Abstract
In recent years, genomic, animal and cell biology studies have implicated deficiencies in retromer-mediated trafficking of proteins in an increasing number of neurodegenerative diseases including Alzheimer’s Disease (AD), Parkinson’s Disease (PD) and Frontotemporal Lobar Degener-ation (FTLD). The retromer complex, which is highly conserved across all eukaryotes, regulates the sorting of transmembrane proteins out of endo-somes to the cell surface or to the trans-Golgi network. Within retromer, cargo selection and binding are performed by a trimer of the Vps26, Vps29 and Vps35 proteins, named the “Cargo-Selective Complex (CSC)”. Sorting of cargo into tubules for distribution to the trans-Golgi network or the cell sur-face is achieved through the dimeric sorting nexin (SNX) component of retromer and accessory proteins such as the WASH complex which medi-ates the formation of discrete endosomal tubules enabling the sorting of cargo into distinct pathways through production of filamentous actin patch-es. In the present article, we review the molecular structure and function of the retromer and summarize the evidence linking retromer dysfunction to neurodegenerative disease.
Keywords: Retromer, Wash complex, Intracellular trafficking, Alzheimer’s disease, Genomics, Cell biology
1. INTRODUCTION
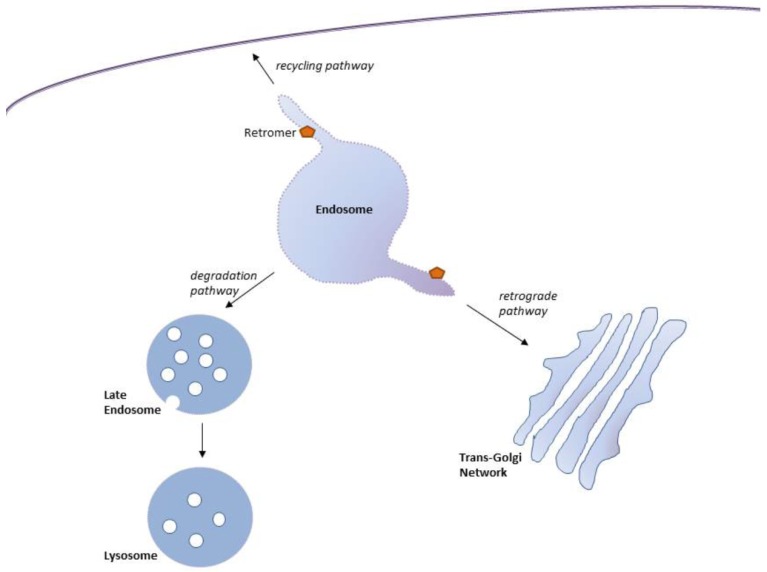
Eukaryotic cells comprise numerous organelles vital for cellular function. To maintain the function of these organelles, cellular homeostasis - achieved through the efficient sorting of specific proteins through the individual intracellular compartments - must be maintained. A crucial sorting machinery organizing the intracellular trafficking of proteins in eukaryotic cells is the endosomal network, a series of linked membrane-bound compartments. A transmembrane protein enters the Early Endosome (EE) - a dynamic tubular-vesicular membranous compartment enriched in phosphatidylinositol 3-monophosphate (PtdIns(3)P) [1, 2] - through endocytosis from the plasma membrane or through trafficking from the Trans-Golgi Network (TGN) [3, 4]. Once in the EE, a protein can either be recycled for reuse or be retained for lysosomal degradation (Fig. 1). Unbalanced sorting of proteins into recycling or degrading pathways can lead to cellular dysfunction and disease. Central to the recycling of cargo proteins from endosomes to the plasma membrane and the TGN is the retromer, a heteropentameric protein complex linked to the endosomal membrane (Fig. 1). In the present article, we review the morphology and function of the retromer and summarize the rapidly growing evidence linking the retromer to loss of cellular homeostasis and neurodegenerative disease.
Fig. (1).
Endosomal transport routes mediated by retromer. Retromer-mediates protein trafficking out of the endosome via the retrograde pathway to the Trans-Golgi Network (TGN), or through the recycling pathway back to the cell surface. The degradation pathway, through which cargo is trafficked from endosomes to lysosomes for degradation, is not mediated by retromer.
2. COMPOSITION OF THE RETROMER COMPLEX
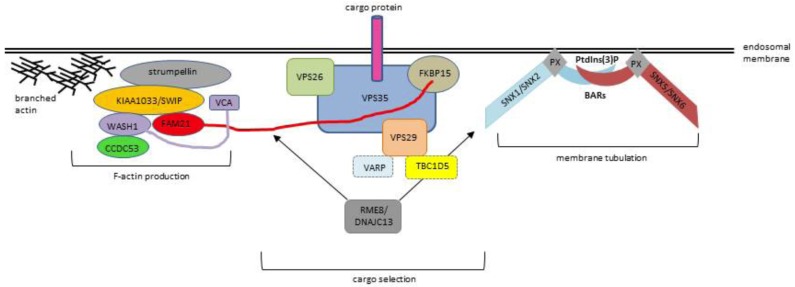
The retromer complex is composed of two main sub-components, the Cargo Selective Complex (CSC) and the SNX-BAR complex. Retromer was first identified in Saccharomyces cerevisiae as a system essential for trafficking the vacuolar protein sorting 10 protein (Vps10p) receptor, a protein transporting soluble acid hydrolases to the yeast vacuole, between the endosomes and the TGN [5, 6]. In yeast, the CSC which recognizes, selects and binds cargo proteins, [6] is composed of a heterotrimer of Vps35-Vps29-Vps26 proteins. A dimer of sorting nexin (SNX) proteins Vps5 and Vps17 [5-7] containing Bin/Amphiphysin/Rvs (BAR) domains constitutes the SNX-BAR complex which induces tubulation of the endosomal membrane [8, 9] thereby enabling distinct trafficking of cargo to the cell surface or the TGN. The Vps35-Vps29-Vps26 CSC is highly conserved between species [10], although as opposed to yeast, in mammals Vps26 is expressed as two orthologs, (Vps26a and Vps26b) [11]. Whether Vps26a and Vps26b form distinct cargo recognition cores [12] or exert a similar function [13] remains to be clarified. How exactly the CSC recognizes cargo, has yet to be disentangled but as both VPS35 and VPS26 are globular, it has been suggested that the cytoplasmic tails of transmembrane cargo proteins play a prominent role in recognition by the CSC [14]. The SNX-BAR complex differs between species: SNX1 and SNX2 are the mammalian homologs of Vps5; SNX5 and SNX6 (and possibly SNX32) are homologs of Vps17. Any combination of SNX1 or SNX2 with SNX5 or SNX6 forms the mammalian heterodimeric subcomplex [15, 16]. The mammalian retromer also functions as a hub for recruiting accessory proteins to endosomal membranes that are crucial to the regulation of membrane tubule dynamics [17], including the WASH complex (consisting of FAM21, strumpellin, KIAA1033 (SWIP) and WASH1), EHD1, FKBP15 and TBC1D5 (see below). Fig. (2) shows a schematic diagram of the retromer complex and associated factors in endosomal protein sorting.
Fig. (2).
Schematic depiction of the role of the retromer complex in mediating endosomal protein sorting. Recruitment and stabilization of the association of retromer to the endosomal membrane is mediated through bivalent recognition of SNX3 and the GTPases RAB5 and RAB7 by Vps35 (not shown).
In mammals, the family of SNX proteins comprises 33 members [2], which can be divided into 17 subfamilies based on the architecture of the C-terminal domain [18]. Common to all SNX proteins is a phospholipid-binding (PX) motif [18], which allows recruitment to the phosphatidylinositol 3-phosphate (PI3P)-rich membranes of the EE. As described above, SNX1, SNX2, SNX5 and SNX6 (and possibly SNX32) [18] form the SNX-BAR subcomplex associating with the CSC of the mammalian retromer [16]. These proteins harbor, in addition to the PX-motif, a C-terminal Bin-Amphiphysin-Rvs (BAR) domain [18]. BAR domains are highly conserved, banana shaped protein dimerization domains that are present in many proteins involved in cell membrane dynamics, [19] bind to membrane via their concave side [19] and are capable of sensing and stabilizing membrane curvature [19]. By bending the endosomal membrane, the SNX-BAR subcomplex prompts tubulation and directs the spatial organization of the endosomal network enabling sorting of cargo for trafficking to the cell surface or the TGN [8, 9]. By associating with the C-terminal domain of p150glued component of dynactin (DCTN1), SNX5 and SNX6 recruit the dynein-dynactin motor complex [20] which facilitates the transport of cargo molecules along the endosomal microtubules [21]. Detachment of cargo from the dynein motor at the TGN is facilitated by negative regulation of the association between SNX5/6 and p150glued through Phosphotidylinositol-4-phosphate (PtdIns(4)P) [22]. In addition to these BAR domain containing members of the SNX family, SNX27 containing a FERM domain, and SNX3 containing only the PX motif are SNX proteins essential for proper retromer function. Both these peptides are crucial for recruitment of retromer to the endosomal membrane (see below) [18].
3. RECRUITMENT OF RETROMER TO THE ENDOSOMAL MEMBRANE
The retromer complex is essential for several protein export pathways out of the endosome. Cargo export occurs throughout endosomal maturation [8], requiring a stable association of retromer to the endosomal membrane throughout the maturation process. Recruitment and stabilization of the association of retromer to the endosomal membrane are mediated through bivalent recognition of SNX3 [23] and the small GTPases RAB5 and RAB7 [24] by Vps35. Rab GTPases play essential roles in several steps of membrane trafficking, including vesicle formation, vesicle movement along actin and tubulin networks, and membrane fusion [25]. Like other GTPases, Rabs switch between two conformations, an inactive form bound to GDP (guanosine diphosphate), and an active form bound to GTP (guanosine triphosphate). A GDP/GTP Exchange Factor (GEF) catalyzes the conversion from GDP-bound to GTP-bound form, thereby activating the Rab. Inherent GTP hydrolysis of Rabs enhanced by a GTPase-Activating Protein (GAP) leads to Rab inactivation. In the EE, Rab5 recruits PI3K to generate PI3P which in turn recruits the retromer recruitment factor SNX3 (see below). As EEs mature into Late Endosomes (LEs), they undergo an Rab conversion in which active Rab7-family GTPases are recruited and Rab5-family GTPases are inactivated and extracted from the membrane [26]. TBC1D5, a Rab7 GAP (GTPase-activating protein) which associates with the CSC by binding to VPS29, causes Rab7 to dissociate from the membrane and downregulates CSC recruitment to the membrane [27] A mutation in VPS29, L152E, inhibits TBC1D5 binding to the CSC [27].
SNX3 is a small protein of 162 amino acids containing only the characteristic PX motif. Linked to Vps35, [24] SNX3 facilitates recruitment of the CSC to the EE through bivalent binding of its PX motif to PI3P (recruited by RAB5) in the endosomal membrane [28]. In addition to recruiting retromer to the EE, SNX3 is essential for retrograde endosome-to-TGN transport of the Wnt-binding protein Wntless (Wls) [23]. Wls transports Wnt proteins - lipid-modified glycoproteins that play a central role in development, adult tissue homeostasis and disease - from the Golgi network to the cell surface for release. SNX3 interacts directly with the CSC to sort Wls into a morphologically distinct retrieval pathway that is independent of the retromer sorting nexins SNX1-SNX2 and SNX5-SNX6, indicating that SNX3 is part of an alternative retromer complex (“SNX3-retromer complex”) that functionally dissociates the retrograde transport of Wls from other retromer cargo [23]. This independence of WLS trafficking from the mammalian retromer SNX-BARs also suggests that the SNX-BAR subcomplex is a retromer accessory rather than a component of the retromer CSC.
4. ADDITIONAL RETROMER ACCESSORY PROTEINS
In addition to the SNX-BAR complex, there are several accessory proteins that are crucial for proper protein sorting mediated by the retromer CSC.
4.1. The Wash Complex
Critical for accurate protein sorting into distinct pathways is the accurate formation of endosomal sub-compartments through tubulation. A vital step for this tubulation is the assembly of actin filaments in the endosomal membrane into branched networks through the Arp2/3 complex [29-31]. This localized actin polymerization provides a structural or force-generating scaffold for the formation of endosomal sub-compartments, which separate the different trafficking pathways enabling differential sorting [32]. Inhibition of actin polymerization results in protein missorting and impaired endosomal maturation [33].
Closely resembling the structure of monomeric actin, ARP2 and ARP3 serve as nucleation sites for new actin filaments. The Arp2/3 complex binds to the sides of existing (“mother”) filaments and initiates the growth of new (“daughter”) filaments at a distinctive 70 degree angle thereby creating branched actin networks [34]. The Arp2/3 complex is activated through Nucleation Promoting Factors (NPFs) [35]. The major NPF on the endosomal surface, and thus a key regulator of F-actin-mediated endosomal trafficking, is the pentameric WASH complex [36] consisting of the five subunits WASH1 featuring a VCA domain promoting actin polymerization by the Arp2/3 complex, Strumpellin (KIAA0196), KIAA1033(SWIP), CCDC53 and FAM21. The WASH complex is recruited to the endosomal membrane through association of FAM21 with the Vps35 subcomponent of the CSC [17], as well as through interaction with SNX27 [37, 38]. Loss of F-actin or WASH results in endosomal enlargement, loss of the compartmentalization, and loss of all three major endosomal trafficking pathways (i.e. endosome-to-TGN, endosome-to-membrane, and endolysosomal pathway) [38-40].
4.2. RME-8
Besides associating with Vps35, the FAM21 component of the WASH complex also binds to the DnaJ Domain-containing receptor-mediated endocytosis-8 (RME-8/DNAJ C13) protein, which in turn also associates with the SNX-BAR complex subunit SNX1 [41]. DnaJ proteins are highly preserved across species and are involved in translation, folding, unfolding, translocation and degradation of proteins, primarily through stimulating the ATPase activity of associated chaperones (Hsp70s) [42]. Loss of RME-8 alters the membrane association of SNX1 and leads to a significant increase in branched retromer-positive endosomal tubules [41]. Localization of membrane proteins to endosomal tubules is dependent on WASH complex activity suggesting that the interaction between RME-8, SNX1 and FAM21 enables coordination of the activity of the WASH complex with the membrane-tubulating function of the sorting nexins. RME-8 colocalizes with several endosomal markers including cation-independent mannose 6-phosphate receptor (CI-MPR) and epidermal growth factor receptor (EGFR); [43, 44] loss of RME-8 disrupts endosmal trafficking.
4.3. VARP
VARP, a VPS9-domain protein with ankyrin repeats, is a Guanine nucleotide Exchange Factor (GEF) for Rab21, a GTPase required for endosome-to-cell surface recycling [45]. Similar to TBC1D5, VARP binds to VPS29 and needs retromer for its membrane association [46]. The fact that the L152E mutation in VPS29, which inhibits TBC1D5 binding, also prevents VARP binding to VPS29 suggests that TBC1D5 and VARP may compete for the same binding site in VPS29 [46]. As binding of TBC1D5 by retromer leads to retromer dissociation from the endosomal membranes through increasing GTP hydrolysis of Rab7A [27], VARP association with retromer may increase stability of retromer association and sorting into the recycling pathway by inhibiting CSC binding of TBC1D5 [46].
4.4. EHD1
The Eps15-homology domain 1 (EHD1) protein has been shown to associate with the CSC although it remains to be clarified how this association is mediated [47]. EHD1 stabilizes SNX1-positive membrane tubules. Loss of function of EHD1, its paralogue EHD3 or the EHD1-interacting protein rabankyrin-5 (Rank-5/ANKFY1) results in a decrease in SNX1-positive membrane tubules and impaired endosome-to-Golgi trafficking [47-49]. As EHD1 is required for endosome-to-cell surface recycling of transferrin receptor and Major Histocompatibility Complex (MHC) class I, neither of which is regarded a retromer cargo molecule, EHD proteins may exert a more generic effect, for example as scaffolding proteins involved in membrane bending contributing to tubule scission or tubule stabilization [50].
5. ROLE OF RETROMER DYSFUNCTION IN DISEASE
In line with the vital role of the retromer complex in endosomal trafficking, it has been shown in animal studies that loss function can be embryonically lethal, and that retromer dysfunction, its subcomponents or accessory factors can result in a variety of disorders (including several neurodegenerative diseases) in humans. Knocking out the CSC components Vps35 or Vps26a in animal models is embryonically fatal [51, 52]. Knockout of Vps26b does not lead to significant health defects although Vps26b-deficient mice show a significant reduction of expression of Vps29 and Vps35 due to absence of the Vps26b-Vps29-Vps35 CSC, and an increase in the expression of sortilin, which is regulated by retromer [53]. These findings indicate that Vp26a and Vp26b may have different functions in vivo.
In the neurodegenerative disease spectrum, over the past 5 years retromer dysfunction has been linked to a number of diseases including Alzheimer’s Disease (AD) and Parkinson’s disease (PD) [52, 54-80]. In AD, the accumulation of extracellular β-amyloid (Aβ) protein in diffuse and neuritic plaques, produced through successive cleavage of the Amyloid Precursor Protein (APP) through β- (BACE1) and ϒ-secretase, is considered a key pathological mechanism [81, 82]. APP is a type-I transmembrane protein that is sorted through multiple membranous compartments of the cell. APP trafficked to the plasma membrane is predominantly cleaved by α-secretase into nontoxic products (soluble APPα (sAPPα) secreted into the extracellular space and an 83 amino-acid membrane-associated C-terminal fragment (C83)). Unprocessed APP, however, can enter the amyloidogenic pathway by internalization into the EE, which harbor BACE1 and feature an optimal pH for BACE1 cleavage. BACE1 cleavage results in generation of a membrane-associated C-terminal fragment (C99), which can subsequently be shuttled back to the endosomal reticulum (ER) to be processed into neurotoxic Aβ peptides by ϒ-secretase [83, 84], sorted to the plasma membrane where ϒ-secretase is also present, or processed to Aβ within the endosome/ lysosome system. The neuronal retromer prevents amyloidogenic processing of APP by directing it away from the endosomes to the TGN or to the cell surface. Retromer cargo molecules through which APP is linked to the CSC are the Vps10-containing transmembrane proteins SORL1, SORCS1 and SORT1. Retromer dysfunction results in reduced trafficking of APP out of the endosomes and increased APP cleavage through BACE1 [85, 86]. In in vitro and in vivo experimental studies, silencing of the cargo molecules SORL1 [87-89] or SORCS1 [90] or the Vps35 [91, 92] or Vps26 [87, 92] CSC components results in increased amounts of toxic Aβ peptides. Manipulation of protein sorting motifs within the SORL1 or SORCS1c cytoplasmic tail interacting with the CSC results in increases Aβ production through missorting of APP and its fragments to endosomal compartments and decreased retrograde TGN trafficking [93, 94]. There is also evidence for an increased secretion of APP C-terminal fragments (APP CTFs) via exosomes, suggesting that retromer dysfunction might redirect trafficking of APP CTFs into exosomes leading to an alternative pathway for secretion of APP fragments and a potential source of extracellular Aβ [95, 96]. Finally, in addition to their role in tubule formation, SNX proteins may negatively regulate retrograde trafficking of BACE1 from EE to the TGN, keeping BACE1 in the EE increasing APP processing [97].
The retromer has also been linked to AD in human studies. In the AD disease process, the entorhinal cortex and the dentate gyrus subregions of the hippocampus are strongly affected. In microarray gene expression analysis of dentate gyrus and entorhinal cortex tissue from AD patients, Vps35 and Vps26 levels are reduced [91]. In several genetic association studies, genes encoding SNX1, SNX3 and RAB7 essential for tubule formation and membrane association of the CSC [98] as well as genes encoding the retromer cargo molecules SORL1 [87, 99-101], SORCS1 [73, 90, 102-104], SORCS2 [105] and SORCS3 [105] have been associated with AD, and sequencing analyses identified three disease-associated coding mutations in SORL1 [101]. The locus for human VPS26A has been genetically associated with type 2 diabetes (T2D) [106], an established risk factor for AD [107, 108].
Retromer dysfunction and genetic variation in retromer-related genes have also been linked to Parkinson’s Disease (PD). The D620N (c.1858G>A) missense variant in VPS35, which shows incomplete, age-dependent penetrance, was originally shown to segregate with PD in Swiss and Austrian families, and has now been identified in a number of PD subjects and families worldwide [78, 109, 110] D620N leads to an autosomal dominant form of PD by blunting WASH recruitment to endosomes leading to abnormal trafficking of the autophagy protein ATG9A and thereby autophagy dysfunction, [111] and impairing trafficking of cathepsin D, a CI-M6PR ligand and protease degrading α-synuclein [60]. In line with this notion, in a viral-mediated gene transfer rat model, [112] VPS35 D620N expression resulted in a marked degeneration of dopaminergic neurons in the substantia nigra and axonal pathology, key pathological changes in PD. A mutation in RME8, DNAJC13 (p.Asn855Ser) coordinating the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation is associated with a toxic gain-of-function and impairs endosomal transport [77]. PD-associated variants in RAB7L1 (the PARK16 locus gene) impair endolysosomal and Golgi apparatus trafficking and lead to Vps35 deficiency [113]. Expression of wild-type Vps35 rescues these defects. Additional mutations that have been reported but remain to be validated in PD include R524W, P316S and p.E787K in VPS35 [114], p.K93E in VPS26A and p.N72H in VPS29 [54, 56, 75, 78, 109].
Finally, genetic variants in retromer-related genes have also been associated with closely related neurodegenerative diseases. In a recent study, the RME-8/DNAJC13 c.2564A>G (p.(N855S)) variant has been linked to essential tremor [115]. A recent experimental study showed that loss of SNX27 contributes to excitatory synaptic dysfunction and learning and memory deficits by modulating glutamate receptor recycling in Down's syndrome [116]. SNX27 interacts with these receptors through its PDZ domain, regulating their recycling to the plasma membrane. Upregulating SNX27 in the hippocampus of Down syndrome mice rescued synaptic and cognitive deficits [116]. Genetic variants in the gene encoding the WASH complex component strumpellin (KIAA0196), cause autosomal dominant Hereditary Spastic Paraplegia (HSP) characterized by degeneration of corticospinal tract axons [117]. Due to the burden on membrane traffic imposed by their exceptionally long axons, corticospinal neurons might be particularly vulnerable to reductions in functional WASH complex. A mutation of the KIAA1033 (Strumpellin And WASH-Interacting Protein (SWIP)) component of the WASH complex (c.3056C > G), which leads to significantly reduced SWIP levels and destabilization of the entire WASH complex, is associated with autosomal recessive intellectual disability [118]. Mutations in RAB7, regulating retromer recruitment to endosomes, have been linked in Charcot-Marie-Tooth hereditary neuropathy [119], a group of disorders characterized by a chronic motor and sensory polyneuropathy. Finally, genetic variants in the retromer-cargo-binding molecule SORCS2 have been reported in psychiatric diseases including schizophrenia [57] and bipolar disorder [57, 120].
6. RETROMER AS A TARGET FOR PREVENTION AND TREATMENT OF NEURODEGENRATIVE DISEASE
The findings summarized above nominate the retromer complex and its associated factors as potential targets for prevention and treatment of neurodegenerative disease characterized by retromer dysfunction. That this notion may hold promise has been demonstrated by recent studies which showed that increasing the levels of the CSC through retromer-stabilizing pharmacological chaperones can enhance retromer function [121]. While there is valid concern whether enhancement of retromer function leads to toxic adverse effects, increasing retromer levels in yeast, neuronal cultures, and animal studies reversed neurotoxic effects of pathogenic mutations without causing adverse effects, suggesting that increasing the normal flow of proteins out of the endosome may be tolerated [58, 85, 113, 121]. These studies further indicate that retromer-enhancing drugs may be beneficial even in the absence of retromer dysfunction. For example, in AD, retromer enhancing drugs might decrease the processing of APP into neurotoxic fragments by reducing the endosomal residing time of APP [121].
CONCLUSION AND FUTURE DIRECTIONS
In recent years, evidence from human, animal and cell biology studies has accumulated linking retromer-mediated intracellular sorting and trafficking of transmembrane proteins as a key pathological mechanism to several age-related neurodegenerative diseases including AD and PD. While recent studies have demonstrated that pharmacological chaperones can stabilize the CSC, increase retromer levels in neurons, and direct disease-causing proteins away from a pathogenic and toward a neuroprotective processing pathway, several points remain to be clarified on how the individual retromer components function in endosomal sorting and what consequences arise when these processes are perturbed. The subcomponents of the CSC, its accessory proteins and its interacting pathways need to be fully characterized, both structurally and functionally. In addition, modulating up- and downstream pathways need to be mapped. It needs to be characterized, how and through which protein domains the cargo recognition at the CSC occurs. Finally, retromer defects seem to differentially target the nervous system suggesting that the nervous system is differentially dependent on retromer for its normal function. Studies are required that clarify the mechanisms by which endosomal transport and trafficking affect the cellular properties of neurons and synaptic biology. It has to be clarified why retromer dysfunction seems to show regional vulnerability, i.e. why it seems to target specific neuronal populations, but at the same time can cause diseases stemming from distinct regions of the nervous system. Identification of causative genomic variants through genomic next generation technologies could help to clarify several of these issues and could further inform in silico and functional studies to identify possible drug-binding sites. If drugs targeting the retromer without harmful adverse effects can be successfully developed, they hold strong therapeutic promise for neurodegenerative diseases characterized by retromer dysfunction.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
Dr. Reitz was supported by NIH grants K23AG034550, P50 AG08702, UF1AG047133 and R01 AG034189.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gillooly D.J., Morrow I.C., Lindsay M., Gould R., Bryant N.J., Gaullier J.M., Parton R.G., Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19(17):4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullen P.J. Endosomal sorting and signalling: An emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 2008;9(7):574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 3.Pearse B.M. Clathrin: A unique protein associated with intracellular transfer of membrane by coated vesicles. Proc. Natl. Acad. Sci. USA. 1976;73(4):1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayor S., Parton R.G., Donaldson J.G. Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol. 2014;6(6):a016758. doi: 10.1101/cshperspect.a016758. http://cshperspectives.cshlp.org/ content/6/6/a016758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaman M.N., Marcusson E.G., Cereghino J.L., Emr S.D. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 1997;137(1):79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seaman M.N., McCaffery J.M., Emr S.D. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 1998;142(3):665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horazdovsky B.F., Davies B.A., Seaman M.N., McLaughlin S.A., Yoon S., Emr S.D. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol. Biol. Cell. 1997;8(8):1529–1541. doi: 10.1091/mbc.8.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Weering J.R., Verkade P., Cullen P.J. SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic. 2012;13(1):94–107. doi: 10.1111/j.1600-0854.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- 9.Carlton J., Bujny M., Peter B.J., Oorschot V.M., Rutherford A., Mellor H., Klumperman J., McMahon H.T., Cullen P.J. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr. Biol. 2004;14(20):1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 10.Koumandou V.L., Klute M.J., Herman E.K., Nunez-Miguel R., Dacks J.B., Field M.C. Evolutionary reconstruction of the retromer complex and its function in Trypanosoma brucei. J. Cell Sci. 2011;124(Pt 9):1496–1509. doi: 10.1242/jcs.081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr M.C., Bennetts J.S., Simpson F., Thomas E.C., Flegg C., Gleeson P.A., Wicking C., Teasdale R.D. A novel mammalian retromer component, Vps26B. Traffic. 2005;6(11):991–1001. doi: 10.1111/j.1600-0854.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 12.Bugarcic A., Zhe Y., Kerr M.C., Griffin J., Collins B.M., Teasdale R.D. Vps26A and Vps26B subunits define distinct retromer complexes. Traffic. 2011;12(12):1759–1773. doi: 10.1111/j.1600-0854.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- 13.Gallon M., Clairfeuille T., Steinberg F., Mas C., Ghai R., Sessions R.B., Teasdale R.D., Collins B.M., Cullen P.J. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc. Natl. Acad. Sci. USA. 2014;111(35):E3604–E3613. doi: 10.1073/pnas.1410552111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukadam A.S., Seaman M.N. Retromer-mediated endosomal protein sorting: The role of unstructured domains. FEBS Lett. 2015;589(19 Pt A):2620–2626. doi: 10.1016/j.febslet.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 15.Rojas R., Kametaka S., Haft C.R., Bonifacino J.S. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol. Cell. Biol. 2007;27(3):1112–1124. doi: 10.1128/MCB.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassmer T., Attar N., Bujny M.V., Oakley J., Traer C.J., Cullen P.J. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J. Cell Sci. 2007;120(Pt 1):45–54. doi: 10.1242/jcs.03302. [DOI] [PubMed] [Google Scholar]
- 17.Harbour M.E., Breusegem S.Y., Antrobus R., Freeman C., Reid E., Seaman M.N. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J. Cell Sci. 2010;123(Pt 21):3703–3717. doi: 10.1242/jcs.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teasdale R.D., Collins B.M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions and roles in disease. Biochem. J. 2012;441(1):39–59. doi: 10.1042/BJ20111226. [DOI] [PubMed] [Google Scholar]
- 19.Peter B.J., Kent H.M., Mills I.G., Vallis Y., Butler P.J., Evans P.R., McMahon H.T. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303(5657):495–499. doi: 10.1126/science.1092586. http://science.sciencemag.org/content/ 303/5657/495/tab-article-info [DOI] [PubMed] [Google Scholar]
- 20.Schroer T.A. 2004 https://www.annualreviews.org/doi/10.1146/
- 21.Wassmer T., Attar N., Harterink M., van Weering J.R., Traer C.J., Oakley J., Goud B., Stephens D.J., Verkade P., Korswagen H.C., Cullen P.J. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev. Cell. 2009;17(1):110–122. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu Y., Zhang C., Sun Z., Hong Z., Li K., Sun D., Yang Y., Tian C., Gong W., Liu J.J. PtdIns(4)P regulates retromer-motor interaction to facilitate dynein-cargo dissociation at the trans-Golgi network. Nat. Cell Biol. 2013;15(4):417–429. doi: 10.1038/ncb2710. [DOI] [PubMed] [Google Scholar]
- 23.Harterink M., Port F., Lorenowicz M.J., McGough I.J., Silhankova M., Betist M.C., van Weering J.R., van Heesbeen R.G., Middelkoop T.C., Basler K., Cullen P.J., Korswagen H.C.A. SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat. Cell Biol. 2011;13(8):914–923. doi: 10.1038/ncb2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison M.S., Hung C.S., Liu T.T., Christiano R., Walther T.C., Burd C.G. A mechanism for retromer endosomal coat complex assembly with cargo. Proc. Natl. Acad. Sci. USA. 2014;111(1):267–272. doi: 10.1073/pnas.1316482111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 26.Rink J., Ghigo E., Kalaidzidis Y., Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122(5):735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 27.Seaman M.N., Harbour M.E., Tattersall D., Read E., Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J. Cell Sci. 2009;122(Pt 14):2371–2382. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y., Hortsman H., Seet L., Wong S.H., Hong W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat. Cell Biol. 2001;3(7):658–666. doi: 10.1038/35083051. [DOI] [PubMed] [Google Scholar]
- 29.Barr F.A., Gruneberg U. Cytokinesis: Placing and making the final cut. Cell. 2007;131(5):847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Engqvist-Goldstein A.E., Drubin D.G. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. https://www.annualreviews.org/ doi/10.1146/annurev.cellbio.19.111401.093127 [DOI] [PubMed] [Google Scholar]
- 31.Goley E.D., Welch M.D. The ARP2/3 complex: An actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 2006;7(10):713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 32.Puthenveedu M.A., Lauffer B., Temkin P., Vistein R., Carlton P., Thorn K., Taunton J., Weiner O.D., Parton R.G., von Zastrow M. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143(5):761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohashi E., Tanabe K., Henmi Y., Mesaki K., Kobayashi Y., Takei K. Receptor sorting within endosomal trafficking pathway is facilitated by dynamic actin filaments. PLoS One. 2011;6(5):e19942. doi: 10.1371/journal.pone.0019942. http://journals.plos.org/plosone/article? id=10.1371/journal.pone.0019942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollard T.D. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. https://www.annualreviews.org/ doi/abs/10.1146/annurev.biophys.35.040405.101936?journalCode=biophys.3 [DOI] [PubMed] [Google Scholar]
- 35.Welch M.D., Mullins R.D. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 2002;18:247–288. doi: 10.1146/annurev.cellbio.18.040202.112133. https://www.annualreviews.org/doi/10.1146/annurev.cellbio.18.040202.112133 [DOI] [PubMed] [Google Scholar]
- 36.Derivery E., Sousa C., Gautier J.J., Lombard B., Loew D., Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev. Cell. 2009;17(5):712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg F., Gallon M., Winfield M., Thomas E.C., Bell A.J., Heesom K.J., Tavare J.M., Cullen P.J. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 2013;15(5):461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Temkin P., Lauffer B., Jager S., Cimermancic P., Krogan N.J., von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 2011;13(6):715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derivery E., Helfer E., Henriot V., Gautreau A. Actin polymerization controls the organization of WASH domains at the surface of endosomes. PLoS One. 2012;7(6):e39774. doi: 10.1371/journal.pone.0039774. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0039774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez T.S., Billadeau D.D.A. FAM21-containing WASH complex regulates retromer-dependent sorting. Dev. Cell. 2009;17(5):699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman C.L., Hesketh G., Seaman M.N. RME-8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J. Cell Sci. 2014;127(Pt 9):2053–2070. doi: 10.1242/jcs.144659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu X.B., Shao Y.M., Miao S., Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 2006;63(22):2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girard M., Poupon V., Blondeau F., McPherson P.S. The DnaJ-domain protein RME-8 functions in endosomal trafficking. J. Biol. Chem. 2005;280(48):40135–40143. doi: 10.1074/jbc.M505036200. [DOI] [PubMed] [Google Scholar]
- 44.Popoff V., Mardones G.A., Bai S.K., Chambon V., Tenza D., Burgos P.V., Shi A., Benaroch P., Urbe S., Lamaze C., Grant B.D., Raposo G., Johannes L. Analysis of articulation between clathrin and retromer in retrograde sorting on early endosomes. Traffic. 2009;10(12):1868–1880. doi: 10.1111/j.1600-0854.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X., He X., Fu X.Y., Chang Z. Varp is a Rab21 guanine nucleotide exchange factor and regulates endosome dynamics. J. Cell Sci. 2006;119(Pt 6):1053–1062. doi: 10.1242/jcs.02810. [DOI] [PubMed] [Google Scholar]
- 46.Hesketh G.G., Perez-Dorado I., Jackson L.P., Wartosch L., Schafer I.B., Gray S.R., McCoy A.J., Zeldin O.B., Garman E.F., Harbour M.E., Evans P.R., Seaman M.N., Luzio J.P., Owen D.J. VARP is recruited on to endosomes by direct interaction with retromer, where together they function in export to the cell surface. Dev. Cell. 2014;29(5):591–606. doi: 10.1016/j.devcel.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gokool S., Tattersall D., Seaman M.N. EHD1 interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic. 2007;8(12):1873–1886. doi: 10.1111/j.1600-0854.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J., Naslavsky N., Caplan S. EHDs meet the retromer: Complex regulation of retrograde transport. Cell. Logist. 2012;2(3):161–165. doi: 10.4161/cl.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J., Reiling C., Reinecke J.B., Prislan I., Marky L.A., Sorgen P.L., Naslavsky N., Caplan S. Rabankyrin-5 interacts with EHD1 and Vps26 to regulate endocytic trafficking and retromer function. Traffic. 2012;13(5):745–757. doi: 10.1111/j.1600-0854.2012.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daumke O., Lundmark R., Vallis Y., Martens S., Butler P.J., McMahon H.T. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449(7164):923–927. doi: 10.1038/nature06173. https://www.nature.com/articles/nature06173 [DOI] [PubMed] [Google Scholar]
- 51.Lee J.J., Radice G., Perkins C.P., Costantini F. Identification and characterization of a novel, evolutionarily conserved gene disrupted by the murine H beta 58 embryonic lethal transgene insertion. Dev. Cell. 1992;115(1):277–288. doi: 10.1242/dev.115.1.277. [DOI] [PubMed] [Google Scholar]
- 52.Wen L., Tang F.L., Hong Y., Luo S.W., Wang C.L., He W., Shen C., Jung J.U., Xiong F., Lee D.H., Zhang Q.G., Brann D., Kim T.W., Yan R., Mei L., Xiong W.C. VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. J. Cell Biol. 2011;195(5):765–779. doi: 10.1083/jcb.201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim E., Lee Y., Lee H.J., Kim J.S., Song B.S., Huh J.W., Lee S.R., Kim S.U., Kim S.H., Hong Y., Shim I., Chang K.T. Implication of mouse Vps26b-Vps29-Vps35 retromer complex in sortilin trafficking. Biochem. Biophys. Res. Commun. 2010;403(2):167–171. doi: 10.1016/j.bbrc.2010.10.121. [DOI] [PubMed] [Google Scholar]
- 54.Lesage S., Condroyer C., Klebe S., Honore A., Tison F., Brefel-Courbon C., Durr A., Brice A. Identification of VPS35 mutations replicated in French families with Parkinson disease. Neurology. 2012;78(18):1449–1450. doi: 10.1212/WNL.0b013e318253d5f2. [DOI] [PubMed] [Google Scholar]
- 55.Ando M., Funayama M., Li Y., Kashihara K., Murakami Y., Ishizu N., Toyoda C., Noguchi K., Hashimoto T., Nakano N., Sasaki R., Kokubo Y., Kuzuhara S., Ogaki K., Yamashita C., Yoshino H., Hatano T., Tomiyama H., Hattori N. VPS35 mutation in Japanese patients with typical Parkinson’s disease. Mov. Disord. 2012;27(11):1413–1417. doi: 10.1002/mds.25145. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y., Chen K., Song W., Chen X., Cao B., Huang R., Zhao B., Guo X., Burgunder J., Li J., Shang H.F. VPS35 Asp620Asn and EIF4G1 Arg1205His mutations are rare in Parkinson disease from southwest China. Neurobiol. Aging. 2013;34(6):e7–e8. doi: 10.1016/j.neurobiolaging.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Christoforou A., McGhee K.A., Morris S.W., Thomson P.A., Anderson S., McLean A., Torrance H.S., Le Hellard S., Pickard B.S., StClair D., Muir W.J., Blackwood D.H., Porteous D.J., Evans K.L. Convergence of linkage, association and GWAS findings for a candidate region for bipolar disorder and schizophrenia on chromosome 4p. Mol. Psychiatry. 2011;16(3):240–242. doi: 10.1038/mp.2010.25. [DOI] [PubMed] [Google Scholar]
- 58.Dhungel N., Eleuteri S., Li L.B., Kramer N.J., Chartron J.W., Spencer B., Kosberg K., Fields J.A., Stafa K., Adame A., Lashuel H., Frydman J., Shen K., Masliah E., Gitler A.D. Parkinson’s disease genes VPS35 and EIF4G1 interact genetically and converge on alpha-synuclein. Neuron. 2015;85(1):76–87. doi: 10.1016/j.neuron.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elias-Sonnenschein L.S., Helisalmi S., Natunen T., Hall A., Paajanen T., Herukka S.K., Laitinen M., Remes A.M., Koivisto A.M., Mattila K.M., Lehtimaki T., Verhey F.R., Visser P.J., Soininen H., Hiltunen M. Genetic loci associated with Alzheimer’s disease and cerebrospinal fluid biomarkers in a Finnish case-control cohort. PLoS One. 2013;8(4):e59676. doi: 10.1371/journal.pone.0059676. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0059676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Follett J., Norwood S.J., Hamilton N.A., Mohan M., Kovtun O., Tay S., Zhe Y., Wood S.A., Mellick G.D., Silburn P.A., Collins B.M., Bugarcic A., Teasdale R.D. The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic. 2014;15(2):230–244. doi: 10.1111/tra.12136. [DOI] [PubMed] [Google Scholar]
- 61.Gagliardi M., Annesi G., Tarantino P., Nicoletti G., Quattrone A. Frequency of the ASP620ASN mutation in VPS35 and Arg1205His mutation in EIF4G1 in familial Parkinson’s disease from South Italy. 2014 doi: 10.1016/j.neurobiolaging.2014.04.020. https://linkinghub.elsevier.com/retrieve/pii/S0197458014003 [DOI] [PubMed]
- 62.He Y., Fang Z., Yu G. Sortilin-related VPS10 domain containing receptor 1 and Alzheimer’s disease-associated allelic variations preferentially exist in female or type 2 diabetes mellitus patients in southern Han Chinese. Psychogeriatrics. 2012;12(4):215–225. doi: 10.1111/j.1479-8301.2012.00405.x. [DOI] [PubMed] [Google Scholar]
- 63.Jin C., Zhang L., Xian Y., Liu X., Wu Y., Zhang F., Zhu J., Zhang G., Chen C., Gong R., Yuan J., Tian L., Wang G., Cheng Z. The SORL1 polymorphism rs985421 may confer the risk for amnestic mild cognitive impairment and Alzheimer’s disease in the Han Chinese population. Neurosci. Lett. 2014;563:80–84. doi: 10.1016/j.neulet.2014.01.029. https://www.sciencedirect.com/science/article/ abs/pii/S0304394014000561 [DOI] [PubMed] [Google Scholar]
- 64.Kimura R., Yamamoto M., Morihara T., Akatsu H., Kudo T., Kamino K., Takeda M. SORL1 is genetically associated with Alzheimer disease in a Japanese population. Neurosci. Lett. 2009;461(2):177–180. doi: 10.1016/j.neulet.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 65.Kolsch H., Jessen F., Wiltfang J., Lewczuk P., Dichgans M., Kornhuber J., Frolich L., Heuser I., Peters O., Schulz J.B., Schwab S.G., Maier W. Influence of SORL1 gene variants: Association with CSF amyloid-beta products in probable Alzheimer’s disease. Neurosci. Lett. 2008;440(1):68–71. doi: 10.1016/j.neulet.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 66.Kolsch H., Jessen F., Wiltfang J., Lewczuk P., Dichgans M., Teipel S.J., Kornhuber J., Frolich L., Heuser I., Peters O., Wiese B., Kaduszkiewicz H., van den Bussche H., Hull M., Kurz A., Ruther E., Henn F.A., Maier W. Association of SORL1 gene variants with Alzheimer’s disease. 2009 doi: 10.1016/j.brainres.2009.01.044. https://www.sciencedirect.com/science/article/pii/ [DOI] [PubMed]
- 67.Lambert J.C., Ibrahim-Verbaas C.A., Harold D., Naj A.C., Sims R., Bellenguez C., DeStafano A.L., Bis J.C., Beecham G.W., Granier-Boley B., Russo G., Thorton-Wells T.A., Jones N., Smith A.V., Chouraki V., Thomas C., Ikram M.A., Zelenika D., Vardarajan B.N., Kamatani Y., Lin C.F., Gerrish A., Schmidt H., Kunkle B., Dunstan M.L., Ruiz A., Bihoreau M.T., Choi S.H., Reitz C., Pasquier F., Cruchaga C., Craig D., Amin N., Berr C., Lopez O.L., De Jager P.L., Deramecourt V., Johnston J.A., Evans D., Lovestone S., Letenneur L., Moron F.J., Rubinsztein D.C., Eiriksdottir G., Sleegers K., Goate A.M., Fievet N., Huentelman M.W., Gill M., Brown K., Kamboh M.I., Keller L., Barberger-Gateau P., McGuiness B., Larson E.B., Green R., Myers A.J., Dufouil C., Todd S., Wallon D., Love S., Rogaeva E., Gallacher J., St George-Hyslop P., Clarimon J., Lleo A., Bayer A., Tsuang D.W., Yu L., Tsolaki M., Bossu P., Spalletta G., Proitsi P., Collinge J., Sorbi S., Sanchez-Garcia F., Fox N.C., Hardy J., Deniz Naranjo M.C., Bosco P., Clarke R., Brayne C., Galimberti D., Mancuso M., Matthews F., Moebus S., Mecocci P., Del Zompo M., Maier W., Hampel H., Pilotto A., Bullido M., Panza F., Caffarra P., Nacmias B., Gilbert J.R., Mayhaus M., Lannefelt L., Hakonarson H., Pichler S., Carrasquillo M.M., Ingelsson M., Beekly D., Alvarez V., Zou F., Valladares O., Younkin S.G., Coto E., Hamilton-Nelson K.L., Gu W., Razquin C., Pastor P., Mateo I., Owen M.J., Faber K.M., Jonsson P.V., Combarros O., O’Donovan M.C., Cantwell L.B., Soininen H., Blacker D., Mead S., Mosley T.H., Jr, Bennett D.A., Harris T.B., Fratiglioni L., Holmes C., de Bruijn R.F., Passmore P., Montine T.J., Bettens K., Rotter J.I., Brice A., Morgan K., Foroud T.M., Kukull W.A., Hannequin D., Powell J.F., Nalls M.A., Ritchie K., Lunetta K.L., Kauwe J.S., Boerwinkle E., Riemenschneider M., Boada M., Hiltuenen M., Martin E.R., Schmidt R., Rujescu D., Wang L.S., Dartigues J.F., Mayeux R., Tzourio C., Hofman A., Nothen M.M., Graff C., Psaty B.M., Jones L., Haines J.L., Holmans P.A., Lathrop M., Pericak-Vance M.A., Launer L.J., Farrer L.A., van Duijn C.M., Van Broeckhoven C., Moskvina V., Seshadri S., Williams J., Schellenberg G.D., Amouyel P. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Rowland C., Catanese J., Morris J., Lovestone S., O’Donovan M.C., Goate A., Owen M., Williams J., Grupe A. SORL1 variants and risk of late-onset Alzheimer’s disease. Neurobiol. Dis. 2008;29(2):293–296. doi: 10.1016/j.nbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng Y., Lee J.H., Cheng R., St George-Hyslop P., Mayeux R., Farrer L.A. Association between SORL1 and Alzheimer’s disease in a genome-wide study. Neuroreport. 2007;18(17):1761–1764. doi: 10.1097/WNR.0b013e3282f13e7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyashita A., Koike A., Jun G., Wang L.S., Takahashi S., Matsubara E., Kawarabayashi T., Shoji M., Tomita N., Arai H., Asada T., Harigaya Y., Ikeda M., Amari M., Hanyu H., Higuchi S., Ikeuchi T., Nishizawa M., Suga M., Kawase Y., Akatsu H., Kosaka K., Yamamoto T., Imagawa M., Hamaguchi T., Yamada M., Moriaha T., Takeda M., Takao T., Nakata K., Fujisawa Y., Sasaki K., Watanabe K., Nakashima K., Urakami K., Ooya T., Takahashi M., Yuzuriha T., Serikawa K., Yoshimoto S., Nakagawa R., Kim J.W., Ki C.S., Won H.H., Na D.L., Seo S.W., Mook-Jung I., St George-Hyslop P., Mayeux R., Haines J.L., Pericak-Vance M.A., Yoshida M., Nishida N., Tokunaga K., Yamamoto K., Tsuji S., Kanazawa I., Ihara Y., Schellenberg G.D., Farrer L.A., Kuwano R. SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese, Koreans and Caucasians. PLoS One. 2013;8(4):e58618. doi: 10.1371/journal.pone.0058618. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0058618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reitz C., Cheng R., Rogaeva E., Lee J.H., Tokuhiro S., Zou F., Bettens K., Sleegers K., Tan E.K., Kimura R., Shibata N., Arai H., Kamboh M.I., Prince J.A., Maier W., Riemenschneider M., Owen M., Harold D., Hollingworth P., Cellini E., Sorbi S., Nacmias B., Takeda M., Pericak-Vance M.A., Haines J.L., Younkin S., Williams J., van Broeckhoven C., Farrer L.A., St George-Hyslop P.H., Mayeux R. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch. Neurol. 2011;68(1):99–106. doi: 10.1001/archneurol.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reitz C., Lee J.H., Rogers R.S., Mayeux R. Impact of genetic variation in SORCS1 on memory retention. PLoS One. 2011;6(10):e24588. doi: 10.1371/journal.pone.0024588. http://journals.plos.org/plosone/article?id= 10.1371/journal.pone.0024588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reitz C., Tokuhiro S., Clark L.N., Conrad C., Vonsattel J.P., Hazrati L.N., Palotas A., Lantigua R., Medrano M. I, Z.J.-V.; Vardarajan, B.; Simkin, I.; Haines, J.L.; Pericak-Vance, M.A.; Farrer, L.A.; Lee, J.H.; Rogaeva, E.; George-Hyslop, P.S.; Mayeux, R. SORCS1 alters amyloid precursor protein processing and variants may increase Alzheimer’s disease risk. Ann. Neurol. 2011;69(1):47–64. doi: 10.1002/ana.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reitz C., Tosto G., Vardarajan B., Rogaeva E., Ghani M., Rogers R.S., Conrad C., Haines J.L., Pericak-Vance M.A., Fallin M.D., Foroud T., Farrer L.A., Schellenberg G.D., George-Hyslop P.S., Mayeux R. Independent and epistatic effects of variants in VPS10-d receptors on Alzheimer disease risk and processing of the amyloid precursor protein (APP). Transl. Psychiatry. 2013;3:e256. doi: 10.1038/tp.2013.13. https://www.nature.com/articles/tp201313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shannon B., Soto-Ortolaza A., Rayaprolu S., Cannon H.D., Labbe C., Benitez B.A., Choi J., Lynch T., Boczarska-Jedynak M., Opala G., Krygowska-Wajs A., Barcikowska M., Van Gerpen J.A., Uitti R.J., Springer W., Cruchaga C., Wszolek Z.K., Ross O.A. Genetic variation of the retromer subunits VPS26A/B-VPS29 in Parkinson’s disease. Neurobiol. Aging. 2014;35(8):e1–e2. doi: 10.1016/j.neurobiolaging.2014.03.004. http://www.neurobiologyofaging.org/article/S0197-4580(14)00 238-3/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheerin U.M., Charlesworth G., Bras J., Guerreiro R., Bhatia K., Foltynie T., Limousin P., Silveira-Moriyama L., Lees A., Wood N. Screening for VPS35 mutations in Parkinson’s disease. Neurobiol. Aging. 2012;33(4):e1–e5. doi: 10.1016/j.neurobiolaging.2011.10.032. http://www.neurobiologyofaging.org/article/S0197-4580(11) 00463-5/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vilarino-Guell C., Rajput A., Milnerwood A.J., Shah B., Szu-Tu C., Trinh J., Yu I., Encarnacion M., Munsie L.N., Tapia L., Gustavsson E.K., Chou P., Tatarnikov I., Evans D.M., Pishotta F.T., Volta M., Beccano-Kelly D., Thompson C., Lin M.K., Sherman H.E., Han H.J., Guenther B.L., Wasserman W.W., Bernard V., Ross C.J., Appel-Cresswell S., Stoessl A.J., Robinson C.A., Dickson D.W., Ross O.A., Wszolek Z.K., Aasly J.O., Wu R.M., Hentati F., Gibson R.A., McPherson P.S., Girard M., Rajput M., Rajput A.H., Farrer M.J. DNAJC13 mutations in Parkinson disease. Hum. Mol. Genet. 2014;23(7):1794–1801. doi: 10.1093/hmg/ddt570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vilarino-Guell C., Wider C., Ross O.A., Dachsel J.C., Kachergus J.M., Lincoln S.J., Soto-Ortolaza A.I., Cobb S.A., Wilhoite G.J., Bacon J.A., Behrouz B., Melrose H.L., Hentati E., Puschmann A., Evans D.M., Conibear E., Wasserman W.W., Aasly J.O., Burkhard P.R., Djaldetti R., Ghika J., Hentati F., Krygowska-Wajs A., Lynch T., Melamed E., Rajput A., Rajput A.H., Solida A., Wu R.M., Uitti R.J., Wszolek Z.K., Vingerhoets F., Farrer M.J. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 2011;89(1):162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H.F., Yu J.T., Zhang W., Wang W., Liu Q.Y., Ma X.Y., Ding H.M., Tan L. SORCS1 and APOE polymorphisms interact to confer risk for late-onset Alzheimer’s disease in a Northern Han Chinese population. 2012 doi: 10.1016/j.brainres.2012.01.067. https://www.sciencedirect.com/science/article/pii/S0006899 [DOI] [PubMed]
- 80.Xu W., Xu J., Wang Y., Tang H., Deng Y., Ren R., Wang G., Niu W., Ma J., Wu Y., Zheng J., Chen S., Ding J. The genetic variation of SORCS1 is associated with late-onset Alzheimer’s disease in Chinese Han population. PLoS One. 2013;8(5):e63621. doi: 10.1371/journal.pone.0063621. http://journals.plos.org/plosone/article?id= 10.1371/journal.pone.0063621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A.Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D.J. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. 1992 doi: 10.1038/360672a0. https://www.nature.com/articles/ [DOI] [PubMed]
- 82.Cai X.D., Golde T.E., Younkin S.G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. 1993 doi: 10.1126/science.8424174. mag.org/content/259/5094/514.long [DOI] [PubMed]
- 83.Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer’s disease. J. Neurochem. 1995;65(4):1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- 84.De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. doi: 10.1038/34910. https://www.nature.com/articles/34910 [DOI] [PubMed] [Google Scholar]
- 85.Bhalla A., Vetanovetz C.P., Morel E., Chamoun Z., Di Paolo G., Small S.A. The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport. Neurobiol. Dis. 2012;47(1):126–134. doi: 10.1016/j.nbd.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vieira S.I., Rebelo S., Esselmann H., Wiltfang J., Lah J., Lane R., Small S.A., Gandy S., da Cruz E.S.E.F., da Cruz E.S.O.A. Retrieval of the Alzheimer’s amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol. Neurodegener. 2010;5:40. doi: 10.1186/1750-1326-5-40. https://molecularneurodegeneration.biomedcentral.com/ articles/10.1186/1750-1326-5-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rogaeva E., Meng Y., Lee J.H., Gu Y., Kawarai T., Zou F., Katayama T., Baldwin C.T., Cheng R., Hasegawa H., Chen F., Shibata N., Lunetta K.L., Pardossi-Piquard R., Bohm C., Wakutani Y., Cupples L.A., Cuenco K.T., Green R.C., Pinessi L., Rainero I., Sorbi S., Bruni A., Duara R., Friedland R.P., Inzelberg R., Hampe W., Bujo H., Song Y.Q., Andersen O.M., Willnow T.E., Graff-Radford N., Petersen R.C., Dickson D., Der S.D., Fraser P.E., Schmitt-Ulms G., Younkin S., Mayeux R., Farrer L.A., St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andersen O.M., Reiche J., Schmidt V., Gotthardt M., Spoelgen R., Behlke J., von Arnim C.A., Breiderhoff T., Jansen P., Wu X., Bales K.R., Cappai R., Masters C.L., Gliemann J., Mufson E.J., Hyman B.T., Paul S.M., Nykjaer A., Willnow T.E. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2005;102(38):13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dodson S.E., Gearing M., Lippa C.F., Montine T.J., Levey A.I., Lah J.J. LR11/SorLA expression is reduced in sporadic Alzheimer disease but not in familial Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006;65(9):866–872. doi: 10.1097/01.jnen.0000228205.19915.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lane R.F., Raines S.M., Steele J.W., Ehrlich M.E., Lah J.A., Small S.A., Tanzi R.E., Attie A.D., Gandy S. Diabetes-associated SorCS1 regulates Alzheimer’s amyloid-beta metabolism: Evidence for involvement of SorL1 and the retromer complex. J. Neurosci. 2010;30(39):13110–13115. doi: 10.1523/JNEUROSCI.3872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Small S.A., Kent K., Pierce A., Leung C., Kang M.S., Okada H., Honig L., Vonsattel J.P., Kim T.W. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann. Neurol. 2005;58(6):909–919. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- 92.Muhammad A., Flores I., Zhang H., Yu R., Staniszewski A., Planel E., Herman M., Ho L., Kreber R., Honig L.S., Ganetzky B., Duff K., Arancio O., Small S.A. Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc. Natl. Acad. Sci. USA. 2008;105(20):7327–7332. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lane R.F., Steele J.W., Cai D., Ehrlich M.E., Attie A.D., Gandy S. Protein sorting motifs in the cytoplasmic tail of SorCS1 control generation of Alzheimer’s amyloid-beta peptide. J. Neurosci. 2013;33(16):7099–7107. doi: 10.1523/JNEUROSCI.5270-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adzhubei I., Jordan D.M., Sunyaev S.R. Predicting functional effect of human missense mutations using PolyPhen-2. 2013 doi: 10.1002/0471142905.hg0720s76. http://onlinelibrary.wiley.com/doi/10.1002/0471142905.hg0720s76/abstract [DOI] [PMC free article] [PubMed]
- 95.Choy R.W., Cheng Z., Schekman R. Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid beta (Abeta) production in the trans-Golgi network. Proc. Natl. Acad. Sci. USA. 2012;109(30):E2077–E2082. doi: 10.1073/pnas.1208635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sullivan C.P., Jay A.G., Stack E.C., Pakaluk M., Wadlinger E., Fine R.E., Wells J.M., Morin P.J. Retromer disruption promotes amyloidogenic APP processing. Neurobiol. Dis. 2011;43(2):338–345. doi: 10.1016/j.nbd.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okada H., Zhang W., Peterhoff C., Hwang J.C., Nixon R.A., Ryu S.H., Kim T.W. Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. FASEB J. 2010;24(8):2783–2794. doi: 10.1096/fj.09-146357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vardarajan B.N., Bruesegem S.Y., Harbour M.E., Inzelberg R., Friedland R., St George-Hyslop P., Seaman M.N., Farrer L.A. Identification of Alzheimer disease-associated variants in genes that regulate retromer function. 2012 doi: 10.1016/j.neurobiolaging.2012.04.020. faging.org/article/S0197-4580(12)00271-0/fulltext [DOI] [PMC free article] [PubMed]
- 99.Miyashita A., Koike A., Jun G., Wang L.S., Takahashi S., Matsubara E., Kawarabayashi T., Shoji M., Tomita N., Arai H., Asada T., Harigaya Y., Ikeda M., Amari M., Hanyu H., Higuchi S., Ikeuchi T., Nishizawa M., Suga M., Kawase Y., Akatsu H., Kosaka K., Yamamoto T., Imagawa M., Hamaguchi T., Yamada M., Morihara T., Takeda M., Takao T., Nakata K., Fujisawa Y., Sasaki K., Watanabe K., Nakashima K., Urakami K., Ooya T., Takahashi M., Yuzuriha T., Serikawa K., Yoshimoto S., Nakagawa R., Kim J.W., Ki C.S., Won H.H., Na D.L., Seo S.W., Mook-Jung I. Alzheimer Disease Genetics, C.; St George-Hyslop, P.; Mayeux, R.; Haines, J.L.; Pericak-Vance, M.A.; Yoshida, M.; Nishida, N.; Tokunaga, K.; Yamamoto, K.; Tsuji, S.; Kanazawa, I.; Ihara, Y.; Schellenberg, G.D.; Farrer, L.A.; Kuwano, R. SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese, Koreans and Caucasians. PLoS One. 2013;8(4):e58618. doi: 10.1371/journal.pone.0058618. http://journals.plos.org/plosone/ article?id=10.1371/journal.pone.0058618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reitz C., Cheng R., Rogaeva E., Lee J.H., Tokuhiro S., Zou F., Bettens K., Sleegers K., Tan E.K., Kimura R., Shibata N., Arai H., Kamboh M.I., Prince J.A., Maier W., Riemenschneider M., Owen M., Harold D., Hollingworth P., Cellini E., Sorbi S., Nacmias B., Takeda M., Pericak-Vance M.A., Haines J.L., Younkin S., Williams J., van Broeckhoven C., Farrer L.A., St George-Hyslop P.H., Mayeux R. Genetic Environmental Risk in Alzheimer Disease, C. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch. Neurol. 2011;68(1):99–106. doi: 10.1001/archneurol.2010.346. https://jamanetwork.com/journals/jamaneurology/ fullarticle/802061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vardarajan B.N., Zhang Y., Lee J.H., Cheng R., Bohm C., Ghani M., Reitz C., Reyes-Dumeyer D., Shen Y., Rogaeva E., St George-Hyslop P., Mayeux R. Coding mutations in SORL1 and Alzheimer’s disease. Ann. Neurol. 2014;77(2):215–227. doi: 10.1002/ana.24305. http://onlinelibrary.wiley.com/doi/10.1002/ ana.24305/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grupe A., Li Y., Rowland C., Nowotny P., Hinrichs A.L., Smemo S., Kauwe J.S., Maxwell T.J., Cherny S., Doil L., Tacey K., van Luchene R., Myers A., Wavrant-De Vrieze F., Kaleem M., Hollingworth P., Jehu L., Foy C., Archer N., Hamilton G., Holmans P., Morris C.M., Catanese J., Sninsky J., White T.J., Powell J., Hardy J., O’Donovan M., Lovestone S., Jones L., Morris J.C., Thal L., Owen M., Williams J., Goate A. A scan of chromosome 10 identifies a novel locus showing strong association with late-onset Alzheimer disease. Am. J. Hum. Genet. 2006;78(1):78–88. doi: 10.1086/498851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li H., Wetten S., Li L., St Jean P.L., Upmanyu R., Surh L., Hosford D., Barnes M.R., Briley J.D., Borrie M., Coletta N., Delisle R., Dhalla D., Ehm M.G., Feldman H.H., Fornazzari L., Gauthier S., Goodgame N., Guzman D., Hammond S., Hollingworth P., Hsiung G.Y., Johnson J., Kelly D.D., Keren R., Kertesz A., King K.S., Lovestone S., Loy-English I., Matthews P.M., Owen M.J., Plumpton M., Pryse-Phillips W., Prinjha R.K., Richardson J.C., Saunders A., Slater A.J., St George-Hyslop P.H., Stinnett S.W., Swartz J.E., Taylor R.L., Wherrett J., Williams J., Yarnall D.P., Gibson R.A., Irizarry M.C., Middleton L.T., Roses A.D. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch. Neurol. 2008;65(1):45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- 104.Liang X., Slifer M., Martin E.R., Schnetz-Boutaud N., Bartlett J., Anderson B., Zuchner S., Gwirtsman H., Gilbert J.R., Pericak-Vance M.A., Haines J.L. Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum. Mutat. 2009;30(3):463–471. doi: 10.1002/humu.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reitz C., Tosto G., Vardarajan B., Rogaeva E., Ghani M., Rogers R.S., Conrad C., Haines J.L., Pericak-Vance M.A., Fallin M.D., Foroud T., Farrer L.A., Schellenberg G.D., George-Hyslop P.S., Mayeux R. Alzheimer’s Disease Genetics, C. Independent and epistatic effects of variants in VPS10-d receptors on Alzheimer disease risk and processing of the amyloid precursor protein (APP). Transl. Psychiatry. 2013;3:e256. doi: 10.1038/tp.2013.13. https://www.nature.com/articles/tp201313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kooner J.S., Saleheen D., Sim X., Sehmi J., Zhang W., Frossard P., Been L.F., Chia K.S., Dimas A.S., Hassanali N., Jafar T., Jowett J.B., Li X., Radha V., Rees S.D., Takeuchi F., Young R., Aung T., Basit A., Chidambaram M., Das D., Grundberg E., Hedman A.K., Hydrie Z.I., Islam M., Khor C.C., Kowlessur S., Kristensen M.M., Liju S., Lim W.Y., Matthews D.R., Liu J., Morris A.P., Nica A.C., Pinidiyapathirage J.M., Prokopenko I., Rasheed A., Samuel M., Shah N., Shera A.S., Small K.S., Suo C., Wickremasinghe A.R., Wong T.Y., Yang M., Zhang F., Abecasis G.R., Barnett A.H., Caulfield M., Deloukas P., Frayling T.M., Froguel P., Kato N., Katulanda P., Kelly M.A., Liang J., Mohan V., Sanghera D.K., Scott J., Seielstad M., Zimmet P.Z., Elliott P., Teo Y.Y., McCarthy M.I., Danesh J., Tai E.S., Chambers J.C. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat. Genet. 2011;43(10):984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ott A., Stolk R.P., Hofman A., van Harskamp F., Grobbee D.E., Breteler M.M. Association of diabetes mellitus and dementia: The Rotterdam study. Diabetologia. 1996;39(11):1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- 108.Luchsinger J.A., Tang M.X., Stern Y., Shea S., Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am. J. Epidemiol. 2001;154(7):635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 109.Zimprich A., Benet-Pages A., Struhal W., Graf E., Eck S.H., Offman M.N., Haubenberger D., Spielberger S., Schulte E.C., Lichtner P., Rossle S.C., Klopp N., Wolf E., Seppi K., Pirker W., Presslauer S., Mollenhauer B., Katzenschlager R., Foki T., Hotzy C., Reinthaler E., Harutyunyan A., Kralovics R., Peters A., Zimprich F., Brucke T., Poewe W., Auff E., Trenkwalder C., Rost B., Ransmayr G., Winkelmann J., Meitinger T., Strom T.M. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharma M., Ioannidis J.P., Aasly J.O., Annesi G., Brice A., Bertram L., Bozi M., Barcikowska M., Crosiers D., Clarke C.E., Facheris M.F., Farrer M., Garraux G., Gispert S., Auburger G., Vilarino-Guell C., Hadjigeorgiou G.M., Hicks A.A., Hattori N., Jeon B.S., Jamrozik Z., Krygowska-Wajs A., Lesage S., Lill C.M., Lin J.J., Lynch T., Lichtner P., Lang A.E., Libioulle C., Murata M., Mok V., Jasinska-Myga B., Mellick G.D., Morrison K.E., Meitnger T., Zimprich A., Opala G., Pramstaller P.P., Pichler I., Park S.S., Quattrone A., Rogaeva E., Ross O.A., Stefanis L., Stockton J.D., Satake W., Silburn P.A., Strom T.M., Theuns J., Tan E.K., Toda T., Tomiyama H., Uitti R.J., Van Broeckhoven C., Wirdefeldt K., Wszolek Z., Xiromerisiou G., Yomono H.S., Yueh K.C., Zhao Y., Gasser T., Maraganore D., Kruger R. A multi-centre clinico-genetic analysis of the VPS35 gene in Parkinson disease indicates reduced penetrance for disease-associated variants. J. Med. Genet. 2012;49(11):721–726. doi: 10.1136/jmedgenet-2012-101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zavodszky E., Seaman M.N., Moreau K., Jimenez-Sanchez M., Breusegem S.Y., Harbour M.E., Rubinsztein D.C. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. 2014 doi: 10.1038/ncomms4828. https://www.nature.com/articles/ [DOI] [PMC free article] [PubMed]
- 112.Tsika E., Glauser L., Moser R., Fiser A., Daniel G., Sheerin U.M., Lees A., Troncoso J.C., Lewis P.A., Bandopadhyay R., Schneider B.L., Moore D.J. Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum. Mol. Genet. 2014;23(17):4621–4638. doi: 10.1093/hmg/ddu178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.MacLeod D.A., Rhinn H., Kuwahara T., Zolin A., Di Paolo G., McCabe B.D., Marder K.S., Honig L.S., Clark L.N., Small S.A., Abeliovich A. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77(3):425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nuytemans K., Bademci G., Inchausti V., Dressen A., Kinnamon D.D., Mehta A., Wang L., Zuchner S., Beecham G.W., Martin E.R., Scott W.K., Vance J.M. Whole exome sequencing of rare variants in EIF4G1 and VPS35 in Parkinson disease. Neurology. 2013;80(11):982–989. doi: 10.1212/WNL.0b013e31828727d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rajput A., Ross J.P., Bernales C.Q., Rayaprolu S., Soto-Ortolaza A.I., Ross O.A., van Gerpen J., Uitti R.J., Wszolek Z.K., Rajput A.H., Vilarino-Guell C. VPS35 and DNAJC13 disease-causing variants in essential tremor. Eur. J. Hum. Genet. 2014;23(6):887–888. doi: 10.1038/ejhg.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X., Zhao Y., Zhang X., Badie H., Zhou Y., Mu Y., Loo L.S., Cai L., Thompson R.C., Yang B., Chen Y., Johnson P.F., Wu C., Bu G., Mobley W.C., Zhang D., Gage F.H., Ranscht B., Zhang Y.W., Lipton S.A., Hong W., Xu H. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat. Med. 2013;19(4):473–480. doi: 10.1038/nm.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Freeman C., Seaman M.N., Reid E. The hereditary spastic paraplegia protein strumpellin: Characterisation in neurons and of the effect of disease mutations on WASH complex assembly and function. Biochim. Biophys. Acta. 2013;1832(1):160–173. doi: 10.1016/j.bbadis.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ropers F., Derivery E., Hu H., Garshasbi M., Karbasiyan M., Herold M., Nurnberg G., Ullmann R., Gautreau A., Sperling K., Varon R., Rajab A. Identification of a novel candidate gene for non-syndromic autosomal recessive intellectual disability: The WASH complex member SWIP. Hum. Mol. Genet. 2011;20(13):2585–2590. doi: 10.1093/hmg/ddr158. [DOI] [PubMed] [Google Scholar]
- 119.Verhoeven K., De Jonghe P., Coen K., Verpoorten N., Auer-Grumbach M., Kwon J.M., FitzPatrick D., Schmedding E., De Vriendt E., Jacobs A., Van Gerwen V., Wagner K., Hartung H.P., Timmerman V. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am. J. Hum. Genet. 2003;72(3):722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ollila H.M., Soronen P., Silander K., Palo O.M., Kieseppa T., Kaunisto M.A., Lonnqvist J., Peltonen L., Partonen T., Paunio T. Findings from bipolar disorder genome-wide association studies replicate in a Finnish bipolar family-cohort. Mol. Psychiatry. 2009;14(4):351–353. doi: 10.1038/mp.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mecozzi V.J., Berman D.E., Simoes S., Vetanovetz C., Awal M.R., Patel V.M., Schneider R.T., Petsko G.A., Ringe D., Small S.A. Pharmacological chaperones stabilize retromer to limit APP processing. Nat. Chem. Biol. 2014;10(6):443–449. doi: 10.1038/nchembio.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]