Abstract
Approximately 33% of melanomas are derived directly from benign, melanocytic nevi. Despite this, the vast majority of melanocytic nevi, which typically form as a result of BRAFV600E-activating mutations, will never progress to melanoma. Herein, we synthesize basic scientific insights and data from mouse models with common observations from clinical practice to comprehensively review melanocytic nevus biology. In particular, we focus on the mechanisms by which growth arrest is established after BRAFV600E mutation. Means by which growth arrest can be overcome and how melanocytic nevi relate to melanoma are also considered. Finally, we present a new conceptual paradigm for understanding the growth arrest of melanocytic nevi in vivo termed stable clonal expansion. This review builds upon the canonical hypothesis of oncogene-induced senescence in growth arrest and tumor suppression in melanocytic nevi and melanoma.
INTRODUCTION
Growth arrest after activation of individual oncogenes can prevent cancer formation. Melanocytic nevi are neoplasms resulting from the proliferation of melanocytes, the normal pigment-producing cells in the skin. Nevi are growth arrested, clonal neoplasms of melanocytes initiated by well-defined oncogenic mutations in the mitogen-activated protein kinase (MAPK) pathway, most commonly by BRAFV600E-activating mutation. In addition, they are pigmented in nature and located in skin, making nevi readily identifiable by visual examination and allowing for monitoring in real time. Given their well-defined genetics and accessibility, nevi have been used as a model by which to study the growth arrest of lesions after oncogene mutation.
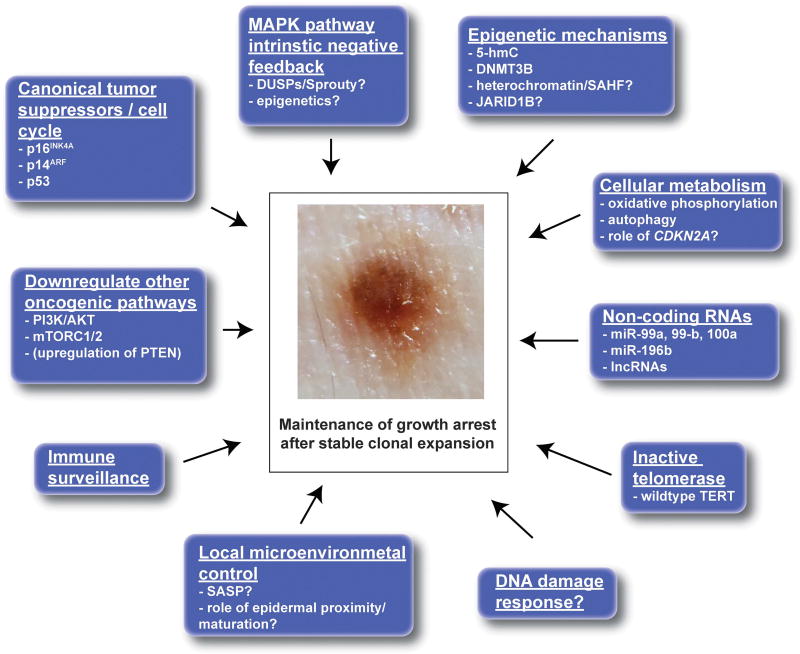
In this review, the fundamental mechanisms regulating growth arrest of nevi will be discussed in the context of clinical features commonly observed in nevi and in light of new observations in mouse models and human tissue. In addition, although nevus growth arrest is very robust and the vast majority of nevi will remain benign over time, a small proportion will progress to melanoma. Mechanisms by which growth arrest of nevi can be overcome and lead to melanoma formation will also be considered. These observations will be integrated into an updated model of growth arrest of melanocytic nevi after oncogene activation, a process we call stable clonal expansion. Stable clonal expansion in nevi is akin to the subclinical clonal expansion observed in other cell types (including in skin) and is also discussed below.
HISTORICAL CONTEXT AND ONCOGENE-INDUCED SENESCENCE
The most well-known hypothesis explaining how individual critical oncogenes can be activated, yet not give rise to cancer, is termed oncogene-induced senescence (OIS). The concept of OIS is based on the phenomenon of replicative senescence (RS), a process during which cultured cells cease proliferation after a finite number of passages in vitro.1,2 During RS, cells lose the ability to re-enter the cell cycle, even in the presence of mitogenic stimuli.3 In culture, senescent cells exhibit a distinct cellular morphology; they become large, flattened, dendritic and often multinucleated. Senescent cells express characteristic markers such as senescence-associated beta-galactosidase (SA-β-gal) and upregulate tumor suppressors including p16INK4A and p21CIP1.4 RS is thought to result from progressive shortening of telomeres and is in part driven by the activation of a DNA damage response that occurs when telomeres reach a critically shortened length.5,6 RS can be overcome by expression of telomerase, which can restore and maintain telomeric DNA.7
The OIS hypothesis dates back to the 1980s, when an interesting phenotype was noted after the introduction of individual oncogenes into non-immortalized-cultured cells. Rather than transforming the cells, oncogene expression instead induced a senescence-like phenotype.8,9 These observations led to the early hypothesis that these senescence-like responses have a tumor-suppressive role in cancer.10,11 More formal support for the OIS hypothesis came in 1997, when Serrano et al.12 showed that expression of oncogenic HRASG12V in cultured primary cells paradoxically induced a permanent G1 cell cycle arrest; with growth arrested cells exhibiting a morphologic phenotype similar to the cells that had undergone RS.
Since this time, it has been shown that cells that have undergone OIS express similar markers to cells that have undergone RS including: SA-β-gal, H3K9Me3, γ-H2AX and p16INK4A, among others (discussed further below).4 However, contrary to RS, in OIS critical shortening of telomeres does not necessarily occur. Accordingly, expression of telomerase is insufficient to bypass OIS in culture.13 These observations suggest that despite morphologic and biomarker similarities, OIS and RS may be fundamentally different processes.
Although OIS is well-defined in vitro, it has been more difficult to identify and study in vivo, where its exact role is debated.14 In tissue, cells with oncogenic changes exhibit some markers of senescence, but do not appear to rigidly adhere to the OIS phenotype as defined in vitro. Further, it has recently been noted that oncogenic mutations are very common in vivo in phenotypically normal tissue such as skin, and result in ‘invisible’ expansion of a quilt work of numerous, overlapping clonal lesions.15 Despite these oncogenic mutations resulting in finite clonal outgrowth, the mutant cells appear to largely maintain their ability to proliferate, differentiate and perform their normal functions. In the following sections, the evidence for and against acquisition of senescence-like features after oncogene activation in melanocytic nevi will be considered.
MELANOCYTIC NEVI
Natural history
Melanocytes are pigment-producing cells in the skin and typically reside within the epidermis, at the dermoepidermal junction and within hair follicles. Several benign neoplasms are derived from melanocytes and are typically the result of individual oncogenic mutations.16 This review will focus on the most common of these lesions, benign-acquired melanocytic nevi (referred to as nevi from here on).
Many adults have nevi, but their abundance varies tremendously from individual to individual, ranging from just a few nevi up to hundreds of lesions per person. Nevi are rarely present at birth and when they are, are known as congenital nevi. Rather, most nevi form later on in life, typically during the first and second decades.17,18 Total nevus number in any given individual is thought to peak during the third decade of life.19 This peak is due to reduced formation of new nevi (which becomes less common after 30 years of age) combined with the clinical regression of some existing nevi. Clinical regression of nevi is a poorly understood process during which nevi involute and can disappear entirely. The frequency of nevus regression increases with advancing age.20,21
Compared with other clinically apparent, benign, but potentially precancerous lesions, melanocytic nevi are unique as they arise relatively early in life. In contrast, for example, actinic keratosis, which can be a precursor of cutaneous squamous cell carcinoma, are uncommon prior to the age of 40 and become much more prevalent with advancing age, even into the 80s and 90s.22 The reason(s) why nevi arise primarily during the first two decades of life and less so with advancing age is unclear. The reason why some individuals get only a few nevi, whereas others get hundreds are also not well understood. In terms of abundance, a combination of inherited causes and ultraviolet radiation and other environmental mutagens, are likely at play.23,24 Germline mutations such as in CDKN2A, which affects both nevus size and total nevus counts, underlie this phenotype in a small subset of patients.25–28 Inherited variation in nevus and melanoma risk will be discussed in more detail below.
Clinical and histopathologic features
Nevi are most often 2–6 mm in size and have a uniform color and symmetric architecture clinically. Nevi are grouped into one of three major categories: junctional (melanocytes confined to the epidermis only), intradermal (confined to the dermis only) and compound (both an epidermal and a dermal component). The relationship among these three different types of nevi and what factor(s) result in the formation of one type versus another are not well understood. BRAFV600E mutations, which are found in the majority of nevi, appear to occur with relatively similar frequencies in all three types, but may be slightly more common in nevi with a dermal component.29–31 Despite the heterogeneity in clinical and histologic appearance of these types of acquired nevi, all are thought to share a relatively similar natural history and relationship to melanoma. For the purposes of this review, all three types will be considered together. It should be noted that additional types of benign melanocytic nevi such as: blue nevi,32 Spitz nevi33 and deep penetrating nevi34 exist, however, are relatively less common and will not be discussed in detail. Dysplastic nevi will be considered separately below.
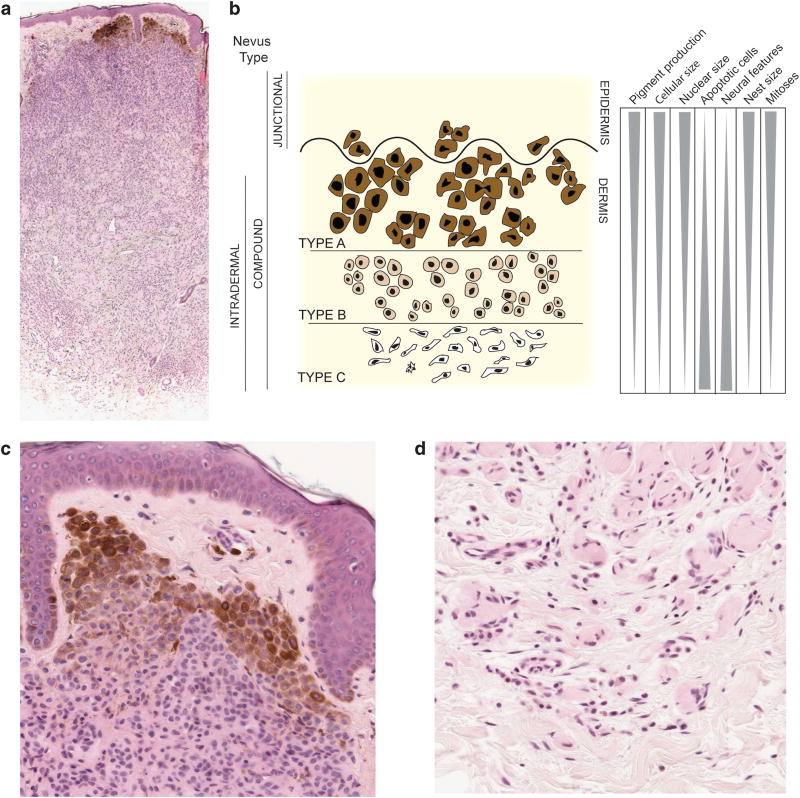
Microscopically, nevi are well circumscribed, symmetric and are composed of melanocytes with a monotonous, banal cytology. Two cardinal histopathological features of nevi are nesting and maturation. Nesting refers to the tendency of nevus melanocytes to form small clusters of cells within tissue (Figure 1). Maturation is a feature of nevi with a dermal component and refers to a gradual and progressive change (from superficial to deep) in nest architecture and melanocyte cytology. As one goes deeper into the lesion, nest size decreases, cell and nuclear volume decreases, pigment production decreases and changes in cell shape occur35 (Figure 1).
Figure 1.
Schematic of melanocytic nevus architecture. (a) Low power image of an intradermal melanocytic nevus stained with hematoxylin and eosin (H&E). The nevus shows features of maturation. (b) Junctional nevi are confined to the epidermis and appear as pigmented macules. Compound nevi have both an intra-epidermal and dermal component. Intradermal nevi are entirely confined to the dermis. Type A, B and C melanocytes are morphologically distinct and found at different depths within the skin. With increasing depth, nevi are less pigmented, smaller, have smaller nuclei, fewer mitoses, increased number of apoptotic cells and increased neural features. Nest size decreases with maturation. (c) High power images of type A melanocytes in the most superficial portion of the nevus. H&E stained section. (d) Type C melanocytes in the deepest portion of the nevus showing neural (Schwannian) differentiation. H&E stained section.
Cytologic features of maturation have been used to divide the melanocytes in individual nevi into three groups, types A, B and C. Type A melanocytes are most similar in morphology to normal epidermal melanocytes and are found in nests in the most superficial portions of nevi, including the epidermis and superficial dermis. Type B melanocytes are found in the mid dermis in relatively smaller nests and are also relatively smaller in size and rounder in shape. Type C melanocytes are found primarily as individual cells in lower portions of the dermis and have a more spindled/fusiform morphology. The complex architecture observed in nevi suggests that both cell intrinsic and extrinsic factors act in concert to shape nevus formation, prevent uncontrolled growth and maintain homeostasis. In melanoma, organized nesting and maturation tend to be lost. It is possible that nesting and/or maturation reflect poorly understood tumor-suppressive interactions within the tissue microenvironment, however, there is currently no data to support what (if any) active role these processes have in constraining nevus growth.
BRAF-ACTIVATING MUTATIONS CAUSE NEVUS FORMATION
Genetics of human nevi
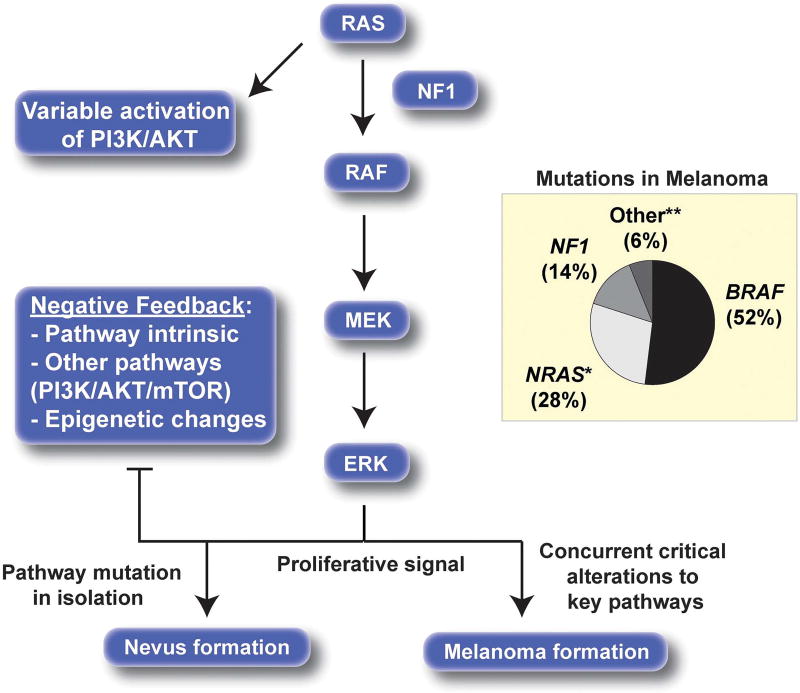
The MAPK pathway is a central activator of cellular proliferation (Figure 2). RAF proteins are serine/threonine kinases, which when activated either by upstream mitogenic signals or via activating mutations, drive signaling through this pathway. In 2002, it was noted that BRAF-activating mutations, which render its kinase function constitutively active, commonly occurred in human cancers, including melanoma.36 BRAF-activating mutations (most commonly V600E) are present in about 50% of melanomas.37 The central role of BRAF as a melanoma oncogene is supported by the marked (albeit typically temporary) responses observed when BRAF inhibitors are used to treat BRAF-mutant melanomas.37 Constitutive MAPK pathway activation is likely a shared feature of most melanomas and is achieved through a variety of mechanisms including mutation of other components of the MAPK pathway, such as NRAS and NF1.16,37
Figure 2.
MAPK pathway alterations in melanoma. RAS (usually NRAS-activating mutation), BRAF-activating mutations and NF1-inactivating mutations are common drivers of constitutive MAPK pathway activation in melanoma. Activation of the MAPK pathway in isolation provides a strong proliferative signal, but ultimately negative feedback loops result in growth arrest. *Small proportion of HRAS, KRAS mutations. **KIT, GNAQ and GNA11 mutations. Mutation data from The Cancer Genome Atlas.37
In 2003, it was noted that BRAF-activating mutations were also present in many nevi.38 Most studies have shown that BRAF is mutated in ∼80% of nevi.39–41 NRAS mutations have been found in about 5.9–18.2% of nevi.40 MAPK-activating mutations appear to be a shared characteristic of most benign cutaneous melanocytic neoplasms. For example, NRAS mutations are common in congenital nevi,42 HRAS mutations and copy number gains are found in in Spitz nevi43 and GNAQ/GNA11 mutations are present in blue nevi.44 As an important aside, MAPK pathway mutations tend to occur in a mutually exclusive fashion in melanocytic (and other) neoplasms,37 suggesting a functional redundancy with no added selective advantage of having multiple mutations in this pathway. In fact, having two different MAPK pathway-activating mutations may confer a proliferative disadvantage.45 A variety of approaches have been used to show that nevi are clonal,16,46–49 suggesting that the formation of nevi in humans occurs as a result of a single MAPK pathway-activating mutation in an individual melanocyte.
The etiology of BRAFV600E mutations is debated. Ultraviolet (UV) light is thought to have a positive role in melanoma pathogenesis (especially in melanomas arising on chronically sun damaged skin), and as a group, melanoma genomes carry a tremendous burden of UV damage.37 Interestingly, however, the T-to-A transversion that underlies the V600E mutation is not a classic direct UV signature mutation (C-to-T or CC-to-TT), and the distribution of nevi clinically does not match the areas of skin with the highest exposure to UV light. Furthermore, xeroderma pigmentosum (XP) patients, who are deficient in nucleotide excision repair needed for optimal repair of UV-induced DNA changes, have a markedly elevated rate of melanoma formation, yet only 11% of XP melanomas contain BRAFV600 mutations.50 However, some authors still implicate UV light, arguing that T-to-A transversions are a rare, but direct byproduct of damage from UV.51 Other authors have suggested nevus formation could be stochastic and due to occasional mistakes during DNA replication, which are then highly selected for and lead to nevus formation.52 Other, unidentified environmental mutagens have been proposed to have a role. For example, papillary thyroid carcinoma also commonly carries BRAFV600E mutations, however, in these neoplasms UV would not be predicted to have a pathogenic role. Interestingly, it has been noted that certain geographic areas have increased rates of both papillary thyroid cancer and melanoma, relative to surrounding areas, suggesting another unknown environmental mutagen may have a role in the formation of BRAFV600 mutations.53 Overall, this issue remains to be resolved.
Overall, the important hypothesis generated from these findings is that although individual MAPK pathway mutations may initiate inappropriate proliferation resulting in nevus formation, they are not sufficient for melanoma formation in isolation. This line of reasoning provided a conceptual link between the formation of nevi and the OIS hypothesis, as in both cases oncogene activation leads ultimately to a growth arrest phenotype rather than cancer formation. A series of important studies examining the effect of the BRAFV600E mutation in the melanocytic lineage both in vitro and in mice followed and will be discussed in the following sections.
Functional evaluation of BRAF-activating mutations
In 2005, Michaloglou et al.54 reported that expression of BRAFV600E in cultured melanocytes resulted in a rapid proliferative arrest. Interestingly, there was no clear initial period of proliferative advantage provided by BRAF mutation, as presumably occurs in vivo and leads to nevus formation (the timing of growth arrest is discussed further below). BRAF-mutant melanocytes were found to exhibit cytologic features and expressed markers of OIS (p16INK4A and SA-β-gal) in this model.54 In vivo correlates of these findings included a panel of congenital melanocytic nevi, which were also shown to stain with OIS markers p16INK4A and SA-β-gal. As would be predicted based on the OIS hypothesis, the melanocytes in this panel of nevi exhibited preserved telomere length.54
In 2006, Gray-Schopfer et al.55 expanded these findings to common acquired nevi, which were also found to stain positively for p16INK4A and SA-β-gal. These findings showed that melanocytes in nevi share some features with melanocytes that have undergone OIS in culture. However, despite these similarities, the cytologic changes exhibited during OIS in vitro (large, flat, dendritic and multinucleate) do not tend to be reflected in nevus melanocytes found in tissue. In contrast, nevus melanocytes tend to be small, mononucleate and relatively less dendritic than normal melanocytes. Altogether, these observations raise the possibility that despite the similarity in marker expression, melanocytes in nevi may be distinct from cultured melanocytes that have undergone OIS.
The first functional evaluation of BRAF-activating mutations in the melanocytic lineage in vivo was performed in 2005 in a zebrafish model. In this model, melanocyte-specific BRAFV600E expression induced the formation of benign melanocytic proliferations called ‘fish nevi’.56 This study provided support for the hypothesis that BRAF activation is sufficient to drive nevus formation, but does not in itself result melanoma formation in vivo. In 2009, multiple groups, observed that melanocyte-specific expression of Braf600E in mice also resulted in the formation of benign melanocytic lesions akin to human nevi.57–59 The melanocytes composing mouse nevi also expressed senescence markers such as SA-β-gal, but similarly to human nevi did not assume the morphologic features of OIS melanocytes in culture. Subsequent work (discussed in more detail below) has shown that although BrafV600E-induced mouse nevi remain in a stable growth-arrested state over time, a small subset will later give rise to melanoma.60 Variability in the penetrance of melanoma with Braf600E in mouse models has been observed and will be discussed further below.58–60
It is now generally accepted that BRAF activation in vitro leads to OIS and in vivo results in the formation of nevi. On the basis of the above data, there is undeniably phenotypic overlap between these two states, however, there are also clear differences. In addition, the observation that nevi serve as precursor lesions in about 25% of melanomas suggests that nevi are not inextricably terminally growth arrested in vivo. Given these differences, it is unclear if at a functional level these two processes are mediated by the same, similar, or different mechanism(s). In the following sections, the relationship between OIS, nevi and melanoma will be discussed.
RELATIONSHIP BETWEEN NEVI AND MELANOMA
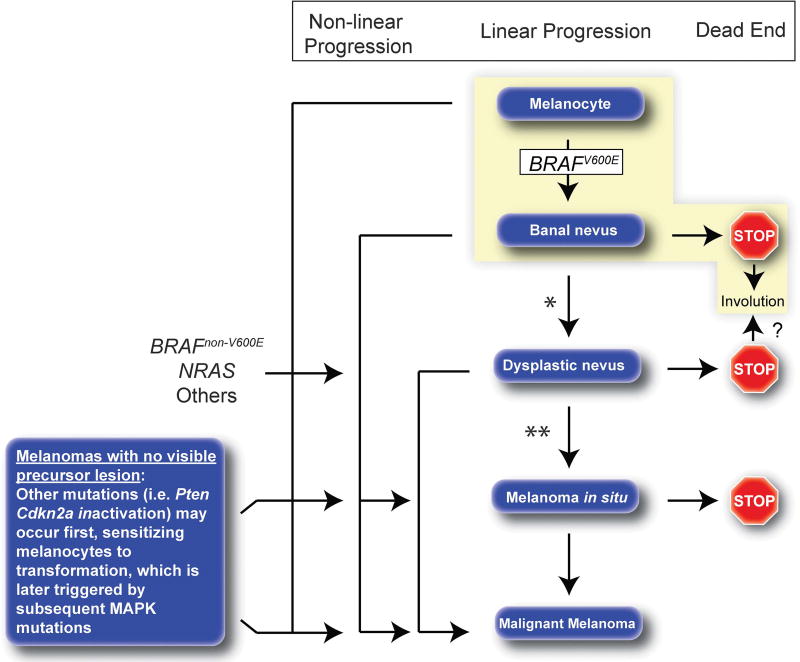
The Clark model of melanoma pathogenesis posits that a series of steps occur during progression from normal melanocyte to melanoma.61 These steps include formation of banal nevi, then dysplastic nevi, then melanoma in situ, and ultimately invasive melanoma; a path thought to be driven by the progressive accumulation of pathogenic genetic/epigenetic changes.62 Although linear, step-wise progression may characterize the natural history of a subset of melanomas, significant evidence suggests that in most melanomas, progression is more complex and includes many distinct paths which may be dictated in part by distinct oncogenic hits (Figure 3).63 Interesting new data from Bastian and colleagues regarding the sequence of different mutations in melanocytic neoplasms is discussed below.
Figure 3.
Natural history of melanocytic lesions. Traditionally progression from normal melanocyte to melanoma has been depicted in a linear fashion (linear progression), however, in individual lesions, certain stages may be skipped or never occur at all (non-linear progression pathways). Linear progression through all stages in any individual lesion is probably fairly uncommon. Melanocytes that acquire a BRAFV600Emutation give rise to banal melanocytic nevi. Melanocytes that acquire NRAS and BRAFnon-V600E mutations may more commonly form de novo dysplastic nevi. Approximately 2/3 of melanomas arise without a known benign precursor lesion, possibly as a result of late acquisition of a MAPK pathway mutation in already sensitized melanocyte(s) with other oncogenic changes such at PTEN and/or CDKN2A inactivation. The vast majority of nevi will never progress to melanoma, many will remain clinically stable over a lifetime, whereas others will regress (dead end pathways). The most common natural history for nevi is highlighted in yellow. *Some banal nevi may later give rise to dysplastic nevi, but this is probably fairly uncommon. **It is not clear that dysplastic nevi progress to melanoma more commonly than banal nevi.
Approximately 25–33% of cutaneous melanomas arise from nevi.64,65 Nevi which do not arise from melanoma are considered further below. In high-risk patients, such as those with numerous nevi, this number may be as high as 50%.66 Dysplastic nevi are also discussed separately below. Transformation of nevi to melanoma has been shown to occur most commonly in non-chronically sun damaged (non-CSD) skin (intermittently sun exposed areas such as the trunk and proximal extremities) in relatively younger patients. Superficial spreading melanoma is the most common histologic subtype in these lesions.51,67 One study suggested that junctional and compound nevi may be relatively more likely to give rise to melanoma than intradermal nevi, but this has not been definitively shown.67
In contrast, melanomas that develop in CSD skin (such as the head and neck) are only rarely associated with nevi.51,67 Some melanomas arising in CSD skin show a pattern termed lentigo maligna melanoma. Bastian and colleagues have proposed that melanomas arising in CSD skin and non-CSD skin are indeed fundamentally different based on divergent genetics. Non-CSD melanomas have more BRAFV600E and PTEN mutations, whereas CSD melanomas have more NF1 and TP53 mutations.51 CSD and non-CSD melanomas likely have distinct natural histories; the subsequent discussion will be more relevant to non-CSD melanoma, given the current data available.
In melanomas that arise from pre-existing nevi, remnants of the original nevus are often evident histologically. Genetic analyses of such histologically contiguous benign nevus-melanoma pairs support the hypothesis that the melanoma cells were derived directly from the nevus cells.31,68–72 Although cases of driver mutation (that is, RAF/RAS) discordance between paired nevus and melanoma have been reported70,73 and are interesting mechanistically, these cases appear to be much less common and some may represent coincidental collision lesions between unrelated nevi and melanomas.
Although a small proportion of nevi will ultimately give rise to melanoma, the vast majority never will. It has been estimated that the annual transformation rate of any single nevus ranges from ∼ 1 in 200 000 in individuals under 40 years old to about 1 in 33 000 in men over 60 years old.74 Extended over a lifetime, the risk of progression of any individual nevus to melanoma is about 1 in 3000 for men and 1 in 11 000 for women.74 For this reason, prophylactic removal of nevi is not part of clinical practice; however, screening for progression of nevi and de novo melanoma development with serial skin examination may result in identification and treatment of melanomas at earlier stages.
Nevi are also an independent marker of overall melanoma risk. There is a well-established, positive, dose-dependent relationship between the total number of melanocytic nevi and the risk of developing melanoma.75 This increased risk is distinct from the risk of progression of any individual nevus to melanoma and applies to (and is additive for) both banal as well as histologically dysplastic nevi.75 The exact explanation for this observation is not completely understood, but likely relates to shared genetic and environmental factors predisposing to melanocyte neoplasia.
The overall low rate of nevus progression to melanoma suggests that robust tumor-suppressive mechanisms are enacted following BRAF and other mutations. Understanding how and why individual nevi progress to melanoma and why individuals with many nevi are at a higher risk for melanoma formation will be considered further below.
DYNAMICS OF GROWTH ARREST
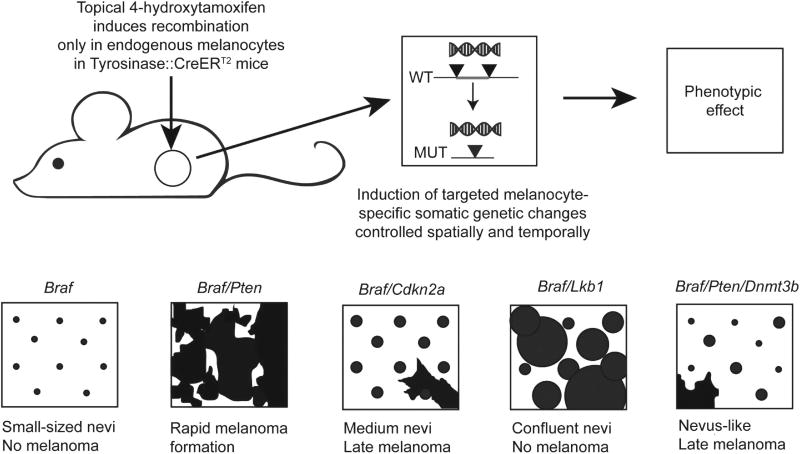
The timing of growth arrest after BRAF activation is different in vitro and in vivo. BRAF activation in vitro leads to nearly immediate growth arrest (within days) with no clear period of initially increased proliferation.54 In contrast, BRAF activation in vivo leads to an initial period of enhanced proliferation leading to nevus formation, but is ultimately followed by clinical growth arrest as a mature nevus.59,60 A similar phenotype is observed after RAS activation, with near immediate induction of a growth arrest in vitro,12 but an initial period of proliferation in vivo followed by growth arrest.76 This same pattern has been noted in other cell types. The reason for this discrepancy in the timing of growth arrest is unknown, but is explored in the following section. In mouse models, the proliferation induced by BrafV600E lasts for 14–21 days, after which lesion expansion ceases and a mature nevus is formed.60 BrafV600E induced nevi remain stable in size over time as mice age.60 When Braf is activated in the context of melanocyte-specific Cdkn2a inactivation (Braf/Cdkn2a model) (Figure 4), nevus area is increased about threefold, with a final area of ∼ 0.75 mm2. A similar model based on NrasQ61K and Cdk4R24C-activating mutations also results in nevi of roughly the same size.77,78 On the basis of these estimates, mouse nevi, which are thought to be derived from an individual parental melanocyte, are composed of ∼1500 to 3000 melanocytes.77
Figure 4.
Mouse models of melanocytic nevi and melanoma. BrafV600E mutation in isolation results in the formation of small, growth-arrested nevi. When Pten in inactivated in the setting of Braf activation (Braf/Pten) no growth arrest is observed; rapid progression to metastatic melanoma ensues. When Cdkn2a is inactivated in the setting of Braf activation larger melanocytic nevi form, but are still stably growth arrested. With increasing age, a small proportion of nevi progress to melanoma at rates similar to human nevi. When Lkb1 is inactivated (constitutive mTORC1 activation) in the setting of Braf activation, growth arrest of nevi is abrogated, but melanoma never forms. When Dnmt3b is inactivated in the Braf/Pten model, most melanocytic lesions growth arrest, with rare progression to melanoma with advancing age.
In humans, it is less clear over what period of time BRAF and NRAS induced nevus formation occurs, but it perhaps can be estimated indirectly based on certain clinical observations. For example, serial nevus photography in children and adolescents (when rates of new nevus formation are highest) has shown that most enlarging nevi grow over a period of months up to a year and then stop.79,80 In a different scenario, eruptive nevi, numerous new nevi develop within a short period of time in individual patients. Studies looking at eruptive nevi suggest that formation of the nevi typically occurs over one to several months.81–83 However, how closely eruptive nevus formation mimics sporadic nevus formation, and the precise mechanism(s) underlying this phenomenon are unclear. Taking these observations together, it could be roughly estimated that nevus formation in humans occurs as quickly as within 1–2 months, but may take a year or more.
Most nevi in adults range in size from 2 to 6 mm and have been estimated to be composed of several tens of thousands up to hundreds of thousands of melanocytes depending on the size and type of nevus.16,84 On the basis of this size estimate, roughly 13–16 rounds of cell division would be required to generate a nevus from a single precursor melanocyte if clonal expansion occurred equally among all daughter cells without any loss of progeny. More rounds of division are likely required as in reality proliferation is probably not perfectly exponential. Nonetheless, this estimated number of divisions is importantly significantly lower than the 60–80 rounds of cell division that would be required to result in critical telomere shortening,85 consistent with the observation that telomere length is preserved in nevi and they do not appear to undergo RS.54,86 If similar logic is applied to murine melanocytes, 10–11 rounds of division would be required to form a mouse nevus. In addition, telomeres are much longer in laboratory mouse strains (50–150 kb) than typically seen in humans (5–6 and 10–12 kb, adults and newborn humans, respectively)87 and significant telomere erosion in mouse nevi is unlikely to occur.
Altogether these data strongly support the hypothesis that at least in a subset of melanocytes that have acquired BRAFV600E mutations, growth arrest has significant latency in vivo and does not occur as quickly as it does in vitro. The reason for this discrepancy is not entirely clear. One hypothesis is that the mechanism of growth arrest in vitro is different from that occurring in vivo. If true, this may be related to the non-physiologic conditions of cell culture, where cells are already constitutively proliferating and are in the presence of favorable concentrations of growth factors and nutrients. This hypothesis is supported by the differences in time frame of growth arrest and differences in cytology between growth-arrested cells in vitro and in vivo.
An alternative hypothesis is that an immediate growth arrest phenotype analogous to that observed in vitro does occur in vivo, but is not routinely appreciated because no clinically apparent lesion develops. In this scenario in order for the formation of a visible nevus to occur, immediate senescence programs would need to be either ineffective or somehow rapidly bypassed. Along these lines, some authors have hypothesized that patients with relatively fewer nevi are more effectively able to enact an immediate senescence response after BRAF mutation in melanocytes. In these hypothetical patients, although activating BRAF mutations occur, they rarely result in formation of clinically visible nevi due to the robust and rapid onset of growth arrest programs. In contrast, patients with less robust immediate growth arrest programs would develop more clinically visible nevi, as they would rely more on secondary mechanisms of growth arrest that act with longer latency.16 However, which tumor-suppressive mechanisms potentially act immediately versus those that are secondary are not well-defined and there is no direct experimental evidence in support of this hypothesis.
The hypothesis that nearly immediate melanocytic growth arrest after BRAF activation occurs in vivo predicts that individual melanocytes or subclinical melanocytic proliferations harboring BRAFV600E mutations should be detectable in skin. Indeed, subclinical melanocytic proliferations are encountered as chance findings in skin excisions for other cutaneous neoplasms.52 However, the frequency with which these lesions occur is not well characterized and it is not known if they contain BRAF mutations. Some authors have suggested that many nevi in humans may never grow larger than 1 mm in size and thus have been largely overlooked by most studies of nevi in humans.88
In another example, eruptive nevi, in which numerous new nevi synchronously appear, BRAFV600E mutations are present in most lesions. This observation suggests that subclinical BRAF-mutant melanocytes may have been present and then triggered to grow. Alternatively, but less likely, new BRAF mutations could be induced in multiple cutaneous locations over a relatively short time period. Last, many BRAF-mutant melanomas do not develop from preexisting nevi, suggesting either that clinically silent BRAF-mutant melanocytes preceded the melanoma, or alternatively that BRAF mutation was instead acquired relatively late in melanomagenesis, leading to de novo melanoma formation (Figure 3). This issue is considered more below.
MECHANISMS OF GROWTH ARREST
Several specific mediators of growth arrest after activation of critical oncogenes have been proposed and are based on both in vitro and in vivo experimental evidence. Although these experiments are numerous and have been performed in many cell types, the following sections will focus primarily on experiments performed in the melanocytic lineage. Where possible in vitro and in vivo data will be discussed together.
Negative feedback within the MAPK pathway
Although BRAF mutation and activation of the MAPK pathway is important in nevogenesis, MAPK pathway activation do not appear to be sustained at high levels in nevi after growth arrest. Time-course studies performed by our group in the Braf/Cdkn2a mouse model of nevus formation show that the MAPK pathway is activated only transiently after Braf mutation and corresponds to the phase of active melanocyte proliferation during nevus formation.60 MAPK activity is significantly lower during stable growth arrest in this model. As might be predicted, the MAPK pathway is re-activated at high levels in melanoma.60 Analysis of human nevi shows a similar pattern, with relatively low levels of MAPK pathway activation in nevi relative to melanoma.89–91 Retention of low levels of pathway activity may be supported by the observation that treatment of patients with BRAF inhibitors results in changes in the appearance of pre-existing BRAF-mutant nevi.92,93 BRAF-mutant melanomas show high levels of MAPK pathway activation and are clearly dependent on BRAF-induced MAPK pathway activation given the efficacy of BRAF and MEK inhibitors.94
The mechanisms by which MAPK signaling in nevi is attenuated during growth arrest are not well characterized. Negative feedback loops involving dual specificity MAPK phosphatases (MKPs or DUSPs) or Sprouty proteins are defined inhibitors of the MAPK pathway generally, but have not been carefully studied in nevi.95–97 Progression to melanoma appears to rely in part upon reactivation of MAPK signaling,60 which may be facilitated by copy number gains and upregulation of mutant BRAF, but ultimately is likely also related to disruption of negative feedback loops.91,98 Concomitant dysregulation of additional pathways (such as PI3K/ AKT and mTOR) appears to facilitate sustained MAPK pathway activation in melanoma59,60 and will be discussed further below.
CDKN2A
The CDKN2A locus encodes two distinct proteins, p16INK4A and p14ARF, both of which are considered bonafide tumor suppressors in melanoma. p16INK4A opposes Cyclin D-Cdk4/6 mediated cell cycle progression through the G1/S restriction point via phosphorylation of pRB.99 More recently, Cyclin D-Cdk4 has been implicated in regulating cellular glucose metabolism independently of cell cycle progression.100 p14ARF (p19Arf in mice) inhibits MDM2-mediated degradation of p53 and may also function as a tumor suppressor by opposing ribosome production.101 Metabolic implications of CDKN2A loss will be discussed in more detail below.
CDKN2A is the prototypic familial melanoma susceptibility locus and accounts for ∼40% of familial melanoma. Multiple different germline mutations resulting in loss-of-function of one copy of p16INK4A and/or p14ARF have been reported in melanoma kindreds.102,103 The clinical phenotype in many patients with germline CDKN2A mutations is characterized by an increased abundance and larger size of nevi25,26 and a significantly increased risk of developing cutaneous melanoma.104,105 However, a subset of these patients do not have elevated nevus counts, yet are still at an increased risk for developing melanoma. The observed alteration in nevus size and number may argue that CDKN2A gene products have a role in rapid induction of growth arrest, and when impaired nevus melanocytes must rely on other mechanisms that act with longer latency. In the nevi in these patients, one normal copy of the CDKN2A locus is still thought to be expressed.106 CDKN2A mutations are also very common in sporadic melanomas. Inactivation of one copy of CDKN2A is also common in melanoma in situ; inactivation of both copies is more commonly found in advanced melanomas.37,51,98 Altogether, these observations suggest that in humans there is a complex and potentially dose-dependent effect of inactivating mutations in CDKN2A in nevus and melanoma biology.
Both p16INK4A and p14ARF are canonical tumor suppressors and have also been implicated in growth arrest after oncogene inactivation at a functional level. p16INK4A in particular is highly upregulated in cells that have undergone both OIS and RS, and is one of the most commonly used markers of these states.107,108 In human melanocytic lesions, p16INK4A staining is typically higher in nevi than in melanomas, where expression tends to be reduced or lost entirely.109–112 Interestingly, at a functional level, neither p16INK4A nor p14ARF appear to be required for induction of OIS phenotypes in melanocytes in vitro.113 Similarly, they do not appear to be required for growth arrest in vivo. For example, simultaneous inactivation of both p16Ink4a and p19Arf in the Braf/ Cdkn2a mouse model does not abrogate BrafV600E-induced growth arrest (Figure 4).60 However, similarly to patients with germline CDKN2A mutations, Braf-induced mouse nevi are both more numerous and larger when Cdkn2a is disrupted.60
The in vivo data from mice and humans suggest that CDKN2A inactivation results in a temporary disruption, but not abrogation of growth arrest, and that in the absence of CDKN2A, other growth arrest programs can still control growth of the lesions. However, in the Braf/Cdkn2a mouse model, the additional loss of Cdkn2a (compared with Braf activation alone) has the important effect of increasing melanoma penetrance from near 0 to 100%.59,60 Interestingly, this increase in melanoma penetrance appears to be independent of growth arrest of nevi in the Braf/Cdkn2a model. Although robust growth arrest of nevi is observed, a small subset of nevi will progress to melanoma as mice age. Interestingly, the progression rate in this model is similar to that observed in other mouse nevus models and in human nevi.60,77 Dhomen and colleagues also noted that in Braf-mutant melanocytes, that p16INK4a loss was not required for senescence in vivo, but its loss did increase tumor penetrance and decrease tumor latency.58 These models and factors regulating progression of nevi to melanoma will be discussed further below.
DNA damage response and p53
The role of DNA damage responses (DDR) have been intensively studied in cells that have undergoing OIS in culture. In 2006, it was shown that introduction of MAPK-activating mutations such as activated HRAS into cultured cells induced a DNA hyper-replication phenotype causing replication stress and resulting in double stranded DNA breaks. In these models, double strand breaks triggered DDR programs, which were themselves required for effective enforcement of OIS.114,115 More recently, multiple groups have shown that natural depletion of cellular nucleotide pools after oncogene-induced hyper-replication may also lead to replication stress and contribute to activation of DDR during OIS.116–118
The functional role of DDR programs in the growth arrest of nevi, however, is less clear. An initial evaluation of dysplatic nevi and melanomas showed that markers of DDR (such as, γ-H2AX and CHK2) were present in both types of lesions, but not normal skin.119 Subsequent analyses show that banal nevi also express γ-H2AX, at levels that appear to be higher than normal melanocytes, but lower than melanoma.120,121 These data can be interpreted in different ways. For example, one could argue that a DDR, which was initially effective in enforcing growth arrest, has become ineffective (but is sustained) in melanoma, which would explain the higher levels in melanoma relative to nevi. Alternatively, is also possible that a stronger, but transient DDR occurs during growth arrest, but is not sustained during homeostatic conditions after growth arrest.
p53 is a potent tumor suppressor and is a central regulator of DDRs.122,123 In melanoma, the TP53 gene is mutated at relatively low rates compared with most other solid malignancies.37,98 TP53 mutations are enriched in melanomas arising on CSD skin and associated with thicker invasive melanomas, but tend to be relatively less common in non-CSD melanomas.37,51,98 Immunohistochemical analysis of histologically contiguous human nevus-melanoma pairs has shown that the melanoma portion of the lesions tend to have higher p53 expression, whereas p53 expression is relatively lower in the nevus portion of the lesion.110 However, these data are difficult to interpret in the absence of knowing the TP53 mutational status.
Studies testing the functional role of p53 loss on nevus formation in mice have been performed. In the Braf/p53 model developed by our group, where p53 is simultaneously inactivated in BrafV600E -mutant melanocytes, stable growth arrest of nevi still occurs despite the absence of functional p53 and an impaired p53-dependent DDR.60 However, similarly to the Cdkn2a/Braf model, inactivation of p53 results in an increased total number of nevi, larger nevi, but nevi that still growth arrest (Figure 4). However, as in the Cdkn2a/Braf model, 1–4 melanomas typically arise within 100 days of life in these mice.60 Viros et al.124 also found that inactivation of p53 in the setting of Braf activation leads to increased melanoma formation in mice. Altogether, these observations suggest that p53 and DDR do not have an obligate role in growth arrest of nevi, but do alter the phenotype of nevi slightly and regulate the rare, stochastic progression to melanoma. This is perhaps not surprising as there are likely multiple levels of protection from transformation after BRAF activation.
Epigenetics
Epigenetics broadly refers to chromosomal alterations that affect processes such as gene expression, but do not change the actual DNA sequence. DNA methylation and histone modifications are common examples of epigenetic alterations. Epigenetics and epigenetic regulators are known to be dysregulated in cancer including melanoma and in many instances contribute to cancer formation and progression.125 In this section, the role of epigenetics in the formation nevi and subsequent progression to melanoma will be discussed.
In nevi, ultrastructural studies using electron microscopy have shown that heterochromatin (more tightly packed, less transcriptionally active) predominates over euchromatin (relatively less condensed, more transcriptionally active). Not surprisingly, in melanoma, euchromatin predominates.126,127 This pattern is common when compared between benign and malignant lesions in other tissues. In fact, one of the most commonly used markers of senescence, senescence-associated heterochromatic foci (SAHF), reflects an epigenetic modification, which leads to heterochromatin formation. SAHF were initially described in vitro and functionally are thought to promote senescence by silencing of E2F target genes by affecting chromatin structure. E2F target genes are critical for cell cycle progression from G1 to S phase.128 SAHF are detected using antibodies specific for trimethylation of lysine-9 of histone H3 (H3K9me3); however, this marker can be difficult to quantitate.
Heterochromatin formation in nevi has been proposed to be mediated by specific factors, including histone deacetylase 1 (HDAC1), the activity of which can be partially inferred by H3K9me3 staining.129 Although initial evidence suggested HDAC1 was upregulated in nevi, subsequent analyses found H3K9me3 staining to be essentially equivalent in nevi and melanomas.120,129 Other studies have implicated the expression of histone variant macroH2A in heterochromatin formation in nevi, with expression of macroH2A tending to be lost with progression to melanoma.130 MacroH2A may promote the senescence-associated secretory phenotype131 (discussed below).
DNA methylation is globally dysregulated in melanoma. For example, tumor suppressor genes are commonly silenced by hypermethylation of GpG islands at promoter sites.132 In fact aberrant DNA methylation may be the most common genomic alteration in melanoma, with certain loci being methylated in >95% of melanomas.133 Several groups have characterized the differences in DNA methylation patterns between nevi and melanoma.134–137 Detection of certain epigenetic marks may be usefully clinically and are being developed as serum biomarkers for melanoma.138
In 2012, Lian et al.139 showed that the specific epigenetic modification, hydroxymethylation at the 5 position of cytosine (5-hmC), was common in nevi, but was nearly universally lost in melanoma.139 Follow-up studies have confirmed this pattern.140 The mechanism by which this epigenetic change is regulated and the functional significance in melanocytic lesions is not completely clear. Isocitrate dehydrogenase 2 (IDH2) and ten-eleven translocation (TET) proteins, such as TET2 may have a role in induction of 5-hmC in nevi.139,141 Functional evaluation of the role of this modification in nevus and melanoma formation in vivo is likely to be very informative. The latency with which growth arrest in nevi occurs after BRAF mutation may also argue that epigenetic modifications (which may take time to take effect), have an important role in constraining growth.
Epigenetic modifications can also have a permissive role in melanomagenesis. For example, DNMT3B is a DNA methyltransferase responsible for de novo DNA methylation. In a study from our group, we found that inactivation of Dnmt3b in the highly penetrant and rapidly lethal Braf/Pten mouse model (Figure 4) markedly impaired melanoma formation and rather, resulted in the formation of nevus-like growths through a mechanism discussed below.142 This observation provides strong evidence to support the hypothesis that epigenetic modifications (specifically de novo methylation of DNA) are required for melanocytic proliferations to grow beyond a nevus-like size, even in the presence of Braf and Pten mutations that would otherwise lead to melanoma formation. DNA methylation may regulate feedback loops that might otherwise limit MAPK and PI3K signaling, which are thought to be required for melanoma growth.
Other epigenetic regulators have been proposed to have a role in melanomagenesis, but will only be mentioned briefly. JARID1B (KDM5B) is expressed at higher levels in melanoma than nevi,143 and has been shown to be required for continuous growth of melanoma in experimental models.144,145 SETDB1 is a methyltransferase responsible for H3K9me3 methylation (as seen in SAHF) that interestingly has actually been shown to be recurrently amplified in melanoma.146 Germline SETDB1 sequence variants have been shown to confer increased susceptibility to melanoma formation.147 The SWI/SNF (switch/sucrose nonfermentable) complex regulates chromatin remodeling via nucleosome sliding; components of this complex have been shown to be recurrently mutated in melanoma.37,148,149 Last, EZH2, a histone methyltransferase is mutated in a small proportion of melanomas,37,149 and has been noted to be upregulated in melanomas compared with nevi;150 however, the functional role of this protein in melanocytic lesions is not well understood yet.
Modification of gene expression by non-coding RNAs is often considered along with epigenetics. Gene product regulation by microRNAs (miRNAs) in particular is likely to have an important role in both establishment and escape from growth arrest. For example, using the Braf/Cdkn2a model, we found that miR-99a, miR-99b and miR-100 are upregulated after Braf activation and likely help to enforce growth arrest via downregulation of mTOR signaling60 (mTOR is discussed in detail below). miR99/100 are expressed at high levels in human nevi relative to melanoma, consistent our observations in mice.151,152 In addition, using the Dnmt3b/Braf/Pten model discussed above, we identified miR-196B as an important suppressor of mTORC2 activation after Pten loss, by targeting mTORC2 component Rictor.142 Other microRNAs also appear to be involved in melanoma pathogenesis at various stages and have been recently reviewed.153 Long non-coding RNAs such as MIR31HG and have been reported to have a role in BRAF-induced OIS in vitro,154,155 but have yet to be studied in nevi.
Cellular metabolism
In recent years, study of metabolic alterations in cancer cells has regained focus. Metabolic reprogramming is central to cancer formation and progression, and is classically referred to as the Warburg effect. In normal cells, under conditions of normoxia, glucose if fully oxidized to carbon dioxide via the citric acid cycle and mitochondrial oxidative phosphorylation. This pathway is very efficient in terms of ATP production. Glycolysis, the alternative pathway of glucose metabolism, is less energetically efficient and most normal cells is only used under conditions of hypoxia. Cancer cells, however, preferentially metabolize glucose via glycolysis regardless of oxygen abundance.156 Although less efficient in terms of ATP production, glycolytic pathways generate molecules useful in nucleotide, amino acid and lipid synthesis, and facilitate generation of biomass.157 Rapid glucose uptake by cancer cells is so conserved that a clinical imaging modality commonly used in cancer patients (fludeoxyglucose positron emission topography or FDG-PET) specifically measures this aberration to localize cancer within the body. Specific mediators of metabolic reprogramming in cancer cells are beginning to be understood and their role in nevus and melanoma formation will be considered in this section.
Early studies in senescence and OIS showed that although senescent cells permanently exit from the cell cycle, they maintain metabolic activity.12,158,159 Since this time, several groups have shown that oxidative phosphorylation favors development and maintenance of OIS, possibly by generating redox stress.160–163 Consistent with this hypothesis, introduction of BRAFV600E into cultured fibroblasts promotes oxidative phosphorylation by inhibiting pyruvate dehydrogenase kinase 1 (PDK1).160 Pyruvate kinase is a second regulator of glycolysis and has also been implicated in metabolic reprogramming of cancer cells. The M2 splice isoform of pyruvate kinase (PKM2) has been shown to be preferentially upregulated in cancers and may specifically induce a Warburg metabolism.164,165 As a side-note, despite this observation, a subset of melanomas appear to maintain and tolerate high levels of oxidative phosphorylation by upregulating reactive oxygen species (ROS) detoxification capacity.166
PDK1 levels tend to be higher in human nevi than in melanoma,167 consistent with the hypothesis that oxidative phosphorylation is the predominate means by which glucose is metabolized in nevi. PKM2 levels have not been specifically compared between nevi and melanomas. Functional analysis of nevi in vivo at a microscopic level in the Cdkn2a/Braf mouse model using an imaging modality analogous to FDG-PET (2-NBD glucose uptake) showed that nevi do not take up glucose at high levels (whereas the melanomas that develop in this model do).60 In our experience in clinical practice, human nevi, including nevi larger than 1 cm2 are also not FDG-PET positive. On the basis of these observations, it is reasonable to hypothesize that restriction of Warburg metabolism is likely an important factor that limits nevus growth after BRAF mutation; however, how specifically this occurs in nevi remains unclear.
In general, several other factors have been proposed to drive metabolic reprogramming in cancer and include C-MYC and HIF-1α.168–170 In melanocytes, over expression of C-MYC has been shown to suppress OIS in vitro,171 though whether or not this effect was related to changes in cellular metabolism was not studied. C-MYC transcriptional activity is thought to be higher in melanomas than in nevi, consistent with this hypothesis.171,172 In terms of HIF-1α, one study found higher levels of HIF-1α in melanomas than in nevi.173 In the Braf/Pten model, inactivation of HIF-1α and HIF2α does not affect primary tumor formation, but does decrease metastasis.174
Interestingly, although in isolation BRAFV600E mutation promotes oxidative phosphorylation, in a fully transformed state, such as melanoma, mutant BRAF alternatively appears to promote glycolysis and support the Warburg effect.175 For example, in patients with BRAF-mutant melanomas treated with BRAF inhibitors, a rapid and marked reduction in glucose uptake (including by FDG-PET) is observed; this change has been shown to correspond to a decrease in volume of melanoma cells.176,177 The differential role of BRAF in these two contexts is likely a reflection of whether BRAF activation occurs in relative isolation (as in nevi) or rather occurs in the context of other cooperative driver mutations, which likely cooperate to coordinately dysregulate cellular metabolism promoting the Warburg effect. For example, dysregulation of the PI3K/AKT and mTOR pathways in melanoma appear to have a central role in the metabolic reprogramming of melanoma cells and allowing outgrowth of BRAF-mutant melanocytes as melanoma (the role of these pathways will be discussed in detail below).
Autophagy and endoplasmic reticulum stress
Several studies have suggested that autophagy has an important role in OIS. Autophagy is a process by which cellular proteins and organelles can be degraded under unfavorable conditions to generate both energy and macromolecular building blocks. When autophagy is activated, cellular substrates are encircled by autophagic vesicles and delivered to lysosomes for bulk degredatation.178 A variety of cellular stressors can activate autophagy, including oncogene activation.178,179
Activation of autophagy has been proposed to promote OIS in part by facilitating the senescence-associated secretory phenotype (SASP) in senescent cells.179 SASP is discussed in more detail below. Interestingly, SA-β-gal which is commonly used as a marker of senescent cells, labels lysosomes and may reflect increased levels of autophagy.180,181 Complicating interpretation of the role of autophagy in melanomagenesis is the observation that autophagy can alternatively promote or repress tumorigenesis in different contexts. For example, autophagy promotes tumor cell survival in the setting of anti-cancer therapy, including during treatment with BRAF-mutant melanomas with BRAF inhibitors.182,183
These seemingly disparate roles for autophagy are perhaps reconcilable if one considers the context in which they occur. For example, a study in mice showed that inactivation of autophagy had opposite effects based on whether or not functional p53 was present. In this study, when p53 is intact (that is, early in tumorigenesis) autophagy has a tumor-suppressive function, however, when p53 is lost (that is, as later in tumor progression), autophagy alternatively promotes tumor progression.184 The role of autophagy in cancer more broadly was recently reviewed.162
Analysis of human melanocytic lesions supports the hypothesis that autophagy has a context-dependent role. Immunohistochemical analyses have shown that relative to early melanomas, nevi show increased staining for markers of autophagy including LC3B, Beclin1 and ATG5.185–187 However, when comparing levels of autophagy in primary versus metastatic melanoma, metastatic lesions appeared to have higher levels of autophagy based on LC3B staining.188,189 These findings appear to support the hypothesis that autophagy correlates with growth arrest in nevi, but may also promote progression of melanoma once it becomes invasive.
Ultrastructural analysis of nevi has shown that the number and size of most cytoplasmic organelles decrease from superficial dermal cells to deeper dermal cells, which correlates with a marked reduction in cell size/volume.190 It could be hypothesized that levels of autophagy increase as a function of depth within the dermis, possibly explaining the decrease in cell size and organelle content. Maturation in nevi is discussed above and summarized in Figure 1. In this scenario, autophagy might be induced in melanocytes as they leave the epidermis/superficial dermis and venture further into the potentially less favorable microenvironmental conditions in the mid and deep dermis. Interestingly, Ivanov et al.191 have shown that increased autophagy-mediated degradation of histones occurs along with maturation and increases in deeper portions of the nevi. However, previous ultrastructural analyses were not able to detect changes in the number of autophagosomes as a function of nevus maturation.190 Further study will be required to more clearly delineate any possible relationship between autophagy and maturation and the potential functional relevance of either process to growth arrest of nevi.
Endoplasmic reticulum (ER) stress occurs in the setting of very high levels of protein translation when misfolded and unfolded proteins accumulate in the ER, leading to an unfolded protein response (UPR).192 Activation of ER stress/UPR promotes cell survival under such adverse conditions by decreasing rates of translation and promoting degradation of misfolded proteins.182 The high levels of protein translation that occur after oncogene activation is one setting in which ER stress can occur.192 ER stress can also activate autophagy, a process which has been implicated in resistance of BRAF-mutant melanomas to BRAF inhibitors.182,192
In 2006, Denoyelle et al.193 reported that HRAS, but not BRAF or NRAS activation triggered ER stress in the melanocytic lineage.193 HRAS mutations are more common in Spitz nevi, but relatively uncommon in acquired nevi. In this study, although evidence of sustained ER stress was noted in Spitz nevi, it was not evident in more typical acquired nevi. Subsequent analysis of melanocytic lesions using GRP78, a marker of ER stress, showed low levels of ER stress in nevi, but much higher levels in melanoma.194 In summary, the role of ER stress in the growth arrest of nevi, if any, remains unclear.
Microenvironmental mediators
A major difference between in vivo and in vitro systems is the presence or absence of a physiologic microenvironment. In vivo, the tissue microenvironment consists of multiple cellular and non-cellular entities, which directly and indirectly interact with melanocytes and undoubtedly influence their behavior. It can be hypothesized that features of nevi observed in tissue, but not in growth-arrested BRAF-mutant melanocytes in culture, such as nesting and maturation, may be a reflection of interactions among nevus melanocytes and with the tissue microenvironment (Figure 1). During maturation, melanocytes become smaller, less pigmented and change their shape. Further, within nests themselves, melanocytes at the edges of the nest tend to be smaller, whereas those centrally tend to be larger. These patterns suggest that the phenotype of an individual melanocyte is influenced by its position within the nevus and within in the skin.
The specific factors regulating nesting and maturation are difficult to study and not well understood. In terms of maturation, a study by Perez et al.195 showed that levels of the matrix metalloproteinase MT1-MMP, an extracellular matrix degradation enzyme, differ as a function of nevus maturation. However, it is unclear if or how MT1-MMP is functionally related to maturation or nesting. Extracellular matrix composition is thought to vary significantly between nevi and melanomas, however the specific factors which influence nevus nesting and maturation are not known.196,197
The likely importance of nesting and maturation in tumor suppression is underscored by the observation that in melanoma these features tend to be disrupted. In fact, patterns of nesting and maturation are key histologic features used by dermatopathologists to distinguish nevi from melanoma in biopsy specimens. In melanoma, nest morphology is altered with nests tending to be larger, irregularly sized and/or more tightly packed, whereas in some melanomas the nesting phenotype is lost almost entirely. Similarly, maturation is lost in melanoma. Although the factors that mediate these processes are poorly understood, they are likely to have an important role in nevus formation, and at least in part, reflect a regulatory influence of the tissue microenvironment.
In skin biopsies, both normal individual melanocytes, as well as nevus melanocytes appear to prefer to be in close association with keratinocytes. Melanocytes in tissue tend to be concentrated in areas adjacent to both basal interfollicular and follicular keratinocytes. This observation also appears to be true in vitro also. Cultured melanocytes prefer to be associated with and grow better in association with keratinocytes. For this, reason a keratinocyte feeder layer is often used in the culture of melanocytes.198 The specific signals that underlie this phenomenon and whether or not proximity to keratinocytes in the epidermis has a role in the process of maturation in nevi is not known.
Nevus melanocytes likely interact with other cells in their microenvironment actively through secreted molecules. For example, senescent cells including those that have undergone OIS are highly secretory, a characteristic termed the SASP.162 SASP has been shown to have a functional role in growth arrest through propagation of this phenotype in an autocrine/paracrine manner. SASP has even been proposed to activate immune surveillance of lesions in tissue.199,200
In the setting of BRAFV600E mutation, specific secreted factors including both inflammatory (IL-1, IL-6 and type I interferons)201,202 and non-inflammatory (IGFBP7)203 factors have been reported to influence growth arrest phenotypes. For example, secretion of IGFBP7 was shown to drive BRAFV600E-induced OIS in melanocytic neoplasms in 2008 by Wajapeyee et al.,203 however, these findings have been debated in the literature.204,205 Interestingly, IGFBP7 can inhibit signaling through the IGF-1 receptor (IGF1R).206 We have found that Igf1r activation and in turn activation of PI3K/AKT signaling is associated with progression of nevi to melanoma in the Cdkn2a/Braf mouse model and hypothesize this is an important oncogenic driver in PTEN wild-type melanomas, by providing an alternate way to activate PI3K/AKT signaling.60 In human specimens, nevi tend to have lower levels of IGF1R expression than melanoma,207 consistent with this hypothesis. This link between IFGBP7 and IGF1R in nevi and melanoma is only hypothetical.
In terms of secreted inflammatory mediators, upregulation of IL-6 and IL-8 have been shown to occur after BRAF activation in vitro and are thought to reinforce senescence in a cell autonomous manner.201 Other inflammatory mediators such as IL-1 have been shown to regulate paracrine senescence in other models.200 Type I interferons have recently been shown to have an important non-cell autonomous role in growth arrest after BRAF activation202 and will be discussed further in the following section.
In human tissue, both IL-1 and IL-6 are upregulated in benign nevi relative to dysplastic nevi and melanoma.208 Altogether, these observations are consistent with a growth suppressive role for these interleukins, however, it remains unclear to what degree in vivo these and other inflammatory mediators act at by inhibiting melanocyte growth directly versus activating immune surveillance. The role of the immune system in suppression of melanocytic neoplasia will be the focus of the following section.
Role of the immune surveillance
The immune system likely has a role in controlling growth of nevi and preventing progression to melanoma. The ability of the immune system to interact with melanocytes in a functionally relevant manner is supported by several clinical observations. For example, vitiligo is a condition characterized by complete loss of melanocytes in affected areas of skin leading to the formation of depigmented patches. Although vitiligo pathogenesis is complex, melanocyte depletion is thought to be at least in part mediated by targeted destruction by CD8+ cytotoxic T cells.209,210 In a similar example, halo nevi, nevus melanocytes are targeted for destruction by the immune system. In halo nevi, nevus melanocytes are recognized and destroyed by CD8+ T cells leading to formation of a depigemented patch of skin around a pre-existing nevus and sometimes disappearance of the nevus altogether.211,212
Melanoma is associated with a relatively high mutation burden and is considered an immunogenic cancer.37,213 It has long been hypothesized that rare cases of spontaneous regression of metastatic melanoma are related to immune-mediated tumor recognition and destruction.214 In the 1970s, it was noted that a subset of patients responded to early immune-based therapies such as bacillus calmette-guerin.215–217 Since this time, we have learned that systemic immune stimulatory therapies such as high dose IL-2 can induce durable tumor remission in a small subset of patients with metastatic melanoma.218 Most recently, blockade of inhibitory immune checkpoints using inhibitors of CTLA-4 and PD-1 have been shown to induce durable anti-tumor responses in a subset of melanoma patients.219 One case of CTLA-4 inhibitor-induced regression of benign nevi has been reported to date, suggesting checkpoint inhibitors can also stimulate recognition and destruction of nevus melanocytes.220 Vitiligo-like depigmentation can also occur in patients with melanoma treated with checkpoint inhibitors (or spontaneously) and is considered a good prognostic sign.221
An additional informative clinical observation from patients relates to immunosuppression. Immunosuppressed patients such as solid organ transplant recipients and patients with chronic lymphocytic leukemia have an approximately twofold increased incidence of invasive melanoma compared with non-immunosuppressed individuals.222–224 This observation suggests that adaptive immunity has a role in suppressing melanoma formation and/or progression. Importantly, though, the increased risk of melanoma in immunosuppressed patients is relatively modest compared with some other malignancies. For example, solid organ transplant recipeints have a 65–100-fold increased risk of developing cutaneous squamous cell carcinoma.225
Matin et al.226 have suggested that the proportion of melanomas developing from nevi may be slightly higher in transplant recipients based on results from a larger study. However, the relative proportion of nevi developing from nevi versus de novo in transplant recipients has not been specifically studied and is already known to be highly variable between different studies.
Although it is clear that the adaptive immune system can recognize and eliminate melanocytes under a variety of conditions, it is unclear to what degree lymphocytes and other immune cells interact with nevi under homeostatic conditions and whether or not these interactions constrain growth and/or prevent transformation to melanoma. Some murine models have shown that in certain circumstances, cells with senescence phenotypes can be recognized and eliminated by both innate and adaptive arms of the immune system,227–229 however, this has not specfically been studied in nevi. In tissue, banal acquired nevi tend to be relatively pauci-inflammatory in contrast to melanomas, which in general show more robust lymphocytic infiltration.230,231 CD8+ T cell infiltration and histologic evidence of cytotoxic responses are not usually observed in nevi.52
Regression is a histologic phenomenon observed in some early melanomas and is characterized by focal areas of apparent tumor cell loss and replacement by fibrosis and inflammation.232 When observed in histologic specimens, regression is usually partial, rather than complete. Regression is typically not observed in nevi other than the outer perimeter of halo nevi. The generally accepted view is that regression reflects prior immune-mediated destruction of a portion of the melanoma, however, this is based mainly on inference from the clinical and histological appearance of regressed melanomas. Interestingly, the T cells found in areas of regression actually differ from the T cells in conditions such as vitiligo and halo nevi.233,234 In regression, primarily CD4+, not CD8 + T cells are present.233 CD8+ T cells predominate in vitiligo and halo nevi. The significance of this observation is unclear. An alternative hypothesis to explain regression is that tumor cell loss is instead driven by genomic crisis occuring in incipient melanomas and leading to apoptosis235–237 (this hypothesis will be discussed further below).
Recently, type I interferon signaling was also shown to have a tumor-suppressive role in the BrafV600E mouse model.202 In this study, inactivation of type 1 interferon receptor, Ifnar1, resulted in impaired growth arrest of BrafV600E-mutant melanocytes and increased melanoma penetrance in vivo. The tumor-suppressive effect of interferon signaling in this model appeared to be partially melanocyte autonomous and partially melanocyte non-autonomous, suggesting one possibility is that interferon could stimulate immune surveillance of nevus melanocytes, however, this was not specifically studied. Interestingly, previous work has shown that secretion of type I interferon by senescent cells is mediated by activation of the DNA damage response.238 Therapeutic interferon α (IFN-α) has been used as an adjuvant agent in melanoma, however, its efficacy has been debated.239
The complex interplay between the immune system and neoplastic cells is underscored by the observation that chronic inflammation, can alternatively promote tumorigenesis over time.240 Other cell types including some myeloid-derived cells and macrophages can support the formation and progression of cancer by multiple mechanisms.241 For example, Gr-1+ myeloid cells have been shown to oppose senescence in a murine model of prostate cancer.242 In melanoma, tumor-associated macrophages have been shown to facilitate melanoma progression by various mechanisms.243,244 The role of chronic inflammation, macrophages and other myeloid-derived cell populations have not been closely studied with respect to nevus biology.
The role of telomeres
Telomeres are protective structures at the ends of chromosomes formed by a repetitive DNA sequence and an associated protein complex (shelterin). The DNA portion of telomeres becomes progressively shorter after each round of cell division and upon reaching a critically shortened length triggers RS (as discussed above). The number of cell divisions required for induction of this process is called the ‘Hayflick limit’ and has been estimated to require ∼60 to 80 population doublings.85 The observations that growth arrest after oncogene activation in vitro occurs rapidly,54 that telomerase expression cannot overcome this growth arrest,13 an estimated 13–16 rounds of cell division are required for formation of a nevus, and that telomere length tends to be preserved in nevi,54,86,245 all support the hypothesis that RS should not contribute to nevus growth arrest.
In 2003, it was reported that the promoter of the telomere reverse transcriptase (TERT) is mutated at very high frequencies in melanomas, but not in nevi.37,246,247 Telomerase can extend shortened telomeres and is often aberrantly re-activated in cancers allowing proliferation beyond the Hayflick limit. Nevi have been shown to have absent or relatively low telomerase expression, but in melanoma telomerase is commonly expressed at relatively high levels, especially in advanced lesions.248–250
TERT promoter mutations are thought to result in increased TERT gene expression by creating binding motifs for ETS/TCF transcription factors.251 Given that these transcription factors are activated downstream of oncogenic pathways such as MAPK and WNT, it is possible that in the presence of TERT promoter mutations, activation of these pathways drives expression of TERT. TERT promoter mutations have been shown to be associated with increased telomerase expression in melanoma.252
In the context of these observations, one might predict that TERT promoter mutations provide a selective advantage in advanced melanomas, but might have relatively less important role in nevi and in situ melanomas. However, recent findings by Shain et al.98 have provided evidence somewhat contrary to this hypothesis.98 They show that TERT promoter mutations are found in combination with BRAF mutations in indeterminate melanocytic lesions (dysplastic-nevus spectrum) and melanoma in situ,98 suggesting TERT promoter mutations provide an early selective advantage.
One hypothesis to reconcile these seemingly disparate observations is that although telomeres are not critically shortened in nevi, they become so during the transition to melanoma. Along these lines, Bastian and colleagues have proposed that histologic regression observed in early melanomas (as discussed above) reflects the aftermath of a catastrophic genetic event that is initiated by critical telomere shortening. In this hypothetical model, melanocytic subpopulations of incipient melanomas that are not able to overcome genetic stress induced by critical telomere shortening undergo apoptosis, resulting in the loss of areas of neoplastic melanocytes.235,236 In continuing with this line of reasoning, this group has also proposed that telomere shortening actually does occur in nevi as patients age and explains the eventual regression of nevi in older patients. In this model, melanocytes in nevi would be predicted to slowly replicate overtime leading to critical telomere shortening that drives disappearance of nevi after RS. Although intriguing, to date, there is little experimental evidence to directly support this hypothesis.
An alternative hypothesis is that TERT expression provides a telomere-independent function that is important in early stages of melanomagenesis. For example, TERT has been shown to promote C-MYC stabilization;253 C-MYC is known to suppress growth arrest phenotypes in melanoma.171 Telomerase has other telomere-independent functions, which may also be important and have been recently reviewed.254 Last, oncogene activation has been shown to cause telomere dysfunction, which can induce growth arrest even in the presence of non-critically shortened telomeres through induction of DNA damage responses.255–257 It is possible that TERT expression may also relate to this observation in some way, however, telomere dysfunction has not been well documented in nevi.
The importance of telomere biology in melanocytic neoplasia is underscored by the observation that inherited mutations conferring an increased risk of melanoma cluster on genes that encode components of the telomere shelterin complex, in addition to TERT itself. These genes include POT1, ACD and TERF2IP.258–261 It has curiously even been reported that inherited variation in telomere length correlates with both total nevus number and nevus size.19 An improved understanding of the role that telomeres and TERT promoter mutations have in melanocytic neoplasia has the potential to significantly advance our understanding of factors regulating early stages of melanoma formation.
The role of PTEN, PI3K and AKT
PTEN is a tumor suppressor in the PI3K/AKT pathway. Functional data from murine models and observations in human lesions strongly implicate PTEN in restricting BRAFV600E-induced melanomagenesis in vivo; perhaps more convincingly that any other proposed mechanism of growth arrest after BRAF activation in melanocytes.
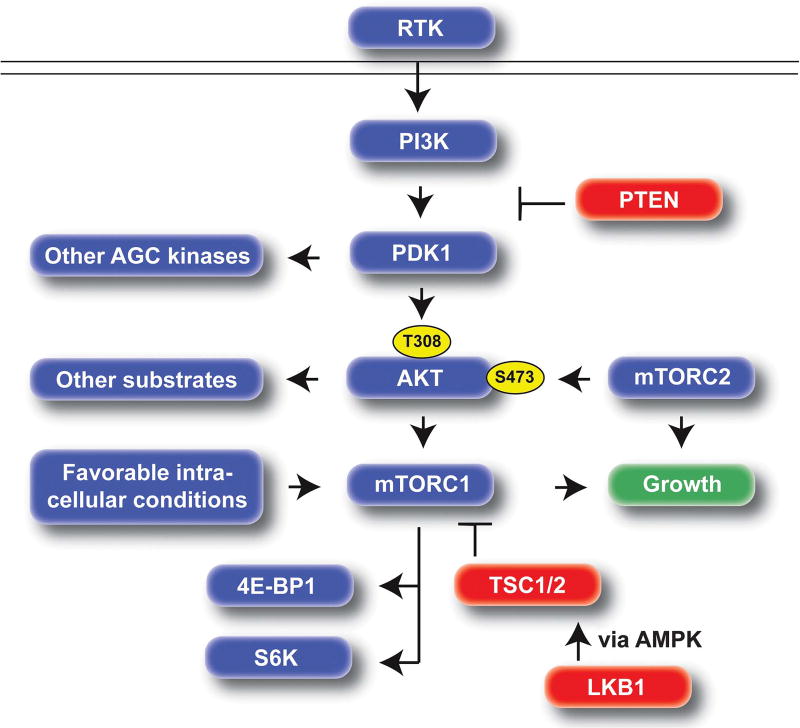
The PI3K/AKT and mTOR signaling pathways are central regulators of cell growth. These pathways are highly conserved and are typically activated downstream of receptor tyrosine kinases, but are also regulated by sensors of intracellular conditions.262 Activation of PI3K signaling results in activation of PDK1, which in turn activates the critical downstream kinase, AKT (Figure 5). PI3K and AKT signaling is also highly integrated with mTOR signaling (discussed in more detail below). PTEN, through its lipid phosphatase activity is a critical inhibitor of the PI3K/AKT pathway and considered a canonical cancer tumor suppressor.263 Although the discussion below will primarily focus on the role of PI3K signaling through AKT, PDK1 also has targets other than AKT, which are likely also important in melanoma pathogenesis.264,265
Figure 5.
Overview of the PI3K/AKT/mTOR pathway. When activated via mutation, this pathway provides a constitutive cellular growth signal. PTEN is a central tumor suppressor upstream of PDK1/AKT. LKB1 can inhibit mTORC1 via AMPK and TSC signaling. mTORC2 activates AKT by phosphorylating the S473 residue, whereas PDK1 activates AKT by phosphorylating T308. Activation of both mTORC1 and mTORC2 are required for progression of nevi to melanoma. Canonical outputs of mTORC1 include S6K and 4E–BP1. Tumor suppressors: red, oncogenic effect: blue. RTK, receptor tyrosine kinase.
In 2004, it was noted by Tsao and colleagues that PTEN inactivation tended to occur more frequently in BRAF than NRAS-mutant melanomas.266 In 2004, it was also noted at a functional level that concurrent dysregulation of the MAPK and PI3K/AKT pathways in cultured fibroblasts resulted in G1 to S progression, whereas activation of either pathway in isolation did not.267 In 2006, Courtois-Cox and colleagues noted that constitutive activation of MAPK signaling, which results in OIS in culture, was associated with induction of negative feedback loops that inhibited both MAPK and PI3K/AKT signaling. These data importantly suggested that after activation of MAPK signaling, negative feedback to PI3K/AKT helps constrain proliferation as part of OIS phenotypes in culture.95 Along these lines, in 2008, Cheung and colleagues reported that BRAFV600E can cooperate with AKT3 to drive early melanoma formation in vitro,268,269 again suggesting coordinated dysregulation of these two pathways is a potent oncogenic driver. Although the exact feedback loops that are important in melanoma need to be more clearly defined, one group has shown that BRAF activation leads to specific negative regulation of AKT signaling by feedback inhibition of the mTORC2 component Rictor (regulation of Akt by mTORC2 is discussed below).270
In 2009, it was noted by our lab that simultaneous BrafV600E mutation and Pten inactivation showed tremendous synergy in driving melanoma development in a mouse model (Braf/Pten model). As discussed above, BrafV600E mutation alone induces formation of mouse nevi, but rarely melanoma, even with long latency. However, when Pten is simultaneously inactivated in this model (Braf/Pten model), detectable growth arrest is no longer observed and unabated melanocytic proliferation ensues without delay, leading to synchronous formation of innumerable melanomas and rapid lethality from overwhelming tumor burden within 3–4 weeks (Figure 4).
The phenotype observed in the Braf/Pten model strongly supports the hypothesis that Pten has a central role in restricting melanoma formation after activation of MAPK signaling. Data published by Vredeveld and colleagues supports and extends this hypothesis. They show that that Pten depletion in already established BrafV600E-induced nevi using an shRNA approach also results in melanoma formation. These data suggest that Pten remains an important tumor suppressor in established nevi and that inactivation of Pten and activation of PI3K/Akt is a mechanism by which nevi may progress to melanoma.271 Activation of PI3K/ Akt signaling was also shown to have a similar effect in a mouse model of pancreatic neoplasia based on activated Ras (RasG12D).272
Analysis of human lesions also supports the important role of PTEN in restricting melanoma formation. Immunohistochemical staining for PTEN tends to be strong and uniform in melanocytic nevi.273,274 In contrast to nevi, PTEN is dysregulated in melanoma. Complete loss of PTEN staining occurs in about ∼ 1/3 of melanomas, with reduced or altered expression found in another ∼1/3.274 PTEN inactivation occurs by mutation in a small subset of melanomas, but is more commonly silenced epigenetically.98,274–277 As would be predicted based on these observations, levels of AKT activation have been found to be lower in nevi relative to melanomas using phospho-specific antibody staining.278–280 Immunohistochemical analysis of contiguous nevus-melanoma pairs also shows that although nevus portions of the lesion tend to have high PTEN expression and low levels of AKT activation; the melanoma portions tended to show reduced PTEN expression and increased levels of activated AKT.271
As discussed above, PTEN inactivation is enriched in BRAF-mutant melanomas suggesting that there may be a particularly potent synergy between BRAFV600E and PTEN loss.37,266,281 These two mutations tend to be enriched in non-CSD melanomas,16,51 suggesting that these mutations may define a biological subset of melanomas that tend to be less related to chronic ultraviolet exposure, more frequently associated with a nevus precursor, and affect relatively young patients.
The role of mTOR signaling
PI3K/AKT signaling is tightly integrated with mTOR signaling, which occurs as part of two distinct complexes, mTORC1 and mTORC2. mTORC1 integrates signals from growth factors, other pathways, and sensors of cellular nutrient, energy, and redox status to control protein synthesis and other anabolic processes.282 PI3K/AKT is a potent upstream regulator of mTORC1 and provides a strong activation signal to the complex via inhibition of TSC and PRAS40.282 mTORC2, is nutrient insensitive, but is also thought to be activated downstream of growth factor signaling. mTORC2 mediated phosphorylation of AKT on S473 is required for full AKT activation282 (Figure 5).
In the Braf/Pten model, constitutive activation of both mTORC1 and mTORC2 is observed.59,60 As mTORC1 is a highly conserved output of activated PI3K/Akt signaling, and we found that the mTORC1 inhibitor rapamycin inhibits melanoma growth in the Braf/Pten model,59,60 we hypothesized that mTORC1 activation was essential to the effect of Pten loss in Braf-mutant melanocytes. To test this hypothesis, we generated mice with Braf activation in the context of either Lkb1 (Braf/Lkb1) or Tsc1 (Braf/Tsc1) inactivation, which result in isolated activation of mTORC1 without directly affecting mTORC2 activity.60 Interestingly, constitutive activation of mTORC1 abrogated growth arrest of nevi, however, full transformation to melanoma did not occur as in the Braf/Pten model (Figure 4). In these models, confluent melanocytic neoplasia, rather than formation of discrete nevi was observed; however, although mice were tracked for >1 year, the melanocytic neoplasms in this model never exhibited malignant behavior such as uncontrolled or invasive growth, a Warburg metabolism, or metastasis.
Lack of a fully malignant behavior in the Braf/Lkb1 and Braf/Tsc1 models is likely at least partially due to well-defined negative feedback loops induced by constitutive activation of mTORC1, which oppose activation of mTORC2.282 Interestingly, we found that Cdkn2a inactivation in the Braf/Lkb1 model enabled simultaneous activation of mTORC1 and mTORC2 leading to rapid melanoma formation.60 The mechanism by which Cdkn2a inactivation permits mTORC2 activation in this model is unclear, but may be related to novel roles of Ckdn2a in regulating cellular metabolism. For example, Cdk4 was recently shown to control cellular glucose uptake in addition to cell cycle control.100 Arf has also been shown to regulate cellular metabolism.101
The central importance of mTORC2 in melanoma pathogenesis was also recently illustrated by our lab using the Pten/Braf/Dnmt3b model in which Dnmt3b mediated repression of miR-196b is required to alleviate inhibition of mTORC2 signaling by targeting of the mTORC2 component Rictor.142 Without this activity of Dnmt3b, full activation of mTORC2 and formation of melanoma does not occur. This work uncovered an unanticipated epigenetic checkpoint that regulates full mTOR activation in the progression of nevi to melanoma. The oncogenic role of Rictor in human melanoma is supported by the observation that the RICTOR gene is frequently amplified in melanoma.148 Rictor has been shown to be essential for melanoma formation in other models as well.283
Analysis of human melanocytic lesions supports the hypothesis that activation of both mTORC1 and mTORC2 are important in melanoma, as the activity of both complexes tends to be relatively low in in nevi, but high in melanoma. For example, activation of mTORC1 substrates such as ribosomal protein S6 are only rarely present in nevi, but frequent in human melanomas.284 Similarly, levels of eukaryotic initiation factor (eIF-4E), which is inhibited by mTORC1 substrate eIF-4E binding protein 1 (4E–BP1) are also significantly lower in nevi than in melanoma.285 mTORC2 activity has similarly been shown to be lower in nevi than in melanoma. For example, levels of phospho-AKT-S473, a phosphorylation event catalyzed by mTORC2, are significantly lower in nevi than in melanoma. 60,278–280
Altogether, these observations support the hypothesis that activation of PI3K/AKT signaling, including mTORC1 and mTORC2, is central to the pathogenesis of BRAFV600E-mutant melanoma. A requirement for simultaneous activation of mTORC1 and mTORC2 has been proposed in other malignancies and is also likely to be important in NRAS and NF1-mutant melanomas. Although the exact reason why concurrent activation is required for a fully malignant phenotype is not completely clear, it has been hypothesized to relate in part to metabolic reprogramming of tumor cells.282,286 In this conceptual model, MAPK pathway mutations would provide the initial proliferative signal, whereas dysregulation of PI3K/AKT/mTOR signaling enables sustained growth. In the absence of PI3K/AKT/mTOR dysregulation, BRAF mutation would only induce transient proliferation as seen during nevus formation. In melanomas that do not develop from a nevus precursor, BRAF mutation may occur relatively later in an already sensitized melanocyte with PI3K/AKT/mTOR dysregulation (Figure 3). Pten loss in our murine models does not induce analagous melanocyte proliferation in isolation.
Separate work has suggested that alternatively mTORC1 may become increasingly activated in senescent cells and may help enforce senescence phenotypes through activation of SASP.287–290 The reason(s) for the discrepancy in these observations with data from mouse models are unclear, but may be related to differences in cell type, in vitro versus in vivo effects, relative levels of mTORC1 activation, and/or difference in subcellular localization of activated mTORC1.
Additional pathways such as Wnt/β-catenin are also important in melanoma pathogenesis. We have shown that stabilization of β-catenin through activating mutation, enhances melanoma metastasis in the Pten/Braf mouse model.63,291 However, we also showed that β-catenin stabilization results in an altered nevus phenotype with more polymorphous lesion size and increased pigment compared to Braf activation alone. In a lung cancer model, β-catenin has been shown to cooperate with activated Braf to suppress OIS via induction of c-Myc expression.292 Wnt/β-catenin activation is thought to occur in ∼1/3 of human melanomas.293 The example of β-catenin illustrates that there are many potential combinations of cooperative oncogenic hits in human melanocytic lesions, which give rise to melanocytic proliferations with heterogeneous and often overlapping clinical and histologic features. The possible sequence and differential contribution of various mutations, as recently described by Shain and colleagues, is discussed below.
DYSPLASTIC NEVI
The above discussion has largely focused on banal acquired melanocytic nevi, lesions for which most, if not all, dermatopathologists would agree on the diagnosis based on histologic grounds. Other melanocytic lesions are not as clear cut and can have some features of melanoma and some features of nevi, either clinically, histologically, or both; creating a diagnostic gray zone. Dysplastic and atypical nevi are terms used by clinicians to describe lesions with concerning histologic or clinical features, respectively. As part of clinical practice, histologically dysplastic nevi are often graded based on the degree abnormality into categories of mild, moderate and severe dysplasia; with severe dysplasia bordering on melanoma, but not quite meeting diagnostic criteria. This system of grading histologic dysplasia and its implications are controversial because of a lack of consensus terminology and disagreement over clinical management of these lesions. Further, this grading system implies that progression through different degrees of dysplasia toward melanoma occurs in a linear, progressive fashion.294,295 However, the natural history and biologic significance of dysplastic nevi is not well characterized. For example, it is not known what proportion of dysplastic nevi develop de novo versus what percentage could represent evolution of previously banal nevi. It should be noted that in the vast majority of cases, definitive histological features of banal nevi and dysplastic nevi are not observed in the same lesion, suggesting progression from one to the other is probably rare.
The relationship of dysplastic nevi to melanoma is also incompletely understood. For example, it is unclear if individual dysplastic nevi progress to melanoma at higher rates than banal nevi. In fact, this seemingly straight forward question is difficult to study directly as to establish a diagnosis of dysplasia, the lesion must be biopsied (usually fully removed). Further, clinical aytpia does not necessarily correlate with histologic dysplasia,296 suggesting these lesions cannot reliably be identified clinically and followed. Several studies have indirectly addressed this question by comparing the frequency with which melanomas are associated with the remnants of banal nevi versus the remnants dysplastic nevi.64,297–301 These analyses have generally shown that dysplastic nevi tend to be associated with melanomas at similar rates as banal nevi, however, this observation may be confounded by the relative abundance of banal nevi relative to dysplastic nevi.294,295
A recent study by Shain et al.98 found that melanocytic lesions in the diagnostic gray zone between nevi and melanoma may be genetically distinct from banal nevi. This group found that whereas banal nevi typically have BRAFV600E mutation only, ambiguous lesions tended to have NRAS mutations and BRAFnon-V600E mutations, as well as TERT promoter mutations. In this excellent work, the authors also address the sequence in which mutations are likely to have occurred. Other studies have also found relatively lower rates (∼60%) of BRAFV600E mutation in dysplastic nevi, compared to ∼80% in banal nevi.38,40,91,302,303 These data suggest that histologic dysplasia may hold a meaningful, lesion intrinsic biologic significance and also implies that dysplastic nevi are often likely not derived from previously banal nevi. It also suggests that BRAFV600E-mutant and non-BRAFV600E-mutant melanomas may show distinct natural histories. Despite these observations by Shain and colleagues, however, it is still not clear that histologically dysplastic lesions have an increased risk of progression to melanoma compared to their banal counterparts, they may just be more morphologically similar to melanoma histologically. Further work will be required to better characterize the relationship among benign nevi, histologically dysplastic nevi and melanoma.
Despite the controversy in this area, a history of a histologically dysplastic nevus is still clinically significant for patients. At a population level, patients with a history of nevi with increasing histologic dysplasia carry a dose-dependent increase in the overall risk of developing melanoma.304,305 This increased risk appears distinct from the individual lesion actually biopsied and diagnosed as dysplastic. It is unclear if this increased risk is related to exposure to mutagens such as ultraviolet light, an inherent genetic susceptibility to melanocytic neoplasia, or a combination of both.
STABLE CLONAL EXPANSION
OIS is a paradigm that has been used to understand growth arrest after oncogene activation and has significantly advanced our understanding of neoplasia broadly, including the importance of cooperation between multiple oncogenes in cancer. However, this terminology is slightly confusing when applied to melanocytic lesions in tissue, as although cells express some markers of senescence, overall they appear to have relatively few phenotypic features of senescent cells (as discussed above). In fact, in 2012 Tran et al.120 found that levels of the most commonly used markers of OIS do not readily distinguish between nevi and melanoma. These markers included p16INK4A, p53, SA-β-gal, PML, SAHF (H3K9Me, 4′-6-diamidino-2-phenylindole) and DNA damage response (γ-H2AX). Further, authors have also noted that one of the most robust OIS markers, SA-β-gal, can be detected not only in nevi but also in some late stage melanomas, including metastasis.55,120,306 Although no individual histologic marker is perfect, these observations suggest that thinking about nevi slightly differently could be useful; we propose that stable clonal expansion may be a more useful term in describing this process moving forward than oncogene-induced senescence (Figure 6).
Figure 6.
Mechanisms of growth arrest during stable clonal expansion. After acquisition of individual oncogenic mutations (BRAFV600E), growth arrest of melanocytic nevi is established and maintained by multiple different, overlapping mechanisms. Progression to melanoma likely requires simultaneous abrogation of multiple growth suppressive pathways.
Several clinical observations suggest that nevi are not static (senescent), even after they reach a seemingly final size. For example, although the majority of nevi do appear to remain relatively stable in size over time, a subset will enlarge. In clinical practice, when enlarging nevi are noted, they are typically biopsied to evaluate for melanoma. But, does enlargement of nevi necessarily mean that growth arrest mechanisms have been bypassed and progression to melanoma has occurred; as would be implied based on the OIS hypothesis? To address this question, Lucas and colleagues carefully tracked individual nevi over time in adults using total body photography and biopsied lesions that had changed appearance (including increased in size). They found that increase in size alone in otherwise non-concerning lesions rarely led to a diagnosis of melanoma.307 Similar studies have also shown that most enlarging nevi, when biopsied, are diagnosed as nevi (both histologically banal and dysplastic) and not melanoma.79,308,309 These observations are not included to suggest that enlarging nevi should not be biopsied to rule out melanoma in clinical practice, but rather emphasize that from a mechanistic standpoint tumor-suppressive mechanisms constraining progression to melanoma remain intact even during periods of clinical growth. Multiple distinct mechanisms are likely overlapping/redundant in the maintenance of benignity and preventing uncontrolled outgrowth of nevi (Figure 6).
Interestingly, nevi can change appearance in other settings, such as during pregnancy and after exposure to ultraviolet radiation, changes that do not necessarily signify progression to melanoma. For example, in pregnant patients, nevi can change color, dermatoscopic appearance,310,311 increase in size312,313 and show increased mitotic rates.314 Although melanoma does rarely develop during pregnancy, this is very rare compared to these common changes in benign nevi. Exposure to ultraviolet radiation induces proliferation of nevus cells,315,316 yet this proliferation does not equate to melanoma formation. In other words, nevi appear to maintain the ability to respond physiologically to various stimuli, including increasing proliferation rates while maintaining their benignity and stability of growth arrest.
Additional observations suggest that, in fact, nevus melanocytes actually retain significant proliferative potential. For example, nevi can regrow in patients when only partially biopsied (incompletely removed) and are known as recurrent nevi. In recurrent nevi, nevi regrow to a similar size within the scar at the biopsy site, but then again stop clinical growth and remain benign.317,318 Data from in vitro work also suggests that nevus melanocytes retain proliferative capacity. In several reports from the 1980s, it was shown that nevus melanocytes derived from clinical specimens can proliferate in culture, where they actually proliferate faster than normal melanocytes grown under the same conditions.319–321
In fact, close histologic examination of nevi shows that even common banal nevi have mitotically active melanocytes, which are present at low, but reproducible rates.322–324 Glatz et al.323 estimated that 0.024 mitoses are present per mm2 in histologically banal melanocytic nevi. Using Ki67, a marker of actively cycling cells, Soyer et al.84 reported that 0.78% of nevus melanocytes, an estimated 2200 cells out of 282 000 cells per mm3 were cycling at the time of biopsy. In nevi, proliferative activity is generally restricted to the most superficial portions of the lesion322–324 (Figure 1).
If the above estimates are correct, and melanocytes in nevi do slowly divide over time, then melanocyte attrition would be need to be present at a similar rate to maintain a relatively stable size over time. Along these lines, low rates of apoptosis have also been reported in melanocytic nevi.325,326 It is unclear whether apoptosis in nevi is a melanocyte intrinsic phenomenon or whether apoptosis is induced by some other factor or cell type in the microenvironment; for example, lymphocytes (though satellite cell apoptosis is not routinely observed in nevi). Interestingly, contrary to mitotic activity, which tends to favor the superficial portion of the lesion, apoptotic cells predominate in deeper portions of the dermis and are almost never found in superficial portions of the lesion.326 Given these observations, it is tempting to hypothesize that in nevi with a dermal component, histologic maturation reflects a process in which melanocytes which are generated in superficial portions of the lesion migrate over time into deeper portions of the lesion where apoptosis occurs (Figure 1). However, to date there is no experimental evidence to support this hypothesis.
Shain et al.98 have proposed that continued proliferation of nevus melanocytes over decades results in progressive and ultimately critical shortening of telomeres, resulting in telomere crisis. In this model, eventual telomere crisis, RS, and subsequent clearance of nevus melanocytes might mechanistically underlie the observed clinical regression of nevi with advancing age.98 Clinical nevus regression is associated with complete disappearance of the lesion and replacement of melanocytes by fatty and fibrotic tissue.20 However, as discussed above, critical shortening of telomeres in nevi has not been documented to date,54,86 despite the apparent early selection for TERT promoter mutations during transition to melanoma (as discussed above).
Overall, these observations suggest that although nevi demonstrate tremendous clinical stability, behind the scenes nevus melanocytes are seemingly fairly dynamic. Microscopically low rates of proliferation are balanced by cell attrition. Nevus melanocytes can respond to environmental stimuli and even increase their proliferation without transforming to melanoma. Stable clonal expansion is maintained by multiple, overlapping, nearly fail-safe mechanisms including pathway intrinsic and extrinsic feedback loops, epigenetic reprogramming, microenvironmental effects, metabolic constraints and others (Figure 6).
These dynamic (rather than static/senescent) features of nevus melanocytes are notable in the setting of the recent observation that in clinically normal skin, individual oncogenic keratinocytic clones are actually fairly ubiquitous.15 In this study, it was shown that potent oncogenic mutations in NOTCH, TP53 and FGFR3 are commonly present in morphologically normal keratinocytes and result in the subclinical expansion of mutant clones, despite the otherwise normal appearance of the skin clinically. These mutant clones provide local selective advantage and ultimately result in formation of a quilt-like pattern of partially overlapping, competing clones. These results are important as they show that despite the presence of potent oncogenic mutations, normal cellular function and tissue viability (including proliferation) is maintained. Constitutive proliferation of keratinocytes is required to continuously turnover skin and maintain epidermal barrier function; a process that seemingly proceeds undisturbed within mutant clones. In keratinocytes at least, a tumor-suppressive response that involved complete and irreversible withdrawal from the cell cycle would be predicted to result in disappearance of the clone over time. Applied to melanocytes and other cell types, these observations suggest that despite oncogenes which result in clonal expansion, typical cellular function can be maintained.
This principle is likely to be important in other tissue types, even those not constantly exposed to a potent mutagen like ultraviolet light. Even in the absence of an outside mutagen, cells are constantly exposed to new oncogenic insults generated during DNA replication. For example, Chandeck and Mooi estimate based on organism wide rates of cell division in humans (5 million every second) and the inherent imperfection in DNA replication which leads to an unrepaired point mutation in 1 out of every 1 billion replicated bases, that by chance activating mutations occurring in any given oncogene (for example, BRAFV600E) occur approximately every 10 min somewhere in the body.52 Although the vast majority of these cells are likely eliminated, never expand, or are shed/lost, a subset likely clonally expand and suggest that the body must deal with a constant barrage of mutant clones, yet maintain tissue function. The importance of this effect is underscored by the more recent observation that differential rates of cancer development in different tissue types correlates with the frequency with which stem cells divide in that tissue. This presumably incidentally leads to enhanced generation of mutant oncogenes, which persist in stem cell populations.327
Taking these various considerations together, we favor the term stable clonal expansion when referring to melanocytic nevi in tissue, rather than oncogene-induced senescence. To us, this term better reflects nevus phenotypes observed in mouse models and in human lesions. In nevi, despite stable lesion size clinically, the melanocytes in nevi are dynamic and multiple cooperative cell intrinsic and extrinsic factors restrain continuous growth (Figure 6). However, this process can be overcome as part of progression to melanoma, with the acquisition of additional pathogenic mutations and failure of a critical mass of growth suppressive programs.
Acknowledgments
We would like to acknowledge the following funding sources: R01 CA196660, P50 CA121974, P01CA128814, the Melanoma Research Alliance, the Melanoma Research Foundation and the Hervey Family Foundation (to MB).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 3.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 6.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 8.Newbold RF, Overell RW. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983;304:648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- 9.Newbold RF, Overell RW, Connell JR. Induction of immortality is an early event in malignant transformation of mammalian cells by carcinogens. Nature. 1982;299:633–635. doi: 10.1038/299633a0. [DOI] [PubMed] [Google Scholar]
- 10.Sager R. Senescence as a mode of tumor suppression. Environ Health Perspect. 1991;93:59–62. doi: 10.1289/ehp.919359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien W, Stenman G, Sager R. Suppression of tumor growth by senescence in virally transformed human fibroblasts. Proc Natl Acad Sci USA. 1986;83:8659–8663. doi: 10.1073/pnas.83.22.8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 13.Wei S, Wei S, Sedivy JM. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 1999;59:1539–1543. [PubMed] [Google Scholar]
- 14.Baek KH, Ryeom S. Detection of oncogene-induced senescence in vivo. Methods Mol Biol. 2017;1534:185–198. doi: 10.1007/978-1-4939-6670-7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–271. doi: 10.1146/annurev-pathol-012513-104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaffer JV. Update on melanocytic nevi in children. Clin Dermatol. 2015;33:368–386. doi: 10.1016/j.clindermatol.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Barnhill RL. Pathology of Melanocytic Nevi and Melanoma. 3. Springer; New York, NY: 2014. [Google Scholar]
- 19.Bataille V, Kato BS, Falchi M, Gardner J, Kimura M, Lens M, et al. Nevus size and number are associated with telomere length and represent potential markers of a decreased senescence in vivo. Cancer Epidemiol Biomarkers Prev. 2007;16:1499–1502. doi: 10.1158/1055-9965.EPI-07-0152. [DOI] [PubMed] [Google Scholar]
- 20.Stegmaier OC. Natural regression of the melanocytic nevus. J Invest Dermatol. 1959;32:413–421. doi: 10.1038/jid.1959.70. [DOI] [PubMed] [Google Scholar]
- 21.MacKie RM, English J, Aitchison TC, Fitzsimons CP, Wilson P. The number and distribution of benign pigmented moles (melanocytic naevi) in a healthy British population. Br J Dermatol. 1985;113:167–174. doi: 10.1111/j.1365-2133.1985.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 22.Eder J, Prillinger K, Korn A, Geroldinger A, Trautinger F. Prevalence of actinic keratosis among dermatology outpatients in Austria. Br J Dermatol. 2014;171:1415–1421. doi: 10.1111/bjd.13132. [DOI] [PubMed] [Google Scholar]
- 23.English DR, Milne E, Simpson JA. Ultraviolet radiation at places of residence and the development of melanocytic nevi in children (Australia) Cancer Causes Control. 2006;17:103–107. doi: 10.1007/s10552-005-0425-0. [DOI] [PubMed] [Google Scholar]
- 24.Luther H, Altmeyer P, Garbe C, Ellwanger U, Jahn S, Hoffmann K, et al. Increase of melanocytic nevus counts in children during 5 years of follow-up and analysis of associated factors. Arch Dermatol. 1996;132:1473–1478. [PubMed] [Google Scholar]
- 25.Bishop JA, Wachsmuth RC, Harland M, Bataille V, Pinney E, Mac KP, et al. Genotype/phenotype and penetrance studies in melanoma families with germline CDKN2A mutations. J Invest Dermatol. 2000;114:28–33. doi: 10.1046/j.1523-1747.2000.00823.x. [DOI] [PubMed] [Google Scholar]
- 26.Florell SR, Meyer LJ, Boucher KM, Porter-Gill PA, Hart M, Erickson J, et al. Longitudinal assessment of the nevus phenotype in a melanoma kindred. J Invest Dermatol. 2004;123:576–582. doi: 10.1111/j.0022-202X.2004.23312.x. [DOI] [PubMed] [Google Scholar]
- 27.Goldgar DE, Cannon-Albright LA, Meyer LJ, Piepkorn MW, Zone JJ, Skolnick MH. Inheritance of nevus number and size in melanoma and dysplastic nevus syndrome kindreds. J Natl Cancer Inst. 1991;83:1726–1733. doi: 10.1093/jnci/83.23.1726. [DOI] [PubMed] [Google Scholar]
- 28.Falchi M, Bataille V, Hayward NK, Duffy DL, Bishop JA, Pastinen T, et al. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat Genet. 2009;41:915–919. doi: 10.1038/ng.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karram S, Novy M, Saroufim M, Loya A, Taraif S, Houreih MA, et al. Predictors of BRAF mutation in melanocytic nevi: analysis across regions with different UV radiation exposure. Am J Dermatopathol. 2013;35:412–418. doi: 10.1097/DAD.0b013e31826db181. [DOI] [PubMed] [Google Scholar]
- 30.Hafner C, Stoehr R, van Oers JM, Zwarthoff EC, Hofstaedter F, Klein C, et al. The absence of BRAF, FGFR3, and PIK3CA mutations differentiates lentigo simplex from melanocytic nevus and solar lentigo. J Invest Dermatol. 2009;129:2730–2735. doi: 10.1038/jid.2009.146. [DOI] [PubMed] [Google Scholar]
- 31.Tschandl P, Berghoff AS, Preusser M, Burgstaller-Muehlbacher S, Pehamberger H, Okamoto I, et al. NRAS and BRAF mutations in melanoma-associated nevi and uninvolved nevi. PLoS ONE. 2013;8:e69639. doi: 10.1371/journal.pone.0069639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugianto JZ, Ralston JS, Metcalf JS, McFaddin CL, Smith MT. Blue nevus and “malignant blue nevus:” A concise review. Semin Diagn Pathol. 2016;33:219–224. doi: 10.1053/j.semdp.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara G, Gianotti R, Cavicchini S, Salviato T, Zalaudek I, Argenziano G. Spitz nevus, Spitz tumor, and spitzoid melanoma: a comprehensive clinicopathologic overview. Dermatol Clin. 2013;31:589–598. doi: 10.1016/j.det.2013.06.012. viii. [DOI] [PubMed] [Google Scholar]
- 34.Strazzula L, Senna MM, Yasuda M, Belazarian L. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234–1240. doi: 10.1016/j.jaad.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 35.Ferringer T. Update on immunohistochemistry in melanocytic lesions. Dermatol Clin. 2012;30:567–579. doi: 10.1016/j.det.2012.06.007. v. [DOI] [PubMed] [Google Scholar]
- 36.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 37.Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 39.Poynter JN, Elder JT, Fullen DR, Nair RP, Soengas MS, Johnson TM, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16:267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 40.Roh MR, Eliades P, Gupta S, Tsao H. Genetics of melanocytic nevi. Pigment Cell Melanoma Res. 2015;28:661–672. doi: 10.1111/pcmr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piris A, Mihm MC, Jr, Hoang MP. BAP1 and BRAFV600E expression in benign and malignant melanocytic proliferations. Hum Pathol. 2015;46:239–245. doi: 10.1016/j.humpath.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Carr J, Mackie RM. Point mutations in the N-ras oncogene in malignant melanoma and congenital naevi. Br J Dermatol. 1994;131:72–77. doi: 10.1111/j.1365-2133.1994.tb08460.x. [DOI] [PubMed] [Google Scholar]
- 43.Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol. 2000;157:967–972. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cisowski J, Sayin VI, Liu M, Karlsson C, Bergo MO. Oncogene-induced senescence underlies the mutual exclusive nature of oncogenic KRAS and BRAF. Oncogene. 2016;35:1328–1333. doi: 10.1038/onc.2015.186. [DOI] [PubMed] [Google Scholar]
- 46.Robinson WA, Lemon M, Elefanty A, Harrison-Smith M, Markham N, Norris D. Human acquired naevi are clonal. Melanoma Res. 1998;8:499–503. doi: 10.1097/00008390-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Yeh I, von Deimling A, Bastian BC. Clonal BRAF mutations in melanocytic nevi and initiating role of BRAF in melanocytic neoplasia. J Natl Cancer Inst. 2013;105:917–919. doi: 10.1093/jnci/djt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hui P, Perkins A, Glusac E. Assessment of clonality in melanocytic nevi. J Cutan Pathol. 2001;28:140–144. doi: 10.1034/j.1600-0560.2001.028003140.x. [DOI] [PubMed] [Google Scholar]
- 49.Harada M, Suzuki M, Ikeda T, Kaneko T, Harada S, Fukayama M. Clonality in nevo-cellular nevus and melanoma: an expression-based clonality analysis at the X-linked genes by polymerase chain reaction. J Invest Dermatol. 1997;109:656–660. doi: 10.1111/1523-1747.ep12337678. [DOI] [PubMed] [Google Scholar]
- 50.Masaki T, Wang Y, DiGiovanna JJ, Khan SG, Raffeld M, Beltaifa S, et al. High frequency of PTEN mutations in nevi and melanomas from xeroderma pigmentosum patients. Pigment Cell Melanoma Res. 2014;27:454–464. doi: 10.1111/pcmr.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shain AH, Bastian BC. From melanocytes to melanomas. Nat Rev Cancer. 2016;16:345–358. doi: 10.1038/nrc.2016.37. [DOI] [PubMed] [Google Scholar]
- 52.Chandeck C, Mooi WJ. Oncogene-induced cellular senescence. Adv Anat Pathol. 2010;17:42–48. doi: 10.1097/PAP.0b013e3181c66f4e. [DOI] [PubMed] [Google Scholar]
- 53.Lott JP, Gross CP, Bosenberg M. County-level association of melanoma and papillary thyroid cancer: evidence of shared environmental risk? Pigment Cell Melanoma Res. 2015;28:120–123. doi: 10.1111/pcmr.12322. [DOI] [PubMed] [Google Scholar]
- 54.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 55.Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 57.Goel VK, Ibrahim N, Jiang G, Singhal M, Fee S, Flotte T, et al. Melanocytic nevuslike hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene. 2009;28:2289–2298. doi: 10.1038/onc.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 59.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damsky W, Micevic G, Meeth K, Muthusamy V, Curley DP, Santhanakrishnan M, et al. mTORC1 activation blocks BrafV600E–induced growth arrest but is insufficient for melanoma formation. Cancer Cell. 2015;27:41–56. doi: 10.1016/j.ccell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark WH, Jr, Elder DE, Guerry D, Epstein MN, Greene MH, Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984;15:1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- 62.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 63.Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014;33:2413–2422. doi: 10.1038/onc.2013.194. [DOI] [PubMed] [Google Scholar]
- 64.Bevona C, Goggins W, Quinn T, Fullerton J, Tsao H. Cutaneous melanomas associated with nevi. Arch Dermatol. 2003;139:1620–1624. doi: 10.1001/archderm.139.12.1620. [DOI] [PubMed] [Google Scholar]
- 65.Lin WM, Luo S, Muzikansky A, Lobo AZ, Tanabe KK, Sober AJ, et al. Outcome of patients with de novo versus nevus-associated melanoma. J Am Acad Dermatol. 2015;72:54–58. doi: 10.1016/j.jaad.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 66.Haenssle HA, Mograby N, Ngassa A, Buhl T, Emmert S, Schon MP, et al. Association of patient risk factors and frequency of nevus-associated cutaneous melanomas. JAMA Dermatol. 2016;152:291–298. doi: 10.1001/jamadermatol.2015.3775. [DOI] [PubMed] [Google Scholar]
- 67.Shitara D, Nascimento MM, Puig S, Yamada S, Enokihara MM, Michalany N, et al. Nevus-associated melanomas: clinicopathologic features. Am J Clin Pathol. 2014;142:485–491. doi: 10.1309/AJCP4L5CJGKTJVDD. [DOI] [PubMed] [Google Scholar]
- 68.Dadzie OE, Yang S, Emley A, Keady M, Bhawan J, Mahalingam M. RAS and RAF mutations in banal melanocytic aggregates contiguous with primary cutaneous melanoma: clues to melanomagenesis. Br J Dermatol. 2009;160:368–375. doi: 10.1111/j.1365-2133.2008.08887.x. [DOI] [PubMed] [Google Scholar]
- 69.Kakavand H, Crainic O, Lum T, O’Toole SA, Kefford RF, Thompson JF, et al. Concordant BRAFV600E mutation status in primary melanomas and associated naevi: implications for mutation testing of primary melanomas. Pathology. 2014;46:193–198. doi: 10.1097/PAT.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 70.Shitara D, Tell-Marti G, Badenas C, Enokihara MM, Alos L, Larque AB, et al. Mutational status of naevus-associated melanomas. Br J Dermatol. 2015;173:671–680. doi: 10.1111/bjd.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bogdan I, Smolle J, Kerl H, Burg G, Boni R. Melanoma ex naevo: a study of the associated naevus. Melanoma Res. 2003;13:213–217. doi: 10.1097/01.cmr.0000056226.78713.99. [DOI] [PubMed] [Google Scholar]
- 72.Demunter A, Stas M, Degreef H, De Wolf-Peeters C, van den Oord JJ. Analysis of N- and K-ras mutations in the distinctive tumor progression phases of melanoma. J Invest Dermatol. 2001;117:1483–1489. doi: 10.1046/j.0022-202x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- 73.Tan JM, Lin LL, Lambie D, Flewell-Smith R, Jagirdar K, Schaider H, et al. BRAF wild-type melanoma in situ arising in a BRAF V600E mutant dysplastic nevus. JAMA Dermatol. 2015;151:417–421. doi: 10.1001/jamadermatol.2014.3775. [DOI] [PubMed] [Google Scholar]
- 74.Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol. 2003;139:282–288. doi: 10.1001/archderm.139.3.282. [DOI] [PubMed] [Google Scholar]
- 75.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: I Common and atypical naevi. Eur J Cancer. 2005;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen M, Viros A, Cook M, Marais R. (G12D) NRAS and kinase-dead BRAF cooperate to drive naevogenesis and melanomagenesis. Pigment Cell Melanoma Res. 2014;27:1162–1166. doi: 10.1111/pcmr.12293. [DOI] [PubMed] [Google Scholar]
- 77.Chai E, Ferguson B, Prow T, Soyer P, Walker G. Three-dimensional modelling for estimation of nevus count and probability of nevus-melanoma progression in a murine model. Pigment Cell Melanoma Res. 2014;27:317–319. doi: 10.1111/pcmr.12195. [DOI] [PubMed] [Google Scholar]
- 78.Wurm EM, Lin LL, Ferguson B, Lambie D, Prow TW, Walker GJ, et al. A blueprint for staging of murine melanocytic lesions based on the Cdk4 ( R24C/R24C ) ::Tyr-NRAS ( Q ) ( 61K ) model. Exp Dermatol. 2012;21:676–681. doi: 10.1111/j.1600-0625.2012.01543.x. [DOI] [PubMed] [Google Scholar]
- 79.Kittler H, Seltenheim M, Dawid M, Pehamberger H, Wolff K, Binder M. Frequency and characteristics of enlarging common melanocytic nevi. Arch Dermatol. 2000;136:316–320. doi: 10.1001/archderm.136.3.316. [DOI] [PubMed] [Google Scholar]
- 80.Menzies SW, Stevenson ML, Altamura D, Byth K. Variables predicting change in benign melanocytic nevi undergoing short-term dermoscopic imaging. Arch Dermatol. 2011;147:655–659. doi: 10.1001/archdermatol.2011.133. [DOI] [PubMed] [Google Scholar]
- 81.Jimenez-Gallo D, Albarran-Planelles C, Linares-Barrios M, Martinez-Rodriguez A, Baez-Perea JM. Eruptive melanocytic nevi in a patient undergoing treatment with sunitinib. JAMA Dermatol. 2013;149:624–626. doi: 10.1001/jamadermatol.2013.263. [DOI] [PubMed] [Google Scholar]
- 82.Alaibac M, Piaserico S, Rossi CR, Foletto M, Zacchello G, Carli P, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020–1022. doi: 10.1016/s0190-9622(03)02482-4. [DOI] [PubMed] [Google Scholar]
- 83.Uhlenhake EE, Watson AC, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:18184. [PubMed] [Google Scholar]
- 84.Soyer HP, Smolle J, Smolle-Juettner FM, Kerl H. Proliferation antigens in cutaneous melanocytic tumors--an immunohistochemical study comparing the transferrin receptor and the Ki 67 antigen. Dermatologica. 1989;179:3–9. doi: 10.1159/000248090. [DOI] [PubMed] [Google Scholar]
- 85.Bastian BC. The longer your telomeres, the larger your nevus? Am J Dermatopathol. 2003;25:83–84. [PubMed] [Google Scholar]
- 86.Miracco C, Margherita De Santi M, Schurfeld K, Santopietro R, Lalinga AV, Fimiani M, et al. Quantitative in situ evaluation of telomeres in fluorescence in situ hybridization-processed sections of cutaneous melanocytic lesions and correlation with telomerase activity. Br J Dermatol. 2002;146:399–408. doi: 10.1046/j.1365-2133.2002.04600.x. [DOI] [PubMed] [Google Scholar]
- 87.Calado RT, Dumitriu B. Telomere dynamics in mice and humans. Semin Hematol. 2013;50:165–174. doi: 10.1053/j.seminhematol.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bauer J, Garbe C. Acquired melanocytic nevi as risk factor for melanoma development. A comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297–306. doi: 10.1034/j.1600-0749.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 89.Oba J, Nakahara T, Abe T, Hagihara A, Moroi Y, Furue M. Expression of c-Kit, p-ERK and cyclin D1 in malignant melanoma: an immunohistochemical study and analysis of prognostic value. J Dermatol Sci. 2011;62:116–123. doi: 10.1016/j.jdermsci.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 90.Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Palmer AA, Zhang XD, et al. Activation of the extracellular signal regulated kinase (ERK) pathway in human melanoma. J Clin Pathol. 2005;58:1163–1169. doi: 10.1136/jcp.2005.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uribe P, Andrade L, Gonzalez S. Lack of association between BRAF mutation and MAPK ERK activation in melanocytic nevi. J Invest Dermatol. 2006;126:161–166. doi: 10.1038/sj.jid.5700011. [DOI] [PubMed] [Google Scholar]
- 92.McClenahan P, Lin LL, Tan JM, Flewell-Smith R, Schaider H, Jagirdar K, et al. BRAFV600E mutation status of involuting and stable nevi in dabrafenib therapy with or without trametinib. JAMA Dermatol. 2014;150:1079–1082. doi: 10.1001/jamadermatol.2014.436. [DOI] [PubMed] [Google Scholar]
- 93.Perier-Muzet M, Thomas L, Poulalhon N, Debarbieux S, Bringuier PP, Duru G, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351–1358. doi: 10.1038/jid.2013.462. [DOI] [PubMed] [Google Scholar]
- 94.Spain L, Julve M, Larkin J. Combination dabrafenib and trametinib in the management of advanced melanoma with BRAFV600 mutations. Expert Opin Pharmacother. 2016;17:1031–1038. doi: 10.1517/14656566.2016.1168805. [DOI] [PubMed] [Google Scholar]
- 95.Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kidger AM, Keyse SM. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs) Semin Cell Dev Biol. 2016;50:125–132. doi: 10.1016/j.semcdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Masoumi-Moghaddam S, Amini A, Morris DL. The developing story of Sprouty and cancer. Cancer Metastasis Rev. 2014;33:695–720. doi: 10.1007/s10555-014-9497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med. 2015;373:1926–1936. doi: 10.1056/NEJMoa1502583. [DOI] [PubMed] [Google Scholar]
- 99.LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res. 2014;12:167–183. doi: 10.1158/1541-7786.MCR-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee Y, Dominy JE, Choi YJ, Jurczak M, Tolliday N, Camporez JP, et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature. 2014;510:547–551. doi: 10.1038/nature13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maggi LB, Jr, Winkeler CL, Miceli AP, Apicelli AJ, Brady SN, Kuchenreuther MJ, et al. ARF tumor suppression in the nucleolus. Biochim Biophys Acta. 2014;1842:831–839. doi: 10.1016/j.bbadis.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 102.FitzGerald MG, Harkin DP, Silva-Arrieta S, MacDonald DJ, Lucchina LC, Unsal H, et al. Prevalence of germ-line mutations in p16, p19ARF, and CDK4 in familial melanoma: analysis of a clinic-based population. Proc Natl Acad Sci USA. 1996;93:8541–8545. doi: 10.1073/pnas.93.16.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamb A, Shattuck-Eidens D, Eeles R, Liu Q, Gruis NA, Ding W, et al. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat Genet. 1994;8:23–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- 104.Aoude LG, Wadt KA, Pritchard AL, Hayward NK. Genetics of familial melanoma: 20 years after CDKN2A. Pigment Cell Melanoma Res. 2015;28:148–160. doi: 10.1111/pcmr.12333. [DOI] [PubMed] [Google Scholar]
- 105.de Snoo FA, Hayward NK. Cutaneous melanoma susceptibility and progression genes. Cancer Lett. 2005;230:153–186. doi: 10.1016/j.canlet.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 106.Liu L, Lassam NJ, Slingerland JM, Bailey D, Cole D, Jenkins R, et al. Germline p16INK4A mutation and protein dysfunction in a family with inherited melanoma. Oncogene. 1995;11:405–412. [PubMed] [Google Scholar]
- 107.Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/ MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sviderskaya EV, Hill SP, Evans-Whipp TJ, Chin L, Orlow SJ, Easty DJ, et al. p16 (Ink4a) in melanocyte senescence and differentiation. J Natl Cancer Inst. 2002;94:446–454. doi: 10.1093/jnci/94.6.446. [DOI] [PubMed] [Google Scholar]
- 109.Talve L, Sauroja I, Collan Y, Punnonen K, Ekfors T. Loss of expression of the p16INK4/CDKN2 gene in cutaneous malignant melanoma correlates with tumor cell proliferation and invasive stage. Int J Cancer. 1997;74:255–259. doi: 10.1002/(sici)1097-0215(19970620)74:3<255::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 110.Radhi JM. Malignant melanoma arising from nevi, p53, p16, and Bcl-2: expression in benign versus malignant components. J Cutan Med Surg. 1999;3:293–297. doi: 10.1177/120347549900300603. [DOI] [PubMed] [Google Scholar]
- 111.Funk JO, Schiller PI, Barrett MT, Wong DJ, Kind P, Sander CA. p16INK4a expression is frequently decreased and associated with 9p21 loss of hetero-zygosity in sporadic melanoma. J Cutan Pathol. 1998;25:291–296. doi: 10.1111/j.1600-0560.1998.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 112.Karim RZ, Li W, Sanki A, Colman MH, Yang YH, Thompson JF, et al. Reduced p16 and increased cyclin D1 and pRb expression are correlated with progression in cutaneous melanocytic tumors. Int J Surg Pathol. 2009;17:361–367. doi: 10.1177/1066896909336177. [DOI] [PubMed] [Google Scholar]
- 113.Haferkamp S, Scurr LL, Becker TM, Frausto M, Kefford RF, Rizos H. Oncogene-induced senescence does not require the p16(INK4a) or p14ARF melanoma tumor suppressors. J Invest Dermatol. 2009;129:1983–1991. doi: 10.1038/jid.2009.5. [DOI] [PubMed] [Google Scholar]
- 114.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 115.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 116.Aird KM, Zhang G, Li H, Tu Z, Bitler BG, Garipov A, et al. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 2013;3:1252–1265. doi: 10.1016/j.celrep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bester AC, Roniger M, Oren YS, Im MM, Sarni D, Chaoat M, et al. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell. 2011;145:435–446. doi: 10.1016/j.cell.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mannava S, Moparthy KC, Wheeler LJ, Natarajan V, Zucker SN, Fink EE, et al. Depletion of deoxyribonucleotide pools is an endogenous source of DNA damage in cells undergoing oncogene-induced senescence. Am J Pathol. 2013;182:142–151. doi: 10.1016/j.ajpath.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 120.Tran SL, Haferkamp S, Scurr LL, Gowrishankar K, Becker TM, Desilva C, et al. Absence of distinguishing senescence traits in human melanocytic nevi. J Invest Dermatol. 2012;132:2226–2234. doi: 10.1038/jid.2012.126. [DOI] [PubMed] [Google Scholar]
- 121.Wasco MJ, Pu RT, Yu L, Su L, Ma L. Expression of gamma-H2AX in melanocytic lesions. Hum Pathol. 2008;39:1614–1620. doi: 10.1016/j.humpath.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 122.Nowsheen S, Yang ES. The intersection between DNA damage response and cell death pathways. Exp Oncol. 2012;34:243–254. [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X, Simpson ER, Brown KA. p53: Protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 2015;75:5001–5007. doi: 10.1158/0008-5472.CAN-15-0563. [DOI] [PubMed] [Google Scholar]
- 124.Viros A, Sanchez-Laorden B, Pedersen M, Furney SJ, Rae J, Hogan K, et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nature. 2014;511:478–482. doi: 10.1038/nature13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 126.Curran RC, McCann BG. The ultrastructure of benign pigmented naevi and melanocarcinomas in man. J Pathol. 1976;119:135–146. doi: 10.1002/path.1711190303. [DOI] [PubMed] [Google Scholar]
- 127.Stolz W, Abmayr W, Schmoeckel C, Landthaler M, Massoudy P, Braun-Falco O. Ultrastructural discrimination between malignant melanomas and benign nevocytic nevi using high-resolution image and multivariate analyses. J Invest Dermatol. 1991;97:903–910. doi: 10.1111/1523-1747.ep12491659. [DOI] [PubMed] [Google Scholar]
- 128.Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 129.Bandyopadhyay D, Curry JL, Lin Q, Richards HW, Chen D, Hornsby PJ, et al. Dynamic assembly of chromatin complexes during cellular senescence: implications for the growth arrest of human melanocytic nevi. Aging Cell. 2007;6:577–591. doi: 10.1111/j.1474-9726.2007.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kapoor A, Goldberg MS, Cumberland LK, Ratnakumar K, Segura MF, Emanuel PO, et al. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature. 2010;468:1105–1109. doi: 10.1038/nature09590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen H, Ruiz PD, McKimpson WM, Novikov L, Kitsis RN, Gamble MJ. MacroH2A1 and ATM play opposing roles in paracrine senescence and the senescence-associated secretory phenotype. Mol Cell. 2015;59:719–731. doi: 10.1016/j.molcel.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sarkar D, Leung EY, Baguley BC, Finlay GJ, Askarian-Amiri ME. Epigenetic regulation in human melanoma: past and future. Epigenetics. 2015;10:103–121. doi: 10.1080/15592294.2014.1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muthusamy V, Duraisamy S, Bradbury CM, Hobbs C, Curley DP, Nelson B, et al. Epigenetic silencing of novel tumor suppressors in malignant melanoma. Cancer Res. 2006;66:11187–11193. doi: 10.1158/0008-5472.CAN-06-1274. [DOI] [PubMed] [Google Scholar]
- 134.Walesch SK, Richter AM, Helmbold P, Dammann RH. Claudin11 promoter hypermethylation is frequent in malignant melanoma of the skin, but uncommon in nevus cell nevi. Cancers. 2015;7:1233–1243. doi: 10.3390/cancers7030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao L, van den Hurk K, Moerkerk PT, Goeman JJ, Beck S, Gruis NA, et al. Promoter CpG island hypermethylation in dysplastic nevus and melanoma: CLDN11 as an epigenetic biomarker for malignancy. J Invest Dermatol. 2014;134:2957–2966. doi: 10.1038/jid.2014.270. [DOI] [PubMed] [Google Scholar]
- 136.Helmbold P, Richter AM, Walesch S, Skorokhod A, Marsch W, Enk A, et al. RASSF10 promoter hypermethylation is frequent in malignant melanoma of the skin but uncommon in nevus cell nevi. J Invest Dermatol. 2012;132:687–694. doi: 10.1038/jid.2011.380. [DOI] [PubMed] [Google Scholar]
- 137.Conway K, Edmiston SN, Khondker ZS, Groben PA, Zhou X, Chu H, et al. DNA-methylation profiling distinguishes malignant melanomas from benign nevi. Pigment Cell Melanoma Res. 2011;24:352–360. doi: 10.1111/j.1755-148X.2011.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martinez-Cardus A, Vizoso M, Moran S, Manzano JL. Epigenetic mechanisms involved in melanoma pathogenesis and chemoresistance. Ann Transl Med. 2015;3:209. doi: 10.3978/j.issn.2305-5839.2015.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rodic N, Zampella J, Sharma R, Burns KH, Taube JM. Diagnostic utility of 5-hydroxymethylcytosine immunohistochemistry in melanocytic proliferations. J Cutan Pathol. 2015;42:807–814. doi: 10.1111/cup.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferreira Gomes CB, Zechin KG, Xu S, Stelini RF, Nishimoto IN, Zhan Q, et al. TET2 negatively regulates nestin expression in human melanoma. Am J Pathol. 2016;186:1427–1434. doi: 10.1016/j.ajpath.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Micevic G, Muthusamy V, Damsky W, Theodosakis N, Liu X, Meeth K, et al. DNMT3b modulates melanoma growth by controlling levels of mTORC2 component RICTOR. Cell Rep. 2016;14:2180–2192. doi: 10.1016/j.celrep.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kuzbicki L, Lange D, Straczynska-Niemiec A, Chwirot BW. JARID1B expression in human melanoma and benign melanocytic skin lesions. Melanoma Res. 2013;23:8–12. doi: 10.1097/CMR.0b013e32835d5d6f. [DOI] [PubMed] [Google Scholar]
- 144.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Held M, Bosenberg M. A role for the JARID1B stem cell marker for continuous melanoma growth. Pigment Cell Melanoma Res. 2010;23:481–483. doi: 10.1111/j.1755-148X.2010.00726.x. [DOI] [PubMed] [Google Scholar]
- 146.Ceol CJ, Houvras Y, Jane-Valbuena J, Bilodeau S, Orlando DA, Battisti V, et al. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–517. doi: 10.1038/nature09806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Macgregor S, Montgomery GW, Liu JZ, Zhao ZZ, Henders AK, Stark M, et al. Genome-wide association study identifies a new melanoma susceptibility locus at 1q21.3. Nat Genet. 2011;43:1114–1118. doi: 10.1038/ng.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kampilafkos P, Melachrinou M, Kefalopoulou Z, Lakoumentas J, Sotiropoulou-Bonikou G. Epigenetic modifications in cutaneous malignant melanoma: EZH2, H3K4me2, and H3K27me3 immunohistochemical expression is enhanced at the invasion front of the tumor. Am J Dermatopathol. 2015;37:138–144. doi: 10.1097/DAD.0b013e31828a2d54. [DOI] [PubMed] [Google Scholar]
- 151.Liu S, Tetzlaff MT, Liu A, Liegl-Atzwanger B, Guo J, Xu X. Loss of microRNA-205 expression is associated with melanoma progression. Lab Invest. 2012;92:1084–1096. doi: 10.1038/labinvest.2012.62. [DOI] [PubMed] [Google Scholar]
- 152.Xu Y, Brenn T, Brown ER, Doherty V, Melton DW. Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br J Cancer. 2012;106:553–561. doi: 10.1038/bjc.2011.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mannavola F, Tucci M, Felici C, Stucci S, Silvestris F. miRNAs in melanoma: a defined role in tumor progression and metastasis. Expert Rev Clin Immunol. 2016;12:79–89. doi: 10.1586/1744666X.2016.1100965. [DOI] [PubMed] [Google Scholar]
- 154.Montes M, Nielsen MM, Maglieri G, Jacobsen A, Hojfeldt J, Agrawal-Singh S, et al. The lncRNA MIR31HG regulates p16(INK4A) expression to modulate senescence. Nat Commun. 2015;6:6967. doi: 10.1038/ncomms7967. [DOI] [PubMed] [Google Scholar]
- 155.Montes M, Lund AH. Emerging roles of lncRNAs in senescence. FEBS J. 2016;283:2414–2426. doi: 10.1111/febs.13679. [DOI] [PubMed] [Google Scholar]
- 156.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 159.Campisi J. Replicative senescence: an old lives’ tale? Cell. 1996;84:497–500. doi: 10.1016/s0092-8674(00)81023-5. [DOI] [PubMed] [Google Scholar]
- 160.Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 161.Li M, Durbin KR, Sweet SM, Tipton JD, Zheng Y, Kelleher NL. Oncogene-induced cellular senescence elicits an anti-Warburg effect. Proteomics. 2013;13:2585–2596. doi: 10.1002/pmic.201200298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Perez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. 2014;14:547–558. doi: 10.1038/nrc3773. [DOI] [PubMed] [Google Scholar]
- 163.Quijano C, Cao L, Fergusson MM, Romero H, Liu J, Gutkind S, et al. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle. 2012;11:1383–1392. doi: 10.4161/cc.19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Christofk HR, Van der Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 165.Hitosugi T, Kang S, Van der Heiden MG, Chung TW, Elf S, Lythgoe K, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K, et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Populo H, Caldas R, Lopes JM, Pardal J, Maximo V, Soares P. Overexpression of pyruvate dehydrogenase kinase supports dichloroacetate as a candidate for cutaneous melanoma therapy. Expert Opin Ther Targets. 2015;19:733–745. doi: 10.1517/14728222.2015.1045416. [DOI] [PubMed] [Google Scholar]
- 168.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, metabolism, and cancer. Cancer Discov. 2015;5:1024–1039. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abildgaard C, Guldberg P. Molecular drivers of cellular metabolic reprogramming in melanoma. Trends Mol Med. 2015;21:164–171. doi: 10.1016/j.molmed.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 171.Zhuang D, Mannava S, Grachtchouk V, Tang WH, Patil S, Wawrzyniak JA, et al. C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene. 2008;27:6623–6634. doi: 10.1038/onc.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kraehn GM, Utikal J, Udart M, Greulich KM, Bezold G, Kaskel P, et al. Extra c-myc oncogene copies in high risk cutaneous malignant melanoma and melanoma metastases. Br J Cancer. 2001;84:72–79. doi: 10.1054/bjoc.2000.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Slominski A, Kim TK, Brozyna AA, Janjetovic Z, Brooks DL, Schwab LP, et al. The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1alpha expression and HIF-dependent attendant pathways. Arch Biochem Biophys. 2014;563:79–93. doi: 10.1016/j.abb.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hanna SC, Krishnan B, Bailey ST, Moschos SJ, Kuan PF, Shimamura T, et al. HIF1alpha and HIF2alpha independently activate SRC to promote melanoma metastases. J Clin Invest. 2013;123:2078–2093. doi: 10.1172/JCI66715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC, et al. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell. 2013;23:302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McArthur GA, Puzanov I, Amaravadi R, Ribas A, Chapman P, Kim KB, et al. Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J Clin Oncol. 2012;30:1628–1634. doi: 10.1200/JCO.2011.39.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Theodosakis N, Held MA, Marzuka-Alcala A, Meeth KM, Micevic G, Long GV, et al. BRAF inhibition decreases cellular glucose uptake in melanoma in association with reduction in cell volume. Mol Cancer Ther. 2015;14:1680–1692. doi: 10.1158/1535-7163.MCT-15-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 179.Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 181.Gerland LM, Peyrol S, Lallemand C, Branche R, Magaud JP, Ffrench M. Association of increased autophagic inclusions labeled for beta-galactosidase with fibroblastic aging. Exp Gerontol. 2003;38:887–895. doi: 10.1016/s0531-5565(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 182.Meng XX, Yao M, Zhang XD, Xu HX, Dong Q. ER stress-induced autophagy in melanoma. Clin Exp Pharmacol Physiol. 2015;42:811–816. doi: 10.1111/1440-1681.12436. [DOI] [PubMed] [Google Scholar]
- 183.Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014;124:1406–1417. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 185.Liu H, He Z, von Rutte T, Yousefi S, Hunger RE, Simon HU. Down-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Sci Transl Med. 2013;5:202ra123. doi: 10.1126/scitranslmed.3005864. [DOI] [PubMed] [Google Scholar]
- 186.Liu H, He Z, Simon HU. Autophagy suppresses melanoma tumorigenesis by inducing senescence. Autophagy. 2014;10:372–373. doi: 10.4161/auto.27163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miracco C, Cevenini G, Franchi A, Luzi P, Cosci E, Mourmouras V, et al. Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Hum Pathol. 2010;41:503–512. doi: 10.1016/j.humpath.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 188.Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18:370–379. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lazova R, Klump V, Pawelek J. Autophagy in cutaneous malignant melanoma. J Cutan Pathol. 2010;37:256–268. doi: 10.1111/j.1600-0560.2009.01359.x. [DOI] [PubMed] [Google Scholar]
- 190.Goovaerts G, Buyssens N. Nevus cell maturation or atrophy? Am J Dermatopathol. 1988;10:20–27. doi: 10.1097/00000372-198802000-00003. [DOI] [PubMed] [Google Scholar]
- 191.Ivanov A, Pawlikowski J, Manoharan I, van Tuyn J, Nelson DM, Rai TS, et al. Lysosome-mediated processing of chromatin in senescence. J Cell Biol. 2013;202:129–143. doi: 10.1083/jcb.201212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pluquet O, Pourtier A, Abbadie C. The unfolded protein response, cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol. 2015;308:C415–C425. doi: 10.1152/ajpcell.00334.2014. [DOI] [PubMed] [Google Scholar]
- 193.Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8:1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- 194.Zhuang L, Scolyer RA, Lee CS, McCarthy SW, Cooper WA, Zhang XD, et al. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–470. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 195.Perez LJ, Penas PF, Atienzar M, Garcia-Diez A. Implication of MT1-MMP in the maturation steps of benign melanocytic nevi. J Cutan Pathol. 2006;33:139–144. doi: 10.1111/j.0303-6987.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 196.Van Duinen CM, Fleuren GJ, Bruijn JA. The extracellular matrix in pigmented skin lesions: an immunohistochemical study. Histopathology. 1994;24:33–40. doi: 10.1111/j.1365-2559.1994.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 197.Nikitovic D, Mytilinaiou M, Berdiaki A, Karamanos NK, Tzanakakis GN. Heparan sulfate proteoglycans and heparin regulate melanoma cell functions. Biochim Biophys Acta. 2014;1840:2471–2481. doi: 10.1016/j.bbagen.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 198.Godwin LS, Castle JT, Kohli JS, Goff PS, Cairney CJ, Keith WN, et al. Isolation, culture, and transfection of melanocytes. Curr Protoc Cell Biol. 2014;63:1–20. doi: 10.1002/0471143030.cb0108s63. 1. 8. [DOI] [PubMed] [Google Scholar]
- 199.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 202.Katlinskaya YV, Katlinski KV, Yu Q, Ortiz A, Beiting DP, Brice A, et al. Suppression of type I interferon signaling overcomes oncogene-induced senescence and mediates melanoma development and progression. Cell Rep. 2016;15:171–180. doi: 10.1016/j.celrep.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Scurr LL, Pupo GM, Becker TM, Lai K, Schrama D, Haferkamp S, et al. IGFBP7 is not required for B-RAF-induced melanocyte senescence. Cell. 2010;141:717–727. doi: 10.1016/j.cell.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 205.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Role for IGFBP7 in senescence induction by BRAF. Cell. 2010;141:746–747. doi: 10.1016/j.cell.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M, et al. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal. 2012;5:ra92. doi: 10.1126/scisignal.2003184. [DOI] [PubMed] [Google Scholar]
- 207.Kanter-Lewensohn L, Dricu A, Girnita L, Wejde J, Larsson O. Expression of insulinlike growth factor-1 receptor (IGF-1R) and p27Kip1 in melanocytic tumors: a potential regulatory role of IGF-1 pathway in distribution of p27Kip1 between different cyclins. Growth Factors. 2000;17:193–202. doi: 10.3109/08977190009001068. [DOI] [PubMed] [Google Scholar]
- 208.Ahmed AA, Nordlind K, Hedblad M, Lagerholm B, Schultzberg M, Liden S. Interleukin (IL)-1 alpha- and −1 beta-, IL-6-, and tumor necrosis factor-alpha-like immunoreactivities in human common and dysplastic nevocellular nevi and malignant melanoma. Am J Dermatopathol. 1995;17:222–229. doi: 10.1097/00000372-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 209.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386:74–84. doi: 10.1016/S0140-6736(14)60763-7. [DOI] [PubMed] [Google Scholar]
- 210.Sanchez-Sosa S, Aguirre-Lombardo M, Jimenez-Brito G, Ruiz-Arguelles A. Immunophenotypic characterization of lymphoid cell infiltrates in vitiligo. Clin Exp Immunol. 2013;173:179–183. doi: 10.1111/cei.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aouthmany M, Weinstein M, Zirwas MJ, Brodell RT. The natural history of halo nevi: a retrospective case series. J Am Acad Dermatol. 2012;67:582–586. doi: 10.1016/j.jaad.2011.11.937. [DOI] [PubMed] [Google Scholar]
- 212.Zeff RA, Freitag A, Grin CM, Grant-Kels JM. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620–624. doi: 10.1016/s0190-9622(97)70181-6. [DOI] [PubMed] [Google Scholar]
- 213.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19:275–282. doi: 10.1097/CMR.0b013e32832eabd5. [DOI] [PubMed] [Google Scholar]
- 215.Morton D, Eilber FR, Malmgren RA, Wood WC. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery. 1970;68:158–163. [PubMed] [Google Scholar]
- 216.Gutterman JU, Mavligit G, McBride C, Frei E3rd, Freireich EJ, Hersh EM. Active immunotherapy with B.C.G for recurrent malignant melanoma. Lancet. 1973;1:1208–1212. doi: 10.1016/s0140-6736(73)90526-6. [DOI] [PubMed] [Google Scholar]
- 217.Baker MA, Taub RN. BCG in malignant melanoma. Lancet. 1973;1:1117–1118. doi: 10.1016/s0140-6736(73)90423-6. [DOI] [PubMed] [Google Scholar]
- 218.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 219.Ascierto ML, Melero I, Ascierto PA. Melanoma: from incurable beast to a curable bet the success of immunotherapy. Front Oncol. 2015;5:152. doi: 10.3389/fonc.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Libon F, Arrese JE, Rorive A, Nikkels AF. Ipilimumab induces simultaneous regression of melanocytic naevi and melanoma metastases. Clin Exp Dermatol. 2013;38:276–279. doi: 10.1111/j.1365-2230.2012.04452.x. [DOI] [PubMed] [Google Scholar]
- 221.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 222.Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol. 2015;135:2657–2665. doi: 10.1038/jid.2015.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Brewer JD, Shanafelt TD, Call TG, Cerhan JR, Roenigk RK, Weaver AL, et al. Increased incidence of malignant melanoma and other rare cutaneous cancers in the setting of chronic lymphocytic leukemia. Int J Dermatol. 2015;54:e287–e293. doi: 10.1111/ijd.12564. [DOI] [PubMed] [Google Scholar]
- 224.Famenini S, Martires KJ, Zhou H, Xavier MF, Wu JJ. Melanoma in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. J Am Acad Dermatol. 2015;72:78–84. doi: 10.1016/j.jaad.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 225.Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–519. [PubMed] [Google Scholar]
- 226.Matin RN, Mesher D, Proby CM, McGregor JM, Bouwes Bavinck JN, del Marmol V, et al. Melanoma in organ transplant recipients: clinicopathological features and outcome in 100 cases. Am J Transplant. 2008;8:1891–1900. doi: 10.1111/j.1600-6143.2008.02326.x. [DOI] [PubMed] [Google Scholar]
- 227.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 228.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D–dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hussein MR, Elsers DA, Fadel SA, Omar AE. Immunohistological characterisation of tumour infiltrating lymphocytes in melanocytic skin lesions. J Clin Pathol. 2006;59:316–324. doi: 10.1136/jcp.2005.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lyle S, Salhany KE, Elder DE. TIA-1 positive tumor-infiltrating lymphocytes in nevi and melanomas. Mod Pathol. 2000;13:52–55. doi: 10.1038/modpathol.3880009. [DOI] [PubMed] [Google Scholar]
- 232.Kang S, Barnhill RL, Mihm MC, Jr, Sober AJ. Histologic regression in malignant melanoma: an interobserver concordance study. J Cutan Pathol. 1993;20:126–129. doi: 10.1111/j.1600-0560.1993.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 233.Botella-Estrada R, Kutzner H. Study of the immunophenotype of the inflammatory cells in melanomas with regression and halo nevi. Am J Dermatopathol. 2015;37:376–380. doi: 10.1097/DAD.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 234.Romano E, Romero P. The therapeutic promise of disrupting the PD-1/PD-L1 immune checkpoint in cancer: unleashing the CD8 T cell mediated anti-tumor activity results in significant, unprecedented clinical efficacy in various solid tumors. J Immunother Cancer. 2015;3:15. doi: 10.1186/s40425-015-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bastian BC. Hypothesis: a role for telomere crisis in spontaneous regression of melanoma. Arch Dermatol. 2003;139:667–668. doi: 10.1001/archderm.139.5.667. [DOI] [PubMed] [Google Scholar]
- 236.Bastian BC. Understanding the progression of melanocytic neoplasia using genomic analysis: from fields to cancer. Oncogene. 2003;22:3081–3086. doi: 10.1038/sj.onc.1206463. [DOI] [PubMed] [Google Scholar]
- 237.Pathak S, Multani AS, McConkey DJ, Imam AS, Amoss MS., Jr Spontaneous regression of cutaneous melanoma in sinclair swine is associated with defective telomerase activity and extensive telomere erosion. Int J Oncol. 2000;17:1219–1224. doi: 10.3892/ijo.17.6.1219. [DOI] [PubMed] [Google Scholar]
- 238.Yu Q, Katlinskaya YV, Carbone CJ, Zhao B, Katlinski KV, Zheng H, et al. DNA-damage-induced type I interferon promotes senescence and inhibits stem cell function. Cell Rep. 2015;11:785–797. doi: 10.1016/j.celrep.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mocellin S, Lens MB, Pasquali S, Pilati P, Chiarion Sileni V. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev. 2013;6:CD008955. doi: 10.1002/14651858.CD008955.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 242.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–137. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 243.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, et al. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 244.Wang T, Feldman GM, Herlyn M, Kaufman RE. The macrophage: Switches from a passenger to a driver during anticancer therapy. Oncoimmunology. 2015;4:e1052929. doi: 10.1080/2162402X.2015.1052929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vagner J, Steiniche T, Stougaard M. In-situ hybridization-based quantification of hTR: a possible biomarker in malignant melanoma. Histopathology. 2015;66:747–751. doi: 10.1111/his.12501. [DOI] [PubMed] [Google Scholar]
- 246.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 247.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Glaessl A, Bosserhoff AK, Buettner R, Hohenleutner U, Landthaler M, Stolz W. Increase in telomerase activity during progression of melanocytic cells from melanocytic naevi to malignant melanomas. Arch Dermatol Res. 1999;291:81–87. doi: 10.1007/s004030050387. [DOI] [PubMed] [Google Scholar]
- 249.Rudolph P, Schubert C, Tamm S, Heidorn K, Hauschild A, Michalska I, et al. Telomerase activity in melanocytic lesions: A potential marker of tumor biology. Am J Pathol. 2000;156:1425–1432. doi: 10.1016/S0002-9440(10)65011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ramirez RD, D’Atri S, Pagani E, Faraggiana T, Lacal PM, Taylor RS, et al. Progressive increase in telomerase activity from benign melanocytic conditions to malignant melanoma. Neoplasia. 1999;1:42–49. doi: 10.1038/sj.neo.7900004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Heidenreich B, Rachakonda PS, Hemminki K, Kumar R. TERT promoter mutations in cancer development. Curr Opin Genet Dev. 2014;24:30–37. doi: 10.1016/j.gde.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 252.Lee S, Opresko P, Pappo A, Kirkwood JM, Bahrami A. Association of TERT promoter mutations with telomerase expression in melanoma. Pigment Cell Melanoma Res. 2016;29:391–393. doi: 10.1111/pcmr.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Koh CM, Khattar E, Leow SC, Liu CY, Muller J, Ang WX, et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J Clin Invest. 2015;125:2109–2122. doi: 10.1172/JCI79134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Maida Y, Masutomi K. Telomerase reverse transcriptase moonlights: Therapeutic targets beyond telomerase. Cancer Sci. 2015;106:1486–1492. doi: 10.1111/cas.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Suram A, Kaplunov J, Patel PL, Ruan H, Cerutti A, Boccardi V, et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, et al. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet. 2014;46:478–481. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shi J, Yang XR, Ballew B, Rotunno M, Calista D, Fargnoli MC, et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet. 2014;46:482–486. doi: 10.1038/ng.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Aoude LG, Pritchard AL, Robles-Espinoza CD, Wadt K, Harland M, Choi J, et al. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J Natl Cancer Inst. 2015;107:1–7. doi: 10.1093/jnci/dju408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Harland M, Petljak M, Robles-Espinoza CD, Ding Z, Gruis NA, van Doorn R, et al. Germline TERT promoter mutations are rare in familial melanoma. Fam Cancer. 2016;15:139–144. doi: 10.1007/s10689-015-9841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bermudez Brito M, Goulielmaki E, Papakonstanti EA. Focus on PTEN regulation. Front Oncol. 2015;5:166. doi: 10.3389/fonc.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Scortegagna M, Lau E, Zhang T, Feng Y, Sereduk C, Yin H, et al. PDK1 and SGK3 contribute to the growth of BRAF-mutant melanomas and are potential therapeutic targets. Cancer Res. 2015;75:1399–1412. doi: 10.1158/0008-5472.CAN-14-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Scortegagna M, Ruller C, Feng Y, Lazova R, Kluger H, Li JL, et al. Genetic inactivation or pharmacological inhibition of Pdk1 delays development and inhibits metastasis of Braf(V600E)::Pten(−/−) melanoma. Oncogene. 2014;33:4330–4339. doi: 10.1038/onc.2013.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mirza AM, Gysin S, Malek N, Nakayama K, Roberts JM, McMahon M. Cooperative regulation of the cell division cycle by the protein kinases RAF and AKT. Mol Cell Biol. 2004;24:10868–10881. doi: 10.1128/MCB.24.24.10868-10881.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–3439. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 270.Chen B, Tardell C, Higgins B, Packman K, Boylan JF, Niu H. BRAFV600E negatively regulates the AKT pathway in melanoma cell lines. PLoS One. 2012;7:e42598. doi: 10.1371/journal.pone.0042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, et al. Abrogation of BRAFV600E–induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, et al. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell. 2011;42:36–49. doi: 10.1016/j.molcel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Deichmann M, Thome M, Benner A, Egner U, Hartschuh W, Naher H. PTEN/ MMAC1 expression in melanoma resection specimens. Br J Cancer. 2002;87:1431–1436. doi: 10.1038/sj.bjc.6600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tsao H, Mihm MC, Jr, Sheehan C. PTEN expression in normal skin, acquired melanocytic nevi, and cutaneous melanoma. J Am Acad Dermatol. 2003;49:865–872. doi: 10.1016/s0190-9622(03)02473-3. [DOI] [PubMed] [Google Scholar]
- 275.Zhou XP, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol. 2000;157:1123–1128. doi: 10.1016/S0002-9440(10)64627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Whiteman DC, Zhou XP, Cummings MC, Pavey S, Hayward NK, Eng C. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer. 2002;99:63–67. doi: 10.1002/ijc.10294. [DOI] [PubMed] [Google Scholar]
- 277.Roh MR, Gupta S, Park KH, Chung KY, Lauss M, Flaherty KT, et al. Promoter methylation of PTEN is a significant prognostic factor in melanoma survival. J Invest Dermatol. 2016;136:1002–1011. doi: 10.1016/j.jid.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 278.Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002;62:7335–7342. [PubMed] [Google Scholar]
- 279.Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473–1482. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 280.Kantrow SM, Boyd AS, Ellis DL, Nanney LB, Richmond A, Shyr Y, et al. Expression of activated Akt in benign nevi, Spitz nevi and melanomas. J Cutan Pathol. 2007;34:593–596. doi: 10.1111/j.1600-0560.2006.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 282.Liko D, Hall MN. mTOR in health and in sickness. J Mol Med. 2015;93:1061–1073. doi: 10.1007/s00109-015-1326-7. [DOI] [PubMed] [Google Scholar]
- 283.Laugier F, Finet-Benyair A, Andre J, Rachakonda PS, Kumar R, Bensussan A, et al. RICTOR involvement in the PI3K/AKT pathway regulation in melanocytes and melanoma. Oncotarget. 2015;6:28120–28131. doi: 10.18632/oncotarget.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Karbowniczek M, Spittle CS, Morrison T, Wu H, Henske EP. mTOR is activated in the majority of malignant melanomas. J Invest Dermatol. 2008;128:980–987. doi: 10.1038/sj.jid.5701074. [DOI] [PubMed] [Google Scholar]
- 285.Khosravi S, Tam KJ, Ardekani GS, Martinka M, McElwee KJ, Ong CJ. eIF4E is an adverse prognostic marker of melanoma patient survival by increasing melanoma cell invasion. J Invest Dermatol. 2015;135:1358–1367. doi: 10.1038/jid.2014.552. [DOI] [PubMed] [Google Scholar]
- 286.Masui K, Cavenee WK, Mischel PS. mTORC2 in the center of cancer metabolic reprogramming. Trends Endocrinol Metab. 2014;25:364–373. doi: 10.1016/j.tem.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–2401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, et al. beta-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20:741–754. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Juan J, Muraguchi T, Iezza G, Sears RC, McMahon M. Diminished WNT −4 beta-catenin −4 c-MYC signaling is a barrier for malignant progression of BRAFV600E–induced lung tumors. Genes Dev. 2014;28:561–575. doi: 10.1101/gad.233627.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER. Frequent nuclear/cytoplasmic localization of beta-catenin without exon 3 mutations in malignant melanoma. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Duffy K, Grossman D. The dysplastic nevus: from historical perspective to management in the modern era: part I. Historical, histologic, and clinical aspects. J Am Acad Dermatol. 2012;67:e1–16. doi: 10.1016/j.jaad.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Duffy K, Grossman D. The dysplastic nevus: from historical perspective to management in the modern era: part II. Molecular aspects and clinical management. J Am Acad Dermatol. 2012;67:19. doi: 10.1016/j.jaad.2012.03.013. e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Annessi G, Cattaruzza MS, Abeni D, Baliva G, Laurenza M, Macchini V, et al. Correlation between clinical atypia and histologic dysplasia in acquired melanocytic nevi. J Am Acad Dermatol. 2001;45:77–85. doi: 10.1067/mjd.2001.114580. [DOI] [PubMed] [Google Scholar]
- 297.Rhodes AR, Harrist TJ, Day CL, Mihm MC, Jr, Fitzpatrick TB, Sober AJ. Dysplastic melanocytic nevi in histologic association with 234 primary cutaneous melanomas. J Am Acad Dermatol. 1983;9:563–574. doi: 10.1016/s0190-9622(83)70171-4. [DOI] [PubMed] [Google Scholar]
- 298.Sagebiel RW. Melanocytic nevi in histologic association with primary cutaneous melanoma of superficial spreading and nodular types: effect of tumor thickness. J Invest Dermatol. 1993;100:322S–325S. doi: 10.1111/1523-1747.ep12470218. [DOI] [PubMed] [Google Scholar]
- 299.Hastrup N, Osterlind A, Drzewiecki KT, Hou-Jensen K. The presence of dysplastic nevus remnants in malignant melanomas. A population-based study of 551 malignant melanomas. Am J Dermatopathol. 1991;13:378–385. doi: 10.1097/00000372-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 300.Black WC. Residual dysplastic other nevi in superficial spreading melanoma. Clinical correlations and association with sun damage. Cancer. 1988;62:163–173. doi: 10.1002/1097-0142(19880701)62:1<163::aid-cncr2820620126>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 301.Goodson AG, Florell SR, Boucher KM, Grossman D. A decade of melanomas: identification of factors associated with delayed detection in an academic group practice. Dermatol Surg. 2011;37:1620–1630. doi: 10.1111/j.1524-4725.2011.02097.x. [DOI] [PubMed] [Google Scholar]
- 302.Papp T, Schipper H, Kumar K, Schiffmann D, Zimmermann R. Mutational analysis of the BRAF gene in human congenital and dysplastic melanocytic naevi. Melanoma Res. 2005;15:401–407. doi: 10.1097/00008390-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 303.Saroufim M, Habib R, Karram S, Youssef Massad C, Taraif S, Loya A, et al. BRAF analysis on a spectrum of melanocytic neoplasms: an epidemiological study across differing UV regions. Am J Dermatopathol. 2014;36:68–73. doi: 10.1097/DAD.0b013e318293f355. [DOI] [PubMed] [Google Scholar]
- 304.Arumi-Uria M, McNutt NS, Finnerty B. Grading of atypia in nevi: correlation with melanoma risk. Mod Pathol. 2003;16:764–771. doi: 10.1097/01.MP.0000082394.91761.E5. [DOI] [PubMed] [Google Scholar]
- 305.Ahmed I, Piepkorn MW, Rabkin MS, Meyer LJ, Feldkamp M, Goldgar DE, et al. Histopathologic characteristics of dysplastic nevi. Limited association of conventional histologic criteria with melanoma risk group. J Am Acad Dermatol. 1990;22:727–733. [PubMed] [Google Scholar]
- 306.Michaloglou C, Vredeveld LC, Mooi WJ, Peeper DS. BRAF(E600) in benign and malignant human tumours. Oncogene. 2008;27:877–895. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- 307.Lucas CR, Sanders LL, Murray JC, Myers SA, Hall RP, Grichnik JM. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663–671. doi: 10.1067/mjd.2003.283. [DOI] [PubMed] [Google Scholar]
- 308.Goodson AG, Florell SR, Hyde M, Bowen GM, Grossman D. Comparative analysis of total body and dermatoscopic photographic monitoring of nevi in similar patient populations at risk for cutaneous melanoma. Dermatol Surg. 2010;36:1087–1098. doi: 10.1111/j.1524-4725.2010.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Tschandl P, Berghoff AS, Preusser M, Pammer J, Pehamberger H, Kittler H. Impact of oncogenic BRAF mutations and p16 expression on the growth rate of early melanomas and naevi in vivo. Br J Dermatol. 2016;174:364–370. doi: 10.1111/bjd.14323. [DOI] [PubMed] [Google Scholar]
- 310.Zampino MR, Corazza M, Costantino D, Mollica G, Virgili A. Are melanocytic nevi influenced by pregnancy? A dermoscopic evaluation. Dermatol Surg. 2006;32:1497–1504. doi: 10.1111/j.1524-4725.2006.32362.x. [DOI] [PubMed] [Google Scholar]
- 311.Rubegni P, Sbano P, Burroni M, Cevenini G, Bocchi C, Severi FM, et al. Melanocytic skin lesions and pregnancy: digital dermoscopy analysis. Skin Res Technol. 2007;13:143–147. doi: 10.1111/j.1600-0846.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- 312.Pennoyer JW, Grin CM, Driscoll MS, Dry SM, Walsh SJ, Gelineau JP, et al. Changes in size of melanocytic nevi during pregnancy. J Am Acad Dermatol. 1997;36:378–382. doi: 10.1016/s0190-9622(97)80212-5. [DOI] [PubMed] [Google Scholar]
- 313.Lee HJ, Ha SJ, Lee SJ, Kim JW. Melanocytic nevus with pregnancy-related changes in size accompanied by apoptosis of nevus cells: a case report. J Am Acad Dermatol. 2000;42:936–938. doi: 10.1016/s0190-9622(00)90277-9. [DOI] [PubMed] [Google Scholar]
- 314.Chan MP, Chan MM, Tahan SR. Melanocytic nevi in pregnancy: histologic features and Ki-67 proliferation index. J Cutan Pathol. 2010;37:843–851. doi: 10.1111/j.1600-0560.2009.01491.x. [DOI] [PubMed] [Google Scholar]
- 315.Rudolph P, Tronnier M, Menzel R, Moller M, Parwaresch R. Enhanced expression of Ki-67, topoisomerase IIalpha, PCNA, p53 and p21WAF1/Cip1 reflecting proliferation and repair activity in UV-irradiated melanocytic nevi. Hum Pathol. 1998;29:1480–1487. doi: 10.1016/s0046-8177(98)90019-3. [DOI] [PubMed] [Google Scholar]
- 316.Tronnier M, Rudolph P, Koser T, Raasch B, Brinckmann J. One single erythemagenic UV irradiation is more effective in increasing the proliferative activity of melanocytes in melanocytic naevi compared with fractionally applied high doses. Br J Dermatol. 1997;137:534–539. doi: 10.1111/j.1365-2133.1997.tb03782.x. [DOI] [PubMed] [Google Scholar]
- 317.King R, Hayzen BA, Page RN, Googe PB, Zeagler D, Mihm MC., Jr Recurrent nevus phenomenon: a clinicopathologic study of 357 cases and histologic comparison with melanoma with regression. Mod Pathol. 2009;22:611–617. doi: 10.1038/modpathol.2009.22. [DOI] [PubMed] [Google Scholar]
- 318.Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842–846. doi: 10.5858/2010-0429-RAR.1. [DOI] [PubMed] [Google Scholar]
- 319.Herlyn M, Thurin J, Balaban G, Bennicelli JL, Herlyn D, Elder DE, et al. Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res. 1985;45:5670–5676. [PubMed] [Google Scholar]
- 320.Herlyn M, Clark WH, Rodeck U, Mancianti ML, Jambrosic J, Koprowski H. Biology of tumor progression in human melanocytes. Lab Invest. 1987;56:461–474. [PubMed] [Google Scholar]
- 321.Mancianti ML, Herlyn M, Weil D, Jambrosic J, Rodeck U, Becker D, et al. Growth and phenotypic characteristics of human nevus cells in culture. J Invest Dermatol. 1988;90:134–141. doi: 10.1111/1523-1747.ep12462099. [DOI] [PubMed] [Google Scholar]
- 322.O’Rourke EA, Balzer B, Barry CI, Frishberg DP. Nevic mitoses: a review of 1041 cases. Am J Dermatopathol. 2013;35:30–33. doi: 10.1097/DAD.0b013e3182587ef8. [DOI] [PubMed] [Google Scholar]
- 323.Glatz K, Hartmann C, Antic M, Kutzner H. Frequent mitotic activity in banal melanocytic nevi uncovered by immunohistochemical analysis. Am J Dermatopathol. 2010;32:643–649. doi: 10.1097/DAD.0b013e3181d7ce6f. [DOI] [PubMed] [Google Scholar]
- 324.Nasr MR, El-Zammar O. Comparison of pHH3, Ki-67, and survivin immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopathol. 2008;30:117–122. doi: 10.1097/DAD.0b013e3181624054. [DOI] [PubMed] [Google Scholar]
- 325.Florell SR, Bowen AR, Hanks AN, Murphy KJ, Grossman D. Proliferation, apoptosis, and survivin expression in a spectrum of melanocytic nevi. J Cutan Pathol. 2005;32:45–49. doi: 10.1111/j.0303-6987.2005.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sprecher E, Bergman R, Meilick A, Kerner H, Manov L, Reiter I, et al. Apoptosis, Fas and Fas-ligand essssxpression in melanocytic tumors. J Cutan Pathol. 1999;26:72–77. doi: 10.1111/j.1600-0560.1999.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 327.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]