Abstract
Background:
Dysregulated stress neurocircuits, caused by genetic and/or environmental changes, underlie the development of many neuropsychiatric disorders. Corticotropin-releasing factor (CRF) is the major physiological activator of the hypothalamic-pituitary-adrenal (HPA) axis and conse-quently a primary regulator of the mammalian stress response. Together with its three family members, urocortins (UCNs) 1, 2, and 3, CRF integrates the neuroendocrine, autonomic, metabolic and behavioral responses to stress by activating its cognate receptors CRFR1 and CRFR2.
Objective:
Here we review the past and current state of the CRF/CRFR field, ranging from pharmacologi-cal studies to genetic mouse models and virus-mediated manipulations.
Results:
Although it is well established that CRF/CRFR1 signaling mediates aversive responses, includ-ing anxiety and depression-like behaviors, a number of recent studies have challenged this viewpoint by revealing anxiolytic and appetitive properties of specific CRF/CRFR1 circuits. In contrast, the UCN/CRFR2 system is less well understood and may possibly also exert divergent functions on physiol-ogy and behavior depending on the brain region, underlying circuit, and/or experienced stress conditions.
Conclusion:
A plethora of available genetic tools, including conventional and conditional mouse mutants targeting CRF system components, has greatly advanced our understanding about the endogenous mecha-nisms underlying HPA system regulation and CRF/UCN-related neuronal circuits involved in stress-related behaviors. Yet, the detailed pathways and molecular mechanisms by which the CRF/UCN-system translates negative or positive stimuli into the final, integrated biological response are not completely un-derstood. The utilization of future complementary methodologies, such as cell-type specific Cre-driver lines, viral and optogenetic tools will help to further dissect the function of genetically defined CRF/UCN neurocircuits in the context of adaptive and maladaptive stress responses.
Keywords: Corticotropin-releasing factor, urocortin, stress, mouse genetic tools, hypothalamic-pituitary-adrenal (HPA), neuropsychiatric disorders
1. Introduction
“It’s not stress that kills us, it is our reaction to it” Hans Selye (1907-1982).
Over the few past decades, growing evidence has linked life stress to various pathologies, including cardiovascular disease, inflammation, metabolic dysfunctions, and most prominently neurodegenerative and psychiatric disorders. In particular, conditions of severe prolonged stress are considered to be the most devastating because they tend to induce long-term or permanent changes in the physiological, emotional and behavioral responses that influence susceptibility to disease. Persistent stress, such as prolonged exposure to war, physical abuse, devastating socioeconomic status or social/psychological surroundings, has been shown to increase the likelihood of developing depression and anxiety disorders, cognitive dysfunction, metabolic conditions such as obesity and diabetes, as well as sleep and cardiovascular disorders, to name just a few [1-7]. However, we tend to forget that stress per se is not a bad thing, but rather the reaction and/or inability to adapt to it that constitutes health or disease. Importantly, acute stress can exert a wide range of positive effects, as it primes the brain towards optimal alertness, behavioral and cognitive performance [8-13]. The reaction to stress represents an adaptive mechanism, triggering the so-called “fight-or-flight” response in order to cope with a dangerous situation, be it a predator, an accident, or a natural disaster. Stress can be discriminated on the one hand into eustress, or “positive” stress, meaning that the succeeding adaptive response is able to re-instate homeostasis, and on the other hand into distress, or “negative” stress resulting in pathological outcomes [14]. In general, we are not equipped to withstand chronic activation of specific stress-pathways, which is increasingly occurring in today’s urbanized social environments partially due to disparities in income, education, occupation and other dimensions of socioeconomic status [2]. But when does stress, or more precisely the response to stress, cross the line from being adaptive to maladaptive? This question is extremely difficult to answer, considering that the threshold of stress-resistance is different for each individual and is influenced to a variable degree by genetic predisposition [15, 16].
Two closely interplaying systems are primarily responsible for orchestrating the stress response: the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis. The SNS is largely responsible for initiating the “flight-or-flight” reaction by stimulating, amongst others, the release of adrenaline and noradrenaline from the adrenal medulla. The latter exerts its commands at multiple sites, including the spinal cord, medulla, pons and higher order centers such as the hypothalamus [17, 18]. The HPA axis is characterized by the release of different neuropeptides and hormones, and is believed to mediate the immediate, as well as the long-lasting effects of stress. As a result of the two interplaying systems, various substances are released in response to stress, which are then orchestrated into a coordinated physiological and behavioral response [19]. These so-called stress-mediators are broadly classified into three groups; the monoamines, neuropeptides and steroids. Importantly, different stressors are processed by distinct circuits and/or in specific brain areas. The non-specific effects of stress are mirrored by the rapid activation of the SNS and the neuroendocrine arm of the stress response, i.e. the HPA axis, which in turn regulates the synthesis and release of glucocorticoids (GCs) from the adrenal glands [19]. Conversely, psychological and anticipatory stressors (stress due to hypothetical events that may/may not occur) are primarily processed by the limbic system, including the hippocampus, amygdala and prefrontal cortex [19, 20]. The complex interaction between these different stress-mediators and pathways has gained substantial interest in the past [21]. However, some play very specific and potentially even opposing roles than others and are especially relevant in dysfunctional stress circuits, which can result in various neuropsychopathologies [19, 22]. Already in 1955, Hans Selye postulated that “through some unknown pathway, the “first mediator” travels directly from the injured target area to the anterior pituitary. It notifies the latter that a condition of stress exists and thus induces it to discharge adrenocorticotropic hormone (ACTH)” [23]. It took another 26 years until Wylie Vale´s group discovered this central stress mediator - the neuropeptide corticotropin-releasing factor (CRF). This major breakthrough contributed significantly to our understanding of the neurobiological mechanisms underlying the stress response [24].
2. CRF modulates the neuroendocrine stress response via the HPA axis
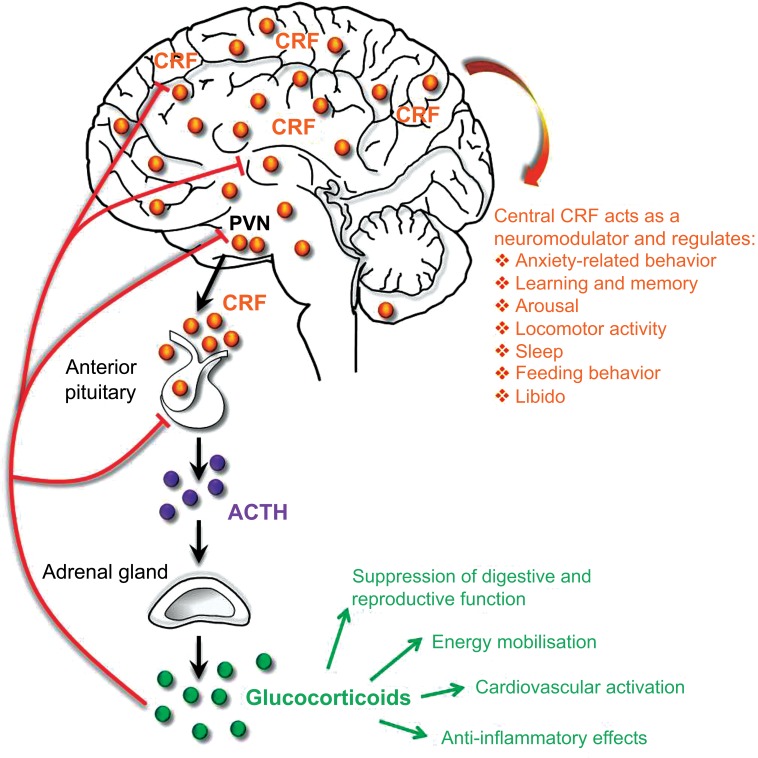
CRF (also referred to as corticotropin-releasing hormone – CRH) is the major physiological activator of the HPA axis, and coordinates the neuroendocrine response to stress. Perception of physical or psychological stress by an organism is followed by a series of events, including the release of CRF from parvocellular neuroendocrine neurons of the paraventricular nucleus of the hypothalamus (PVN). These neurons project via the external zone of the median eminence and release CRF into the hypophysial portal vasculature, which transports the neuropeptide to secretory corticotrope cells of the anterior pituitary, which express the CRF receptor type 1 (CRFR1; Fig. 1). The activation of CRFR1 stimulates the release of ACTH and other pro-opiomelanocortin (POMC) -derived peptides [25]. ACTH, in turn, triggers the synthesis and release of GCs from the adrenal cortex (cortisol in humans, corticosterone in rodents), which mediate numerous physiological and metabolic reactions and ultimately prepare the organism to deal with the stressful situation. These responses to GCs include cardiovascular activation, energy mobilization, anti-inflammatory effects and suppression of reproductive and digestive functions, [26-32]. In order to restore the HPA axis to its normal state and to protect it from overshooting, GCs signal back via glucocorticoid (GR) and mineralocorticoid receptors (MR) at various feedback levels (e.g. pituitary, hippocampus, PVN and amygdala), which ultimately inhibit the secretion of CRF and consequently ACTH (Fig. 1). Similar to many physiological processes in the body, GCs exhibit a circadian rhythm, with increased levels toward the active phase of the light/dark cycle, which is regulated by the main circadian pacemaker in the suprachiasmatic nucleus [33, 34]. At this point it is also important to note that the HPA axis is not exclusively activated during aversive stressful situations. In fact, the physiological stress-response to appetitive, rewarding stimuli (which are generally not considered as stressors) can be as large as the response to a negative stimulus. For example, positive experiences such as sexual encounter, wheel running and social victory in rats induce a similar degree of HPA axis activation as an aversive footshock, social defeat and restraint stress [35, 36]. Consequently, HPA axis activation and enhanced cortisol levels are not solely indicative of an aversive stressful state.
Fig. (1).
CRF regulates neuroendocrine and behavioral responses to stress. CRF integrates neuroendocrine and higher-order behavioral responses by regulating peripheral HPA axis function and modulating synaptic transmission in the CNS. ACTH: Adrenocorticotropic hormone; CRF: corticotropin-releasing factor, PVN: hypothalamic paraventricular nucleus.
3. The family of CRF-related neuropeptides and their receptors
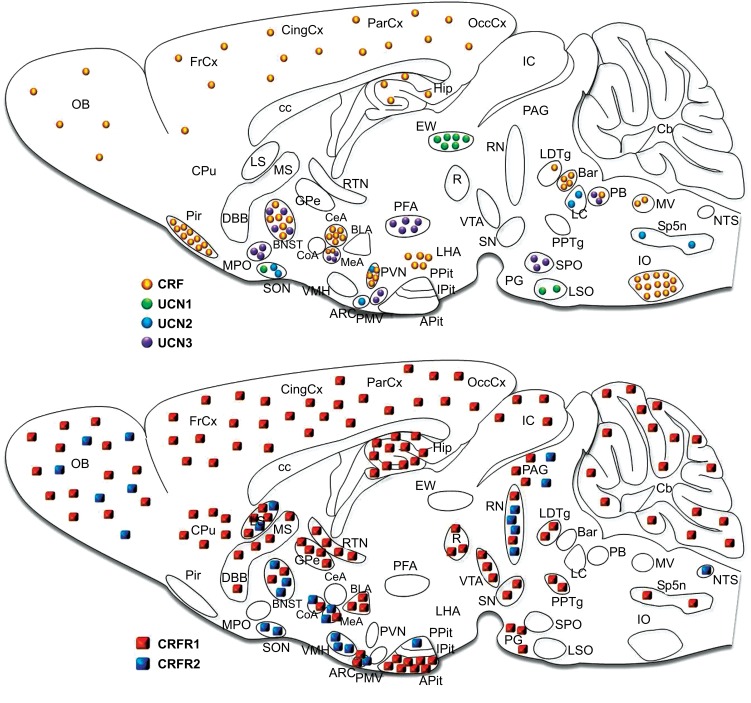
The actions of CRF are not confined to the neuroendocrine HPA system. The anatomical distribution of CRF in the brain suggests that this peptide not only acts as a key neuroendocrine stress mediator, but is also able to regulate neuronal activity in a neuromodulatory fashion. In fact, CRF is expressed throughout the central nervous system (CNS) including in most limbic and cortical structures (Fig. 2), where it has been shown to regulate the emotional and cognitive components of the stress response. The mature and biologically active form of CRF is a 41-amino acid peptide generated by proteolytic cleavage from a 196-amino acid precursor. To date, the mammalian CRF family comprises three additional peptides (Fig. 3). Urocortin (UCN) 1 was initially described in 1995 by Vaughan and colleagues [37] followed by the discovery of UCN2 (or stresscopin-related peptide) and UCN3 (or stresscopin) shortly afterwards [38-40]. CRF is most closely related to UCN1, sharing 43% amino acid homology, whereas CRF sequence identity with UCN2 and UCN3 is 34% and 26% respectively [41]. In comparison to CRF, urocortin-expressing neurons are found in more discrete regions and nuclei of the CNS (Fig. 2). In rodents, UCN1 is mainly expressed in the Edinger-Westphal nucleus and sparsely distributed in the lateral superior olive and supraoptic nucleus [37]. UCN2 is found in the rodent PVN, supraoptic nucleus, arcuate nucleus, locus coeruleus and brainstem, whereas UCN3 is mainly expressed in the medial amygdala, rostral perifornical area of the hypothalamus, the bed nucleus of the stria terminalis (BNST), superior paraolivary nucleus, nucleus parabrachialis and the premammillary nucleus (Fig. 2); [38-40, 42]. All four neuropeptides have also been detected in the periphery, in particular UCN2 and UCN3, which have recently been recognized as novel modulators of centrally- and peripherally-controlled metabolic function [43-45].
Fig. (2).
Schematic illustrations of the spatial distribution and relative expression of CRF family peptides and their receptors in the mouse brain. Abbreviations: Anterior pituitary (APit), arcuate nucleus (ARC), basolateral nucleus of the amygdala (BLA), bed nucleus of the stria terminalis (BNST), caudate putamen (CPu), central nucleus of the amygdala (CeA), cerebellum (Cb), cingulate cortex (CingCx), corpus callosum (cc), cortical nucleus of the amygdala (CoA), Barrington’s nucleus (Bar), diagonal band of Broca (DBB), Edinger Westphal nucleus (EW), frontal cortex (FrCx), globus pallidus (GPe), inferior colliculi (IC), inferior olive (IO), intermediate lobe of the pituitary (IPit), locus coeruleus (LC), lateral septum (LS), laterodorsal tegmental nucleus (LDTg), lateral hypothalamic area (LHA), lateral superior olive (LSO), medial nucleus of the amygdala (MeA), medial preoptic area (MPO), medial septum (MS), medial vestibular nucleus (MV), nucleus tractus solitarii (NTS), olfactory bulb (OB), occipital cortex (OccCx), parietal cortex (ParCx), parabrachial nucleus (PB), periaquaductal gray (PAG), perifornical area (PFA), piriform cortex (Pir), pontine gray (PG), posterior pituitary (Ppit), pedunculopontine tegmental nucleus (PPTg), premammillary nucleus (PMV), paraventricular nucleus of the hypothalamus (PVN), red nucleus (R), raphe nuclei (RN), reticular thalamic nucleus (RTN), superior colliculi (SC), substantia nigra (SN), supraoptic nucleus (SON), spinal trigeminal nucleus (Sp5n), superior paraolivary nucleus (SPO), ventral medial hypothalamus (VMH), ventral tegmental area (VTA). Modified from [88].
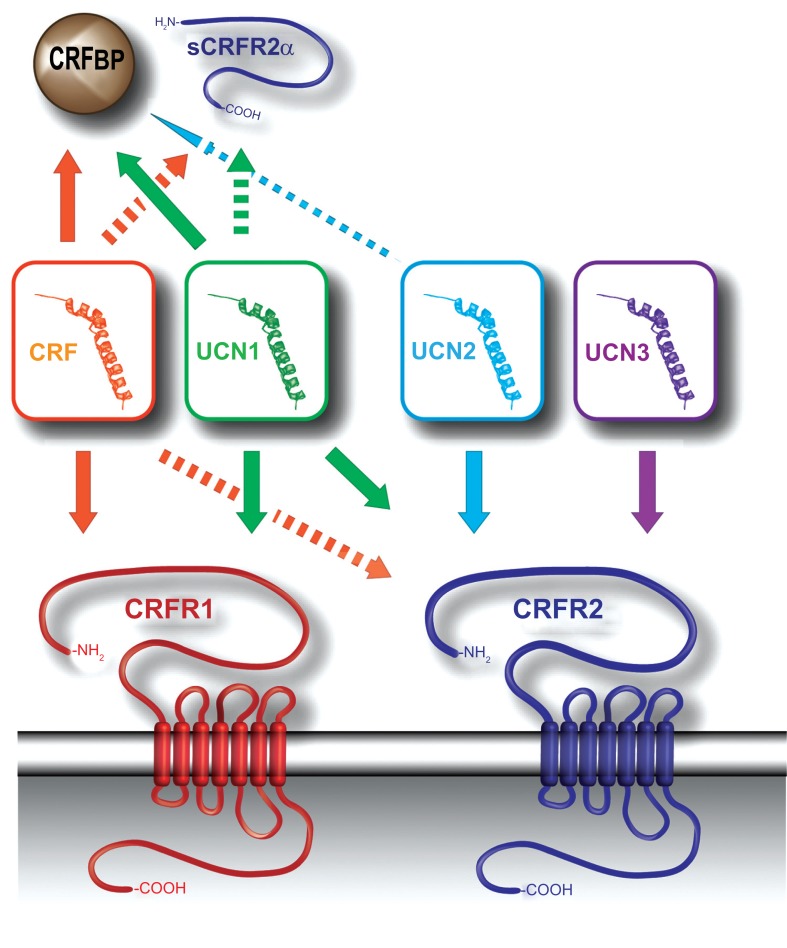
Fig. (3).
CRF family members, their receptors and binding proteins. CRF and the UCNs signal through one of two CRF receptors (CRFR1 and CRFR2). The arrows represent ligand-receptor or ligand-binding protein interactions. Dashed arrows indicate low-affinity binding, compared to solid arrow-lines. CRF displays a relatively high affinity for CRFR1 and a low affinity for CRFR2, while UCN1 binds to both receptors with equal affinity. UCN2 and UCN3 are selective ligands for CRFR2. CRFBP and sCRFR2α are able to sequester both CRF and UCN1, while CRFBP exerts a low affinity for UCN2. Abbreviations: corticotropin-releasing factor (CRF), CRF receptor 2 (CRFR2), CRF binding protein (CRFBP), soluble variant of CRFR2 (sCRFR2α).
In contrast to the detailed expression profiles in rodents, data is more limited with respect to CRF and UCN1-3 distribution within the human CNS. However, a few studies have examined CRF/UCN1 expression in human postmortem brains. In accordance with the observations in mice and rats, CRF immunoreactivity and mRNA levels in humans were demonstrated in the PVN, pituitary stalk, hypothalamus, and cortex [46-50]. As in rodents, the most abundant expression of UCN1 in humans is found in the Edinger-Westphal nucleus [51, 52]. However UCN1 expression was also detected in the human anterior pituitary and Purkinje cells of the cerebellar cortex, which has not been observed in the mouse brain [52, 53].
CRF and the urocortins signal through the activation of two, class B1, membrane-bound, G-protein-coupled receptors (GPCRs), CRFR1 and CRFR2, which share 70% amino acid identity [54-58]. The lowest degree of homology exists in their N-terminal extracellular domains (40% identity). In contrast, the transmembrane domains of CRFR1 and CRFR2 are highly homologous (80-85% amino acid identity) [41]. CRF shows a much higher affinity for the CRFR1 than for the CRFR2 while UCN1 displays equal affinities for both receptors (Fig. 3). UCN2 and UCN3, on the other hand, appear to be selective ligands of CRFR2 [38, 41, 56]. Similarly to its main ligand CRF, CRFR1 mRNA is found throughout the rodent CNS including the cortex, cerebellum and limbic forebrain (Fig. 2). It is also highly expressed in anterior pituitary corticotropes where it initiates HPA axis activity in response to CRF binding [59, 60]. CRFR1 has only one known functional splice variant (α) expressed in the CNS [61, 62]. Particularly in the skin, numerous additional splice variants have been identified resulting in soluble or membrane bound isoforms that might potentially affect receptor activity via dimerization [62-64]. In contrast, CRFR2 has three functional splice variants in humans (α, β and γ) and two in rodents (α and β) [56, 61, 65-68]. CRFR2α (the major splice variant in rodents and from here on referred to as CRFR2), displays a more confined but partially overlapping expression with CRFR1, with high densities in the olfactory bulb, BNST, lateral septum (LS), ventromedial hypothalamic nucleus, and dorsal raphe nucleus (Fig. 2); [60, 69-71]. In mice, CRFR2β is primarily expressed in peripheral tissue (with the highest levels of expression in skeletal muscle, heart and skin), as well as in the choroid plexus of the brain [56, 69].
CRFR1 and CRFR2 are also highly expressed in the human brain, although their expression patterns deviate to some extent from the rodent brain [72-75]. In general, CRFR1 represents the major receptor in the brain of humans and non-human primates, while CRFR2 shows a predominant expression in peripheral tissues [72]. Both receptors are abundantly expressed in the pituitary, which is in contrast to the distribution in rodents, where mainly CRFR1 is found in the pituitary [72, 73]. CRFR1 and CRFR2 are also present in the amygdala, thalamus, and hippocampus. In addition, significant levels of CRFR1 have been detected in the cerebellum and cortex of humans and non-human primates [73, 75]. Another discrepancy between rodents and primates is the presence of CRFR2 in different cortical regions [73]. The absence of CRFR2 from the cerebellum, and a strong expression in the choroid plexus of the human brain is again in line with observations in the rodent brain [73].
The activity of CRF and UCN1 can be regulated additionally by the CRF binding protein (CRF-BP) [41, 76]. Past research has largely ignored the presence of the CRF-BP, which is thought to act as an endogenous buffer, possibly by regulating the availability of active CRF and UCN1 [76-78]. The complexity of the CRF-system is further increased by the recently discovered soluble splice variant of CRFR2 (sCRFR2α), which encodes the extracellular receptor ligand-binding domain, but terminates before the first transmembrane domain [79]. sCRFR2α has been proposed to act as a decoy receptor, mimicking the ability of the CRF-BP to sequester free CRF [79]. Alternatively, Evens and colleagues suggested that the unproductive splicing of CRFR2 pre-mRNA to sCRFR2 may selectively alter the cellular levels of full-length CRFR2 mRNA and consequently affect the number of functional CRFR2 receptors, a mechanism common to GPCRs of the secretin family [80, 81]. The diverse and broad expression patterns of CRF-related peptides and receptors, as well as the high level of signaling complexity, enable this circuitry to effectively integrate neuroendocrine, autonomic and behavioral responses of stress.
4. Neuromodulatory effects of central CRF-CRFR1 signaling
As previously introduced, CRF coordinates the physiological/neuroendocrine responses to stress via the HPA axis. In addition, CRF and its high affinity receptor, CRFR1, are widely distributed throughout the brain, which allows them to orchestrate autonomic and behavioral stress responses. Consequently, hyperactivity of the CRF/CRFR1 system has been linked to stress-related psychiatric disorders that involve a strong emotional component such as depression and anxiety [6, 82-87]. Alterations in HPA axis function, such as impaired negative feedback, which results in hypercortisolemia, have been reported repeatedly in a subset of depressed patients, and attributed to centrally elevated CRF levels [82, 88]. Increased CRF levels have also been detected in the CSF of untreated depressed patients [87]. Furthermore, reduced CRF binding sites have been identified in the frontal cortex of suicide victims, which was attributed to an adaptive downregulation in response to CRF hypersecretion [89].
Shortly after its isolation in 1981, a number of rodent studies demonstrated that intracerebroventricular (i.c.v) administration of CRF results in behavioral responses that are similar to those observed in stressed animals. These include increased arousal and anxiety-related behavior, altered locomotor activity and social behavior, diminished sexual behavior and food consumption as well as sleep disturbances [90-99]. Importantly, many of these effects were independent of downstream GC effects, defining the ability of central CRF to coordinate behavioral responses independent of, or in synergism with peripheral HPA axis function [87, 100, 101]. Evidently, all of these studies support a role of CRF hyperactivity in stress-related neuropathophysiologies. In order to further elucidate the brain regions responsible for mediating the effects of CRF on behavior, a large body of research focused on site-specific CRF administration and pharmacological manipulations [99]. Naturally the involvement of the limbic system was investigated given its modulatory role in emotion, motivation and cognition.
4.1. Limbic System: Hippocampus and Extended Amygdala
The effects of CRF on hippocampal function and integrity with respect to learning and memory were repeatedly investigated in the past. The hippocampus contains scattered CRF-expressing GABAergic interneurons and numerous CRFR1-expressing excitatory pyramidal neurons [59, 102-106]. It is generally proposed that a short-lived increase in CRF facilitates hippocampus-dependent learning and memory (similarly to acute stress), whereas prolonged exposure to elevated CRF impairs cognitive performance [107-109]. However, this is largely based on electrophysiological studies in slice cultures and requires more validation in vivo. CRF exerts its effect by potentiating excitatory neurotransmission in the hippocampus, providing direct evidence for an interaction with the glutamatergic system [106, 110-112]. Yet it remains largely unknown how CRF is able to modulate excitatory neurotransmission in the hippocampus considering that it is primarily released from GABAergic interneurons, in which co-release of GABA would presumably induce inhibition. Local diffusion, or long-distance signaling via volume transmission has been proposed as a mode of action by which CRF is able to target receptors in the vicinity of its release site [107]. Knowledge about the precise cellular localization of the receptor would advance our understanding in this regard, but has so far been hampered by the lack of specific CRFR1 antibodies [106]. At present, CRFR1 has been found on cell bodies, dendritic shafts and dendritic spines of hippocampal neurons [105, 113, 114]. The adverse effects of chronic CRF release (as they occur during persistent stress) are proposed to result from CRF-induced dendritic spine loss on CRFR1-expressing neurons in the hippocampus [107, 115, 116]. Interestingly, only a subpopulation of co-called “thin spines” seem to be lost following excessive CRF release, but their absence results in profound memory impairments [117]. Along these lines, stress-induced dendritic atrophy has also been linked to excessive release of CRF [114, 117]. Accordingly, CRFR1-antagonists were shown to reduce hippocampus-dependent deficits in memory and synaptic long-term potentiation [117]. At the same time, CRF is required for fear memory formation given that acute injections into the dorsal hippocampus enhance contextual and auditory fear memory [118]. This is also supported by previous experiments with CRF receptor antagonists, which have resulted in fear memory impairments [119, 120]. In addition, CRF injections into the ventral hippocampus were shown to increase anxiety-related behavior in rats [121].
The amygdala plays a prominent role in fear memory acquisition and expression, and modulates aspects of anxiety-related behavior. In rodents, CRF is highly expressed in the central nucleus of the amygdala (CeA), whereas CRFR1 is primarily located in the basolateral amygdala (BLA) [59, 102, 103, 106, 122, 123]. CRF application into the BLA enhances anxiety-related behavior and reduces social interaction in rats [124]. CeA-infusion of CRF receptor antagonists ameliorates stress-induced anxiety and freezing behavior [91, 125], which is likely due to blockage of receptors in the BLA caused by diffusion of the antagonists. Similarly, intra-BLA administration of antalarmin, a CRFR1-antagonist, counteracts social defeat-induced defensive behavior in mice [126]. Along these lines, viral-mediated knockdown of CRFR1 in the BLA of mice mimicked the anxiolytic effect of environmental enrichment [127]. Another CRF-expressing brain region which has attracted increasing attention in the recent years is the BNST. Often referred to as the extended amygdala, the BNST is heavily innervated by the amygdala and projects to the PVN and brainstem monoaminergic nuclei including the locus coeruleus and ventral tegmental area [128-133]. Recent optogenetic studies in mice have clearly implicated the BNST in the modulation of anxiety [134, 135]. Consequently, some of these effects might be modulated via CRF, but so far only few studies have investigated the role of BNST-CRF neurons in emotional behavior. Microinfusion of CRF into the BNST enhanced the startle amplitude, and retention in an inhibitory avoidance task in rats [136, 137]. Similarly, intra-BNST administration of CRF in rats elicited a dose-dependent increase in anxiety-related behavior, which could be reversed upon CRFR1 antagonist treatment [138]. In addition, CRF in the BNST has repeatedly been related to addiction, more specifically stress-induced relapse [139, 140]. This is interesting considering that 30-40% of individuals suffering from addictive disorders have a comorbid mood or anxiety disorder [141]. Intra-BNST injection of CRF in rats can induce reinstatement (relapse to drug-seeking behavior in animals), which can be blocked by application of CRF antagonists [142, 143]. Numerous studies have consequently linked the CRF-pathway to dopaminergic signaling, which is primarily involved in addiction-related processes [139, 140, 144-147]. Direct and indirect mechanisms were proposed by which CRF is able to enhance dopaminergic firing in order to drive stress- or cue-induced drug- and alcohol seeking behaviors.
4.2. Modulation of Catecholamines: Ventral Tegmental Area (VTA) and the Locus Coeruleus (LC)
CRF-dopamine interactions in the context of stress and addiction have been extensively examined [144, 148, 149]. The CRF/CRFR1 system has been repeatedly associated with stress-induced drug reinforcement, where it acts to facilitate relapse and increase anxiety during acute and chronic withdrawal [139]. Accordingly, CRF was shown to increase dopamine neuron firing and dopamine release [106, 150-155], although opposite results were also observed depending on the study-design and the investigated release sites [150, 151]. A recent study by Lemos and colleagues demonstrated that CRF acts in the nucleus accumbens (NAc) to increase dopamine release and promote appetitive behavior in mice; an effect which is lost following previous stress exposure [150]. This suggests that CRF might differentially affect the reward circuitry under basal and stress conditions. CRFR1 is expressed in dopaminergic neurons of the VTA and substantia nigra pars compacta [106] while the source of CRF is believed to derive from axons that originate in the forebrain [147, 150, 156-160]. However, Grieder and colleagues recently identified a subpopulation of VTA dopaminergic neurons that express CRF, which is upregulated following chronic nicotine exposure [161]. The ability of CRF to modulate dopaminergic neurotransmission has recently prompted investigations in other behavioral domains, revealing an important role for CRF/CRFR1-dopamine interactions in the context of anxiety and social behavior [106, 162].
CRF is also viewed as a potential mediator of stress-elicited locus coeruleus (LC) activation, the brain’s major noradrenergic nucleus [163, 164]. The LC sends noradrenergic projections throughout the brain, including the brain-stem, cortical, limbic and hypothalamic structures, and is consequently able to modulate various behavioral, endocrine and autonomic responses [164-167]. Dysregulated noradrenergic circuits via excessive CRF have been proposed to underlie pathological hyperarousal observed in numerous stress-related psychiatric disorders [168-170]. CRF is able to induce LC neuronal firing, which is believed to modulate behavioral arousal and attention during stressful situations [163, 164, 171]. Hence, CRF not only facilitates activation of glutamatergic neurotransmission in the hippocampus, and dopaminergic firing in the VTA, but also noradrenergic firing in the LC. In addition, CRF can indirectly regulate endocrine responses via activation of the noradrenergic system, which in turn regulates components of the HPA axis [164, 169, 172, 173]. CRF-immunoreactive fibers innervate the LC [174], although unequivocal evidence for the presence of CRFR1 or CRFR2 in the LC is still lacking, in particular in mice [60, 164]. Moreover, the sources of CRF afferents to the LC which modulate specific behavioral effects have not been clearly identified.
5. Neuromodulatory effects of central UCN/CRFR2-signaling
Whereas the role of CRF/CRFR1 in the modulation of HPA axis activity, stress-induced behavior and cognitive functions is well established, the role of CRFR2 and the urocortins still remains controversial. Although still debated, it is postulated that CRF/CRFR1 signaling mediates the initial reaction to stress, whereas UCN/CRFR2 activation controls the later adaptive phase [51, 88, 175]. UCN1 neurons are mainly localized in the Edinger Westphal (EW) nucleus where they constitute the centrally-projecting part of the nucleus (EWcp). UCN1 neurons are recruited following chronic stress exposure, and stay active for a prolonged period of time, suggesting that this peptide plays a prominent role in the later adaptive phase of the stress response [176-178]. Evidence for a possible role of UCN1 in the regulation of mood comes from enhanced UCN1 expression in the EWcp of depressed male suicide victims [53]. Similar to the i.c.v. administration of CRF, UCN1 application was also shown to result in behavioral responses similar to those observed in stressed animals [99, 179], which include increased grooming, locomotion and anxiety-related behavior [180, 181]. However, it is often not clear whether these effects result from CRFR1 or CRFR2 activation. Contradictory is also the observation that UCN1 knockout mice display enhanced anxiety-related behavior (discussed later on). The behavioral outcomes of central UCN2 or UCN3 application have yielded contrasting results depending on the dose and site of application. Previous studies have demonstrated anxiolytic-like effects of CRFR2 activation following i.c.v. administration of UCN2 [182] or UCN3 [183-185] in mice and rats. Surprisingly, CRFR2 antagonism with antisauvagine-30 was also shown to produce anxiolytic responses in rats [186]. The interpretation of these results is further complicated by additional studies reporting no changes in behavioral arousal and anxiety, while others revealed anxiogenic effects of UCN2 administration or CRFR2 activation in the LS [187, 188] and dorsal raphe nucleus [189]. Consequently, CRFR2-activation might exert differential behavioral and neuroendocrine effects depending on the brain region and experienced stress conditions.
5.1. Modulation of Serotonergic System: Raphe Nuclei
A growing body of literature is implicating CRFR2 activation in the modulation of the serotonergic system, although some of the effects are not solely attributable to the UCNs, but also to CRF [190-196]. CRFR2 is abundant in the midbrain raphe nuclei [60, 69], where it regulates firing rates of serotonin (5-HT) neurons and 5-HT release in efferent stress-related nuclei of the forebrain [191, 193-195]. I.c.v. administration of UCN2 in mice induces enhanced c-fos immunoreactivity in serotonergic neurons of the DRN [193, 197]. Site-specific injection of UCN2 into the DRN was shown to increase c-fos expression in a subpopulation of serotonergic neurons [198], induce 5-HT release in the BLA [190] and potentiate conditioned fear and escape deficits in a model of learned helplessness [189]. On the other hand, injection of the CRFR2 antagonist, antisauvagine-30, resulted in anxiolytic effects [186] including reversal of the potentiation of conditioned fear and the escape deficits following exposure to inescapable stress [189]. These effects were not observed upon CRFR1-antagonist application, suggesting that CRFR2 on serotonergic neurons in the DRN is conveying anxiety in response to uncontrollable stress. However, the identity and expression sites of CRF/UCN-producing neurons targeting CRFR2 in the DRN remain largely elusive.
5.2. Modulation of Energy Balance and Feeding Behavior
Chronic stress has repeatedly been associated with altered metabolic function, in both animal and human studies. Along these lines, growing evidence indicates that mediators of the stress response represent a key locus for gene-environment interactions in the shared biology of depression and obesity [199]. It is well documented that members of the CRF family, and more prominently the UCNs, are capable of suppressing food intake and altering energy expenditure following central or peripheral administration [183, 200-205]. Employing specific pharmacological and genetic tools, a number of studies have established a predominant role for CRFR2 in mediating the anorexigenic effects of CRF and UCNs [43, 206]. The major CRFR2-expressing brain structures identified to play a role in CRFR2-mediated anorexigenic responses are the ventromedial hypothalamus (VMH), LS, PVN, medial amygdala and DRN [207-212]. CRFR2 signaling pathways in the VMH are starting to gain increasing attention, which is not surprising considering the significant role of this brain region in metabolic regulation. Microinjections of UCN3 into the PVN or VMH were shown to induce satiety [207, 213], and site-specific knockdown of CRFR2 in the VMH resulted in enhanced food intake under basal conditions and following food-deprivation [214]. These results are in part supported by CRFR2 knockout mice, which display increased nocturnal food intake of normal chow [215] and consumed more high fat food compared to littermate controls [216]. In support of this, mice lacking UCN3 showed elevated basal feeding and increased nocturnal food intake after overnight fasting [214], while mice overexpressing UCN3 show leaner body composition and are protected against diet-induced obesity and hyperglycaemia. On the other hand, UCN1 and UCN2 knockout mice display normal spontaneous food intake, which could be the result of a functional compensation by the other two family members, CRF and UCN3 [217, 218]. However, the mechanisms underlying CRFR2/UCN-induced anorexia are not fully understood. Several concepts have been proposed including suppression of gastric emptying and induction of hyperglycemia by CRF/UCNs, as well as direct effects on ghrelin and leptin, the main orexigenic and anorectic peptides in the brain [43, 206]. In addition, CRFR1, CRFR2, UCN1 and UCN3 were shown to be expressed in human adipose tissue, which might indicate a direct effect on fat cell function in addition to the central effects on weight regulation [219]. More recent work has additionally demonstrated that UCN2 and UCN3 also act as autocrine and/or paracrine regulators of glucose homeostasis in the periphery by modulating insulin sensitivity in skeletal muscles or by regulating glucose-induced insulin secretion in beta-cells of the pancreas, respectively [43, 206]. UCN3 is expressed by both beta and alpha cells in human islets [44, 220, 221] and was shown to be markedly depleted in human diabetic islets [222]. Together, CRFR2 and the UCNs help to maintain energy homeostasis in the presence of diverse stressors, through numerous adaptive responses in both the CNS and peripheral tissues [43].
6. Genetically Engineered Mice Targeting CRF-Family Members and their Receptors
Although pharmacological studies have provided valuable insights into the function of the CRF/UCN system, they also face certain limitations. Comparability amongst many of the studies is difficult due to the use of mouse versus rat models. More importantly however, is the fact that they assessed effects of acute or repeated administration of exogenous peptides which might not necessarily mimic normal patterns of endogenous CRF/UCN1-3 release and signaling. Moreover, these experiments provide little insight into the outcomes of long-lasting CRF system dysregulations as they might occur in stress-related mood and anxiety disorders. In addition, it is not always clear whether the effects of centrally administered CRF/UCN1 were mediated by CRFR1 or CRFR2. Although some studies applied CRFR1 and CRFR2 antagonists to tackle this question, many of these compounds are not fully discriminative at the applied concentrations. The generation of transgenic mice, overexpressing or lacking different CRF-family members, has provided valuable insights into the involvement of the CRF/UCN system in stress-related physiology and behavior (Table 1).
Table 1.
Summary of genetic mouse models targeting CRF family members and their receptors.
| Transgenic Line | Targeting Strategy | CNS-Related Phenotype | References |
|---|---|---|---|
| CRF overexpression | |||
|
CRF-OEMt Developmental / ubiquitous |
Non-selective OE of rat CRF under murine metallothionein promoter | Cushing-like phenotype (↑ ACTH & CORT levels, = CORT stress response), adrenal hypertrophy, ↓ general locomotion, ↑ anxiety, ↓ immobility FST, deficits in learning and spatial memory |
[108, 223-225] |
|
CRH-OEThy1.2 Developmental |
OE of rat CRF under Thy-1
promoter |
Cushing-like phenotype at 6 months (↑ CORT levels, marginal increase in ACTH, = CORT stress response), adrenal hypertrophy, nonsupression of DXM, ↓locomotion, ↓startle reactivity & habituation, ↓PPI, = anxiety |
[227, 228] |
|
CRH-COECNS Developmental / CNS-specific |
Conditional Nestin-Cre induced OE of murine CRF driven by the Rosa26-promoter | Stress-induced hypersecretion of CORT, = basal CORT levels, ↓ immobility FST/TST, ↑ REM sleep & slightly suppressed non-REM sleep | [231, 233] |
|
CRH-COEFB Postnatal / forebrain-specific |
Conditional Camk2α-Cre induced OE of murine CRF driven by the Rosa26-promoter | = basal HPA axis & CORT stress response, = immobility FST, ↑ REM sleep |
[231, 233] |
|
CRH-COEGABA Developmental / restricted to GABAergic neurons |
Conditional Dlx5/6-Cre induced OE of murine CRF driven by the Rosa26-promoter | = basal HPA axis & CORT stress response, = immobility FST |
[231, 233] |
|
FB-CRH (Camk2α-rtTA/tetO-Crf) Inducible forebrain-specific |
Camk2α-Cre mediated COE of rat CRF driven by the CMV promoter (tet-on system; DOX induces expression) |
DOX administration at P56 for 3 weeks, ↑ CORT at circadian nadir, = ACTH levels & CORT stress response, ↓thymus size, = dextamethason suppression |
[229] |
|
FBCRHOE (Camk2α-rTA/tetop-CRH) Inducible forebrain-specific |
Camk2α-Cre mediated COE of CRF driven by the CMV promoter (tet-off system; DOX represses expression) |
Early life forebrain CRF OE (off DOX E15-P21) causes ↑ CORT levels only during development & long-lasting anxiogenic & despair-like alterations; Lifetime CRF OE induces Cushing-like phenotype at 8 weeks & ↑ CORT & ACTH levels only at circadian nadir |
[230] |
| Transgenic Line | Targeting Strategy | CNS-Related Phenotype | References |
|
CRH-COECamkCreERT2 Inducible forebrain-specific |
Conditional Camk2α-CreERT2 induced OE of murine CRF driven by the Rosa26-promoter; tamoxifen induces expression | Tamoxifen administration at P 56, ↑ anxiety, = locomotion |
[106] |
|
CRH-COEDel Developmental /ubiquitous |
Conditional Deleter-Cre induced OE of murine CRF driven by the Rosa26-promoter | Cushing-like phenotype at 3 weeks, ↑ body weight, adrenal hypertrophy, ↑ CORT levels at circadian peak & trough, = CORT stress response, = locomotion, ↑ anxiety, ↓ immobility FST |
[234] |
|
CRF-COEApit Developmental /anterior pituitary-specific |
Conditional Pomc-Cre induced OE of murine CRF driven by the Rosa26-promoter | Mild Cushing-like phenotype at 5-6 months, ↓ body weight, adrenal hypertrophy, thymus atrophy, ↑ CORT levels at circadian trough, = CORT levels at circadian peak, = CORT stress response (↓ in females), = locomotion, = anxiety, slightly ↓ immobility FST, ↑ NREM sleep |
[234] |
|
Crh-120/+ Developmental / ubiquitous |
ENU-induced gain-of-function mutation in the CRF promoter region |
Cushing-like phenotype including obesity, muscle wasting, thin skin, hair loss, ↑ CORT levels, hyperglycemia, hyperinsulinemia | [235] |
| UCN2 overexpression | |||
|
UCN2-COE Developmental / conditional |
SF-1-Cre induced OE of murine UCN2 driven by the Rosa26
promoter |
Down-regulation of adrenal and ovarian steroidogenesis | [232] |
| UCN3 overexpression | |||
|
UCN3OE Developmental / ubiquitous |
Constitutive OE of mouse UCN3 under the Rosa26 promoter | Leaner body composition, protected against diet-induced obesity and hypoglycaemia, = basal & response CORT levels, ↓ ACTH response to acute stress, ↑ anxiety, ↓ spatial memory following restraint stress, stress-induced alterations in tissue serotonin levels in the DRN |
[196, 258] |
| CRF knockout | |||
|
CRH-KO Developmental / constitutive |
Replacement of the CRF coding region with a neomycin cassette | Blunted HPA axis activity (↓ basal & stress-induced CORT levels), = anxiety, = anxiety post restraint stress, = locomotion, = ASR & learning |
[236, 237] |
| CRFR1 knockout | |||
|
CRHR1-KO Developmental / constitutive |
Replacement of exons 8-13 with a neomycin cassette | Blunted HPA axis activity (↓ basal & stress-induced CORT levels), ↓ anxiety, ↑ locomotion, ↑ & delayed stress-induced alcohol intake, ↓ remote fear memory consolidation |
[120, 238] |
|
CRFR1-KO Developmental / constitutive |
Replacement of exons 5-8 with a neomycin cassette | Blunted HPA axis activity (↓ basal and stress-induced CORT levels), ↓ anxiety, ↓ spatial memory performance |
[239, 240] |
|
Crhr1FB-CKO Postnatal / forebrain-specific inactivation |
Conditional Camk2α-Cre mediated CRFR1 inactivation (floxed exons 9-13) |
= basal HPA axis activity, slightly enhanced CORT after acute stress, ↓ anxiety, ↓ chronic-stress induced cognitive deficits, ↓ dendritic atrophy & spine loss, ↓ remote fear memory consolidation |
[120, 241-244] |
| Transgenic Line | Targeting Strategy | CNS-Related Phenotype | References |
|
Crhr1Glu-CKO Developmental / inactivation in glutamatergic neurons |
Conditional Nex-Cre mediated CRFR1 inactivation (floxed exons 9-13) |
= basal & stress-induced HPA activity, ↑ locomotion, ↓ anxiety, = immobility FST, = auditory fear conditioning, impaired glutamatergic neurotransmission in the amygdala and hippocampus |
[106, 112] |
|
Crhr1GABA-CKO Developmental / inactivation in GABAergic neurons |
Conditional Dlx5/6-Cre mediated CRFR inactivation (floxed exons 9-13) |
= basal & stress-induced HPA activity, = locomotion, = anxiety, = immobility FST, = auditory fear conditioning, = CRF induced neuronal excitability in the hippocampus |
[106, 112] |
|
Crhr15HT-CKO Developmental / inactivation in serotonergic neurons |
Conditional ePet-Cre mediated CRFR1 inactivation (floxed exons 9-13) |
= basal & stress-induced HPA activity, = locomotion, = anxiety, = immobility FST, = auditory fear conditioning |
[106] |
|
Crhr1DA-CKO Inducible / inactivation in dopaminergic neurons |
Conditional DAT-CreERT2 mediated CRFR1 inactivation (floxed exons 9-13), tamoxifen induces knockout |
Tamoxifen administration at P56, = basal & stress-induced HPA activity, ↓ locomotion, ↑ anxiety, = immobility FST, = auditory fear conditioning, ↓ footshock-induced dopamine release in the prefrontal cortex |
[106] |
|
Crhr1CNS-CKO Developmental / CNS-specific |
Conditional Nes-Cre mediated CRFR1 inactivation (floxed exons 9-13) |
= locomotion & anxiety | [106] |
|
Crhr1ΔEgfp Developmental CRFR1 reporter allele |
Knockin of EGFP into exon 2 resulting in a null allele |
Designed to visualize 1CRFR1-expression, with the ability to conditionally restore expression of a GFP tagged full-length CRFR1, which can be deleted via Cre recombinase, blunted HPA axis activity (↓ basal & stress-induced CORT levels) |
[106] |
|
Crhr1tZ Conditional multifunctional CRFR1 allele |
Knockin of tau-LacZ (tZ) into endogenous CRFR1 locus resulting in a null allele |
Designed to genetically label CRFR1-expressing cells with the ability to conditionally restore or delete CRFR1 with Flp and Cre recombinase, Crhr1tZ reporter mice revealed novel aspects of CRFR1 expression, blunted HPA axis activity (↓ basal & stress-induced CORT levels) |
[59] |
| CRFR2 knockout | |||
|
CRHR2-KO Developmental / constitutive |
Replacement of transmembrane domains 3-5 with a neomycin cassette |
↓ ACTH & CORT response to stress & early termination of ACTH release, = anxiety, ↑ social discrimination |
[42, 246] |
|
CRFR2-KO Developmental / constitutive |
Replacement of exons 10-12 with a neomycin cassette | ↓ ACTH & CORT response to stress & early termination of ACTH release, ↑ anxiety & immobility FST, ↑ basal & high-fat diet food consumption | [215, 216, 247] |
|
CRHR2-null Developmental / constitutive |
Replacement of 3rd cytoplasmic region with a neomycin cassette | = HPA axis activity, = locomotion, ↑ anxiety & immobility FST | [248] |
| Transgenic Line | Targeting Strategy | CNS-Related Phenotype | References |
| CRFR1/CRFR2 double knockout | |||
|
CRHR1/CRHR2 dKO Developmental / constitutive |
Crossbreeding | ↓ HPA stress response | [251] |
|
CRFR1/CRFR2 dKO Developmental / constitutive |
Crossbreeding | ↓ HPA stress response, ↓ anxiety only in females | [250] |
| UCN1 knockout | |||
|
UCN1-KO Developmental / constitutive |
Replacement of coding region with neomycin cassette | = HPA axis and feeding, ↑ anxiety, impaired hearing | [217] |
|
UCN1-null Developmental / constitutive |
Replacement of exon 2 with eGFP-LacZ reporter cassette | = HPA axis, = locomotion & anxiety, ↓ impaired ASR | [252] |
|
UCN1-/- Developmental / constitutive |
Replacement of exon 2 with a neomycin cassette | = basal HPA axis, ↓ HPA adaptation to repeated restraint stress | [253] |
| UCN2 knockout | |||
|
UCN2-KO Developmental / constitutive |
Replacement of exon 2 with a neomycin cassette | ↑ nocturnal ACTH & CORT levels, ↓ FST immobility only in females, = anxiety & locomotion & fear conditioning in males and females | [218] |
|
Ucn2tz/tz Developmental / constitutive |
Replacement of open reading frame with tau-LacZ reporter cassette | = HPA axis, = anxiety,= immobility FST, = social discrimination, ↓ aggressiveness |
[42, 254] |
| UCN3 knockout | |||
|
UCN3-KO Developmental / constitutive |
Replacement of coding region with a neomycin cassette | ↓glucose-induced insulin secretion, ↓ basal glucose and insulin secretion under high-fat diet, ↑ basal feeding & following food deprivation, ↑ ethanol intake & preference | [256, 257] |
|
Ucn3tz/tz Developmental / constitutive |
Replacement of open reading frame with tau-LacZ reporter cassette | = HPA axis, = anxiety & immobility FST, ↑ social discrimination |
[42] |
| UCN1/UCN2 double knockout | |||
|
UCN1-KO/UCN2-KO Developmental / constitutive |
Crossbreeding | ↑ stress-induced HPA response only in males, ↓ anxiety | [192] |
| UCN1/UCN2/UCN3 triple knockout | |||
|
UCN1-KO/UCN2-KO/UCN3-KO Developmental / constitutive |
Crossbreeding | = HPA activity, ↓ locomotion, ↑ anxiety 24h after acute stress but not under basal conditions | [255] |
6.1. CRF Overexpressing Mice
In order to study the role of chronic CRF hyperdrive in the context of mood and anxiety-disorders, independent models of CRF excess were developed (Table 1). The first CRF overexpressing mouse line was generated via classical pronuclear injection applying the broadly active metallothionine 1 (Mt1) promoter [223]. These mice (CRF-OEMt1) showed strong CRF overexpression in the brain and peripheral organs including lung, adrenal, heart, and testis. CRF overproduction resulted in elevated plasma corticosterone levels and Cushing’s-like symptoms. Of note: the Mt1 promoter harbors a GC response element and thus might react in a feed-forward manner upon elevated corticosterone levels. CRF-OEMt1 mice showed increased anxiety-related behavior, which was reversible with the CRF receptor antagonist α-helical CRF [224]. Moreover, these mice displayed deficits in learning, decreased immobility in the forced swim test (FST) and reduced attention [108, 225]. Another CRF overexpressing mouse line was developed using the Thy1.2 promoter driving CRF expression in postnatal and adult neurons of the brain [226]. However, CRH-OEThy1.2 mice did not show an altered stress response or phenotype indicative of changes in anxiety behaviors [226, 227]. Instead, CRH-OEThy1.2 mice displayed reduced startle reactivity as well as reduced freezing following fear conditioning [226, 227]. With some delay CRH-OEThy1.2 mice also developed a mild cushingoid phenotype [228]. Finally, different conditional CRF-overexpressing mouse lines have been established in recent years. Two studies applied the “tet-on/tet-off” system, which allows for reversible and inducible overexpression of CRF [229, 230]. Although both made use of the forebrain-specific Camk2α promoter combined with a tet-operator driven CRF-construct, the behavioral and neuroendocrine consequences of CRF excess were rather specific for each mouse strain (Table 1). Taken together, these examples illustrate the difficulties of comparing results from different transgenic mouse lines even if they are based on similar constructs. A mouse model which permits conditional CRF overexpression avoiding common uncertainties of classical transgenesis, such as unpredictable influences of the site of transgene insertion and the number of inserted transgene copies, is the CRH-COE mouse line [231]. This mouse line was generated by introducing a CRF expression unit into the ubiquitously expressed Rosa26 (R26) locus. Expression of exogenous CRF driven by the R26 promoter is prevented unless a loxP flanked transcriptional terminator is deleted via a Cre recombinase, which determines the spatial and temporal pattern of CRF overexpression. A similar strategy was recently applied to generate a model which enables conditional overexpression of UCN2 [232]. This model of CRF overexpression demonstrated that CNS-restricted CRF overexpression in CRH-COECNS mice, achieved by breeding to Nestin-Cre mice, leads to HPA axis hyperactivity, increased active stress-coping behavior in the FST and altered sleep regulation [231, 233]. Importantly, reduced immobility in the FST was not observed in mice overexpressing CRF specifically in forebrain Camk2α-positive or forebrain GABAergic neurons, suggesting an involvement of hindbrain-regions in CRF-induced active-stress coping behavior. The same model was consequently used to induce CRF overexpression specifically in the forebrain during adulthood by breeding CRH-COE mice to the tamoxifen-inducible Camk2α-CreERT2 driver line (CRH-COECamkCreERT2). Behavioral assessment of these animals revealed an anxiogenic phenotype, supporting earlier findings in conditional CRFR1 knockout mice that anxiety-related behavior is regulated by forebrain CRF/CRFR1 during adulthood [106]. In order to discriminate the direct effects of centrally hypersecreted CRF from those resulting from HPA axis activation, two additional conditional CRF-overexpressing mouse lines were created [234]. CRH-COEDel mice overexpress CRF in a ubiquitous manner, while CRH-COEApit mice selectively overexpress CRF in the anterior and intermediate lobes of the pituitary. Both mouse lines displayed increased basal plasma corticosterone levels and consequently signs of Cushing’s syndrome. However, alterations in anxiety were only observed upon ubiquitous CRF overexpression, suggesting that chronic hypercorticosteroidism alone is not sufficient to alter emotional behavior. The study implies that central CRF hyperdrive on its own or in combination with elevated GCs is responsible for the observed behavioral alterations in CRH-COEDel mice [234]. With respect to clinical findings and in order to fully understand the effects of CRF hyperdrive in the context of stress-related neuropathologies, the generation of mice overexpressing CRF under its endogenous promoter represents a matter of particular importance. As discussed later on, this goal could ultimately be achieved by breeding conditional CRF overexpressing mice with the recently generated CRF-Cre driver lines. Along these lines, a recent study utilized a mouse N-ethyl-N-nitrosourea (ENU)-screen, and identified a point mutation in the CRF promoter region, which results in a gain-of-function mutation, i.e. CRF overexpression and consequently development of Cushing’s syndrome [235].
6.2. CRF Knockout Mice
Although CRF overexpressing mice represent valuable disease models with respect to chronic CRF and HPA axis hyperactivity, they are confounded by ectopic expression in non-endogenous brain regions/neurons and peripheral organs. Consequently a loss-of-function approach is more likely to reveal physiologically relevant effects of CRF on behavior. The development of constitutive CRF knockout mice (CRH-KO) by Muglia and colleagues has been important in addressing this issue [236]. CRH-KO mice displayed severely reduced plasma corticosterone levels indicative of blunted HPA axis activity (Table 1). Importantly, this study revealed fetal GC requirement for lung maturation, which was severely impaired in CRF-deficient mice obtained from homozygous breedings [236]. Surprisingly, CRH-KO mice displayed no gross alterations in emotional behavior and CRFR1 antagonists were still able to exert an anxiolytic effect in these animals [237]. The discrepancy between constitutive CRF and CRFR1 null mutants (discussed below) with respect to behavioral outcomes could be due to a number of reasons: 1) UCN1, the only other CRFR1 ligand, might compensate for the loss of CRF; 2) early deletion of CRF might trigger general compensatory processes; 3) corticosterone deficiency might mask potential phenotypes; 4) CRF might exert its action primarily under conditions of chronic or severe stress; 5) CRFR1 might comprise ligand-independent activity, e.g. due to constitutive activity or heteromerization with other receptors; 6) and last but not least, it might suggest the presence of a yet unidentified CRFR1-ligand. The generation of conditional CRF knockout mice would significantly help to shed light on some of these issues.
6.3. CRFR1 Knockout Mice
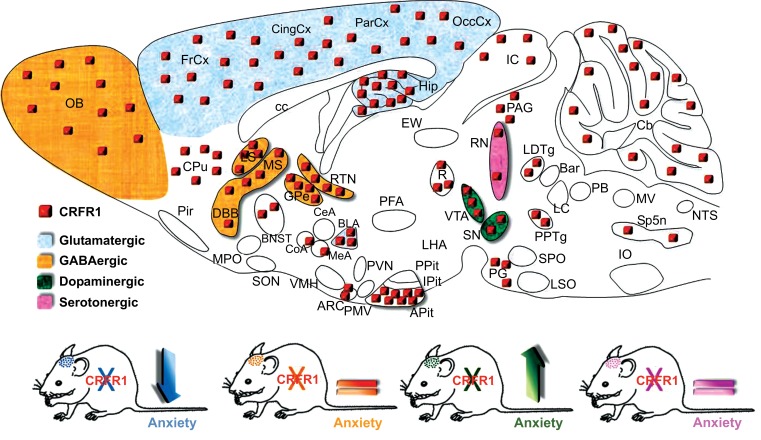
The contribution of CRFR1 to the modulation of stress-related behaviors was addressed by conventional and conditional CRFR1 knockout mice (Table 1). Expectedly, CRFR1 null mice exhibited a chronic GC deficiency due to disrupted HPA axis activity, which was observed in two independently generated CRFR1-deficient mouse lines; CRHR1-KO and CRFR1-KO [238, 239]. Furthermore, both mouse lines displayed reduced anxiety-related behavior [238-240]. In order to exclude the possibility that the GC deficit is mediating the observed behavioral effects, a conditional forebrain-specific CRFR1 knockout mouse line (Crhr1FB-CKO) was generated [241]. In this mouse model, Cre-mediated deletion of CRFR1 is initiated in the second week of postnatal life, and is primarily restricted to cortical and limbic forebrain regions including the amygdala, hippocampus, BNST, but not the anterior pituitary. Crhr1FB-CKO mice displayed reduced anxiety-related behavior and normal GC levels under basal conditions, supporting the notion that limbic CRFR1 can regulate emotional behavior independent of HPA axis alterations [241]. However, corticosterone levels were slightly elevated in Crhr1FB-CKO mice 30 and 90 min following a 5 min restraint stress, suggesting that limbic CRFR1 itself is partially involved in HPA axis feedback regulation [241]. Both CRHR1-KO and Crhr1FB-CKO mice displayed impairments in remote fear memory consolidation, suggesting that cognitive processes are also mediated by CRFR1 in forebrain cortical and limbic structures [120]. In addition, more recent work demonstrated that forebrain CRFR1 deficiency prevents cognitive deficits induced by early-life stress and chronic stress during adulthood [242-244]. Moreover, stress-induced dendritic remodeling and spine loss was attenuated in Crhr1FB-CKO mice [242, 244]. Although all these studies clearly implicate CRFR1 in the modulation of emotional and cognitive responses, a fundamental question remained unsolved: Which are the underlying neurotransmitter circuits controlled by CRFR1 that modulate anxiety-related behavior? In order to address this question the neurochemical identity of CRFR1-expressing neurons was established by sensitive neurochemical methods and genetic tools [106]. CRFR1 expression was demonstrated in forebrain glutamatergic and GABAergic neurons as well as in midbrain dopaminergic neurons, and in a few serotonergic cells of the dorsal and median raphe nucleus [106]. In order to dissect the underlying neurotransmitter circuits, previously generated floxed CRFR1 mutants [241] were bred to a set of neurotransmitter-specific Cre-driver lines resulting in selective deletion of the receptor from glutamatergic (Crhr1Glu-CKO), GABAergic (Crhr1GABA-CKO), dopaminergic (Crhr1DA-CKO) and serotonergic (Crhr15-HT-CKO) neurons, respectively (Fig. 4). Selective deletion of CRFR1 in forebrain glutamatergic circuits reduced anxiety, which is in agreement with the previously established phenotype of forebrain-specific CRFR1 knockout mice, and the anxiolytic properties of CRFR1 antagonists. Moreover, CRF-induced changes in glutamatergic neurotransmission in the amygdala and hippocampus were absent in Crhr1Glu-CKO mice [106, 112]. Remarkably, specific deletion from midbrain dopaminergic neurons enhanced anxiety-related behavior, highlighting a previously unrecognized negative effect of CRFR1-ablation on emotional behavior. Importantly, this anxiety-inducing effect was associated with reduced dopamine release in the prefrontal cortex, establishing the relevance of dopamine-CRF interactions in behaviors other than addiction [106]. These results defined a novel bidirectional role of CRFR1 in anxiety, suggesting that CRF/CRFR1-controlled glutamatergic and dopaminergic systems might function in a concerted but antagonist manner to keep emotional responses to stressful situations in balance. This was further supported by the absence of an anxiety phenotype in Crhr1CNS-CKO animals, in which the CRFR1 is absent from both neurotransmitter systems [106]. HPA axis activity, FST behavior and auditory fear condition were not differently affected in the investigated mouse lines, suggesting that the bidirectional role of CRFR1 might be specific for anxiety-related behavior. However, additional cognitive parameters and behavioral domains need to be investigated in the future to further substantiate the selectivity of the observed effects. An anxiolytic role for CRFR1 was also demonstrated shortly afterwards in the globus pallidus, a central component of the basal ganglia circuitry which is indirectly controlled by dopaminergic substantia nigra-striatal projections [245]. As previously mentioned, Lemos and colleagues recently demonstrated a novel appetitive effect of CRF in the nucleus accumbens under basal conditions resulting from CRF´s ability to positively regulate dopamine release [150]. However, severe stress was shown to induce a persistent dysregulation of CRF-dopamine interactions that normally produce a positive affective state, resulting in an aversive phenotype. Whether stress is able to induce a switch in CRF-neurotransmitter interactions in the conditional CRFR1 mutants analyzed by Refojo and colleagues remains to be investigated. An important insight from all three studies is that we can no longer regard CRF as a generally “aversive” stress neuropeptide.
Fig. (4).
CRFR1 modulates anxiety-related behavior in a bidirectional manner. CRFR1 is expressed in diverse neuronal subpopulations. Selective deletion of the receptor in glutamatergic neurons reduces anxiety-related behavior while deletion in dopaminergic neurons produces the opposite effect [106].
6.4. CRFR2 Knockout Mice
In contrast to the consistent and reproducible phenotype of CRFR1 knockout mice, a number of discrepancies have been observed in CRFR2 knockout mice (Table 1). Until now, three conventional CRFR2 knockout mouse models have been generated [246-248]. Two studies reported enhanced ACTH and corticosterone release in response to stress, but an early termination of ACTH release, suggesting that CRFR2 is involved in maintaining HPA drive [246, 247]. In addition, Coste and colleagues observed an overall blunted corticosterone recovery in CRHR2-KO mice, implying an involvement in HPA feedback function. The effects of
Overexpressing mouse lines were either generated via classical pronuclear DNA injection or via targeted insertion in embryonic stem cells. All knockout lines were generated by means of targeted deletion via homologous recombination in embryonic stem cells. Nomenclature of mouse lines is based on the first publication or laboratory of origin. ↑ indicates an increase, ↓ indicates a decrease, = indicates no difference compared to control animals. Abbreviations: Acoustic startle response (ASR), adrenocorticotropic hormone (ACTH), corticosterone (CORT), dexamethasone (DXE), overexpression (OE), conditional overexpression (COE), doxycycline (DOX), forced swim test (FST), hypothalamic-pituitary-adrenal (HPA) axis. Italics highlight the original publications.
CRFR2 deficiency on anxiety-related behavior are less clear. Whereas Coste and colleagues observed no changes in anxiety-related behavior, Bale et al. and Kishimoto et al. detected increased anxiety-related behavior. The absence of a baseline anxiety phenotype in CRFR2-KO mice was also confirmed in a later study in which it appeared that the constitutive deletion of CRFR2 enhances anxiety 24 h after restraint stress, but not immediately or following prolonged periods of stress [249]. Importantly, Bale and colleagues reported a compensatory upregulation of CRF in the central amygdala of CRFR2-KO mice, which might have influenced the anxiogenic phenotype. Two of the studies also reported increased immobility of CRFR2-deficient mice in the FST [247, 248]. In addition, enhanced social discrimination was observed in CRHR2-KO mice [42]. Based on the above studies, CRFR2 was initially proposed to exert opposite functions compared to CRFR1, but this simplistic view has been rejected by more recent research. Differences in strain background might underlie some of the discrepancies observed between the mouse lines. In addition different exons were targeted in the individual knockout lines, which leads to the speculation that different truncated splice variants might still be present and affect the phenotypic outcome of the genetic manipulation. Along these lines, the soluble variant of CRFR2 (sCRFR2α) should be intact in all three CRFR2-KO mouse lines. Double CRFR1/CRFR2 knockout mice have also been generated, but only mild behavioral alterations were observed in one of the lines, which were specific for female mice [250, 251]. However, both lines displayed impaired stress-induced HPA axis activation. A more detailed analysis of both double knockout lines, as well as the utilization of respective conditional alleles, might provide additional insights with regards to the behavioral discrepancies. Importantly, assessment of feeding behavior in CRFR2 knockout mice supports a role for the receptor in anorexigenic responses and centrally controlled metabolic functions [215, 216]. The generation of conditional CRFR2 knockout mice will be mandatory in order to uncover the precise involvement of CRFR2 in stress-related behaviors, HPA axis activity, as well as metabolic function.
6.5. Genetically Modified Mice Targeting the Urocortins
Three different UCN1 knockout lines have been independently generated (Table 1); however their phenotypes remain controversial [217, 252, 253]. Vetter and colleagues reported increased anxiety-related behavior in their mouse model, which was not confirmed by the study of Wang et al. and so far not investigated in the UCN-deficient mice generated by Zalutskaya and colleagues. Consequently, UCN1 seems an unlikely candidate to compensate for CRF deficiency in CRH-KO mice. This is also supported by the restricted expression pattern of UCN1 compared to CRF (Fig. 3). Importantly, all UCN1 knockout lines exhibited normal basal and stress-induced GC levels, supporting the notion that UCN1 plays a minor role in control of HPA axis function. However, Zalutskaya and colleagues observed that corticosterone levels in male UCN1-deficient mice did not adapt to repeated restraint stress. The role of the other two urocortin members has been assessed in UCN2 and UCN3 knockout mice. Female UCN2 knockout mice display mild alterations in their basal circadian rhythm of ACTH and corticosterone secretion [218]; whereas no differences were observed in basal and stress-induced corticosterone levels in male mice [218, 254]. Male and female UCN2-KO mice generated by Chen et al. exhibited no alterations in locomotion, anxiety and contextual fear conditioning. However, only female UCN2-KO mice displayed reduced immobility in the FST [218]. Male UCN2-deficient mice developed by Deussing and colleagues (Ucn2tz/tz) displayed reduced aggressiveness, but showed no changes in anxiety, immobility in the FST and social discrimination [42, 254]. Differences in HPA axis activity and anxiety were also not observed in UCN3-deficient mice (Ucn3tz/tz) [42]. However, male and female Ucn3tz/tz mice showed enhanced social discrimination abilities, which was also observed in CRHR2-KO mice, and attributed to UCN3-expressing neurons of the BNST and medial amygdala - nuclei functionally connected to the accessory olfactory system. These data suggest an involvement of the UCN3/CRFR2-system in social memory.
The relevance of the UCN family members in stress-related behavior was additionally assessed in recently generated UCN1/UCN2 double and UCN1/UCN2/UCN3 (tKO) triple knockout mice. UCN1/UCN2 knockout mice displayed no changes in basal HPA axis activity, but exhibited elevated corticosterone levels following acute stress exposure [192]. On the other hand, HPA axis function was indistinguishable in tKO mice compared to controls [255]. UCN1/UCN2 knockout mice displayed decreased anxiety under basal and acute-stress conditions, which was accompanied by elevated serotonin concentrations in a number of brain regions including the DRN, hippocampus, BLA and subiculum [192]. In contrast, tKO mice exhibited increased anxiety-like behavior, but only 24 h following restraint-stress. Moreover, tKO mice displayed an increased stress-induced startle response [255]. As opposed to UCN1/UCN2 knockout mice, the behavioral phenotype in tKO mice was associated with decreased serotonergic metabolism in regions such as the septum, central and basolateral amygdala [255]. Again, the effect of compensatory changes in CRF expression on emotional behavior cannot be excluded in many of the urocortin mouse models, as shown in UCN1/UCN2 knockout mice [192]. Overall the majority of data suggests that the urocortins and CRFR2 are able to regulate specific aspects of stress-related emotional behavior complementing effects of CRF and CRFR1. As previously mentioned, more recent studies are starting to implicate the UCNs, especially UCN3, in the modulation of glucose homeostasis and metabolic function. Both, UCN3 knockout mice [256, 257] and UCN3 overexpressing mice [258] display alterations in glucose metabolism, although the results of the former have to be interpreted with caution considering the uncertainties that arise with ectopic gene expression.
7. Viral-based gain- and loss-of-function studies
In addition to gene targeting in mice, viral-mediated gene transfer is another popular method used to study neuronal function in the rodent brain. Adeno-associated viruses (AAVs) and lentiviruses (LVs) are the most commonly used viral vectors to infect adult neurons in vivo, which can be achieved via stereotactic injections in any given brain-region. AAVs and LVs can be used to e.g. express fluorescent markers, genes of interest, fusion proteins, Cre recombinases or short-hairpin RNAs (shRNAs) [259-268]. Spatial restriction can be achieved by utilizing cell type-specific promoters to drive gene expression. Consequently, AAVs or LVs represent a versatile tool that can be used for a number of applications including gain- or loss-of-function approaches, tracing studies, or simply to label neurons. For example, delivery of AAVs, expressing the Cre recombinase, into a specific brain region of mice with a floxed gene of interest results in Cre-mediated deletion of the floxed gene. Another possibility is to generate floxed viral constructs (Cre-dependent viral vectors), which are only active upon the presence of Cre recombinase, for example in region- or cell type-specific Cre-driver mouse lines. Tracing and mapping studies of neuronal circuits have also heavily relied on viral-mediated delivery of fluorescent proteins [269-273]. For instance, AAVs can be designed to harbor synaptic proteins fused to fluorescent markers, which will be actively transported to the synapses enabling the visualization of axonal projections. Specific neuronal circuits can be targeted by expressing these “tracers” in a Cre-dependent manner. One important issue is variable transduction efficiencies between different viral serotypes [274-277]. In addition, serotypical variations are also observed with respect to toxicity and immune responses triggered by the viral capsule [278-280]. Nonetheless, the combination of mouse genetics and recombinant AAVs/LVs has greatly improved our ability to map, monitor and manipulate neurons and circuits. For instance optogenetics, one of the latest technological leaps in neuroscience, greatly benefits from these combinatorial approaches. As a result, an increasing number of studies have started to make use of viral-mediated gene transfer to study the CRF system. The most prominent examples are listed in Table 2. As opposed to conventional pharmacological administrations (i.c.v. or microinjection pumps), viral-based methods have greatly improved the spatial and temporal precision of neuropeptide delivery into the CNS. This was effectively demonstrated by a recently described viral-mediated genetic approach for i.c.v. delivery of CRF to the CSF. Applying the choroid plexus-specific CRFR2 promoter, a LV-based system was established which enables a doxycycline-inducible and hence reversible delivery of CRF into the CSF [267]. The induction of CRF overexpression in the choroid plexus resulted in enhanced anxiety-related behavior, which is in accordance with previous findings in mice treated with i.c.v. injections of CRF [reviewed in 99], CRF overexpressing mice [224], and depressed individuals that displayed enhanced CRF levels in the CSF [87]. A number of additional studies investigated the effects of LV-based CRF hyperdrive in the CeA. Keen-Rhinehart and colleagues reported increased despair-like behavior in the FST and an enhanced acoustic startle response, which is indicative of enhanced anxiety [281]. Along these lines, Regev et al. demonstrated that short-term inducible overexpression of CRF in the CeA enhances stress-induced effects on anxiety-like behavior [282] however, prolonged overexpression produced the opposite effect [283]. This possibly suggests that acute and chronic CRF hyperactivity might be able to differentially regulate basal and stress-induced emotional behaviors. Regev and colleagues additionally investigated the effects of site-specific CRF overexpression in the BNST, but observed no effects on basal and stress-induced anxiety [283]. An important drawback of the above mentioned viral studies is the lack of overexpression specificity. In all cases, exogenous CRF expression is driven by ubiquitously active promoters, resulting in transgene expression that extends beyond the cell population of interest – neurons endogenously expressing CRF. Thus the observed effects may partially originate from unintentionally targeted cells. These problems can be circumvented by designing viral vectors with CRF-specific promoters, as demonstrated by two recent studies [284, 285]. Both made use of the same LV-construct which contains a 3.0 kb CRF promoter region, previously utilized for the generation of transgenic CRFp3.0Cre mice [286]. Applying this overexpression model, Sink and colleagues demonstrated that CRF neurons within the lateral BNST modulate conditioned anxiety-like behaviors, thus suggesting that enhanced CRF expression within these neurons may contribute to inappropriate regulation of emotional memories. However, the expression of the exploited CRF promoter does not fully recapitulate the endogenous CRF expression pattern, possibly due to missing regulatory elements, which might be located further up- or downstream of the promoter sequence. In fact, the size restriction of the DNA/RNA which can be incorporated into the desired viruses is a major hurdle when it comes to transgene expression driven from cell type-specific promoters. An alternative strategy is the delivery of Cre-dependent viral vectors into cell type-specific Cre-driver lines, which will be discussed later on.
Table 2.
Viral-mediated gain- and loss-of-function approaches targeting CRF and its receptors.
| Viral Construct | Target Site | Phenotype | References |
|---|---|---|---|
| Viral-based gain-of-function studies | |||
| LV - tet-on based CRF OE in mice driven by a choroid plexus-specific promoter | Choroid plexus | ↑ anxiety, = home cage activity | [267] |
| LV - CMV driven CRF OE, study in females rats |
CeA | ↑ CRF & AVP expression in PVN, ↓ HPA axis feedback, ↑ ASR, ↑ immobility in FST, ↓ GnRH expression in CeA, altered sexual behavior | [281] |
| LV - CMV driven CRF OE in mice, injection at week 7; tested 4 months later |
CeA BNST |
= basal anxiety, ↓ stress-induced anxiety, = immobility in FST, = HPA axis activity, = ASR, ↓ reaction time to startle stimuli = basal & stress induced anxiety, = ASR = immobility in FST, = HPA axis activity |
[283] |
| LV - tet-on based, short-term CRF OE in mice driven by a CMV promoter; analyses 3 days post DOX induction | CeA | = basal anxiety, ↑ stress-induced anxiety, = immobility in TST, = fear conditioning, = HPA axis activity |
[282] |
| LV - CRF OE driven by a 3kb CRF promoter fragment, analyses in rats 2 weeks post injection | CeA BNST |
↑ CRF & AVP expression in PVN, HPA axis hyperactivity, ↑ anxiety = basal anxiety & ASR, = HPA axis activity, alterations in conditioned anxiety |
[284] [285] |
| LV - CRFR2 OE in rats driven by a pCSC minimal promoter | BNST | ↓ PTSD-like symptoms | [290] |
| Viral-based loss-of-function studies | |||
| LV - shRNA based CRFR1 KD in mice | BlA | ↓ anxiety, = general locomotion | [127] |
| LV - shRNA based CRF KD in mice | CeA | ↓ stress-induced anxiety, ↑ basal CORT levels, = home cage activity, = immobility in TST, ↑ CRFR1 expression in BNST | [282] |
| LV - shRNA based CRFR1 KD in mice | Globus pallidus | ↓ anxiety, = general locomotion | [245] |
| LV - siRNA based CRF KD in mice | PVN | = basal social avoidance, ↓ stress-induced social avoidance | [287] |
| LV - shRNA based CRFR2 KD in mice | BNST | ↓ susceptibility to PTSD-like behavior | [289] |
| LV - shRNA based CRF KD in mice | VTA | Prevents aversive effects of nicotine withdrawal, ↓escalation of nicotine intake | [161] |
| LV-shRNA based CRFR1 KD in mice | Blocks acute food deprivation stress-induced reinstatement of cocaine seeking, ↓ cue-induced cocaine seeking | [288] | |
↑ indicates an increase, ↓ indicates a decrease, = indicates no difference compared to control animals. Abbreviations: Acoustic startle response (ASR), arginine vasopressin (Avp), basolateral nucleus of the amygdala (BlA), bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), corticosterone (CORT), doxycycline (DOX), forced swim test (FST), gonadotropin-releasing hormone (GnRH), hypothalamic-pituitary-adrenal (HPA) axis, knockdown (KD), lentivirus (LV), overexpression (OE), paraventricular nucleus of the hypothalamus (PVN), post-traumatic stress disorder (PTSD), tail-suspension test (TST), ventral tegmental area (VTA).
In addition to the gain-of-function procedures, viral-mediated loss-of-function approaches have also been employed to study aspects of the CRF system (Table 2). LV-mediated knockdown of CRF in the CeA attenuated stress-induced anxiety-like behaviors and altered HPA axis activity, reinforcing a role for amygdalar CRF in the modulation of fear and anxiety [282]. In support, LV-based knockdown of CRFR1 in the BLA decreases anxiety-related behavior [127]. Elliott and colleagues targeted CRF in the PVN via stereotactic delivery of LV-constructs, carrying short hairpin RNAs (shRNA) against CRF. The knockdown attenuated chronic stress-induced social avoidance, which was shown to result from demethylation of the CRF promotor [287]. Similar viral-mediated loss-of-function tools were used to knockdown CRFR1 in the globus pallidus, revealing a previously unknown anxiolytic effect of the receptor in this brain region, which was further confirmed with site-specific CRFR1 antagonist administration [245]. The relevance of VTA CRFR1 receptors in addiction and reward processes was recently investigated via LV-mediated knockdown in this region, which attenuated cue- and acute food deprivation stress-induced cocaine seeking, but had no effect on self-administration behavior. Consequently, the authors propose that CRFR1 signaling in the VTA presents a target for convergent effects of both cue- and stress-induced cocaine-seeking pathways [288]. The previously mentioned, newly described population of CRF neurons within the VTA was investigated, amongst others, via AAV2-mediated knockdown, which prevented the aversive effects of nicotine withdrawal in these mice [161]. Similar to CRFR1 manipulations, virus-based tools have been used to investigate the CRFR2/UCN-system. LV-mediated knockdown and overexpression of CRFR2 in the BNST revealed an important role of this receptor in PTSD-like behavior [289, 290], while knockdown in the VMH further emphasized CRFR2 as a critical mediator in regulating feeding and lipid metabolism [214].
LVs and AAVs have clearly become indispensable tools in neuroscience research, and will help to further elucidate the involvement of the CRF system in healthy and pathological stress-responses. Yet it will be important to enable more precise comparability between the different studies, which is currently problematic considering the diversity of employed viruses and serotypes, behavioral paradigms, testing conditions, and rodent models (mouse versus rat). In addition, the utilized promoters driving transgene expression, as well as the duration of viral expression, varied across studies, which might have resulted in different intensities of gene expression depending on the respective construct.
8. Indispensable tools in CRF research – past, present and future
The pharmacological, genetic and virus-based studies reviewed above clearly support a role for the CRF/CRFR system in stress-related neuroendocrine, autonomic and behavioral alterations, which is of relevance to a number of psychiatric disorders. In particular, the involvement of CRF/CRFR1 in the regulation of HPA axis function and emotional behavior is well established. On the other hand, the role of the central UCN/CRFR2-system in the context of emotional behavior is less clearly defined, while the impact of this circuit on metabolic function is just starting to evolve. The field of stress research has advanced considerably, yet little is known about specific CRF/UCN-neurotransmitter interactions during normal and pathological conditions. Although the neurochemical identity of CRFR1-expressing neurons in the mouse brain has largely been dissected, the same cannot be stated for CRF, CRFR2 and the UCNs. In addition, the projection sites of neurons expressing CRF and the UCNs have scarcely been investigated, as well as the precise subcellular localization of CRFR1 and CRFR2 in different neuronal subpopulations. This gap of knowledge can be ascribed to the lack of specific antibodies and genetic tools targeting CRF system components. The necessity to further dissect the CRF system at the circuit level becomes evident in view of the fact that CRFR1 was shown to regulate anxiety-related behavior in opposite directions in glutamatergic and dopaminergic neurons [106]. Based on this and other recent studies, it can be implied that the CRF/CRFR1-system does not solely modulate aversive responses similar to those observed after stress. However, a detailed analysis of “aversive” vs “appetitive” CRF pathways is still missing. Consequently, researchers have to increase their focus on the physiological role of CRF and the UCNs, and assess behavioral effects not only in response to site/region-specific manipulations of the system, but also at the circuit level. The future generation of conditional CRF, UCN1-3 and CRFR2 knockout mice, as well as specific Cre-driver lines reflecting the expression of the CRF ligands and their receptors, is mandatory for such an endeavor. The latter is of utter importance, considering the limited availability of specific and high-quality antibodies, and the low expression level of CRF-related peptides and receptors in certain brain regions, which also challenges their detection at the mRNA level by in situ or double in situ hybridization. In addition, the combination of Cre-dependent optogenetic tools with specific Cre-driver lines (e.g. CRF- or CRFR1-Cre) would permit selective, bidirectional control of neural activity in genetically defined cell populations [291-293], enabling direct analysis of endogenous circuits related to emotion, cognition and addiction. With respect to the CRF/UCN system, this was elegantly demonstrated by Anthony and colleagues, who made use of optogentics to specifically activate or inhibit CRFR2-expressing neurons in the LS. This was achieved by generating a transgenic CRFR2-Cre drive line and subsequently injecting Cre-dependent AAVs expressing either activating channelrhodopsin or inhibiting halorhodopsin into the LS [294]. The study revealed that CRFR2-positive cells of the LS include GABAergic projection neurons that innervate the anterior hypothalamus. Surprisingly enough, these CRFR2 outputs were shown to promote, rather than suppress, stress-induced behavioral measures of anxiety and corticosterone levels [294]. However, the utility of specific CRF family-related Cre-lines is much more versatile, especially when bred to Cre-dependent reporter strains. This makes the cells of interest easily accessible for different purposes: 1) visualization and morphological characterization, 2) co-expression analyses, 3) mRNA isolation and RT-PCR, 4) laser capture dissection, 5) fluorescence-activated cell sorting, 6) and electrophysiological assessment. In addition, breeding the specific Cre-lines to mouse strains that contain “floxed” genes of interest or expression cassettes, facilitates gain- or loss-of-function of specific genes within Cre-expressing cells. Spatial and temporal restriction can further be enhanced by the use of Cre-dependent viruses to deliver and express a gene of interest, which is also of relevance for neuronal tracing approaches. An alternative to the Cre-line × reporter approach are transgenic mice expressing fluorescent proteins under the direct control of the CRF and CRFR1 promoters [103, 123]. In addition, CRFR1, UCN1, UCN2 and UCN3 knockout mice that have been generated via replacement of the coding region by EGFP, LacZ or tau-LacZ reporter cassettes, can also be exploited to visualize the desired neurons [42, 59, 252]. However, the specificity of transgene expression in Cre-, or reporter-lines is a major issue and strongly dependent on the utilized promoter fragment, site of transgene insertion and the number of inserted transgene copies. As a result, Cre recombinase activity might only be detected in a subset of the targeted cell population, or in the worst case, be expressed ectopically [295, 296]. Thus, it is of utter importance to confirm that the transgene expression accurately recapitulates that of the gene of interest. Besides the mentioned CRFR2-Cre mice, two UCN3-Cre and four CRF-Cre mouse lines have been generated to date (Table 3). Both UCN3-Cre lines (KF43 and KF31) are available from the Gene Expression Nervous System Atlas (GENSAT) project (http://www.gensat.org) but have not been fully characterized yet. In contrast, all four available CRF-Cre mice have partially been investigated (Table 3). A recent study convincingly demonstrated a partially divergent pattern of CRF-Cre mediated reporter expression in two of these lines (CRFp3.0Cre and Crh-IRES-Cre mice), highlighting the importance of confirming the cell-type specificity of the utilized Cre-driver [297]. Cre-activated LacZ expression in transgenic CRFp3.0Cre mice, generated by Martin and colleagues, was demonstrated in the PVN, CeA and BNST, three major CRF-expression sites in the brain [286]. A small amount of LacZ expressing neurons were scattered within the cortex, consistent with the pattern of cortical CRF expression. However, the authors reported lack of LacZ expression throughout the remainder of the brain and brainstem, which is not in accordance with the endogenous CRF expression, which is additionally found in other brain structures (e.g. piriform cortex, hippocampus, Barrington’s nucleus and inferior olive). Thus, CRFp3.0Cre mice seem to reflect the expression of a subset of CRF neurons in the brain. Importantly, CRFp3.0Cre-dependent GFP expression colocalized to some extent with endogenous CRF in the CeA [286], but was not confirmed for other CRF-expressing brain regions. Two additional studies made use of the CRFp3.0Cre-driver to study the role GABA(A)α1 and the Grin1 receptor (glutamate receptor, ionotropic, N-methyl D-aspartate 1) specifically in CRF neurons [298, 299]. Deletion of the GABA(A)α1 in CRF neurons was shown to enhance anxiety and disrupt fear extinction [298], while deletion of Grin1 enhanced fear memory [299]. In addition to the CRFp3.0Cre-line, GENSAT has also produced a CRF-Cre
Table 3.
Cre-driver mouse lines related to the CRF/CRFR system.
| Cre-driver lines | Construct | Expression pattern | Reference |
|---|---|---|---|
| CRF-Cre lines | |||
| CRFp3.0Cre | Transgenic - 3.0 kb of CRF promoter were amplified and cloned in front of the Cre coding sequence | Reflects a subset of the endogenous expression pattern; CRF-specific expression in the PVN, CeA, BNST, hypothalamus and scattered neurons of the cortex; lack of expression in other CRF-containing nuclei | [286, 298, 299] |
| CRH-Cre (KN282) |
BAC transgenic - insertion of the Cre gene, followed by a polyA sequence, at the initiating ATG codon of the 2nd CRF exon | Only partially reflects the endogenous expression pattern e.g. PVN; absence of Cre activity in the CeA and BNST, ectopic expression throughout the thalamus, olfactory bulb, & hippocampal pyramidal neurons | [300, 301] http://www.gensat.org/cre.jsp |
| Crh-IRES-Cre | Knockin - insertion of an ires-Cre targeting cassette immediately after the translational STOP codon of the endogenous CRF gene | Cre expression is regulated by the endogenous CRF promoter and thus largely recapitulates the endogenous CRF expression pattern | [147, 302-307] |
| Chr-ires-Cre | Knockin - insertion of an ires-Cre targeting cassette after the coding region of the endogenous CRF gene | Cre expression is regulated by the endogenous CRF promoter and thus largely recapitulates the endogenous CRF expression pattern | [308] |
| CRH-creERT2 | BAC transgenic - insertion of CreERT2 into the human CRF gene; BAC was inserted into the Hprt locus | Cre expression is induced by tamoxifen and regulated by CRF promoter elements; very few scattered Cre expressing cells in regions of endogenous CRF expression |
http://www.informatics.jax.org/allele/MGI:5568222 Simpson & Deussing unpublished data |
| UCN3-Cre lines | |||
|
Ucn3-Cre (KF31) |
BAC transgenic - insertion of the Cre gene, followed by a polyA sequence, at the initiating ATG codon of the first coding exon of Ucn3 | Expression in specific hypothalamic nuclei, moderate to strong expression in the medial amygdala; scattered probably ectopic expression in the caudate putamen and ventral striatum | http://www.gensat.org/cre.jsp |
|
Ucn3-Cre (KF43) |
BAC transgenic - insertion of Cre gene, followed by a polyA sequence, at the initiating ATG codon of the first coding exon of Ucn3 | Expression largely reflects endogenous Ucn3 expression pattern | http://www.gensat.org/cre.jsp |
| CRFR2-Cre lines | |||
| Crfr2α-eGFPCre | BAC transgenic - eGFPCre fusion protein driven by the CRFR2α promoter | ~ 92% of Cre-expressing cells co-localize with endogenous CRFR2 mRNA in the lateral septum; no detailed analysis of other expression domains | [294] |
|
Crhr2-Cre (RT30-Cre) |
BAC transgenic - insertion of Cre gene, followed by a polyA sequence, at the initiating ATG codon of the first coding exon of CRFR2 | Not fully characterized, little specificity for CRFR2-neurons | http://www.gensat.org/cre.jsp |
strain (KN282), which was previously used by Sarkar and colleagues to investigate the effect of the stress-derived neurosteroid tetrahydrodeoxycorticosterone (THDOC) on CRF neurons in the PVN, in the context of HPA axis regulation [300]. Although Cre-expression in the PVN was verified to recapitulate that of endogenous CRF [300, 301], it is absent in the CeA and BNST, and ectopically expressed throughout the thalamus, olfactory bulb, and pyramidal neurons of the hippocampus (http://www.gensat.org). Cre expression in unintentionally targeted cells in the KN282 CRF-Cre transgenic mouse line could confound the interpretation of cell-type specific experiments, and should be used with caution. In order to circumvent the uncertainties that arise due to random integration of DNA constructs in transgenic Cre-lines, targeted knockin strategies in embryonic stem cells have been used. The recently generated CRH-ires-Cre mouse line relies on this principle [302]. In this case, a targeting cassette containing an internal ribosome entry site (ires), followed by the Cre recombinase coding region, was inserted immediately after the translational STOP codon of the endogenous CRF gene [302]. As a result, Cre expression is regulated and directed by the endogenous CRF promoter/enhancer elements, without compromising CRF expression. CRH-ires-Cre recombinase activity is reported as highly specific and efficient, and largely recapitulates the endogenous CRF expression pattern [302], including the PVN, BNST, CeA, piriform cortex, inferior olive and scattered neurons of the cortex and hippocampus (images of Cre-dependent reporter expression are available at the Allen Institute for Brain Science website - http://brain-map.org/). Consequently, an increasing number of studies has started to employ the Crh-ires-Cre driver line to analyze different populations of CRF neurons [147, 303-307]. For example, Cusulin and colleagues functionally characterized CRF-expressing PVN neurons [303], while Silberman et al. examined the electrophysiological properties of CRF neurons in the BNST and CeA in the context of addiction [147]. The latter demonstrate that CRF-expressing BNST neurons regulate ethanol-seeking behavior by potentiating glutamatergic transmission in a population of VTA-projecting BNST neurons [147]. Recently, a similar strategy was applied to generate an independent Crh-ires-Cre knockin line, which also strongly reflects the endogenous CRF expression pattern [308]. Attempts to generate an inducible CreERT2-based CRF Cre driver line, undertaken within the Pleiades Promoter Project and CanEuCre [309, 310], were of limited success, i.e. a CRF-specific BAC construct introduced into the Hprt locus showed only limited and partially ectopic Cre activity (E.M. Simpson & J.M. Deussing, unpublished results). However, the generation of an adequate CRF-Cre counterpart, a CRFR1-Cre driver, has not yet been realized. A CRFR1-Cre line would significantly contribute to our understanding of CRF/CRFR1-pathways in the brain, as it would enable more precise circuit analysis via retrograde tracing approaches and optogenetic manipulations. With the current development and expansion of CRISPR/Cas technology, this goal might be achieved in the near future.
Although a number transgenic Cre-driver lines have been generated for a subset of CRF family members and their receptors, most still lack extensive anatomical, molecular, and functional characterization. The necessity for this was recently demonstrated for Cre-driver lines that target dopaminergic neurons, some of which exhibited highly non-specific expression patterns [295, 296]. In the end, complementary methodologies such as pharmacological manipulations, immunohistochemical and in situ hybridization studies, will considerably strengthen the conclusions drawn from the use of transgenic Cre-driver mouse lines.
Concluding Remarks
Stress-related mental disorders including depression, anxiety-disorders, as well as alcohol and substance abuse, represent some of the most common and escalating health problems in today’s society. Depression tops the estimated financial burden of all psychiatric disorders, and represents the most prevalent mental illness and third leading contributor to the global disease burden [311-313]. The lack of effective treatments or preventive interventions for most mental disorders partially reflects our limited understanding of the underlying brain-circuitries. This is largely owed to the substantial amount of overlapping symptoms of many psychiatric illnesses, which makes accurate diagnosis often very difficult [314]. This overlap in disease etiology partially arises from shared genetic susceptibility factors [315]; however environmental perturbations, such as trauma and chronic stress, are additional, well-established risk factors [6, 316, 317]. Chronic stress represents a strong proximal predictor of major depressive disorder onset and can also induce recurrence of depressive symptoms [318-321]. Similarly, stressful life events are associated with substance and drug abuse and are frequently reported to trigger relapse [141, 322]. Even adverse experiences in utero or during early childhood are increasingly associated with lifelong health disparities [323, 324]. But why does stress cause disease in some individuals but not in others? A common perception is that adverse environments might trigger disease onset in genetically predisposed individuals. Evidence for such gene-environment interactions have been provided by a number of studies [325-327]. Consequently, altered stress-neurocircuits, either caused by genetic, and/or environmental changes, constitute a common domain of many mental disorders, and highlight the necessity to functionally dissect the brains’ most prominent stress system – the CRF/UCN/CRFR system. As summarized above, a large body of evidence has implicated CRF-family members and their receptors in the neuroendocrine and behavioral responses to stress. The ability of the CRF system to interact and modulate monoaminergic circuits is of particular importance considering the involvement of serotonergic, dopaminergic and noradrenergic systems in essentially all aspects of emotion, motivation, reward and cognition. The interaction of the CRF/CRFR-system with different neurotransmitters possibly accounts for the generation of highly specific molecular, circuit and behavioral effects under both, basal and stress conditions. Furthermore, the identity and release sites of activated CRF/UCN neurons, as well as the identity and expression sites of the responding postsynaptic CRFR neurons are likely determining specific behavioral outcomes in response to stress. Although we have substantially advanced our understanding of functional CRF circuits and their effects on stress-related behaviors, we still lack detailed knowledge about the underlying molecular mechanisms and pathways by which the brain translates stressful stimuli into the final integrated biological response under physiological and pathological conditions. Based on recent genetic and pharmacological studies, it becomes evident that CRF is losing its reputation as an “all-aversive” peptide, which is not entirely surprising considering that CRF-mediated HPA axis activation also occurs during eustress - the positive stress-response. The CRF/CRFR1 system modulates very specific and partially opposing physiological and behavioral effects depending on the underlying neuronal circuits, brain regions and environmental conditions, which also most likely holds true for CRFR2. The generation of more specific mouse genetic models, viral and optogenetic tools will enhance our understanding of CRF/UCN-CRFR1/2 circuit dynamics in adaptive and maladaptive stress-related behaviors, and aid in the development of more effective treatment modalities in psychiatry.
Acknowledgements
A.C. is the head of the Max Planck Society - Weizmann Institute of Science Laboratory for Experimental Neuropsychiatry and Behavioral Neurogenetics. His work is supported by: an FP7 Grant from the European Research Council (260463); Research Grant from the Israel Science Foundation (803/11); a Research support from Roberto and Renata Ruhman; Nella and Leon Benoziyo Center for Neurological Diseases; the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics; the Perlman Family Foundation, Founded by Louis L. and Anita M. Perlman; the Adelis Foundation and the Irving I. Moskowitz Foundation; I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (grant No 1916/12). J.D.´s work has been supported by the German Federal Ministry of Education and Research within the framework of the e:Med research and funding concept (IntegraMent: Integrated Understanding of Causes and Mechanisms in Mental Disorders; FKZ 01ZX1314H), by the program for medical genome research within the framework of NGFN-Plus (FKZ: 01GS08151 and FKZ: 01GS08155), by the Max Planck Institute for Psychiatry and the Helmholtz Zentrum München with their Clinical Cooperation Group (CCG), and by the Initiative and Networking Fund of the Helmholtz Association in the framework of the Helmholtz Alliance for Mental Health in an Ageing Society (HA-215).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Cohen S., Janicki-Deverts D., Miller G.E. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B.S., Gianaros P.J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrousos G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 4.Adler N.E., Ostrove J.M. Socioeconomic status and health: what we know and what we don’t. Ann. N. Y. Acad. Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 5.McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet E.R., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 7.Steptoe A., Kivimaki M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 8.Smeets T., Wolf O.T., Giesbrecht T., Sijstermans K., Telgen S., Joels M. Stress selectively and lastingly promotes learning of context-related high arousing information. Psychoneuroendocrinology. 2009;34:1152–1161. doi: 10.1016/j.psyneuen.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Roozendaal B., Okuda S., de Quervain D.J., McGaugh J.L. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Sandi C., Pinelo-Nava M.T. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joels M., Pu Z., Wiegert O., Oitzl M.S., Krugers H.J. Learning under stress: how does it work? Trends Cogn. Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Kirby E.D., Muroy S.E., Sun W.G., Covarrubias D., Leong M.J., Barchas L.A., Kaufer D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. eLife. 2013;2:e00362. doi: 10.7554/eLife.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 14.Selye H. Changing Distress Into Eustress - Selye,Hans Voices Theories on Stress. Tex. Med. 1980;76:78–80. [Google Scholar]
- 15.Klengel T., Binder E.B. Epigenetics of Stress-related psychiatric disorders and gene x environment interactions. Neuron. 2015;86:1343–1357. doi: 10.1016/j.neuron.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S., Powers A., Bradley B., Ressler K. Gene x environment determinants of stress- and anxiety-related disorders. Annu. Rev. Psychol. 2016;67:239–261. doi: 10.1146/annurev-psych-122414-033408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen A.S., Nguyen X.V., Karpitskiy V., Mettenleiter T.C., Loewy A.D. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein D.S. Stress-induced activation of the sympathetic nervous system. Baillieres Clin. Endocrinol. Metab. 1987;1:253–278. doi: 10.1016/s0950-351x(87)80063-0. [DOI] [PubMed] [Google Scholar]
- 19.Joels M., Baram T.Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 21.Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Pol A.N. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selye H. Stress and disease. Science. 1955;122:625–631. doi: 10.1126/science.122.3171.625. [DOI] [PubMed] [Google Scholar]
- 24.Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 25.Vale W., Rivier C., Brown M.R., Spiess J., Koob G., Swanson L., Bilezikjian L., Bloom F., Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog. Horm. Res. 1983;39:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 26.Sapolsky R.M., Romero L.M., Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 27.Munck A., Guyre P.M., Holbrook N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 28.Picard M., Juster R.P., McEwen B.S. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 2014;10:303–310. doi: 10.1038/nrendo.2014.22. [DOI] [PubMed] [Google Scholar]
- 29.Stahn C., Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008;4:525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 30.Quax R.A., Manenschijn L., Koper J.W., Hazes J.M., Lamberts S.W., van Rossum E.F., Feelders R.A. Glucocorticoid sensitivity in health and disease. Nat. Rev. Endocrinol. 2013;9:670–686. doi: 10.1038/nrendo.2013.183. [DOI] [PubMed] [Google Scholar]
- 31.Buttgereit F., Burmester G.R., Lipworth B.J. Inflammation, glucocorticoids and risk of cardiovascular disease. Nat. Clin. Pract. Rheumatol. 2009;5:18–19. doi: 10.1038/ncprheum0963. [DOI] [PubMed] [Google Scholar]
- 32.de Kloet E.R. Hormones and the stressed brain. Ann. N. Y. Acad. Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- 33.Qian X., Droste S.K., Lightman S.L., Reul J.M., Linthorst A.C. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153:4346–4353. doi: 10.1210/en.2012-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieger D.T., Allen W., Rizzo F., Krieger H.P. Characterization of the normal temporal pattern of plasma corticosteroid levels. J. Clin. Endocrinol. Metab. 1971;32:266–284. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- 35.Koolhaas J.M., Bartolomucci A., Buwalda B., de Boer S.F., Flugge G., Korte S.M., Meerlo P., Murison R., Olivier B., Palanza P., Richter-Levin G., Sgoifo A., Steimer T., Stiedl O. van, D.G.; Wohr, M.; Fuchs, E. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Koolhaas J.M., de Boer S.F., De Rutter A.J., Meerlo P., Sgoifo A. Social stress in rats and mice. Acta Physiol. Scand. Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- 37.Vaughan J., Donaldson C., Bittencourt J., Perrin M.H., Lewis K., Sutton S., Chan R., Turnbull A.V., Lovejoy D., Rivier C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 38.Hsu S.Y., Hsueh A.J. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 39.Lewis K., Li C., Perrin M.H., Blount A., Kunitake K., Donaldson C., Vaughan J., Reyes T.M., Gulyas J., Fischer W., Bilezikjian L., Rivier J., Sawchenko P.E., Vale W.W. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyes T.M., Lewis K., Perrin M.H., Kunitake K.S., Vaughan J., Arias C.A., Hogenesch J.B., Gulyas J., Rivier J., Vale W.W., Sawchenko P.E. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dautzenberg F.M., Hauger R.L. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol. Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- 42.Deussing J.M., Breu J., Kuhne C., Kallnik M., Bunck M. Glasl. L.; Yen, Y.C.; Schmidt, M.V.; Zurmuhlen, R.; Vogl, A.M.; Gailus-Durner, V.; Fuchs, H.; Holter, S.M.; Wotjak, C.T.; Landgraf, R.; de Angelis, M.H.; Holsboer, F.; Wurst, W. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J. Neurosci. 2010;30:9103–9116. doi: 10.1523/JNEUROSCI.1049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuperman Y., Chen A. Urocortins: emerging metabolic and energy homeostasis perspectives. Trends Endocrinol. Metab. 2008;19:122–129. doi: 10.1016/j.tem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Li C., Chen P., Vaughan J., Blount A., Chen A., Jamieson P.M., Rivier J., Smith M.S., Vale W. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- 45.Chen A., Blount A., Vaughan J., Brar B., Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145:2445–2457. doi: 10.1210/en.2003-1570. [DOI] [PubMed] [Google Scholar]
- 46.Nieuwenhuyzen Kruseman A.C., Linton E.A., Ackland J., Besser G.M., Lowry P.J. Heterogeneous immunocytochemical reactivities of oCRF-41-like material in the human hypothalamus, pituitary and gastrointestinal tract. Neuroendocrinology. 1984;38:212–216. doi: 10.1159/000123893. [DOI] [PubMed] [Google Scholar]
- 47.Pelletier G., Desy L., Cote J., Vaudry H. Immunocytochemical localization of corticotropin-releasing factor-like immunoreactivity in the human hypothalamus. Neurosci. Lett. 1983;41:259–263. doi: 10.1016/0304-3940(83)90460-3. [DOI] [PubMed] [Google Scholar]
- 48.Suda T., Tomori N., Tozawa F., Mouri T., Demura H., Shizume K. Distribution and characterization of immunoreactive corticotropin-releasing factor in human tissues. J. Clin. Endocrinol. Metab. 1984;59:861–866. doi: 10.1210/jcem-59-5-861. [DOI] [PubMed] [Google Scholar]
- 49.Mouri T., Itoi K., Takahashi K., Suda T., Murakami O., Yoshinaga K., Andoh N., Ohtani H., Masuda T., Sasano N. Colocalization of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of the human hypothalamus. Neuroendocrinology. 1993;57:34–39. doi: 10.1159/000126339. [DOI] [PubMed] [Google Scholar]
- 50.Merali Z., Du L., Hrdina P., Palkovits M., Faludi G., Poulter M.O., Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J. Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozicz T. On the role of urocortin 1 in the non-preganglionic Edinger-Westphal nucleus in stress adaptation. Gen. Comp. Endocrinol. 2007;153:235–240. doi: 10.1016/j.ygcen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Ryabinin A.E., Tsivkovskaia N.O., Ryabinin S.A. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience. 2005;134:1317–1323. doi: 10.1016/j.neuroscience.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 53.Kozicz T., Tilburg-Ouwens D., Faludi G., Palkovits M., Roubos E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 2008;152:1015–1023. doi: 10.1016/j.neuroscience.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 54.Chen R., Lewis K.A., Perrin M.H., Vale W.W. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrin M.H., Donaldson C.J., Chen R., Lewis K.A., Vale W.W. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- 56.Perrin M., Donaldson C., Chen R., Blount A., Berggren T., Bilezikjian L., Sawchenko P., Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. USA. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang C.P., Pearse R.V., O’Connell S., Rosenfeld M.G. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- 58.Vita N., Laurent P., Lefort S., Chalon P., Lelias J.M., Kaghad M., Le F.G., Caput D., Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993;335:1–5. doi: 10.1016/0014-5793(93)80427-v. [DOI] [PubMed] [Google Scholar]
- 59.Kuhne C., Puk O., Graw J., de Angelis M.H., Schutz G., Wurst W., Deussing J.M. Visualizing Corticotropin-Releasing Hormone Receptor Type 1 Expression and Neuronal Connectivities in the Mouse Using a Novel Multifunctional Allele. J. Comp. Neurol. 2012;520:3150–3180. doi: 10.1002/cne.23082. [DOI] [PubMed] [Google Scholar]
- 60.Van Pett K., Viau V., Bittencourt J.C., Chan R.K., Li H.Y., Arias C., Prins G.S., Perrin M., Vale W., Sawchenko P.E. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 61.Grammatopoulos D.K., Dai Y., Randeva H.S., Levine M.A., Karteris E., Easton A.J., Hillhouse E.W. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol. Endocrinol. 1999;13:2189–2202. doi: 10.1210/mend.13.12.0391. [DOI] [PubMed] [Google Scholar]
- 62.Pisarchik A., Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur. J. Biochem. 2004;271:2821–2830. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slominski A.T., Zmijewski M.A., Zbytek B., Tobin D.J., Theoharides T.C., Rivier J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zmijewski M.A., Slominski A.T. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J. Cell. Physiol. 2009;218:593–602. doi: 10.1002/jcp.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lovenberg T.W., Liaw C.W., Grigoriadis D.E., Clevenger W., Chalmers D.T., De Souza E.B., Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stenzel P., Kesterson R., Yeung W., Cone R.D., Rittenberg M.B., Stenzel-Poore M.P. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol. Endocrinol. 1995;9:637–645. doi: 10.1210/mend.9.5.7565810. [DOI] [PubMed] [Google Scholar]
- 67.Chen A., Perrin M., Brar B., Li C., Jamieson P., Digruccio M., Lewis K., Vale W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol. Endocrinol. 2005;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- 68.Kostich W.A., Chen A., Sperle K., Largent B.L. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol. Endocrinol. 1998;12:1077–1085. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- 69.Lovenberg T.W., Chalmers D.T., Liu C., De Souza E.B. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 70.Day H.E., Greenwood B.N., Hammack S.E., Watkins L.R., Fleshner M., Maier S.F., Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J. Comp. Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lukkes J.L., Staub D.R., Dietrich A., Truitt W., Neufeld-Cohen A., Chen A., Johnson P.L., Shekhar A., Lowry C.A. Topographical distribution of corticotropin-releasing factor type 2 receptor-like immunoreactivity in the rat dorsal raphe nucleus: co-localization with tryptophan hydroxylase. Neuroscience. 2011;183:47–63. doi: 10.1016/j.neuroscience.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hiroi N., Wong M.L., Licinio J., Park C., Young M., Gold P.W., Chrousos G.P., Bornstein S.R. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol. Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez M.M., Young L.J., Plotsky P.M., Insel T.R. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J. Comp. Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- 74.Sullivan G.M., Parsey R.V., Kumar J.S., Arango V., Kassir S.A., Huang Y.Y., Simpson N.R., Van Heertum R.L., Mann J.J. PET Imaging of CRF1 with [11C]R121920 and [11C]DMP696: is the target of sufficient density? Nucl. Med. Biol. 2007;34:353–361. doi: 10.1016/j.nucmedbio.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kostich W.A., Grzanna R., Lu N.Z., Largent B.L. Immunohistochemical visualization of corticotropin-releasing factor type 1 (CRF1) receptors in monkey brain. J. Comp. Neurol. 2004;478:111–125. doi: 10.1002/cne.20271. [DOI] [PubMed] [Google Scholar]
- 76.Seasholtz A.F., Burrows H.L., Karolyi I.J., Camper S.A. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22:743–751. doi: 10.1016/s0196-9781(01)00387-4. [DOI] [PubMed] [Google Scholar]
- 77.Seasholtz A.F., Valverde R.A., Denver R.J. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J. Endocrinol. 2002;175:89–97. doi: 10.1677/joe.0.1750089. [DOI] [PubMed] [Google Scholar]
- 78.Behan D.P., De Souza E.B., Lowry P.J., Potter E., Sawchenko P., Vale W.W. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front. Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- 79.Chen A.M., Perrin M.H., Digruccio M.R., Vaughan J.M., Brar B.K., Arias C.M., Lewis K.A., Rivier J.E., Sawchenko P.E., Vale W.W. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc. Natl. Acad. Sci. USA. 2005;102:2620–2625. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evans R.T., Seasholtz A.F. Soluble corticotropin-releasing hormone receptor 2alpha splice variant is efficiently translated but not trafficked for secretion. Endocrinology. 2009;150:4191–4202. doi: 10.1210/en.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Markovic D., Grammatopoulos D.K. Focus on the splicing of secretin GPCRs transmembrane-domain 7. Trends Biochem. Sci. 2009;34:443–452. doi: 10.1016/j.tibs.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J. Psychiatr. Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- 83.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 84.Arborelius L., Owens M.J., Plotsky P.M., Nemeroff C.B. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 85.Binder E.B., Nemeroff C.B. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol. Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gillespie C.F., Nemeroff C.B. Hypercortisolemia and depression. Psychosom. Med. 2005;67(Suppl. 1):S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 87.Nemeroff C.B., Widerlov E., Bissette G., Walleus H., Karlsson I., Eklund K., Kilts C.D., Loosen P.T., Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 88.Reul J.M., Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr. Opin. Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- 89.De Bellis M.D., Gold P.W., Geracioti T.D., Jr, Listwak S.J., Kling M.A. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am. J. Psychiatry. 1993;150:656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- 90.Eaves M., Thatcher-Britton K., Rivier J., Vale W., Koob G.F. Effects of corticotropin releasing factor on locomotor activity in hypophysectomized rats. Peptides. 1985;6:923–926. doi: 10.1016/0196-9781(85)90323-7. [DOI] [PubMed] [Google Scholar]
- 91.Heinrichs S.C., Pich E.M., Miczek K.A., Britton K.T., Koob G.F. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- 92.Dunn A.J., Berridge C.W. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res. Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 93.Sutton R.E., Koob G.F., Le M.M., Rivier J., Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 94.Koob G.F., Swerdlow N., Seeligson M., Eaves M., Sutton R., Rivier J., Vale W. Effects of alpha-flupenthixol and naloxone on CRF-induced locomotor activation. Neuroendocrinology. 1984;39:459–464. doi: 10.1159/000124021. [DOI] [PubMed] [Google Scholar]
- 95.Dunn A.J., File S.E. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm. Behav. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- 96.Sirinathsinghji D.J., Rees L.H., Rivier J., Vale W. Corticotropin-releasing factor is a potent inhibitor of sexual receptivity in the female rat. Nature. 1983;305:232–235. doi: 10.1038/305232a0. [DOI] [PubMed] [Google Scholar]
- 97.Krahn D.D., Gosnell B.A., Grace M., Levine A.S. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Res. Bull. 1986;17:285–289. doi: 10.1016/0361-9230(86)90233-9. [DOI] [PubMed] [Google Scholar]
- 98.Heinrichs S.C., Koob G.F. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 99.Sztainberg Y., Chen A. Neuropeptide Regulation of Stress-Induced Behavior: Insights from the CRF/Urocortin Family. In: Fink G., Pfaff D., Levine J., editors. Handbook of Neuroendocrinology. London, Waltham, San Diego: Academic press, Elsevier; 2012. pp. 355–375. [Google Scholar]
- 100.Fossey M.D., Lydiard R.B., Ballenger J.C., Laraia M.T., Bissette G., Nemeroff C.B. Cerebrospinal fluid corticotropin-releasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol. Psychiatry. 1996;39:703–707. doi: 10.1016/0006-3223(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 101.Carpenter L.L., Tyrka A.R., McDougle C.J., Malison R.T., Owens M.J., Nemeroff C.B., Price L.H. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- 102.Swanson L.W., Sawchenko P.E., Rivier J., Vale W.W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 103.Justice N.J., Yuan Z.F., Sawchenko P.E., Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: Implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J. Comp. Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan X.X., Toth Z., Schultz L., Ribak C.E., Baram T.Z. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: Light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Y., Brunson K.L., Adelmann G., Bender R.A., Frotscher M., Baram T.Z. Hippocampal corticotropin releasing hormone: Pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Refojo D., Schweizer M., Kuehne C., Ehrenberg S., Thoeringer C., Vogl A.M., Dedic N., Schumacher M. von, W.G.; Avrabos, C.; Touma, C.; Engblom, D.; Schutz, G.; Nave, K.A.; Eder, M.; Wotjak, C.T.; Sillaber, I.; Holsboer, F.; Wurst, W.; Deussing, J.M. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y., Andres A.L., Frotscher M., Baram T.Z. Tuning synaptic transmission in the hippocampus by stress: The CRH system. Front. Cell. Neurosci. 2012;6:13. doi: 10.3389/fncel.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heinrichs S.C., Stenzel-Poore M.P., Gold L.H., Battenberg E., Bloom F.E., Koob G.F., Vale W.W., Pich E.M. Learning impairment in transgenic mice with central overexpression of corticotropin-releasing factor. Neuroscience. 1996;74:303–311. doi: 10.1016/0306-4522(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 109.Radulovic J., Ruhmann A., Liepold T., Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: Differential roles of CRF receptors 1 and 2. J. Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aldenhoff J.B., Gruol D.L., Rivier J., Vale W., Siggins G.R. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- 111.von Wolff G., Avrabos C., Stepan J., Wurst W., Deussing J.M., Holsboer F., Eder M. Voltage-sensitive dye imaging demonstrates an enhancing effect of corticotropin-releasing hormone on neuronal activity propagation through the hippocampal formation. J. Psychiatr. Res. 2011;45:256–261. doi: 10.1016/j.jpsychires.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 112.Kratzer S., Mattusch C., Metzger M.W., Dedic N., Noll-Hussong M., Kafitz K.W., Eder M., Deussing J.M., Holsboer F., Kochs E., Rammes G. Activation of CRH receptor type 1 expressed on glutamatergic neurons increases excitability of CA1 pyramidal neurons by the modulation of voltage-gated ion channels. Front. Cell. Neurosci. 2013;7:91. doi: 10.3389/fncel.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y., Rex C.S., Rice C.J., Dube C.M., Gall C.M., Lynch G., Baram T.Z. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc. Natl. Acad. Sci. USA. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y., Bender R.A., Brunson K.L., Pomper J.K., Grigoriadis D.E., Wurst W., Baram T.Z. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y., Dube C.M., Rice C.J., Baram T.Z. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J. Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Y., Kramar E.A., Chen L.Y., Babayan A.H., Andres A.L., Gall C.M., Lynch G., Baram T.Z. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol. Psychiatry. 2013;18:485–496. doi: 10.1038/mp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ivy A.S., Rex C.S., Chen Y., Dube C., Maras P.M., Grigoriadis D.E., Gall C.M., Lynch G., Baram T.Z. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radulovic J., Blank T., Eckart K., Radulovic M., Stiedl O., Spiess J. CRF and CRF receptors. Results Probl. Cell Differ. 1999;26:67–90. doi: 10.1007/978-3-540-49421-8_4. [DOI] [PubMed] [Google Scholar]
- 119.Hikichi T., Akiyoshi J., Yamamoto Y., Tsutsumi T., Isogawa K., Nagayama H. Suppression of conditioned fear by administration of CRF receptor antagonist CP-154,526. Pharmacopsychiatry. 2000;33:189–193. doi: 10.1055/s-2000-7587. [DOI] [PubMed] [Google Scholar]
- 120.Thoeringer C.K., Henes K., Eder M., Dahlhoff M., Wurst W., Holsboer F., Deussing J.M., Moosmang S., Wotjak C.T. Consolidation of remote fear memories involves Corticotropin-Releasing Hormone (CRH) receptor type 1-mediated enhancement of AMPA receptor GluR1 signaling in the dentate gyrus. Neuropsychopharmacology. 2012;37:787–796. doi: 10.1038/npp.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pentkowski N.S., Litvin Y., Blanchard D.C., Vasconcellos A., King L.B., Blanchard R.J. Effects of acidic-astressin and ovine-CRF microinfusions into the ventral hippocampus on defensive behaviors in rats. Horm. Behav. 2009;56:35–43. doi: 10.1016/j.yhbeh.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keegan C.E., Karolyi I.J., Knapp L.T., Bourbonais F.J., Camper S.A., Seasholtz A.F. Expression of corticotropin-releasing hormone transgenes in neurons of adult and developing mice. Mol. Cell. Neurosci. 1994;5:505–514. doi: 10.1006/mcne.1994.1062. [DOI] [PubMed] [Google Scholar]
- 123.Alon T., Zhou L., Perez C.A., Garfield A.S., Friedman J.M., Heisler L.K. Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter. Endocrinology. 2009;150:5626–5632. doi: 10.1210/en.2009-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sajdyk T.J., Schober D.A., Gehlert D.R., Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav. Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 125.Swiergiel A.H., Takahashi L.K., Kalin N.H. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- 126.Robison C.L., Meyerhoff J.L., Saviolakis G.A., Chen W.K., Rice K.C., Lumley L.A.A. CRH1 antagonist into the amygdala of mice prevents defeat-induced defensive behavior. Ann. N. Y. Acad. Sci. 2004;1032:324–327. doi: 10.1196/annals.1314.052. [DOI] [PubMed] [Google Scholar]
- 127.Sztainberg Y., Kuperman Y., Tsoory M., Lebow M., Chen A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol. Psychiatry. 2010;15:905–917. doi: 10.1038/mp.2009.151. [DOI] [PubMed] [Google Scholar]
- 128.Kudo T., Uchigashima M., Miyazaki T., Konno K., Yamasaki M., Yanagawa Y., Minami M., Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J. Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Georges F., Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: A novel excitatory amino acid input to midbrain dopamine neurons. J. Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van Bockstaele E.J., Peoples J., Valentino R.J.A.E. Bennett Research Award. Anatomic basis for differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol. Psychiatry. 1999;46:1352–1363. doi: 10.1016/s0006-3223(99)00213-9. [DOI] [PubMed] [Google Scholar]
- 131.Dong H.W., Swanson L.W. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp. Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- 132.Dong H.W., Petrovich G.D., Watts A.G., Swanson L.W. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J. Comp. Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 133.Mulders W.H., Meek J., Hafmans T.G., Cools A.R. Plasticity in the stress-regulating circuit: Decreased input from the bed nucleus of the stria terminalis to the hypothalamic paraventricular nucleus in Wistar rats following adrenalectomy. Eur. J. Neurosci. 1997;9:2462–2471. doi: 10.1111/j.1460-9568.1997.tb01663.x. [DOI] [PubMed] [Google Scholar]
- 134.Kim S.Y., Adhikari A., Lee S.Y., Marshel J.H., Kim C.K., Mallory C.S., Lo M., Pak S., Mattis J., Lim B.K., Malenka R.C., Warden M.R., Neve R., Tye K.M., Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jennings J.H., Sparta D.R., Stamatakis A.M., Ung R.L., Pleil K.E., Kash T.L., Stuber G.D. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee Y., Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J. Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liang K.C., Chen H.C., Chen D.Y. Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin. J. Physiol. 2001;44:33–43. [PubMed] [Google Scholar]
- 138.Sahuque L.L., Kullberg E.F., Mcgeehan A.J., Kinder J.R., Hicks M.P., Blanton M.G., Janak P.H., Olive M.F. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: Role of CRF receptor subtypes. Psychopharmacology (Berl.) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.George O., Le M.M., Koob G.F. Allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiol. Behav. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haass-Koffler C.L., Bartlett S.E. Stress and addiction: Contribution of the corticotropin releasing factor (CRF) system in neuroplasticity. Front. Mol. Neurosci. 2012;5:91. doi: 10.3389/fnmol.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Erb S., Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang J., Fang Q., Liu Z., Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl.) 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- 144.Koob G.F., Zorrilla E.P. Neurobiological mechanisms of addiction: Focus on corticotropin-releasing factor. Curr. Opin. Investig. Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- 145.LeMoal M., Koob G.F. Drug addiction: Pathways to the disease and pathophysiological perspectives. Eur. Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 146.Silberman Y., Winder D.G. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front. Psychiatry. 2013;4:42. doi: 10.3389/fpsyt.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Silberman Y., Matthews R.T., Winder D.G. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J. Neurosci. 2013;33:950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spanagel R., Noori H.R., Heilig M. Stress and alcohol interactions: Animal studies and clinical significance. Trends Neurosci. 2014;37:219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 149.Gold P.W., Chrousos G.P. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol. Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 150.Lemos J.C., Wanat M.J., Smith J.S., Reyes B.A., Hollon N.G., Van Bockstaele E.J., Chavkin C., Phillips P.E. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wanat M.J., Hopf F.W., Stuber G.D., Phillips P.E., Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bagosi Z., Jaszberenyi M., Bujdoso E., Telegdy G. The effects of corticoptropin-releasing factor and the urocortins on striatal dopamine release induced by electrical stimulation-an in vitro superfusion study. Neurochem. Res. 2006;31:209–213. doi: 10.1007/s11064-005-9010-x. [DOI] [PubMed] [Google Scholar]
- 153.Kalivas P.W., Duffy P., Latimer L.G. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J. Pharmacol. Exp. Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- 154.Muramatsu T., Inoue K., Iwasaki S., Yamauchi T., Hayashi T., Kiriike N. Corticotropin-releasing factor receptor type 1, but not type 2, in the ventromedial hypothalamus modulates dopamine release in female rats. Pharmacol. Biochem. Behav. 2006;85:435–440. doi: 10.1016/j.pbb.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 155.Wang B., Shaham Y., Zitzman D., Azari S., Wise R.A., You Z.B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: A role in stress-induced relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wanat M.J., Bonci A., Phillips P.E. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Boyson C.O., Miguel T.T., Quadros I.M., Debold J.F., Miczek K.A. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl.) 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Blacktop J.M., Seubert C., Baker D.A., Ferda N., Lee G., Graf E.N., Mantsch J.R. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vranjkovic O., Gasser P.J., Gerndt C.H., Baker D.A., Mantsch J.R. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J. Neurosci. 2014;34:12504–12514. doi: 10.1523/JNEUROSCI.0680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rodaros D., Caruana D.A., Amir S., Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 161.Grieder T.E., Herman M.A., Contet C., Tan L.A., Vargas-Perez H., Cohen A., Chwalek M., Maal-Bared G., Freiling J., Schlosburg J.E., Clarke L., Crawford E., Koebel P., Repunte-Canonigo V., Sanna P.P., Tapper A.R., Roberto M., Kieffer B.L., Sawchenko P.E., Koob G.F., van der Kooy D., George O. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat. Neurosci. 2014;17:1751–1758. doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Walsh J.J., Friedman A.K., Sun H., Heller E.A., Ku S.M., Juarez B., Burnham V.L., Mazei-Robison M.S., Ferguson D., Golden S.A., Koo J.W., Chaudhury D., Christoffel D.J., Pomeranz L., Friedman J.M., Russo S.J., Nestler E.J., Han M.H. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat. Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Valentino R.J., Foote S.L., Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- 164.Valentino R.J., Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swanson L.W., Hartman B.K. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J. Comp. Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 166.Foote S.L., Aston-Jones G., Bloom F.E. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. USA. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chamberlain S.R., Robbins T.W. Noradrenergic modulation of cognition: Therapeutic implications. J. Psychopharmacol. 2013;27:694–718. doi: 10.1177/0269881113480988. [DOI] [PubMed] [Google Scholar]
- 168.Bissette G., Klimek V., Pan J., Stockmeier C., Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- 169.Gold P.W., Chrousos G.P. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol. Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 170.Wong M.L., Kling M.A., Munson P.J., Listwak S., Licinio J., Prolo P., Karp B., McCutcheon I.E., Geracioti T.D., Jr, DeBellis M.D., Rice K.C., Goldstein D.S., Veldhuis J.D., Chrousos G.P., Oldfield E.H., McCann S.M., Gold P.W. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: Relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sci. USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Valentino R.J., Wehby R.G. Corticotropin-releasing factor: Evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology. 1988;48:674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- 172.Hwang K.R., Chan S.H., Chan J.Y. Noradrenergic neurotransmission at PVN in locus ceruleus-induced baroreflex suppression in rats. Am. J. Physiol. 1998;274:H1284–H1292. doi: 10.1152/ajpheart.1998.274.4.H1284. [DOI] [PubMed] [Google Scholar]
- 173.Herman J.P., Cullinan W.E. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 174.Valentino R.J., Page M., Van B.E., Aston-Jones G. Corticotropin-releasing factor innervation of the locus coeruleus region: Distribution of fibers and sources of input. Neuroscience. 1992;48:689–705. doi: 10.1016/0306-4522(92)90412-u. [DOI] [PubMed] [Google Scholar]
- 175.Kozicz T., Sterrenburg L., Xu L. Does midbrain urocortin 1 matter? A 15-year journey from stress (mal)adaptation to energy metabolism. Stress. 2011;14:376–383. doi: 10.3109/10253890.2011.563806. [DOI] [PubMed] [Google Scholar]
- 176.Xu L., Bloem B., Gaszner B., Roubos E.W., Kozicz T. Stress-related changes in the activity of cocaine- and amphetamine-regulated transcript and nesfatin neurons in the midbrain non-preganglionic Edinger-Westphal nucleus in the rat. Neuroscience. 2010;170:478–488. doi: 10.1016/j.neuroscience.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 177.Ryabinin A.E., Tsoory M.M., Kozicz T., Thiele T.E., Neufeld-Cohen A., Chen A., Lowery-Gionta E.G., Giardino W.J., Kaur S. Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol. 2012;46:349–357. doi: 10.1016/j.alcohol.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Korosi A., Schotanus S., Olivier B., Roubos E.W., Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res. 2005;1046:172–179. doi: 10.1016/j.brainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 179.Koob G.F., Heinrichs S.C. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 180.Moreau J.L., Kilpatrick G., Jenck F. Urocortin, a novel neuropeptide with anxiogenic-like properties. Neuroreport. 1997;8:1697–1701. doi: 10.1097/00001756-199705060-00027. [DOI] [PubMed] [Google Scholar]
- 181.Spina M.G., Merlo-Pich E., Akwa Y., Balducci C., Basso A.M., Zorrilla E.P., Britton K.T., Rivier J., Vale W.W., Koob G.F. Time-dependent induction of anxiogenic-like effects after central infusion of urocortin or corticotropin-releasing factor in the rat. Psychopharmacology (Berl.) 2002;160:113–121. doi: 10.1007/s00213-001-0940-y. [DOI] [PubMed] [Google Scholar]
- 182.Valdez G.R., Inoue K., Koob G.F., Rivier J., Vale W., Zorrilla E.P. Human urocortin II: Mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- 183.Ohata H., Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides. 2004;25:1703–1709. doi: 10.1016/j.peptides.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 184.Valdez G.R., Zorrilla E.P., Rivier J., Vale W.W., Koob G.F. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- 185.Venihaki M., Sakihara S., Subramanian S., Dikkes P., Weninger S.C., Liapakis G., Graf T., Majzoub J.A. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: Modulation by stress and attenuation of some anxiety-like behaviours. J. Neuroendocrinol. 2004;16:411–422. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- 186.Takahashi L.K., Ho S.P., Livanov V., Graciani N., Arneric S.P. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- 187.Henry B., Vale W., Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J. Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bakshi V.P., Smith-Roe S., Newman S.M., Grigoriadis D.E., Kalin N.H. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J. Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hammack S.E., Schmid M.J., LoPresti M.L., Der-Avakian A., Pellymounter M.A., Foster A.C., Watkins L.R., Maier S.F. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J. Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Amat J., Tamblyn J.P., Paul E.D., Bland S.T., Amat P., Foster A.C., Watkins L.R., Maier S.F. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 191.Pernar L., Curtis A.L., Vale W.W., Rivier J.E., Valentino R.J. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J. Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Neufeld-Cohen A., Evans A.K., Getselter D., Spyroglou A., Hill A., Gil S., Tsoory M., Beuschlein F., Lowry C.A., Vale W., Chen A. Urocortin-1 and -2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol. Psychiatry. 2010;15:426–441. doi: 10.1038/mp.2009.115. [DOI] [PubMed] [Google Scholar]
- 193.Staub D.R., Evans A.K., Lowry C.A. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF2) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 194.Lukkes J.L., Forster G.L., Renner K.J., Summers C.H. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur. J. Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Forster G.L., Pringle R.B., Mouw N.J., Vuong S.M., Watt M.J., Burke A.R., Lowry C.A., Summers C.H., Renner K.J. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur. J. Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Neufeld-Cohen A., Kelly P.A., Paul E.D., Carter R.N., Skinner E., Olverman H.J., Vaughan J.M., Issler O., Kuperman Y., Lowry C.A., Vale W.W., Seckl J.R., Chen A., Jamieson P.M. Chronic activation of corticotropin-releasing factor type 2 receptors reveals a key role for 5-HT1A receptor responsiveness in mediating behavioral and serotonergic responses to stressful challenge. Biol. Psychiatry. 2012;72:437–447. doi: 10.1016/j.biopsych.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Staub D.R., Spiga F., Lowry C.A. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 198.Hale M.W., Stamper C.E., Staub D.R., Lowry C.A. Urocortin 2 increases c-Fos expression in serotonergic neurons projecting to the ventricular/periventricular system. Exp. Neurol. 2010;224:271–281. doi: 10.1016/j.expneurol.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bornstein S.R., Schuppenies A., Wong M.L., Licinio J. Approaching the shared biology of obesity and depression: The stress axis as the locus of gene-environment interactions. Mol. Psychiatry. 2006;11:892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- 200.Yakabi K., Noguchi M., Ohno S., Ro S., Onouchi T., Ochiai M., Takabayashi H., Takayama K., Harada Y., Sadakane C., Hattori T. Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am. J. Physiol. Endocrinol. Metab. 2011;301:E72–E82. doi: 10.1152/ajpendo.00695.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zorrilla E.P., Tache Y., Koob G.F. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol. Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 202.Spina M., Merlo-Pich E., Chan R.K., Basso A.M., Rivier J., Vale W., Koob G.F. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 203.Richard D., Lin Q., Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: Their roles in the regulation of energy balance. Eur. J. Pharmacol. 2002;440:189–197. doi: 10.1016/s0014-2999(02)01428-0. [DOI] [PubMed] [Google Scholar]
- 204.Pelleymounter M.A., Joppa M., Ling N., Foster A.C. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 205.Inoue K., Valdez G.R., Reyes T.M., Reinhardt L.E., Tabarin A., Rivier J., Vale W.W., Sawchenko P.E., Koob G.F., Zorrilla E.P. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J. Pharmacol. Exp. Ther. 2003;305:385–393. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- 206.Stengel A., Tache Y. CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front. Neurosci. 2014;8:52. doi: 10.3389/fnins.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fekete E.M., Zorrilla E.P. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front. Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ohata H., Suzuki K., Oki Y., Shibasaki T. Urocortin in the ventromedial hypothalamic nucleus acts as an inhibitor of feeding behavior in rats. Brain Res. 2000;861:1–7. doi: 10.1016/s0006-8993(99)02378-1. [DOI] [PubMed] [Google Scholar]
- 209.Currie P.J., Coscina D.V., Bishop C., Coiro C.D., Koob G.F., Rivier J., Vale W. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain Res. 2001;916:222–228. doi: 10.1016/s0006-8993(01)02851-7. [DOI] [PubMed] [Google Scholar]
- 210.Bakshi V.P., Newman S.M., Smith-Roe S., Jochman K.A., Kalin N.H. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: Functional homology to CRF1 receptors in basolateral amygdala. J. Neurosci. 2007;27:10568–10577. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen P., Hover C.V., Lindberg D., Li C. Central urocortin 3 and type 2 corticotropin-releasing factor receptor in the regulation of energy homeostasis: Critical involvement of the ventromedial hypothalamus. Front. Endocrinol. (Lausanne) 2012;3:180. doi: 10.3389/fendo.2012.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Weitemier A.Z., Ryabinin A.E. Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience. 2006;137:1439–1445. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 213.Fekete E.M., Inoue K., Zhao Y., Rivier J.E., Vale W.W., Szucs A., Koob G.F., Zorrilla E.P. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: Intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chao H., Digruccio M., Chen P., Li C. Type 2 corticotropin-releasing factor receptor in the ventromedial nucleus of hypothalamus is critical in regulating feeding and lipid metabolism in white adipose tissue. Endocrinology. 2012;153:166–176. doi: 10.1210/en.2011-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tabarin A., Diz-Chaves Y., Consoli D., Monsaingeon M., Bale T.L., Culler M.D., Datta R., Drago F., Vale W.W., Koob G.F., Zorrilla E.P., Contarino A. Role of the corticotropin-releasing factor receptor type 2 in the control of food intake in mice: A meal pattern analysis. Eur. J. Neurosci. 2007;26:2303–2314. doi: 10.1111/j.1460-9568.2007.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bale T.L., Anderson K.R., Roberts A.J., Lee K.F., Nagy T.R., Vale W.W. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144:2580–2587. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- 217.Vetter D.E., Li C., Zhao L., Contarino A., Liberman M.C., Smith G.W., Marchuk Y., Koob G.F., Heinemann S.F., Vale W., Lee K.F. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat. Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- 218.Chen A., Zorrilla E., Smith S., Rousso D., Levy C., Vaughan J., Donaldson C., Roberts A., Lee K.F., Vale W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J. Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Seres J., Bornstein S.R., Seres P., Willenberg H.S., Schulte K.M., Scherbaum W.A., Ehrhart-Bornstein M. Corticotropin-releasing hormone system in human adipose tissue. J. Clin. Endocrinol. Metab. 2004;89:965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- 220.Benner C., van der Meulen T., Caceres E., Tigyi K., Donaldson C.J., Huising M.O. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.van der Meulen T., Xie R., Kelly O.G., Vale W.W., Sander M., Huising M.O. Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic alpha and beta cells. PLoS One. 2012;7:e52181. doi: 10.1371/journal.pone.0052181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.van der Meulen T., Donaldson C.J., Caceres E., Hunter A.E., Cowing-Zitron C., Pound L.D., Adams M.W., Zembrzycki A., Grove K.L., Huising M.O. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 2015;21:769–776. doi: 10.1038/nm.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stenzel-Poore M.P., Cameron V.A., Vaughan J., Sawchenko P.E., Vale W. Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- 224.Stenzel-Poore M.P., Heinrichs S.C., Rivest S., Koob G.F., Vale W.W. Overproduction of corticotropin-releasing factor in transgenic mice: A genetic model of anxiogenic behavior. J. Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.van Gaalen M.M., Stenzel-Poore M.P., Holsboer F., Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur. J. Neurosci. 2002;15:2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- 226.Dirks A., Groenink L., Verdouw M.P., Schipholt M., Gugten J.d., Hijzen T., Olivier B. Behavioural analysis of transgenic mice overexpressing corticotropin-releasing hormone in paradigms emulating aspects of stress, anxiety, and depression. Int. J. Comp. Psychol. 2001:123–135. [Google Scholar]
- 227.Groenink L., Dirks A., Verdouw P.M., Schipholt M., Veening J.G., Van Der G.J., Olivier B. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol. Psychiatry. 2002;51:875–881. doi: 10.1016/s0006-3223(02)01334-3. [DOI] [PubMed] [Google Scholar]
- 228.Dirks A., Groenink L., Bouwknecht J.A., Hijzen T.H., Van Der G.J., Ronken E., Verbeek J.S., Veening J.G., Dederen P.J., Korosi A., Schoolderman L.F., Roubos E.W., Olivier B. Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. Eur. J. Neurosci. 2002;16:1751–1760. doi: 10.1046/j.1460-9568.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- 229.Vicentini E., Arban R., Angelici O., Maraia G., Perico M., Mugnaini M., Ugolini A., Large C., Domenici E., Gerrard P., Bortner D., Mansuy I.M., Mangiarini L., Merlo-Pich E. Transient forebrain over-expression of CRF induces plasma corticosterone and mild behavioural changes in adult conditional CRF transgenic mice. Pharmacol. Biochem. Behav. 2009;93:17–24. doi: 10.1016/j.pbb.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 230.Kolber B.J., Boyle M.P., Wieczorek L., Kelley C.L., Onwuzurike C.C., Nettles S.A., Vogt S.K., Muglia L.J. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J. Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lu A., Steiner M.A., Whittle N., Vogl A.M., Walser S.M., Ableitner M., Refojo D., Ekker M., Rubenstein J.L., Stalla G.K., Singewald N., Holsboer F., Wotjak C.T., Wurst W., Deussing J.M. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol. Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- 232.Spyroglou A., Riester A., Mueller-Peltzer K., Lu A., Rohde J., Hantel C., Kuehne C., Kulle A., Riepe F., Deussing J.M., Beuschlein F. Adrenal and ovarian phenotype of a tissue specific urocortin 2 overexpressing mouse model. Endocrinology. 2015;156:2646–2656. doi: 10.1210/en.2014-1971. [DOI] [PubMed] [Google Scholar]
- 233.Kimura M., Muller-Preuss P., Lu A., Wiesner E., Flachskamm C., Wurst W., Holsboer F., Deussing J.M. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol. Psychiatry. 2010;15:154–165. doi: 10.1038/mp.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dedic N., Touma C., Romanowski C.P., Schieven M., Kuhne C., Ableitner M., Lu A., Holsboer F., Wurst W., Kimura M., Deussing J.M. Assessing behavioural effects of chronic HPA axis activation using conditional CRH-overexpressing mice. Cell. Mol. Neurobiol. 2012;32:815–828. doi: 10.1007/s10571-011-9784-0. [DOI] [PubMed] [Google Scholar]
- 235.Bentley L., Esapa C.T., Nesbit M.A., Head R.A., Evans H., Lath D., Scudamore C.L., Hough T.A., Podrini C., Hannan F.M., Fraser W.D., Croucher P.I., Brown M.A., Brown S.D., Cox R.D., Thakker R.V. An N-ethyl-N-nitrosourea induced corticotropin-releasing hormone promoter mutation provides a mouse model for endogenous glucocorticoid excess. Endocrinology. 2014;155:908–922. doi: 10.1210/en.2013-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Muglia L., Jacobson L., Dikkes P., Majzoub J.A. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 237.Weninger S.C., Dunn A.J., Muglia L.J., Dikkes P., Miczek K.A., Swiergiel A.H., Berridge C.W., Majzoub J.A. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc. Natl. Acad. Sci. USA. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Timpl P., Spanagel R., Sillaber I., Kresse A., Reul J.M., Stalla G.K., Blanquet V., Steckler T., Holsboer F., Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat. Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 239.Smith G.W., Aubry J.M., Dellu F., Contarino A., Bilezikjian L.M., Gold L.H., Chen R., Marchuk Y., Hauser C., Bentley C.A., Sawchenko P.E., Koob G.F., Vale W., Lee K.F. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 240.Contarino A., Dellu F., Koob G.F., Smith G.W., Lee K.F., Vale W., Gold L.H. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- 241.Muller M.B., Zimmermann S., Sillaber I., Hagemeyer T.P., Deussing J.M., Timpl P., Kormann M.S., Droste S.K., Kuhn R., Reul J.M., Holsboer F., Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 242.Wang X.D., Chen Y., Wolf M., Wagner K.V., Liebl C., Scharf S.H., Harbich D., Mayer B., Wurst W., Holsboer F., Deussing J.M., Baram T.Z., Muller M.B., Schmidt M.V. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol. Dis. 2011;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang X.D., Rammes G., Kraev I., Wolf M., Liebl C., Scharf S.H., Rice C.J., Wurst W., Holsboer F., Deussing J.M., Baram T.Z., Stewart M.G., Muller M.B., Schmidt M.V. Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. J. Neurosci. 2011;31:13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang X.D., Su Y.A., Wagner K.V., Avrabos C., Scharf S.H., Hartmann J., Wolf M., Liebl C., Kuhne C., Wurst W., Holsboer F., Eder M., Deussing J.M., Muller M.B., Schmidt M.V. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat. Neurosci. 2013;16:706–713. doi: 10.1038/nn.3395. [DOI] [PubMed] [Google Scholar]
- 245.Sztainberg Y., Kuperman Y., Justice N., Chen A. An anxiolytic role for CRF receptor type 1 in the globus pallidus. J. Neurosci. 2011;31:17416–17424. doi: 10.1523/JNEUROSCI.3087-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Coste S.C., Kesterson R.A., Heldwein K.A., Stevens S.L., Heard A.D., Hollis J.H., Murray S.E., Hill J.K., Pantely G.A., Hohimer A.R., Hatton D.C., Phillips T.J., Finn D.A., Low M.J., Rittenberg M.B., Stenzel P., Stenzel-Poore M.P. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 247.Bale T.L., Contarino A., Smith G.W., Chan R., Gold L.H., Sawchenko P.E., Koob G.F., Vale W.W., Lee K.F. Mice, deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 248.Kishimoto T., Radulovic J., Radulovic M., Lin C.R., Schrick C., Hooshmand F., Hermanson O., Rosenfeld M.G., Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 249.Issler O., Carter R.N., Paul E.D., Kelly P.A., Olverman H.J., Neufeld-Cohen A., Kuperman Y., Lowry C.A., Seckl J.R., Chen A., Jamieson P.M. Increased anxiety in corticotropin-releasing factor type 2 receptor-null mice requires recent acute stress exposure and is associated with dysregulated serotonergic activity in limbic brain areas. Biol. Mood Anxiety Disord. 2014;4:1. doi: 10.1186/2045-5380-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bale T.L., Picetti R., Contarino A., Koob G.F., Vale W.W., Lee K.F. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J. Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Preil J., Muller M.B., Gesing A., Reul J.M., Sillaber I., van Gaalen M.M., Landgrebe J., Holsboer F., Stenzel-Poore M., Wurst W. Regulation of the hypothalamic-pituitary-adrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology. 2001;142:4946–4955. doi: 10.1210/endo.142.11.8507. [DOI] [PubMed] [Google Scholar]
- 252.Wang X., Su H., Copenhagen L.D., Vaishnav S., Pieri F., Shope C.D., Brownell W.E., De B.M., Paylor R., Bradley A. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol. Cell. Biol. 2002;22:6605–6610. doi: 10.1128/MCB.22.18.6605-6610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zalutskaya A.A., Arai M., Bounoutas G.S., Abou-Samra A.B. Impaired adaptation to repeated restraint and decreased response to cold in urocortin 1 knockout mice. Am. J. Physiol. Endocrinol. Metab. 2007;293:E259–E263. doi: 10.1152/ajpendo.00616.2006. [DOI] [PubMed] [Google Scholar]
- 254.Breu J., Touma C., Holter S.M., Knapman A., Wurst W., Deussing J.M. Urocortin 2 modulates aspects of social behaviour in mice. Behav. Brain Res. 2012;233:331–336. doi: 10.1016/j.bbr.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 255.Neufeld-Cohen A., Tsoory M.M., Evans A.K., Getselter D., Gil S., Lowry C.A., Vale W.W., Chen A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc. Natl. Acad. Sci. USA. 2010;107:19020–19025. doi: 10.1073/pnas.1013761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li C., Chen P., Vaughan J., Lee K.F., Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc. Natl. Acad. Sci. USA. 2007;104:4206–4211. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Smith M.L., Li J., Ryabinin A.E. Increased alcohol consumption in urocortin 3 knockout mice is unaffected by chronic inflammatory pain. Alcohol Alcohol. 2015;50:132–139. doi: 10.1093/alcalc/agu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jamieson P.M., Cleasby M.E., Kuperman Y., Morton N.M., Kelly P.A., Brownstein D.G., Mustard K.J., Vaughan J.M., Carter R.N., Hahn C.N., Hardie D.G., Seckl J.R., Chen A., Vale W.W. Urocortin 3 transgenic mice exhibit a metabolically favourable phenotype resisting obesity and hyperglycaemia on a high-fat diet. Diabetologia. 2011;54:2392–2403. doi: 10.1007/s00125-011-2205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Davidson B.L., Breakefield X.O. Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci. 2003;4:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 260.Osakada F., Mori T., Cetin A.H., Marshel J.H., Virgen B., Callaway E.M. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011;71:617–631. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bartel M.A., Weinstein J.R., Schaffer D.V. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther. 2012;19:694–700. doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- 262.Hommel J.D., Sears R.M., Georgescu D., Simmons D.L., Dileone R.J. Local gene knockdown in the brain using viral-mediated RNA interference. Nat. Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 263.Tenenbaum L., Chtarto A., Lehtonen E., Velu T., Brotchi J., Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J. Gene Med. 2004;6(Suppl. 1):S212–S222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- 264.Wong L.F., Goodhead L., Prat C., Mitrophanous K.A., Kingsman S.M., Mazarakis N.D. Lentivirus-mediated gene transfer to the central nervous system: Therapeutic and research applications. Hum. Gene Ther. 2006;17:1–9. doi: 10.1089/hum.2006.17.1. [DOI] [PubMed] [Google Scholar]
- 265.Betley J.N., Sternson S.M. Adeno-associated viral vectors for mapping, monitoring, and manipulating neural circuits. Hum. Gene Ther. 2011;22:669–677. doi: 10.1089/hum.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lentz T.B., Gray S.J., Samulski R.J. Viral vectors for gene delivery to the central nervous system. Neurobiol. Dis. 2012;48:179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Regev L., Ezrielev E., Gershon E., Gil S., Chen A. Genetic approach for intracerebroventricular delivery. Proc. Natl. Acad. Sci. USA. 2010;107:4424–4429. doi: 10.1073/pnas.0907059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Atasoy D., Aponte Y., Su H.H., Sternson S.M. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Li L., Tasic B., Micheva K.D., Ivanov V.M., Spletter M.L., Smith S.J., Luo L. Visualizing the distribution of synapses from individual neurons in the mouse brain. PLoS One. 2010;5:e11503. doi: 10.1371/journal.pone.0011503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Grinevich V., Brecht M., Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. J. Neurosci. 2005;25:8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Harris J.A., Oh S.W., Zeng H. Adeno-associated viral vectors for anterograde axonal tracing with fluorescent proteins in nontransgenic and cre driver mice. 2012. [DOI] [PubMed] [Google Scholar]
- 272.Lo L., Anderson D.J.A. Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72:938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ugolini G. Advances in viral transneuronal tracing. J. Neurosci. Methods. 2010;194:2–20. doi: 10.1016/j.jneumeth.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 274.Burger C., Gorbatyuk O.S., Velardo M.J., Peden C.S., Williams P., Zolotukhin S., Reier P.J., Mandel R.J., Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 275.Baekelandt V., Eggermont K., Michiels M., Nuttin B., Debyser Z. Optimized lentiviral vector production and purification procedure prevents immune response after transduction of mouse brain. Gene Ther. 2003;10:1933–1940. doi: 10.1038/sj.gt.3302094. [DOI] [PubMed] [Google Scholar]
- 276.Baekelandt V., Claeys A., Eggermont K., Lauwers E., De S.B., Nuttin B., Debyser Z. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum. Gene Ther. 2002;13:841–853. doi: 10.1089/10430340252899019. [DOI] [PubMed] [Google Scholar]
- 277.Cearley C.N., Vandenberghe L.H., Parente M.K., Carnish E.R., Wilson J.M., Wolfe J.H. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol. Ther. 2008;16:1710–1718. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 279.Bessis N. GarciaCozar, F.J.; Boissier, M.C. Immune responses to gene therapy vectors: Influence on vector function and effector mechanisms. Gene Ther. 2004;11(Suppl. 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 280.Mingozzi F., High K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Keen-Rhinehart E., Michopoulos V., Toufexis D.J., Martin E.I., Nair H., Ressler K.J., Davis M., Owens M.J., Nemeroff C.B., Wilson M.E. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol. Psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Regev L., Tsoory M., Gil S., Chen A. Site-Specific Genetic Manipulation of Amygdala Corticotropin-Releasing Factor Reveals Its Imperative Role in Mediating Behavioral Response to Challenge. Biol. Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 283.Regev L., Neufeld-Cohen A., Tsoory M., Kuperman Y., Getselter D., Gil S., Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol. Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- 284.Flandreau E.I., Ressler K.J., Owens M.J., Nemeroff C.B. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sink K.S., Walker D.L., Freeman S.M., Flandreau E.I., Ressler K.J., Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol. Psychiatry. 2013;18:308–319. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Martin E.I., Ressler K.J., Jasnow A.M., Dabrowska J., Hazra R., Rainnie D.G., Nemeroff C.B., Owens M.J. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol. Psychiatry. 2010;67:1212–1216. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Elliott E., Ezra-Nevo G., Regev L., Neufeld-Cohen A., Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- 288.Chen N.A., Jupp B., Sztainberg Y., Lebow M., Brown R.M., Kim J.H., Chen A., Lawrence A.J. Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice. J. Neurosci. 2014;34:11560–11570. doi: 10.1523/JNEUROSCI.4763-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lebow M., Neufeld-Cohen A., Kuperman Y., Tsoory M., Gil S., Chen A. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J. Neurosci. 2012;32:6906–6916. doi: 10.1523/JNEUROSCI.4012-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Elharrar E., Warhaftig G., Issler O., Sztainberg Y., Dikshtein Y., Zahut R., Redlus L., Chen A., Yadid G. Overexpression of corticotropin-releasing factor receptor type 2 in the bed nucleus of stria terminalis improves posttraumatic stress disorder-like symptoms in a model of incubation of fear. Biol. Psychiatry. 2013;74:827–836. doi: 10.1016/j.biopsych.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 291.Boyden E.S., Zhang F., Bamberg E., Nagel G., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 292.Deisseroth K. Controlling the brain with light. Sci. Am. 2010;303:48–55. doi: 10.1038/scientificamerican1110-48. [DOI] [PubMed] [Google Scholar]
- 293.Deisseroth K. Optogenetics. Nat. Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Anthony T.E., Dee N., Bernard A., Lerchner W., Heintz N., Anderson D.J. Control, of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell. 2014;156:522–536. doi: 10.1016/j.cell.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Stuber G.D., Stamatakis A.M., Kantak P.A. Considerations when using cre-driver rodent lines for studying ventral tegmental area circuitry. Neuron. 2015;85:439–445. doi: 10.1016/j.neuron.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lammel S., Steinberg E.E., Foldy C., Wall N.R., Beier K., Luo L., Malenka R.C. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85:429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chen Y., Molet J., Gunn B.G., Ressler K., Baram T.Z. Diversity of Reporter Expression Patterns in Transgenic Mouse Lines Targeting Corticotropin-Releasing Hormone-Expressing Neurons. Endocrinology. 2015;156:4769–4780. doi: 10.1210/en.2015-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gafford G.M., Guo J.D., Flandreau E.I., Hazra R., Rainnie D.G., Ressler K.J. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc. Natl. Acad. Sci. USA. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gafford G., Jasnow A.M., Ressler K.J. Grin1 receptor deletion within CRF neurons enhances fear memory. PLoS One. 2014;9:e111009. doi: 10.1371/journal.pone.0111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sarkar J., Wakefield S., MacKenzie G., Moss S.J., Maguire J. Neurosteroidogenesis is required for the physiological response to stress: Role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lee V., Sarkar J., Maguire J. Loss of Gabrd in CRH neurons blunts the corticosterone response to stress and diminishes stress-related behaviors. Psychoneuroendocrinology. 2014;41:75–88. doi: 10.1016/j.psyneuen.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Taniguchi H., He M., Wu P., Kim S., Paik R., Sugino K., Kvitsiani D., Fu Y., Lu J., Lin Y., Miyoshi G., Shima Y., Fishell G., Nelson S.B., Huang Z.J. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wamsteeker Cusulin J.I., Fuzesi T., Watts A.G., Bains J.S. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS One. 2013;8:e64943. doi: 10.1371/journal.pone.0064943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhu Y., Xu J., Hauswirth W.W., DeVries S.H. Genetically targeted binary labeling of retinal neurons. J. Neurosci. 2014;34:7845–7861. doi: 10.1523/JNEUROSCI.2960-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Smith J.A., Wang L., Hiller H., Taylor C.T., de Kloet A.D., Krause E.G. Acute hypernatremia promotes anxiolysis and attenuates stress-induced activation of the hypothalamic-pituitary-adrenal axis in male mice. Physiol. Behav. 2014;136:91–96. doi: 10.1016/j.physbeh.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Herman A.M., Huang L., Murphey D.K., Garcia I., Arenkiel B.R. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin-2. eLife. 2014;3:e01481. doi: 10.7554/eLife.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pleil K.E., Rinker J.A., Lowery-Gionta E.G., Mazzone C.M., McCall N.M., Kendra A.M., Olson D.P., Lowell B.B., Grant K.A., Thiele T.E., Kash T.L. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat. Neurosci. 2015;18:545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Krashes M.J., Shah B.P., Madara J.C., Olson D.P., Strochlic D.E., Garfield A.S., Vong L., Pei H., Watabe-Uchida M., Uchida N., Liberles S.D., Lowell B.B. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Portales-Casamar E., Swanson D.J., Liu L., de Leeuw C.N., Banks K.G., Ho Sui S.J., Fulton D.L., Ali J., Amirabbasi M., Arenillas D.J., Babyak N., Black S.F., Bonaguro R.J., Brauer E., Candido T.R., Castellarin M., Chen J., Chen Y., Cheng J.C., Chopra V., Docking T.R., Dreolini L., D’Souza C.A., Flynn E.K., Glenn R., Hatakka K., Hearty T.G., Imanian B., Jiang S., Khorasan-zadeh S., Komljenovic I., Laprise S., Liao N.Y., Lim J.S., Lithwick S., Liu F., Liu J., Lu M., McConechy M., McLeod A.J., Milisavljevic M., Mis J., O’Connor K., Palma B., Palmquist D.L., Schmouth J.F., Swanson M.I., Tam B., Ticoll A., Turner J.L., Varhol R., Vermeulen J., Watkins R.F., Wilson G., Wong B.K., Wong S.H., Wong T.Y., Yang G.S., Ypsilanti A.R., Jones S.J., Holt R.A., Goldowitz D., Wasserman W.W., Simpson E.M. A regulatory toolbox of MiniPromoters to drive selective expression in the brain. Proc. Natl. Acad. Sci. USA. 2010;107:16589–16594. doi: 10.1073/pnas.1009158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Yang G.S., Banks K.G., Bonaguro R.J., Wilson G., Dreolini L., de Leeuw C.N., Liu L., Swanson D.J., Goldowitz D., Holt R.A., Simpson E.M. Next generation tools for high-throughput promoter and expression analysis employing single-copy knock-ins at the Hprt1 locus. Genomics. 2009;93:196–204. doi: 10.1016/j.ygeno.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 311.Smith K. Trillion-dollar brain drain. Nature. 2011;478:15. doi: 10.1038/478015a. [DOI] [PubMed] [Google Scholar]
- 312.Collins P.Y., Patel V., Joestl S.S., March D., Insel T.R., Daar A.S., Anderson W., Dhansay M.A., Phillips A., Shurin S., Walport M., Ewart W., Savill S.J., Bordin I.A., Costello E.J., Durkin M., Fairburn C., Glass R.I., Hall W., Huang Y., Hyman S.E., Jamison K., Kaaya S., Kapur S., Kleinman A., Ogunniyi A., Otero-Ojeda A., Poo M.M., Ravindranath V., Sahakian B.J., Saxena S., Singer P.A., Stein D.J. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lepine J.P., Briley M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011;7:3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Adam D. Mental health: On the spectrum. Nature. 2013;496:416–418. doi: 10.1038/496416a. [DOI] [PubMed] [Google Scholar]
- 315.Lee S.H., Ripke S., Neale B.M., Faraone S.V., Purcell S.M., Perlis R.H., Mowry B.J., Thapar A., Goddard M.E., Witte J.S., Absher D., Agartz I., Akil H., Amin F., Andreassen O.A., Anjorin A., Anney R., Anttila V., Arking D.E., Asherson P., Azevedo M.H., Backlund L., Badner J.A., Bailey A.J., Banaschewski T., Barchas J.D., Barnes M.R., Barrett T.B., Bass N., Battaglia A., Bauer M., Bayes M., Bellivier F., Bergen S.E., Berrettini W., Betancur C., Bettecken T., Biederman J., Binder E.B., Black D.W., Blackwood D.H., Bloss C.S., Boehnke M., Boomsma D.I., Breen G., Breuer R., Bruggeman R., Cormican P., Buccola N.G., Buitelaar J.K., Bunney W.E., Buxbaum J.D., Byerley W.F., Byrne E.M., Caesar S., Cahn W., Cantor R.M., Casas M., Chakravarti A., Chambert K., Choudhury K., Cichon S., Cloninger C.R., Collier D.A., Cook E.H., Coon H., Cormand B., Corvin A., Coryell W.H., Craig D.W., Craig I.W., Crosbie J., Cuccaro M.L., Curtis D., Czamara D., Datta S., Dawson G., Day R., de Geus E.J., Degenhardt F., Djurovic S., Donohoe G.J., Doyle A.E., Duan J., Dudbridge F., Duketis E., Ebstein R.P., Edenberg H.J., Elia J., Ennis S., Etain B., Fanous A., Farmer A.E., Ferrier I.N., Flickinger M., Fombonne E., Foroud T., Frank J., Franke B., Fraser C., Freedman R., Freimer N.B., Freitag C.M., Friedl M., Frisen L., Gallagher L., Gejman P.V., Georgieva L., Gershon E.S., Geschwind D.H., Giegling I., Gill M., Gordon S.D., Gordon-Smith K., Green E.K., Greenwood T.A., Grice D.E., Gross M., Grozeva D., Guan W., Gurling H., De H.L., Haines J.L., Hakonarson H., Hallmayer J., Hamilton S.P., Hamshere M.L., Hansen T.F., Hartmann A.M., Hautzinger M., Heath A.C., Henders A.K., Herms S., Hickie I.B., Hipolito M., Hoefels S., Holmans P.A., Holsboer F., Hoogendijk W.J., Hottenga J.J., Hultman C.M., Hus V., Ingason A., Ising M., Jamain S., Jones E.G., Jones I., Jones L., Tzeng J.Y., Kahler A.K., Kahn R.S., Kandaswamy R., Keller M.C., Kennedy J.L., Kenny E., Kent L., Kim Y., Kirov G.K., Klauck S.M., Klei L., Knowles J.A., Kohli M.A., Koller D.L., Konte B., Korszun A., Krabbendam L., Krasucki R., Kuntsi J., Kwan P., Landen M., Langstrom N., Lathrop M., Lawrence J., Lawson W.B., Leboyer M., Ledbetter D.H., Lee P.H., Lencz T., Lesch K.P., Levinson D.F., Lewis C.M., Li J., Lichtenstein P., Lieberman J.A., Lin D.Y., Linszen D.H., Liu C., Lohoff F.W., Loo S.K., Lord C., Lowe J.K., Lucae S., MacIntyre D.J., Madden P.A., Maestrini E., Magnusson P.K., Mahon P.B., Maier W., Malhotra A.K., Mane S.M., Martin C.L., Martin N.G., Mattheisen M., Matthews K., Mattingsdal M., McCarroll S.A., McGhee K.A., McGough J.J., McGrath P.J., McGuffin P., McInnis M.G., McIntosh A., McKinney R., McLean A.W., McMahon F.J., McMahon W.M., McQuillin A., Medeiros H., Medland S.E., Meier S., Melle I., Meng F., Meyer J., Middeldorp C.M., Middleton L., Milanova V., Miranda A., Monaco A.P., Montgomery G.W., Moran J.L., Moreno-De-Luca D., Morken G., Morris D.W., Morrow E.M., Moskvina V., Muglia P., Muhleisen T.W., Muir W.J., Muller-Myhsok B., Murtha M., Myers R.M., Myin-Germeys I., Neale M.C., Nelson S.F., Nievergelt C.M., Nikolov I., Nimgaonkar V., Nolen W.A., Nothen M.M., Nurnberger J.I., Nwulia E.A., Nyholt D.R., O’Dushlaine C., Oades R.D., Olincy A. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ising M., Holsboer F. Genetics of stress response and stress-related disorders. Dialogues Clin. Neurosci. 2006;8:433–444. doi: 10.31887/DCNS.2006.8.4/mising. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 318.Kendler K.S., Kessler R.C., Walters E.E., Maclean C., Neale M.C., Heath A.C., Eaves L.J. Stressful Life Events, Genetic Liability, and Onset of An Episode of Major Depression in Women. Am. J. Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- 319.Kendler K.S., Prescott C.A. A population-based twin study of lifetime major depression in men and women. Arch. Gen. Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- 320.Hammen C. Stress generation in depression: Reflections on origins, research, and future directions. J. Clin. Psychol. 2006;62:1065–1082. doi: 10.1002/jclp.20293. [DOI] [PubMed] [Google Scholar]
- 321.Burcusa S.L., Iacono W.G. Risk for recurrence in depression. Clin. Psychol. Rev. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Koob G.F. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Huizink A.C., Mulder E.J., Buitelaar J.K. Prenatal stress and risk for psychopathology: Specific effects or induction of general susceptibility? Psychol. Bull. 2004;130:115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- 324.Buss C., Entringer S., Wadhwa P.D. Fetal programming of brain development: Intrauterine stress and susceptibility to psychopathology. Sci. Signal. 2012;5:t7. doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 326.Mehta D., Klengel T., Conneely K.N., Smith A.K., Altmann A., Pace T.W., Rex-Haffner M., Loeschner A., Gonik M., Mercer K.B., Bradley B., Muller-Myhsok B., Ressler K.J., Binder E.B. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl. Acad. Sci. USA. 2013;110:8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J.C., Pariante C.M., Pace T.W., Mercer K.B., Mayberg H.S., Bradley B., Nemeroff C.B., Holsboer F., Heim C.M., Ressler K.J., Rein T., Binder E.B. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]