ABSTRACT
Ebola virus (EBOV) infection is a major public health concern due to high fatality rates and limited effective treatments. Statins, widely used cholesterol-lowering drugs, have pleiotropic mechanisms of action and were suggested as potential adjunct therapy for Ebola virus disease (EVD) during the 2013–2016 outbreak in West Africa. Here, we evaluated the antiviral effects of statin (lovastatin) on EBOV infection in vitro. Statin treatment decreased infectious EBOV production in primary human monocyte-derived macrophages and in the hepatic cell line Huh7. Statin treatment did not interfere with viral entry, but the viral particles released from treated cells showed reduced infectivity due to inhibition of viral glycoprotein processing, as evidenced by decreased ratios of the mature glycoprotein form to precursor form. Statin-induced inhibition of infectious virus production and glycoprotein processing was reversed by exogenous mevalonate, the rate-limiting product of the cholesterol biosynthesis pathway, but not by low-density lipoprotein. Finally, statin-treated cells produced EBOV particles devoid of the surface glycoproteins required for virus infectivity. Our findings demonstrate that statin treatment inhibits EBOV infection and suggest that the efficacy of statin treatment should be evaluated in appropriate animal models of EVD.
KEYWORDS: Ebola virus, Filoviridae, antiviral, hemorrhagic fever
IMPORTANCE
Treatments targeting Ebola virus disease (EVD) are experimental, expensive, and scarce. Statins are inexpensive generic drugs that have been used for many years for the treatment of hypercholesterolemia and have a favorable safety profile. Here, we show the antiviral effects of statins on infectious Ebola virus (EBOV) production. Our study reveals a novel molecular mechanism in which statin regulates EBOV particle infectivity by preventing glycoprotein processing and incorporation into virus particles. Additionally, statins have anti-inflammatory and immunomodulatory effects. Since inflammation and dysregulation of the immune system are characteristic features of EVD, statins could be explored as part of EVD therapeutics.
INTRODUCTION
Ebola virus (EBOV) poses a threat to people throughout Africa, and, as the 2013–2016 outbreak demonstrated, to the rest of the world (1). The 2013–2016 outbreak was unprecedented in the history of the virus, with over 28,000 cases and more than 11,000 deaths (1). Despite the devastating consequences of EBOV infection, treatment options remain limited and experimental (2). Ebola virus disease (EVD) is associated with systemic inflammation, endothelial dysfunction, coagulopathy, vascular leakage, shock, and organ failure (3, 4). Statins, well-known cholesterol-lowering drugs, have potential beneficial effects beyond their ability to reduce cholesterol levels, including anti-inflammatory and immunomodulatory functions and the ability to reverse endothelial abnormalities (5, 6). For example, statins have been implicated in improving survival in sepsis patients (7–9); like EVD, sepsis is characterized by inflammation, endothelial dysfunction, and coagulopathy (6). Statins are already FDA approved for reducing high cholesterol, have a favorable safety profile, and are inexpensive. Thus, they were suggested as a possible adjunct therapy for EVD patients during the 2013–2016 outbreak (10).
Statins block 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme that catalyzes the conversion of HMG-CoA to mevalonate, a key intermediate for synthesis of cholesterol and isoprenoids (11). Since cholesterols play important roles in membrane fluidity, organization, and signaling (12, 13), they serve as important platforms for viruses to enter cells (14, 15). Statins have been widely reported to block infection of many enveloped viruses by inhibiting the cholesterol/isoprenoid pathway (16–22). Cholesterol likewise contributes to the EBOV life cycle, including viral entry, fusion, and budding (23–29); EBOV has been reported to utilize cholesterol-enriched rafts as a platform for cell entry, as well as for assembly and budding from cells (25, 30–32). In addition, cholesterol-dependent interactions between EBOV glycoproteins (GPs) are essential for virus assembly (15). This further suggests that drugs lowering cholesterol levels, like statins, could be useful therapeutics for EVD patients.
EBOV virions project glycoprotein (GP) spikes that are synthesized and inserted into the host cell-derived envelope during budding (33). EBOV GP is synthesized in several forms. The most abundant form of GP is a secreted protein (sGP) translated from an unedited mRNA, whereas the structural GP is a product of the edited mRNA. The monomeric EBOV GP, a type I transmembrane glycoprotein, is processed by a complex series of events (34–36). An N-glycosylated, endoplasmic reticulum (ER)-resident form of GP precursor (preGP) undergoes N,O-glycosylation maturation in the Golgi apparatus to become GP0 (36). GP0 is then transported further into the trans-Golgi network, where the proprotein convertase furin or a furin-like protease cleaves GP0 at a multibasic motif that is conserved in all EBOV strains (36). Cleavage results in the mature N,O-glycosylated GP1 and in the GP2 subunit linked by disulfide bond (34–38). These subunits interact to form GP1,2, present on virions as trimeric spikes; GP1 mediates receptor binding while GP2 is critical for fusion of the EBOV envelope with the endosomal/lysosomal membrane (39, 40). However, unlike other viruses, cleavage of GP0 by furin is not required for fusion (41, 42) or glycoprotein incorporation into virions (43–46).
Here, we report that a statin (lovastatin) suppresses infectious EBOV production in a human hepatoma cell line (Huh7) and in primary monocyte-derived macrophages, cell types that are in vivo targets for EBOV replication. Statin treatment inhibited processing of preGP into GP1 in EBOV-infected cells or cells transfected with plasmids encoding GP1,2; the effect was reversed by adding mevalonate. EBOV particles produced in statin-treated cells were depleted of the essential glycoprotein subunit GP1 required for virus entry, suggesting that statins reduce EBOV infectivity by inhibiting glycoprotein maturation and incorporation into virions. In addition, we have tested the effect of 5 other types of statins, fluvastatin, simvastatin, atorvastatin, rosuvastatin, and pitavastatin, on EBOV replication. Of all the statins, simvastatin and pitavastatin were the most potent in reducing EBOV infectivity. Our results suggest that statins selectively inhibit preGP maturation and should be further investigated in in vivo models for EBOV infection.
RESULTS
Statin treatment inhibits EBOV infection.
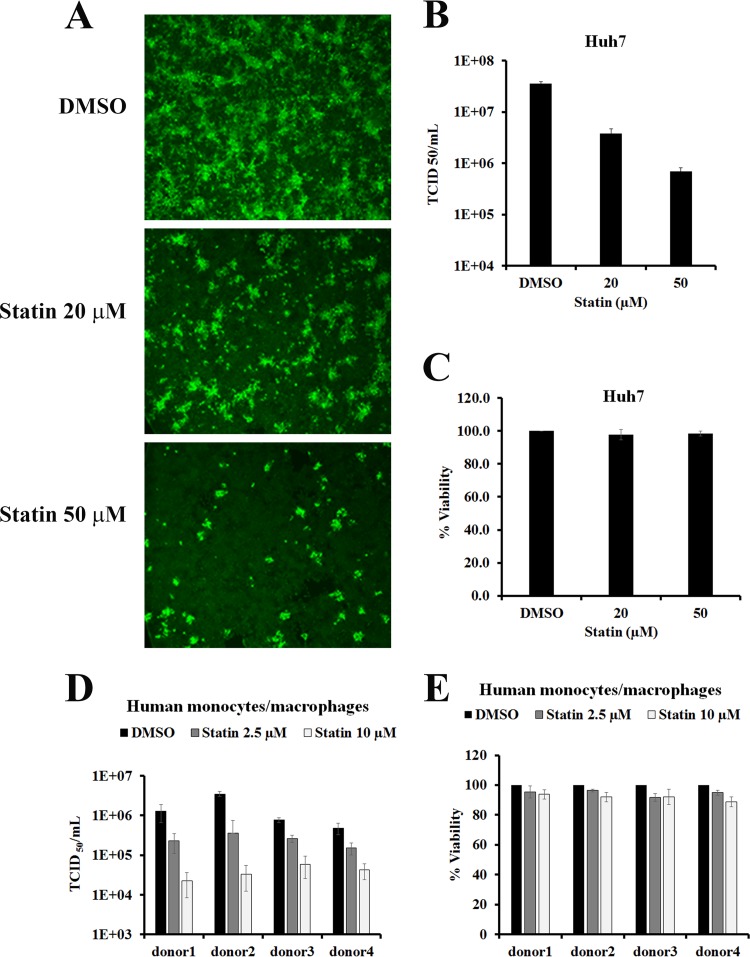
To test if statins affect EBOV replication, Huh7 cells were infected with the EBOV variant Mayinga (Ebola virus/H. sapiens-tc/COD/1976/Yambuku-Mayinga) at a multiplicity of infection (MOI) of 0.05. After 1 h of virus adsorption, the cells were treated with dimethyl sulfoxide (DMSO) (vehicle control) or with 20 µM or 50 µM lovastatin (referred to as “statin” here unless stated otherwise), the first clinically approved statin, in medium supplemented with lipoprotein-deficient serum (LPDS). LPDS eliminates the possible uptake of cholesterol from the medium (47). After 72 h postinfection (hpi), cells were fixed and viral antigen expression was evaluated by immunofluorescence assays using polyclonal anti-EBOV serum. As shown in Fig. 1A, EBOV antigen-positive staining was seen throughout infected Huh7 cells treated with DMSO only. However, EBOV-positive staining was reduced compared to controls in cells treated with statin at either concentration. To ensure that statin-mediated reduction in EBOV-positive staining was not due to cytotoxicity, cell viability was assayed after 72 h of treatment. Cell viability was unaffected by either concentration of statin (Fig. 1C). These results suggest that statin reduced EBOV infection.
FIG 1 .
Statin inhibits Ebola virus infection. (A) Huh7 cells were infected with Ebola virus (EBOV) at an MOI of 0.05. After infection, cells were washed and then treated with various concentrations of statin or with DMSO (control). At 72 hpi, the cells were fixed, permeabilized, and stained with anti-EBOV rabbit polyclonal antibody. (B) Culture supernatants of Huh7 cells infected with EBOV and treated with statin or DMSO as in panel A were harvested 72 hpi, and viral titers were quantified by 50% tissue culture infective dose (TCID50) determination. (C) Viability (percent) of statin-treated Huh7 cells was determined after 72 h of treatment. Values were normalized to DMSO-treated controls. (D) Human monocyte-derived macrophages from 4 separate donors were infected with EBOV at an MOI of 0.05, and cells were washed and then treated with various concentrations of statin or DMSO. Cell supernatants were harvested 72 hpi, and viral titers were quantified by TCID50 determination. The results shown are means ± standard deviations from triplicate wells and representative of two independent experiments. (E) Viability (percent) of statin-treated and mock-infected human monocytes/macrophages was determined after 72 h of treatment. Values were normalized to DMSO controls.
To determine if statin treatment can inhibit infectious EBOV production, we examined viral titers in supernatants of infected cells. High titers of infectious virus (1.5 × 107/ml) were detected at 72 hpi in vehicle control-treated cell culture supernatants supplemented with LPDS. Treatment with statin under the same cell culture conditions reduced EBOV titers; 20 µM statin decreased the production of infectious EBOV titers by >1.1 log, and 50 µM decreased EBOV titers by 1.5 log (Fig. 1B). In contrast, statin treatment under similar conditions did not affect titers of adenovirus type 5, a nonenveloped virus (see Fig. S1 in the supplemental material).
Statin does not affect adenovirus type 5 titers. Huh7 cells were infected with human adenovirus type 5 (Ad5) at an MOI of 0.05. Three days postinfection, titers of infectious virus in cell supernatants were determined by a standard TCID50 titration method. Download FIG S1, TIF file, 22.6 MB (23.2MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
The antiviral potency of statin treatment was also evaluated in primary human monocyte-derived macrophages, since these cells represent a major target of EBOV infection. To account for donor variations, cells from 4 different donors were tested. Cell viability of macrophages treated with 10 µM statin was >80% for all the donors (Fig. 1E). Untreated cells yielded infectious titers ranging between 5 × 105 and 4 × 106 (Fig. 1D). Statin treatment efficiently reduced EBOV titers in macrophages from each donor; 2.5 µM statin reduced infectious EBOV titers by 0.5 to 1.0 log, and 10 µM statin reduced EBOV titers by 1 to 2 log (Fig. 1D).
Statin inhibition of EBOV infection is reversed by exogenous mevalonate but not by LDL.
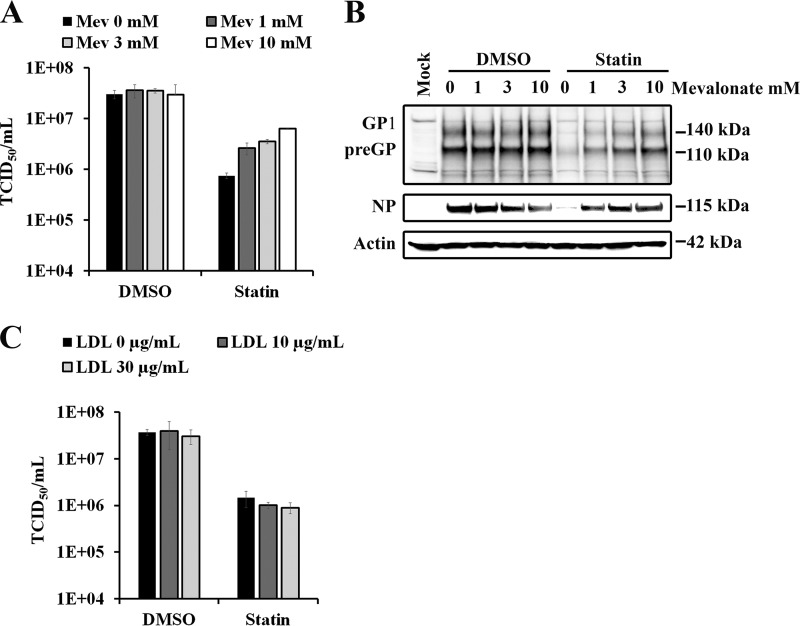
Statin blocks mevalonate generation and subsequent cholesterol biosynthesis by competitively inhibiting HMG-CoA reductase (11). To investigate whether the anti-EBOV effect of statin was dependent on its ability to specifically inhibit mevalonate production, we added mevalonate during statin treatment. Since inhibition of cholesterol synthesis can be compensated for by import of low-density lipoprotein (LDL)-derived cholesterol from outside the cells, we also looked at the effects of LDL supplementation during statin treatment. Huh7 cells were infected with EBOV as described above and then treated with statin with or without the indicated concentrations of LDL or mevalonate. As shown in Fig. 2A, addition of mevalonate reversed statin-mediated reduction in EBOV titers in a dose-dependent manner, while mevalonate alone had no effect on viral titers. Expression of viral glycoprotein GP1/preGP and nucleoprotein (NP) was also restored when mevalonate was added during statin treatment (Fig. 2B). In contrast to mevalonate, adding LDL did not reverse the effects of statin treatment on viral titers (Fig. 2C). Altogether, we showed that inhibition of EBOV infection by statin treatment was reversed by mevalonate, the immediate downstream product of the reaction catalyzed by HMG-CoA reductase. These findings are consistent with statin reducing EBOV infectivity by inhibiting HMG-CoA reductase and not via off-target effects.
FIG 2 .
Mevalonate, but not low-density lipoproteins, restores the antiviral effect of statin. (A) Huh7 cells infected with EBOV as in Fig. 1A were treated with DMSO or 50 µM statin in the presence of indicated concentrations of mevalonate (Mev). Culture supernatants of infected cells were harvested 72 hpi, and viral titers were quantified by determining TCID50. (B) Glycoprotein (GP), nucleoprotein (NP), and actin expression was analyzed by Western blotting in lysates of Huh7 cells infected with EBOV and treated with DMSO or 50 µM statin in the presence of indicated concentrations of Mev. (C) Huh7 cells infected with EBOV as in Fig. 1A were treated with DMSO or statin (50 µM) in the presence of various concentrations of low-density lipoprotein (LDL). Culture supernatants of infected cells were harvested 72 hpi, and viral titers were quantified by TCID50 determination.
Statin treatment does not affect EBOV entry.
To assess whether reduced production of infectious virus was due to the inhibition of EBOV entry into cells, we measured the levels of cell-associated EBOV NP RNA at 3 hpi. Huh7 cells pretreated with statin were infected with EBOV at an MOI of 3 to ensure synchronous infection. Levels of viral NP RNA in the lysed cells were measured by quantitative reverse transcription PCR (qRT-PCR) and normalized to cellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels. As shown in Fig. 3A, NP RNA levels did not significantly differ among the samples, indicating that statin did not affect the levels of internalized EBOV genome. On the other hand, treatment with the positive control U18666A, a Niemann-Pick C1 protein (NPC1) inhibitor previously shown to prevent EBOV glycoprotein-dependent entry (25), reduced NP RNA copy numbers in a dose-dependent manner. These results are consistent with statin not affecting EBOV entry.
FIG 3 .
Statin inhibits specific infectivity of EBOV but does not affect entry. (A) Huh7 cells pretreated with the indicated concentrations of statin or DMSO for 48 h were infected with EBOV (MOI = 3.0). For a positive control, cells were pretreated for 1 h with various concentrations of U18666A before infection with EBOV. After 1 h, infected cells were washed with serum-free medium; fresh medium with or without statin or U18666A was then added back to the cells. After 3 h of incubation at 37°C, NP gene RNA copy numbers were determined by qRT-PCR and normalized to GAPDH mRNA. Results represent mean percent normalized NP RNA levels, with error bars indicating standard deviations calculated from 3 independent experiments. *, P < 0.005. (B) Culture supernatants of cells infected with EBOV (MOI = 2.0) and treated with DMSO or statin were harvested 48 hpi, and viral titers were quantified by TCID50 determination. RNA was extracted from supernatants of cells, and absolute quantification of viral RNA copy numbers was done using a standard curve with known viral titers. (C) Ratios were calculated using TCID50-per-milliliter values from panel B divided by the extracellular viral RNA copy numbers from panel A. Mean specific infectivity was calculated as a percentage of DMSO-treated samples. Mean values from triplicate wells are shown, and error bars indicate standard deviations. The graph shown is representative of 3 independent experiments.
Statin treatment impairs EBOV infectivity.
To further examine the mechanism by which statin reduces the levels of EBOV released from infected cells, the specific infectivity of virions made in the presence of statin was determined by comparing 50% tissue culture infective doses (TCID50) with copy numbers of viral RNA released into culture supernatants of infected cells at 48 hpi. To maximize the initial number of infected cells, Huh7 cells were infected with EBOV at an MOI of 2.0; MOIs higher than 2.0 caused a significant drop in cell viability at 48 hpi (data not shown). Infected cells were then treated either with DMSO (vehicle control) or with 20 µM or 50 µM statin. After 48 h, no differences were noted in extracellular EBOV RNA copy numbers released from cells treated with statin or with vehicle control (Fig. 3B). In contrast, yield of infectious virus (measured as TCID50 per milliliter) in supernatants from statin-treated cells was approximately ~4 to 9 times lower than that in DMSO-treated samples (Fig. 3B). The ratio of TCID50 per milliliter to viral RNA copy numbers (Fig. 3C) in statin-treated cells was reduced to ~5 to 10% of controls. Taken together, these data indicate that postinfection statin treatment decreased infectivity of newly synthesized EBOV particles.
Statin treatment inhibits EBOV glycoprotein maturation.
To explore the mechanism responsible for reduced particle infectivity in statin-treated cells, we examined the impact of statins on the viral proteins involved in virus assembly and budding: the matrix protein VP40 and the envelope glycoprotein GP1,2. We first determined whether statin treatment affected VP40 expression. Huh7 cells were transfected with plasmid expressing VP40 and then treated with statin or vehicle control; cell lysates were analyzed by Western blotting. As shown in Fig. S2, VP40 expression levels were similar in statin-treated and vehicle-treated cells. We then examined GP1 expression in cells transfected with plasmid expressing EBOV GP1,2 and treated with statin. Two forms of GP were detectable: a 110-kDa form sensitive to both endoglycosidase H (endo H) and peptide-N-glycosidase F (PNGase F) digestion that had previously been identified as the N-glycosylated precursor present in the endoplasmic reticulum (designated preGP) and the 140-kDa form that was sensitive only to PNGase F digestion and was identified as the mature GP1 (Fig. 4B ), consistent with the presence of complex N- and O-glycans (36). Most of the GP synthesized in statin-treated cells was the immature precursor glycoprotein (preGP) containing high-mannose sugar chains sensitive to endo H treatment (compare Fig. 4A with B). In contrast, the expression of mature N,O-glycosylated glycoprotein (GP1), which was resistant to endo H treatment (compare Fig. 4A with B), decreased upon statin treatment. Statin did not similarly affect the glycosylation pattern of NPC1 (Fig. S3), suggesting that the observed effect on GP1 was specific.
FIG 4 .
Statin inhibits EBOV GP processing. (A) Huh7 cells were transfected with a plasmid expressing EBOV GP1,2 and treated with statin or DMSO. GP and actin expression in cell lysates was then analyzed by Western blotting. To determine the extent of GP1 cleavage, blot images were subjected to densitometry analysis. The percentage of GP1 was determined by dividing the signal of GP1 over the total amount of glycoprotein recognized by GP1 MAb [GP1/(GP1 + preGP)]. (B) Cell lysates of EBOV GP1,2-transfected cells either were left untreated or were digested with endo H or PNGase. GP and actin expression was then visualized by Western blotting. (C) Huh7 cells transfected with a plasmid expressing EBOV GP1,2 were treated with 50 µM statin or DMSO in the presence of various concentrations of mevalonate. GP and actin expression in cell lysates was analyzed by Western blotting. The percentage of GP1 was determined as in panel A. (D) Huh7 cells were transfected with a plasmid expressing either wild-type EBOV GP1,2 or GP1,2 in which the furin cleavage motif (furin-site mutant GP1,2) had been mutated and were treated with statin (left panel), proprotein convertase inhibitor (PC inh, right panel), or DMSO control. GP and actin expression in cell lysates was then analyzed by Western blotting. To determine the extent of GP0 cleavage, blot images were subjected to densitometry analysis. Percentage of GP1 or GP0 was determined by dividing the signal of GP1 or GP0 over the total amount of glycoprotein recognized by GP1,0 MAb [GP1,0/(GP1,0 + preGP)].
Statin does not affect VP40 expression level. Huh7 cells were transfected with a plasmid expressing EBOV Flag-tagged VP40 and treated with statin or DMSO. VP40 and actin expression in cell lysates was analyzed by Western blotting. Download FIG S2, TIF file, 22.7 MB (23.3MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Statin does not affect Niemann-Pick C1 protein levels. Huh7 cells were treated with indicated concentrations of statins or DMSO. Levels of Niemann-Pick C1 protein (NPC1) and actin were analyzed by Western blotting. Download FIG S3, TIF file, 22.9 MB (23.5MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
We next evaluated the effect of mevalonate on EBOV preGP maturation. Cells were treated with increasing concentrations of mevalonate, and GP1 and preGP expression levels were determined. As shown in Fig. 4C, increasing mevalonate concentrations at least partially reversed statin-mediated inhibition of mature GP1 expression. In parallel, a decrease in immature preGP expression was observed. Densitometry analysis indicated an increase in the GP1/(GP1 + preGP) ratio, indicating restoration of preGP maturation to GP1. This ratio did not change in cells treated only with mevalonate. Partial rescue of preGP processing efficiency and viral titers (Fig. 2A) by mevalonate in statin-treated cells is consistent with statin reducing viral titers by a mechanism impeding preGP maturation.
Statin treatment inhibits preGP glycan maturation.
EBOV GP1,2 is cleaved into subunits GP1 and GP2 by the proprotein convertase furin at the RRTRR501 site. To investigate whether statin inhibits maturation of preGP glycan, we investigated the effect of statin on mutant EBOV GP1,2 resistant to furin cleavage. To generate this mutant, we replaced the RRTRR cleavage site with AGTAA, as described previously (44, 45). Huh7 cells were transfected with plasmids encoding wild-type or furin-resistant mutant EBOV GP1,2 and treated with either statin or furin-like protease inhibitor (proprotein convertase inhibitor). GP1,2 levels were determined in cell lysates by Western blotting. Unlike wild-type EBOV GP1,2, which was processed into GP1, mutant GP1,2 was observed as a higher-molecular-weight form of preGP consistent with the molecular weight of GP0 (36) (Fig. 4D). Treating mutant EBOV GP1,2-transfected cells with statin resulted in a dose-dependent decrease in GP0 level compared to vehicle control, similar to samples with wild-type GP1,2. No changes in preGP levels were detected in cells expressing either wild-type or furin-cleavage-resistant EBOV GP1,2. In addition, the GP0/(GP0 + preGP) ratio in cells expressing the mutant EBOV GP1,2 was similar to that in cells expressing wild-type GP1,2, indicating that statin treatment affected GP1 glycan maturation independently of GP0 cleavage by furin.
Statin treatment results in GP1-deficient virions.
To confirm the effect of statin treatment on EBOV GP maturation, we infected Huh7 cells with EBOV, treated the cells with DMSO or statin, and partially purified the viral particles released from infected cells. GP1,2 and VP40 expression in cell lysates and corresponding viral particles from statin-treated cells was analyzed by Western blotting. As shown in Fig. 5, blotting cell lysate samples from EBOV-infected, vehicle-treated cells showed bands of preGP (~110 kDa) and GP1 (~140 kDa), as expected. Treatment with statin resulted in a selective decrease in GP1 levels compared to preGP and VP40. These results are consistent with statin inhibiting preGP maturation of GP1, as was observed in GP1,2-transfected cells.
FIG 5 .
Statin inhibits GP processing and incorporation of GP1 into EBOV particles. Huh7 cells infected with EBOV at an MOI of 2.0 were treated with DMSO or statin. Supernatants and cell lysates were collected, and EBOV particles were purified from supernatants by ultracentrifugation through a sucrose cushion. Purified EBOV particles were resuspended in 2× sample lysis buffer, and levels of GP and VP40 were analyzed by Western blotting. Percentage of GP1 was determined by dividing the signal of GP1 over the total amount of glycoprotein recognized by GP1 MAb [GP1/(GP1 + preGP)].
Analysis of viral particles from supernatants showed GP1 migrating at ~140 kDa in the pelleted virions, and no uncleaved preGP or GP0 was detected (Fig. 5). In cells treated with statin, GP1 levels decreased in a dose-dependent manner while VP40 levels were unchanged. These results are consistent with the concept that statin treatment resulted in production of VP40-containing EBOV particles deficient in GP1. Taken together, these results indicate that the lowered expression of GP1 in statin-treated, EBOV-infected cells results in reduced incorporation of GP1 into EBOV particles, leading to lower infectivity.
Multiple statins show antiviral activity against EBOV.
To investigate the efficacy of other commonly prescribed statins, we compared the antiviral activities of lovastatin, fluvastatin, simvastatin, atorvastatin, rosuvastatin, and pitavastatin in Huh7 cells. As shown in Fig. 6A, at all doses tested, simvastatin and pitavastatin reduced EBOV infectious particle production most potently: 50 µM simvastatin or pitavastatin reduced viral titers by 2.5 log. Rosuvastatin inhibited EBOV production least effectively, reducing viral titers by ~1 log, while lovastatin, atorvastatin, and fluvastatin were moderately effective, reducing titers by 1.7, 1.5, and 1.4 log, respectively. The viability of untreated cells was similar to that of cells treated with each statin (Fig. 6B).
FIG 6 .
Antiviral activity of other statins against EBOV. (A) Huh7 cells were infected with Ebola virus (EBOV) at an MOI of 0.05. After infection, cells were washed and then treated with various concentrations of lovastatin, fluvastatin, simvastatin, atorvastatin, rosuvastatin, and pitavastatin or DMSO (control). Culture supernatants were harvested 72 hpi, and viral titers were quantified by 50% tissue culture infective dose (TCID50) determination. (B) Viability (percentage) of Huh7 cells treated with lovastatin, fluvastatin, simvastatin, atorvastatin, rosuvastatin, and pitavastatin or DMSO (control) was determined after 72 h of treatment. Values were normalized to DMSO-treated controls.
DISCUSSION
Statins, well-known cholesterol-lowering drugs, have been proposed as therapeutic agents against certain viruses (16–18, 21, 22, 48–59). Statins are known for their anti-inflammatory and immunomodulatory effects as well as for preserving endothelial integrity; inflammation, immune system dysregulation, and endothelial dysfunction are major contributors of EVD pathogenesis (3, 60–62). Statin use was suggested as an adjunct therapy for EVD during the 2013–2016 outbreak (63). A clinical trial evaluating atorvastatin for use in EVD was registered with clinicaltrials.gov (NCT02380625), but the trial was never initiated, presumably because the outbreak was waning prior to the scheduled study start date. Here, we provide evidence of the antiviral effects of statin treatment in a human liver cell line and in primary human macrophages, both major target cells of EBOV. The antiviral activity of statin in Huh7 cells was due to loss in particle infectivity rather than inhibition of viral entry (Fig. 3). Statin reduced the levels of GP1, the envelope glycoprotein responsible for receptor binding and entry into cells, in GP1,2-transfected (Fig. 4) and EBOV-infected (Fig. 5) cells. Finally, we found that virus particles produced in statin-treated cells had lower levels of GP1 relative to VP40 matrix protein than did control cells. Thus, statin’s antiviral activity was due to its interference in GP1 maturation, leading to production of EBOV particles with impaired infectivity.
While GP1 levels were reduced in infected cells and in released EBOV particles, VP40 levels were not (see Fig. S2 in the supplemental material). Similarly, qRT-PCR analysis showed no changes in the extracellular levels of EBOV RNA after statin treatment, while TCID50 values were reduced upon statin treatment (Fig. 3). Interaction of VP40 with minigenome RNA has been reported to be sufficient for packaging RNA into virus-like particles (64). Our observation that the levels of extracellular VP40 and genomic RNA did not change even in the presence of little GP1 is consistent with this report (64) and supports the idea that an interaction between the ribonucleoprotein components and VP40 is a critical step for the budding and release of viral particles (65).
Despite the abundant preGP present in cells transfected with furin-cleavage-resistant EBOV GP1,2, statin treatment resulted in decreased GP0 levels similar to those seen in cells transfected with wild-type EBOV GP1,2 (Fig. 4D). This indicates that statin affects a step prior to GP0 cleavage, possibly by blocking transport of preGP out of the ER (36, 38). The observed decrease in the GP1 steady-state levels could be due to degradation of GP1 (via ER-associated or proteasomal degradation) resulting from its prolonged ER residency because of improper or insufficient maturation of preGP. Since cholesterol levels are lowest in the ER in the secretory pathway (66), ER-resident events involving transmembrane proteins might be particularly sensitive to very small deviations in cholesterol levels from a critical threshold. While most of statins’ effect is associated with lowering cellular cholesterol levels, statins also blunt the nonsterol branch of the mevalonate pathway, decreasing formation of isoprenoids and altering protein prenylation, an often critical event in posttranslational modulation of proteins (67). Inhibitors of isoprenoid intermediates, such as geranylgeranyltransferase inhibitor (GGTI), which inhibits prenylation of Rho proteins, or farnesyltransferase inhibitor (FTI), which inhibits the prenylation of the Ras proteins geranyltransferase and farnesyltransferase, have been effective against certain viruses (19, 51). Whether statin’s effects on GP1 processing are mediated through the isoprenoid pathway is currently unclear and needs further investigation.
All statins tested in our study reduced EBOV titers, although with variable efficacy. Simvastatin and pitavastatin inhibited EBOV production most potently (0.5- to 2.5-log reduction at 5 to 50 µM concentrations). Differences in the antiviral effects of individual statins may be due to many factors, such as chemical structures of each compound affecting pharmacokinetics and pharmacodynamics (68). One limitation of our study is that higher concentrations of statins were required to inhibit EBOV replication in vitro than are achievable in humans using current dosing regimens, as plasma levels of statins are usually low (maximum concentration of drug in serum [Cmax], 0.019 to 0.031 µM for simvastatin and 0.005 µM for lovastatin, based on 40-mg oral dose [69]). Although statin concentrations are likely to be much higher in the liver (69), a major site of EBOV replication, comprehensive in vivo studies in appropriate animal models are required. Unfortunately, statins do not reliably decrease circulating cholesterol concentrations in rodents (70–72), and thus, such studies would require nonhuman primate models that recapitulate human EVD signs (73).
In summary, we provide evidence that statin treatment decreases production of infectious EBOV virions in a human liver cell line (Huh7) and primary human macrophages, both of which are primary target cells for EBOV infection. Statin reduced production of infectious EBOV particles in Huh7 cells by interfering with GP processing and reducing the amount of GP1 incorporated into virus particles. The results of this study clearly show that statin inhibits EBOV infection. Our results, combined with statins’ known role in suppressing inflammation (74) and preserving endothelial integrity (75), pathways that are impaired in EVD, argue for a potential benefit of using statins as adjunctive therapy in patients with EVD. Ideally, the use of an antiviral that exhibits additional effects in combination with a statin has the potential both to block virus replication and to decrease the deleterious effects of inflammation on the host. Clearly, the next step for evaluating statins for use in EVD would require testing in a nonhuman primate model of disease to ensure both safety and potential efficacy.
MATERIALS AND METHODS
Biosafety.
All work with infectious virus was conducted in a biosafety level 4 laboratory at the Centers for Disease Control and Prevention (CDC; Atlanta, GA) according to the guidelines of CDC standard operating procedures.
Cells, virus, plasmids, reagents, and antibodies.
Huh7 cells were from Apath, LLC (Brooklyn, NY), and were propagated in Dulbecco’s modified Eagle’s medium (DMEM) with 10% (vol/vol) fetal calf serum (FCS; HyClone, Thermo Fisher Scientific, Waltham, MA) and 1× nonessential amino acids (Life Technologies, Grand Island, NY, USA). For statin treatment, sterol-depleted medium (LPDS; Sigma-Aldrich, St. Louis, MO, USA; not heat inactivated) was used instead of medium with FCS to eliminate the possible uptake of cholesterol from the medium, as previously reported (47). Human monocyte-derived macrophages were isolated as described previously (76). Vero-E6 cells were obtained from the CDC core facility and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) FCS and 1% penicillin-streptomycin (Life Technologies). Wild-type EBOV variant Mayinga (Ebola virus/H. sapiens-tc/COD/1976/Yambuku-Mayinga) was from the CDC Viral Special Pathogens Branch reference collection. Lovastatin was from Calbiochem (Billerica, MA). Human LDL was purchased from Sigma-Aldrich (St. Louis, MO, USA). Mevalonate was synthesized according to the method of Goldstein et al. (77).
The plasmid encoding EBOV GP1,2 was designed to express a human codon-optimized synthetic gene (GPco) corresponding to that of the EBOV Mayinga isolate. Briefly, the GPco (without RNA editing site) gene was purchased from GenScript (Piscataway, NJ) and cloned into the polymerase II (PolII) expression vector pCAGGS (78). EBOV GP resistant to furin cleavage (RRTRR501 cleavage site replaced with AGTAA) was purchased from GenScript as described previously (44, 45). The plasmid encoding Flag-tagged VP40 has been described previously (79).
The following antibodies were used in this study: rabbit polyclonal antibody against EBOV NP (IBT Bioservices, Rockville, MD), rabbit polyclonal antibody against EBOV GP (IBT Bioservices), and rabbit polyclonal antibody against EBOV for immunofluorescence (in-house reagent 70331; Viral Special Pathogens Branch, CDC, Atlanta, GA). The anti-Flag antibody and mouse monoclonal anti-actin antibody were from Sigma (Sigma-Aldrich, St. Louis, USA). NPC1 antibody was from Abcam (Cambridge, MA).
Transfection and infection.
To determine the effects of statin on GP processing or VP40 expression, 2.0 × 105 Huh7 cells plated in 12-well plates were transiently transfected with plasmids expressing either EBOV GP or Flag-tagged VP40; transfections were done using LT-1 reagent according to the manufacturer’s instructions (Mirus, Madison, WI). After 24 h, cells were treated with statin in LPDS-containing DMEM; cells were harvested 48 h posttransfection. For EBOV infection, Huh7 cells were plated at 2 × 105 cells per well in 12-well plates. The next day, cells were infected with EBOV at the indicated MOI for 1 h. For control experiments, Huh7 cells were infected with adenovirus type 5 (ATCC, Manassas, VA) at an MOI of 0.05 for 1 h. Virus inoculum was removed, and cells were washed with serum-free medium. Fresh medium containing 10% LPDS and with or without statin was then added. Culture supernatants and cell lysates were harvested and analyzed as indicated.
TCID50 and cell viability determination.
Supernatants from EBOV-infected Huh7 cells and monocyte-derived macrophages were harvested 72 hpi, and virus titrations were performed in Vero-E6 cells. Three days postinfection, the cells were fixed, permeabilized, and stained to visualize viral proteins. For adenovirus type 5 titer, Vero-E6 cells were treated with 8 serial 10-fold dilutions of supernatants of infected Huh7 cells. After 10 days, the wells with cytopathic effects were counted for each dilution after crystal violet staining. Endpoint viral titers were determined, and TCID50 was calculated as described previously (80). Results represent mean titers, with error bars indicating standard deviations calculated from 3 independent experiments. For human monocyte-derived macrophages, results represent mean titers with standard deviations from 3 replicate wells, representative of 2 independent experiments.
Cell viability was determined on statin-treated and mock-infected cells, using CellTiter-Glo (Promega) according to the manufacturer’s instructions.
qRT-PCR.
Huh7 cells were infected with EBOV for the indicated times, and then RNA was isolated from cells or from supernatants of infected cells using the MagMAX-96 total RNA isolation kit (Thermo Fisher Scientific). To determine viral RNA copy numbers, RNA was extracted from supernatants of infected cells. Absolute quantification of viral RNA copy numbers was done by measuring EBOV NP copy numbers using a standard curve with known viral titers serially diluted 5-fold. qRT-PCR was performed with the EBOV NP assay (81). To measure cell-associated EBOV NP gene levels, Huh7 cells pretreated with statin for 48 h or with U18666A (positive control) for 1 h were infected with EBOV at an MOI of 3. Cells were harvested after 3 h, and RNA was isolated from the cells. NP RNA levels were measured by qRT-PCR as described above and normalized to GAPDH mRNA. Results represent mean percent normalized NP RNA levels, with error bars indicating standard deviations calculated from 3 independent experiments.
Western blotting.
At indicated times after transfection or infection, cell lysates were harvested by adding lysis buffer containing 50 mM NaCl, 5 mM EDTA, 1% NP-40, 1.0% SDS, and 0.5% sodium deoxycholate supplemented with a protease inhibitor cocktail (Sigma-Aldrich). Lysates from infected cells were gamma irradiated at 2 × 106 rads using a high-energy 60Co source to ensure complete virus inactivation, allowing work at biosafety level 2. Proteins were electrophoretically separated on either 3 to 8% Tris-acetate or 4 to 12% NuPAGE gels (Invitrogen) and transferred to nitrocellulose membranes. The membranes were blocked for 1 h with buffer containing Tris-buffered saline, 0.1% Tween 20, and 5% nonfat dry milk and then probed overnight at 4°C with primary antibodies. Membranes were developed using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence. After detection of primary antibodies, the membranes were stripped and reprobed with antiactin antibody as a loading control. Results shown are representative of 3 independent experiments.
Immunofluorescence.
At 72 hpi, the cells were washed twice with phosphate-buffered saline (PBS) and fixed with 10% formalin at room temperature for 20 min. After formalin fixation, the cells were washed three times with PBS and permeabilized with 0.1% Triton X-100 for intracellular staining. The primary antibodies were added at a 1:1,000 dilution in 1% bovine serum albumin in PBS for 1 h. The cells were then washed 3 times with PBS and incubated for 30 min with the secondary antibodies diluted 1:1,000 in 1% bovine serum albumin in PBS. Multiple final washes were done, and the images were taken using a Nikon Eclipse Ti-S.
Virion purification.
EBOV virions were partially purified similarly to the procedure reported for Lassa virus (82). Briefly, supernatants from DMSO- or statin-treated and EBOV-infected cells were clarified by centrifugation at 1,500 × g for 30 min. Clarified supernatants were subjected to ultracentrifugation (100,000 × g for 90 min at 4°C) through a 20% sucrose cushion to collect EBOV virions. Virions were suspended in 2× Western lysis buffer, gamma irradiated at 5 × 106 rads using a high-energy 60Co source, and analyzed by Western blotting to detect GP and VP40.
Endo H and PNGase F treatment.
In order to investigate the modifications of EBOV glycoproteins, cell lysates were digested with endo H or PNGase F (New England Biolabs, Ipswich, MA) according to the manufacturer’s instructions. The digested proteins were resolved by SDS-PAGE under reducing conditions and were analyzed by Western blotting.
Statistical analysis.
Error bars in graphs represent standard deviations of the means from comparing Student’s t tests for paired samples. Differences were considered significant for P values of <0.005.
ACKNOWLEDGMENTS
We thank Tanya Klimova for her excellent assistance with editing the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This work was conducted while Anita K. McElroy held a Burroughs Wellcome Career Award for Medical Scientists (1013362.01) and an NIH K08 (AI119448).
Footnotes
Citation Shrivastava-Ranjan P, Flint M, Bergeron É, McElroy AK, Chatterjee P, Albariño CG, Nichol ST, Spiropoulou CF. 2018. Statins suppress Ebola virus infectivity by interfering with glycoprotein processing. mBio 9:e00660-18. https://doi.org/10.1128/mBio.00660-18.
REFERENCES
- 1.Bixler SL, Duplantier AJ, Bavari S. 2017. Discovering drugs for the treatment of Ebola virus. Curr Treat Options Infect Dis 9:299–317. doi: 10.1007/s40506-017-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayden FG, Friede M, Bausch DG. 2017. Experimental therapies for Ebola virus disease: what have we learned? J Infect Dis 215:167–170. doi: 10.1093/infdis/jiw496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElroy A. 2015. Understanding bleeding in Ebola virus disease. Clin Adv Hematol Oncol 13:29–31. [PMC free article] [PubMed] [Google Scholar]
- 4.Muñoz-Fontela C, McElroy AK. 2017. Ebola virus disease in humans: pathophysiology and immunity. Curr Top Microbiol Immunol 411:141–169. doi: 10.1007/82_2017_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yende S, Milbrandt EB, Kellum JA, Kong L, Delude RL, Weissfeld LA, Angus DC. 2011. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med 39:1871–1878. doi: 10.1097/CCM.0b013e31821b8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus DC, van der Poll T. 2013. Severe sepsis and septic shock. N Engl J Med 369:2063. doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- 7.Ouellette DR, Moscoso EE, Corrales JP, Peters M. 2015. Sepsis outcomes in patients receiving statins prior to hospitalization for sepsis: comparison of in-hospital mortality rates between patients who received atorvastatin and those who received simvastatin. Ann Intensive Care 5:9. doi: 10.1186/s13613-015-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansur A, Steinau M, Popov AF, Ghadimi M, Beissbarth T, Bauer M, Hinz J. 2015. Impact of statin therapy on mortality in patients with sepsis-associated acute respiratory distress syndrome (ARDS) depends on ARDS severity: a prospective observational cohort study. BMC Med 13:128. doi: 10.1186/s12916-015-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao F, Linhartova L, Johnston AM, Thickett DR. 2008. Statins and sepsis. Br J Anaesth 100:288–298. doi: 10.1093/bja/aem406. [DOI] [PubMed] [Google Scholar]
- 10.Fedson DS, Jacobson JR, Rordam OM, Opal SM. 2015. Treating the host response to Ebola virus disease with generic statins and angiotensin receptor blockers. mBio 6:e00716-15. doi: 10.1128/mBio.00716-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein JL, Brown MS. 1990. Regulation of the mevalonate pathway. Nature 343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 12.Simons K, Toomre D. 2000. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 13.Heaton NS, Randall G. 2011. Multifaceted roles for lipids in viral infection. Trends Microbiol 19:368–375. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzon M, Mercer J. 2014. Lipid interactions during virus entry and infection. Cell Microbiol 16:1493–1502. doi: 10.1111/cmi.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bavari S, Bosio CM, Wiegand E, Ruthel G, Will AB, Geisbert TW, Hevey M, Schmaljohn C, Schmaljohn A, Aman MJ. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med 195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt AA, Yan P, Bonilla H, Abou-Samra AB, Shaikh OS, Simon TG, Chung RT, Rogal SS, ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) Study Team . 2015. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: results from ERCHIVES. Hepatology 62:365–374. doi: 10.1002/hep.27835. [DOI] [PubMed] [Google Scholar]
- 17.Gower TL, Graham BS. 2001. Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro. Antimicrob Agents Chemother 45:1231–1237. doi: 10.1128/AAC.45.4.1231-1237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange PT, Darrah EJ, Vonderhaar EP, Mboko WP, Rekow MM, Patel SB, Sidjanin DJ, Tarakanova VL. 2016. Type I interferon counteracts antiviral effects of statins in the context of gammaherpesvirus infection. J Virol 90:3342–3354. doi: 10.1128/JVI.02277-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordier BB, Marion PL, Ohashi K, Kay MA, Greenberg HB, Casey JL, Glenn JS. 2002. A prenylation inhibitor prevents production of infectious hepatitis delta virus particles. J Virol 76:10465–10472. doi: 10.1128/JVI.76.20.10465-10472.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordier BB, Ohkanda J, Liu P, Lee SY, Salazar FH, Marion PL, Ohashi K, Meuse L, Kay MA, Casey JL, Sebti SM, Hamilton AD, Glenn JS. 2003. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J Clin Invest 112:407–414. doi: 10.1172/JCI17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amet T, Nonaka M, Dewan MZ, Saitoh Y, Qi X, Ichinose S, Yamamoto N, Yamaoka S. 2008. Statin-induced inhibition of HIV-1 release from latently infected U1 cells reveals a critical role for protein prenylation in HIV-1 replication. Microbes Infect 10:471–480. doi: 10.1016/j.micinf.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Rothwell C, Lebreton A, Young Ng C, Lim JY, Liu W, Vasudevan S, Labow M, Gu F, Gaither LA. 2009. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology 389:8–19. doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Argüello MB, Goñi FM, Pereira FB, Nieva JL. 1998. Phosphatidylinositol-dependent membrane fusion induced by a putative fusogenic sequence of Ebola virus. J Virol 72:1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller EH, Obernosterer G, Raaben M, Herbert AS, Deffieu MS, Krishnan A, Ndungo E, Sandesara RG, Carette JE, Kuehne AI, Ruthel G, Pfeffer SR, Dye JM, Whelan SP, Brummelkamp TR, Chandran K. 2012. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J 31:1947–1960. doi: 10.1038/emboj.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR. 2011. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soni SP, Stahelin RV. 2014. The Ebola virus matrix protein VP40 selectively induces vesiculation from phosphatidylserine-enriched membranes. J Biol Chem 289:33590–33597. doi: 10.1074/jbc.M114.586396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbert AS, Davidson C, Kuehne AI, Bakken R, Braigen SZ, Gunn KE, Whelan SP, Brummelkamp TR, Twenhafel NA, Chandran K, Walkley SU, Dye JM. 2015. Niemann-Pick C1 is essential for Ebolavirus replication and pathogenesis in vivo. mBio 6:e00565-15. doi: 10.1128/mBio.00565-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hacke M, Björkholm P, Hellwig A, Himmels P, Ruiz de Almodóvar C, Brügger B, Wieland F, Ernst AM. 2015. Inhibition of Ebola virus glycoprotein-mediated cytotoxicity by targeting its transmembrane domain and cholesterol. Nat Commun 6:7688. doi: 10.1038/ncomms8688. [DOI] [PubMed] [Google Scholar]
- 29.Spence JS, Krause TB, Mittler E, Jangra RK, Chandran K. 2016. Direct visualization of Ebola virus fusion triggering in the endocytic pathway. mBio 7:e01857-15. doi: 10.1128/mBio.01857-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoemaker CJ, Schornberg KL, Delos SE, Scully C, Pajouhesh H, Olinger GG, Johansen LM, White JM. 2013. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. PLoS One 8:e56265. doi: 10.1371/journal.pone.0056265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu F, Liang Q, Abi-Mosleh L, Das A, De Brabander JK, Goldstein JL, Brown MS. 2015. Identification of NPC1 as the target of U18666a, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife 4:e12177. doi: 10.7554/eLife.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J. 2011. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldmann H, Volchkov VE, Volchkova VA, Klenk HD. 1999. The glycoproteins of Marburg and Ebola virus and their potential roles in pathogenesis. Arch Virol Suppl 15:159–169. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez A, Yang ZY, Xu L, Nabel GJ, Crews T, Peters CJ. 1998. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J Virol 72:6442–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci U S A 93:3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volchkov VE, Feldmann H, Volchkova VA, Klenk HD. 1998. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc Natl Acad Sci U S A 95:5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiley MP, Regnery RL, Johnson KM. 1980. Ebola virus: identification of virion structural proteins. J Gen Virol 49:333–341. doi: 10.1099/0022-1317-49-2-333. [DOI] [PubMed] [Google Scholar]
- 38.Jeffers SA, Sanders DA, Sanchez A. 2002. Covalent modifications of the Ebola virus glycoprotein. J Virol 76:12463–12472. doi: 10.1128/JVI.76.24.12463-12472.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aman MJ. 2016. Chasing Ebola through the endosomal labyrinth. mBio 7:e00346-16. doi: 10.1128/mBio.00346-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White JM, Schornberg KL. 2012. A new player in the puzzle of filovirus entry. Nat Rev Microbiol 10:317–322. doi: 10.1038/nrmicro2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazarowitz SG, Choppin PW. 1975. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology 68:440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- 42.McCune JM, Rabin LB, Feinberg MB, Lieberman M, Kosek JC, Reyes GR, Weissman IL. 1988. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 53:55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- 43.Lenz O, ter Meulen J, Klenk HD, Seidah NG, Garten W. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc Natl Acad Sci U S A 98:12701–12705. doi: 10.1073/pnas.221447598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann G, Feldmann H, Watanabe S, Lukashevich I, Kawaoka Y. 2002. Reverse genetics demonstrates that proteolytic processing of the Ebola virus glycoprotein is not essential for replication in cell culture. J Virol 76:406–410. doi: 10.1128/JVI.76.1.406-410.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumann G, Geisbert TW, Ebihara H, Geisbert JB, Daddario-DiCaprio KM, Feldmann H, Kawaoka Y. 2007. Proteolytic processing of the Ebola virus glycoprotein is not critical for Ebola virus replication in nonhuman primates. J Virol 81:2995–2998. doi: 10.1128/JVI.02486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ströher U, Willihnganz L, Jean F, Feldmann H. 2007. Blockage of filoviral glycoprotein processing by use of a protein-based inhibitor. J Infect Dis 196(Suppl 2):S271–S275. doi: 10.1086/520592. [DOI] [PubMed] [Google Scholar]
- 47.Gbelcová H, Svéda M, Laubertová L, Varga I, Vítek L, Kolář M, Strnad H, Zelenka J, Böhmer D, Ruml T. 2013. The effect of simvastatin on lipid droplets accumulation in human embryonic kidney cells and pancreatic cancer cells. Lipids Health Dis 12:126. doi: 10.1186/1476-511X-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng J, Zhang D, Ma Y, Wang G, Guo Z, Lu J. 2014. Protective effect of fluvastatin on influenza virus infection. Mol Med Rep 9:2221–2226. doi: 10.3892/mmr.2014.2076. [DOI] [PubMed] [Google Scholar]
- 49.Mehrbod P, Hair-Bejo M, Tengku Ibrahim TA, Omar AR, El Zowalaty M, Ajdari Z, Ideris A. 2014. Simvastatin modulates cellular components in influenza A virus-infected cells. Int J Mol Med 34:61–73. doi: 10.3892/ijmm.2014.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hui KP, Kuok DI, Kang SS, Li HS, Ng MM, Bui CH, Peiris JS, Chan RW, Chan MC. 2015. Modulation of sterol biosynthesis regulates viral replication and cytokine production in influenza A virus infected human alveolar epithelial cells. Antiviral Res 119:1–7. doi: 10.1016/j.antiviral.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Ye J, Wang C, Sumpter R Jr., Brown MS, Goldstein JL, Gale M Jr.. 2003. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci U S A 100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohan KV, Muller J, Atreya CD. 2008. Defective rotavirus particle assembly in lovastatin-treated MA104 cells. Arch Virol 153:2283–2290. doi: 10.1007/s00705-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazière JC, Landureau JC, Giral P, Auclair M, Fall L, Lachgar A, Achour A, Zagury D. 1994. Lovastatin inhibits HIV-1 expression in H9 human T lymphocytes cultured in cholesterol-poor medium. Biomed Pharmacother 48:63–67. doi: 10.1016/0753-3322(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 54.Martínez-Gutierrez M, Castellanos JE, Gallego-Gómez JC. 2011. Statins reduce dengue virus production via decreased virion assembly. Intervirology 54:202–216. doi: 10.1159/000321892. [DOI] [PubMed] [Google Scholar]
- 55.Kim SS, Peng LF, Lin W, Choe WH, Sakamoto N, Kato N, Ikeda M, Schreiber SL, Chung RT. 2007. A cell-based, high-throughput screen for small molecule regulators of hepatitis C virus replication. Gastroenterology 132:311–320. doi: 10.1053/j.gastro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 56.Bajimaya S, Hayashi T, Frankl T, Bryk P, Ward B, Takimoto T. 2017. Cholesterol reducing agents inhibit assembly of type I parainfluenza viruses. Virology 501:127–135. doi: 10.1016/j.virol.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehrbod P, El Zowalaty M, Omar AR, Hair-Bejo M, Ideris A. 2012. Statins reduce the expression of proinflammatory cytokines in influenza A virus infected CrFK cells. Acta Virol 56:353–355. doi: 10.4149/av_2012_04_353. [DOI] [PubMed] [Google Scholar]
- 58.del Real G, Jiménez-Baranda S, Mira E, Lacalle RA, Lucas P, Gómez-Moutón C, Alegret M, Peña JM, Rodríguez-Zapata M, Alvarez-Mon M, Martínez-A C, Mañes S. 2004. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med 200:541–547. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montoya CJ, Jaimes F, Higuita EA, Convers-Páez S, Estrada S, Gutierrez F, Amariles P, Giraldo N, Peñaloza C, Rugeles MT. 2009. Antiretroviral effect of lovastatin on HIV-1-infected individuals without highly active antiretroviral therapy (the LIVE study): a phase-II randomized clinical trial. Trials 10:41. doi: 10.1186/1745-6215-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McElroy AK, Erickson BR, Flietstra TD, Rollin PE, Nichol ST, Towner JS, Spiropoulou CF. 2014. Ebola hemorrhagic fever: novel biomarker correlates of clinical outcome. J Infect Dis 210:558–566. doi: 10.1093/infdis/jiu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McElroy AK, Akondy RS, Davis CW, Ellebedy AH, Mehta AK, Kraft CS, Lyon GM, Ribner BS, Varkey J, Sidney J, Sette A, Campbell S, Ströher U, Damon I, Nichol ST, Spiropoulou CF, Ahmed R. 2015. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci U S A 112:4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lyon GM, Mehta AK, Varkey JB, Brantly K, Plyler L, McElroy AK, Kraft CS, Towner JS, Spiropoulou C, Ströher U, Uyeki TM, Ribner BS, Emory Serious Communicable Diseases Unit . 2014. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med 371:2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 63.Fedson DS. 2016. Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola. Ann Transl Med 4:421. doi: 10.21037/atm.2016.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson RF, McCarthy SE, Godlewski PJ, Harty RN. 2006. Ebola virus VP35-VP40 interaction is sufficient for packaging 3E-5E minigenome RNA into virus-like particles. J Virol 80:5135–5144. doi: 10.1128/JVI.01857-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spiegelberg L, Wahl-Jensen V, Kolesnikova L, Feldmann H, Becker S, Hoenen T. 2011. Genus-specific recruitment of filovirus ribonucleoprotein complexes into budding particles. J Gen Virol 92:2900–2905. doi: 10.1099/vir.0.036863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikonen E. 2008. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol 9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 67.Greenwood J, Steinman L, Zamvil SS. 2006. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schachter M. 2005. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19:117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 69.Björkhem-Bergman L, Lindh JD, Bergman P. 2011. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br J Clin Pharmacol 72:164–165. doi: 10.1111/j.1365-2125.2011.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Endo A. 1992. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res 33:1569–1582. doi: 10.1016/j.atherosclerosissup.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 71.Björkhem-Bergman L, Acimovic J, Torndal UB, Parini P, Eriksson LC. 2010. Lovastatin prevents carcinogenesis in a rat model for liver cancer. Effects of ubiquinone supplementation. Anticancer Res 30:1105–1112. [PubMed] [Google Scholar]
- 72.Endo A, Tsujita Y, Kuroda M, Tanzawa K. 1979. Effects of ML-236B on cholesterol metabolism in mice and rats: lack of hypocholesterolemic activity in normal animals. Biochim Biophys Acta 575:266–276. doi: 10.1016/0005-2760(79)90028-6. [DOI] [PubMed] [Google Scholar]
- 73.Geisbert TW, Strong JE, Feldmann H. 2015. Considerations in the use of nonhuman primate models of Ebola virus and Marburg virus infection. J Infect Dis 212(Suppl 2):S91–S97. doi: 10.1093/infdis/jiv284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blake GJ, Ridker PM. 2000. Are statins anti-inflammatory? Curr Control Trials Cardiovasc Med 1:161–165. doi: 10.1186/cvm-1-3-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sandhu K, Mamas M, Butler R. 2017. Endothelial progenitor cells: exploring the pleiotropic effects of statins. World J Cardiol 9:1–13. doi: 10.4330/wjc.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McElroy AK, Nichol ST. 2012. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology 422:6–12. doi: 10.1016/j.virol.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldstein JL, Basu SK, Brown MS. 1983. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol 98:241–260. [DOI] [PubMed] [Google Scholar]
- 78.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199. doi: 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
- 79.Guito JC, Albariño CG, Chakrabarti AK, Towner JS. 2017. Novel activities by Ebolavirus and Marburgvirus interferon antagonists revealed using a standardized in vitro reporter system. Virology 501:147–165. doi: 10.1016/j.virol.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohr EL, McMullan LK, Lo MK, Spengler JR, Bergeron É, Albariño CG, Shrivastava-Ranjan P, Chiang CF, Nichol ST, Spiropoulou CF, Flint M. 2015. Inhibitors of cellular kinases with broad-spectrum antiviral activity for hemorrhagic fever viruses. Antiviral Res 120:40–47. doi: 10.1016/j.antiviral.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Towner JS, Sealy TK, Ksiazek TG, Nichol ST. 2007. High-throughput molecular detection of hemorrhagic fever virus threats with applications for outbreak settings. J Infect Dis 196(Suppl 2):S205–S212. doi: 10.1086/520601. [DOI] [PubMed] [Google Scholar]
- 82.Shrivastava-Ranjan P, Bergeron É, Chakrabarti AK, Albariño CG, Flint M, Nichol ST, Spiropoulou CF. 2016. 25-Hydroxycholesterol inhibition of Lassa virus infection through aberrant GP1 glycosylation. mBio 7:e01808-16. doi: 10.1128/mBio.01808-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statin does not affect adenovirus type 5 titers. Huh7 cells were infected with human adenovirus type 5 (Ad5) at an MOI of 0.05. Three days postinfection, titers of infectious virus in cell supernatants were determined by a standard TCID50 titration method. Download FIG S1, TIF file, 22.6 MB (23.2MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Statin does not affect VP40 expression level. Huh7 cells were transfected with a plasmid expressing EBOV Flag-tagged VP40 and treated with statin or DMSO. VP40 and actin expression in cell lysates was analyzed by Western blotting. Download FIG S2, TIF file, 22.7 MB (23.3MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Statin does not affect Niemann-Pick C1 protein levels. Huh7 cells were treated with indicated concentrations of statins or DMSO. Levels of Niemann-Pick C1 protein (NPC1) and actin were analyzed by Western blotting. Download FIG S3, TIF file, 22.9 MB (23.5MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.