Abstract
Purpose: This study was performed to compare the outcome of pleurectomy/decortication (P/D) with that of extrapleural pneumonectomy (EPP) for patients with malignant pleural mesothelioma (MPM).
Methods: Patients with MPM underwent either P/D or EPP from August 2008 to December 2014. Various clinicopathological factors were analyzed to identify differences between the two procedures.
Results: P/D was performed in nine patients and EPP in 30 patients. Most of the patients’ background characteristics were not significantly different between the groups. The surgery time (680 vs. 586 min, p = 0.0034) and bleeding volume (4050 vs. 2110 mL, p = 0.002) were significantly greater in P/D than in EPP; however, grade ≥3 complications (44% vs. 33%, p = 0.54) and length of postoperative hospital stay (29 vs. 37 days, p = 0.26) were not significantly different. The median survival time and 2- and 3-year survival rates in all patients were 16.7 months, 28.5%, and 15.3%, respectively. The median survival time and 2- and 3-year survival in the P/D and EPP groups were 22.5 months, 43.8%, and 43.8% and 16.5 months, 24.0%, and 14.4%, respectively (p = 0.13).
Conclusion: Survival of patients with MPM remains poor despite multidisciplinary treatment. P/D is comparable with EPP and could be a safe and another surgical treatment for patients with MPM.
Keywords: pleurectomy/decortication, extrapleural pneumonectomy, malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is a rare malignant tumor with an extremely poor prognosis. After asbestos exposure, a mean latency period of 20–40 years can be seen before the disease becomes apparent.1) The number of patients with MPM in Japan has been increasing2) due to the delay in the ban on asbestos use compared with Western countries. Cure of MPM is almost impossible with surgical resection alone; therefore, multimodal treatment involving neoadjuvant chemotherapy followed by extrapleural pneumonectomy (EPP) and adjuvant radiotherapy has been performed in patients with MPM with radical intent. This multimodal therapy is effective in highly selected patients.3) However, EPP is one of the most invasive procedures in thoracic surgery, and high morbidity and mortality rates have been reported.4) In the MARS 1 trial,5) all patients underwent induction platinum-based chemotherapy and were randomly assigned to either EPP followed by hemithorax irradiation or no EPP. Surprisingly, radical surgery in the form of EPP within trimodal therapy offered no benefit and was associated with a risk of harming patients. Notably, however, this study had several flaws such as a small number of patients, an unusual high mortality rate in the EPP arm, and others.
Pleurectomy/decortication (P/D) is a lung-sparing surgery that has recently received attention as a less invasive procedure than EPP. Many reports6–8) have compared the outcomes of P/D and EPP in terms of morbidity, mortality, and survival, suggesting that P/D is comparable with EPP in terms of various surgical outcomes. Taioli et al.9) recommend P/D because of its 2.5-fold lower short-term mortality. A clinical trial in Japan was also conducted to evaluate the feasibility of induction chemotherapy followed by P/D aimed at macroscopic complete resection (MCR), which is the essential objective of surgery.10) The primary endpoint was the MCR rate, and the secondary endpoints included the P/D rate, MCR rate by P/D, pulmonary function at 3 months postoperatively, and other variables.
These reports indicate a recent trend that P/D is preferred when technically feasible, suggesting that MCR could be achieved.9) Thus, P/D comprises about 80% of procedures performed among surgically treated patients with MPM.11) However, each procedure has its advantages and disadvantages, and which procedure is superior remains controversial.12) This single-institutional retrospective study was performed to compare the morbidity, mortality, and survival rates of P/D and EPP for patients with MPM to identify which procedure is more effective.
Patients and Methods
Patients
This study was approved by the research review board of Nagasaki University Hospital in accordance with the Declaration of Helsinki (No. 17082120). From January 1998 to March 2017, a total of 39 patients with MPM underwent either P/D or EPP at Nagasaki University Hospital. Various clinicopathological factors were evaluated, including age, sex, histology, location of tumor (right/left), stage, Charlson comorbidity index (CCI), Glasgow prognostic score (GPS), neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and the European Organization for Research and Treatment of Cancer (EORTC) prognostic score.
The following formula was used for calculation of the EORTC prognostic score:13) (0.550) a + (0.60) b + (0.52) c + (0.67) d + (0.60) e, where a = white blood cell count of >8.3 × 109/L, b = Eastern Cooperative Oncology Group performance status (ECOG PS) of 1 or 2, c = probable histology, d = sarcomatous histology, and e = male sex.
The surgery time, bleeding volume, comorbidities, mortality, types of recurrence, treatment for recurrence, and prognosis of both treatments were also were analyzed to identify differences between the two procedures. The guideline of the International Mesothelioma Interest Group (IMIG) version 7 was applied in this study.
Surgical indications and preoperative examination of patients with MPM
All diagnoses of MPM were confirmed by preoperative histology (n = 37) or cytology (n = 2). Surgical indications for MPM were an age of <80 years and IMIG clinical stage I to III (T1-3, N0-2, M0) considered to be resectable. Preoperative staging routinely included chest X-rays, chest computed tomography (CT) scans, magnetic resonance imaging of the brain, and positron emission tomography/CT scans. The clinical status of lymph nodes was radiographically evaluated by chest CT using a criterion of a short axis of <1 cm and accumulation of fluorodeoxyglucose on positron emission tomography/CT scans from routine use by 2008. Complete evaluation of cardiac and respiratory function, including the forced expiratory volume in 1 second (FEV1.0), FEV1.0%, vital capacity, and percent vital capacity, was performed to ensure that patients could tolerate pulmonary resection. A predicted FEV1.0 of >1000 mL was required for both surgeries. A good ECOG PS of 0 to 1 was also required.
P/D and EPP
P/D and EPP were performed by posterolateral thoracotomy at the floor of the 6th rib bed. The skin incisions for pleural biopsy were completely resected. An additional 10th thoracotomy was performed to facilitate resection of the tumor and reconstruction of the diaphragm. Combined resection of the pericardium, diaphragm, and chest wall was added when achievement of MCR was needed. These resections were reconstructed with DUALMESH (W.L. Gore and Associates, Inc., Flagstaff, AZ, USA), which is a pure and unique expanded polytetrafluoroethylene prosthesis. One chest tube was inserted during EPP, and two or three were inserted during P/D. In EPP, the chest tube was removed soon after hemostasis was confirmed. Prolonged air leakage for >7 days in patients who underwent P/D was treated with chemical pleurodesis using minocycline and/or OK-432. EPP was the procedure of choice until September 2012, and then we have begun performing P/D from October 2012. When intraoperative bleeding and massive air leakage were uncontrolled during P/D, the procedure was converted to EPP for safety. Perioperative complications were assessed according to Common Terminology Criteria for Adverse Events version 4, and grade ≥3 complications were regarded as severe complications in this study.
Statistical analysis
The patients’ baseline characteristics are presented as frequency and percentage for categorical data and median with range for continuous data. The associations between P/D and EPP were assessed using Fisher’s exact test for categorical data and the Wilcoxon rank sum test for continuous data. Overall survival was calculated from the day of definitive diagnosis of MPM to death and analyzed using the Kaplan–Meier method and log-rank test. To assess the relationship of each parameter with prognostic factors, the value determined by the receiver operating characteristic curve was decided and divided into two groups. The hazard ratio and 95% confidence interval for survival were calculated using a Cox proportional hazard regression model. A p-value of <0.05 was considered statistically significant. JMP version 13 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Characteristics of patients who underwent P/D
In the P/D group (n = 9), all patients were males and their median age was 69 years (range, 55–77 years). Eight (88.9%) of the nine patients had an ECOG PS of 0. The histological types were epithelial in five (55.6%) patients, biphasic in three patients (33.3%), and sarcomatous in one patient (11.1%). Neoadjuvant chemotherapy including cisplatin regimens was administered to eight (88.9%) patients. Combined lobectomy was required in two patients during P/D to achieve MCR; thus, all patients achieved MCR. Postoperative complications included persistent air leakage in two (22.2%) patients, pyothorax in one patient (11.1%), and redothoracotomy in three patients (33.3%) (postoperative bleeding in two patients and chylothorax in one patient). One (11.1%) patient received adjuvant chemotherapy because the patient was in good condition after P/D. Five (55.6%) patients had stage III disease and three (33.3%) patients had stage II disease according to the IMIG classification.
Characteristics of patients who underwent EPP
In the EPP group (n = 30), 24 patients were males and 6 were females; their median age was 63 years (range, 47–78 years). Twenty-seven (90.0%) of the 30 patients had an ECOG PS of 0. The histological types were epithelial in 19 (63.3%) patients, biphasic and sarcomatous in 5 (16.7%) patients, and desmoplastic in 1 (3.3%) patient. Neoadjuvant chemotherapy including cisplatin regimens was administered to 21 (70.0%) patients. All patients achieved MCR. Postoperative complications included arrhythmia in nine (30.0%) patients, retention of sputum in six (20.0%) patients, pneumonia in two (6.7%) patients, and others. Three patch dislocations (diaphragm, n = 2; pericardium, n = 1) occurred in the early phase of this study. Adjuvant radiotherapy was administered to 20 (66.7%) patients; the other patients could not receive radiotherapy because of their poor PS. Nineteen (63.3%) patients completed trimodal therapy. Twenty-three (76.7%) patients had stage III disease, and six (20.0%) patients had stage II disease.
Comparison of P/D and EPP
Table 1 shows the characteristics of all surgically treated patients with MPM in this study. We divided the patients’ characteristics into four categories: patient, tumor, perioperative, and survival factors. With respect to patient factors, we found no significant differences in age (p = 0.13), CCI (p = 0.16), GPS (p = 0.47), NLR (p = 0.24), PLR (p = 0.14), or EORTC prognostic score (p = 0.7) between the two surgical procedures. For tumor factors, we found no significant differences in histological type (p = 0.67), tumor location (p = 0.65), or IMIG stage (p = 0.41). With respect to preoperative factors, the surgery time (680 vs. 586 min, p = 0.0034) and bleeding volume (4050 vs. 2110 mL, p = 0.002) were significantly larger in P/D than in EPP; however, grade ≥3 complications (44% vs. 33%, p = 0.54) and length of the postoperative hospital stay (29 vs. 97 days, p = 0.26) were not significantly different. No patients died in either group. In terms of survival factors, the rate of local recurrence was not significantly different between the two groups (n = 6, all local recurrences in P/D; n = 16, 76.2% of all recurrences in EPP; p = 0.19). Treatment after recurrence was performed in 2 (33.3%) of the 6 patients who developed recurrence in the P/D group and in 1 (4.8%) of the 21 patients who developed recurrence in the EPP group, with a significant difference (p = 0.0495). Treatment after recurrence in our study was severely restricted due to the poor PS after surgery, especially in the EPP group. Most causes of death in patients with MPM were cancerrelated, but without a significant difference (100% vs. 76%, p = 0.22). Among the other five deaths in the EPP group, two were related to the adjuvant radiotherapy, two were related to debility, and one was related to another malignancy.
Table 1. Characteristics of patients with malignant pleural mesothelioma in the present study.
| P/D | EPP | p value | ||
|---|---|---|---|---|
| (n = 9) | (n = 30) | |||
| Patient factors | Sex, male/female | 9/0 | 24/6 | 0.14 |
| Age, years | 69 (55–77) | 63 (47–78) | 0.13 | |
| CCI | 1 (0–2) | 0 (0–3) | 0.16 | |
| GPS | 0 (0–2) | 0 (0–2) | 0.47 | |
| NLR | 2.5 (1.3–4.6) | 2.8 (1.1–11.6) | 0.24 | |
| PLR | 86 (72–108) | 111 (49–629) | 0.14 | |
| EORTC score | 1.12 (0.52–2.34) | 1.12 (1.12–1.79) | 0.70 | |
| Tumor factors | Histology, epi/others | 5/4 | 19/11 | 0.67 |
| Tumor location, right/left | 7/2 | 21/9 | 0.65 | |
| IMIG stage, I/II/III | 1/3/5 | 1/6/23 | 0.41 | |
| Perioperative factors | Operative time, min | 680 (596–818) | 586 (383–780) | 0.0034* |
| Bleeding volume, mL | 4050 (2500–5680) | 2110 (724–6268) | 0.0017* | |
| Grade ≥3 morbidity, yes/no | 4/5 | 10/20 | 0.54 | |
| Postoperative stay, days | 29 (25–135) | 37 (17–301) | 0.26 | |
| Survival factors | Recurrence, local/distant | 6/0 | 16/5 | 0.16 |
| Therapy after recurrence, yes/no | 2/4 | 1/20 | 0.0495* | |
| Cause of death, cancer/other | 5/0 | 19/6 | ||
| Median survival time, mos | 22.5 | 16.5 | 0.13 | |
Data are presented as number of patients or median (range). *Statistically significant. P/D: pleurectomy/decortication; EPP: extrapleural pneumonectomy; CCI: Charlson comorbidity index; GPS: Glasgow prognostic score; NLR: neutrophil/lymphocyte ratio; PLR: platelet/lymphocyte ratio; EORTC: European Organization for Research and Treatment of Cancer; IMIG: International Mesothelioma Interest Group; G3: grade 3 (Common Terminology Criteria for Adverse Events, version 4); epi: epithelial
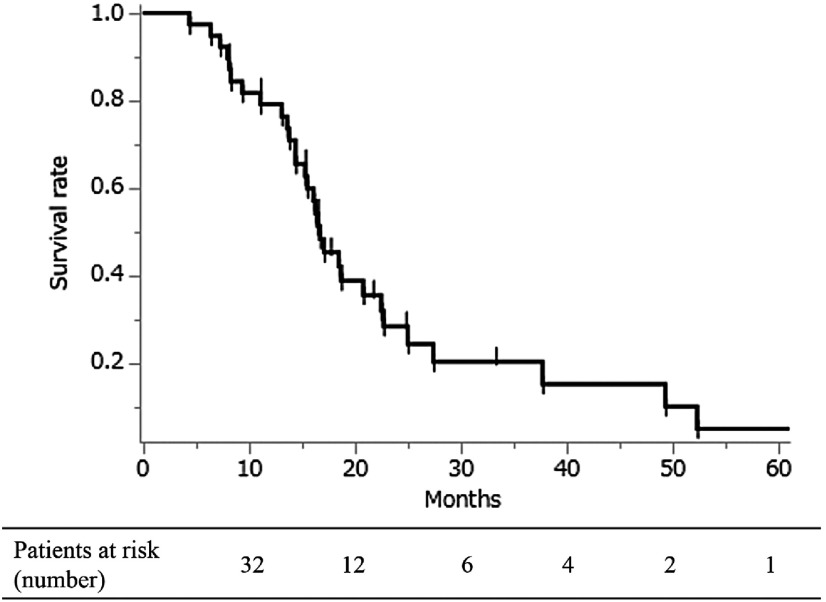
The median survival time of all patients (n = 39) was 16.7 months, and the 2- and 3-year survival were 28.5% and 15.3%, respectively (Fig. 1). The median survival time and 2- and 3-year survival rates in the P/D and EPP groups were 22.5 months, 43.8%, and 43.8% and 16.5 months, 24.0%, and 14.4%, respectively, without significant differences (p = 0.13) (Fig. 2).
Fig. 1. Overall survival of surgically treated patients with malignant pleural mesothelioma (n = 39). The median survival time after diagnosis was 16.7 months. The 2- and 3-year survival rates were 28.5% and 15.3%, respectively. P/D: pleurectomy/decortication; EPP: extrapleural pneumonectomy.
Fig. 2. Comparison of overall survival after P/D versus EPP among patients with malignant pleural mesothelioma. The median survival time of P/D and EPP was 22.5 and 16.5 months, respectively, and the 2- and 3-year survival rates were 43.8% and 24.0% for P/D and 43.8% and 14.4% for EPP with no significant difference (p = 0.13). P/D: pleurectomy/decortication; EPP: extrapleural pneumonectomy.
The results of the univariate and multivariate analyses for prognostic factors in all patients with MPM are shown in Table 2. In the univariate analysis, a low PLR of <98 and low EORTC score of <1.13 were found to be prognostic factors; however, the multivariate analysis revealed no significant prognostic factors.
Table 2. Univariate and multivariate analyses of overall survival in patients with malignant pleural mesothelioma.
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Reference | Hazard ratio | p value | 95% CI | Hazard ratio | p value | 95% CI | ||
| P/D | EPP | 0.48 | 0.11 | 0.16 | 1.17 | ||||
| Age ≥65 years | Age <65 years | 0.92 | 0.83 | 0.43 | 2.04 | ||||
| Male | Female | 0.44 | 0.10 | 0.19 | 1.21 | ||||
| Epithelioid | Others | 0.62 | 0.21 | 0.30 | 1.33 | ||||
| Stage I + II | Stage III | 0.54 | 0.18 | 0.20 | 1.30 | ||||
| CCI 0 | CCI ≥1 | 0.58 | 0.21 | 0.27 | 1.38 | ||||
| GPS 0 | GPS ≥1 | 0.61 | 0.20 | 0.29 | 1.31 | ||||
| NLR <2.8 | NLR ≥2.8 | 0.56 | 0.15 | 0.25 | 1.23 | ||||
| PLR <98 | PLR ≥98 | 0.34 | 0.009* | 0.14 | 0.77 | 0.43 | 0.06 | 0.98 | 6.08 |
| EORTC score <1.13 | EORTC score ≥1.13 | 0.37 | 0.016* | 0.17 | 0.83 | 0.52 | 0.13 | 0.22 | 1.21 |
| Trimodal therapy | No trimodal therapy | 1.25 | 0.55 | 0.60 | 2.63 | ||||
*Statistically significant. CI: confidence interval; P/D: pleurectomy/decortication; EPP: extrapleural pneumonectomy; Others: other types of histology such as biphasic and sarcomatoid; CCI: Charlson comorbidity index; GPS: Glasgow prognostic score; NLR: neutrophil/lymphocyte ratio; PLR: platelet/lymphocyte ratio; EORTC: European Organization for Research and Treatment of Cancer
Discussion
This retrospective analysis of the morbidity, mortality, and survival rates of P/D versus EPP for patients with MPM showed that P/D was comparable with EPP and could be a safe and another surgical treatment. However, survival of patients with MPM was still poor despite multimodal treatment.
MPM is one of the most highly malignant tumors. Surgical resection is the most effective approach for malignant tumors; for MPM, however, secure surgical margins are impossible to attain because of the tumor characteristics. P/D and EPP are among the most invasive types of thoracic surgery. In one meta-analysis, the short-term mortality rate (perioperatively and within 30 days) was 4.5% in EPP and 1.7% in P/D.9) In other studies, the median survival durations of patients who had undergone P/D and EPP were 14.6–32.0 months and 16–23 months, respectively,6–8,11) which are not satisfactory results in large-scale studies. Additionally, no consensus regarding which procedure is superior to the other has yet been reached.12) In fact, the researchers of the MARS 1 trial5) doubted the usefulness of EPP surgery itself for MPM. Conversely, Nelson et al.14) reported that surgery-based multimodal therapy was associated with improved survival compared with no therapy and single-modality treatment in 6645 propensity score-matched patients. Thus, other modalities including chemotherapy and radiotherapy were definitely required, that is, multimodal treatment is fundamental for MPM. Appropriate patient selection is also important when undertaking multimodal therapy.
Many recent reports have described the use of prognostic factors involving inflammatory markers such as combinations of neutrophils, platelets, and lymphocytes;1,2) the C-reactive protein level; and the nutritional status as indicated by the serum albumin and cholesterol levels15) in both lung cancer16,17) and MPM although the cutoff values have not been fully investigated. The EORTC prognostic score13) has also been assessed. Systemic inflammation is considered to be involved in multiple stages of cancer progression,18) that is, cancer growth and eventual damage of local tissue, which disrupts homeostasis and incites a systemic acute-phase response. Cancer progression simultaneously induces the release of proinflammatory cytokines and promotes the immunovascular system, and neutrophils, lymphocytes, platelets, albumin, and C-reactive protein are affected.16,17) The detailed mechanism and clinical significance of the inflammatory response have been fully described elsewhere.15,18) In the present study, only the PLR and EORTC prognostic score were identified as prognostic factors in the univariate analysis. Tagawa et al.18) reported that sex and the PLR were independent predictors of overall survival in patients who underwent EPP for treatment of MPM and advocated the use of a new prognostic score using the PLR. Our study has shown the importance of these parameters; however, these factors disappeared in the multivariate analysis. We believe that the small number of patients might have affected these results. A further large-scale study of the use of inflammation-based markers as prognostic factors is required.
There are several reasons why the patients with MPM in this study had poor survival. First, most of them (n = 28, 71.8%) had stage III disease. Early detection of this disease is very difficult, and it is essential for clinicians to improve the early detection rate and perform precise annual examinations and aggressive biopsy in patients with pleural effusion of unknown origin among the high-risk population (those with exposure to asbestos). Second, with respect to histology, the sarcomatoid type (n = 6) was associated with significantly poorer survival than the epithelial type (n = 24) and biphasic type (n = 8) (p = 0.018, data not shown) in our study. We consider that the sarcomatoid type should not be an indication for surgical resection like many centers.12) Of course, these factors are closely related to the high grade of malignancy of MPM itself. Third, treatment after recurrence was strictly limited because of the patients’ poor postoperative ECOG PS. This poor PS was related to the large lung volume loss especially in the EPP group and sometimes to postoperative complications. Evidence-based second-line therapy including chemotherapy and radiotherapy has not yet been established. In our institutional experience, the quality of life of patients who underwent P/D tended to be better than that of patients who underwent EPP because of the lung preservation; in fact, patients in the P/D group had significantly more chances for treatment with chemotherapy after recurrence than did patients in the EPP group. Finally, the number of surgical cases of P/D per surgeon was very limited, and technical problems might have existed. We believe that the significant differences of bleeding between P/D and EPP were due to the bleeding from lung parenchyma during decortication, resulted that surgery time was also significantly longer for P/D than EPP, although there were no significant differences in other factors including the rate of severe postoperative complications, mortality, length of hospital stay, local recurrence, and survival. Thus, we recommend P/D as another surgical treatment when technically feasible in terms of the lack of significant differences in morbidity, mortality, and prognosis.
The surgical outcomes of MPM remain unsatisfactory, as noted above. However, the addition of nintedanib to pemetrexed plus cisplatin showed good progression-free survival,19) as did the use of bevacizumab in the phase-III MAPS trial.20) Moreover, the use of immune checkpoint inhibitors21) and multimodal therapy including intraoperative photodynamic therapy22) has also been reported. Clinical use of these promising treatments is anticipated to improve the survival of patients with MPM.
A precise cancer staging system is also important for both patients and clinicians. The IMIG staging system was revised to version 8 in 2017.23) In addition, the maximum thickness and sum of three-level thickness24) and CT volume of the tumor25) were introduced to more precisely reflect the prognosis of patients with MPM. In Japan, data regarding newly diagnosed MPM are being prospectively collected from 2017 to 2018 by the Japanese Joint Committee for Lung Cancer Registration not only to investigate epidemiology and treatment choices, but also in anticipation of the validation of version 8 of the IMIG staging system.
This study had several limitations. First, this was a retrospective study with several biases and with a small sample from a single institution, which could have affected the statistical accuracy. Because of the rarity of MPM, the accumulation of cases was performed not domestically but globally. Second, technical problems were encountered, especially in P/D, as described above; each surgeon’s surgical experience was limited. We might still be in the learning curve period. To resolve these problems, technical improvement of MPM surgery and larger prospective randomized controlled studies are required although enrollment of such clinical trials is very difficult due to the rarity of MPM.
Conclusion
Survival of patients with MPM remains poor despite multimodal treatment. P/D was comparable with EPP and could be a safe and feasible treatment for patients with MPM in our study. P/D could be another surgical treatment when technically feasible.
Disclosure Statement
The authors declare no competing financial conflicts of interest.
References
- 1).Tural Onur S, Sokucu SN, Dalar L, et al. Are neutrophil/ lymphocyte ratio and platelet/lymphocyte ratio reliable parameters as prognostic indicators in malignant mesothelioma? Ther Clin Risk Manag 2016; 12: 651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).The prevalence of malignant pleural mesothelioma http://ganjoho.jp/public/cancer/mesothelioma/index.html. Accessed 11 Oct 2017. (in Japanese)
- 3).Sugarbaker DJ, Wolf AS, Chirieac LR, et al. Clinical and pathological features of three-year survivors of malignant pleural mesothelioma following extrapleural pneumonectomy. Eur J Cardiothorac Surg 2011; 40: 298-303. [DOI] [PubMed] [Google Scholar]
- 4).Hasegawa S, Okada M, Tanaka F, et al. Trimodality strategy for treating malignant pleural mesothelioma: results of a feasibility study of induction pemetrexed plus cisplatin followed by extrapleural pneumonectomy and postoperative hemithoracic radiation (Japan Mesothelioma Interest Group 0601 Trial). Int J Clin Oncol. 2016; 21: 523-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011; 12: 763-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Kostron A, Friess M, Inci I, et al. Propensity matched comparison of extrapleural pneumonectomy and pleurectomy/decortication for mesothelioma patients†. Interact Cardiovasc Thorac Surg 2017; 24: 740-6. [DOI] [PubMed] [Google Scholar]
- 7).Infante M, Morenghi E, Bottoni E, et al. Comorbidity, postoperative morbidity and survival in patients undergoing radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016; 50: 1077-82. [DOI] [PubMed] [Google Scholar]
- 8).Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016; 151: 478-84. [DOI] [PubMed] [Google Scholar]
- 9).Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015; 99: 472-80. [DOI] [PubMed] [Google Scholar]
- 10).Shimokawa M, Hasegawa S, Fukuoka K, et al. A feasibility study of induction pemetrexed plus cisplatin followed by pleurectomy/decortication aimed at macroscopic complete resection for malignant pleural mesothelioma. Jpn J Clin Oncol 2013; 43: 575-8. [DOI] [PubMed] [Google Scholar]
- 11).Verma V, Ahern CA, Berlind CG, et al. National cancer database report on pneumonectomy versus lung-sparing surgery for malignant pleural mesothelioma. J Thorac Oncol 2017; 12: 1704-14. [DOI] [PubMed] [Google Scholar]
- 12).Takuwa T, Hasegawa S. Current surgical strategies for malignant pleural mesothelioma. Surg Today 2016; 46: 887-94. [DOI] [PubMed] [Google Scholar]
- 13).Fennell DA, Parmar A, Shamash J, et al. Statistical validation of the EORTC prognostic model for malignant pleural mesothelioma based on three consecutive phase II trials. J Clin Oncol 2005; 23: 184-9. [DOI] [PubMed] [Google Scholar]
- 14).Nelson DB, Rice DC, Niu J, et al. Long-term survival outcomes of cancer-directed surgery for malignant pleural mesothelioma: propensity score matching analysis. J Clin Oncol 2017; 35: 3354-62. [DOI] [PubMed] [Google Scholar]
- 15).Takamori S, Toyokawa G, Taguchi K, et al. The controlling nutritional status score is a significant independent predictor of poor prognosis in patients with malignant pleural mesothelioma. Clin Lung Cancer 2017; 18: e303-13. [DOI] [PubMed] [Google Scholar]
- 16).Miyazaki T, Yamasaki N, Tsuchiya T, et al. Inflammation-based scoring is a useful prognostic predictor of pulmonary resection for elderly patients with clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2015; 47: e140-5. [DOI] [PubMed] [Google Scholar]
- 17).Miyazaki T, Yamasaki N, Tsuchiya T, et al. Ratio of C-reactive protein to albumin is a prognostic factor for operable non-small-cell lung cancer in elderly patients. Surg Today 2017; 47: 836-43. [DOI] [PubMed] [Google Scholar]
- 18).Tagawa T, Anraku M, Morodomi Y, et al. Clinical role of a new prognostic score using platelet-to-lymphocyte ratio in patients with malignant pleural mesothelioma undergoing extrapleural pneumonectomy. J Thorac Dis 2015; 7: 1898-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Grosso F, Steele N, Novello S, et al. Nintedanib plus pemetrexed/cisplatin in patients with malignant pleural mesothelioma: phase II results from the randomized, placebo-controlled LUME-Meso trial. J Clin Oncol 2017; 35: 3591-600. [DOI] [PubMed] [Google Scholar]
- 20).Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016; 387: 1405-14. [DOI] [PubMed] [Google Scholar]
- 21).Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017; 18: 623-30. [DOI] [PubMed] [Google Scholar]
- 22).Friedberg JS, Simone CB, Culligan MJ, et al. Extended pleurectomy-decortication-based treatment for advanced stage epithelial mesothelioma yielding a median survival of nearly three years. Ann Thorac Surg 2017; 103: 912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Pass H, Giroux D, Kennedy C, et al. The IASLC mesothelioma staging project: improving staging of a rare disease through international participation. J Thorac Oncol 2016; 11: 2082-8. [DOI] [PubMed] [Google Scholar]
- 24).Nowak AK, Chansky K, Rice DC, et al. The IASLC mesothelioma staging project: proposals for revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for pleural mesothelioma. J Thorac Oncol 2016; 11: 2089-99. [DOI] [PubMed] [Google Scholar]
- 25).Rusch VW, Gill R, Mitchell A, et al. A multicenter study of volumetric computed tomography for staging malignant pleural mesothelioma. Ann Thorac Surg 2016; 102: 1059-66. [DOI] [PMC free article] [PubMed] [Google Scholar]