 Study Team. Changing clinician practices and attitudes regarding the use of antiretroviral therapy for HIV treatment and prevention. J Int Assoc Provid AIDS Care 2017; 16:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. New York Department of Health and Mental Hygiene. The Bronx knows HIV testing initiative final report Available at: http://www.nyc.gov/html/doh/downloads/pdf/ah/bronx-knows-summary-report.pdf. Accessed 12 April 2017.
- 20. District of Columbia Department of Health. Annual epidemiology and surveillance report Available at: http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/2012AESRFINAL.pdf. Accessed 12 April 2017.
- 21. Castel AD, Magnus M, Peterson J et al. Implementing a novel citywide rapid HIV testing campaign in Washington, D.C.: findings and lessons learned. Public Health Rep 2012; 127:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hudepohl NJ, Lindsell CJ, Hart KW et al. Effect of an emergency department HIV testing program on the proportion of emergency department patients who have been tested. Ann Emerg Med 2011; 58:S140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goetz MB, Hoang T, Knapp H et al. ; QUERI-HIV/Hepatitis Program. Central implementation strategies outperform local ones in improving HIV testing in Veterans Healthcare Administration facilities. J Gen Intern Med 2013; 28:1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGuire R, Moore E. Using a configurable EMR and decision support tools to promote process integration for routine HIV screening in the emergency department. J Am Med Inform Assoc 2016; 23:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilbur L, Huffman G, Lofton S, Finnell JT. The use of a computer reminder system in an emergency department universal HIV screening program. Ann Emerg Med 2011; 58(1 Suppl 1): S71–3.e1. [DOI] [PubMed] [Google Scholar]
- 26. Avery AK, Del Toro M, Caron A. Increases in HIV screening in primary care clinics through an electronic reminder: an interrupted time series. BMJ Qual Saf 2014; 23:250–6. [DOI] [PubMed] [Google Scholar]
- 27. Schackman BR, Eggman AA, Leff JA et al. Costs of expanded rapid HIV testing in four emergency departments. Public Health Rep 2016; 131 (Suppl 1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hankin A, Freiman H, Copeland B, Travis N, Shah B. A comparison of parallel and integrated models for implementation of routine HIV screening in a large, urban emergency department. Public Health Rep 2016; 131(Suppl 1):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Signer D, Peterson S, Hsieh YH et al. Scaling up HIV testing in an academic emergency department: an integrated testing model with rapid fourth-generation and point-of-care testing. Public Health Rep 2016; 131(Suppl 1):82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacobson KR, Arora S, Walsh KB et al. High feasibility of empiric HIV treatment for patients with suspected acute HIV in an emergency department. J Acquir Immune Defic Syndr 2016; 72:242–5. [DOI] [PubMed] [Google Scholar]
- 31. US Preventive Services Task Force. Screening for HIV: recommendation statement. Ann Intern Med 2005; 143:32–7. [DOI] [PubMed] [Google Scholar]
- 32. O’Connell DA, Martin EG, Cutler B, Birkhead GS. The evolution of HIV testing requirements in New York State, 1989–2013. J Acquir Immune Defic Syndr 2015; 68(Suppl 1):S5–9. [DOI] [PubMed] [Google Scholar]
- 33. Wing C. Effects of written informed consent requirements on HIV testing rates: evidence from a natural experiment. Am J Public Health 2009; 99:1087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mimiaga MJ, Johnson CV, Reisner SL, Vanderwarker R, Mayer KH. Barriers to routine HIV testing among Massachusetts community health center personnel. Public Health Rep 2011; 126:643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dietz PM, Krueger AL, Wolitski RJ et al. CDC state HIV prevention progress report, 2014 Available at: http://www.cdc.gov/hiv/policies/progressreports/index.html. Accessed 4 August 2017.
- 36. LaCalle E, Rabin E. Frequent users of emergency departments: the myths, the data, and the policy implications. Ann Emerg Med 2010; 56:42–8. [DOI] [PubMed] [Google Scholar]
- 37. Flash CA, Pasalar S, Hemmige V et al. Benefits of a routine opt-out HIV testing and linkage to care program for previously diagnosed patients in publicly funded emergency departments in Houston, TX. J Acquir Immune Defic Syndr 2015; 69(Suppl 1):S8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mignano JL, Miner L, Siedl K et al. Results and implications of routine HIV testing in the inpatient setting: a descriptive analysis. Popul Health Manag 2017. doi:10.1089/pop.2017.0012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.