Abstract
Aim of Study:
Respiratory infections account for significant morbidity, mortality and expenses to patients getting admitted to ICU. Antibiotic resistance is a major worldwide concern in ICU, including India. It is important to know the antibiotic prescribing pattern in ICU, organisms and its resistance pattern as there is sparse data on Indian ICUs.
Materials and Methods:
We conducted a prospective study from August 2015 to February 2016. All patients getting admitted to RICU with respiratory infection who were treated with antibiotics were included into study. Demographic details, comorbidities, Clinco-pathological score (CPI) on day1 and 2 of admission, duration of ICU admission, number of antibiotics used, antibiotic prescription, antimicrobial resistance pattern of patients were collected using APRISE questionnaire.
Results:
During study period 352 patients were screened and 303 patients were included into study. Mean age was 56.05±16.37 and 190 (62.70%) were men. Most common diagnosis was Pneumonia (66%). Piperacillin-tazobactam was most common empirical antibiotic used. We found 60% resistance to piperacillin-tazobactam. Acinetobacter baumanii was the most common organism isolated (29.2%) and was highly resistant to Carbapenem (60%). Klebsiella pneumoniae was resistant to Amikacin (45%), piperacillin (55%) and Ceftazidime (50%).
Conclusion:
Piperacillin-tazobactam was the most common antibiotic prescribed to patients with respiratory infection admitted to ICU. More than half of patients (60%) had resistance to the empirical antibiotic used in our ICU, highlighting the need for antibiogram for each ICU. Thirty six percent of patient had prior antibiotic use and had mainly gram negative organisms with high resistance to commonly used antibiotics.
Keywords: Antibiotic resistance, mortality, pneumonia, respiratory infection
INTRODUCTION
Respiratory infections account for significant morbidity, mortality, and healthcare-related expenditure in patients admitted to the Intensive Care Unit (ICU). Respiratory infections account for 3.5 million deaths worldwide and 79 million loss of disability-adjusted life-years.[1] Antibiotics form the main stay of treatment of various respiratory infections which are often initiated empirically based on their previous experiences, hence, leading to the inappropriate use of antibiotics and antimicrobial resistance.[2] Resistance to antibiotics has emerged recently due to misuse of antibiotics and is a threat to health-care system, especially in developing countries like India where there are no antimicrobial stewardship programs in most ICUs.[3] Antimicrobial resistance results in increased economic burden on patients due to the higher cost of antibiotics, prolonged ICU stay, and increased mortality.[2,4,5,6] Prescribing appropriate antibiotics for the right duration is very important to prevent drug resistance. Local knowledge regarding the most common organisms and their resistance pattern in various infections will greatly assist clinicians in choosing appropriate initial antibiotic therapy.[7] Hence, it is important to know the antibiotic prescribing pattern and resistance patterns as there is sparse data on Indian ICUs.
METHODOLOGY
Study design
We conducted a prospective, observational study in the Department of Pulmonary Medicine in an 1800 bedded tertiary care University Medical College Hospital from August 2015 to February 2016. The study was part of an Indian multicentric study “Antibiotic Prescribing practices and prevalence of antibiotic resistance in various ICUs across multiple centers in India.” (APRISE Study). All patients admitted to respiratory ICU (RICU) (10 bedded, open ICU) with respiratory infection who had been treated with antibiotics for more than 3 days and patients in whom adequate respiratory specimen was obtained were included in the study after obtaining informed consent. Data, on demographic variables such as name, age, gender, diagnosis, and reason for ICU admission, were recorded. Other variables such as the presence of comorbidities such as diabetes, ischemic heart disease (IHD), chronic obstructive pulmonary disease (COPD), asthma, and hypertension were noted. Clinicopathological Indicator score (CPI score) was obtained on day 1 and day 2. The previous history of antibiotic usage, duration of ICU admission, antibiotics used and their duration, organisms isolated, antimicrobial resistance pattern, and outcome of patients were collected. Exclusion criteria were patients who were HIV positive, who could not provide adequate respiratory specimen, patients who were not on antibiotics or received antibiotics for <3 days, and patients who died within 48 h after admission.
Sputum collection
In conscious patients who were able to expectorate, a single sputum sample was obtained within 24 h of admission to ICU in a sterile wide mouthed container. A sample was considered adequate if the quantity was 15 ml and was mucopurulent on visual examination. Sputum samples were graded following the criteria of Lentino and Lucks.[8] A portion of the sputum with most purulent or clotted part of sputum was chosen in case of nonpurulent sputum for culture. Blood agar and MacConkey agar were used for quantitative cultures and >106 CFU/ml on quantitative culture was considered pathological. Anaerobic cultures were not done in any of these patients.
In patients who could not expectorate or sample provided was inadequate, induced sputum was used. Sample was considered adequate if it was >5 ml and appeared turbid on visual examination. For patients who were obtunded or had weak cough, tracheal aspirate was obtained.
Endotracheal aspirate
In patients who were in respiratory failure and needed immediate mechanical ventilation, endotracheal secretions were collected within 24 h of ventilation. Samples were processed similar to sputum specimen. A quantitative culture >106 CFU/ml was considered pathological.
Bronchoalveolar lavage
Bronchoscopy and bronchoalveolar lavage (BAL) were done in selected patients in whom there was suspicion of malignancy and in whom microbiologic diagnosis was not established. BAL was performed as per ATS guidelines.[9] BAL was collected from affected bronchopulmonary segment and in patients with diffuse lung involvement, BAL was taken from the right middle lobe or lingula.[9] BAL samples were processed similar to sputum samples. BAL samples were considered satisfactory if there were ciliated cells, alveolar macrophages, and <5% squamous epithelial cells.[10] BAL quantitative culture >104 CFU/ml was considered pathological.[11]
Blood culture
Blood culture was done in patients with suspected bacteremia and sepsis.
We defined sepsis as life-threatening organ dysfunction with increase of two points or more in sequential organ function assessment score.[12]
We defined prior antibiotic use as “usage of antibiotics within 1 year before current admission”. Details of previous antibiotic usage were collected from the patients and from their hospital records.
The Institutional Ethics Committee approved the study IEC no-JSS/MC/IEC/05/5238/2015-16. Informed consent from patient/patient's legally authorized representative was taken before inclusion in the study.
Statistical analysis
Descriptive data are presented as frequencies (percentages) for discrete variables and as means (standard deviations) or medians (interquartile ranges) for continuous variables. All statistical tests were two-tailed, and factors were considered statistically significant at P < 0.05. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. and CDC Epi Info version 7 software were used for the analysis.
RESULTS
During the study period, we screened 352 patients admitted to RICU and 49 patients were excluded from the study [Figure 1], Three hundred and three patients satisfied inclusion and exclusion criteria and were included in the study. Mean age of our study population was 56.05 ± 16.37 and 190 (62.70%) were men. The most common diagnosis was pneumonia (66% [200/303]) followed by acute exacerbation of COPD (43.5% [132/303]), pleural infections (12.5% [38/303]), bronchiectasis (4.6% [14/303]), Interstitial lung disease (ILD) (4.29% [13/303]), and TB (2.6% [8/303]). Most common reason for shifting to ICU was respiratory distress. COPD was the most common comorbidity 48%, followed by diabetes 33%, hypertension 28.38%, IHD 5.2%, and asthma 3.9%.
Figure 1.
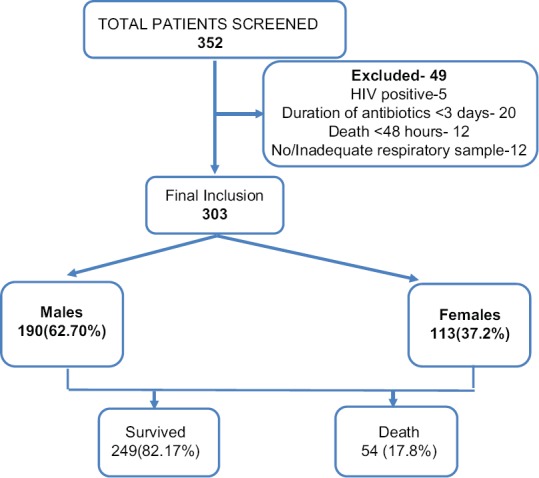
Flowchart depicting number of patients screened, included in the study and number of survival and deaths
Penicillin group of antibiotics were the most commonly used antibiotic (53.13%), followed by lincosamide (36.96%), cephalosporins (29.70%), Carbapenems (24.75%), Macrolides (23.43%), polymyxin (17.4%) and quinolones (12.2%). Among the penicillin group of antibiotics, piperacillin-tazobactam was the most commonly used. Mean duration of antibiotics usage was 5.88 ± 3.39 days. Seventy-eight percent of patients were given combination of two or more antibiotics at admission. The causative organisms were isolated in 46.2% (140/303) of patients. Isolation rates were different for different biologic specimens; blood culture (5/40; 12.5%), sputum culture (47/152; 30.92%), tracheal aspirate (62/120; 51.6%), throat swab (19/75; 25.3%), pleural fluid (13/35; 37.14%), and BAL (6/8; 75%). Acinetobacter baumannii was the most common organism isolated (41/140), followed by Klebsiella (20/140), Streptococcus pneumonia (20/140), H1N1 (19/140), Pseudomonas aeruginosa (15/140), and Staphylococcus aureus (10/140). Other organisms isolated were tuberculosis (7/140), Stenotrophomonas maltophilia (3/140), Peptostreptococcus (3/140), Serratia Fonticola (1/140), Burkholderia cepacia (1/140), Staphylococcus haemolyticus (1/140), Staphylococcus hominis (1/140), and Candida tropicalis (1/140). Most common organisms isolated in patients with pneumonia were Acinetobacter (29/200), followed by Klebsiella (17/200) and Streptococcus (15/200) [Figure 2a]. Acinetobacter (15/132) was the most common organism isolated in patients with COPD followed by Klebsiella (9/132) and Streptococcus (9/132) [Figure 2b]. Acinetobacter (5/38) followed by S. aureus (3/38) were the most common organisms isolated in patients with Empyema [Figure 2c].
Figure 2.
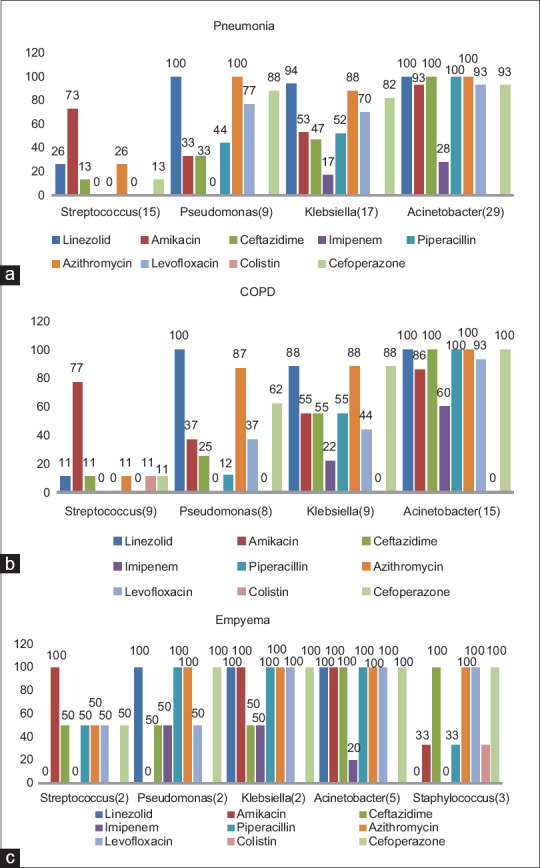
(a) Common organisms isolated and their resistance pattern of organisms isolated in patients with pneumonia. (b) Common organisms isolated and their resistance pattern of organisms isolated in patients with COPD. (c) Common organisms isolated and their resistance pattern of organisms isolated in patients with empyema
One hundred and ten patients had used antibiotics previously. In patients with prior antibiotic use, we found a higher proportion of Gram-negative organisms (78.4% vs 50%). The most common organism isolated was Acinetobacter (21.5%) followed by Klebsiella (17.6%), Pseudomonas (13.7%), and Streptococcus (11.7%). Acinetobacter was found to be highly resistant to most of the antibiotics except Colistin (100% sensitivity). We found higher resistance of Klebsiella isolated from patients who had prior antibiotic usage, to amikacin, ceftazidime, piperacillin-tazobactam, imipenem, azithromycin, and cefoperazone compared to patients with no prior antibiotic usage. However, there was lower resistance to levofloxacin in patients with prior antibiotics [Figure 3a and b]. Patients with underlying lung disease (COPD, bronchiectasis, ILD, and asthma) had a higher proportion of Gram-negative infections compared to patients with underlying normal lung [Figure 4]. Two hundred and ten patients needed mechanical ventilation. Ventilator-associated pneumonia (VAP) was observed in 17.5% of patients. A. baumannii (52.1%) was the most common organism isolated in patients with VAP. Mean duration of hospital stay was 6.29 ± 3.86 days. Mortality rate of patients on a ventilator was higher at 22% compared to overall mortality rate which was 17.8% (54/303).
Figure 3.
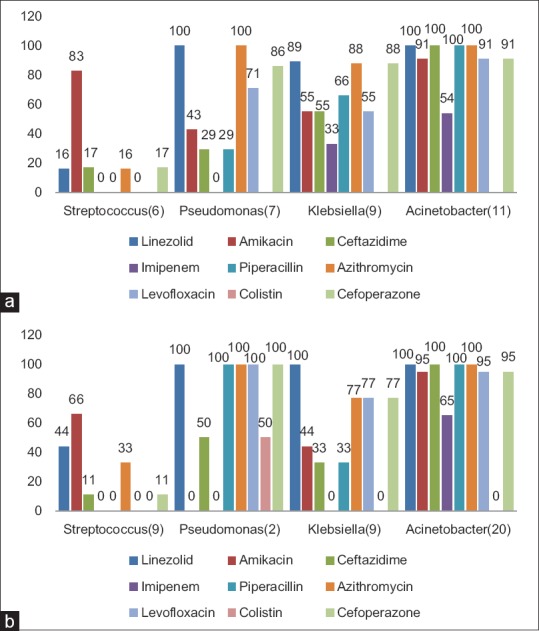
(a) Common organisms isolated and their antibiotic resistance in patients with prior antibiotic use. (b) Common organisms isolated and their antibiotic resistance in patients with no prior antibiotic use
Figure 4.
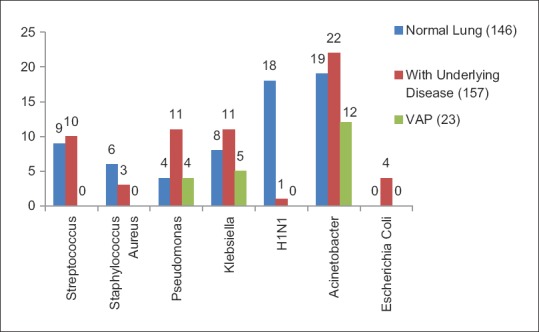
Common organisms and their resistance pattern in patients with normal lung, underlying diseased lung, and ventilator-associated pneumonia
Penicillin group of antibiotics were most commonly used for the treatment of pneumonia, followed by lincosamide and macrolide group of antibiotics. For treatment of pleural infections, such as empyema and lincosamide groups were most commonly used followed by penicillins. In patients with COPD with exacerbation and bronchiectasis, penicillin group followed by cephalosporin antibiotics were most commonly used [Figure 5]. Single antibiotic was used in 65 patients; poly antimicrobials were given in 216 patients (2 antibiotics-126, 3 antibiotics-81, 4 antibiotics-4, and 5 antibiotics-5 patients). Antibiotic resistance pattern is depicted in Tables 1 and 2. In patients where piperacillin-tazobactam was used empirically, 60% of cases, isolated organism was found to be resistant to it. In 34% of all patients, the empirical antibiotic was found to be sensitive. Subgroup analysis revealed resistance to Piperacillin-Tazobactam was higher in patients with a history of prior antibiotic use [Figure 6]. However, we did not find any difference in mortality rates among patients with and without prior antibiotic use (p-0.87).
Figure 5.
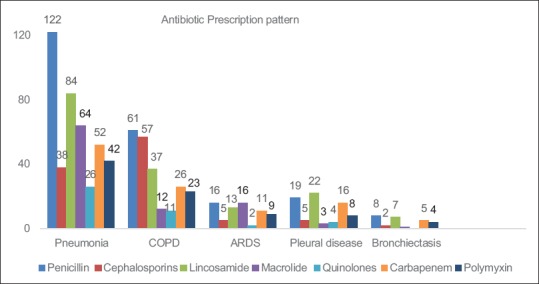
Antibiotic prescription pattern to patients admitted to respiratory Intensive Care Unit with respiratory infection
Table 1.
Drug sensitivity pattern among patients with respiratory infection admitted to Intensive Care Unit
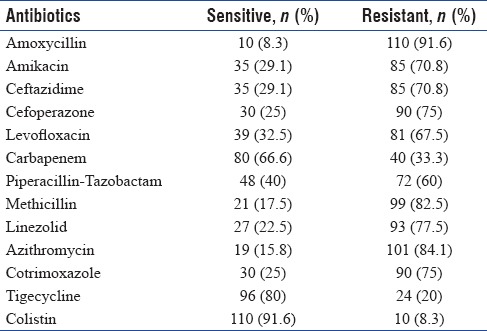
Table 2.
Sensitivity to specific antibiotics in isolates from the Intensive Care Unit with respiratory infection
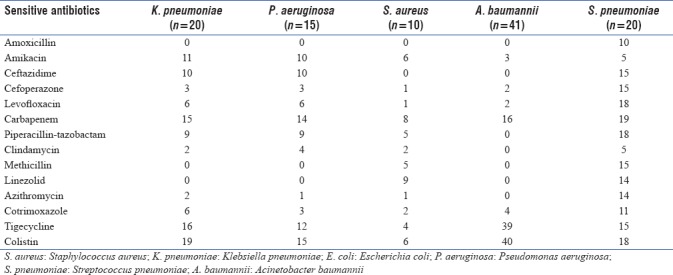
Figure 6.
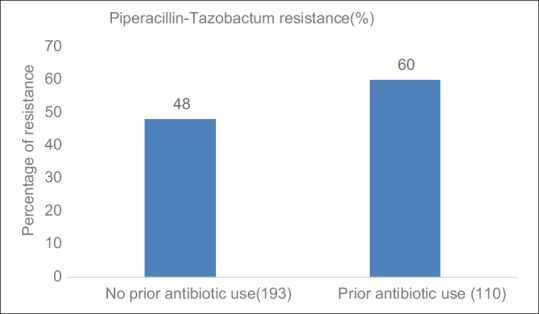
Comparison of proportion of resistance to Piperacillin-tazobactum in relation to prior antibiotic use
DISCUSSION
Respiratory infections cause significant morbidity and mortality in patients admitted to the ICU worldwide. Admission to ICU has immense economic burden on the patient of which cost for antibiotics forms a major component.[5] Nosocomial infections are very common in patients admitted to ICU. Inappropriate use of antibiotics has led to antimicrobial resistance further increasing the health-care cost and increased mortality. Worldwide incidence rate of antibiotic-resistant pathogen in ICU is 23.7 infection per 1000 patient days.[8]
We found in our study, A. baumannii was the most common organism isolated in patients with respiratory infection admitted to ICU and piperacillin-tazobactam was the most common empirical antibiotic used in our ICU. We observed around 60% of organisms isolated had resistance to empirical antibiotic piperacillin-tazobactam. Another important observation was patients with prior antibiotic use had higher proportion of infection with Gram-negative organisms and had higher resistance to organisms compared to those with no prior antibiotic use. Causative organisms vary in different geographical areas and antibiotic prescribing patterns. Studies done in US during period between 1992 and 1997 found Pseudomonas (12.4%–21%) to be most common organism isolated in ICU with respiratory infections followed by S. aureus (12.3%)[13,14,15] However, a recent study (2015) showed S. aureus (17%) to be most common organism.[16] A number of studies from Asian countries such as Indonesia, Thailand found Pseudomonas (26%–50%) to be most common organism in ICU followed by Klebsiella (15.3%) and Staphylococcus epidermidis (14.9%).[17,18] In contrast to Western studies, recent Indian studies from Chandigarh (34.8.7%), Puducherry (41.8%), and Jaipur (21%) found Acinetobacter as the most common organism followed by Pseudomonas (14%–24%), Klebsiella (19.7%), and Escherichia coli (15%–18%).[19,20,21] This is similar to our findings. Rate of Acinetobacter infection differs across various countries with highest rate in Asia (19.2%) followed by Eastern Europe (17.1%), Africa (14.8%), South America (13.8%), and least in North America (3.7%).[22] The possible explanation for high prevalence of resistant Acinetobacter species in developing countries could be due to noncompliance in infection control regulations, inadequate hand hygiene, and lack of or noncompliance with antimicrobial policy. Most common organisms isolated among patients with respiratory infection worldwide in ICUs are depicted in Table 3.
Table 3.
Most common organsims isolated among patients with respiratory infection across the world in Intensive Care Units
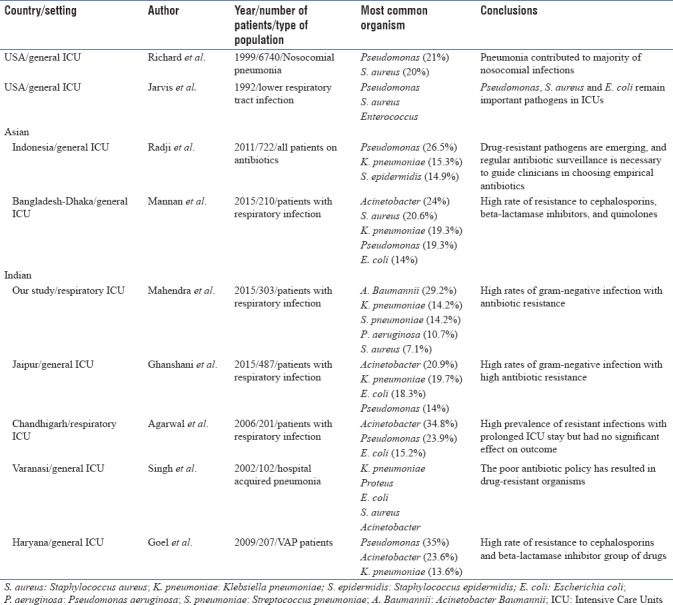
Antibiotic resistance is a major worldwide concern in ICU, including India. ICUs are the major foci of antimicrobial resistance within the hospital.[23,24] Antibiotic resistance is driven due to lack of hospital hygiene and overuse of antibiotics which leads to selection pressure on organisms.[25] Antibiotic prescription in an ICU is largely empirical and based on past experiences; hence, patients who receive inappropriate empirical antibiotic therapy substantially increases hospital stay and increases mortality.[2] Nosocomial infections resulting from drug-resistant pathogens further add to the existing problem. The most common organism isolated in our study, Acinetobacter was highly resistant to Carbapenem (60%) and was higher compared to a North Indian study (25.6%).[26] A possible reason was the empirical use of Carbapenem as the first choice in our ICU while it was the last choice in their study. The study was done 8 years ago when carbapenems was reserved as a last choice. This further emphasizes the need for judicious use of antibiotics in ICU, preferably based on the local antibiogram. Recent studies in India have shown high prevalence of multidrug resistance Gram-negative infections (40%–70%) including Acinetobacter which may necessitate earlier use of higher antibiotics empirically, especially in an ICU setting.[27,28,29,30] We observed lower drug resistance of Klebsiella to amikacin (45%), piperacillin (55%), and ceftazidime (50%) compared to a study done by Kumari et al.,[31] who observed higher levels at 50%, 91%, and 90%, respectively. An Indonesian study by Radji et al.[17] observed lower resistance of Pseudomonas to amikacin (16%) but higher resistance to ceftazidime (42%) compared to our study. A noteworthy observation that can be done from above studies is that different centers have varying pattern of resistance among different organisms, thus substantiating the need for antibiogram in each ICU. It is alarming to note that compared to early 2000s, there is a shift in most common organisms isolated from respiratory specimen from Klebsiella and Pseudomonas to Acinetobacter. Acinetobacter is highly resistant to antibiotics,[19,21,26,32] and antibiotic resistance has also significantly increased compared to previous decade in India.[26,31,33] Antibiotic resistance pattern to specific organisms among different ICUs in India are summarized in Table 4.
Table 4.
Antibiotic resistance pattern to specific organisms among different Intensive Care Units in India
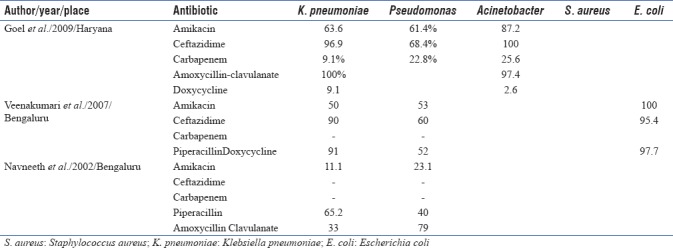
Choosing appropriate empirical antibiotic plays an important role in reducing mortality of patients admitted to ICU. Although Indian guidelines by ICMR recommend beta-lactam plus beta lactam inhibitor (e.g., piperacillin-tazobactam) for empirical treatment of Gram-negative infections,[34] we found very high resistance to piperacillin-tazobactam (76.3%) among Gram-negative organisms. Our study highlights the importance of having an antibiogram for each ICU that helps the clinician to understand local susceptibility patterns and help them to make an informed decision about the initial empirical antibiotic.
There are some limitations in this study. First, it is done in a single ICU and hence cannot be generalized to other treatment settings. Anaerobic cultures were not performed in any of the patients. Modified CPI score system, which we have used, needs further validation in larger studies.
CONCLUSION
Piperacillin-tazobactam was the most common empirical antibiotic prescribed to patients with respiratory infection admitted to ICU. More than half of the patients (60%) had resistance to the empirical antibiotic used in our ICU, highlighting the need for antibiogram for each ICU. More than one-third of the patients had prior antibiotic use in the last year and had mainly Gram-negative organisms isolated from their respiratory secretions, with high degree of resistance to commonly used antibiotics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.The Burden of Lung Disease-ERS White book. 2013. [Last cited on 2017 Jul 25]. Available from: http://www.erswhitebook.org/chapters/the-burden-of-lung-disease/
- 2.Esposito S, Leone S. Antimicrobial treatment for intensive care unit (ICU) infections including the role of the infectious disease specialist. Int J Antimicrob Agents. 2007;29:494–500. doi: 10.1016/j.ijantimicag.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Poor Compliance with the Antibiotic Policy in the Intensive Care Unit (ICU) of a Tertiary care Hospital in India. The Journal of Infection in Developing Countries. 2013. [Last cited on 2017 Dec 29]. Available from: https://jidc.org/index.php/journal/article/view/24334948 . [DOI] [PubMed]
- 4.Weber RJ, Kane SL, Oriolo VA, Saul M, Skledar SJ, Dasta JF, et al. Impact of Intensive Care Unit (ICU) drug use on hospital costs: A descriptive analysis, with recommendations for optimizing ICU pharmacotherapy. Crit Care Med. 2003;31:S17–24. doi: 10.1097/00003246-200301001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Tavallaee M, Fahimi F, Kiani S. Drug-use patterns in an intensive care unit of a hospital in iran: An observational prospective study. Int J Pharm Pract. 2010;18:370–6. doi: 10.1111/j.2042-7174.2010.00065.x. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–11. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 7.Micek ST, Ward S, Fraser VJ, Kollef MH. A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator-associated pneumonia. Chest. 2004;125:1791–9. doi: 10.1378/chest.125.5.1791. [DOI] [PubMed] [Google Scholar]
- 8.Lentino JR, Lucks DA. Nonvalue of sputum culture in the management of lower respiratory tract infections. J Clin Microbiol. 1987;25:758–62. doi: 10.1128/jcm.25.5.758-762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Thoracic Society-Bronchoalveolar Lavage. 2012. [Last cited on 2018 Jan 04]. Available from: https://www.thoracic.org/professionals/clinical-resources/criticalcare/clinical-education/critical-care-procedures/bronchoalveolarlavage.php .
- 10.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. An official American thoracic society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–14. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 11.Search Results CDC. 2017. [Last cited on 2018 Jan 04]. Available from: https://www.cdc.gov/search/index.html .
- 12.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 13.Fridkin SK. Increasing prevalence of antimicrobial resistance in intensive care units. Crit Care Med. 2001;29:N64–8. doi: 10.1097/00003246-200104001-00002. [DOI] [PubMed] [Google Scholar]
- 14.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National nosocomial infections surveillance system. Crit Care Med. 1999;27:887–92. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis WR, Martone WJ. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29(Suppl A):19–24. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 16.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 17.Radji M, Fauziah S, Aribinuko N. Antibiotic sensitivity pattern of bacterial pathogens in the intensive care unit of fatmawati hospital, Indonesia. Asian Pac J Trop Biomed. 2011;1:39–42. doi: 10.1016/S2221-1691(11)60065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Refdanita R. The Sensitivity pattern of microorganisms against antibiotics at the intensive care unit of fatmawati hospital Radji M, Aribinuko N, Pauline E. Jakarta 2001–2002. Makara J Health Res. 2010;8:41–8. [Google Scholar]
- 19.Agarwal R, Gupta D, Ray P, Aggarwal AN, Jindal SK. Epidemiology, risk factors and outcome of nosocomial infections in a respiratory intensive care unit in North India. J Infect. 2006;53:98–105. doi: 10.1016/j.jinf.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Prashanth K, Badrinath S. Nosocomial infections due to Acinetobacter species: Clinical findings, risk and prognostic factors. Indian J Med Microbiol. 2006;24:39–44. doi: 10.4103/0255-0857.19893. [DOI] [PubMed] [Google Scholar]
- 21.Ghanshani R, Gupta R, Gupta BS, Kalra S, Khedar RS, Sood S. Epidemiological study of prevalence, determinants, and outcomes of infections in medical ICU at a tertiary care hospital in India. Lung India Off Organ Indian Chest Soc. 2015;32:441–8. doi: 10.4103/0970-2113.164155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). Nosocomial enterococci resistant to vancomycin--United States, 1989-1993. MMWR Morb Mortal Wkly Rep. 1993;42:597–9. [PubMed] [Google Scholar]
- 24.Archibald L, Phillips L, Monnet D, McGowan JE, Jr, Tenover F, Gaynes R, et al. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: Increasing importance of the intensive care unit. Clin Infect Dis. 1997;24:211–5. doi: 10.1093/clinids/24.2.211. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein RA. Controlling antimicrobial resistance in hospitals: Infection control and use of antibiotics. Emerg Infect Dis. 2001;7:188–92. doi: 10.3201/eid0702.010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel N, Chaudhary U, Aggarwal R, Bala K. Antibiotic sensitivity pattern of gram negative bacilli isolated from the lower respiratory tract of ventilated patients in the intensive care unit. Indian J Crit Care Med. 2009;13:148–51. doi: 10.4103/0972-5229.58540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathai AS, Oberoi A, Madhavan S, Kaur P. Acinetobacter infections in a tertiary level intensive care unit in northern India: Epidemiology, clinical profiles and outcomes. J Infect Public Health. 2012;5:145–52. doi: 10.1016/j.jiph.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Gopalakrishnan R, Sureshkumar D. Changing trends in antimicrobial susceptibility and hospital acquired infections over an 8 year period in a tertiary care hospital in relation to introduction of an infection control programme. J Assoc Physicians India. 2010;58(Suppl):25–31. [PubMed] [Google Scholar]
- 29.Patwardhan RB, Dhakephalkar PK, Niphadkar KB, Chopade BA. A study on nosocomial pathogens in ICU with special reference to multiresistant Acinetobacter baumannii harbouring multiple plasmids. Indian J Med Res. 2008;128:178–87. [PubMed] [Google Scholar]
- 30.Mannan MA, Kashem MA, Mohammed FR, Rabbani R, Islam MM. Microbiological Profile of severe lower respiratory tract infection in intensive care unit of a tertiary care center of Dhaka, Bangladesh. Bangladesh Crit Care J. 2015;2:53–6. [Google Scholar]
- 31.Kumari HBV, Nagarathna S, Chandramuki A. Antimicrobial resistance pattern among aerobic gram-negative bacilli of lower respiratory tract specimens of intensive care unit patients in a neurocentre. Indian J Chest Dis Allied Sci. 2007;49(1):19–22. [PubMed] [Google Scholar]
- 32.Singh AK, Sen MR, Anupurba S, Bhattacharya P. Antibiotic sensitivity pattern of the bacteria isolated from nosocomial infections in ICU. J Commun Dis. 2002;34:257–63. [PubMed] [Google Scholar]
- 33.Navaneeth BV, Belwadi MR. Antibiotic resistance among gram-negative bacteria of lower respiratory tract secretions in hospitalized patients. Indian J Chest Dis Allied Sci. 2002;44:173–6. [PubMed] [Google Scholar]
- 34.Treatment Guidelines for Antimicrobial Use in Common Syndromes. ICMR Guidelines. 2017. [Last cited on 2017 Dec 06]. Available from: http://icmr.nic.in/About_Us/Guidelines.html .


