ABSTRACT
Bifidobacteria are mutualistic intestinal bacteria, and their presence in the human gut has been associated with health-promoting activities. The presence of antibiotic resistance genes in this genus is controversial, since, although bifidobacteria are nonpathogenic microorganisms, they could serve as reservoirs of resistance determinants for intestinal pathogens. However, until now, few antibiotic resistance determinants have been functionally characterized in this genus. In this work, we show that Bifidobacterium breve CECT7263 displays atypical resistance to erythromycin and clindamycin. In order to delimit the genomic region responsible for the observed resistance phenotype, a library of genomic DNA was constructed and a fragment of 5.8 kb containing a gene homologous to rRNA methylase genes was able to confer erythromycin resistance in Escherichia coli. This genomic region seems to be very uncommon, and homologs of the gene have been detected in only one strain of Bifidobacterium longum and two other strains of B. breve. In this context, analysis of shotgun metagenomics data sets revealed that the gene is also uncommon in the microbiomes of adults and infants. The structural gene and its upstream region were cloned into a B. breve-sensitive strain, which became resistant after acquiring the genetic material. In vitro conjugation experiments did not allow us to detect gene transfer to other recipients. Nevertheless, prediction of genes potentially acquired through horizontal gene transfer events revealed that the gene is located in a putative genomic island.
IMPORTANCE Bifidobacterium breve is a very common human intestinal bacterium. Often described as a pioneer microorganism in the establishment of early-life intestinal microbiota, its presence has been associated with several beneficial effects for the host, including immune stimulation and protection against infections. Therefore, some strains of this species are considered probiotics. In relation to this, because probiotic bacteria are used for human and animal consumption, one of the safety concerns over these bacteria is the presence of antibiotic resistance genes, since the human gut is a densely populated habitat that could favor the transfer of genetic material to potential pathogens. In this study, we analyzed the genetic basis responsible for the erythromycin and clindamycin resistance phenotype of B. breve CECT7263. We were able to identify and characterize a novel gene homologous to rRNA methylase genes which confers erythromycin and clindamycin resistance. This gene seems to be very uncommon in other bifidobacteria and in the gut microbiomes of both adults and infants. Even though conjugation experiments showed the absence of transferability under in vitro conditions, it has been predicted to be located in a putative genomic island recently acquired by specific bifidobacterial strains.
KEYWORDS: Bifidobacterium, Bifidobacterium breve, clindamycin resistance, erythromycin resistance, microbiota, probiotics
INTRODUCTION
Bifidobacterium breve is a mutualistic microorganism very commonly found in humans. It is one of the most prevalent species in the infant gut microbiota (1), being especially abundant in the intestine and feces of breast-fed individuals, in which it is believed that it plays an important role in the maturation of the immune system and the development of other physiological functions, as well as, although to a lesser extent, in adults (1, 2). Notably, members of the B. breve species have been shown to be maternally inherited through a vertical transmission route (3–5). Some strains of this species are used in functional foods and food supplements and are considered probiotics, that is, live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (6). In relation to this, one of the more important safety concerns of probiotic microorganisms is their potential to harbor and transfer antibiotic resistance genes (7). In this regard, international regulatory agencies recommend the absence of transferable antibiotic resistance determinants before the introduction of probiotic bacteria into the market (8, 9). However, nontransferable resistance to clinically relevant antibiotics could be considered a desired trait for those probiotics that are intended to be administered to treat or prevent antibiotic-associated secondary effects and could be useful to repopulate the gut microbiota during and after antibiotic treatment (7). In fact, some of the most common and widely commercialized probiotic bifidobacteria possess genetic determinants of antibiotic resistance (10, 11).
Following the broth microdilution antibiotic testing methods recommended by the guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance (8), we found that B. breve CECT7263 displays high MICs for erythromycin and clindamycin. This is an atypical resistance phenotype for Bifidobacterium species, which are, in general, very sensitive to macrolides and lincosamides (12, 13). This prompted us to look deeper into the genetic basis responsible for this phenotype. Identification of the genetic determinant was followed by a survey of all the sequenced bifidobacterial genomes and the gut microbiomes of both adults and infant individuals for this genetic determinant.
RESULTS AND DISCUSSION
Identification and characterization of the genetic determinant responsible for antibiotic resistance.
B. breve CECT7263 is resistant to erythromycin and clindamycin in broth culture, with MICs of 128 and >512, respectively (Table 1). Simultaneous resistance to these two drugs is commonly due to the acquisition of erythromycin ribosome methylase (erm) genes (14), which are responsible for the enzymatic modification of the nucleotide sequence of the 23S rRNA gene by adding methyl groups, thus preventing the binding of macrolides, lincosamides, and streptogramin B (MLS), conferring the so-called MLS resistance phenotype. Since the genome of B. breve CECT7263 is available in GenBank under accession number MWVR00000000 (BioProject accession number PRJNA377846), the genome sequence and the open reading frames (ORFs) were compared through BLAST analysis with those in a wide antibiotic resistance gene database installed locally and in the NCBI database. However, none of the retrieved results showed the presence of known genes responsible for the MLS resistance phenotype. Furthermore, we searched for mutations previously associated with erythromycin and clindamycin resistance. In this regard, mutations in the 23S rRNA gene were previously described as being responsible for macrolide resistance in bacteria (14, 15). However, we could not identify any nucleotide mutation in the 23S rRNA gene that would explain the resistance phenotype of our strain. These results suggest that a genetic determinant not described previously could be responsible for the atypical resistance phenotype in B. breve CECT7263.
TABLE 1.
MICs for the strains analyzed in this work
| Strain | Descriptionc | Source | MIC (μg/ml) |
Reference or source | |
|---|---|---|---|---|---|
| Erythromycin | Clindamycin | ||||
| B. breve CECT7263a | Commercial strain, Eryr | Biosearch S.A. collection | 128 | >512 | 37 |
| B. breve CECT8606a | Commercial strain, Erys | Biosearch S.A. collection | <1 | <1 | 38 |
| L. lactis NZ9000b | Culture collection, plasmid free, Erys | NIZO collection | <1 | <1 | 39 |
| L. lactis/pNZ8048b | NZ9000 carrying pNZ8048, a broad-host-range shuttle vector, Cmr | This work | <1 | <1 | This work |
| L. lactis/pAN1b | NZ9000 carrying pAN1, a pNZ8048-derived plasmid with the erm(49) gene | This work | 16 | >512 | This work |
| B. breve NCIMB8807a | Culture collection, plasmid free, Erys | NCIMB collection | <1 | <1 | 40 |
| B. breve 8807/pNZ8048a | NCIMB8807 carrying pNZ8048 | This work | <1 | <1 | This work |
| B. breve 8807/pAN1a | NCIMB8807 carrying pAN1 | This work | 32 | >512 | This work |
Iso-Sensitest–MRS culture broth was used.
Iso-Sensitest culture broth was used.
Eryr, erythromycin resistant; Erys, erythromycin sensitive; Cmr, chloramphenicol resistant.
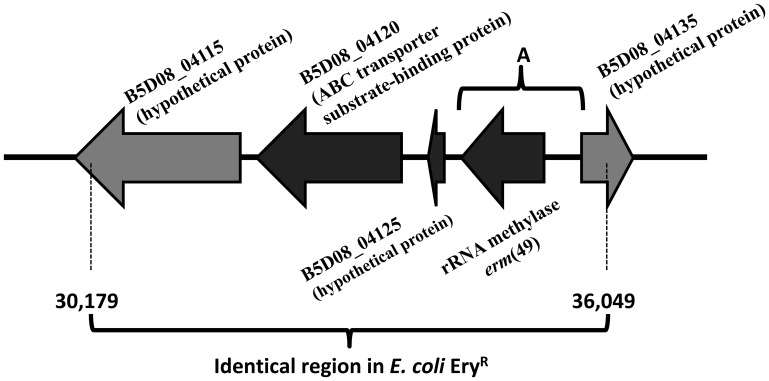
Thus, in order to identify the DNA fragment conferring this resistance, we constructed in Escherichia coli a genomic library for B. breve CECT7263. We obtained and sequenced 23 E. coli clones with different EcoRI restriction profile fragments, 18 of which had an identical DNA region of approximately 5.8 kb. This region is located between base numbers 30,179 and 36,049 in contig 9 of the B. breve CECT7263 genome (Fig. 1). The DNA sequence of the 5.8-kb fragment was used as a template to perform a BLAST homology search using the NCBI database, and, surprisingly, we found 100% homology with a 2.6-kb DNA genomic region (lacking an annotation and including the B5D08_04125, B5D08_04130, and B5D08_04135 loci; Fig. 1) of the strain Bifidobacterium longum subsp. infantis CECT7210, a bifidobacterium active against rotavirus infection for which the erythromycin MIC was higher than 256 μg/ml (16, 17). No other significant homologies were found in the BLASTN search. A further analysis with BLASTP showed that the protein encoded by the B5D08_04130 gene [for which we propose the gene name erm(49), according to the nomenclature for macrolide-lincosamide-streptogramin B resistance determinants described by Roberts and coworkers (18); this name has been approved by the MLS nomenclature center (http://faculty.washington.edu/marilynr)] has homology with rRNA (adenine-N6)-methyltransferases. This was further confirmed by domain prediction through the PFAM database (19), which revealed the presence of a complete RrnaAD domain, characterized as an rRNA adenine dimethylase involved in antibiotic resistance (20). This link between a higher erythromycin resistance phenotype and the presence of an identical DNA fragment [containing the gene erm(49); Fig. 1] in two different Bifidobacterium species led us to believe that the gene(s) present in this DNA region could be a potential candidate to be further studied as the genetic determinant(s) responsible for the observed phenotype.
FIG 1.
Identical DNA fragments in 18 erythromycin-resistant E. coli clones (base numbers from 30,179 to 36,049 in contig 9; GenBank accession number MWVR01000009.1). Black arrows, ORFs that were complete in all the fosmids analyzed; gray arrows, ORFs that were not complete in all the fosmids analyzed; A, DNA fragment cloned into the vector pNZ8048. The annotations of the ORFs in the genome of B. breve CECT7263 are B5D08_04115, B5D08_04125, B5D08_04130 [rRNA methylase erm(49)], and B5D08_04135 (hypothetical proteins) and B5D08_04120 (ABC transporter substrate-binding protein).
To prove the involvement of erm(49) in the antibiotic resistance phenotype of B. breve, we cloned the structural gene and its adjacent upstream region in the host Lactococcus lactis NZ9000, which is naturally susceptible to erythromycin. A single clone, named L. lactis/pAN1, which contains the plasmid pAN1 and which is resistant to chloramphenicol and erythromycin, was selected, and its plasmid was transferred to B. breve NCIMB8807 (Cms Erys). The resulting strain, which was named B. breve 8807/pAN1 and which contained the plasmid pAN1, was further characterized. We determined the erythromycin and clindamycin MICs for the parental strains (L. lactis NZ9000 and B. breve NCIMB8807), the strains carrying the empty plasmid (L. lactis/pNZ8048 and B. breve 8807/pNZ8048), and the strains carrying the pAN1 plasmid (L. lactis/pAN1 and B. breve 8807/pAN1). The parental strains showed sensitivity to both antibiotics with MICs of <1 μg/ml. However, the two strains containing the plasmid pAN1 [carrying erm(49)] had significantly increased resistance levels compared with the respective controls (L. lactis/pNZ8048 and B. breve 8807/pNZ8048 harboring the empty plasmid pNZ8048; Table 1). Remarkably, the MICs of erythromycin and clindamycin were 32 and >512 μg/ml, respectively, for B. breve 8807/pAN1, and the MICs of both antibiotics were less than 1 μg/ml for strain B. breve 8807/pNZ8048. This result demonstrates that the cloned DNA fragment containing the gene erm(49) and its upstream region confers resistance to erythromycin and clindamycin in B. breve, although the erythromycin resistance level seems to be lower in B. breve NCIMB8807 than in the parental strain, B. breve CECT7263, likely due to the different physiological characteristics and genetic backgrounds of the strains. In this regard, some erythromycin resistance genes were previously described in bifidobacteria. Van Hoek and coworkers found a resistance determinant, erm(X), in the transposon Tn5432 in Bifidobacterium thermophilum and Bifidobacterium animalis subsp. lactis (21), and Margolles and coworkers showed evidence for the involvement of a multidrug resistance membrane protein from B. breve conferring moderate resistance to macrolides (22). However, this study reports a specific erythromycin resistance gene in B. breve and provides solid evidence of the involvement of an rRNA methylase in conferring macrolide-lincosamide resistance in bifidobacteria. The protein sequence encoded by erm(49) displays different degrees of homology and phylogenetic distances with Erm proteins (Fig. 2), showing the highest homology (47% identity; query coverage, 92%) with Erm(42), a protein from Pasteurella multocida able to confer macrolide and lincosamide resistance (23).
FIG 2.
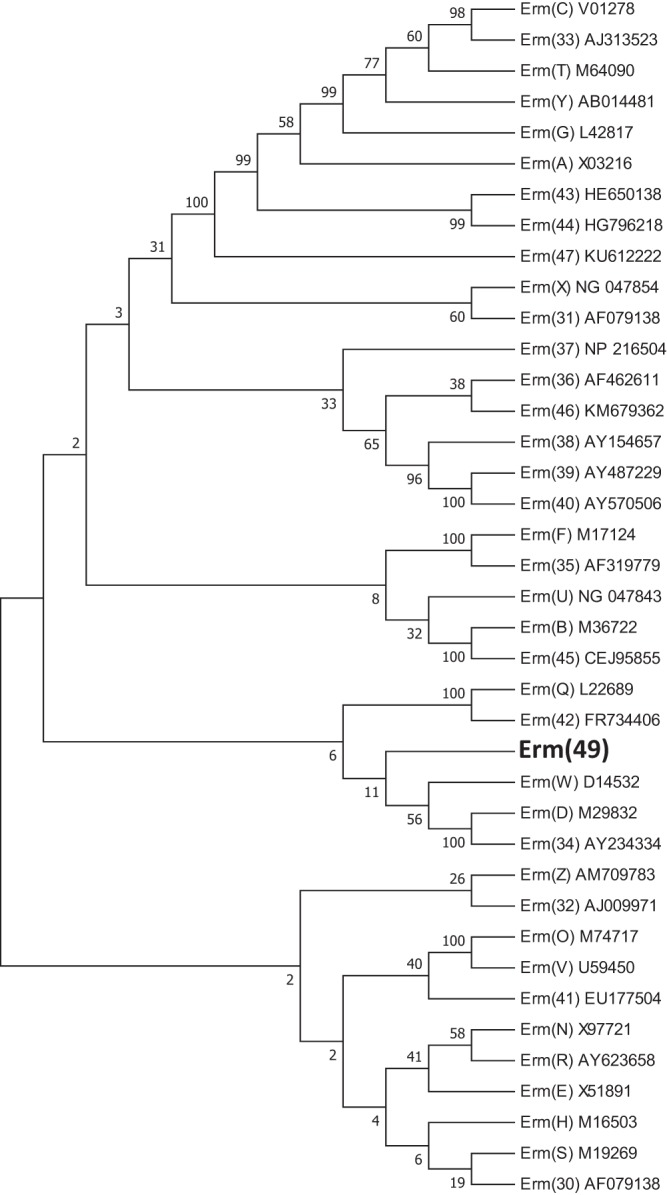
Phylogenetic tree of Erm proteins. Protein names and GenBank accession numbers are shown on the right. The evolutionary history was inferred using the neighbor-joining method (33). The bootstrap consensus tree inferred from 500 replicates (34) is taken to represent the evolutionary history of the taxa analyzed (34). Branches corresponding to partitions reproduced in less than 50% of the bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to the branches (34). The evolutionary distances were computed using the p-distance method (35) and are in units of the number of amino acid differences per site. The analysis involved 39 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 138 positions in the final data set. Evolutionary analyses were conducted in the MEGA7 program (36). An updated list of Erm proteins is available at http://faculty.washington.edu/marilynr/ermweb1.pdf.
In vitro transferability assays.
In the genome of B. breve CECT7263, the gene erm(49) is not close to DNA sequences annotated as mobile genetic elements, such as transposons or phage-related sequences. To shed some light on its potential transferability, horizontal transfer of erythromycin resistance was examined by filter mating experiments. We used B. breve CECT7263 and two lactobacilli (Lactobacillus plantarum LMG21684 and Lactobacillus plantarum LMG21687) as donor strains and Enterococcus faecalis LMG19456 and Staphylococcus aureus LMG21674 as recipient strains. Since the two lactobacilli were previously shown to transfer tetracycline resistance to the recipient strains [they both contain a transferable tet(M)-carrying plasmid (24, 25)], they were used as positive controls for the conjugation experiments. Both lactobacillus strains were able to transfer tetracycline resistance to E. faecalis LMG19456 and S. aureus LMG21674, as previously described (24). However, B. breve CECT7263 was not able to transfer erythromycin resistance to the recipient strains (data not shown). Thus, under the experimental conditions used in the present work, transfers between B. breve CECT7263 and the recipient strains E. faecalis LMG19456 and S. aureus LMG21674 were not detected.
Screening for erm(49) homologs in all the publicly available bifidobacterial genomes.
The genomic sequences of 337 bifidobacterial strains (see Table S1 in the supplemental material) were retrieved from the most updated version of the NCBI genome database available at the time of writing of the manuscript (11 November 2017). To ensure the same high-quality standard for gene prediction and functional annotation, all the 337 genomes were submitted to analysis with MEGAnnotator software (26). Interestingly, screening of the predicted genes for homologs to erm(49) revealed the presence of genes with 100% identity in B. longum subsp. infantis CECT7210 as well as B. breve BR-14 and B. breve DPC6330 (Table 2), while no identical gene was found in the genomes of the remaining 88 B. longum and 48 B. breve strains analyzed.
TABLE 2.
Homologous genes found in the 337 publicly available bifidobacterial genomes
| Species | Strain | BLASTP E value | % identity | % similarity | PFAM domain | Corresponding amino acid sequence |
|---|---|---|---|---|---|---|
| Bifidobacterium longum subsp. infantis | CECT7210 | 0 | 100 | 100 | Complete RrnaAD domain | MRNIKDTQNFLHSKELVRHLIGICNIKLDDVVIEIGPGKGIITNELAHKARKVVAIEFDEELYEKLKNKFQSNNKVDIIYGDILNYTPRIPSYCVFSNIPFNITSEILNKFLSDKKNEKMFLIMQYEPFIKYAGNPYGAETLRSMLYKPFFDMDLKYRFDPSDFKPAPQARIVLASFERKQFPDVKKEEEKLYKDFLAYIYTNKGETFFAKIKTLFSSNQIKRVWGQIKIDKTTKISEVPYESILKVFKLFFLYGTDANKQLVVNSFNNMNKQNNKLQKNHRNNSKAKSWNSNRKRKPYHRNNV |
| Bifidobacterium breve | CECT7263 | 0 | 100 | 100 | Complete RrnaAD domain | MRNIKDTQNFLHSKELVRHLIGICNIKLDDVVIEIGPGKGIITNELAHKARKVVAIEFDEELYEKLKNKFQSNNKVDIIYGDILNYTPRIPSYCVFSNIPFNITSEILNKFLSDKKNEKMFLIMQYEPFIKYAGNPYGAETLRSMLYKPFFDMDLKYRFDPSDFKPAPQARIVLASFERKQFPDVKKEEEKLYKDFLAYIYTNKGETFFAKIKTLFSSNQIKRVWGQIKIDKTTKISEVPYESILKVFKLFFLYGTDANKQLVVNSFNNMNKQNNKLQKNHRNNSKAKSWNSNRKRKPYHRNNV |
| Bifidobacterium breve | BR-14 | 0 | 100 | 100 | Complete RrnaAD domain | MRNIKDTQNFLHSKELVRHLIGICNIKLDDVVIEIGPGKGIITNELAHKARKVVAIEFDEELYEKLKNKFQSNNKVDIIYGDILNYTPRIPSYCVFSNIPFNITSEILNKFLSDKKNEKMFLIMQYEPFIKYAGNPYGAETLRSMLYKPFFDMDLKYRFDPSDFKPAPQARIVLASFERKQFPDVKKEEEKLYKDFLAYIYTNKGETFFAKIKTLFSSNQIKRVWGQIKIDKTTKISEVPYESILKVFKLFFLYGTDANKQLVVNSFNNMNKQNNKLQKNHRNNSKAKSWNSNRKRKPYHRNNV |
| Bifidobacterium breve | DPC6330 | 0 | 100 | 100 | Complete RrnaAD domain | MRNIKDTQNFLHSKELVRHLIGICNIKLDDVVIEIGPGKGIITNELAHKARKVVAIEFDEELYEKLKNKFQSNNKVDIIYGDILNYTPRIPSYCVFSNIPFNITSEILNKFLSDKKNEKMFLIMQYEPFIKYAGNPYGAETLRSMLYKPFFDMDLKYRFDPSDFKPAPQARIVLASFERKQFPDVKKEEEKLYKDFLAYIYTNKGETFFAKIKTLFSSNQIKRVWGQIKIDKTTKISEVPYESILKVFKLFFLYGTDANKQLVVNSFNNMNKQNNKLQKNHRNNSKAKSWNSNRKRKPYHRNNV |
| Bifidobacterium breve | BR-I29 | 3.00E−29 | 30 | 51 | Complete RrnaAD domain | MNKNIKYSQNFLTSEKVLNQIIKQLNLKETDNVYEIGTGKGHLTTKLAKISKQVTSIELDSHLFNLSSEKLKLNTRVTLIHQDILQFQFPNKQRYKIVGSIPYHLSTQIIKKVVFESHASDIYLIVAEGFYKRTVNIHRTLGLLLHTQVSIQQLLKLPAECFHPKPKVNSVLIKLTRHTTDVPDKYWKLYTYFVSKWVNREYRQLFTKNQFHQAMKHAKVNNLSTVTYEQVLSIFNSYLLFNGRK |
Nevertheless, a putative homolog with 30% identity and 51% similarity was also identified in B. breve BR-I29 (Table 2). Domain prediction through the PFAM database (19) revealed that the putative homologous gene present in B. breve BR-I29 encodes a complete RrnaAD domain involved in antibiotic resistance (20), as previously observed for erm(49) (Table 2).
Altogether, data collected through screening of all the publicly available bifidobacterial genomes evidenced the limited distribution of this gene across the pangenome of the genus Bifidobacterium.
Profiling of erm(49) in the gut microbiomes of adults and infants.
The nucleotide sequence of erm(49) was also used to perform a survey of its abundance in metagenomics data sets of both adult and infant individuals. Notably, mapping of metagenomics reads was performed using stringent settings that allowed alignment of reads with >99% identity with respect to the sequence of erm(49). Analysis of data sets corresponding to the microbiomes of 30 adults randomly selected among those sequenced in the framework of the Human Microbiome Project (BioProject accession number PRJNA48479) revealed the absence of reads corresponding to erm(49). This result is in accordance with the identification of erm(49) only in bifidobacterial species that typically colonize the infant gut, i.e., B. longum subsp. infantis and B. breve.
In contrast, profiling of erm(49) in 64 shotgun metagenomics data sets obtained from 20 healthy infants at multiple time points (BioProject accession number PRJNA63661) revealed the presence of mapping reads in infant 30081, who was sampled at days 76, 162, and 336 of age. Notably, data sets for the same infant sampled at days 6, 10, and 17 of age did not reveal reads corresponding to erm(49), thus suggesting a (bifido)bacterial strain carrying erm(49) that probably colonized this infant or grew above the limit of detection at between days 17 and 76 of age. Analysis of additional metatranscriptomics data sets available for infant 30081 (BioProject accession number PRJNA63661) also allowed the observation that the erm(49) gene was expressed at 162 days of age.
Despite the widespread distribution of bifidobacteria, the presence of erm(49) across healthy infants seems to be infrequent. Nevertheless, these data are in accordance with the small number of bifidobacterial strains in which erm(49) was found, e.g., 4 out of 337 screened genomes, thus suggesting that the acquisition of this gene happened recently in the evolution of bifidobacteria.
Analysis of the genomic region containing erm(49) for genes acquired by HGT events.
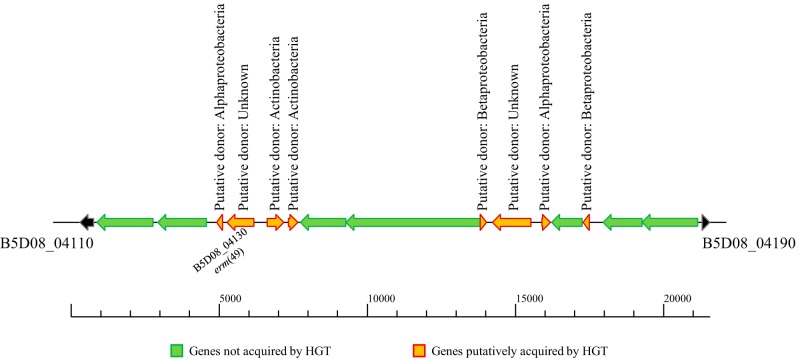
In order to evaluate if erm(49) and adjacent genes have been acquired by horizontal transmission events, Colombo software (27) was used for analysis of the assembled contig of B. breve CECT7263 encompassing the erm(49) gene (contig 9; GenBank accession number MWVR01000009.1). In detail, Colombo exploits hidden Markov models and detection of codon usage bias to predict the presence of genomic islands and genes acquired by horizontal gene transfer (HGT). Nevertheless, ancient gene acquisition events may be overlooked due to the amelioration of bacterial genomes (28). The data collected revealed that erm(49) is located in a putative genomic island characterized by multiple genes predicted to be acquired through HGT events (Fig. 3). While it was not possible to predict the donor of erm(49), adjacent genes suggest possible acquisition from Alphaproteobacteria or other Actinobacteria (Fig. 3).
FIG 3.
Genes putatively acquired by HGT events in the genomic region encompassing erm(49). The schematic representation of the genomic region encompassing erm(49) reports in green the genes predicted not to be involved in HGT events and in orange the genes putatively acquired by HGT.
Conclusions.
In summary, in this work we characterized a gene from B. breve CECT7263, erm(49), that is homologous to rRNA methyltransferase genes and that confers a high level of resistance to erythromycin and clindamycin. The gene is scarcely represented in bacterial genomes, since an extensive homology search showed that the only homologs found were present in the genomes of four bifidobacteria, and it was not detected in other genera. The limited distribution of erm(49) was also highlighted by its profiling in a total of 94 shotgun metagenomics data sets representing the fecal microbiota of 30 adult and 20 infant individuals. The results of in vitro conjugation experiments suggest that the gene is not transferable under the experimental conditions used in the current work and point to a genomic stability of the DNA region containing erm(49) in the original host. Nevertheless, in silico prediction of HGT events revealed that erm(49) is located in a putative genomic island that may have been acquired through horizontal gene transfer events. Thus, in view of the lack of identical sequences in bacteria, except in other bifidobacteria, the results support the suggestion that although erm(49) has probably been acquired by horizontal transfer, it seems to be restricted to the genus Bifidobacterium.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. B. breve strains were grown at 37°C in MRS broth (Difco, BD Diagnostic Systems, Sparks, MD, USA) supplemented with 0.05% (wt/vol) l-cysteine (Sigma, St. Louis, MO, USA) (MRSc) in an anaerobic chamber (Mac 500; Don Whitley Scientific, West Yorkshire, UK) with an atmosphere of 5% CO2, 5% H2, and 90% N2. Lactococcus lactis was grown at 32°C under aerobic static conditions in GM17 (M17 broth [Oxoid Limited, Hampshire, UK] supplemented with 0.5% d-glucose [Merck KGaA, Darmstadt, Germany]). Escherichia coli was grown in LB broth according to the instructions of the manufacturer of the CopyControl fosmid library production kit (Epicentre, Madison, WI, USA). Where appropriate, antibiotics were added to the broth or agar for selection or growth of resistant strains. The antibiotic concentration for each specific condition is described elsewhere in the article.
DNA extraction.
Total DNA of B. breve CECT7263 was extracted using a QIAamp DNeasy blood and tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations for sequencing assays and by using standard techniques (29) for cloning assays, with the incorporation of an initial lysis step involving suspension of the cells in lysis buffer (20% sucrose, 10 mM Tris-HCl, pH 8.1, 10 mM EDTA, 50 mM NaCl) supplemented with lysozyme (10 mg/ml) and 50 units/ml mutanolysin, followed by incubation at 37°C for 3 h.
Genomic library and sequence analysis.
A genomic library for B. breve CECT7263 was constructed using a CopyControl fosmid library production kit (Epicentre) according to the manufacturer's instructions. Briefly, genomic DNA was mechanically sheared into approximately 40-kb fragments. Both ends of the size-fractionated DNA were repaired to create blunt 5′-phosphorylated ends and were ligated into the pCC1FOS fosmid vector (Epicentre). Ligated DNA mixtures were then packaged by using the supplied bacteriophage lambda packaging extracts and were transformed into an EPI300-T1R phage T1-resistant E. coli host. Transformants were selected on LB agar plates with 12.5 μg/ml of chloramphenicol (Sigma) and 300 μg/ml of erythromycin (Sigma). Clones resistant to both antibiotics were inoculated in LB broth containing chloramphenicol and erythromycin, and the fosmids were isolated and sequenced using primers FP (5′-GGATGTGCTGCAAGGCGATTAAGTTGG-3′) and RP (5′-CTCGTATGTTGTGTGGAATTGTGAGC-3′) as described in the CopyControl fosmid library production kit.
Cloning of B5D08_04130 [erm(49)] in L. lactis and B. breve.
B5D08_04130 [named erm(49) (GenBank accession number MH015334)] and its adjacent region were amplified from B. breve CECT7263 using Phusion high-fidelity DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA) and the primer pair A1-XbaI (5′-GGGTCTAGACATTATACATTATTTCTATGATAAGGC-3′; forward primer [the XbaI restriction site is underlined]) and A2-BglII (5′-GGGAGATCTCATAATGGCCTAGCTTTCCC-3′; reverse primer [the BglII restriction site is underlined]). The PCR product and plasmid pNZ8048, a plasmid able to replicate in L. lactis and B. breve (30), were digested with XbaI and BglII (EURx, Przyrodnikow, Poland), and then the vector was dephosphorylated using alkaline phosphatase (Promega, Madison, WI, USA). Digestion products were ligated using T4 DNA ligase (Promega) and then transformed into L. lactis NZ9000 according to previously described methods (22). Clones were selected in GM17 agar plates containing chloramphenicol (5 μg/ml) and erythromycin (2.5 μg/ml) as selective agents. A single clone, named L. lactis/pAN1, resistant to both antibiotics was selected. Sequencing of the resulting plasmid was carried out in order to ensure that undesirable mutations were not generated. Plasmid pAN1, obtained from L. lactis/pAN1, was transferred by electroporation into B. breve NCIMB8807 (Cms Erys) according to previously described protocols (31). Transformants were selected on MRSc agar plates containing chloramphenicol (3 μg/ml) and erythromycin (5 μg/ml) as selective agents. A single clone, named B. breve 8807/pAN1, resistant to both antibiotics was selected for further studies. Sequencing of plasmid pAN1 obtained from this clone was carried out to guarantee the absence of mutations.
MICs of erythromycin and clindamycin.
MIC determinations were performed in broth microdilution 96-well plates according to EFSA guidelines (8). In brief, Iso-Sensitest broth (Oxoid) supplemented with 10% MRS (Difco) and Iso-Sensitest broth were used for the Bifidobacterium and Lactococcus strains, respectively. Twofold dilutions from 512 μg/ml downwards were tested for erythromycin and clindamycin. For each strain, assays were carried out at least in triplicate. As controls, the parental strains and clones containing the empty plasmid pNZ8048 were used in each assay.
Horizontal transfer of erythromycin resistance in vitro.
Horizontal transfer of erythromycin resistance was examined by filter mating as described by Gevers and coworkers (24). B. breve CECT7263, Lactobacillus plantarum LMG21684, and Lactobacillus plantarum LMG21687 were used as donor strains. The rifampin-resistant strains Enterococcus faecalis LMG19456 and Staphylococcus aureus LMG21674 were used as recipients. The donor and recipient strains were grown in nonselective broth medium to mid-exponential phase of growth, and then the cell populations were mixed in the same proportions. Mixtures were filtered through MF-Millipore membrane filters (pore size, 0.45 μm; Millipore, Darmstadt, Germany), and the filters were incubated overnight in nonselective medium, brain heart infusion (BHI; Oxoid). Assays were performed at least in triplicate, and all the experiments were carried out under both aerobic and anaerobic conditions. Furthermore, in order to mimic the conditions of the gut, experiments with filters soaked in fecal water prior to the filter mating were also carried out. Cells were recovered and spread onto BHI agar plates with rifampin at 50 μg/ml as the selective agent for recipient strains and tetracycline at 10 μg/ml or erythromycin at 2.5 μg/ml as the selective agents for lactobacilli or Bifidobacterium strains, respectively.
Screening of all sequenced bifidobacterial strains for erm(49) homologs.
The sequences of 337 bifidobacterial genomes retrieved from the NCBI genome database (see Table S1 in the supplemental material) were processed with MEGAnnotator software (26) in order to ensure the same high-quality ORF prediction and functional annotation for all the analyzed strains. BLASTP software (32) was used to search all the predicted genes for homologs to erm(49) using default settings.
Profiling of erm(49) in the microbiomes of adults and infants.
Bowtie2 software was used to evaluate the abundance of reads corresponding to erm(49) in 30 shotgun metagenomics data sets for 30 adult individuals sequenced in the framework of the Human Microbiome Project (BioProject accession number PRJNA48479) and 64 data sets corresponding to 20 healthy infants sampled at multiple time points (BioProject accession number PRJNA63661). Four additional metatranscriptomics data sets obtained from infant 30081 (BioProject accession number PRJNA63661) were also screened. Bowtie2 was run using stringent settings (–score-min C,−13,0) in order to allow mapping only of reads with identities of >99%.
Prediction of genes acquired through HGT events.
Identification of genes putatively acquired through horizontal gene transfer (HGT) events was performed using the software Colombo (27) with a sensitivity of 0.95.
Accession number(s).
Data are available in the NCBI database under GenBank accession number MH015334.
Supplementary Material
ACKNOWLEDGMENTS
This research was carried out thanks to the grant METASIN (Investigación, Desarrollo e Innovación en Nuevos Alimentos Multifuncionales para Síndrome Metabolic), funded by the Centro Para el Desarrollo Tecnológico Industrial (CDTI) and Biosearch S.A. This work was also funded by the EU Joint Programming Initiative A Healthy Diet for a Healthy Life (JPI HDHL, http://www.healthydietforhealthylife.eu/) and MIUR to M.V. This research benefits from the HPC (High-Performance Computing) facility of the University of Parma, Parma, Italy.
Roberto Luque and Oscar Bañuelos are employees of Biosearch S.A., company owner of a patent (41) including the B. breve CECT7263 strain.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02888-17.
REFERENCES
- 1.Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, Gueimonde M, Margolles A, De Bellis G, O'Toole PW, van Sinderen D, Marchesi JR, Ventura M. 2012. Diversity of bifidobacteria within the infant gut microbiota. PLoS One 7:e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turroni F, Marchesi JR, Foroni E, Gueimonde M, Shanahan F, Margolles A, van Sinderen D, Ventura M. 2009. Microbiomic analysis of the bifidobacterial population in the human distal gut. ISME J 3:745–751. doi: 10.1038/ismej.2009.19. [DOI] [PubMed] [Google Scholar]
- 3.Milani C, Mancabelli L, Lugli GA, Duranti S, Turroni F, Ferrario C, Mangifesta M, Viappiani A, Ferretti P, Gorfer V, Tett A, Segata N, van Sinderen D, Ventura M. 2015. Exploring vertical transmission of bifidobacteria from mother to child. Appl Environ Microbiol 81:7078–7087. doi: 10.1128/AEM.02037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duranti S, Lugli GA, Mancabelli L, Armanini F, Turroni F, James K, Ferretti P, Gorfer V, Ferrario C, Milani C, Mangifesta M, Anzalone R, Zolfo M, Viappiani A, Pasolli E, Bariletti I, Canto R, Clementi R, Cologna M, Crifò T, Cusumano G, Fedi S, Gottardi S, Innamorati C, Masè C, Postai D, Savoi D, Soffiati M, Tateo S, Pedrotti A, Segata N, van Sinderen D, Ventura M. 2017. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome 5:66. doi: 10.1186/s40168-017-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turroni F, Milani C, Duranti S, Mahony J, van Sinderen D, Ventura M. 28 October 2017. Glycan utilization and cross-feeding activities by bifidobacteria. Trends Microbiol. doi: 10.1016/j.tim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 7.Gueimonde M, Sánchez B, de los Reyes-Gavilán CG, Margolles A. 2013. Antibiotic resistance in probiotic bacteria. Front Microbiol 4:202. doi: 10.3389/fmicb.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP). 2012. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10:2740. [Google Scholar]
- 9.Sanders ME, Akkermans LMA, Haller D, Hammerman C, Heimbach J, Hörmannsperger G, Huys G, Levy DD, Lutgendorff F, Mack D, Phothirath P, Solano-Aguilar G, Vaughan E. 2010. Safety assessment of probiotics for human use. Gut Microbes 1:164–185. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gueimonde M, Flórez AB, van Hoek AH, Stuer-Lauridsen B, Strøman P, de los Reyes-Gavilán CG, Margolles A. 2010. Genetic basis of tetracycline resistance in Bifidobacterium animalis subsp. lactis. Appl Environ Microbiol 76:3364–3369. doi: 10.1128/AEM.03096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duranti S, Lugli GA, Mancabelli L, Turroni F, Milani C, Mangifesta M, Ferrario C, Anzalone R, Viappiani A, van Sinderen D, Ventura M. 2017. Prevalence of antibiotic resistance genes among human gut-derived bifidobacteria. Appl Environ Microbiol 83:e02894-16. doi: 10.1128/AEM.02894-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammor MS, Flórez AB, van Hoek AH, de Los Reyes-Gavilán CG, Aarts HJ, Margolles A, Mayo B. 2008. Molecular characterization of intrinsic and acquired antibiotic resistance in lactic acid bacteria and bifidobacteria. J Mol Microbiol Biotechnol 14:6–15. doi: 10.1159/000106077. [DOI] [PubMed] [Google Scholar]
- 13.Masco L, van Hoorde K, de Brandt E, Swings J, Huys G. 2006. Antimicrobial susceptibility of Bifidobacterium strains from humans, animals and probiotic products. J Antimicrob Chemother 58:85–94. doi: 10.1093/jac/dkl197. [DOI] [PubMed] [Google Scholar]
- 14.Roberts MC. 2008. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol Lett 282:147–159. doi: 10.1111/j.1574-6968.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 15.Vester B, Douthwaite S. 2001. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob Agents Chemother 45:1–12. doi: 10.1128/AAC.45.1.1-12.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chenoll E, Rivero M, Codoñer FM, Martinez-Blanch JF, Ramón D, Genovés S, Moreno-Muñoz JA. 2015. Complete genome sequence of Bifidobacterium longum subsp. infantis strain CECT 7210, a probiotic strain active against rotavirus infections. Genome Announc 3(2):e00105-15. doi: 10.1128/genomeA.00105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz JA, Chenoll E, Casinos B, Bataller E, Ramón D, Genovés S, Montava R, Ribes JM, Buesa J, Fàbrega J, Rivero M. 2011. Novel probiotic Bifidobacterium longum subsp. infantis CECT7210 strain active against rotavirus infections. Appl Environ Microbiol 77:8775–8783. doi: 10.1128/AEM.05548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts MC, Sutcliffe J, Courvalin P, Jensen LB, Rood J, Seppala H. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother 43:2823–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnhammer EL, Eddy SR, Durbin R. 1997. Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins 28:405–420. doi:. [DOI] [PubMed] [Google Scholar]
- 20.Yu L, Petros AM, Schnuchel A, Zhong P, Severin JM, Walter K, Holzman TF, Fesik SW. 1997. Solution structure of an rRNA methyltransferase (ErmAM) that confers macrolide-lincosamide-streptogramin antibiotic resistance. Nat Struct Biol 4:483–489. doi: 10.1038/nsb0697-483. [DOI] [PubMed] [Google Scholar]
- 21.van Hoek AH, Mayrhofer S, Domig KJ, Aarts HJ. 2008. Resistance determinant erm(X) is borne by transposon Tn5432 in Bifidobacterium thermophilum and Bifidobacterium animalis subsp lactis. Int J Antimicrob Agents 31:544–548. doi: 10.1016/j.ijantimicag.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Margolles A, Moreno JA, van Sinderen D, de los Reyes-Gavilán CG. 2005. Macrolide resistance mediated by a Bifidobacterium breve membrane protein. Antimicrob Agents Chemother 49:4379–4381. doi: 10.1128/AAC.49.10.4379-4381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadlec K, Brenner Michael G, Sweeney MT, Brzuszkiewicz E, Liesegang H, Daniel R, Watts JL, Schwarz S. 2011. Molecular basis of macrolide, triamilide, and lincosamide resistance in Pasteurella multocida from bovine respiratory disease. Antimicrob Agents Chemother 55:2475–2477. doi: 10.1128/AAC.00092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gevers D, Huys G, Swings J. 2003. In vitro conjugal transfer of tetracycline resistance from Lactobacillus isolates to other Gram-positive bacteria. FEMS Microbiol Lett 225:125–130. doi: 10.1016/S0378-1097(03)00505-6. [DOI] [PubMed] [Google Scholar]
- 25.Huys G, D'Haene K, Swings J. 2006. Genetic basis of tetracycline and minocycline resistance in potentially probiotic Lactobacillus plantarum strain CCUG43738. Antimicrob Agents Chemother 50:1550–1551. doi: 10.1128/AAC.50.4.1550-1551.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lugli GA, Milani C, Mancabelli L, van Sinderen D, Ventura M. 2016. MEGAnnotator: a user-friendly pipeline for microbial genomes assembly and annotation. FEMS Microbiol Lett 363:fnw049. doi: 10.1093/femsle/fnw049. [DOI] [PubMed] [Google Scholar]
- 27.Waack S, Keller O, Asper R, Brodag T, Damm C, Fricke WF, Surovcik K, Meinicke P, Merkl R. 2006. Score-based prediction of genomic islands in prokaryotic genomes using hidden Markov models. BMC Bioinformatics 7:142. doi: 10.1186/1471-2105-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence JG, Ochman H. 1997. Amelioration of bacterial genomes: rates of change and exchange. J Mol Evol 44:383–397. doi: 10.1007/PL00006158. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 30.Ruiz L, Zomer A, O'Connell-Motherway M, van Sinderen D, Margolles A. 2012. Discovering novel bile protection systems in Bifidobacterium breve UCC2003 through functional genomics. Appl Environ Microbiol 78:1123–1131. doi: 10.1128/AEM.06060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz L, O'Connell-Motherway M, Zomer A, de los Reyes-Gavilán CG, Margolles A, van Sinderen D. 2012. A bile-inducible membrane protein mediates bifidobacterial bile resistance. Microb Biotechnol 5:523–535. doi: 10.1111/j.1751-7915.2011.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 33.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 34.Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 35.Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY. [Google Scholar]
- 36.Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez N, Luque R, Olivares MM, Margolles A, Bañuelos O. 2017. Resequencing the genome of Bifidobacterium breve strain CECT7263. Genome Announc 5(18):e00299-17. doi: 10.1128/genomeA.00299-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sañudo AI, Criado R, Rodríguez-Nogales A, Garach A, Olivares M, Gálvez J, de la Escalera S, Duarte JM, Zarzuelo A, Bañuelos O. June 2016. Probiotic strains having cholesterol absorbing capacity, methods and uses thereof. Patent WO/2016/092032.
- 39.Kuipers OP, Beerthuyzen MM, Siezen RJ, de Vos WM. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem 216:281–291. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz L, Sánchez B, de Los Reyes-Gavilán CG, Gueimonde M, Margolles A. 2009. Coculture of Bifidobacterium longum and Bifidobacterium breve alters their protein expression profiles and enzymatic activities. Int J Food Microbiol 133:148–153. doi: 10.1016/j.ijfoodmicro.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Olivares M, Jimenez EA, Sierra S, Marín ML, Lara F, Arroyo R, Martín R, Maldonado A, Martin V, Boza J, Jiménez J, Fernández L, Rodríguez JM, Xaus J. 2008. Breast milk bifidobacteria, compositions thereof, their use and a novel culture media to obtain them. EP patent EP1997880A1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.