ABSTRACT
A highly virulent Serratia proteamaculans strain, AGR96X, exhibiting specific pathogenicity against larvae of the New Zealand grass grub (Costelytra giveni; Coleoptera: Scarabaeidae) and the New Zealand manuka beetle (Pyronota festiva and P. setosa; Coleoptera: Scarabaeidae), was isolated from a diseased grass grub larva. A 12-day median lethal dose of 4.89 × 103 ± 0.92 × 103 cells per grass grub larva was defined for AGR96X, and death occurred within 5 to 12 days following the ingestion of a high bacterial dose. During the infection period, the bacterium rapidly multiplied within the insect host and invaded the hemocoel, leading to a mean bacterial load of 8.2 × 109 cells per larva at 6 days postingestion. Genome sequencing of strain AGR96X revealed the presence of a variant of the Serratia entomophila antifeeding prophage (Afp), a tailocin designated AfpX. Unlike Afp, AfpX contains two Afp16 tail-length termination protein orthologs and two putative toxin components. A 37-kb DNA fragment encoding the AfpX-associated region was cloned, transformed into Escherichia coli, and fed to C. giveni and Pyronota larvae, causing mortality. In addition, the deletion of the afpX15 putative chaperone component abolished the virulence of AGR96X. Unlike S. entomophila Afp, the AfpX tailocin could be induced by mitomycin C. Transmission electron microscopy analysis revealed the presence of Afp-like particles of various lengths, and when the purified AfpX tailocin was fed to grass grub or manuka beetle larvae, they underwent phenotypic changes similar to those of larvae fed AGR96X.
IMPORTANCE Serratia proteamaculans strain AGR96X shows dual activity against larvae of endemic New Zealand pasture pests, the grass grub (Costelytra giveni) and the manuka beetle (Pyronota spp.). Unlike Serratia entomophila, the causal agent of amber disease, which takes 3 to 4 months to kill grass grub larvae, AGR96X causes mortality within 5 to 12 days of ingestion and invades the insect hemocoel. AGR96X produces a unique variant of the S. entomophila antifeeding prophage (Afp), a cell-free phage-like entity that is proposed to deliver protein toxins to the grass grub target site, causing a cessation of feeding activity. Unlike other Afp variants, AGR96X Afp, named AfpX, contains two tail-length termination proteins, resulting in greater variability in the AfpX length. AfpX shows dual activity against both grass grub and manuka beetle larvae. AGR96X is a viable alternative to S. entomophila for pest control in New Zealand pasture systems.
KEYWORDS: antifeeding prophage, Serratia entomophila, Serratia proteamaculans, pesticide, tailocin, biocontrol
INTRODUCTION
Amber disease of the New Zealand grass grub, Costelytra giveni (Coleoptera: Scarabaeidae) (formerly C. zealandica) (1), is caused by several strains of Serratia entomophila and Serratia proteamaculans (Yersiniaceae) (2). The disease is highly host specific, affecting only a single species of scarab that is indigenous to New Zealand (3). Feeding ceases within 2 to 3 days of ingestion of these pathogenic Serratia strains, and clearing of the normally dark larval gut results in the characteristic amber coloration (4). During this time, no destruction or blebbing of the insect gut epithelium is observed (4). Within 6 days of ingestion, a maximum population density of 1 × 107 S. entomophila cells per larva is reached (5). Amber disease is chronic, with death occurring 1 to 3 months after the ingestion of the bacterium. Death is thought to result from a gradual weakening of the larval gut barrier, from where bacteria invade the hemocoel, causing generalized septicemia (4, 5).
The main virulence determinants of S. entomophila and S. proteamaculans are encoded on a 153-kb conjugative plasmid named pADAP, for amber disease-associated plasmid (6, 7). pADAP contains two virulence-associated regions. The first region encodes a grass grub-active member of the toxin complex (TC) family (8), designated S. entomophila pathogenicity (sepABC) (9). The second is the antifeeding prophage (Afp)-encoding region, a cluster of 18 genes whose products combine to form a virus-like structure. The ingestion of this prophage by grass grub larvae causes a cessation of feeding (10, 11). The translated products of afp1 to afp16 form the Afp phage-like particle, while afp18 is proposed to encode the toxin components carried by Afp (10). Orthologs of Afp, termed Photorhabdus virulence cassettes (PVCs), were identified in the Photorhabdus luminescens TTO1 genome (10). PVCs carry a range of toxin genes, including those encoding Mcf and LopT (10, 12). Based on bioinformatic, structural, and predicted-function data, Afp has been described as a cell-free variant of the type VI secretion system (13, 14). Recently, the term tailocin, defined as a defective phage that lacks a head and contains no DNA, has been used to describe pyocins and Afp-like structures (15). In addition, phylogenetic analyses by Sarris et al. (16) identified Afp-like variants in a diverse range of bacterial species, including members of the Archaea. Outside the Serratia species, the closest Afp ortholog is encoded by Yersinia ruckeri (7, 17), which causes enteric redmouth disease of trout (18). The Y. ruckeri Afp region encodes a distinct but similarly named Afp18 toxin that disrupts RhoA GTPase-dependent actin organization (17).
The S. entomophila Afp16 protein has been identified as a tail-length termination protein (TrP) (19). The TrP terminates the elongation of the Afp tube when it reaches its requisite length and is proposed to stabilize the formation of the sheath around the tube (19). The length of Afp is determined by the tape measure protein Afp14, which is absent from the mature Afp tailocin. Afp14 is theorized to stretch along the length of the tube and protect it from the premature attachment of the TrP, thereby regulating tail length (20). Afp is proposed to target the host cell via the Afp13 tail fiber protein. This protein shows amino acid similarity to adenovirus tail fiber proteins, which impart host specificity (10, 21, 22).
S. proteamaculans strain AGR96X was isolated from a field-collected C. giveni larva that exhibited an atypical nonamber coloration. Bioassay assessments of AGR96X showed that it not only killed C. giveni larvae but also was pathogenic toward larvae of another endemic New Zealand beetle, the manuka beetle (Pyronota spp.; Coleoptera: Scarabaeidae), which has been recognized as an emerging pastoral pest (23, 24).
In this paper, we describe the identification, mutagenesis, nucleotide sequence analysis, and in vivo pathogenicity of the plasmid-encoded Afp variant (termed AfpX) of S. proteamaculans strain AGR96X and, where appropriate, compared it with the disease characteristics of S. entomophila.
RESULTS
Isolation and characterization of a grass grub- and manuka beetle-active pathogen.
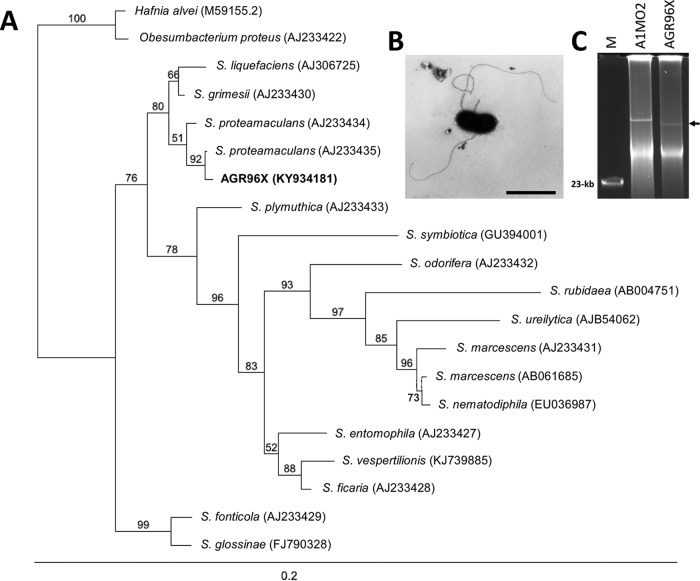
A diseased larva containing a pathogenic strain of S. proteamaculans, designated AGR96X, was isolated from the same geographic location (Lake Coleridge region, New Zealand) and at the same time as the previously identified broad-host-range entomopathogen Yersinia entomophaga (25). When fed to grass grub larvae, reisolated, and refed to healthy larvae, AGR96X caused disease symptoms identical to those induced by the initial isolate, fulfilling Koch's postulates. Analysis of the 16S rRNA gene sequence identified AGR96X as a S. proteamaculans strain, with the highest nucleotide identity (99.85%) to S. proteamaculans subsp. quinovora (26) (Fig. 1A). Electron microscopy revealed the bacterium was rod shaped and ∼1.5 to 3 μm in length, with a diameter of ∼0.6 to 1 μm. Some of the cells were flagellated (Fig. 1B). AGR96X contained a single plasmid of approximately 120 kb (Fig. 1C), which we designated pAGR96X.
FIG 1.
(A) Maximum likelihood phylogenetic tree based on the 16S rRNA gene sequences of S. proteamaculans strain AGR96X and other members of the genus Serratia as well as the type species of closely related genera (GenBank accession numbers are indicated). The significance of each branch is indicated by a bootstrap value calculated as a percentage of 1,000 replications. Bar, 2 nucleotide substitutions per 100 nucleotides. (B) Electron micrograph of S. proteamaculans strain AGR96X. Bar, 2 μm. (C) Kado and Liu plasmid visualization of S. proteamaculans strain AGR96X AfpX-encoding plasmid pAGR96X (denoted by a left-pointing arrow) and S. entomophila strain A1MO2 plasmid pADAP (153 kb). M denotes the λ HindIII marker, and a 23-kb band is indicated.
Similar phenotypic changes were observed in grass grub and manuka beetle larvae following the ingestion of AGR96X. Within 24 to 72 h of ingestion, the larvae regurgitated and changed from a healthy gray color to creamy amber. By 4 to 7 days postingestion, the larvae took on a brownish purple coloration, which was more pronounced at the leading edge of each thoracic segment (Fig. 2B). The larvae then became brown, before blackening completely (Fig. 2), with death typically occurring within 5 to 12 days of ingestion. Although slight maceration of the carrot was noted, no cessation of feeding activity after the ingestion of AGR96X was observed. This contrasted with phenotypic changes that occurred following the ingestion of S. entomophila, wherein the grass grub larvae took on an amber coloration (Fig. 2E), while the manuka beetle larvae remained unaffected (Tables 1 and 2). Host range testing showed that AGR96X was not pathogenic toward any of the other tested insect species belonging to the family Scarabaeidae (Table 1).
FIG 2.
Photographs of representative phenotypes. (A) Manuka beetle. Shown is disease progression from the untreated control (far left) to dead larvae (far right). (B) Grass grub. Shown is disease progression from the untreated control (far left) to dead larvae (far right). The red arrow indicates the purple coloration at the leading edge of each thoracic segment. (C and D) Grass grub larvae challenged with AGR96X::17 (C) or AGR96X::18 (D) (disease progression from left to right). (E) Typical appearance of grass grub larvae challenged with either S. entomophila A1MO2 or AGR96X at 5 days postchallenge.
TABLE 1.
Disease and mortality rates 12 days after maximum challenge with S. proteamaculans AGR96X and S. entomophila A1MO2 bioassayed against each of the listed insect species belonging to the family Scarabaeidae
| Scarabaeidae insect species | A1MO2 |
AGR96X |
||
|---|---|---|---|---|
| Mean % diseased larvae ± SE (P value) | Mean % dead larvae ± SE (P value) | Mean % diseased larvae ± SE (P value) | Mean % dead larvae ± SE (P value) | |
| Costelytra giveni | 97.6 ± 2.4 (<0.001)a | 2.3 ± 2.3 (0.990) | 10.7 ± 4.2 (<0.001) | 87.5 ± 4.5 (<0.001)a |
| Pyronota spp. | 0 (1.000) | 0 (1.000) | 6.3 ± 4.3 (0.546) | 90.6 ± 5.2 (<0.001)a |
| Odontria sp. | 0 (1.000) | 0 (1.000) | 0 (1.000) | 0 (1.000) |
| Adoryphorus couloni | 0 (1.000) | 0 (1.000) | 0 (1.000) | 0 (1.000) |
| Acrossidius tasmaniae | 0 (1.000) | 0 (1.000) | 0 (1.000) | 0 (1.000) |
| Heteronychus arator | 0 (1.000) | 0 (1.000) | 0 (1.000) | 0 (1.000) |
Significance (P) at the 5% level (95% confidence level).
TABLE 2.
Disease and mortality rates 12 days after maximum challenge with S. proteamaculans AGR96X or S. entomophila A1MO2
| Challenge inoculum |
C. giveni |
Pyronota spp. |
||
|---|---|---|---|---|
| Mean % diseased larvae ± SE (P value) | Mean % dead larvae ± SE (P value) | Mean % diseased larvae ± SE (P value) | Mean % dead larvae ± SE (P value) | |
| AGR96X and mutated AfpX derivatives | ||||
| AGR96X | 16.7 ± 4.8 (0.001)a | 81.7 ± 5.7 (<0.001)a | 0 (1.000) | 91.7 ± 4.6 (<0.001)a |
| AGR96XΔAFP1516a | 2.8 ± 2.7 (0.990) | 3.3 ± 2.3 (0.496) | 0 (1.000) | 0 (1.000) |
| AGR96X::17 | 15 ± 4.6 (0.003)a | 51.7 ± 6.5 (<0.001)a | 8.3 ± 4.6 (0.239) | 83.3 ± 6.2 (<0.001)a |
| AGR96X::18 | 51.7 ± 6.5 (<0.001)a | 40.0 ± 6.3 (<0.001)a | 8.3 ± 4.6 (0.239) | 88.9 ± 5.2 (<0.001)a |
| Control | 0 | 2.8 ± 2.8 | 0 | 5.6 ± 3.8 |
| Escherichia coli containing AfpX-encoding clones pAFPXQ and pAFPR | ||||
| E. coli/pAFPXQ | 35.0 ± 6.4 (<0.001)a | 65.7 ± 6.2 (<0.001)a | 19.4 ± 6.6 (0.011)a | 69.4 ± 7.7 (<0.001)a |
| E. coli/pAFPXR | 51.7 ± 6.5 (<0.001)a | 41.3 ± 5.2 (<0.001)a | 13.9 ± 5.8 (0.054)a | 61.1 ± 8.1 (<0.001)a |
| AGR96X | 13.3 ± 4.4 (<0.001)a | 81.7 ± 4.8 (<0.001)a | 0 (1.000) | 97.2 ± 2.7 (<0.001)a |
| Control | 0 | 6.3 ± 3.5 | 0 | 2.8 ± 2.7 |
| Mitomycin-induced AGR96X and S. entomophila A1MO2 concentrated culture filtratesb | ||||
| AGR96Xc | 0 (1.000) | 0 (1.000) | 0 (1.000) | 0 (1.000) |
| A1MO2 | 3.0 ± 3.0 (0.496) | 0 (1.000) | 0 (1.000) | 0 (1.000) |
| AGR96X | 64.4 ± 3.7 (<0.001)a | 12.2 ± 4.3 (0.081) | 82.7 ± 4.1 (<0.001)a | 15.7 ± 5.2 (0.003)a |
| AGR96XΔAFP1516a | 2.4 ± 2.2 (0.124) | 0 (1.000) | 0 (1.000) | 0 (1.000) |
| Control | 0 | 0 | 0 | 0 |
Significance (P) at the 5% level (95% confidence level).
Bioassay assessed at day 6.
Non-mitomycin C-treated culture.
A dose-response analysis of S. proteamaculans AGR96X against grass grub larvae determined that the median lethal 12-day dose was 4.89 × 103 ± 0.92 × 103 CFU per grass grub larva (Fig. 3A).
FIG 3.
(A) Twelve-day mortality rates versus AGR96X dose for C. giveni larvae. (B) Bacterial load in CFU per C. giveni larva for S. proteamaculans AGR96X and S. entomophila A1MO2 over 7 days postchallenge.
Pathobiology of S. proteamaculans AGR96X and S. entomophila A1MO2.
Attempts to use green fluorescent protein-labeled AGR96X(pGFPuvsp) were hindered by a notable delay in disease progression, with the strain taking 2 to 3 days longer to cause disease than wild-type AGR96X. In addition, the onset of disease symptoms correlated with a loss of the pGFPuvsp plasmid and, therefore, fluorescence (data not shown). For this reason, we assessed pathobiology using a combination of light microscopy and bacterial enumeration from whole-grub macerates following challenge with either AGR96X or S. entomophila A1MO2. Forty-eight hours after the ingestion of a maximum dose of 5 × 107 S. proteamaculans AGR96X bacteria, the bacterial load in AGR96X-challenged grass grub macerates was approximately 6.45 × 107 (±2.43 × 107) CFU per larva, which increased to 3.03 × 108 (±1.15 × 108) CFU per larva by 72 h postingestion (Fig. 3B). Thereafter, bacteria were observed in the hemocoel, which coincided with a gradual deterioration of the larval hemocytes and an increase in phenol oxidase (PO) activity (Fig. 4A). The AGR96X-challenged larvae reached a peak cell density of 8.19 × 109 (±3.94 × 109) CFU per larva at 144 h postinfection, which was reflected in the high cell density observed by light microscopy (Fig. 4C). Direct plating of hemocoelic fluid and assessment of the resultant colonies by BOX-PCR validated the bacteria as AGR96X (data not shown). Parallel assessments of S. entomophila strain A1MO2 found that from 72 to 168 h postingestion, the cell density remained relatively consistent, at approximately 2.67 × 107 (±1.08 × 107) CFU per larva (Fig. 3B). Over the same time period, the PO levels did not significantly differ from those of the untreated control (Fig. 4B).
FIG 4.
(A and B) Assessment of the phenol oxidase activity (OD490 per minute) in the hemocoelic fluid of C. giveni following oral challenge with either AGR96X (A) or A1MO2 (B), compared with the nonchallenged control. **, P ≤ 0001. (C) Light microscopy of C. giveni larval hemolymph following a maximum oral challenge with either A1MO2 or AGR96X. (i) Untreated control. (ii) A1MO2 at 3 days postchallenge (3.18 × 107 ± 1.54 × 107 CFU per larva). (iii) AGR96X at 96 h postchallenge (3.33 × 107 ± 1.25 × 107 CFU per larva). At this time point, larvae showed purple coloration at the leading edge of each thoracic segment (Fig. 2B). Red arrows denote bacteria. (iv) AGR96X at 6 days postchallenge (8.19 × 109 ± 1.15 × 109 CFU per larva). Bacteria are readily observed. Bars, 20 μm.
DNA sequence analysis.
Based on the known virulence determinants of amber disease, we searched the Illumina reads for contigs showing nucleotide sequence identity to the sep and afp virulence-associated regions (9, 10). DNA sequence analysis of the assembled contigs identified a cluster of 20 genes, the translated products of which had high amino acid similarity to components of S. entomophila Afp (Table 3 and Fig. 5). The predicted AGR96X tailocin was designated AfpX. The AfpX-encoding genes afpX1 to afpX12 and afpX14 to afpX16 showed at least 93% nucleotide sequence identity and correspondingly high amino acid similarity to their S. entomophila Afp orthologs (Table 3). Similar to Afp, the AfpX virulence-associated region contained the anfA1 and anfA2 loci, the anfA1-associated ops element (5′-GGCGGTAGCAT-3′), and the phage-like lysis cassette (enp1, hol1, and mur1), positioned upstream of the AfpX-encoding gene cluster (Table 3; see also Fig. S1 in the supplemental material).
TABLE 3.
Similarities of products of putative S. proteamaculans strain AGR96X ORFs to translated amino acid sequences in the database detected by using BLASTP
| ORF (aa length) | GenBank accession no. | Locus tag(s), species (aa length) | Degree of amino acid similaritya | Ortholog GenBank accession no. |
|---|---|---|---|---|
| Product 1 | ANV21590 | Hypothetical protein LC20_02642, Yersinia enterocolitica LC20 (142) | 82/89, 147 (1–142) | AHM73895.1 |
| AnfA1 (148) | ANV21591 | AnfA1, Serratia entomophila (148) | 96/97, 148 (1–148) | WP_010895796.1 |
| AnfA2 (98) | ANV21592 | AnfA2, S. entomophila (98) | 99/98, 95 (1–95) | WP_010895797.1 |
| Sea23 (37) | ANV21593 | Sea23, S. entomophila (42) | 92/97, 37 (6–42) | WP_010895799.1 |
| Product 5 (94) | ANV21594 | No similarity | ||
| Enp1 (153) | ANV21595 | Enp1 S. entomophila (153) | 97/99, 149 (5–153) | WP_010895800.1 |
| Hol1 (67) | ANV21596 | Hol1, S. entomophila (67) | 91/92, 67 (1–67) | WP_010895801.1 |
| Mur1 (141) | ANV21598 | Mur1, S. entomophila (141) | 99/99, 141(1–141) | WP_010895802.1 |
| AfpX1 (149) | ANV21600 | Afp1, S. entomophila (140) | 100/100, 149 (1–149) | WP_010895803.1 |
| AfpX2 (354) | ANV21601 | Afp2, S. entomophila (354) | 100/100, 354 (1–354) | WP_010895804.1 |
| AfpX3 (451) | ANV21602 | Afp3, S. entomophila (451) | 99/100, 451 (1–451) | WP_010895805.1 |
| AfpX4 (415) | ANV21603 | Afp4, S. entomophila (417) | 99/99, 415 (3–417) | WP_010895806.1 |
| AfpX5 (149) | ANV21604 | Afp5, S. entomophila (149) | 98/99, 149 (1–149) | WP_010895807.1 |
| AfpX6 (55) | ANV21605 | Afp6, S. entomophila (55) | 100/100, 55 (1–55) | WP_010895808.1 |
| AfpX7 (229) | ANV21606 | Afp7, S. entomophila (229) | 98/98, 229 (1–229) | WP_010895809.1 |
| AfpX8 (529) | ANV21607 | Afp8, S. entomophila (529) | 99/100, 529 (1–529) | WP_010895810.1 |
| AfpX9 (140) | ANV21608 | Afp9, S. entomophila (140) | 97/98, 140, (1–140) | WP_010895811.1 |
| AfpX10 (140) | ANV21609 | Afp10, S. entomophila (140) | 99/98, 140 (1–140) | WP_010895812.1 |
| AfpX11 (602) | ANV21610 | Afp11, S. entomophila (607) | 84/88, 608 (1–607) | WP_010895813.1 |
| AfpX12 (964) | ANV21611 | Afp12, S. entomophila (963) | 84/89, 965 (1–963) | WP_010895814.1 |
| AfpX13 (590) | ANV21612 | Afp13, S. entomophila (636) | 61/73, 656 (1–636) | WP_010895815.1 |
| AfpX14 (535) | ANV21613 | Afp14, S. entomophila (554) | 84/88, 535 (20–554) | WP_010895816.1 |
| AfpX15 (696) | ANV21614 | Afp15, S. entomophila (695) | 93/95, 696 (1–695) | WP_010895817.1 |
| AfpX16a (293) | ANV21615 | Afp16, S. entomophila (295) | 88/91, 293 (3–295) | WP_010895818.1 |
| AfpX18 (2,285) | ANV21616 | Hypothetical protein, Yersinia ruckeri (2,123) | 69/80, 2,075 (50–2116) | WP_038276419.1 |
| Afp18, S. entomophila (2,366) | 46/57, 906 (535–1404) | WP_010895820.1 | ||
| 35/49, 230 (2042–2267) | ||||
| 32/51, 102 (2274–2356) | ||||
| Product 28 (71) | ANV21617 | Hypothetical protein, Vibrio sp. HI00D65 (963) | 59/71, 71 (102–165) | WP_063522665.1 |
| AfpX16b (259) | ANV21618 | Afp16, S. entomophila (295) | 82/83, 293 (3–295) | WP_010895818.1 |
| AfpX17 (913) | ANV21619 | PAU_03033, Photorhabdus asymbiotica (932) | 69/80, 886 (2–887) | CAQ85121.1 |
| Afp17, S. entomophila (358) | 95/95, 341 (1–341) | WP_010895819.1 | ||
| VIP2, Vibrio harveyi (963) | 59/74, 888 (51–937) | WP_045488056.1 |
Percent identity/percent similarity over the given number (span) of amino acid residues (in relation to the subject amino acid sequence). Similarities were considered potentially significant if the BLASTP score exceeded e−5.
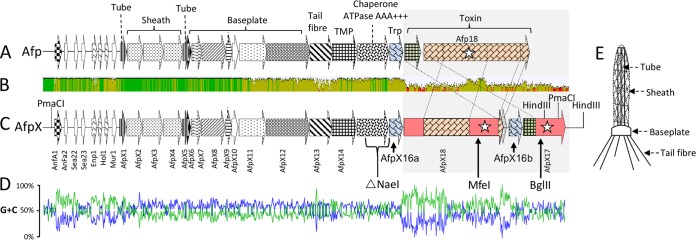
FIG 5.
(A and C) Schematic representations of the S. entomophila Afp (A) and S. proteamaculans AGR96X AfpX (C) gene clusters. ORFs are denoted by arrows, with their designations listed vertically below. Amino acid regions with high similarity are indicated by similar shading (see Table 3 for the percent amino acid similarity/identity of the respective AfpX proteins). Stars indicate ORFs for which the translated product shows similarity to a documented virulence factor. Predicted and ascribed protein domains are listed above the S. entomophila afp gene cluster in panel A. (TMP denotes tape measure protein and Trp denotes tail-length termination protein.) The NaeI region deleted in the AGR96XΔAFP1516a deletion variant (Table 4) is indicated below the ORFs corresponding to AfpX15 and AfpX16 in panel C. The MfeI and BglII sites used to insert markers to generate AGR96X::18 and AGR96X::17 (Table 4) are indicted in panel C. (B) Mean pairwise afp and afpX nucleotide identities over all pairs in the column. Green, 100% identity; olive, ≥30 to 99% identity; red, <30% identity. Angled dashed lines denote shared areas of amino acid similarity between Afp17 and Afp18 and their AfpX orthologs. (D) G+C skew plot of nucleotide identity shared between Afp- and AfpX-coding regions. (E) Schematic representation of Afp with the structural parts, namely, sheath, tube, tail fibers, and baseplate, designated.
Aside from the AfpX13-encoding region, the more divergent areas of nucleotide composition were located in the intergenic regions between anfA1 and enp1, upstream of afpX1. Increased nucleotide divergence was also observed downstream of nucleotide 1021 of the afp17 and afpX17 genes (Fig. 5 and Fig. S2). Assessment of the G+C plot of the afpX-associated region identified a G+C skew in the predicted regulatory region 5′ of afpX1 and in the N- and C-terminal encoding regions of the putative AfpX18 toxin (Fig. 5D). In addition to the high level of nucleotide identity to components of S. entomophila Afp, the afpX17 and afpX18 toxin-encoding genes shared 72% and 77% nucleotide identities to the afp18 genes of Photorhabdus asymbiotica ATCC 43949 (PAU_03033) and Y. ruckeri strain PB-H2 (CSF007_16415), respectively.
Unlike the S. entomophila Afp-encoding region, the AfpX gene cluster encoded two Afp16 orthologs, AfpX16a and AfpX16b. afpX16a and afpX16b were located 5′ of afpX18 and afpX17, respectively (Fig. 5), and shared 87% nucleotide sequence identity. Relative to afpx16a, afpx16b contained an internal 101-bp deletion, corresponding to 34 amino acid (aa) residues (residues 50 to 85 of Afp16 and AfpX16a) (Fig. S3).
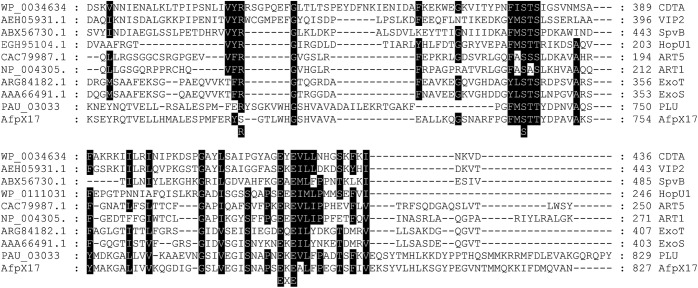
The N terminus of AfpX17 shared >98% amino acid identity with the corresponding region of S. entomophila Afp17 (Table 3). Over its entirety, AfpX17 showed significant amino acid identity to the ADP-ribosyltransferase of P. asymbiotica subsp. australis (PAU_03033) (Table 3). The C-terminal region of AfpX17 contained a predicted S-S-EXE motif that spanned the requisite secondary structural features shared across members of the ADP-ribosyltransferase family, including the insect-active Bacillus cereus vegetative insecticidal protein VIP2 (Fig. 5) (27). Over its entire length, AfpX18 showed 80% amino acid similarity to the Y. ruckeri Afp18 tyrosine glycosylation toxin, with a predicted glycosyltransferase domain in the C-terminal region (Fig. S4) (17). Amino acid alignment of AfpX18 with its S. entomophila and Y. ruckeri Afp18 orthologs identified two areas of high amino acid identity: between AfpX18 residues 494 and 1331 and in the distal C-terminal region (AfpX18 residues 2096 to 2271) (Fig. S4).
The predicted AfpX tail fiber protein AfpX13 shared 61% amino acid similarity with the shorter (by 46 aa residues) S. entomophila Afp13 protein (Table 3 and Fig. S5). With reference to Fig. S6 and S7 in the supplemental material, secondary structure prediction for Afp13X and Afp13 identified a single α-helix positioned approximately 40 to 45 aa residues from the N terminus. AfpX13 contained a series of 24 β-strand repeats of approximately 15 aa residues and shared a common pattern of hydrophobicity. This pattern aligned in part with 14 repeats identified in S. entomophila Afp13, which could be extended to a total of 18 degenerate repeats. We identified a common tryptophan residue followed by a series of cysteine residues located distal to the β-strand repeats of both AfpX13 and Afp13.
Cloning and targeted mutagenesis of the S. proteamaculans AGR96X virulence-associated region.
To experimentally assess whether the AfpX gene cluster is the main virulence determinant in AGR96X, we first restriction enzyme digested the AGR96X genome with PmaCI and then shotgun cloned this fragment into pBRminicos17H (Table 4). Assessment of the restriction enzyme profiles of transformants derived from the ligations identified two PmaCI clones containing DNA regions in opposing orientations (pAFPXR and pAFPXQ) (Table 4). pAFPXQ contained the complete AfpX gene cluster, and when transformed into E. coli, the resulting transformant showed a 65.7% mortality rate compared with wild-type S. proteamaculans strain AGR96X, which had a mortality rate of 81.7% (Table 2). When C. giveni larvae were fed E. coli transformed with pAFPXR, in which the translated AfpX17 protein was truncated by 299 aa residues, only 41.3% of the challenged larvae died (Table 2).
TABLE 4.
Bacteria and plasmids used in the study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| DH10B | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80dlacZΔM15 ΔlacX74 endA1 recA1 deoR Δ(ara leu)7697 araD139 galU galK nupG rpsL λ− | 53 |
| S17-1 λpir | hsdR Pro ΔrecA RP4-2 Tc::Mu Kn::Tn7 integrated into the chromosomal pir gene | 54 |
| XL1-BlueMRA | ΔmcrA183 ΔmcrCB-hsdSMR-mrr173 endA1 supE44 thi-1 recA1 gyrA96 relA1 | Stratagene |
| Serratia proteamaculans | ||
| AGR96X | pAGR96X; C. giveni, Pyronota spp. active; isolated from a diseased grass grub larva collected from the Lake Coleridge area of New Zealand, 1996 | This study |
| AGR96XΔAFP1516a | AfpX15-16 mutant; NaeI-flanked kanamycin resistance amplicon KNNAE inserted into the NaeI restriction site, deleting a 1,745-bp fragment corresponding to the DNA region encoding aa residue 205 of AfpX15 to aa residue 70 of AfpX16a; Knr | This study |
| AGR96X::17 | BglII-flanked spectinomycin resistance amplicon SPBGL inserted into the BglII site located 1,478 bp 3′ of the afpX17 initiation codon; Spr | This study |
| AGR96X::18 | MfeI-flanked kanamycin resistance amplicon KNMFE inserted into the MfeI site located 6,132 bp 3′ of the afpX18 initiation codon; Knr | This study |
| AGR96X(pGFPuvsp) | AGR96X containing pGFPuvsp; Amr Spr | This study |
| Serratia entomophila A1MO2 | pADAP; C. giveni active | 55 |
| Plasmids | ||
| pAGR96X | 120-kb pADAP variant; carries the AfpX gene cluster | |
| pAFPXQ | 36,883-bp clone carrying the AfpX gene cluster; Amr | This study |
| pAFPXR | 36,883-bp clone carrying the AfpX gene cluster; 35,483-bp PmaCI fragment in opposite orientation, leading to a 299-aa truncation of the 913-aa-residue AfpX17 protein; Amr | This study |
| pGEM-T Easy | Amr | Promega Corporation |
| pGEM1516 | 4,539-bp amplicon encompassing the AfpX genes afpX15 and afpX16 cloned into pGEM-T Easy; Amr | This study |
| pGEM1516KN | 1,201-bp EcoRV-flanked kanamycin resistance amplicon KNRV cloned into the NaeI site of pGEM1516, deleting a 1,745-bp region corresponding to aa residue 205 of Afp15 to aa residue 70 of Afp16; Amr Knr | This study |
| pGEM17 | 2,329-bp amplicon 17BGL encompassing afpX17 cloned into pGEM-T Easy; Amr | This study |
| pGEM18 | 2,219-bp amplicon 18MFE encompassing afpX18 cloned into pGEM-T Easy; Amr | This study |
| pGEM17SP | 1,379-bp MfeI-flanked spectinomycin resistance amplicon SPMFE cloned into the MfeI site of pGEM17; Amr Spr | |
| pGEM18KN | 1,201-bp BglII-flanked kanamycin resistance amplicon KNBGL cloned into the BglII site of pGEM18; Amr Knr | |
| pGFPuvsp | pGFPuv variant; Amr Spr | 56 |
| pADAP | S. entomophila, amber disease-associated plasmid; encodes Sep and Afp virulence-associated regions | 6 |
| pAGR96X | S. proteamaculans AGR96X pADAP variant encoding the AfpX virulence-associated region | This study |
| pBRminicospac | pBR322 containing the pLAFR3-derived BglII cos site inserted into the BamHI site; Amr | 10 |
| pBRminicos17H | 1,439-bp HindIII fragment containing the terminal 945 bp of afpx17 and 494 bp 3′ of the afpX17 termination codon, inserted into the HindIII site of pBRminicos; Amr Knr | This study |
| pJP5608 | R6K-based suicide plasmid; Tcr | 57 |
| pJP5608ΔAFP1516a | EcoRI-associated kanamycin resistance fragment of pGEM1516KN ligated into the EcoRI site of pJP5608; Knr Tcr | This study |
| pJP5608::17 | EcoRI, 17BGL-associated spectinomycin resistance fragment of pGEM17SP ligated into the EcoRI site of pJP5608; Spr Tcr | This study |
| pJP5608::18 | EcoRI, 18MFE-associated kanamycin resistance fragment of pGEM18KN ligated into the EcoRI site of pJP5608; Knr Tcr | This study |
To further validate that AfpX is the main S. proteamaculans AGR96X virulence determinant, afpX15, encoding the putative chaperone ATPase required for Afp assembly (10, 19), was targeted for site-directed mutagenesis. Bioassays using the resultant S. proteamaculans AGR96XΔAFP1516 strain showed that the recombinant strain was nonpathogenic in both C. giveni and Pyronota larvae (Table 2). Challenging grass grub larvae with either AGR96X::18 or AGR96X::17 showed that at day 12, the strains caused 40% and 51.7% mortality rates, respectively, with 51.7% of AGR96X::18-challenged larvae appearing diseased, exhibiting an amber to brown coloration. When bioassayed against manuka beetle larvae, both AGR96X::18 and AGR96X::17 caused levels of disease similar to that caused by wild-type strain AGR96X, with >83% mortality being noted (Table 2).
Mitomycin C induction of AfpX.
When grass grub larvae were fed concentrated culture supernatant filtrates from S. proteamaculans AGR96X that had been treated with mitomycin C, the larvae displayed a disease phenotype similar to that of larvae that had ingested AGR96X (Tables 1 and 2). Culture supernatants derived from similarly treated S. entomophila bacteria had no effect on grass grub larvae (Tables 1 and 2). Because of the bioactivity of the mitomycin C-treated AGR96X culture supernatants, the concentrated supernatants were subjected to transmission electron microscopy (TEM) analysis. High numbers of Afp-like particles were observed in the supernatants, and measurements showed that the AfpX particles had various lengths, ranging from 70 to 140 nm, with a mean length of 110 nm (Fig. 6).
FIG 6.
(A) Plot showing the distribution of particle lengths of AfpX, where the numbers shown above each bar denote the number of measured particles (159 in total). (B) Electron micrographs of observed AfpX particles showing the different sizes. Bar, 100 nm.
Assessment of Afp orthologs.
Assessment of gene synteny across the selected members of the Afp paraphyletic group revealed that afp1 to afp15 are typically conserved with regard to gene order. A single example of an Afp variant, encoding two Afp16-like proteins, was identified in the Serratia marcescens MSU97 genome sequence. In Chania multitudinisentens RB-25, the Afp16 ortholog was positioned five open reading frames (ORFs) downstream of Afp14. Two- and four-sheathed Afp variants were identified in the benthos bacterium Shewanella psychrophila WP2 and in the plant endophyte Erwinia oleae strain DAPP-PG531, respectively (see Fig. S8 in the supplemental material). Phylogenetic assessment of the putative AfpX15 ATPase chaperone, which is essential for Afp assembly, revealed a strong similarity between Afp and AfpX of members of the genus Yersinia (Y. ruckeri and Y. similis) and lesser similarity to the more distant species Pseudomonas fluorescens and Burkholderia rhizoxinica (endosymbiont of Rhizopus microsporus) (Fig. S9).
DISCUSSION
Strain AGR96X is the first S. proteamaculans strain with documented activity against grass grub and manuka beetle larvae. Host range testing of AGR96X against other members of the family Scarabaeidae found no activity. It is therefore tempting to speculate that similar to S. entomophila and C. giveni, AGR96X may have coevolved with manuka beetle and grass grub. In contrast to the grass grub-specific species S. entomophila, the ingestion of 5 × 107 AGR96X cells by grass grub larvae results in the invasion of the hemocoel and subsequent death within 5 to 12 days. In addition, the median 12-day lethal dose of AGR96X was 5 × 103 CFU per larva. Also, unlike larvae challenged with either S. entomophila or Y. entomophaga, the color of larvae challenged with AGR96X transitioned from cream to slightly purple, which was more prominent at the leading edge of each thoracic segment.
Postchallenge, the S. entomophila cell density plateaued at 2.67 × 107 ± 1.08 × 107 CFU per grass grub larva over the 7-day assessment period, while AGR96X reached a maximum cell density of 8.19 × 109 ± 3.94 × 109 CFU per larva at 6 days postchallenge, which was similar to the cell density of Y. entomophaga (8.57 × 109 ± 2.41 × 109 CFU per larva) observed 3 days after oral challenge (28). The higher cell density likely reflects the access to and the availability of a hemolymph nutrient source. We observed a slow deterioration of the larval hemocytes and an increase in PO activity after hemocoelic invasion. In line with the chronic and noninvasive lifestyle of S. entomophila, hemocytes, but no increase in PO activity, were observed over the 7-day assessment period (Fig. 4).
By using a combination of bioinformatic analyses, targeted mutagenesis, and DNA cloning, a new variant of Afp, termed AfpX, was identified. AfpX differs from the previously documented Afp/PVC and other tailocin variants (16) by the presence of two TrP Afp16 proteins and an additional toxin protein. When fed to grass grub or manuka beetle larvae, E. coli containing the AfpX-encoding pAFPXQ clone induced AGR96X-like symptoms. The decreased mortality caused by these E. coli strains may be related to an altered efficiency of virulence induction or the survival dynamics of the strain. Bioassays of the AGR96XΔAFP1516a deletion mutant against grass grub or manuka beetle larvae showed that the mutant was avirulent (Tables 1 and 2). The similar gene organizations of AfpX and Afp, combined with data from previous studies that have shown that the full composite of Afp proteins is required for Afp assembly and bioactivity (10, 19, 20), suggest that the AfpX tailocin is the main virulence determinant of AGR96X.
The high degrees of amino acid similarity between AfpX18 and the actin-disrupting Y. ruckeri Afp18 protein (Table 3) (17) and between AfpX17 and the ADP-ribosylating toxins, including the insecticidal VIP toxin (Fig. 7), suggest that AfpX17 and AfpX18 have similar modes of action. When ingested by grass grub larvae, E. coli containing pAFPXR (containing truncated AfpX17) caused approximately 41.3% mortality. Bioassays assessing the activity of AGR96X::18 and AGR96X::17 found them to be less effective than the wild-type AGR96X strain, with mortality rates of only ∼40 to 51%, with the remaining larvae only macerating the surface of the carrot. Both AGR96X::18 and AGR96X::17 were as effective as AGR96X in the killing of manuka beetle larvae. The decreased pathogenicity of AGR96X::18 and AGR96X::17 against grass grub larvae may reflect the larger larva size and/or different host species. Combined, these results suggest that both the AfpX17 and AfpX18 toxins are important for efficacy in grass grub larvae but may have a degree of redundancy against larvae of the manuka beetle.
FIG 7.
Amino acid alignments of the AfpX17 C-terminal region with selected members of the ADP-ribosyltransferase family. The amino acid location of the VIP2 R-S-EXE arginine-specific ADP-ribosyltransferase motif is indicated.
Visual assessment of mitomycin C-induced AfpX particles by TEM identified AfpX particles of various lengths, with some measuring up to 140 nm. As would be predicted based on the similar sizes of the Afp14 and AfpX14 tape measure proteins (Tables 1 and 2), and despite a greater size distribution than that observed for Afp (19), the mean AfpX particle length was 110 nm. Rybakova et al. (19) demonstrated previously that when afp16 is expressed in trans with afp1 to afp15, Afp particles of various lengths are obtained. Those authors theorized that these aberrant particles are the result of the mistimed binding of Afp16 to the polymerizing tube. It is plausible, then, that the variability in the length of AfpX is linked to the presence of two AfpX16 TrPs competing for the tube substrate. The ability to induce AfpX but not S. entomophila Afp with mitomycin C suggests that these tailocins are under the control of different regulatory systems. Based on these results, the influence of the noted nucleotide differences in the intergenic regions between the anfA1 regulator and the afpX1 genes in S. entomophila and S. proteamaculans (see Fig. S1 in the supplemental material) on gene expression warrants further investigation.
With the exception of AfpX13, the AfpX1 to AfpX18 proteins show high amino acid sequence identity to components of Y. ruckeri Afp. The variation shown by Afp13 may therefore confer host range specificity, as has been shown for the tail fibers of phage T4 (29) and R-type pyocins (30). Unlike S. entomophila grass grub-specific Afp, AfpX is active against both grass grub and manuka beetle larvae. Both Afp13 and Afp13X have architectures similar to those of adenovirus tail fibers, comprising a predicted N-terminal region required for the attachment of the fiber to the baseplate and a central shaft composed of a series of hydrophobic β-spiral-based repeats, ∼15 residues in length (31) (Fig. S6 and S7). Although dissimilar in amino acid compositions, the phage T4 short and long fiber shafts also comprise a series of hydrophobic β-strand repeats (32). The adenovirus tail fiber-targeting “knob” region is located in the C terminus, distal to the repeat region (22). Amino acid alignments of the sequences corresponding to the C-terminal tip of S. entomophila Afp13 and AfpX13 reveal few sequence differences (Fig. S5). However, we note that Afp13 and Afp13X differ in amino acid lengths and repeat structures. Differing lengths and the inherent flexibility of the adenovirus tail fibers can alter the infective properties of an adenovirus (33, 34). Therefore, the mechanism of Afp target cell tail fiber attachment warrants further investigation.
The identification of Afp variants in a range of bacterial species sourced from various habitats, including plants, nematodes, and insects, as well as free-living bacteria further alludes to a distant origin of these tailocins, which was supported by results of phylogenetic analyses. This study reports an Afp variant encoding two Afp16-like proteins (AfpX16a and AfpX16b). However, database analyses identified a second example in the S. marcescens MSU97 genome. In this instance, a putative type III secretion effector is located between Afp15 and Afp16, with another putative effector being located 3′ of the second Afp16 ortholog (Fig. S8). In the C. multitudinisentens RB-25 genome, the Afp16 ortholog is positioned several ORFs 3′ of the Afp14 ortholog. This information, combined with the AfpX data and previous trans-based expression data for afp16 with afp1 to afp15 (19), suggests that there may be some degree of fluidity in the regions involved in toxin acquisition. Nucleotide alignments of afp17 and afpX17 identified no significant sequence identity after nucleotide 1021 of these genes. Liquid chromatography-tandem mass spectrometry analysis of purified S. entomophila Afp did not detect Afp17 in mature Afp (19). Based on this information, as well as the high nucleotide identity to components of S. entomophila Afp, it is tempting to speculate that the S. entomophila afp17 gene is an inactive truncated remnant, with afp18 then possibly being acquired by horizontal gene transfer and Afp18 being responsible for the cessation of feeding activity. It is interesting that the afpX17 and afpX18 genes share high nucleotide identities with the corresponding genes in P. luminescens and Y. ruckeri, alluding to their acquisition by horizontal gene transfer.
Based on the data presented in this study, we speculate that the relatively rapid death of the insects following the ingestion of S. proteamaculans AGR96X results from the production of AfpX en masse. In this context, a median dose of 500 S. entomophila Afp particles is required to cause a cessation of feeding within 3 days of ingestion by grass grub larvae (19), while the ingestion of an excess of purified Afp particles causes rapid larva death in C. giveni (10). This information, combined with the chronic nature of S. entomophila-induced amber disease and the lower cell density, suggests that the virulence factors of amber disease are produced at low levels. As was observed for Y. entomophaga, the penetration of fast-acting pathogens such as AGR96X into the hemocoel is the critical step.
The isolation of a highly virulent strain of S. proteamaculans, from the same field site and at the same time as the isolation of the fast-acting broad-host-range entomopathogen Y. entomophaga, raises further questions about the ecology of these insect pathogens. Of particular interest is the requirement for grass grub-active strains with different virulence attributes (i.e., slow versus rapid killing). As noted above, S. entomophila causes a chronic infection, which may facilitate the long-term survival of the bacterium in the grass grub larvae by protecting S. entomophila from external competition. On the other hand, the rapid-acting pathogen S. proteamaculans AGR96X multiplies rapidly in the insect cadaver, which possibly limits its capacity to infect other insects. In future studies, we will endeavor to elucidate the natural ecology of these bacteria, define the histological processes, and identify the potential site(s) of binding of AfpX to the insect gut.
MATERIALS AND METHODS
Bacterial strains, isolation, and culture methods.
Table 4 lists bacterial strains and plasmids used in this study. S. proteamaculans strain AGR96X was isolated, as previously described, from a diseased larva collected near Lake Coleridge, Canterbury, New Zealand (25). Isolates were grown in Luria-Bertani (LB) broth or on LB agar at 37°C (Escherichia coli) or 30°C (S. entomophila and S. proteamaculans) with shaking at 250 rpm in a Ratek orbital incubator (model OM11). The following antibiotics were used: kanamycin at 100 μg/ml, spectinomycin at 100 μg/ml, and tetracycline at 30 μg/ml for Serratia and kanamycin at 50 μg/ml, spectinomycin at 100 μg/ml, tetracycline at 15 μg/ml, and ampicillin at 100 μg/ml for E. coli. Serial dilutions of the LB broth cultures were performed in 10-ml volumes of 0.1% Bacto peptone, and appropriate dilutions (from 10−2 to 10−7) were plated onto LB agar.
DNA isolation, manipulation, and sequencing.
Standard DNA techniques were performed as described previously by Sambrook et al. (35). Plasmids were electroporated into E. coli and S. proteamaculans strains by using a Gene Pulser instrument (Bio-Rad, Hercules, CA, USA) at 25 μF, 2.5 kV, and 200 Ω (36). PCR primers used in this study are listed in Table 5, along with the sizes of the corresponding amplicons. PCR products and plasmid templates were purified by using High Pure PCR product and High Pure plasmid isolation kits (Roche Diagnostics GmbH, Mannheim, Germany), respectively. Large plasmid DNA was visualized according to the method of Kado and Liu (37). pAGR96X DNA was isolated from a 50-ml LB broth culture of AGR96X grown overnight by using a Plasmid Maxi kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions.
TABLE 5.
PCR primer sets, amplicons, and sizes
| Primer | Primer sequence (5′–3′)a | Amplicon | Amplicon size (bp) |
|---|---|---|---|
| AfpX-derived amplicons | |||
| AFPF | AAAAAGCTTTTGTGAAATTGCTGGCTCAG | AFPX17CH | 1,439 |
| AFPR | AAAAAGCTTGAATTCGCGTTAGATACCCCCA | ||
| AFP15F | CCAAACGGCTTGCACTACCGCGGG | AFPX1516 | 4,539 |
| AFP16R | TGAGACTCATGAGGTCAAGCGTTCC | ||
| 18MFEF | AAACAATTGGGGCATCTCGCCTCATGAGGG | 18MFE | 2,219 |
| 18MFER | AAACAATTGGCGAGCGATATACTCCAACGC | ||
| 17BGLF | AAAAGATCTGGGTGGAGCAGCGATAACGCG | 17BGL | 2,329 |
| 17BGLR | AAAAGATCTGCAGTGCGTCATCCATCGCCTC | ||
| Antibiotic cassettes | |||
| KNRVF | AAAGATATCGTAAGCCAGTATACACTCCGCTAGCGC | KNRV | 1,201 |
| KNRVR | AAAGATATCAGTGTTACAACCAATTAACCAATTC | ||
| KNBGLF | AAAAGATCTGTAAGCCAGTATACACTCCGCTAGCGC | KNBGL | 1,201 |
| KNBGLR | AAAAGATCTGAGTGTTACAACCAATTAACCAATTC | ||
| KNNAEF | AAAGCCGGCTAAGCCAGTATACACTCCGCTAGCGC | KNNAE | 1,201 |
| KNNAER | AAAGCCGGCAGTGTTACAACCAATTAACCAATTC | ||
| KNMFEF | AAACAATTGTAAGCCAGTATACACTCCGCTAGCGC | KNMFE | 1,201 |
| KNMFER | AAACAATTGGAGTGTTACAACCAATTAACCAATTC | ||
| SPBGLF | AAAAGATCTATGCCCGTTCCATACAGAAGCT | SPBGL | 1,379 |
| SPBGLR | AAAAGATCTGACATTATTTGCCGACTACCT | ||
| SPMFEF | AAACAATTGATGCCCGTTCCATACAGAAGCT | SPMFE | 1,379 |
| SPMFER | AAACAATTGCGACATTATTTGCCGACTACCT | ||
| Primers for validation of homologous recombination | |||
| 1516F | GAGTCGTTGTTTACAACGCCAGG | 4,814 | |
| 1516R | GTGCGCATCTGCATTTACCGG | ||
| 18F | GTTAGATTACTCCCACCTTCCGGATG | 2,441 | |
| 18R | GATAATTAGACATCCTCGTTCCTC | ||
| 17F | TTAACCACTTGGGTAACGACCTCC | 2,632 | |
| 17R | ACTGGTACTGATTGCGGGTCCAAGG | ||
| Diagnostic primers | |||
| AFP8F | CTGGACATAGCGGGACAGCGCAGCAC | 909 | |
| AFP8R | ACTCAGGGTGATACTCTCGCCCG | ||
| AFPX17F | GCCGACTAACACACCACAGTTACAGC | 2,600 | |
| AFPX17R | TTAACGCATGATCATTTGCGCTTCCGG | ||
| AFPX18F | CAACTGGAGCAGTTCGAGCTGGC | 805 | |
| AFPX18R | GTCATAAGACTCATGCCAAGAGTC | ||
| REPA | TGCAGGGGAACGATCTTCTTGAGG | 893 | |
| REPR | GCCCCACTTTCTTGTACCATCCAG | ||
| BOXA1R | CTACGGCAAGGCGACGCTGACG |
Underlining denotes the restriction enzyme site.
Whole-genome sequencing of AGR96X was undertaken using the Illumina HiSeq 2500 system by Macrogen Sequencing Services (Macrogen Inc., Seoul, Republic of Korea). DNA sequences were trimmed by using Trim_Galore (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) and assembled by using A5-miseq (38) (see Table S1 in the supplemental material for genome assembly data). When required, sequencing gaps were closed by using Sanger sequencing of PCR amplicons. PCR primer sets were designed manually or by using Primer3 (39). Sequences were then assembled by using SeqMan Pro (DNASTAR Inc., Madison, WI, USA).
Bioinformatics and phylogenetic analyses.
The resulting sequence contigs were then analyzed by using Basic Local Alignment Search Tool BLASTN, BLASTX, and BLASTP searches against the nonredundant National Center for Biotechnology Information databases (40). EDITSEQ (DNASTAR Inc.) and GeneMark (41) were used to identify ORFs, and translated ORFs with significant similarity to proteins of a defined function were designated by using the appropriate nomenclature based on the closest validated orthologs. Secondary structures were predicted by using Phyre2 (42), and amino acid sequences were aligned by using DNAMAN (Lynnon Corporation, Quebec, Canada) and Geneious version 8.1 (43).
The 16S rRNA gene sequences of the Serratia strains isolated from the diseased larvae were aligned with those of Serratia type strains, the accession numbers of which are listed in Fig. 1, by using ClustalX (44). Phylogenetic trees were generated in MEGA5.10 (45), using the maximum likelihood method (46) with 1,000 bootstrap replications. Amino acid sequence alignments were undertaken by using Muscle Trees constructed with PhyML, using the LG model and 500 bootstrap replicates (47, 48).
Cloning and targeted mutagenesis of the S. proteamaculans AGR96X virulence-associated region.
Restriction enzyme analysis of the S. proteamaculans AGR96X AfpX-encoding region revealed that it could be cloned in near entirety by using PmaCI restriction enzyme sites located 5,817 bp upstream of the afp1 initiation codon and at nucleotide 1844 of afpX17. To clone the putative AfpX-encoding region, amplicon AFPX17CH, containing a unique PmaCI site within the afpX17 C terminus, was digested with HindIII and ligated into the analogous site of pBRminicospac, generating pBRminicos17H (Tables 1 and 2). Purified pAGR96X DNA was digested with PmaCI and ligated into the analogous site of pBRminicos17H. The ligation was then packaged by using Gigapack III XL packaging extract (Stratagene, La Jolla, CA, USA).
To construct a partial afpX15-afpX16a deletion derivative, 4,539-bp amplicon AFPX1516 (Table 5), encompassing afpX15 and afpX16, was cloned into pGEM-T Easy to generate pGEM1516. The kanamycin resistance amplicon KNRV (Table 5) was then digested with EcoRV and ligated into the NaeI site of pGEM1516, deleting a 1,745-bp region from aa residue 205 of Afp15 to residue 70 of Afp16. This new plasmid, termed pGEM1516KN, was then digested with EcoRI, and the region to be recombined was cloned into the analogous site of suicide vector pJP5608, to form pJP5608ΔAFP1516a. pJP5608ΔAFP1516a was then conjugated to S. proteamaculans AGR96X. Potential recombinants were validated by PCR using inward-facing primers 1516F and 1516R (Table 5), positioned external to the recombined region, followed by DNA sequencing of the resultant amplicon. The validated recombinant was designated S. proteamaculans AGR96XΔAFP1516 (Table 4).
To generate AfpX variants containing insertions in either afpX17 or afpX18, constructs pJP5608::17 and pJP5608::18 (Table 4) were independently conjugated into AGR96X. Recombinants were validated by using the appropriate inward-facing primer sets 17F/R and 18F/R (Table 5), followed by DNA sequencing of the resultant amplicons.
Mitomycin induction.
Aliquots (1 ml) of cultures of S. proteamaculans AGR96X and S. entomophila A1MO2 grown overnight were used to inoculate 50 ml of LB broth in 250-ml conical flasks. The cultures were grown at 30°C with shaking at 250 rpm to an optical density at 600 nm (OD600) of 1.1. Cells were then pelleted by centrifugation at 2,268 × g for 10 min, and the pellets were resuspended in diluted (0.4× strength) LB broth. Mitomycin C was added to the resuspended cells to a final concentration of 0.2 μg/ml, and the flasks were placed on an orbital platform and incubated at ambient temperature (∼22°C) with rotation at 40 rpm for 18 h. Afp particles were extracted by using chloroform and ultracentrifugation, as previously described (11), and purified by size exclusion chromatography when required (19). AfpX samples were maintained in Tris-buffered saline (130 mM NaCl, 25 mM Tris-HCl [pH 7.5]).
Transmission electron microscopy.
Purified and semipurified samples produced in the various expression experiments were applied to a freshly glow-discharged plastic-coated 300-mesh copper grid that was overlaid with a carbon film and then negatively stained and examined with a Morgagni 268D transmission electron microscope, as previously described (19). The lengths of the AfpX particles from micrographs recorded at a nominal magnification of ×89,000 were measured by using ImageJ software (49). The length of an Afp particle was measured from the end of the baseplate to the tip of the sheath. A total of 159 random particles from three independent experiments were measured.
Bioassays.
The pathogenicities of S. entomophila, S. proteamaculans, and their derivatives toward the larvae of a range of the species belonging to the family Scarabaeidae, specifically the New Zealand grass grub (C. giveni), the manuka beetle (P. festiva and P. setosa), the African black beetle (Heteronychus arator), the redheaded cockchafer (Adoryphorus couloni), the Tasmanian grass grub (Acrossidius tasmaniae), and the chafer beetle (Odontria sp.), were bioassayed as follows. Healthy third-instar larvae were individually fed carrot cubes (∼3 mm3 for grass grub larvae and ∼2 mm3 for manuka beetle larvae) that had been rolled in a lawn of bacterial colonies grown overnight on LB agar, resulting in approximately 107 CFU/carrot cube. Twelve larvae were used for each treatment. Larvae were fed treated carrot on day 1 and were transferred to fresh trays containing untreated carrot on days 3 and 6. The occurrence of disease symptoms, including a change in coloration from amber through to brown/black and feeding activity, was monitored on days 3, 6, and 12. S. entomophila strain A1MO2 and, when required, S. proteamaculans strain AGR96X were used in all bioassays as positive controls, with negative-control larvae being fed untreated carrot.
To satisfy Koch's postulates for AGR96X, bacteria isolated from diseased larvae were reisolated and bioassayed against healthy larvae to confirm their pathogenicity, and the process was repeated. Dose-response bioassays against C. giveni larvae were performed by pipetting 5-μl aliquots of serial dilutions (corresponding to 1 × 101 to 1 × 106 CFU) of AGR96X onto the surfaces of carrot cubes (3 mm3). Bioassays were then undertaken as outlined above, except that larvae were transferred to new trays containing freshly cut carrot cubes after 24 h. Negative controls were treated with 5 μl of LB broth. For the assessment of mitomycin C-induced Afp and AfpX samples, 5-μl aliquots of filter-sterilized semipurified samples were pipetted onto the surfaces of carrot cubes (3 mm3) and bioassayed as outlined above. Three independent bioassays were conducted for each treatment. Because of the accessibility, vigor, and consistent feeding activity of C. giveni larvae, this species was used in preference to manuka beetle larvae for assessments of the pathobiology of AGR96X.
Enumeration and microscopy of bacteria from challenged larvae and assessment of phenol oxidase activity.
Bioassays using C. giveni larvae were set up as described above, with an inoculum comprising a 5-μl aliquot of a culture grown overnight (typically corresponding to 1.2 × 1010 CFU for both A1MO2 and AGR96X) on the surface of each 3-mm3 carrot cube. At various time points (48, 72, 94, 120, 144, and 168 h) throughout the bioassays, a random subset of three whole larvae, each weighing approximately 0.11 to 0.17 g and of similar colorations, were removed from each treatment group and assessed for the presence of the infective bacteria and PO activity. Each larva was briefly rinsed with water in a 15-ml centrifuge tube and then placed in 70% ethanol for 1 min before the solution was removed and fresh water was added. The tube was inverted several times at each step. After the final water wash, the larvae were removed and placed on tissue paper to drain excess fluid. Each larva was then weighed and placed into a microcentrifuge tube. The volume was made up to 1 ml with water, and the larvae were macerated by using a pestle. Serial dilutions of the larval homogenates were plated onto caprylate-thallous agar (CTA) selective for Serratia species. The resultant colonies were then patched onto both adonitol agar and DNase agar, allowing the differentiation of S. proteamaculans and S. entomophila (50). When required, a subset of bacteria (typically 10 of 100 patched Serratia-positive isolates from each larva) was validated by BOX-PCR, using the BOX A1R primer as outlined previously by Versalovic et al. (51), and targeted PCR assessing the pADAP repA gene and components of the Afp or AfpX gene cluster (Table 5). PO activity was assessed by collecting hemolymph from the larvae by cutting off a leg and collecting 15 μl of outflowing liquid (hemolymph). This liquid was immediately transferred to a prechilled microcentrifuge tube and processed as previously described (52). When required, 10-μl aliquots of hemolymph were assessed for bacterial content by using serial dilutions, as described above, and assessed by light microscopy using an Olympus BX50 light microscope and an Olympus DP-72 digital camera.
Statistical analysis.
Bioassays were assessed by using a generalized linear model with group-specific binomial distributions through a logit link function using Minitab version 16. PO OD490 values were loge transformed to stabilize variation between phenotype groups and then assessed by using analysis of variance (ANOVA) with SAS version 9.3. (2017; SAS Institute Inc.).
Accession number(s).
The nucleotide sequences of the S. proteamaculans AGR96X 16S rRNA gene and the pAGR96X AfpX-associated region have been deposited in GenBank under accession numbers KY934181 and KU559315, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank James Vernon and Duane Harland (AgResearch, Lincoln, New Zealand) for their guidance on transmission electron microscopy and Richard Townsend (AgResearch, Lincoln, New Zealand) for providing grass grub and manuka beetle larvae.
This research was funded by the Ministry of Business, Innovation and Employment, New Zealand (Next Generation Bio-pesticides, grant number C10X1310).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02739-17.
REFERENCES
- 1.Coca-Abia MM, Romero-Samper J. 2016. Establishment of the identity of Costelytra zealandica (White 1846) (Coleoptera: Scarabaeidae: Melolonthinae) a species commonly known as the New Zealand grass grub. N Z Entomol 39:129–146. doi: 10.1080/00779962.2016.1230254. [DOI] [Google Scholar]
- 2.Adeolu M, Alnajar S, Naushad S, Gupta R. 2016. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’. Int J Syst Evol Microbiol 66:5575–5599. doi: 10.1099/ijsem.0.001485. [DOI] [PubMed] [Google Scholar]
- 3.Jackson TA, Glare TR, O'Callaghan M. 1991. Pathotypic boundaries for Serratia spp. causing amber disease in the New Zealand grass grub, Costelytra zealandica, p 148–152. In Smits PH. (ed), Proceedings of the 3rd European Meeting of Microbial Control of Pests. International Organization for Biological Control of Noxious Plants and Animals, Wageningen, The Netherlands. [Google Scholar]
- 4.Jackson TA, Huger AM, Glare TR. 1993. Pathology of amber disease in the New Zealand grass grub, Costelytra zealandica Coleoptera: Scarabaeidae. J Invertebr Pathol 61:123–130. doi: 10.1006/jipa.1993.1024. [DOI] [Google Scholar]
- 5.Jackson TA, Boucias DG, Thaler JO. 2001. Pathobiology of amber disease, caused by Serratia spp., in the New Zealand grass grub, Costelytra zealandica. J Invertebr Pathol 78:232–243. doi: 10.1006/jipa.2002.5078. [DOI] [PubMed] [Google Scholar]
- 6.Glare TR, Corbett GE, Sadler AJ. 1993. Association of a large plasmid with amber disease of the New Zealand grass grub, Costelytra zealandica, caused by Serratia entomophila and Serratia proteamaculans. J Invertebr Pathol 62:165–170. doi: 10.1006/jipa.1993.1091. [DOI] [Google Scholar]
- 7.Hurst MRH, Becher A, O'Callaghan M. 2011. Nucleotide sequence of the Serratia entomophila plasmid pADAP and the Serratia proteamaculans pU143 plasmid virulence associated region. Plasmid 65:32–41. doi: 10.1016/j.plasmid.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Bowen D, Rocheleau TA, Blackburn M, Andreev O, Golubeva E, Bhartia R, ffrench-Constant RH. 1998. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280:2129–2132. doi: 10.1126/science.280.5372.2129. [DOI] [PubMed] [Google Scholar]
- 9.Hurst MR, Glare TR, Jackson TA, Ronson CW. 2000. Plasmid-located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. J Bacteriol 182:5127–5138. doi: 10.1128/JB.182.18.5127-5138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst MR, Glare TR, Jackson TA. 2004. Cloning Serratia entomophila antifeeding genes—a putative defective prophage active against the grass grub Costelytra zealandica. J Bacteriol 186:5116–5128. doi: 10.1128/JB.186.15.5116-5128.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurst MRH, Beard SS, Jackson TA, Jones SM. 2007. Isolation and characterization of the Serratia entomophila antifeeding prophage. FEMS Microbiol Lett 270:42–48. doi: 10.1111/j.1574-6968.2007.00645.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang G, Dowling AJ, Gerike U, ffrench-Constant RH, Waterfield NR. 2006. Photorhabdus virulence cassettes confer injectable insecticidal activity against the wax moth. J Bacteriol 188:2254–2261. doi: 10.1128/JB.188.6.2254-2261.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bönemann G, Pietrosiuk A, Mogk A. 2010. Tubules and donuts: a type VI secretion story. Mol Microbiol 76:815–821. doi: 10.1111/j.1365-2958.2010.07171.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, de Souza RF, Anantharaman V, Lyer LM, Aravind L. 2012. Polymorphic toxin systems: comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct 7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill JJ, Young RF III (ed). 2011. Therapeutic applications of phage biology: history, practice and recommendations. Caister Academic Press, Norwich, United Kingdom. [Google Scholar]
- 16.Sarris PF, Ladoukakis ED, Panopoulos NJ, Scoulica EV. 2014. A phage tail-derived element with wide distribution among both prokaryotic domains: a comparative genomic and phylogenetic study. Genome Biol Evol 6:1739–1747. doi: 10.1093/gbe/evu136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jank T, Eckerle S, Steinemann M, Trillhaase C, Schimpl M, Wiese S, van Aalten DM, Driever W, Aktories K. 2015. Tyrosine glycosylation of Rho by Yersinia toxin impairs blastomere cell behaviour in zebrafish embryos. Nat Commun 6:7807. doi: 10.1038/ncomms8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar G, Menanteau-Ledouble S, Saleh M, El-Matbouli M. 2015. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Vet Res 1546:103. doi: 10.1186/s13567-015-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybakova D, Radjainia M, Turner A, Sen A, Mitra AK, Hurst MRH. 2013. Role of antifeeding prophage (Afp) protein Afp16 in terminating the length of the Afp tailocin and stabilizing its sheath. Mol Microbiol 89:702–714. doi: 10.1111/mmi.12305. [DOI] [PubMed] [Google Scholar]
- 20.Rybakova D, Schramm P, Mitra AK, Hurst MRH. 2015. Afp14 is involved in regulating the length of anti-feeding prophage (Afp). Mol Microbiol 96:815–826. doi: 10.1111/mmi.12974. [DOI] [PubMed] [Google Scholar]
- 21.Bewley MC, Springer K, Zhang Y-B, Freimuth P, Flanagan JM. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 22.Nicklin SA, Wu E, Nemerow GR, Baker AH. 2005. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol Ther 12:384–393. doi: 10.1016/j.ymthe.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Thomson NA, Miln AJ, Kain WM. 1979. Biology of manuka beetle in Taranaki. Proc N Z Weed Pest Control Conf 32:80–85. [Google Scholar]
- 24.Jackson TA, Townsend RJ, Dunbar JE, Ferguson CM, Marshall SDG, Zydenbos SM. 2012. Anticipating the unexpected—managing pasture pest outbreaks after large-scale land conversion. Proc N Z Grass Assoc 74:147–152. [Google Scholar]
- 25.Hurst MR, Becher SA, Young SD, Nelson TL, Glare TR. 2011. Yersinia entomophaga sp. nov. isolated from the New Zealand grass grub Costelytra zealandica. Int J Syst Evol Microbiol 61:844–849. doi: 10.1099/ijs.0.024406-0. [DOI] [PubMed] [Google Scholar]
- 26.Ashelford KE, Fry JC, Bailey MJ, Day MJ. 2002. Characterization of Serratia isolates from soil, ecological implications and transfer of Serratia proteamaculans subsp. quinovora Grimont et al. 1983 to Serratia quinivorans corrig., sp. nov. Int J Syst Evol Microbiol 52:2281–2289. doi: 10.1099/00207713-52-6-2281. [DOI] [PubMed] [Google Scholar]
- 27.Laing S, Unger M, Koch-Nolte F, Haag F. 2011. ADP-ribosylation of arginine. Amino Acids 41:257–269. doi: 10.1007/s00726-010-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurst MR, van Koten C, Jackson TA. 2014. Pathology of Yersinia entomophaga MH96 towards Costelytra zealandica (Coleoptera; Scarabaeidae) larvae. J Invertebr Pathol 115:102–107. doi: 10.1016/j.jip.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Bartual SG, Otero JM, Garcia-Doval C, Llamas-Saiz AL, Kahn R, Fox GC, van Raaij MJ. 2010. Structure of the bacteriophage T4 long tail fiber receptor-binding tip. Proc Natl Acad Sci U S A 107:20287–20292. doi: 10.1073/pnas.1011218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams SR, Gebhart D, Martin DW, Scholl D. 2008. Retargeting R-type pyocins to generate novel bactericidal protein complexes. Appl Environ Microbiol 74:3868–3876. doi: 10.1128/AEM.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Raaij MJ, Mitraki A, Lavigne G, Cusack S. 1999. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 401:395–398. doi: 10.1038/44880. [DOI] [PubMed] [Google Scholar]
- 32.Cerritelli ME, Walls JS, Simon MN, Conway JF, Steven AC. 1996. Stoichiometry and dominant organization of the long-fiber of bacteriophage T4: a hinged viral adhesion. J Mol Biol 260:767–780. doi: 10.1006/jmbi.1996.0436. [DOI] [PubMed] [Google Scholar]
- 33.Wu E, Pache L, Von Seggern DJ, Mullen TM, Mikyas Y, Stewart PL, Nemerow GR. 2003. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J Virol 77:7225–7235. doi: 10.1128/JVI.77.13.7225-7235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shayakhmetov DM, Lieber A. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J Virol 74:10274–10286. doi: 10.1128/JVI.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 36.Dower WJ, Miller JF, Ragsdale CW. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res 16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kado CI, Liu ST. 1981. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol 145:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coil D, Jospin GG, Darling AE. 2015. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics 31:587–589. doi: 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 39.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. 2012. Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST, a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Besemer J, Borodovsky M. 2005. GeneMark: Web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res 33(Suppl 2):W451–W454. doi: 10.1093/nar/gki487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. 2015. The Phyre2 Web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers JS, Swofford DL. 1998. A fast method for approximating maximum likelihoods of phylogenetic trees from nucleotide sequences. Syst Biol 47:77–89. doi: 10.1080/106351598261049. [DOI] [PubMed] [Google Scholar]
- 47.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 49.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Callaghan M, Jackson TA. 1993. Isolation and enumeration of Serratia entomophila—a bacterial pathogen of the New Zealand grass grub, Costelytra zealandica. J Appl Bacteriol 75:307–314. doi: 10.1111/j.1365-2672.1993.tb02781.x. [DOI] [Google Scholar]
- 51.Versalovic J, Schneider M, De Bruijn FJ, Lupski JR. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol 5:25–40. [Google Scholar]
- 52.Hurst MRH, Beattie A, Jones S, Hsu P-C, Calder J, van Koten C. 2015. Temperature-dependent Galleria mellonella mortality as a result of Yersinia entomophaga infection. Appl Environ Microbiol 81:6404–6414. doi: 10.1128/AEM.00790-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorow D, Jesse J. 1990. Max efficiency DH10B, a new host for cloning methylated DNA. Focus 12:19–20. [Google Scholar]
- 54.Miller H, Mekalanos JJ. 1998. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trought TET, Jackson TA, French RA. 1982. Incidence and transmission of a disease of grass grub Costelytra zealandica in Canterbury. N Z J Exp Agric 10:79–82. [Google Scholar]
- 56.Hurst MRH, Jackson TA. 2002. Use of green fluorescent protein to monitor the fate of Serratia entomophila causing amber disease in the New Zealand grass grub, Costelytra zealandica. J Microbiol Methods 50:1–8. doi: 10.1016/S0167-7012(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 57.Penfold RJ, Pemberton JM. 1992. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118:145–146. doi: 10.1016/0378-1119(92)90263-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.