Abstract
Background:
Dengue fever is an important tropical infection causing significant mortality. The pathophysiology of hematological abnormalities in dengue remains poorly studied. In this study, we analyzed the hematological abnormalities by thromboelastography (TEG).
Methods:
This cross-sectional study evaluated complicated dengue patients with TEG. Thromboelastographic variables were categorized into six patterns: factor deficiency, platelet dysfunction, enzymatic hypercoagulability, combined enzymatic and platelet hypercoagulability, primary fibrinolysis, and secondary hyperfibrinolysis.
Results:
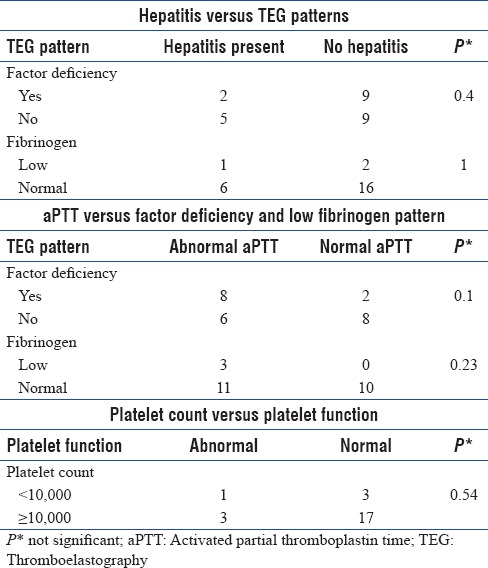
Twenty-five patients were analyzed for coagulation abnormalities by TEG. Coagulation factor deficiency pattern was noted in 11 patients (44%) whereas 3 patients (12%) were found to have low fibrinogen level pattern. Low platelet function was noted in 4 (16%) patients. Enzymatic hypercoagulability and combined enzymatic and platelet hypercoagulability were noted in one patient each (4.5%). Secondary fibrinolysis was noted in 1 patient (5%) and primary fibrinolysis in 3 (15.8%) patients. Factor deficiency pattern and low fibrinogen pattern were not significantly associated with hepatitis (P > 0.05). Activated partial thromboplastin time (aPTT) was not found to be significantly associated with factor deficiency pattern (P = 0.10) and low fibrinogen pattern (P = 0.20). Platelet count was not found to be significantly associated with platelet function (P = 0.54).
Conclusion:
Factor deficiency pattern was the major abnormality noted in dengue patients followed by platelet dysfunction and primary fibrinolysis. Platelet count did not show significant association with platelet function. aPTT did not show significant association with factor deficiency and low fibrinogen patterns. Factor deficiency pattern and low fibrinogen pattern did not show significant association with hepatitis.
Keywords: Dengue, hematological abnormality, thromboelastography
INTRODUCTION
Dengue is endemic in at least 100 countries in the world. The World Health Organization estimates that 50–100 million infections occur annually, including 500,000 dengue hemorrhagic fever (DHF) cases and 22,000 deaths, mostly in children.[1] India also remains as an endemic area, and the highest number of cases have been reported in 2016 with the highest death rate in the last 7 years. In 2017, so far, 87,018 dengue cases were reported in India, and Kerala is the most affected state with highest death rate.[2] The pathophysiology of coagulation abnormalities in dengue fever remains poorly understood. Abnormalities of conventional coagulation tests have been reported routinely in dengue patients. Viscoelastic property analysis of dengue fever patients admitted in an Intensive Care Unit (ICU) has not yet been studied. In this trial, we tried to analyze the prevalence of coagulation abnormalities in dengue fever patients and the correlation between conventional coagulation tests and thromboelastography (TEG) parameters.
TEG is a global viscoelastic property analysis of whole blood clot formation. Different phases of clot formation, namely, clot formation, clot propagation, clot strength, and clot lysis are tested in TEG. Specific TEG parameters that represent the different phases of hemostasis are reaction time (R time) which represent time from start of test to initial fibrin formation, alpha angle which represents the speed at which fibrin build up and cross-linking takes place, maximum amplitude (MA) which represents clot stability, percentage of clot lysis after 30 min (LY30) which represents the percent reduction in amplitude at 30 min post-MA indicating the degree of fibrinolysis and coagulation index (CI) which is a calculated index of overall coagulation.
METHODS
This cross-sectional evaluation was a prospective study done in a multidisciplinary ICU of a tertiary level hospital in South Kerala for 1 year. This study was approved by the Hospital Ethical Committee and funded by the Hospital Research Committee without any financial burden on patients. Written informed consent was taken from the patient or surrogate.
We studied the coagulation profile of dengue patients getting admitted in the ICU for various complications using TEG. Sample size of 30 was calculated from the previous admissions of dengue fever in the ICU for the last 2 years. Dengue fever was confirmed either by nonstructural protein 1 (NS1) antigen if the patient was symptomatic for three or <3 days or by IgM immunoassay if presented after 3 days of the symptom onset. TEG was done for patients following admission to ICU. Patients who were having preexisting coagulation and bleeding disorders, those who were on antiplatelets and anticoagulants, and those who were received platelet transfusion or blood products were excluded from the study. TEG was done by a trained technician. Various TEG parameters including reaction time (R Time), alpha angle, MA, CI, and clot lysis (CL 30) were recorded for each patient. Normal reference range of TEG parameters is shown in Table 1.[3] Using these TEG parameters, patients were categorized to have six coagulation patterns as per the flowchart shown in Figure 1. Patients having R time higher than the upper limit of normal reference range were categorized as having factor deficiency pattern. Those with normal R time and normal MA but alpha angle below the lower limit of normal reference range were categorized as having low fibrinogen level pattern. Patients with a normal R time but with a MA below the lower limit of normal reference range were categorized as having low platelet function pattern. Those with CI above the upper limit of normal reference range with R time below the lower limit of normal reference range and normal MA were categorized as having enzymatic hypercoagulability. Patients with CI above the upper limit of normal reference range with R time below the lower limit of normal reference range and having a MA higher than the upper limit of normal reference range were categorized as combined platelet and enzymatic hypercoagulability pattern. Those with LY30 and CI higher than the upper limit of normal reference range were categorized as secondary fibrinolysis. Finally patients with LY 30 higher than upper limit of normal reference range with coagulation index below the lower limit of normal reference range were categorized as having primary fibrinolysis. Data analysis was done with Epi-info version 7.1.3.2. developed by Centers for Disease Control and Prevention, Atlanta.
Table 1.
Normal range of thromboelastography variables

Figure 1.
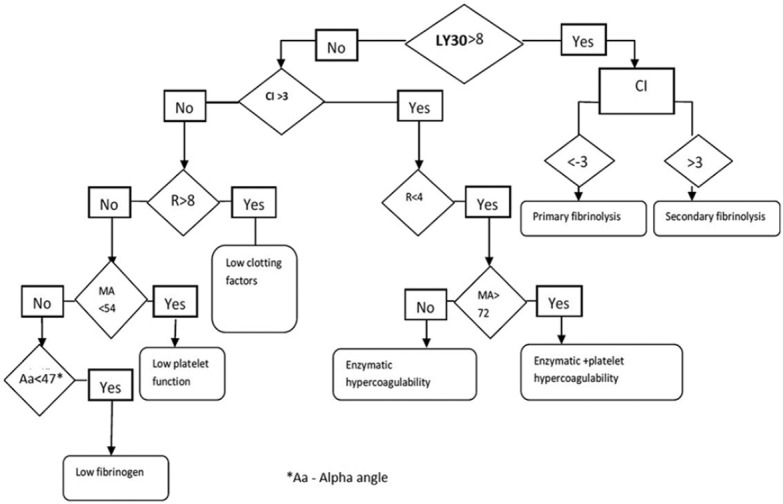
Decision flow chart for different thromboelastography patterns
Statistical analysis
Continuous variables were expressed as a mean and standard deviation and categorical variables were expressed as frequencies with 95% confidence limits. Significance of proportions between groups was tested by Chi-square test wherever applicable and if the number of variables was inadequate Fisher's exact test was done.
RESULTS
A total of 25 patients were analyzed for coagulation abnormalities by TEG. Of this, 10 (40%) were male and 15 (60%) were female. Mean age was 41 years with standard deviation of 16.6. Among them, IgM was positive in 6 (24%) patients and NS1 antigen was positive in 18 (72%) patients and both were positive in one patient (4%). Platelet count (n = 24) below 10000/mm3 was found in 4 (16.66%) patients. Mean platelet count was 42,166 with standard deviation of 43,036 and median of 22,000. Bleeding was found in 2 (8%), hepatitis in 7 (28%), and encephalitis in 2 (8%) patients.
Distribution of different TEG parameters and different TEG patterns are shown in Tables 2 and 3, respectively. Factor deficiency pattern was noted in 11 patients (44%). Three patients (12%) were found to have low fibrinogen level pattern. Low platelet function was noted 4 patients (16%). Enzymatic hypercoagulability (n = 22) and combined enzymatic and platelet hypercoagulability (n = 22) were noted in one patient each (4.5%). Secondary fibrinolysis (N = 19) was noted in one patient (5%) and primary fibrinolysis (n = 19) in three patients (15.8%). Factor deficiency pattern and low fibrinogen pattern in TEG were not significantly associated with hepatitis (P = 0.4 and 1, respectively) [Table 4]. Activated partial thromboplastin time (aPTT) was not found to be significantly associated with factor deficiency pattern (P = 0.10) and low fibrinogen pattern (P = 0.20) in TEG [Table 4]. Platelet count was not found to be significantly associated with platelet function (P = 0.54) [Table 4].
Table 2.
Frequency of abnormal thromboelastography variables and activated partial thromboplastin time
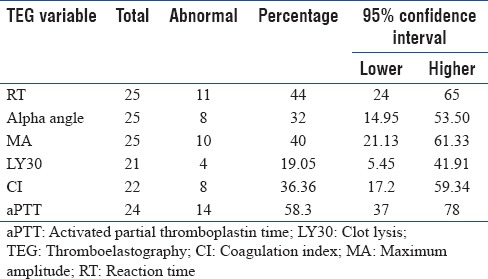
Table 3.
Frequency distribution of different thromboelastography patterns
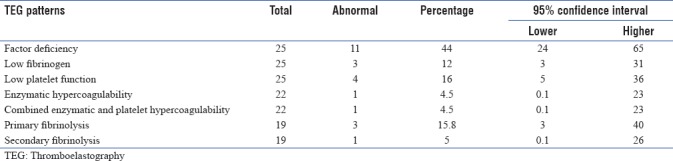
Table 4.
Association of hepatitis, activated partial thromboplastin time, and platelet count with thromboelastography patterns
DISCUSSION
Dengue infection remains a high threat to tropical and subtropical regions and in India. Kerala was the most affected state in 2017 epidemic with the highest reported death rate. However, the pathophysiological basis of coagulation abnormalities in dengue remains unexplored. Coagulation factor deficiency pattern was the predominant coagulation abnormality noted followed by low platelet function and then primary fibrinolysis in this trial. Variable reduction in the activation of coagulation factors including factors V, VII, VIII, IX, and prothrombin have been demonstrated during the acute phase of DHF.[4,5,6] In this study, there was no significant association between platelet number and platelet function tested by TEG. A study done by Nimmannitya has shown poor correlation between platelet count and bleeding risk.[7] In another study done among 106 pediatric patients with dengue shock syndrome with thrombocytopenia and coagulopathy, there was no difference in the incidence of hemorrhage between patients who received preventive transfusion compared to those who did not receive.[8] There was no significant association between factor deficiency pattern and low fibrinogen pattern with hepatitis in this trial. The conventional coagulation test partial thromboplastin time is found to have no significant association with low clotting factor pattern and low fibrinogen pattern in this trial. This seems to be important because an abnormal aPTT can be due to deficient coagulation factors or due to fibrinogen deficiency. This finding is similar to the findings in the study done by Piza et al. which showed that conventional coagulation tests such as PT, PTT, thrombin time, and plasma fibrinogen had no significant association with thromboelastometry (ROTEM) parameters.[9] However, Kulasinghe et al. in a prospective study done on DHF has shown that aPTT done on 4th and 5th day was a strong predictor of bleeding.[10]
Coagulation abnormalities in the form of primary fibrinolysis, reduced levels of protein C, S, and antithrombin with concurrent increase in the levels of thrombomodulin, tissue factor, and plasminogen activator inhibitor type 1 were detected in a study done in 48 Vietnamese children with dengue shock syndrome. The same study also showed moderate reduction in the level of fibrinogen in contrast to our observation, in which the low fibrinogen level pattern was found in 3 patients (12%) only.[6] However, another cross-sectional study done in thrombocytopenic dengue patients by TEG had shown a maintained fibrinogen level as demonstrated by FIBTEM analysis.[9] In our study, primary fibrinolysis also noticed among 3 patients (12%) which correlates with the study done in Vietnamese children suggesting that dengue virus can activate primary fibrinolysis.[6] A reduction in fibrinogen level with increased fibrin degradation products has been demonstrated in other studies also.[4,11,12] In that case, antifibrinolytic may have a therapeutic role which has to be confirmed by controlled trials.
Major advantages of this study are that probably this is the first of this kind of evaluation done among dengue patients getting admitted in a critical care unit. Routine coagulation tests and platelet count fails to show an association with different TEG patterns. Primary fibrinolysis is also found in a small percentage of dengue fever patients which has got a therapeutic implication. Unlike conventional tests of coagulation pathway, TEG is a point-of-care test which can clearly differentiate various abnormalities of coagulation pathway. Hence, a targeted therapy based on TEG abnormalities may be more effective. Moreover, this may avoid potential complications related to unnecessary transfusion of blood and blood products. Thus, a TEG-based therapeutic strategy may have a potential role in dengue fever with hemorrhagic complications. More studies with larger sample size and multiple settings, especially among dengue patients with hemorrhagic complications, may bring out more clarity in the issue.
Major drawback of this study is that we could not achieve the initially planned sample size. The sample size was limited as the study setting was a Level 3 ICU and the number of patients satisfying the inclusion and exclusion criteria in this setup was low. Furthermore, this evaluation was done at the time of admission, and coagulation abnormalities may change as the disease condition progresses. We analyzed using TEG only; however, future studies may use extended viscoelastic technologies such as FIBTEM which will differentiate platelet contribution to clot firmness from contribution by fibrinogen in a better way. We studied all complicated dengue patients admitted in the ICU; however, studying only patients with hemorrhagic complications may give a better understanding about the coagulation abnormalities. Apart from this, contribution of vasculopathy to bleeding is not amenable to measurement by TEG.
CONCLUSION
Coagulation factor deficiency pattern was the major abnormality noted in dengue patients analyzed by TEG followed by platelet dysfunction and primary fibrinolysis. Platelet count was not found to have a significant association with platelet function. Routine coagulation test aPTT was found to have no significant association with factor deficiency pattern and low fibrinogen pattern in TEG.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Sanjeev Nair, Associate Professor, Department of Pulmonary Medicine, Medical College, Trivandrum, for his valuable opinion and advice.
REFERENCES
- 1.Cdc.gov. Epidemiology|Dengue|CDC. 2017. [Last accessed on 2017 Nov 02]. Available from: https://www.cdc.gov/dengue/epidemiology/index.html .
- 2.Nvbdcp.gov.in. NVBDCP|National Vector Borne Disease Control Programme. 2017. [Last accessed on 2017 Nov 02]. Available from: http://www.nvbdcp.gov.in/den-cd.html .
- 3.2017. [Last accessed on 2017 Nov 21]. Haemonetics.com. Available from: http://www.haemonetics.com/~/media/sharepoint/devices/teg/marketing/brochures/teg5000_brochure/col-pp-000078.us_teg_brochure.pdf.ashx .
- 4.Sosothikul D, Seksarn P, Pongsewalak S, Thisyakorn U, Lusher J. Activation of endothelial cells, coagulation and fibrinolysis in children with dengue virus infection. Thromb Haemost. 2007;97:627–34. [PubMed] [Google Scholar]
- 5.Mitrakul C, Poshyachinda M, Futrakul P, Sangkawibha N, Ahandrik S. Hemostatic and platelet kinetic studies in dengue hemorrhagic fever. Am J Trop Med Hyg. 1977;26:975–84. doi: 10.4269/ajtmh.1977.26.975. [DOI] [PubMed] [Google Scholar]
- 6.Wills BA, Oragui EE, Stephens AC, Daramola OA, Dung NM, Loan HT, et al. Coagulation abnormalities in dengue hemorrhagic fever: Serial investigations in 167 Vietnamese children with dengue shock syndrome. Clin Infect Dis. 2002;35:277–85. doi: 10.1086/341410. [DOI] [PubMed] [Google Scholar]
- 7.Nimmannitya S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health. 1987;18:392–7. [PubMed] [Google Scholar]
- 8.Lum LC, Abdel-Latif Mel-A, Goh AY, Chan PW, Lam SK. Preventive transfusion in dengue shock syndrome-is it necessary? J Pediatr. 2003;143:682–4. doi: 10.1067/s0022-3476(03)00503-1. [DOI] [PubMed] [Google Scholar]
- 9.Piza FM, Corrêa TD, Marra AR, Guerra JC, Rodrigues RD, Villarinho AA, et al. Thromboelastometry analysis of thrombocytopenic dengue patients: A cross-sectional study. BMC Infect Dis. 2017;17:89. doi: 10.1186/s12879-017-2204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulasinghe S, Ediriweera R, Kumara P. Association of abnormal coagulation tests with dengue virus infection and their significance as early predictors of fluid leakage and bleeding. Sri Lanka J Child Health. 2016;45:184–8. [Google Scholar]
- 11.Marchi R, Nagaswami C, Weisel JW. Fibrin formation and lysis studies in dengue virus infection. Blood Coagul Fibrinolysis. 2009;20:575–82. doi: 10.1097/MBC.0b013e32832fb1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YH, Liu CC, Wang ST, Lei HY, Liu HL, Lin YS, et al. Activation of coagulation and fibrinolysis during dengue virus infection. J Med Virol. 2001;63:247–51. doi: 10.1002/1096-9071(200103)63:3<247::aid-jmv1008>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]


