Introduction
Aging of the population and the increase in patients on dialysis have led to an increasing frequency of “porcelain aorta” (subtotal circumferential calcification of the ascending aorta) in patients undergoing cardiac surgery.1) Porcelain aorta is reported to be associated with higher morbidity and mortality, especially related to stroke.2,3) Cardiac surgery is more complex in patients with porcelain aorta and their management can be difficult because of the increased risk of perioperative atheroembolism and aortic dissection. Selection of the operative procedure can be problematic, as well as deciding the appropriateness of aortic cross-clamping, identifying the arterial cannulation site, performing proximal anastomosis for coronary artery bypass grafting (CABG), and devising an aortotomy procedure for aortic valve replacement (AVR). Recently, transcatheter aortic valve replacement (TAVR) has become feasible in patients with porcelain aorta for whom conventional AVR is a high-risk procedure.4) This review summarizes the published data on strategies for coping with porcelain aorta during cardiac surgery.
Definition of porcelain aorta and clinical implications
In older patients, calcification is frequently observed in the ascending aorta, the aortic arch, and the coronary ostia. Plain chest computed tomography (CT) can easily demonstrate the distribution of calcification in the aorta. Severe circumferential calcification of the thoracic aorta is called “porcelain aorta,” and its presence can preclude safe aortic cross-clamping, arterial cannulation, and other procedures during cardiac surgery. However, there is still no consensus about the definition porcelain aorta. In the US PARTNER Trial (Placement of AoRTic TraNscathetER Valves Trial), which was the first prospective randomized trial of transcatheter aortic valve replacement (TAVR),5,6) porcelain aorta was defined as nearly or completely circumferential calcification of the ascending aorta and/or aortic arch precluding safe aortic crossclamping or cannulation or requiring circulatory arrest with ascending aorta/arch replacement.4) The prevalence of porcelain aorta has been reported as 0.7%–7.5% in patients requiring cardiac surgery,7–11) while Faggiano et al.3) found porcelain aorta in 7.5% (18/240) of the patients they evaluated for aortic stenosis (AS). In addition, Makkar et al.4) performed TAVR in 85 (23%) of 369 patients with AS who were technically inoperable, with the most common reason being porcelain aorta in 20 (42%) of these 85 patients.
Cardiac surgery and porcelain aorta
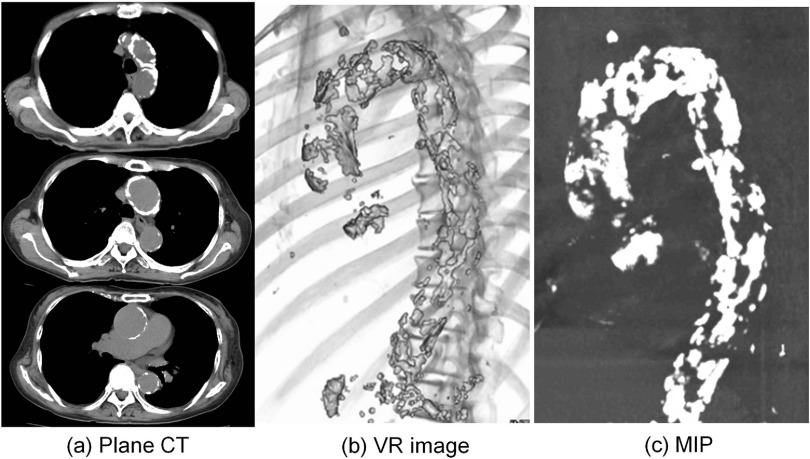
In patients with porcelain aorta, manipulation of the thoracic aorta during cardiac surgery, such as incision, cross-clamping, or cannulation, leads to an increased risk of perioperative embolic stroke.9,10) Therefore, porcelain aorta needs to be diagnosed before cardiac surgery. While severe calcification of the ascending aorta can easily be detected on a chest X-ray film or by cine angiography, these modalities are not able to determine whether there is nearly or completely circumferential calcification as required for porcelain aorta. Plain chest CT can easily demonstrate calcification in the aorta, but it does not adequately evaluate the three-dimensional distribution of calcification. On the other hand, multidetector-row CT (MD-CT) with maximum intensity projection (MIP) and volume-rendered (VR) images can readily evaluate the three-dimensional localization of calcification (Fig. 1). MD-CT allows creation of three-dimensional images that can be rotated 360° and viewed from any angle to visualize calcification in the ascending aorta. Images can also be reconstructed by postprocessing, if necessary. This technique only involves plain CT and injection of contrast medium is not necessary.
Fig. 1. CT shows a heavily calcified ascending aorta and aortic arch. (a) Plain CT, (b) MD-CT with VR image, and (c) MD-CT with MIP. MD-CT: multidetector-row computed tomography; MIP: maximum intensity projection; VR: volume-rendered.
It is well established that porcelain aorta affecting the ascending aorta can be diagnosed by palpation during cardiac surgery, but palpation underestimates the incidence of severe atherosclerotic change. Epi-aortic ultrasound should be performed to reduce the risk of perioperative atheroembolization.
Amorim et al.12) suggested the following classification of aortic calcification and how to approach porcelain aorta in cardiac surgery. Type I is circumferential calcification in the ascending aorta. Type IA means there is no possibility of clamping the calcified aorta. Porcelain ascending aorta is characterized by calcifications of the entire circumference, making it impossible to perform ascending aortic clamping and other aortic treatments. Type IB means clamping may be possible at increased risk, and defined as the ratio of the circumferential length of calcification to the entire ascending aortic circumference below 75%. Porcelain ascending aorta have areas without calcification in the circumference of approximately a finger’s width, making it possible to perform ascending aortic clamping and aortic treatments such as arterial cannulation, proximal anastomosis for CABG, and aortotomy for aortic valve replacement (AVR). Type II is calcification of the descending aorta including or not the aortic arch, without the involvement of the ascending aorta.
Arterial cannulation
In patients with porcelain aorta, an appropriate cannulation site should be selected for safe surgery,13) but each cannulation technique has its advantages and disadvantages.
Arterial perfusion via the common femoral artery is a classic approach for cardiac surgery in patients with calcification of the ascending aorta. While it can be employed easily, this method carries the risk of embolization by atherosclerotic plaque or thrombus from the thoracic and abdominal aorta due to retrograde perfusion,14) or may be undesirable because of iliac artery atherosclerosis due to peripheral arterial disease. Nevertheless, the use of retrograde perfusion via the femoral artery has not decreased at present because of the increasing popularity of minimally invasive cardiac surgery (MICS) via right minithoracotomy. The New York University School of Medicine group15) investigated complications of femoral artery perfusion in 714 patients undergoing minimally invasive mitral surgery and reported a 2.9% incidence of permanent neurologic deficits although retrograde perfusion was not a risk factor for neurologic sequelae according to multivariable analysis. In addition, Gammie et al.16) studied the Society of Thoracic Surgeons database and reported that femoral cannulation was not an independent predictor of stroke associated with minimally invasive mitral surgery. However, Grossi et al.17) demonstrated that retrograde perfusion had no significant influence on the incidence of stroke in patients <50 years old, but was a significant risk factor for occurrence of neurologic events in high-risk patients with aortic disease.
Perfusion via the axillary artery is an alternative approach for cardiac surgery in patients with calcification of the ascending aorta.18–21) Villard et al.21) first reported direct axillary artery cannulation in 1976, but it was used infrequently until the Cleveland Clinic group published a report on 35 patients.19) Since then, axillary artery cannulation has become increasingly popular and positive results have been described in several reports.22–24) The axillary artery is less affected by atherosclerosis than either the ascending aorta or the femoral artery and the major advantage of axillary artery perfusion is a lower risk of cerebral atheroembolization. It can be used for selective antegrade cerebral perfusion to avoid cannulation in patients with severe atheroma of the brachiocephalic artery. In addition, the axillary vessels have abundant collaterals, which lowers the risk of severe distal ischemia-reperfusion injury or embolization after cannulation.25) Hillebrand et al.26) reported that right axillary artery cannulation can provide balanced cerebral oxygenation and might reduce the risk of neurological injury. However, direct axillary artery cannulation can be associated with several local complications, including axillary artery dissection, thrombosis, and brachial plexus injury. Sabik et al.19) compared cannulation-related morbidity between 212 patients receiving direct axillary artery cannulation and 187 patients with side graft cannulation, and demonstrated that side graft cannulation was associated with fewer complications. Cannulation-related morbidity was infrequent in both groups, including brachial plexus injury in 1.8% (7/399 patients), axillary artery damage in 1.8% (7/399 patients), and aortic dissection in 0.8% (3/399 patients). However, propensity-matched analysis revealed complications of cannulation in 1% (2/140 patients) of the side graft group versus 8% (11/140 patients) of the direct cannulation group (p = 0.02). In particular, brachial plexus injury did not occur in the side graft group (0%, 0/140 patients), but was found in 3.6% (5/140 patients) of the direct cannulation group (p = 0.06). Likewise, there was no axillary artery injury (0%, 0/140 patients) in the side graft group, but it also occurred in 3.6% (5/140 patients) of the direct cannulation group (p = 0.03). After propensity matching, the odds ratio was 0.15 (p = 0.002) for reducing the risk of cannulation-related morbidity by employing a side graft, and they recommended routine use of a side graft whenever axillary artery cannulation is performed. Svensson et al.13) investigated complications of perfusion via an axillary side graft versus the femoral artery in 674 patients undergoing cardiac surgery with hypothermic circulatory arrest (HCA). There was no significant difference of the neurologic outcome. Stroke occurred in 4.0% (12/299 patients) of the axillary side graft group and 6.7% (25/375 patients) of the femoral artery group (p = 0.1; p = 0.4 among propensity-matched patients). The risk of hospital mortality was higher with femoral perfusion (11%, 42/375 patients) than axillary side graft perfusion (7.0%, 21/299) (p = 0.06; p = 0.02 among propensity-matched patients). To avoid malperfusion and perform axillary artery cannulation safety, the brachiocephalic and subclavian arteries should be evaluated preoperatively. Measurement of the bilateral brachial artery pressures is also necessary, preferably by determining the ankle-brachial artery pressure index. If the pressure of one brachial artery is significantly lower, MD-row CT or magnetic resonance (MR) angiography should be performed to detect stenosis or severe atherosclerosis of the arch vessels. A known contraindication for axillary artery cannulation is severe atheroma affecting the axillary artery or the subclavian artery. Radial artery pressure monitoring is important to assess distal arm perfusion as well as the perfusion pressure during selective antegrade cerebral perfusion, so bilateral radial artery lines should be placed for intraoperative pressure monitoring.
Cannulation of the brachiocephalic artery was reported to be useful in patients with severe porcelain aorta.27–29) Banbury et al.27) stated that using the brachiocephalic artery for cardiopulmonary bypass has the advantage of allowing central cannulation with the standard ascending aortic cannulation technique in patients whose aorta cannot be clamped, such as those with dissection or an aneurysm. Perfusion via the brachiocephalic artery can avoid the difficulties associated with making a second incision (axillary artery cannulation) or the problems of retrograde perfusion (femoral artery cannulation).
Transapical aortic cannulation is an alternative method of central cannulation. While numerous reports have been published about the usefulness of transapical aortic perfusion for avoiding malperfusion in patients with acute aortic dissection30) or for avoiding retrograde femoral artery perfusion in patients with distal arch aneurysm and thoraco-abdominal aneurysm undergoing surgery via left thoracotomy,31) its clinical impact in patients with porcelain aorta is not clear.
CABG and porcelain aorta
If a patient with a porcelain aorta undergoes CABG, off-pump surgery with the “aortic no touch CABG” technique is recommended to avoid cannulation and clamping of the ascending aorta.32–34) The “no touch” technique can be accomplished by arterial grafting using bilateral internal mammary grafts with addition of radial artery or saphenous veincomposite grafts. If there is a small non-calcified area, proximal anastomosis with a non-clamp technique can be performed using a Heartstring device11,34) or the PASPORT system35) with epi-aortic ultrasound. Lev-Ran et al.34) found a lower incidence of stroke when patients who had porcelain aorta was managed by the off-pump technique with “aortic no touch CABG” compared to standard CABG using cardiopulmonary bypass and femoral artery cannulation. However, revascularization was disadvantageously incomplete in 24.3% of the off-pump patients.
Hypothermic fibrillatory arrest without clamping is also useful.9,36) Salenger et al.36) retrospectively examined the outcome of a non-clamp technique for coronary revascularization in 71 consecutive patients with severe calcification of the ascending aorta. Distal revascularization was accomplished using mildly hypothermic (30–32°C) noncardioplegic myocardial preservation with elective ventricular fibrillation, while proximal anastomoses were performed during brief periods of circulatory arrest. They compared these 71 patients with 615 patients who underwent CABG using partial side clamping. There was only one perioperative stroke in each group (1.5% and 0.2%), showing no significant difference. They suggested that this technique could safely achieve full revascularization in patients with a problematic ascending aorta while minimizing the risk of cerebral embolism.
Valve surgery and porcelain aorta
In patients who have porcelain aorta, it is preferable to perform mitral valve surgery without aortic crossclamping by employing hypothermia and a fibrillating heart.37,38) Loulmet et al.39) reported excellent patient outcomes after mitral valve surgery on the fibrillating heart via right thoracotomy without aortic clamping. Mitral valve procedure can also be performed using endoaortic balloon occlusion and retrograde cardioplegia.40,41)
There are several possible strategies for surgical aortic valve replacement (SAVR) in patients with porcelain ascending aorta. In 1984, Jacobowitz et al.42) first reported surgical AVR under total HCA.
Coselli et al.43) and Byrne et al.44) described a “no-touch” technique for performing AVR with HCA in patients who had a porcelain ascending aorta although this technique requires a longer HCA time than other techniques. A retrospective study by Kaneko and Aranki45) showed excellent outcomes in patients under 80 years old with porcelain aorta who underwent deep HCA for AVR, but the stroke and mortality rates were 3- to 4-fold higher in patients over 80 years old.
There have been some reports of successful aortic endarterectomy combined with HCA for AVR in patients with a calcified ascending aorta.7,46–49) However, Stern et al.48) reported that the postoperative stroke rate was 34.9% following aortic endarterectomy in this clinical setting. In addition, there has been limited long-term follow-up after endarterectomy, and the risk of aneurysmal degeneration is unknown.
Several authors have described excellent results after replacement of the ascending aorta in patients with porcelain ascending aorta who required AVR.7,10,49–51) The advantages of this technique include no need for aortic manipulation before HCA, and it can be performed with relatively brief HCA.
AVR with balloon occlusion of the ascending aorta is an alternative technique7,52) that only requires a brief period of HCA, but there is still the risk of embolization.
Gillinov et al.7) reported excellent results of AVR in 62 patients with porcelain aorta. The overall hospital mortality rate was 14% with a 10% stroke rate. Among 24 patients (39%) who had AVR with HCA, the overall mortality rate was 12% and the stroke rate was 17%. In addition, 16 patients underwent aortic endarterectomy with an overall mortality rate of 19% and a stroke rate of 12%, while 12 patients had ascending aorta replacement with an overall mortality rate of 25% and a stroke rate of 0%. Furthermore, six patients had aortic inspection and cross-clamping with no mortality or stroke, and four patients underwent balloon occlusion with no mortality or stroke. Overall, the incidence of neurologic events and the operative mortality rate were higher compared with the results of standard AVR in patients without porcelain aorta.
Another option for AVR in patients with porcelain aorta is an apico-aortic conduit,53) but there is a risk of thrombus formation or stagnation due to competition between antegrade and retrograde flow.54)
TAVR and porcelain aorta
TAVR has been established as a reproducible and safe technique for treating severe aortic stenosis in high-risk patients.4–6) Several authors have reported acceptable outcomes of TAVR in patients with porcelain ascending aorta.2,4,55–57) Rodés-Cebau et al.55) reported on the results obtained with a balloon expandable Edwards valve (Edwards Life sciences, Inc., Irvine, CA, USA) in 339 patients, including 61 patients (18%) with a porcelain ascending aorta (28 transfemoral, 45.9%; 33 transapical, 54.1%). The procedure was successful in 98.4% of the patients with porcelain aorta, but valve malposition requiring implantation of a second valve tended to be more frequent, probably due to difficulty in achieving the correct valve position or displacement of the valve during balloon inflation because of the highly calcified aorta. The stroke rate and 30-day mortality rate were 1.6% (1/61 patients) and 11.5% (7/61 patients), respectively, showing no differences from patients without porcelain aorta. Pascual et al.56) described the results of implanting a Medtronic CoreValve (Medtronic CoreValve, Irvine, CA, USA) in 449 patients, including 36 patients (8%) with a porcelain ascending aorta (27 transfemoral, 75%; 9 transaxillary, 25%). The procedure was successful in 94.4%. One patient (2.8%) required implantation of a second valve due to malposition. The stroke rate and 30-day mortality rate were 2.8% (1/36 patients) and 5.6% (2/36 patients), respectively, with no differences compared to patients without porcelain aorta. Zahn et al.57) reported the results from a large German registry (1,374 TAVR procedures at 27 hospitals), including 147 patients (10.7%) with porcelain aorta and 1227 patients (89.3%) without porcelain aorta. The patients with porcelain aorta were treated by implantation of a balloon expandable Edwards valve (16.3%, 24/147 patients) or a CoreValve (83.7%, 123/147 patients) via the transfemoral, transsubclavian, transaortic, or transapical approach. The procedure was successful in 97.3% of the patients. There was a higher stroke rate in the patients with porcelain aorta (5.5% vs. 2.8%, p = 0.08), as well as a higher mortality rate (10.9% vs. 8.1%, p = 0.24), and a higher combined death/stroke rate (14.4% vs. 10.2%, p = 0.12).
In the USA, Brennan et al.58) compared TAVR with SAVR in 9464 propensity-matched intermediate- and high-risk (Society of Thoracic Surgeons Predicted Risk of Mortality score ≥3%) patients. There were no significant differences of mortality, stroke, survival time, and out-of-hospital survival up to 1 year, but patients who underwent TAVR were more likely to be discharged. There have not been any randomized controlled studies.
Cross-clamping a porcelain aorta
Several techniques for cross-clamping a porcelain aorta have also been reported. Hartert et al.59) reported the results of aortic cross-clamping with an “open proximal ascending aorta” in 42 patients with porcelain aorta undergoing AVR or mitral valve replacement (MVR). After arterial cannulation via the distal arch or femoral artery, the ascending porcelain aorta was clamped slowly with a special Forgarty clamp (Aesculap Inc., Center Valley, PA, USA), while mobilized atherosclerotic material left the aorta through the open incision. After de-airing, the aorta was gradually declamped while flushing out plaque via the open aortotomy. The stroke rate and 30-day mortality rate were 7.1% (3/42 patients) and 7.1% (3/42 patients), respectively. Aortic dissection did not occur. They suggested that cross-clamping with an “open proximal ascending aorta” is effective and associated with a low incidence of stroke and systemic embolization in patients with porcelain aorta.
Isoda et al.60) reported a “stepwise aortic clamp procedure” for AVR in a patient with porcelain aorta. After arterial cannulation via the right axillary artery and right femoral artery, moderate hypothermia was initiated. Aortotomy was performed during brief circulatory arrest (8 min), and a Foley catheter was inserted and inflated. Endarterectomy was accomplished at the aortic clamp site using an ultrasonic surgical aspirator during low-flow cardiopulmonary bypass. Then, the ascending aorta was slowly clamped with a Forgarty clamp and AVR was carried out. The patient recovered without any thromboembolic events. They suggested that this procedure could be effective to avoid embolic events and reduce the risk of cerebral ischemia.
Conclusion
In patients with porcelain ascending aorta, cardiac surgery is still challenging. Cardiac surgeons need to recognize the growing importance of this condition to increase the possibility of preoperative diagnosis and optimization of the therapeutic approach. MD-CT with MIP and VR provides valuable information for preoperative evaluation in patients with porcelain aorta and it is a useful strategy for preoperative planning to devise a suitable surgical procedure for these patients. To reduce the risk of stroke and aortic dissection, appropriate preoperative evaluation and adoption of a suitable strategy are required for cardiac surgery in patients with porcelain ascending aorta.
Disclosure Statement
We declare that we have no conflicts of interest.
References
- 1).Gaudino M, Glieca F, Alessandrini F, et al. The unclampable ascending aorta in coronary artery bypass patients: A surgical challenge of increasing frequency. Circulation 2000; 102: 1497-502. [DOI] [PubMed] [Google Scholar]
- 2).Kempfert J, Van Linden A, Linke A, et al. Transapical aortic valve implantation: therapy of choice for patients with aortic stenosis and porcelain aorta? Ann Thorac Surg 2010; 90: 1457-61. [DOI] [PubMed] [Google Scholar]
- 3).Faggiano P, Frattini S, Zilioli V, et al. Prevalence of comorbidities and associated cardiac diseases in p™ients with valve aortic stenosis. Potential implications for the decision-making process. Int J Cardiol 2012; 159: 94-9. [DOI] [PubMed] [Google Scholar]
- 4).Makkar RR, Jilaihawi H, Mack M, et al. Stratification of outcomes after transcatheter aortic valve replacement according to surgical inoperability for technical versus clinical reasons. J Am Coll Cardiol 2014; 63: 901-11. [DOI] [PubMed] [Google Scholar]
- 5).Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363: 1597-607. [DOI] [PubMed] [Google Scholar]
- 6).Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364: 2187-98. [DOI] [PubMed] [Google Scholar]
- 7).Gillinov AM, Lytle BW, Hoang V, et al. The atherosclerotic aorta at aortic valve replacement: surgical strategies and results. J Thorac Cardiovasc Surg 2000; 120: 957-63. [DOI] [PubMed] [Google Scholar]
- 8).Wareing TH, Davila-Roman VG, Barzilai B, et al. Management of the severely atherosclerotic ascending aorta during cardiac operations. A strategy for detection and treatment. J Thorac Cardiovasc Surg 1992; 103: 453-62. [PubMed] [Google Scholar]
- 9).Leyh RG, Bartels C, Nötzold A, et al. Management of porcelain aorta during coronary artery bypass grafting. Ann Thorac Surg 1999; 67: 986-8. [DOI] [PubMed] [Google Scholar]
- 10).Zingone B, Rauber E, Gatti G, et al. Diagnosis and management of severe atherosclerosis of the ascending aorta and aortic arch during cardiac surgery: focus on aortic replacement. Eur J Cardiothorac Surg 2007; 31: 990-7. [DOI] [PubMed] [Google Scholar]
- 11).Sirin G, Sarkislali K, Konakci M, et al. Extraanatomical coronary artery bypass grafting in patients with severely atherosclerotic (Porcelain) aorta. J Cardiothorac Surg 2013; 8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Amorim PA, Penov K, Lehmkuhl L, et al. Not all porcelain is the same: classification of circular aortic calcifications (porcelain aorta) according to the impact on therapeutic approach. Thorac Cardiovasc Surg 2013; 61: 559-63. [DOI] [PubMed] [Google Scholar]
- 13).Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg 2004; 78: 1274-84; discussion 1274-84. [DOI] [PubMed] [Google Scholar]
- 14).Price DL, Harris J. Cholesterol emboli in cerebral arteries as a complication of retrograde aortic perfusion during cardiac surgery. Neurology 1970; 20: 1209-14. [DOI] [PubMed] [Google Scholar]
- 15).Grossi EA, Galloway AC, LaPietra A, et al. Minimally invasive mitral valve surgery: a 6-year experience with 714 patients. Ann Thorac Surg 2002; 74: 660-3; discussion 663-4. [DOI] [PubMed] [Google Scholar]
- 16).Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell chamberlain memorial paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the society of thoracic surgeons adult cardiac surgery database. Ann Thorac Surg 2010; 90: 1401-8, 14–0.e1; discussion 1408-10. [DOI] [PubMed] [Google Scholar]
- 17).Grossi EA, Loulmet DF, Schwartz CF, et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg 2012; 143: S68-70. [DOI] [PubMed] [Google Scholar]
- 18).Strauch JT, Spielvogel D, Lauten A, et al. Axillary artery cannulation: routine use in ascending aorta and aortic arch replacement. Ann Thorac Surg 2004; 78: 103-8; discussion 103-8. [DOI] [PubMed] [Google Scholar]
- 19).Sabik JF, Nemeh H, Lytle BW, et al. Cannulation of the axillary artery with a side graft reduces morbidity. Ann Thorac Surg 2004; 77: 1315-20. [DOI] [PubMed] [Google Scholar]
- 20).Hosono M, Shibata T, Murakami T, et al. Right axillary artery cannulation in aortic valve replacement. Ann Thorac Cardiovasc Surg 2016; 22: 84-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Villard J, Froment JC, Milleret R, et al. [Type I, complete, acute aortic dissection. Value of arterial perfusion by the axillary route (author’s transl)]. Ann Chir Thorac Cardiovasc 1976; 15: 133-5. [PubMed] [Google Scholar]
- 22).Isomura T, Hisatomi K, Satoh T, et al. Axillary artery cannulation for cardiopulmonary bypass in the presence of diseased ascending aorta. Eur J Cardiothorac Surg 1996; 10: 481. [DOI] [PubMed] [Google Scholar]
- 23).Halkos ME, Kerendi F, Myung R, et al. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg 2009; 138: 1081-9. [DOI] [PubMed] [Google Scholar]
- 24).Bassin L, Mathur MN. Axillary artery cannulation for aortic and complex cardiac surgery. Heart Lung Circ 2010; 19: 726-9. [DOI] [PubMed] [Google Scholar]
- 25).Kouchoukos NT. Adjuncts to reduce the incidence of embolic brain injury during operations on the aortic arch. Ann Thorac Surg 1994; 57: 243-5. [DOI] [PubMed] [Google Scholar]
- 26).Hillebrand J, Zheng Z, Ploss A, et al. Axillary artery cannulation provides balanced cerebral oxygenation. Heart Vessels 2016; 31: 1077-83. [DOI] [PubMed] [Google Scholar]
- 27).Banbury MK, Cosgrove DM. Arterial cannulation of the innominate artery. Ann Thorac Surg 2000; 69: 957. [DOI] [PubMed] [Google Scholar]
- 28).Di Eusanio M, Quarti A, Pierri MD, et al. Cannulation of the brachiocephalic trunk during surgery of the thoracic aorta: a simplified technique for antegrade cerebral perfusion. Eur J Cardiothorac Surg 2004; 26: 831-3. [DOI] [PubMed] [Google Scholar]
- 29).Preventza O, Garcia A, Tuluca A, et al. Innominate artery cannulation for proximal aortic surgery: outcomes and neurological events in 263 patients. Eur J Cardiothorac Surg 2015; 48: 937-42; discussion 942. [DOI] [PubMed] [Google Scholar]
- 30).Matsushita A, Manabe S, Tabata M, et al. Efficacy and pitfalls of transapical cannulation for the repair of acute type A aortic dissection. Ann Thorac Surg 2012; 93: 1905-9. [DOI] [PubMed] [Google Scholar]
- 31).Shiiya N, Yasuda K, Murashita T, et al. Transapical aortic cannulation for hypothermic aortic operation through a left thoracotomy: an alternative to avoid retrograde arterial perfusion. J Thorac Cardiovasc Surg 1997; 113: 1113-4. [DOI] [PubMed] [Google Scholar]
- 32).Lev-Ran O, Braunstein R, Sharony R, et al. No-touch aorta off-pump coronary surgery: the effect on stroke. J Thorac Cardiovasc Surg 2005; 129: 307-13. [DOI] [PubMed] [Google Scholar]
- 33).Emmert MY, Seifert B, Wilhelm M, et al. Aortic no-touch technique makes the difference in off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 2011; 142: 1499-506. [DOI] [PubMed] [Google Scholar]
- 34).Lev-Ran O, Ben-Gal Y, Matsa M, et al. ‘No touch’ techniques for porcelain ascending aorta: comparison between cardiopulmonary bypass with femoral artery cannulation and off-pump myocardial revascularization. J Card Surg 2002; 17: 370-6. [DOI] [PubMed] [Google Scholar]
- 35).Dohmen G, Hatam N, Goetzenich A, et al. PAS-Port® clampless proximal anastomotic device for coronary bypass surgery in porcelain aorta. Eur J Cardiothorac Surg 2011; 39: 49-52. [DOI] [PubMed] [Google Scholar]
- 36).Salenger R, Rodriquez E, Efird JT, et al. Clampless technique during coronary artery bypass grafting for proximal anastomoses in the hostile aorta. J Thorac Cardiovasc Surg 2013; 145: 1584-8. [DOI] [PubMed] [Google Scholar]
- 37).Pasic M, Sündermann S, Unbehaun A, et al. Beating heart mitral valve surgery: results in 120 consecutive patients considered unsuitable for conventional mitral valve surgery. Interact Cardiovasc Thorac Surg 2017; 25: 541-7. [DOI] [PubMed] [Google Scholar]
- 38).Takami Y, Tajima K, Terazawa S, et al. Safer aortic crossclamping during short-term moderate hypothermic circulatory arrest for cardiac surgery in patients with a bad ascending aorta. J Thorac Cardiovasc Surg 2009; 137: 875-80. [DOI] [PubMed] [Google Scholar]
- 39).Loulmet DF, Patel NC, Jennings JM, et al. Less invasive intracardiac surgery performed without aortic clamping. Ann Thorac Surg 2008; 85: 1551-5. [DOI] [PubMed] [Google Scholar]
- 40).Reichenspurner H, Detter C, Deuse T, et al. Video and robotic-assisted minimally invasive mitral valve surgery: a comparison of the Port-Access and transthoracic clamp techniques. Ann Thorac Surg 2005; 79: 485-90; discussion 490-1. [DOI] [PubMed] [Google Scholar]
- 41).Ward AF, Loulmet DF, Neuburger PJ, et al. Outcomes of peripheral perfusion with balloon aortic clamping for totally endoscopic robotic mitral valve repair. J Thorac Cardiovasc Surg 2014; 148: 2769-72. [DOI] [PubMed] [Google Scholar]
- 42).Jacobowitz IJ, Rose DM, Shevede K, et al. Use of profound hypothermia and circulatory arrest for the calcified aorta. Chest 1984; 85: 288-9. [DOI] [PubMed] [Google Scholar]
- 43).Coselli JS, Crawford ES. Aortic valve replacement in the patient with extensive calcification of the ascending aorta (the porcelain aorta). J Thorac Cardiovasc Surg 1986; 91: 184-7. [PubMed] [Google Scholar]
- 44).Byrne JG, Aranki SF, Cohn LH. Aortic valve operations under deep hypothermic circulatory arrest for the porcelain aorta: “no-touch” technique. Ann Thorac Surg 1998; 65: 1313-5. [DOI] [PubMed] [Google Scholar]
- 45).Kaneko T, Neely RC, Shekar P, et al. The safety of deep hypothermic circulatory arrest in aortic valve replacement with unclampable aorta in non-octogenarians. Interact Cardiovasc Thorac Surg 2015; 20: 79-84. [DOI] [PubMed] [Google Scholar]
- 46).Svensson LG, Sun J, Cruz HA, et al. Endarterectomy for calcified porcelain aorta associated with aortic valve stenosis. Ann Thorac Surg 1996; 61: 149-52. [DOI] [PubMed] [Google Scholar]
- 47).Vogt PR, Hauser M, Schwarz U, et al. Complete thromboendarterectomy of the calcified ascending aorta and aortic arch. Ann Thorac Surg 1999; 67: 457-61. [DOI] [PubMed] [Google Scholar]
- 48).Stern A, Tunick PA, Culliford AT, et al. Protruding aortic arch atheromas: risk of stroke during heart surgery with and without aortic arch endarterectomy. Am Heart J 1999; 138: 746-52. [DOI] [PubMed] [Google Scholar]
- 49).Aranki SF, Nathan M, Shekar P, et al. Hypothermic circulatory arrest enables aortic valve replacement in patients with unclampable aorta. Ann Thorac Surg 2005; 80: 1679-86; discussion 1686-7. [DOI] [PubMed] [Google Scholar]
- 50).Girardi LN, Krieger KH, Mack CA, et al. No-clamp technique for valve repair or replacement in patients with a porcelain aorta. Ann Thorac Surg 2005; 80: 1688-92. [DOI] [PubMed] [Google Scholar]
- 51).Kouchoukos NT, Wareing TH, Daily BB, et al. Management of the severely atherosclerotic aorta during cardiac operations. J Card Surg 1994; 9: 490-4. [DOI] [PubMed] [Google Scholar]
- 52).Cosgrove DM. Management of the calcified aorta: an alternative method of occlusion. Ann Thorac Surg 1983; 36: 718-9. [DOI] [PubMed] [Google Scholar]
- 53).Lockowandt U. Apicoaortic valved conduit: potential for progress? J Thorac Cardiovasc Surg 2006; 132: 796-801. [DOI] [PubMed] [Google Scholar]
- 54).Kawahito K, Kimura N, Komiya K, et al. Blood flow competition after aortic valve bypass: an evaluation using computational fluid dynamics. Interact Cardiovasc Thorac Surg 2017; 24: 670-6. [DOI] [PubMed] [Google Scholar]
- 55).Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010; 55: 1080-90. [DOI] [PubMed] [Google Scholar]
- 56).Pascual I, Avanzas P, Muñoz-García AJ, et al. Percutaneous implantation of the CoreValve® self-expanding valve prosthesis in patients with severe aortic stenosis and porcelain aorta: medium-term follow-up. Rev Esp Cardiol (Engl Ed) 2013; 66: 775-81. [DOI] [PubMed] [Google Scholar]
- 57).Zahn R, Schiele R, Gerckens U, et al. Transcatheter aortic valve implantation in patients with “porcelain” aorta (from a Multicenter Real World Registry). Am J Cardiol 2013; 111: 602-8. [DOI] [PubMed] [Google Scholar]
- 58).Brennan JM, Thomas L, Cohen DJ, et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol 2017; 70: 439-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Hartert M, Conzelmann LO, Mehlhorn U, et al. Cross-clamping a porcelain aorta: an alternative technique for high-risk patients. J Cardiovasc Surg 2014; Feb 13: 1-15. [DOI] [PubMed] [Google Scholar]
- 60).Isoda S, Osako M, Kimura T, et al. A stepwise aortic clamp procedure to treat porcelain aorta associated with aortic valve stenosis and hemodialysis. Ann Thorac Cardiovasc Surg 2014; 20: 725-9. [DOI] [PubMed] [Google Scholar]