Abstract
Background
The neutrophil to lymphocyte ratio (NLR) is an indicator of systemic inflammation and a prognostic marker in patients with acute coronary syndrome (ACS). This study aims to investigate the value of NLR to predict the in-hospital and long-term prognosis in patients with ST segment elevation myocardial infarction (STEMI) after percutaneous coronary intervention (PCI) by meta-analysis.
Method
The studies related to the prognosis of NLR and STEMI patients published in the Pubmed, Embase, and Ovid databases before June 2017 were retrieved. The relevant data were extracted. Review Manager Version 5.3 was used for meta-analysis.
Results
A total of 14 studies of 10,245 patients with STEMI after PCI were included. A significant difference was observed for mortality (P < 0.001; relative risk (RR) 3.32; 95% confidence interval (CI) 2.45–4.49), hospital cardiac mortality(P < 0.001; RR 3.22; 95% CI 2.25–4.60), all mortality (P < 0.001; RR 3.23; 95% CI 2.28–4.57), major adverse cardiovascular events (MACE) (P < 0.001; RR 2.00; 95% CI 1.62–2.46), in-stent thrombosis (P < 0.001; RR 2.72 95% CI 1.66–4.44), nonfatal myocardial infarction(MI) (P < 0.001; RR 1.93; 95%CI 1.43–2.61), angina (P = 0.007; RR 1.67; 95%CI 1.15–2.41), advanced heart failure (AHF) (P < 0.001; RR 1.81; 95% CI 1.48–2.21), arrhythmia (P = 0.002; RR 1.38; 95% CI 1.13–1.69), no reflow (P < 0.001; RR 2.28; 95% CI 1.46–3.57), long-term all mortality (P < 0.001; RR 3.82; 95% CI 2.94–4.96), cardiac mortality (P = 0.004; RR 3.02; 95% CI 1.41–6.45), MACE (P < 0.001; RR 2.49; 95% CI 1.47–4.23), and nonfatal MI (P = 0.46; RR 1.32; 95% CI 0.63–2.75).
Conclusions
Meta-analysis shows that NLR is a predictor of hospitalization and long-term prognosis in patients with STEMI after PCI, but requires further confirmation by large randomized clinical trials.
Electronic supplementary material
The online version of this article (10.1186/s12872-018-0812-6) contains supplementary material, which is available to authorized users.
Keywords: Neutrophil to lymphocyte ratio, ST segment elevation myocardial infarction, Percutaneous coronary intervention, Inflammation, Mortality, Prognosis
Background
Inflammation plays a key role in the occurrence and development of atherosclerosis [1]. The neutrophil to lymphocyte ratio (NLR) is an indicator of systemic inflammation and a prognostic marker in patients undergoing percutaneous coronary intervention (PCI) [2, 3]. Furthermore, in previous studies, the NLR has been demonstrated to be related to in-hospital cardiovascular mortality and long-term mortality in patients with ST segment elevation myocardial infarction (STEMI) [4, 5]. However, the results of individual studies were different. Our meta-analysis aimed to evaluate the relationship between NLR and in-hospital or long-term prognosis of patients with STEMI after PCI.
Methods
Study search strategy
A systematic search was conducted in the English-language databases of Pubmed, Embase, and Ovid (from inception to June 2017) for studies regarding in-hospital or long-term prognosis of patients with NLR and STEMI after PCI. According to the language of the databases, we used the appropriate search strategy. (“Neutrophils to lymphocytes ratio,” or “NLR” or “neutrophils lymphocytes ratio”) and (“ST Segment Elevation Myocardial Infarction,” or “ST Elevated Myocardial Infarction,” or “STEMI”) and (“Percutaneous coronary intervention,” or “PCI”) in the title or abstract terms were used as key words for the English-language databases.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) the subjects were STEMI receiving primary PCI; (2) the study types were prospective cohort study or retrospective cohort study; and (3) risk estimates of association between NLR levels and cardiovascular related events occurring during hospital or follow-up were studied experimentally. Tests that did not meet all of the above criteria were excluded.
Data extraction
Study searches and the data extraction of the included studies were conducted independently by two researchers. The quality of each study was evaluated independently using the Newcastle-Ottawa Scale [6]. This scale uses a star system to evaluate nonrandomized studies regarding 3 criteria: patient selection, comparability of study groups, and outcome assessment. Studies achieving a rating of 6 stars or higher were considered to be of the highest quality. The basic information include domains of authors, year of publication, age of target participant, comparison of NLR, number of events, sample size, and the follow-up period. If the articles were ambiguous or lacking of outcomes, we contacted the author. If the author was not available, we extracted the relevant data through consensus.
Statistical analysis
Data analyses were performed using Version 5.3 Review Manager statistical software. Heterogeneity among studies was assessed using χ2 and I2 statistics. I2 ≤ 50% suggests that heterogeneity is not statistically significant. I2 > 50% indicates the presence of heterogeneity, and heterogeneity analysis will be further performed. If there was no significant heterogeneity between studies, the fixed-effect model was used. Otherwise, the random effects model was applied. Publication bias was indicated by a funnel plot. The value P < 0.05 was considered to be statistically significant.
Results
Search results
A total of 265 potential relevant studies were selected from electronic databases, 251 were excluded as they did not meet the inclusion criteria. 14 studies were finally considered for this study. The studies selection process is shown in Fig. 1.
Fig. 1.
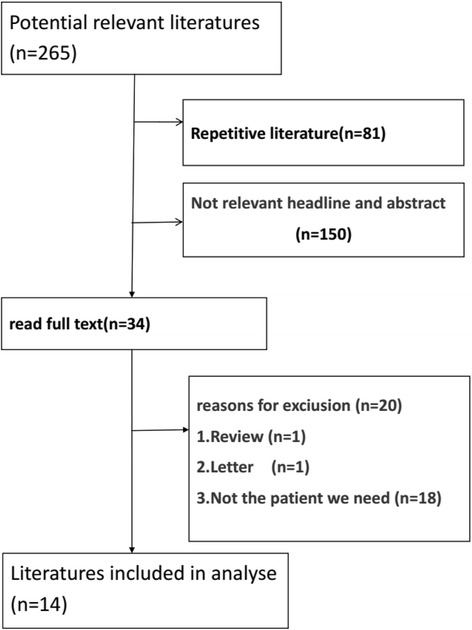
Flow chart of literatures screening and the reasons for exclusion
Study characteristics
Table 1 demonstrates the basic features of the studies. A total of 14 studies from 6 countries were included in our analysis, of which 12 [7–18] were prospective cohort studies, and 2 [19, 20] were retrospective cohort studies. Han [9], He [10] and Ergelen [19] have less than six stars. Akpek [7] and Kaya [13] are multi-country study and the others are single-country study. A total of 10,245 patients were enrolled, including 7908 males. The follow-up time was 3.8 days to 3395 days.
Table 1.
Characteristics of included studies and quality evaluation of documents
| Study | Study | Country | male,% | Age mean(SD) |
NLR grouping |
Follow-up (days) |
Observation index | Quality score |
|---|---|---|---|---|---|---|---|---|
| Akpek, [7] 2012 | Prospective cohort study | Turkey, China, USA |
327(78.0) | 59.4 ± =12.4 | ≤3.3 > 3.3 |
6.7(mean) | In-hospital: in-stent thrombosis, MACE, nonfatal MI, no reflow, all-cause mortality |
6 |
| Arbel, [8] 2014 | Prospective cohort study | Israel | 436(81.0) | 61 ± 13 | < 6.5 ≥6.5 |
1044 (median) | In-hospital: arrhythmia Long-term: all-cause mortality |
6 |
| Han, [9] 2013 |
Prospective cohort study | Korea | 247(75.8) | 61.9 ± 12.3 | ≤3.30 3.31–6.52 > 6.53 |
360(total) | In-hospital: MACE, nonfatal MI, no reflow, all-cause mortality Long-term: nonfatal MI, MACE, all-cause mortality |
5 |
| He, [10] 2014 |
Prospective cohort study | China | 546(78.9) | 60.27 | < 3.16 3.16–4.75 > 4.75 |
3395 (median) | In-hospital: arrhythmia, no reflow, cardiac mortality, all-cause mortality Long-term: cardiac mortality, all-cause mortality, MACE |
5 |
| Her, [11] 2017 |
Prospective cohort study | Korea | 140(81.4) | 57.1 ± 12.4 | < 5.8 ≥5.8 |
1230(median) | In-hospital: MACE 12 months follow-up: MACE, Long-term: all-cause mortality, nonfatal MI |
6 |
| Park JinJoo, 2013 [12] |
Prospective cohort study | Korea | 235(72.0) | 60.9 ± 13.9 | < 5.44 ≥5.44 |
1092(median) | Long-term: all-cause mortality | 6 |
| Kaya, [13] 2013 | Prospective cohort study | Turkey, China, USA |
535(78.4) | 60.8 | < 2.3 2.3–4.4 > 4.4 |
1299(median) | In-hospital: in-stent thrombosis, MACE, non-fatal MI, no reflow, cardiac mortality, Long-term: Non-fatal MI,MACE, cardiac mortality |
6 |
| Pan, [14] 2015 |
Prospective cohort study | China | 496(78.0) | 59.27 ± 11.27 | < 3.0 3.0–6.40 > 6.40 |
360(total) | In-hospital: angina, arrhythmia, cardiac mortality, 12 months follow-up: all-cause mortality |
6 |
| Sen, [15] 2013 | Prospective cohort study | Turkey | 176(86.3) | 55.8 | < 3.30 3.30–4.56 > 4.56 |
1140 (total) | In-hospital: all-cause mortality, MACE, no reflowAt three-year follow up, all-cause mortality, MACE | 6 |
| Shen, [16] 2010 |
Prospective cohort study | China | 329(59.7) | 61 | 1.44–3.45 3.45–4.81 4.82–6.46 6.47–22.57 |
1898 (median) | In-hospital: all-cause mortality Long-term: all-cause mortality |
6 |
| Tanriverdi, [17] 2017 | Prospective cohort study | Turkey | 285(77.4) | 59.6 | < 5.47 ≥ 5.47 |
3.8(mean) | In-hospital: all-cause mortality | 6 |
| Zuin, [18] 2017 | Prospective cohort study | Italy | 1724(71.8) | 64.5 | < 2.1 3.4–4.1 > 4.1 |
363(median) | Long-term: cardiovascular mortality | 6 |
| Ergelen, [19] 2014 | Retrospective cohort study | Turkey | 2015(83.6) | 56.4 | ≤6.97 > 6.97 |
630 (median) | In hospital: AHF, MACE, nonfatal MI, no reflow, cardiovascular mortality, Long-term: MACE, nonfatal MI, cardiovascular mortality |
5 |
| Gazi, [20] 2015 | Retrospective cohort study | Turkey | 417(79.9) | 62.57 | ≤5.77 > 5.77 |
5.7(mean) | In-hospital: AHF, angina, arrhythmia, all-cause mortality, nonfatal MI | 6 |
Incidence of mortality and cardiovascular events
Based on heterogeneity, the fixed effects model was applied to in-hospital cardiac mortality (P < 0.001; relative risk (RR) 3.22; 95% confidence interval (CI) 2.25–4.60), in-hospital all mortality (P < 0.001; RR3.23; 95% CI 2.28–4.57), long-term all mortality (P < 0.001; RR 3.82; 95% CI 2.94–4.96), in-hospital major adverse cardiovascular events (MACE) (P < 0.001; RR 2.00; 95%CI 1.62–2.46), in-stent thrombosis (P < 0.001; RR2.72; 95% CI 1.66–4.44), in-hospital nonfatal MI (P < 0.001; RR 1.93; 95% CI 1.43–2.61), angina (P = 0.007; RR 1.67; 95% CI 1.15–2.41), AHF (P < 0.001; RR 1.81; 95% CI 1.48–2.21), and arrhythmia (P = 0.002; RR 1.38; 95% CI 1.13–1.69). Meanwhile, the random effects model was used for mortality (P < 0.001; RR 3.32; 95%CI 2.45–4.49), long-term cardiac mortality (P = 0.004; RR 3.02; 95% CI 1.41–6.45), long-term MACE (P < 0.001; RR 2.49; 95% CI 1.47–4.23), long-term nonfatal MI (P = 0.46; RR 1.32; 95% CI 0.63–2.75), and no reflow (P < 0.001; RR 2.28; 95% CI 1.46–3.57). No significant difference was observed for long-term nonfatal MI (P = 0.46; RR 1.32; 95% CI 0.63–2.75) (Figs. 2, 3; Table 2, and Additional file 1, Additional file 2, Additional file 3, Additional file 4, Additional file 5, Additional file 6, Additional file 7, Additional file 8, Additional file 9, Additional file 10, Additional file 11, Additional file 12).
Fig. 2.
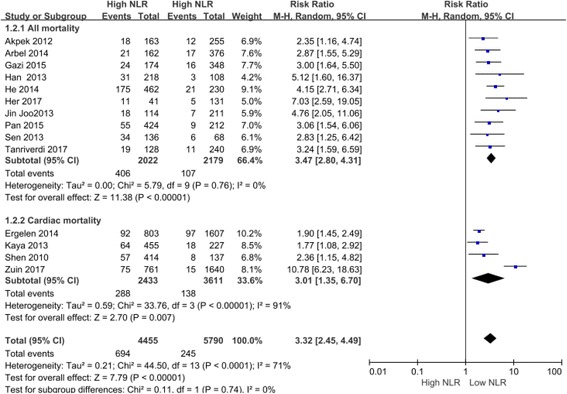
Comparing the mortality between high NLR groups and the low NLR groups
Fig. 3.
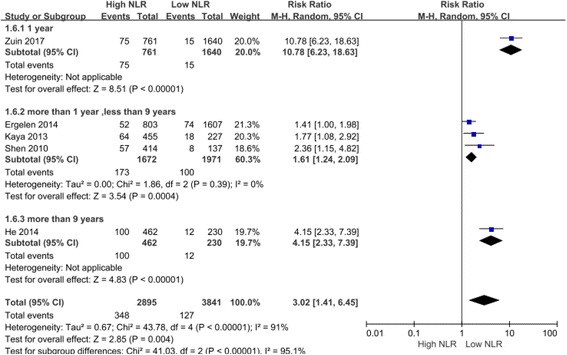
Comparing the long-term cardiac mortality between high NLR groups and the low NLR group
Table 2.
Results of high NLR and low NLR mortality rates and cardiovascular events
| Outcomes | Included studies | Heterogeneity | Test for overall effect | ||||
|---|---|---|---|---|---|---|---|
| χ2 | P | I2(%) | Z | P | RR 95%CI | ||
| In-hospital | |||||||
| AHF | 19,20 | 1.54 | 0.22 | 35 | 5.81 | < 0.00001 | 1.81[1.48,2.21] |
| Angina | 14,20 | 0.08 | 0.78 | 0 | 2.71 | 0.007 | 1.67[1.15,2.41] |
| Arrhythmia | 8, 10,14,20 | 0.82 | 0.84 | 0 | 3.14 | 0.002 | 1.38[1.13,1.69] |
| In-stent thrombosis | 7,13 | 0.03 | 0.86 | 0 | 3.99 | < 0.0001 | 2.72[1.66,4.44] |
| MACE | 7,9,11,13,15,19 | 3.83 | 0.57 | 0 | 6.53 | < 0.00001 | 2.00[1.62,2.46] |
| Nonfatal MI | 7,9,13,19,20 | 4.88 | 0.30 | 18 | 4.32 | < 0.0001 | 1.93[1.43,2.61] |
| No reflow | 7,9,10,13,15,19 | 40.00 | < 0.00001 | 87 | 3.63 | 0.0003 | 2.28[1.46,3.57] |
| All mortality | 7,9,10,15,17,20 | 1.87 | 0.87 | 0 | 6.61 | < 0.00001 | 3.23[2.28,4.57] |
| Cardiac mortality | 10,13,14,16,19 | 1.77 | 0.78 | 0 | 6.42 | < 0.00001 | 3.22[2.25,4.60] |
| Long-term | |||||||
| Nonfatal MI | 9, 11,13,19, | 10.90 | 0.01 | 72 | 0.75 | 0.46 | 1.32[0.63,2.75] |
| MACE | 9,10,11,13,15,19 | 53.33 | < 0.00001 | 91 | 3.39 | 0.0007 | 2.49[1.47,4.23] |
| All mortality | 8,9,10,11,12,14,15 | 3.85 | 0.70 | 0 | 10.06 | < 0.00001 | 3.82[2.94,4.96] |
| Cardiac mortality | 10,13,16,18,19 | 43.78 | < 0.00001 | 91 | 2.85 | 0.004 | 3.02[1.41,6.45] |
Heterogeneity analysis
Analyses with I2 > 50%, including death, no reflow, long term nonfatal MI, MACE, and cardiac mortality, we conducted a subgroup analysis. The results showed that heterogeneity may come from the time of patient follow- up, race, type of study, etc.
Publication bias
No bias was found in the results of 14 studies on funnel plots of mortality risk.(Fig. 4).
Fig. 4.
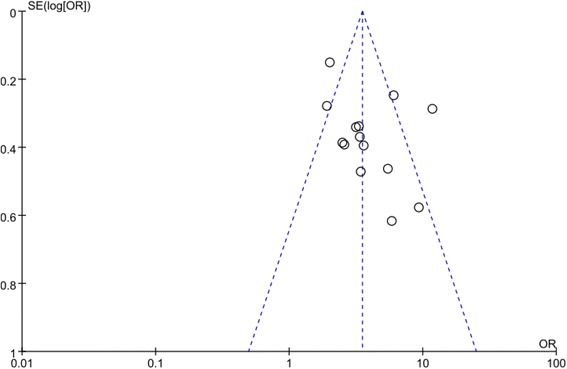
Funnel plot results of 14 studies according to the risk of mortality
Discussion
The results of our meta-analysis show that the high NLR group has a higher risk of angina, advanced HF, arrhythmia, MACE, cardiac mortality, all mortality, in-stent thrombosis, nonfatal MI, and no reflow than the low NLR group during hospitalization. In addition, the risk of long-term mortality, cardiac mortality and MACE in the high NLR group was higher than that of the low NLR group. However, no significant difference was observed for long-term nonfatal MI.
Wang [21] and Zhang [22] reported a meta-analysis, which discussed the predictive value of NLR in patients after angiography or cardiac revascularization. Similar conclusions were drawn from long-term all-cause mortality and several other results. However, this study is significantly different from their studies with regards to the literature inclusion criteria. The trials they selected did not include all patients with acute STEMI who underwent emergency interventional therapy. The time and method of opening blood vessels may affect the prognosis of patients, so we reduce the impact of these factors.
A large number of experiments have demonstrated that inflammatory response plays an important role in the development and progression of acute coronary syndrome (ACS) [23, 24]. White blood cell plays a vital role in the progression of atherosclerosis and destabilization, leading to thrombotic events [25, 26]. Increased white blood cell count is associated with an increased mortality for STEMI patients [27]. However, the total number of leukocytes is easily influenced by race and sex, and it is difficult to establish a reference range for the whole population [28].
In recent years, in-depth study of the clinical effect of inflammatory reaction in occurrence and development of coronary heart disease observed that different subtypes of leukocytes compared with the total leukocyte count and specific subtype, including neutrophils, lymphocytes and monocytes, have more predictive value in the risk assessment of cardiovascular disease [29].
Neutrophils are the first white blood cell subsets to be observed in the damaged myocardium and move away from the myocardium after phagocytizing debris [16]. Neutrophils play an important role in the destabilization of atherosclerotic plaques [30]. CD4 + T lymphocytes belong to the regulatory arm of the immune system, playing a key role in regulating the inflammatory response at different stages of atherogenesis and acute MI [31–33]. However, neutrophils count is easily affected by the patient’s own state, such as blood volume. The CD4 + T lymphocyte counts are not readily available for immediate blood testing.
In recent years, studies have observed that NLR, by combining the change in neutrophils and lymphocytes in the course of inflammation, is a more powerful predictor of cardiovascular disease than any other leukocyte subtypes [18, 34, 35]. NLR can be obtained from routine blood tests and used as a cost effective biomarker for inflammation. Previous studies observed that NLR as a new inflammatory marker is a good prognostic factor for patients with ACS [36, 37]. Many studies have observed that high NLR levels have a very important relationship with cardiovascular and all-cause mortality in STEMI patients, during hospitalization or long-term [16, 20, 38]. In addition, several studies have shown evidence that a higher NLR is associated with a lower ejection fraction after STEMI [8, 11]. Several studies have also observed that high NLR levels are often associated with complex coronary arteries in patients [39, 40]. Left ventricular apical thrombus and remodeling were also found to be related to high NLR [41, 42].
Hence, NLR as a new marker of inflammation might be for the assessment of risk levels in STEMI patients with PCI. However, in order to reduce the incidence of complications and deaths, active treatment may be needed to reduce NLR levels in patients with STEMI, which requires a more rigorous multi-center clinical trial to confirm. There are several limitations in our analysis. First, less relevant experiments was included. Second, different methods may be used for different cell counts. There is no uniform counting standard. Third, the observation indexes of the experiments are different. Several experiments only provided hospital events. Several studies were followed for a short time, while other experiments used telephone follow-up may affect accuracy of the data. Finally, our analysis failed to correct for interference from a number of factors.
Conclusions
Our meta-analysis showed that NLR levels were related to the hospital and long-term prognosis of patients with STEMI after PCI.
Additional files
The result of advanced HF (P < 0.001; RR 1.81; 95%CI 1.48–2.21). (DOCX 41 kb)
The result of Angina(P = 0.007; RR 1.67; 95%CI 1.15–2.41). (DOCX 41 kb)
The result of arrhythmia (P = 0.002; RR 1.38; 95% CI 1.13–1.69). (DOCX 47 kb)
The result of in-stent thrombosis (P < 0.001; RR 2.72; 95%CI 1.66–4.44). (DOCX 41 kb)
The result of in-hospital MACE (P < 0.001; RR 2.00; 95%CI 1.62–2.46). (DOCX 55 kb)
The result of in-hospital nonfatal MI(P < 0.001; RR 1.93; 95%CI 1.43–2.61). (DOCX 51 kb)
The result of no reflow (P < 0.001; RR 2.28; 95%CI 1.46–3.57). (DOCX 95 kb)
The result of in-hospital all mortality (P < 0.001; RR 3.23; 95% CI 2.28–4.57). (DOCX 54 kb)
The result of in-hospital cardiac mortality (P < 0.001; RR 3.22;95% CI 2.25–4.60). (DOCX 51 kb)
The result of long-term nonfatal MI (P = 0.46; RR 1.32; 95%CI 0.63–2.75). (DOCX 77 kb)
The result of long-term MACE (P < 0.001; RR 2.49; 95%CI 1.47–4.23). (DOCX 86 kb)
The result of long-term all mortality (P < 0.001; RR 3.82; 95% CI 2.94–4.96). (DOCX 57 kb)
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Abbreviations
- ACS
Acute coronary syndrome
- AHF
Advanced heart failure
- CI
Confidence interval
- MACE
Major adverse cardiovascular events
- MI
Myocardial infarction
- NLR
Neutrophil to lymphocyte ratio
- PCI
Percutaneous coronary intervention
- RR
Relative risk
- STEMI
ST segment elevation myocardial infarction
Authors’ contributions
WHW and SZ contributed to conception and design; SZ, WHW, JD, CMQ, JJJ, LL and XJG contributed to acquisition of data, or analysis and interpretation of data; SZ, WHW, JD, CMQ, JJJ and LL were involved in drafting the manuscript or critically revising it for important intellectual content; all authors gave final approval of the version to be published.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12872-018-0812-6) contains supplementary material, which is available to authorized users.
Contributor Information
Sai Zhang, Email: zhangsaijs@163.com.
Jun Diao, Email: 1297403115@qq.com.
Chunmei Qi, Email: 1242436142@qq.com.
Jingjing Jin, Email: 1216603463@qq.com.
Li Li, Email: lilyrwyao@126.com.
Xingjuan Gao, Email: 1172944436@qq.com.
Lei Gong, Email: lg2707@qq.com.
Weiheng Wu, Email: wwheng118@163.com.
References
- 1.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawant AC, Adhikari P, Narra SR, et al. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiology journal. 2014;21:500–508. doi: 10.5603/CJ.a2013.0148. [DOI] [PubMed] [Google Scholar]
- 3.Sarli B, Baktir AO, Saglam H, et al. Neutrophil-to-lymphocyte ratio is associated with severity of coronary artery ectasia. Angiology. 2014;65:147–151. doi: 10.1177/0003319713488932. [DOI] [PubMed] [Google Scholar]
- 4.Nunez J, Nunez E, Bodi V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Ayca B, Akin F, Celik O, et al. Neutrophil to lymphocyte ratio is related to stent thrombosis and high mortality in patients with acute myocardial infarction. Angiology. 2015;66:545–552. doi: 10.1177/0003319714542997. [DOI] [PubMed] [Google Scholar]
- 6.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 7.Akpek M, Kaya MG, Lam YY, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110:621–627. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Arbel Y, Shacham Y, Ziv-Baran T, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long-term all-cause mortality in ST-elevation myocardial infarction patients. The Canadian journal of cardiology. 2014;30:1177–1182. doi: 10.1016/j.cjca.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Han YC, Yang TH, Kim DI, et al. Neutrophil to lymphocyte ratio predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Korean Circ J. 2013;43:93–99. doi: 10.4070/kcj.2013.43.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Li J, Wang Y, et al. Neutrophil-to-lymphocyte ratio (NLR) predicts mortality and adverse-outcomes after ST-segment elevation myocardial infarction in Chinese people. Int J Clin Exp Pathol. 2014;7:4045–4056. [PMC free article] [PubMed] [Google Scholar]
- 11.Her AY, Cho KI, Singh GB et al. Plaque characteristics and inflammatory markers for the prediction of major cardiovascular events in patients with ST-segment elevation myocardial infarction. The international journal of cardiovascular imaging. 2017;33:1445-54. [DOI] [PubMed]
- 12.Park JJ, Jang HJ, Oh IY, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–642. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Kaya MG, Akpek M, Lam YY, et al. Prognostic value of neutrophil/lymphocyte ratio in patients with ST-elevated myocardial infarction undergoing primary coronary intervention: a prospective, multicenter study. Int J Cardiol. 2013;168:1154–1159. doi: 10.1016/j.ijcard.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 14.Pan W, Zhao D, Zhang C, et al. Application of neutrophil/lymphocyte ratio in predicting coronary blood flow and mortality in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention. J Cardiol. 2015;66:9–14. doi: 10.1016/j.jjcc.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Sen N, Afsar B, Ozcan F, et al. The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis. 2013;228:203–210. doi: 10.1016/j.atherosclerosis.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Shen XH, Chen Q, Shi Y, Li HW. Association of neutrophil/lymphocyte ratio with long-term mortality after ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Chin Med J. 2010;123:3438–3443. [PubMed] [Google Scholar]
- 17.Tanriverdi Z, Colluoglu T, Dursun H, Kaya D. The relationship between neutrophil-to-lymphocyte ratio and fragmented QRS in acute STEMI patients treated with primary PCI. J Electrocardiol.2017;50:876-83. [DOI] [PubMed]
- 18.Zuin M, Rigatelli G, Picariello C et al. Correlation and prognostic role of neutrophil to lymphocyte ratio and SYNTAX score in patients with acute myocardial infarction treated with percutaneous coronary intervention: a six-year experience. Cardiovascular revascularization medicine : including molecular interventions. 2017;18:565-71. [DOI] [PubMed]
- 19.Ergelen M, Uyarel H, Altay S, et al. Predictive value of elevated neutrophil to lymphocyte ratio in patients undergoing primary angioplasty for ST-segment elevation myocardial infarction. Clin appl thromb hemost. 2014;20:427–432. doi: 10.1177/1076029612473516. [DOI] [PubMed] [Google Scholar]
- 20.Gazi E, Bayram B, Gazi S, et al. Prognostic value of the neutrophil-lymphocyte ratio in patients with ST-elevated acute myocardial infarction. Clin appl thromb hemost. 2015;21:155–159. doi: 10.1177/1076029613492011. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Zhang G, Jiang X, et al. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis. 2014;234:206–213. doi: 10.1016/j.atherosclerosis.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Zhu H, Zhao R, et al. Value of neutrophils/lymphocytes ratio on predicting the prognosis of patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention:a meta-analysis. Zhonghua xin xue guan bing za zhi. 2015;43:264–268. [PubMed] [Google Scholar]
- 23.Palmerini T, Mehran R, Dangas G, et al. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction trial. Circulation. 2011;123:2829–2837. doi: 10.1161/CIRCULATIONAHA.110.985564. [DOI] [PubMed] [Google Scholar]
- 24.Smit JJ, Ottervanger JP, Slingerland RJ, et al. Successful reperfusion for acute ST elevation myocardial infarction is associated with a decrease in WBC count. J Lab Clin Med. 2006;147:321–326. doi: 10.1016/j.lab.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Zernecke A, Bot I, Djalali-Talab Y, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 26.Mozos I, Borzak G, Caraba A, Mihaescu R. Arterial stiffness in hematologic malignancies. OncoTargets and therapy. 2017;10:1381–1388. doi: 10.2147/OTT.S126852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung S, Song YB, Hahn JY, et al. Impact of white blood cell count on myocardial salvage, infarct size, and clinical outcomes in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a magnetic resonance imaging study. Int J Cardiovasc Imaging. 2014;30:129–136. doi: 10.1007/s10554-013-0303-x. [DOI] [PubMed] [Google Scholar]
- 28.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49:664–666. doi: 10.1136/jcp.49.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 30.Guasti L, Dentali F, Castiglioni L, et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost. 2011;106:591–599. doi: 10.1160/TH11-02-0096. [DOI] [PubMed] [Google Scholar]
- 31.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 32.Widmer A, Linka AZ, Attenhofer Jost CH, et al. Mechanical complications after myocardial infarction reliably predicted using C-reactive protein levels and lymphocytopenia. Cardiology. 2003;99:25–31. doi: 10.1159/000068448. [DOI] [PubMed] [Google Scholar]
- 33.Blum A, Sclarovsky S, Rehavia E, Shohat B. Levels of T-lymphocyte subpopulations, interleukin-1 beta, and soluble interleukin-2 receptor in acute myocardial infarction. Am Heart J. 1994;127:1226–1230. doi: 10.1016/0002-8703(94)90040-X. [DOI] [PubMed] [Google Scholar]
- 34.Yu XY, Li XS, Li Y, et al. Neutrophil-lymphocyte ratio is associated with arterial stiffness in postmenopausal women with osteoporosis. Arch Gerontol Geriatr. 2015;61:76–80. doi: 10.1016/j.archger.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–460. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Bajari R, Tak S. Predictive prognostic value of neutrophil-lymphocytes ratio in acute coronary syndrome. Indian Heart J. 2017;69(Suppl 1):S46–s50. doi: 10.1016/j.ihj.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou D, Wan Z, Fan Y, et al. A combination of the neutrophil-to-lymphocyte ratio and the GRACE risk score better predicts PCI outcomes in Chinese Han patients with acute coronary syndrome. Anatol J Cardiol. 2015;15:995–1001. doi: 10.5152/AnatolJCardiol.2015.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gul U, Kayani AM, Munir R, Hussain S. Neutrophil lymphocyte ratio: APrognostic marker in acute ST elevation myocardial infarction. J Coll Physicians Surg Pak. 2017;27:4–7. [PubMed] [Google Scholar]
- 39.Zhang GY, Chen M, Yu ZM, et al. Relation between neutrophil-to-lymphocyte ratio and severity of coronary artery stenosis. Genet Mol Res. 2014;13:9382–9389. doi: 10.4238/2014.November.11.4. [DOI] [PubMed] [Google Scholar]
- 40.Kurtul S, Sarli B, Baktir AO, et al. Neutrophil to lymphocyte ratio predicts SYNTAX score in patients with non-ST segment elevation myocardial infarction. Int Heart J. 2015;56:18–21. doi: 10.1536/ihj.14-175. [DOI] [PubMed] [Google Scholar]
- 41.Ertem AG, Ozcelik F, Kasapkara HA, et al. Neutrophil lymphocyte ratio as a predictor of left ventricular apical Thrombus in patients with myocardial infarction. Korean Circ J. 2016;46:768–773. doi: 10.4070/kcj.2016.46.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borekci A, Gur M, Turkoglu C, et al. Neutrophil to lymphocyte ratio predicts left ventricular remodeling in patients with ST elevation myocardial infarction after primary percutaneous coronary intervention. Korean Circ J. 2016;46:15–22. doi: 10.4070/kcj.2016.46.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The result of advanced HF (P < 0.001; RR 1.81; 95%CI 1.48–2.21). (DOCX 41 kb)
The result of Angina(P = 0.007; RR 1.67; 95%CI 1.15–2.41). (DOCX 41 kb)
The result of arrhythmia (P = 0.002; RR 1.38; 95% CI 1.13–1.69). (DOCX 47 kb)
The result of in-stent thrombosis (P < 0.001; RR 2.72; 95%CI 1.66–4.44). (DOCX 41 kb)
The result of in-hospital MACE (P < 0.001; RR 2.00; 95%CI 1.62–2.46). (DOCX 55 kb)
The result of in-hospital nonfatal MI(P < 0.001; RR 1.93; 95%CI 1.43–2.61). (DOCX 51 kb)
The result of no reflow (P < 0.001; RR 2.28; 95%CI 1.46–3.57). (DOCX 95 kb)
The result of in-hospital all mortality (P < 0.001; RR 3.23; 95% CI 2.28–4.57). (DOCX 54 kb)
The result of in-hospital cardiac mortality (P < 0.001; RR 3.22;95% CI 2.25–4.60). (DOCX 51 kb)
The result of long-term nonfatal MI (P = 0.46; RR 1.32; 95%CI 0.63–2.75). (DOCX 77 kb)
The result of long-term MACE (P < 0.001; RR 2.49; 95%CI 1.47–4.23). (DOCX 86 kb)
The result of long-term all mortality (P < 0.001; RR 3.82; 95% CI 2.94–4.96). (DOCX 57 kb)


