ABSTRACT
Microgreens, like sprouts, are relatively fast-growing products and are generally consumed raw. Moreover, as observed for sprouts, microbial contamination from preharvest sources may also be present in the production of microgreens. In this study, two Salmonella enterica serovars (Hartford and Cubana), applied at multiple inoculation levels, were evaluated for survival and growth on alfalfa sprouts and Swiss chard microgreens by using the most-probable-number (MPN) method. Various abiotic factors were also examined for their effects on Salmonella survival and growth on sprouts and microgreens. Community-level physiological profiles (CLPPs) of sprout/microgreen rhizospheres with different levels of S. enterica inoculation at different growth stages were characterized by use of Biolog EcoPlates. In the seed contamination group, the ability of S. enterica to grow on sprouting alfalfa seeds was affected by both seed storage time and inoculation level but not by serovar. However, the growth of S. enterica on Swiss chard microgreens was affected by serovar and inoculation level. Seed storage time had little effect on the average level of Salmonella populations in microgreens. In the irrigation water contamination group, the growth of Salmonella on both alfalfa sprouts and microgreens was largely affected by inoculation level. Surprisingly, the growth medium was found to play an important role in Salmonella survival and growth on microgreens. CLPP analysis showed significant changes in the microbial community metabolic diversity during sprouting for alfalfa sprouts, but few temporal changes were seen with microgreens. The data suggest that the change in rhizosphere bacterial functional diversity was dependent on the host but independent of Salmonella contamination.
IMPORTANCE Sprouts and microgreens are considered “functional foods,” i.e., foods containing health-promoting or disease-preventing properties in addition to normal nutritional values. However, the microbial risk associated with microgreens has not been well studied. This study evaluated Salmonella survival and growth on microgreens compared to those on sprouts, as well as other abiotic factors that could affect Salmonella survival and growth on microgreens. This work provides baseline data for risk assessment of microbial contamination of sprouts and microgreens. Understanding the risks of Salmonella contamination and its effects on rhizosphere microbial communities enables a better understanding of host-pathogen dynamics in sprouts and microgreens. The data also contribute to innovative preventive control strategies for Salmonella contamination of sprouts and microgreens.
KEYWORDS: growth, alfalfa sprouts, Swiss chard microgreens, Salmonella enterica, CLPP
INTRODUCTION
Sprouts, mainly originating from the Leguminosae family, and microgreens, a new class of edible vegetables harvested and consumed at an immature stage, are both gaining popularity across the world as a significant source of vitamins, minerals, and phytochemicals (1–4). Thus, they are considered “functional foods,” i.e., foods containing health-promoting or disease-preventing properties in addition to normal nutritional values (5, 6). Traditionally, sprouts are produced entirely in water, but they can also be grown in soil and hydroponically. Sprouts are germinated or partially germinated seeds which, depending on the species, are typically eaten with the roots intact. Microgreens are harvested just above the roots when the cotyledons are fully formed or the first true leaves have emerged. They can be grown in soil or soil substitutes or hydroponically and require high-light conditions for efficient growth (7). Microgreens are halfway in size between sprouts and their older counterparts, such as baby spinach, but deliver the most in terms of flavor and nutritional values compared to the other two types of crops (3).
Since 2000, the number of sprout-related foodborne outbreaks has been on the rise, catalyzing a recurrent problem both in the United States and around the world. Between 2000 and 2016, for example, at least 17 salmonellosis outbreaks linked to the consumption of raw sprouts were documented internationally (8; http://www.outbreakdatabase.com; https://www.cdc.gov/foodsafety/outbreaks/index.html), nine of them in the United States. Most involved alfalfa sprouts, but cress, mung bean, and clover sprouts were also implicated. Although various routes of contamination have been noted, contaminated seeds appear to be the source of most sprout-associated foodborne illnesses and are considered the most common source of contamination (9–13). Irrigation water is another potential source of contamination during preharvest growth. Seed sprouting provides an excellent environment for the growth of microorganisms, including foodborne pathogens (14, 15). In fact, a level as low as 4 most-probable-number (MPN) Salmonella organisms per kg of seed was determined to be capable of causing a foodborne outbreak associated with sprouts (16). Unlike sprouts, microgreens so far have not been associated with any foodborne outbreaks. Nevertheless, considering that microgreens are consumed raw, like sprouts, and are relatively fast-growing products, similar microbial contamination risks from preharvest or postharvest sources may be present in the cultivation, harvesting, and marketing of microgreens as well. However, the microbial risk associated with microgreens has not been well studied (17, 18).
In order to determine the risk of microbial contamination and the survival of Salmonella on microgreens, Salmonella growth differences on alfalfa sprouts and Swiss chard microgreens were examined. The effects of Salmonella enterica serovar, initial inoculum dose, source of contamination, seed storage time, and growth medium on Salmonella levels on sprouts and microgreens were also examined in this study. Finally, community-level physiological profiles (CLPPs) were analyzed and compared to assess differences in microbial richness during the sprouting of alfalfa seeds and production of Swiss chard microgreens.
RESULTS
Salmonella enterica survival and growth on alfalfa sprouts and Swiss chard microgreens via contaminated seeds.
S. enterica serovars, inoculation levels, and seed storage times were examined to determine the factors affecting Salmonella growth on alfalfa sprouts and Swiss chard microgreens via contaminated seeds (SC group) (Table 1). Uninoculated sprouts and microgreens were used as plant controls. S. enterica serovar Cubana and S. Hartford isolates used in this study were also monitored in vitro by examining the growth curves for individual isolates at 25°C. No significant growth differences were found between these two isolates. At a seed inoculation level of ∼10 CFU/g, S. Hartford levels in most sprout samples (6/8 samples) were below the detection level (−0.447 log MPN/g sprouts) after contaminated seeds were stored for 7 days at 4°C. The Salmonella population was able to increase by at least 1 log in 1/8 alfalfa sprout samples. Even after the seeds were stored for 28 days at 4°C, 3 of 8 sprout samples were still positive with S. Hartford, at ∼2 log MPN/g sprouts. Similarly, S. Cubana was capable of surviving through 28-day storage and grew to 2 log MPN/g sprouts on germinating alfalfa sprouts in 2/8 sprout samples. As the inoculation level was increased to ∼102 CFU/g, the numbers of S. Hartford- and S. Cubana-positive sprout samples also increased for seeds stored at 4°C for 7 days (9/16 and 11/16 samples, respectively) and 28 days (5/16 and 5/16 samples, respectively). Control sprout samples were all negative for S. enterica. Collectively, as shown in Table 2, the mean levels of Salmonella populations did not differ significantly between serovars (P = 0.7631). However, significant differences in Salmonella growth were observed between seed storage times (P < 0.05) and between inoculation levels (P < 0.05). Analysis of variance (ANOVA) indicated that the differences between the inoculation levels with respect to the mean levels of S. enterica populations on sprout samples (mean log MPN per gram of sprouts) were dependent on the storage time (P < 0.001).
TABLE 1.
Salmonella enterica survival and growth on alfalfa sprouts and Swiss chard microgreens via contaminated seeds
| Commodity | S. enterica serovar | Inoculation level (CFU/g seed) | Contaminated seed storage time (days) | No. of positive samples/total no. of samples | Maximum log MPN/g fresh wt |
|---|---|---|---|---|---|
| Alfalfa sprouts | Cubana | 10 | 7 | 0/8 | 0 |
| 28 | 2/8 | 2.04 | |||
| 102 | 7 | 11/16 | 3.04 | ||
| 28 | 5/16 | 2.04 | |||
| Hartford | 10 | 7 | 2/8 | 2.04 | |
| 28 | 3/8 | 2.04 | |||
| 102 | 7 | 9/16 | 2.04 | ||
| 28 | 5/16 | 2.04 | |||
| Swiss chard microgreensa | Cubana | 10 | 7 | 4/8 | 2.04 |
| 28 | 2/8 | 3.04 | |||
| 102 | 7 | 11/12 | 2.04 | ||
| 28 | 11/16 | 3.04 | |||
| Hartford | 10 | 7 | 8/8 | 2.04 | |
| 28 | 3/8 | 3.04 | |||
| 102 | 7 | 12/12 | 2.04 | ||
| 28 | 16/16 | 3.04 |
Microgreens were grown in soil A.
TABLE 2.
Factors affecting S. enterica growth on alfalfa sprouts and Swiss chard microgreens via seed contamination
| Plant type and factor(s) | df | Sum of squares | Mean square value | F value | P(>F) value | Statistical significancea |
|---|---|---|---|---|---|---|
| Alfalfa sprouts | ||||||
| Inoculation level | 1 | 4.57 | 4.572 | 6.542 | 0.0122 | * |
| Storage time | 1 | 2.92 | 2.917 | 4.173 | 0.0441 | * |
| Serovar | 1 | 0.06 | 0.064 | 0.091 | 0.7631 | |
| Inoculation level × storage time | 1 | 12.35 | 12.347 | 17.667 | 6.3E−05 | *** |
| Inoculation level × serovar | 1 | 0.03 | 0.028 | 0.040 | 0.8427 | |
| Storage time × serovar | 1 | 1.20 | 1.196 | 1.711 | 0.1943 | |
| Inoculation level × storage time × serovar | 1 | 0.05 | 0.055 | 0.079 | 0.7798 | |
| Residuals | 88 | 61.50 | 0.699 | |||
| Swiss chard microgreens | ||||||
| Inoculation level | 1 | 10.06 | 10.056 | 11.375 | 0.00120 | ** |
| Storage time | 1 | 0.10 | 0.105 | 0.119 | 0.73140 | |
| Serovar | 1 | 8.44 | 8.438 | 9.544 | 0.00285 | ** |
| Part | 1 | 0.01 | 0.012 | 0.014 | 0.90767 | |
| Inoculation level × storage time | 1 | 1.16 | 1.163 | 1.315 | 0.25521 | |
| Inoculation level × serovar | 1 | 0.07 | 0.066 | 0.075 | 0.78505 | |
| Storage time × serovar | 1 | 1.53 | 1.529 | 1.730 | 0.19261 | |
| Inoculation level × part | 1 | 0.03 | 0.028 | 0.031 | 0.85977 | |
| Storage time × part | 1 | 0.03 | 0.028 | 0.031 | 0.85989 | |
| Serovar × part | 1 | 0.00 | 0.001 | 0.001 | 0.97014 | |
| Inoculation level × storage time × serovar | 1 | 1.42 | 1.424 | 1.611 | 0.20850 | |
| Inoculation level × storage time × part | 1 | 0.32 | 0.315 | 0.356 | 0.55238 | |
| Inoculation level × serovar × part | 1 | 0.67 | 0.673 | 0.762 | 0.38567 | |
| Storage time × serovar × part | 1 | 0.19 | 0.188 | 0.212 | 0.64641 | |
| Inoculation level × storage time × serovar × part | 1 | 0.00 | 0.000 | 0.000 | 0.99396 | |
| Residuals | 72 | 63.65 | 0.884 |
*, P < 0.05; **, P < 0.01; ***, P < 0.001.
In the case of Swiss chard microgreens, mean log MPN of Salmonella per gram of microgreens were significantly different between the two serovars (P < 0.01) and between inoculation levels (P < 0.01). However, seed storage time did not affect Salmonella growth on microgreens (P > 0.05), and no significant interaction was found among all the factors tested (Table 2). Additionally, at a seed inoculation level of ∼10 CFU/g, all eight microgreen samples from the seeds stored for 7 days at 4°C (100%) were positive for S. Hartford, while only four samples (50%) were positive for S. Cubana. After seeds were stored for 28 days, the percentages of Salmonella-positive seeds decreased to similar levels for both serovars. Also, the differences in the percentages of Salmonella-positive seeds and the average levels of Salmonella populations on microgreens due to serovar were diminished after the seed inoculation level was increased to ∼102 CFU/g. The control microgreen samples were all negative for S. enterica.
S. enterica growth on alfalfa sprouts and Swiss chard microgreens via irrigation water contamination.
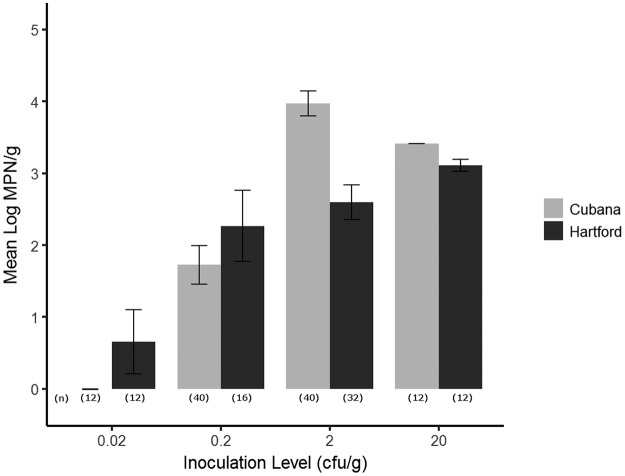
Various factors, including two S. enterica serovars and four inoculation levels, were examined to evaluate Salmonella growth on alfalfa sprouts and Swiss chard microgreens via contaminated irrigation water (IC group). In the alfalfa sprout trial (Table 3), similar to the results for Salmonella growth in the SC group, the mean levels of Salmonella populations in contaminated sprout samples did not differ significantly between serovars (P > 0.05). The growth of S. enterica on sprouting seeds was largely affected by inoculation level (P < 0.0001). However, analysis of variance signaled a significant interaction between inoculation level and serovar with respect to the level of the S. enterica population in sprout samples (MPN per gram of sprouts) (P < 0.001). Different patterns for the relationship between growth of Salmonella and inoculation level were observed for S. Hartford and S. Cubana, as shown in Fig. 1. It is interesting that at the level of 0.02 CFU/g seed (i.e., 0.002 CFU/ml H2O), S. Hartford was still able to proliferate in sprouts, to up to 3.9 log MPN/g sprouts in 2/12 samples, while S. Cubana was only able to grow on sprouts at −0.04 log MPN/g sprouts, in 1/12 samples. When the inoculation level was increased to 0.2 CFU/g seed (i.e., 0.02 CFU/ml), both serovars were able to grow in more than 50% of the sprout samples, and the population surged to up to 4.8 log MPN/g sprouts. At the level of 2 CFU/g seed (i.e., 0.2 CFU/ml), however, S. Cubana proliferated on all the sprout samples, and the maximum level of the Salmonella population was 5.0 log MPN/g sprouts in 41% of the sprout samples (13/32 samples). In contrast to S. Cubana, S. Hartford was present in 75% of the sprout samples (18/24 samples), and only 2/24 samples had Salmonella populations at 5.0 log MPN/g sprouts.
TABLE 3.
Factors influencing S. enterica growth on alfalfa sprouts via irrigation water contamination
| Factor(s) | df | Sum of squares | Mean square value | F value | P(>F) value | Statistical significancea |
|---|---|---|---|---|---|---|
| Inoculation level | 3 | 177.53 | 59.18 | 31.771 | 5.32E−16 | *** |
| Serovar | 1 | 4.49 | 4.49 | 2.409 | 0.1227 | |
| Inoculation level × serovar | 3 | 32.05 | 10.68 | 5.735 | 0.00096 | *** |
| Residuals | 152 | 283.13 | 1.86 |
*, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 1.
Salmonella enterica growth on alfalfa sprouts via irrigation water contamination. Sprouts were grown in a Conviron E7/2 climate-controlled growth chamber for the duration of the experiment. Before germination, growth chamber temperatures were maintained at 24°C (daytime) and 22°C (nighttime) with no light. Temperatures were maintained for 2 days at a constant 22°C day and night, with light, after germination. Growth chambers were kept at a constant relative humidity of 65%. At harvest, the mean log MPN per gram of fresh weight (y axis) was calculated against 4 different inoculation levels (x axis) for S. enterica serovars Cubana (gray) and Hartford (black). Numbers in parentheses show the numbers of replicated samples examined at each inoculation level. The detection limit for Salmonella in this experiment was 0.3 MPN/g.
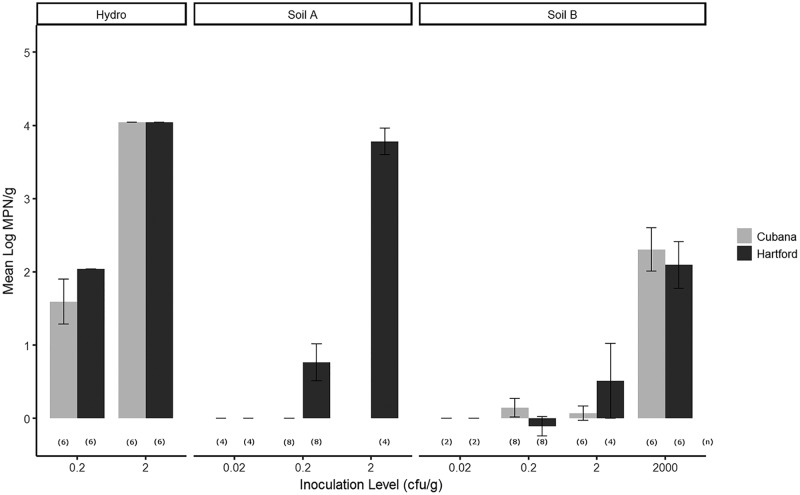
In the case of Swiss chard microgreens, the growth medium was also examined, along with other factors influencing the growth of Salmonella (Table 4). Overall, the growth of S. enterica on microgreens was significantly affected by the inoculation level (P < 0.0001). However, the difference in inoculation levels with respect to the mean level of S. enterica populations in microgreen samples was serovar and growth medium dependent. Specifically, at the level of 0.02 CFU/g seed, S. Hartford could not be detected in Swiss chard microgreen samples (i.e., was below the detection limit), while S. Cubana was found to be able to survive and grow to up to 2 log MPN/g in soil A. The percentage of Salmonella-positive samples largely increased with increases in inoculation level, and the difference between serovars was minimized as well. No significant difference (P > 0.05) was found between edible and nonedible parts of Swiss chard microgreens. Interestingly, the maximum level of Salmonella in the samples varied among different growth meda. For both serovars, compared to the levels in microgreens grown in soil B, the level of Salmonella could be 2 to 4 log higher per gram of sample for growth in soil A or hydroponically (Fig. 2).
TABLE 4.
Factors affecting S. enterica growth on Swiss chard microgreens grown hydroponically, in soil A, and in soil B via irrigation water contamination
| Growth condition and factor(s) | df | Sum of squares | Mean square value | F value | P(>F) value | Statistical significancea |
|---|---|---|---|---|---|---|
| Hydroponic system | ||||||
| Inoculation level | 1 | 39.56 | 39.56 | 211.448 | 1.42E−14 | *** |
| Serovar | 1 | 0.4 | 0.4 | 2.132 | 0.155 | |
| Inoculation level × serovar | 1 | 0.4 | 0.4 | 2.132 | 0.155 | |
| Residuals | 28 | 5.24 | 0.19 | |||
| Soil A | ||||||
| Inoculation level | 2 | 77.61 | 38.8 | 73.397 | 1.11E−12 | *** |
| Serovar | 1 | 2.4 | 2.4 | 4.542 | 0.0409 | * |
| Inoculation level × serovar | 1 | 0.72 | 0.72 | 1.363 | 0.2517 | |
| Residuals | 32 | 16.92 | 0.53 | |||
| Soil B | ||||||
| Inoculation level | 3 | 77.14 | 25.712 | 46.11 | 5.42E−16 | *** |
| Serovar | 1 | 0.2 | 0.201 | 0.361 | 0.55 | |
| Inoculation level × serovar | 3 | 1.15 | 0.383 | 0.687 | 0.563 | |
| Residuals | 64 | 35.69 | 0.558 |
*, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 2.
Salmonella enterica growth on Swiss chard microgreens via irrigation water contamination. Microgreens were grown in a Conviron E7/2 climate-controlled growth chamber for the duration of the experiment. Before germination, growth chamber temperatures were maintained at 24°C (daytime) and 22°C (nighttime) with no light. Temperatures were maintained for 4 days at a constant 24°C day and night, with light, after germination. Growth chambers were kept at a constant relative humidity of 65%. At harvest, the mean log MPN per gram of fresh weight (y axis) was calculated against various inoculation levels (x axis) for S. enterica serovars Cubana (gray) and Hartford (black) for different growth media, including hydroponic growth, soil A, and soil B. Numbers in parentheses show the numbers of replicated samples examined at each inoculation level. The detection limit for Salmonella in this experiment was 0.3 MPN/g.
Microbial richness during sprouting of alfalfa seeds and production of Swiss chard microgreens.
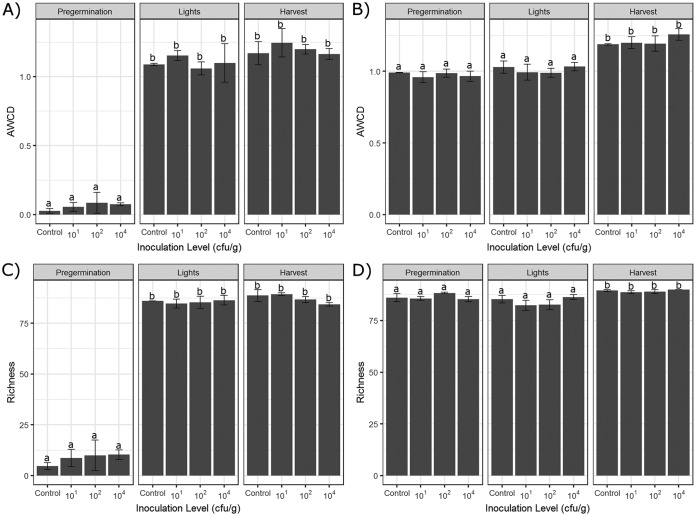
Average well color development (AWCD), used as an estimate of general bacterial activity, was calculated based on the results obtained with Biolog EcoPlates on day 6, when the saturation point of color development was reached. As shown in Fig. 3A and B, no significant difference in bacterial community metabolic activity was observed among control and different Salmonella inoculation levels at any sampling time point for rhizosphere samples from either alfalfa sprouts or Swiss chard microgreens. However, significant differences in bacterial communities during seed sprouting were indicated after comparison of rhizosphere samples pregermination, after light exposure, and at harvest for both control and Salmonella treatment groups. This is also clearly shown with a principal coordinate analysis (PCoA) plot (Fig. 4A), in which a total of 12 samples taken pregermination from the three replicates for each control and Salmonella inoculation group cluster separately from the 12 samples taken after light exposure or at harvest. This clear temporal shift was diminished for the rhizosphere samples from Swiss chard microgreens, as shown by the reduction of separation along the first principal component, from 70.3% to 26.8% (Fig. 4B).
FIG 3.
Average well color development (AWCD) (A and B) and richness (R) (C and D) of metabolized substrates in Biolog EcoPlates after inoculation with different levels of S. enterica pregermination, after light exposure, and at the harvest stage. Data for sprouts are presented in panels A and C, and data for microgreens are presented in panels B and D. Each sample had three replicates. The data shown here are the means of 31 substrate well absorbance values at day 6. Error bars represent standard deviations (n = 3), and different letters indicate a significant difference (P < 0.05).
FIG 4.
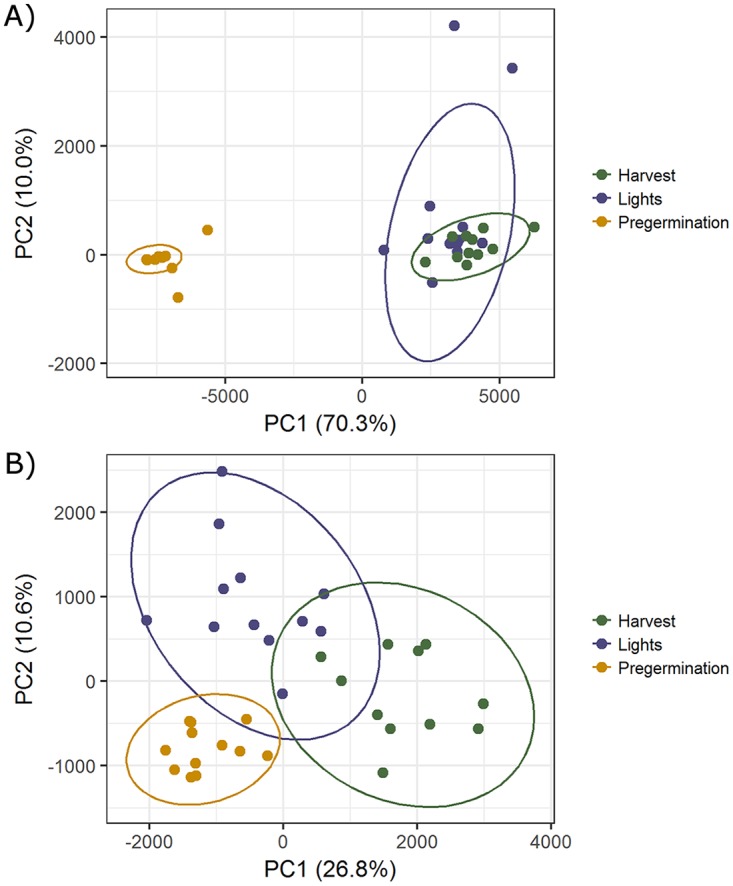
Principal coordinate analysis (PCoA) of bacterial community metabolic profiles for different rhizosphere samples from alfalfa sprouts (A) and Swiss chard microgreens (B). The percentage of total variance explained by each axis is shown parenthetically. All values are based on AWCD data. The various samples from different growth stages are represented by symbols of different colors. Ellipses were drawn with a confidence limit of 0.95.
The microbial community metabolic diversity, as determined by richness (R) based on Biolog EcoPlate profiles, showed significant differences between rhizosphere samples from alfalfa sprouts and those from Swiss chard microgreens (P < 0.05). Additionally, metabolic diversity was drastically increased in sprouts after germination (Fig. 3C and D), but little change was shown during the production of microgreens.
DISCUSSION
Compared to other fresh produce, sprouts are a unique food safety challenge in that foodborne pathogens on the sprouting seeds may multiply by several logs during the short growing period (19, 20). A recent review of sprout-associated outbreaks reported that 85% of the outbreaks in the United States were caused by S. enterica (13). Compared to Escherichia coli, S. enterica was able to grow to significantly higher levels on sprouting seeds (21) and adhered significantly better to alfalfa roots and seed coats (22). Factors that affect the growth of Salmonella during sprouting of contaminated seeds were examined by several groups (21, 23–25). The ability of S. enterica to grow on sprouting seeds was affected by the initial inoculum dose, incubation temperature, and length of exposure but was independent of the serovar, isolation source, virulence of the strain, and day of exposure to the sprouting seeds. An increase in the frequency of irrigation water exchange will not reduce the levels of Salmonella, and a decreased irrigation frequency will increase the Salmonella population. In this study, in addition to various levels of initial inoculation, different Salmonella serovars, and the time of exposure (seed or irrigation water at day 1), contaminated seed storage time was also examined for its effect on Salmonella population levels on sprouting seeds. Salmonella enterica serovars Cubana and Hartford, used in this study, belong to different O groups (O13 for S. Cubana and O7 for S. Hartford) and divergent phylogenetic lineages (26). However, they have both caused salmonellosis outbreaks, associated with alfalfa sprouts and chia seeds, respectively (12, 14). Moreover, S. Cubana was the serovar most frequently recovered from sprout samples collected by the U.S. Department of Agriculture Microbiological Data Program (MDP) between 2002 and 2012 (27). As shown in previous studies, initial inoculation levels played a significant role in the growth of Salmonella on sprouts. Additionally, the overall Salmonella positivity rate with the same inoculation levels (20 CFU/g seed and 2 CFU/g seed) was much higher among the sprout samples from the IC group (60/66 samples [90.9%]) than among those from the SC group (37/81 samples [45.7%]), and the presence of Salmonella was much more homogeneous in sprout samples from the IC group than in those from the SC group. Furthermore, the relationship between inoculation level and mean Salmonella population on alfalfa sprouts was also dependent on seed storage time (in the SC group) and serovar (in the IC group). In general, serovar was not considered to be a factor affecting Salmonella growth on germinating alfalfa seeds, as observed in previous studies. However, serovar might play a role in relation to the population of Salmonella on sprouts when the contamination is introduced via irrigation water.
CLPP analysis revealed that the change in rhizosphere bacterial functional diversity was host dependent but Salmonella contamination independent, which explained why S. enterica was found growing epiphytically on sprout surfaces without producing obvious signs of contamination (9, 28). The exponential increase in microbial richness from times pregermination to postgermination and at sprouting, as shown by CLPP analysis, was probably due to the release of micronutrients from germinating seeds. The fairly low microbial richness (R = 5 to 10) at the pregermination stage also provided a nice niche in which Salmonella could colonize and grow, by up to 4 orders of magnitude.
Compared to their growth on sprouts, E. coli O157:H7 and O104:H4 strains have been shown to grow, but to a lesser extent, on microgreens from contaminated seeds grown in soil and in a hydroponic production system (17, 18). This phenomenon was also observed with S. enterica in our current study. The trivial differences in microbial richness between rhizosphere samples from microgreens from times pregermination to postgermination and at harvest may explain why harvested microgreens carried fewer pathogens than those carried by sprouts. Niche competition with other microorganisms in microgreen growth media may also slow down the growth of pathogens. Factors affecting Salmonella growth on Swiss chard microgreens were also examined in this study. For the SC group, unlike the results for alfalfa sprouts, in addition to the initial inoculation level, the proliferation of Salmonella on Swiss chard microgreens was also serovar dependent. Overall, S. Hartford survived and grew better on microgreens than S. Cubana when seeds were contaminated at a low level. Although a direct comparison between S. enterica and E. coli O157:H7 strains cannot be made because the initial inoculation level was much lower in the current study, the increase in the Salmonella population from the initial inoculation level was much higher on microgreens than that for E. coli O157:H7. For the IC group, consistent with a previous study of E. coli O157:H7, the type of growth medium played a significant role in Salmonella survival and growth during microgreen production, with the hydroponic system having the highest percentage of Salmonella-positive samples and the highest Salmonella population level on microgreens (18). This suggests that the microbial risk associated with hydroponic production systems is potentially much higher than those associated with other forms of production. No significant difference was observed between edible and nonedible parts of Swiss chard microgreens, indicating that the contamination was systemic (18), irrespective of the point of contamination introduction. This may potentially pose a serious microbial safety risk for the production of microgreens. Clearly, these data will contribute to new and innovative preventive control strategies for Salmonella contamination of sprouts and microgreens.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. enterica serovar Cubana strain CFSAN055271, isolated from alfalfa sprouts, and S. Hartford strain NY20, isolated from chia seeds, were obtained from the stock culture collection of the Division of Microbiology, Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, College Park, MD. Stock cultures were stored in brain heart infusion (BHI) broth containing 25% glycerol at −80°C and maintained on tryptic soy agar (TSA) plates.
Germination and growth of alfalfa sprouts and Swiss chard microgreens.
Seeds for sprouting were obtained from West Coast Seeds (British Columbia, Canada). For alfalfa seeds, 5 to 6 g of seeds was sown onto a 13-cm by 13-cm Micro-Mats hydroponic growing pad (Handy Pantry) which was fit into a large (250 ml) square weighing boat to create individual hydroponic systems for all the experiments. For Swiss chard microgreen seeds, 5 to 6 g of seeds was sown onto premoistened potting soil and then covered with a paper towel in a G35 (17 × 10 × 5 cm) mini-seed tray (Garland Products Ltd., UK) placed in an aluminum foil folded tray to serve as a water reservoir or onto hydroponic growing pads, based on the experimental design. Potting soil A (75 to 85% sphagnum peat moss, peat humus, perlite, earthworm castings, and dolomitic limestone), which was used for both the SC and IC groups, and potting soil B (70 to 80% Canadian sphagnum peat moss, perlite, dolomite lime, and a wetting agent [yucca extract]), which was used only for the IC group, were purchased from Amazon, Inc. (Seattle, WA). All the trays were placed in a Conviron E7/2 climate-controlled growth chamber for the duration of the study. Before germination, growth chamber temperatures were maintained at 24°C (daytime) and 22°C (nighttime) with no light for both alfalfa sprouts and Swiss chard microgreens. Temperatures were maintained at a constant 22°C for alfalfa sprouts and 24°C for microgreens day and night, with light, after germination (2 days for alfalfa sprouts and 4 days for Swiss chard microgreens). Growth chambers were kept at a constant relative humidity of 65%. Sprouts and microgreens were irrigated from overhead daily with 40 to 50 ml of tap water (to saturation) for a total of 7 days and 14 days, respectively.
Inoculation of seeds and irrigation water.
A single colony of each culture was transferred to 5 ml of tryptic soy broth (TSB) and grown with shaking at 36°C for 18 to 20 h. Each culture was harvested by centrifugation at 5,000 × g for 10 min, followed by washing with 0.01 M phosphate-buffered saline (PBS) (pH 7.2) three times and then resuspension in 5 ml of TSB. For seed inocula, the culture was further diluted in sterile double-distilled water (ddH2O) to two desired levels (∼101 and ∼102 CFU/g seed). Fifty grams of seeds was soaked in 200 ml inoculum for 20 min, drained, and allowed to air dry at room temperature in a Lumina hood. Seeds were stored at 4°C until use. For irrigation water inocula, the culture suspended in PBS was further diluted to five different levels (∼0.02, ∼0.2, ∼2, ∼20, and ∼2,000 CFU/g seed). A one-time inoculation was made through initial irrigation with a 50-ml inoculum. The inoculation levels for each strain were determined by plate counts immediately following inoculation.
Recovery and enumeration of Salmonella from alfalfa sprouts and Swiss chard microgreens.
Alfalfa sprouts and Swiss chard microgreens were harvested on day 7 and day 14, respectively. Sprouts were sampled whole, and each tray of sprouts was split into two sprout samples by weight. The microgreens were cut above the soil line with a pair of sterilized scissors. Both edible and nonedible parts (i.e., rhizosphere samples) of the microgreens were harvested for evaluation. Salmonella was recovered from the samples and enumerated by use of a miniature three-tube MPN procedure (29), with incorporation of standard enrichment and selection procedures for the pathogen. In short, the sampled sprouts and microgreens were weighed and submerged in modified buffered peptone water (mBPW) at a 1:3 sample-to-broth ratio in individual sterile Whirl-Pak filter bags (Fort Atkinson, WI). Sample bags were mixed for 1 min at 230 rpm with a Seward stomacher 400 circulator, and 4 ml of the mixed solution was then transferred to a sterile 5-ml 48-deep-well plate (Axygen Scientific, Tewksbury, MA), with each sample run in triplicate. An aliquot of 0.4 ml of the original mixed sample was added to a well in the second row of 6-well series, which was prefilled with 3.6 ml of mBPW. The well in the second row was mixed using a disposable serological pipette before transfer of 0.4 ml of sample to a well with 3.6 ml mBPW in the third row. This process was repeated, with changing of pipettes between each transfer, to create a serial 10-fold dilution series. The 48-well plate was then incubated for 24 h at 35°C. An aliquot of 0.1 ml from each well was transferred to 10 ml of Rappaport-Vassiliadis (RV) broth for incubation at 42°C. After 24 h, the selective enrichment tubes were each streaked (10 μl) onto XLT-4 agar plates by use of sterile loops. Plates were incubated for 24 h at 35°C. The presence of colonies with morphologies typical of S. enterica on XLT-4 agar was considered to be a positive result, and random colonies were chosen for confirmation as Salmonella by use of somatic group antisera (Statens Serum Institute, Copenhagen, Denmark) and a Vitek 2 system (bioMérieux, Hazelwood, MO). MPN calculations were performed as previously described (30).
Community-level physiological profiling assay.
Community-level physiological profiles (CLPPs) of each rhizosphere were obtained by use of Biolog EcoPlates (Biolog, Inc., Hayward, CA) as previously described (31), with some modifications. Briefly, 3-g samples of rhizospheres from different growth stages of sprouts and microgreens were transferred to individual sterile Whirl-Pak filter bags filled with 30 ml of saline solution (0.85% NaCl). Sample bags were mixed for 2 min at 200 rpm by use of a stomacher, and then 25 ml of mixed sample was transferred to a 50-ml sterile centrifuge tube. Every suspension was centrifuged at 50 × g for 5 min, and the supernatant was then transferred to a sterile 50-ml centrifuge tube and centrifuged again at 130 × g for 5 min. Three EcoPlates per treatment and one for each replicate sample were filled with 100 μl of suspension per well, followed by incubation at 23°C. The rate of utilization of the carbon sources was monitored using MicroLog 3.0 software with a Biolog MicroStation reader (Biolog, Inc., Hayward, CA). Color development was analyzed at days 0, 1, 3, and 6, when the curve of average well color development (AWCD) reached the saturation point.
Statistical analysis.
All Salmonella growth data were analyzed using analysis of variance (ANOVA) and Tukey's mean separation test, with P values of <0.05 denoting significance. Most-probable-number (MPN) data were logarithmically transformed prior to statistical analysis.
For CLPP data, AWCD was calculated as the sum of the corrected optical density for each well divided by the number of carbon substrates, and richness (R) was calculated as any oxidized carbon substrate. CLPPs were analyzed by principal coordinate analysis (PCoA) to determine the levels of similarity between treatments and time points. Data on AWCD and R were also analyzed by ANOVA, with P values of <0.05 denoting significance.
REFERENCES
- 1.Martinez-Villaluenga C, Frias J, Gulewicz P, Gulewicz K, Vidal-Valverde C. 2008. Food safety evaluation of broccoli and radish sprouts. Food Chem Toxicol 46:1635–1644. doi: 10.1016/j.fct.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Pinto E, Almeida AA, Aguiar AA, Ferreira IMPLVO. 2015. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J Food Compost Anal 37:38–43. doi: 10.1016/j.jfca.2014.06.018. [DOI] [Google Scholar]
- 3.Mir SA, Shah MA, Mir MM. 2017. Microgreens: production, shelf life, and bioactive components. Crit Rev Food Sci 57:2730–2736. doi: 10.1080/10408398.2016.1144557. [DOI] [PubMed] [Google Scholar]
- 4.Guo XB, Li T, Tang KX, Liu RH. 2012. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J Agric Food Chem 60:11050–11055. doi: 10.1021/jf304443u. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe M, Ayugase J. 2010. Effects of buckwheat sprouts on plasma and hepatic parameters in type 2 diabetic db/db mice. J Food Sci 75:H294–H299. doi: 10.1111/j.1750-3841.2010.01853.x. [DOI] [PubMed] [Google Scholar]
- 6.Xiao ZL, Lester GE, Park E, Saftner RA, Luo YG, Wang Q. 2015. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: microgreens. Postharvest Biol Technol 110:140–148. doi: 10.1016/j.postharvbio.2015.07.021. [DOI] [Google Scholar]
- 7.Xiao ZL, Lester GE, Luo YG, Xie ZH, Yu LL, Wang Q. 2014. Effect of light exposure on sensorial quality, concentrations of bioactive compounds and antioxidant capacity of radish microgreens during low temperature storage. Food Chem 151:472–479. doi: 10.1016/j.foodchem.2013.11.086. [DOI] [PubMed] [Google Scholar]
- 8.Yang YS, Meier F, Lo JA, Yuan WQ, Sze VLP, Chung HJ, Yuk HG. 2013. Overview of recent events in the microbiological safety of sprouts and new intervention technologies. Compr Rev Food Sci Food Saf 12:265–280. doi: 10.1111/1541-4337.12010. [DOI] [Google Scholar]
- 9.Proctor ME, Hamacher M, Tortorello ML, Archer JR, Davis JP. 2001. Multistate outbreak of Salmonella serovar Muenchen infections associated with alfalfa sprouts grown from seeds pretreated with calcium hypochlorite. J Clin Microbiol 39:3461–3465. doi: 10.1128/JCM.39.10.3461-3465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winthrop KL, Palumbo MS, Farrar JA, Mohle-Boetani JC, Abbott S, Beatty ME, Inami G, Werner SB. 2003. Alfalfa sprouts and Salmonella Kottbus infection: a multistate outbreak following inadequate seed disinfection with heat and chlorine. J Food Prot 66:13–17. doi: 10.4315/0362-028X-66.1.13. [DOI] [PubMed] [Google Scholar]
- 11.van Duynhoven YT, Widdowson MA, de Jager CM, Fernandes T, Neppelenbroek S, van den Brandhof W, Wannet WJ, van Kooij JA, Rietveld HJ, van Pelt W. 2002. Salmonella enterica serotype Enteritidis phage type 4b outbreak associated with bean sprouts. Emerg Infect Dis 8:440–443. doi: 10.3201/eid0804.010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey RR, Heiman Marshall KE, Burnworth L, Hamel M, Tataryn J, Cutler J, Meghnath K, Wellman A, Irvin K, Isaac L, Chau K, Locas A, Kohl J, Huth PA, Nicholas D, Traphagen E, Soto K, Mank L, Holmes-Talbot K, Needham M, Barnes A, Adcock B, Honish L, Chui L, Taylor M, Gaulin C, Bekal S, Warshawsky B, Hobbs L, Tschetter LR, Surin A, Lance S, Wise ME, Williams I, Gieraltowski L. 2017. International outbreak of multiple Salmonella serotype infections linked to sprouted chia seed powder—USA and Canada, 2013–2014. Epidemiol Infect 145:1535–1544. doi: 10.1017/S0950268817000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dechet AM, Herman KM, Chen Parker C, Taormina P, Johanson J, Tauxe RV, Mahon BE. 2014. Outbreaks caused by sprouts, United States, 1998–2010: lessons learned and solutions needed. Foodborne Pathog Dis 11:635–644. doi: 10.1089/fpd.2013.1705. [DOI] [PubMed] [Google Scholar]
- 14.Taormina PJ, Beuchat LR, Slutsker L. 1999. Infections associated with eating seed sprouts: an international concern. Emerg Infect Dis 5:626–634. doi: 10.3201/eid0505.990503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SA, Kim OM, Rhee MS. 2013. Changes in microbial contamination levels and prevalence of foodborne pathogens in alfalfa (Medicago sativa) and rapeseed (Brassica napus) during sprout production in manufacturing plants. Lett Appl Microbiol 56:30–36. doi: 10.1111/lam.12009. [DOI] [PubMed] [Google Scholar]
- 16.European Food Safety Authority Panel on Biological Hazards. 2011. Scientific opinion on the risk posed by Shiga toxin producing Escherichia coli (STEC) and other pathogenic bacteria in seeds and sprouted seeds. EFSA J 9:2424. doi: 10.2903/j.efsa.2011.2424. [DOI] [Google Scholar]
- 17.Xiao Z, Nou X, Luo Y, Wang Q. 2014. Comparison of the growth of Escherichia coli O157:H7 and O104:H4 during sprouting and microgreen production from contaminated radish seeds. Food Microbiol 44:60–63. doi: 10.1016/j.fm.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Z, Bauchan G, Nichols-Russell L, Luo Y, Wang Q, Nou X. 2015. Proliferation of Escherichia coli O157:H7 in soil-substitute and hydroponic microgreen production systems. J Food Prot 78:1785–1790. doi: 10.4315/0362-028X.JFP-15-063. [DOI] [PubMed] [Google Scholar]
- 19.Stewart DS, Reineke KF, Ulaszek JM, Tortorello ML. 2001. Growth of Salmonella during sprouting of alfalfa seeds associated with salmonellosis outbreaks. J Food Prot 64:618–622. doi: 10.4315/0362-028X-64.5.618. [DOI] [PubMed] [Google Scholar]
- 20.Stewart D, Reineke K, Ulaszek J, Fu T, Tortorello M. 2001. Growth of Escherichia coli O157:H7 during sprouting of alfalfa seeds. Lett Appl Microbiol 33:95–99. doi: 10.1046/j.1472-765x.2001.00957.x. [DOI] [PubMed] [Google Scholar]
- 21.Charkowski AO, Barak JD, Sarreal CZ, Mandrell RE. 2002. Differences in growth of Salmonella enterica and Escherichia coli O157:H7 on alfalfa sprouts. Appl Environ Microbiol 68:3114–3120. doi: 10.1128/AEM.68.6.3114-3120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barak JD, Whitehand LC, Charkowski AO. 2002. Differences in attachment of Salmonella enterica serovars and Escherichia coli O157:H7 to alfalfa sprouts. Appl Environ Microbiol 68:4758–4763. doi: 10.1128/AEM.68.10.4758-4763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard MB, Hutcheson SW. 2003. Growth dynamics of Salmonella enterica strains on alfalfa sprouts and in waste seed irrigation water. Appl Environ Microbiol 69:548–553. doi: 10.1128/AEM.69.1.548-553.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu TJ, Reineke KF, Chirtel S, VanPelt OM. 2008. Factors influencing the growth of Salmonella during sprouting of naturally contaminated alfalfa seeds. J Food Prot 71:888–896. doi: 10.4315/0362-028X-71.5.888. [DOI] [PubMed] [Google Scholar]
- 25.Ge C, Rymut S, Lee C, Lee J. 2014. Salmonella internalization in mung bean sprouts and pre- and postharvest intervention methods in a hydroponic system. J Food Prot 77:752–757. doi: 10.4315/0362-028X.JFP-13-370. [DOI] [PubMed] [Google Scholar]
- 26.Timme RE, Pettengill JB, Allard MW, Strain E, Barrangou R, Wehnes C, Van Kessel JS, Karns JS, Musser SM, Brown EW. 2013. Phylogenetic diversity of the enteric pathogen Salmonella enterica subsp. enterica inferred from genome-wide reference-free SNP characters. Genome Biol Evol 5:2109–2123. doi: 10.1093/gbe/evt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy SP, Wang H, Adams JK, Feng PC. 2016. Prevalence and characteristics of Salmonella serotypes isolated from fresh produce marketed in the United States. J Food Prot 79:6–16. doi: 10.4315/0362-028X.JFP-15-274. [DOI] [PubMed] [Google Scholar]
- 28.Beuchat LR, Ryu JH. 1997. Produce handling and processing practices. Emerg Infect Dis 3:459–465. doi: 10.3201/eid0304.970407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berghaus RD, Thayer SG, Law BF, Mild RM, Hofacre CL, Singer RS. 2013. Enumeration of Salmonella and Campylobacter spp. in environmental farm samples and processing plant carcass rinses from commercial broiler chicken flocks. Appl Environ Microbiol 79:4106–4114. doi: 10.1128/AEM.00836-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garthright WE, Blodgett RJ. 2003. FDA's preferred MPN methods for standard, large or unusual tests with a spreadsheet. Food Microbiol 20:439–445. doi: 10.1016/S0740-0020(02)00144-2. [DOI] [Google Scholar]
- 31.Bonilla N, Vida C, Martinez-Alonso M, Landa BB, Gaju N, Cazorla FM, de Vicente A. 2015. Organic amendments to avocado crops induce suppressiveness and influence the composition and activity of soil microbial communities. Appl Environ Microbiol 81:3405–3418. doi: 10.1128/AEM.03787-14. [DOI] [PMC free article] [PubMed] [Google Scholar]