Abstract
In 2012, the European Society of Cardiology (ESC) guidelines provided recommendations on the management of ST-elevation myocardial infarction (STEMI). The recommendation from these guidelines is restricted to the European subcontinent. To adapt the updated recommendations for Indian subset of STEMI patients, a panel of experts in the management of STEMI provided their expert opinions. This document provides expert consensus on adapting 2012 ESC STEMI guidelines recommendations in Indian setting. Document also discussed “India-specific” relevant literature to support the consensus opinions provided in the management of STEMI.
Keywords: European Society of Cardiology guidelines, expert consensus, India, ST-elevation myocardial infarction
INTRODUCTION
Coronary artery disease (CAD) is a major contributor to morbidity and mortality in India, and its overall prevalence has risen dramatically over the past two decades.[1] It has been seen that patients in India who suffer from Acute Coronary Syndrome (ACS) are younger (mean age, 56.3 years) and ST-elevation myocardial infarction (STEMI), constitute a higher proportion (60.6%) of ACS compared to non-ST elevation MI (NSTEMI).[2] Various evidence-based guidelines published internationally from time to time provide new evidence-based recommendations for diagnosis and the management of STEMI.[3,4,5,6] The European Society of Cardiology (ESC) guidelines released in 2012 provided recommendations on the management of acute MI (AMI) in patients presenting with ST-segment elevation.[7] While these are erudite and exhaustive, they are tailored to the situation in developed countries. Although no evidence-based guidelines in India exists, an expert consensus on the management of STEMI provide some India specific recommendations.[8] As there are differences between European and Indian settings regarding the availability of percutaneous coronary intervention (PCI) centers, initial delay in reperfusion, acceptability of concept of pre-hospital thrombolysis, 24 × 7 availability of trained staff, patient factors and financial challenges, the applicability of the recent ESC guidelines need to be critically looked at to adapt the recommendations in Indian setting. The objective of this expert consensus was to take the ESC 2012 STEMI guideline and tweak it with available Indian evidence by the help of a group of acclaimed Indian experts on the subject to provide a consensus document relevant for Indian STEMI patients which can provide a framework to guide Indian physicians and healthcare providers in making decisions for the optimal management of patients with STEMI.
THE EXPERT PANEL
The expert panel consisted of 19 cardiologists and physicians involved in the management of STEMI from various parts of India and meeting of these experts was held to discuss on this specific issue. Due to the geographical and cultural differences in India, experts from different locations were involved in the discussion. The mini-Delphi or Estimate-Talk-Estimate (ETE) technique was incorporated for generating the consensus. Delphi method is a structured communication technique or method, originally developed as a systematic, interactive forecasting method which relies on a panel of experts. The Delphi technique can be adapted for use in a face-to-face meeting and is then called mini-Delphi or ETE. This consensus technique is widely used in the field of health as a mean of collecting experts’ opinions for guiding health decision-making. This technique is frequently implemented to reach agreement on the classification of diagnostic criteria, the development of clinical guidelines, and the identification of health professionals’ needs. The experts provided India-specific consensus opinions for the management of STEMI after discussion of the recent ESC guideline recommendations about following issues:
Definition and classification of MI
Diagnosis of AMI and role of biomarkers (Troponin T)
Risk stratification of patients with STEMI
Pre-hospital care
Reperfusion therapy
Comparison of fibrinolytic agents.
Experts were allowed to present clarifications and arguments based on their viewpoints. The opinions of experts were counted on majority and consensus was adopted. Following sections provide with practically applicable ESC recommendations with expert opinions on each of them for adaptation in an Indian setting.
Definition of myocardial infarction
The 2012 ESC guidelines on the management of AMI in patients presenting with ST-segment elevation defined MI on the basis of following criteria:[7]
Detection of rise and/or fall of cardiac biomarker values (preferably troponin) with at least one value above the 99th percentile of the upper reference limit and with at least one of the following:
Symptoms of ischemia
New or presumably new significant ST-T changes or new left bundle branch block (LBBB)
Development of pathological Q waves in the electrocardiogram (ECG)
Imaging evidence of new loss of viable myocardium, or new regional wall motion abnormality (RWMA)
Identification of an intracoronary thrombus by angiography or autopsy.
Panelists expressed that the situation of early diagnosis of STEMI is already very complicated in India, where few doctors face challenges in diagnosis by ECG and may not be able to decide the choice of reperfusion (i.e., thrombolysis or PCI).
Expert Consensus: it will be very challenging to adapt the guidelines recommended by ESC 2012 for the definition of MI in Indian settings. Therefore, diagnosing MI patients as STEMI or non-ST elevation ACS to keep it simple and if required supported with a troponin or echocardiogram appears to be the right approach for Indian settings.
Diagnosis of ST-elevation myocardial infarction and biomarkers as predictors
Initial diagnosis
Guidelines suggest a working diagnosis of MI to be made by the history of chest pain lasting for 20 min or more and radiation of the pain to the neck, lower jaw, or left arm being important clues for diagnosis. However, according to a previous registry, it was observed that 30% of patients with STEMI presented with atypical symptoms.[9] The recommendations proposed by the guideline suggest that ECG monitoring should be initiated as soon as possible in all patients with suspected STEMI to detect life-threatening arrhythmias and allow prompt defibrillation if indicated. The guidelines state that a 12-lead ECG should be obtained as soon as possible at the point of first medical contact (FMC), with a target delay of ≤10 min (Class 1B).[7]
The panel discussed the four ways of diagnosing MI:
Symptoms
Elevated troponin
ECG
Imaging.
They felt that typical ischemic symptom of more than 20 min duration and ST segment change either in the form of ST-elevation or dynamic ST-segment change should be sufficient for the diagnosis [Figure 1]. Panelists opined that only for those patients who have ST-segment changes which are nondiagnostic of STEMI; we should look for troponin which is in contrast to the ESC guidelines which suggests that blood sampling for serum markers is recommended routinely in the acute phase. They also suggested to perform echocardiography (ECHO) in conditions where there is a doubt in diagnosis by symptoms, ECG, and troponin. However, for patients who have ST, EMI panelists suggested to go for reperfusion therapy, preferably primary angioplasty in a PCI capable hospital which is in line with the ESC guidelines which suggests that one should not wait for the results before initiating reperfusion treatment.[7] In such cases of clear STEMI, biochemical marker assessment is not required to go for thrombolysis or even for interventions.
Figure 1.
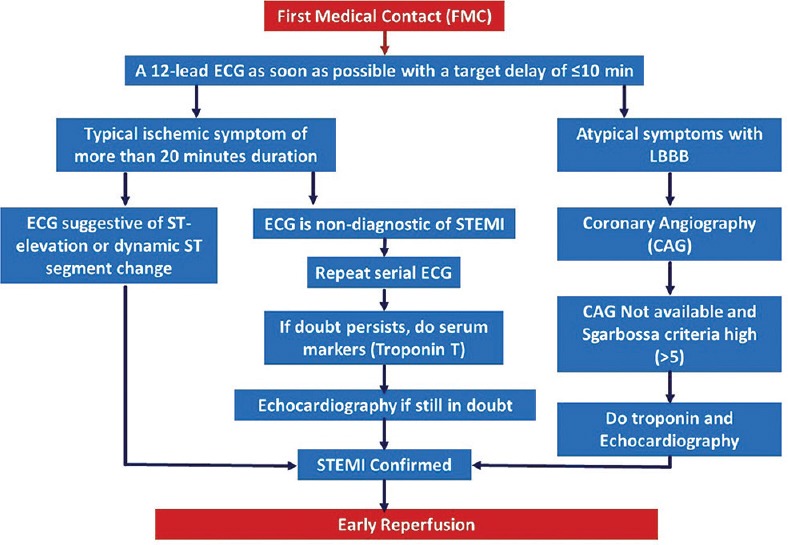
Consensus algorithm of diagnostic approach before proceeding to thrombolysis/percutaneous coronary intervention
Expert consensus
Typical ischemic symptoms with ST elevation are sufficient for initiating reperfusion therapy preferably PCI when possible, and troponin is recommended only in cases of doubtful or difficult to diagnose cases of STEMI.
Conditions with difficult diagnosis by electrocardiogram
Atypical presentations that deserve prompt management in patients with signs and symptoms of ongoing ischemia as per the guidelines are LBBB, ventricular paced rhythm, patients without diagnostic ST elevation but with persistent ischemic symptoms, isolated posterior MI, and ST-segment elevation in lead augmented vector right.
Left bundle branch block
Guidelines suggest that a previous ECG may assist in evaluating whether the LBBB is new (and therefore, the suspicion of ongoing MI is high), and immediate reperfusion therapy preferably using emergency coronary angiography (CAG) with a view to perform primary PCI or, if unavailable, intravenous (i.v.) thrombolysis in such patients should be done with clinical suspicion of ongoing myocardial ischemia with new or presumed new LBBB. Majority of panel members had experienced that most of the patients with MI in India do not come with their previous ECG. The panel was of the opinion that in new LBBB which is considered as STEMI equivalent, diagnosis of RWMA by imaging (echocardiogram) to rule out/confirm STEMI is not easy. For the choice of therapy, every patient with new LBBB patient who comes with chest pain should be preferably sent to CAG. In patients with LBBB of uncertain origin, the guidelines suggest a positive point-of-care troponin test 1–2 h after symptom onset, may help in deciding whether to perform emergency angiography with a view to primary PCI. Panel members discussed the option of applying the Sgarbossa criteria in LBBB with atypical chest pain.[10] They discussed the scenario in which a patient comes with an atypical pain having a high Sgarbossa score (>5) with nonavailability or anticipated delay in CAG; in such a case echocardiographic evidence of RWMA might be helpful before opting for thrombolysis. However, if still there is any confusion after echo and CAG cannot be performed, its better not to thrombolyse until the diagnosis is confirmed. The panel also mentioned the importance of troponin test in this condition. The positive and the negative predictive value of troponin becomes more than 90% only after about 8 h or so. Hence if the patient comes in that window, conventional or qualitative troponin can be done, and if it is high, the panel recommended to perform angiography.
However, for patients presenting early, the panel recommended that the high-sensitivity troponin can be used, and angiography can be performed if the troponin is positive. However, in a condition of typical pain, with Sgarbossa score >5, LBBB, and the troponin is corroborative, thrombolysis can be performed (PCI unavailable). Thrombolysis is however not recommended when troponin is noncorroborative. Panelists also discussed the need to understand that new onset right bundle branch block (RBBB) also should not be missed, the opinion of the panelists is well supported by a study which has also shown that patients with MI and RBBB have a poor prognosis.[11]
Expert consensus
In Indian settings, a previous ECG is not available most of the times to diagnose whether the LBBB is new or old. In conditions of atypical pain with LBBB, its better to do a CAG. If CAG is unavailable and Sgarbossa criteria high (>5) troponin and echocardiogram may be done before proceeding for thrombolysis or referral for PCI.
Patients without diagnostic electrocardiogram
Few patients with genuine acute occlusion of a coronary artery and ongoing MI (such as those with an occluded circumflex coronary artery, acute occlusion of a vein graft, or left main disease), can present without ST-segment elevation and be denied reperfusion therapy, resulting in larger infarction and worse outcomes. In such conditions of doubtful ECG, it is recommended by the guidelines to repeat the ECG or monitor the ST segment. If the doubt persists even after serial ECG, panelists recommended echocardiogram (for RWMA) and troponin levels for facilitating diagnosis of STEMI. In any case, it is recommended that ongoing suspicion of myocardial ischemia despite medical therapy is an indication for emergency CAG to revascularization, even in patients without diagnostic ST-segment elevation.
Expert consensus
Panelists agreed to the ESC recommendations that repeated serial ECG should be performed in case of doubt in diagnosis. ECHO and troponin T can be performed still there is a doubt after serial ECG. They also agreed to the ESC recommendations that CAG should be performed in cases with a high likelihood of ongoing ischemia.
Biomarkers as predictors
ESC 2012 guidelines suggest that though blood sampling for serum markers should be routinely carried out in the acute phase, the initiation of reperfusion treatment should not be dependent on their results. Majority of the panel members agreed to the guidelines that typical ischemic symptoms along with the ST elevation are sufficient to opt for management of STEMI without waiting for the serum markers. Diagnosis of STEMI based on ECG is challenging for most of the inexperienced practitioners, and therefore treatment decisions cannot be taken effectively and promptly. Therefore, in such cases of doubtful ECG findings, the panel recommended troponin test to be done for precise diagnosis of STEMI and timely treatment decision. Troponin (T or I) have proved to be the biomarkers of choice, because of its high sensitivity and specificity for myocardial necrosis.[12] Thus, in patients with a clinically low or intermediate likelihood of ongoing myocardial ischemia with a long prior duration of symptoms, a negative troponin test may help to avoid unnecessary emergency angiography in some patients.[7] Panel members felt that the qualitative biomarker is actually semi-quantitative in nature and is very well validated. They also felt that the quantitative troponin should also be highlighted and with high sensitivity troponin to be available soon, these tests may have a value in special situations like patients with comorbidities, post-PCI, and post-coronary artery bypass graft. It is also recommended in guidelines that in cases of doubt regarding the possibility of acute evolving MI, emergency imaging (as opposed to waiting for the biomarkers to become elevated) allows the provision of timely reperfusion therapy to these patients, which was well accepted by the majority of panel members. Considering the guidelines for management of STEMI, if locally available, emergency CAG is the modality of choice, which can be followed immediately by primary PCI if the diagnosis is confirmed.[7] However, in conditions where CAG is not immediately available, two-dimensional ECHO may assist in deciding for emergency transfer to a PCI center, since regional wall motion abnormalities occur within minutes following coronary occlusion, well before necrosis. This was well accepted by the majority of panel members.
PROBLEMS WITH TROPONIN T ACCORDING TO THE PANEL
Quantitative troponin may not be available in many parts of India. Troponin T is not positive in <4 h from symptom onset, hence not mandatory to begin treatment in STEMI since time is the most important determinant of outcome. Qualitative tests are not sensitive, so there are chances that it might not diagnose STEMI and treating physician might have to face legal issues for misdiagnosis. Although, high sensitivity troponin can be a much better alternative to qualitative troponin with rapid results in a shorter time window.
Expert consensus
Panelists came to a consensus that time is the most important determinant of outcome in the management of STEMI. A symptom of chest pains along with ST elevation on ECG is sufficient to go ahead with the treatment, preferably PCI in conditions possible and the management on STEMI cannot be relied on biomarkers like troponin even if it is positive. However, despite its limitations, qualitative troponin estimation by stick methods may be useful for diagnosis of STEMI, especially in peripheral settings in cases where the diagnosis of STEMI based on ECG is in doubt. Panel members agreed to the guidelines that in conditions where CAG is not immediately available, two dimensional ECHO may assist in making a diagnosis helping in decision for emergency transfer to a PCI center.
Early risk assessment for patients with ST-elevation myocardial infarction
Various clinical indicators of high risk in the acute phase mentioned in the guidelines include older age, sinus tachycardia, hypotension, Killip class, anterior infarction, previous infarction, elevated initial serum creatinine and history of heart failure. Malignant arrhythmias, persistent chest pain and early angina on minimal exertion are also associated with worse outcome. However, majority of the panel members suggested that it cannot be concluded that inferior MI has a better prognosis than anterior wall MI; even though, it is not mentioned as a high risk factor in the guidelines. Similar findings were observed in shock registry which has shown that 51% of the patients developing shock had inferior wall MI.[13] Several risk scores have been developed, based on readily identifiable parameters in the acute phase before reperfusion.[14,15,16] The American Heart Association guidelines have also mentioned some of the independent predictors of early death from STEMI which include age, Killip class, time to reperfusion, cardiac arrest, tachycardia, hypotension, anterior infarct location, prior infarction, diabetes mellitus, smoking status, renal function, and biomarker findings.[17] Subsequently, the thrombolysis in MI (TIMI) risk score was developed specifically for patients with STEMI.[14] Panelists opined that risk stratification is made mostly to find out the appropriate strategy of management and definitely has an important role in NSTEMI for not only estimating the prognosis but also to assess the outcome following interventions. However, they felt that in STEMI such risk stratifications are unable to predict the outcome of these patients after angioplasty unless we understand the coronary anatomy at that point of time. Hence, clinical risk stratifications do not offer any advantage in assessing the outcome after the procedure of revascularization either by thrombolysis or by interventions. Guidelines have also mentioned about stress imaging, perfusion scintigraphy, stress ECHO, computed tomography angiography, additional electrophysiological testing before discharge if arrhythmia is the main concern, and metabolic risk markers including total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting triglycerides and plasma glucose, as well as renal function. The panelists agreed to the guidelines that obviously a recent onset inferior wall MI without any ST-elevation in precordial leads has a better prognosis compared to a person who has an extensive anterior wall MI, RBBB, significant ST-depression, or with hypertension and diabetes. Killip classification, TIMI risk score, and Global Registry of Acute Coronary Events (GRACE) risk scoring were well accepted by most of the panelists for risk stratification out of which they preferred Killip class being easy and very well validated.[14,18,19] However, further stratification between Killip class 1 and 2 not possible. Panel members opined that the risk stratification is more important to study proper strategy of management and deciding better outcome during discharge. However, immediate risk stratification is not necessary for confirmed diagnosis of STEMI as any ST elevation can be a high risk, requiring management as early as possible.
Expert consensus
In Indian perspective, risk stratification is more important at the time of discharge for assessment of long-term prognosis. However, they are of less importance to predict outcome after revascularization either by thrombolysis or interventions. Killip class, TIMI risk score, and GRACE scoring were well accepted by the panel members with preference to Killip class being very well validated.
Prehospital logistics of care
Delays
It has been established in the guidelines that the prevention of delays is critical in STEMI for two reasons: first, the most critical time of an AMI is the very early phase, during which the patient is often in severe pain and liable to cardiac arrest and second is the provision of therapy, particularly reperfusion therapy, is critical to its benefit. Different delays were mentioned in the guidelines like patient delay, the delay between FMC and diagnosis, the delay between FMC and reperfusion therapy [Table 1].[7] Panel members discussed that in western countries, FMC is usually at home due to the availability of ambulance services, and hence the diagnosis is done early by ECG at home with early transfer to hospital for reperfusion. However, in India, the FMC is usually the hospital, which is even observed in treatment and outcomes of acute coronary syndromes in India (CREATE) registry (where >90% have come in their own vehicle to the hospital due to patient delay, delay in diagnosis, transport delay, as well as decision for management.[20] Majority of panel members felt that this delay is not due to unavailability of cath laboratories as concluded usually. An article by British Medical Journal recommends 3 cath laboratories/million population for a better service to the common population and 5 cath laboratories/million if the risk of CAD in population is high.[21] According to the panelists, many of the states in India (e.g., Kerala) do have sufficient cath laboratories. However, the lack of awareness among people causes a delay in approaching for medical help. Hence, it is very important to create awareness among the lay people as well as in the treating physicians to evaluate the entire time-span of the disease, not only from the FMC but also from the first appearance of symptoms, i.e., the total ischemic time. Definitely, a patient arriving after 10 h of symptom onset is not equivalent to one arriving at 3 h or 4 h. Delays in diagnosis of >24 h after onset of symptoms can also occur due to patients first visiting unqualified doctors or registered medical practitioner, who may not be adequately qualified, and hence may face challenges in ECG diagnosis of STEMI. Various studies have mentioned that if the reperfusion therapy is primary PCI, the goal should be a delay (FMC to wire passage into the culprit artery) of ≤90 min (and, in high-risk cases with large anterior infarcts and early presenters within 2 h, it should be ≤60 min).[22,23] Whereas, as shown Table 1, if the reperfusion therapy is fibrinolysis, the goal is to reduce this delay (FMC to the needle) to ≤30 min. In PCI-capable hospitals, the goal should be to achieve a “door-to-balloon” delay ≤60 min between presentation in the hospital and primary PCI (defined as wire passage into the culprit artery). Majority of the panel members were in agreement with the management guidelines in case of delay. However, they felt that from the patient's perspective, total ischemic time, i.e., the delay between symptom onset and provision of reperfusion therapy (either starting fibrinolysis or passing a wire through the culprit's vessel) is possibly the most important and should be reduced as much as possible.
Table 1.
European Society of Cardiology 2012 summary of important delays and treatment goals in the management of acute ST-segment elevation myocardial infarction
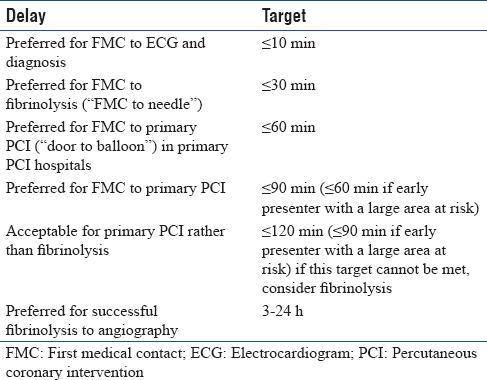
Expert consensus
Panelists agreed to the guidelines with greater emphasis on the total ischemic time which needs to be highlighted. They also recommended that awareness needs to be created among the patients and even treating physicians regarding diagnosis of STEMI; although, it has not been mentioned in the guidelines. In this regard, at population level, media can play a crucial role in creating awareness of symptoms of MI to shorten the time for diagnosis and management.
Reperfusion therapy
Restoring coronary flow and myocardial tissue reperfusion
Guidelines suggest that for patients with the clinical presentation of STEMI within 12 h of symptom onset and with persistent ST-segment elevation or new or presumed new LBBB, early mechanical (PCI) or pharmacological reperfusion should be performed as early as possible. However, there is no consensus as to whether PCI is also beneficial in patients presenting >12 h from symptom onset in the absence of clinical and/or electrocardiographic evidence of ongoing ischemia. A study done by Ndrepepa et al. showed that an invasive strategy based on coronary stenting reduces infarct size better than conservative treatment in patients with acute STEMI without persistent symptoms presenting 12–48 h after symptom onset.[24]
Expert consensus
Panel members agreed on the guidelines for management of STEMI either with PCI or thrombolysis for <12 h; however, majority of them were of the consensus that PCI should be performed in patients presenting >12 h from the symptom onset.
Selection of a strategy for reperfusion
Guidelines recommend that primary PCI without previous fibrinolytic treatment is the preferred reperfusion strategy in patients with STEMI, provided it can be performed expeditiously (i.e., within guideline-mandated times), by an experienced team, and regardless of whether the patient presents to a PCI-capable hospital.[7] In settings where primary PCI cannot be performed within 120 min of FMC by an experienced team, fibrinolysis should be considered, particularly if it can be given prehospital (e.g., in the ambulance) and within the first 120 min of symptom onset which should be followed by consideration of rescue PCI or routine angiography.[7] Guidelines suggest that primary PCI (wire passage) should be performed within 90 min after FMC in all cases.[7] In patients presenting early, with a large amount of myocardium at risk, the delay should be shorter (<60 min). In patients presenting directly in a PCI-capable hospital, the goal should also be to achieve primary PCI within 60 min of FMC.[7] Panel members suggested that in India, if the patient arrives at a PCI capable center or at non-PCI center in >3 h, PCI is recommended treatment option and the patient should be transferred to a PCI capable center for the same in case of arrival of the patient to a non-PCI center. A PCI approach is still recommended if the patient arrives at a non-PCI capable center with surety to reach PCI center within 30 min [Figure 2]. In a case where the time taken to reach the PCI center is between 30 and 60 min, panel recommended to take the total ischemic time from the symptom onset, if it is <3 h, thrombolysis should be performed and if it is >3 h, patient should be transferred to the PCI center [Table 2]. However, the factors which have to be taken into consideration in India is that the timings to reach hospitals may vary according to the conditions. In India, there are certain PCI equipped centers where PCI is performed only in daytime and are not available in night time which may be due to unavailability of cardiologists at night. Hence, panel suggested 3 categories of hospitals, i.e., non-PCI, PCI in daytime only and PCI round the clock. Panelists felt that unlike western countries, in India, every single patient of MI should be sent to a PCI capable center, as even in cases where there is a considerable delay, a delayed PCI is better than not performing a PCI. Also, mechanical complications are detected better by this approach as most non-PCI centers are physician-managed while the PCI centers are managed by cardiologists. However, if the time required to reach a PCI center is >30 min then pharmacoinvasive approach which involves initial thrombolysis followed by PCI is recommended as also observed in the strategic reperfusion early after myocardial infarction trial.[25] The Indian data from the tenecteplase facilitated PCI versus primary PCI in Indian patients with STEMI (STEPP-AMI) study also shows that the pharmacoinvasive strategy compares well with primary PCI in reducing overall morbidity and mortality.[26] The pilot Kovai Erode Study and the subsequent Pilot Tamil Nadu STEMI program have shown the feasibility of combining the two strategies of primary PCI and thrombolysis.[27,28] Pharmacoinvasive approach is also a preferred option in rural areas where nearby PCI capable center is not available. In India, almost 60% of the population lives in rural areas which lack PCI capable hospitals, and hence, pharmaco-invasive approach becomes very important in a country like India. Fibrinolytic therapy is recommended within 12 h of symptom onset if primary PCI cannot be performed within 90 min of being able to administer fibrinolysis and within 120 min from FMC and there are no contraindications. It is recommended that patients undergoing primary PCI should receive a combination of dual anti-platelet therapy with aspirin and an adenosine diphosphate receptor blocker, as early as possible before angiography, and a parenteral anticoagulant.
Figure 2.
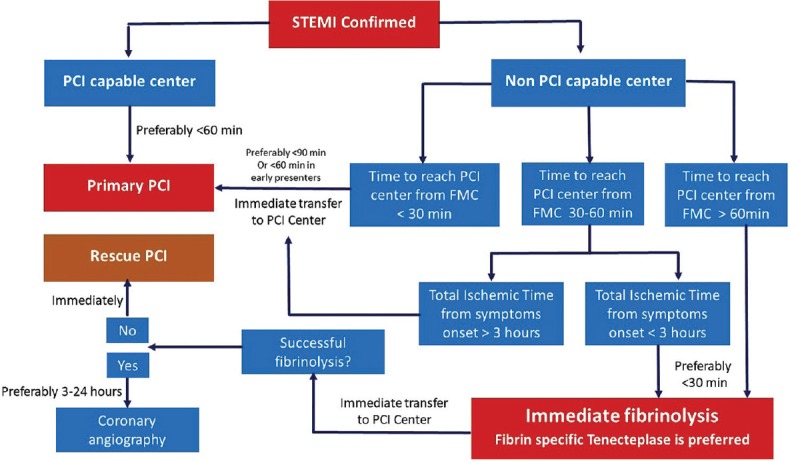
Consensus algorithm of reperfusion strategies
Table 2.
Guidelines for the management of ST-elevation myocardial infarction
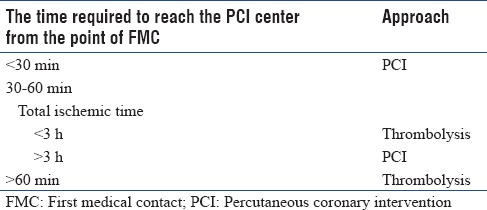
Expert consensus
The panel members agreed to the guidelines with PCI as the choice of reperfusion therapy, whereas fibrinolysis, especially the pharmacoinvasive approach to be recommended in those settings where primary PCI cannot be offered to STEMI patients within the recommended timelines.
Comparison of fibrinolytic agents
Guidelines have discussed the global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries trial, which has observed that tissue plasminogen activator (tPA; alteplase) with concomitant activated partial thromboplastin time adjusted i.v. ultra-fractionated heparin resulted in 10 fewer deaths per 1000 patients treated when compared to streptokinase.[29] However, a double-bolus r-PA (reteplase) does not offer any advantage over accelerated tPA, except for its ease of administration, as observed in a previous study.[30] Comparatively, a single-bolus, weight-adjusted TNK-tPA (tenecteplase) is equivalent to accelerated tPA for 30-day mortality with a significantly lower rate of noncerebral bleedings and lower need for blood transfusion.[31] Furthermore, bolus fibrinolytic therapy is easier to use in the prehospital setting.
Panelists discussed the choice of the fibrinolytic. They discussed a cross-sectional retrospective analysis where streptokinase was used in 93% of patients of the 20% of STEMI patients who were thrombolysed, which have shown lower overall 1 year mortality rate (2.5%) with interventional approach or pharmacotherapy.[32] They were of the opinion that although streptokinase is cheap and tenecteplase is costly, the use of tenecteplase as a single bolus has better TIMI scores which will especially be useful in a country India where already a greater delay is observed from symptom onset to management. It has also been observed in a previous study that better TIMI scores have resulted in better protection of myocardium.[33] They also mentioned the consensus document by Thygesen K et al., which highlights the importance of thrombolysis.[34] As shown in Table 3, along with the lower efficacy, streptokinase also takes about 30–60 min for administration, while tenecteplase can be administered in 5 s as a single bolus, hence saving a lot of time.[35,36] Panelists considered tenecteplase to be a safe and efficacious option for thrombolysis in STEMI [Table 4].[7]
Table 3.
Recommended doses of fibrinolytics
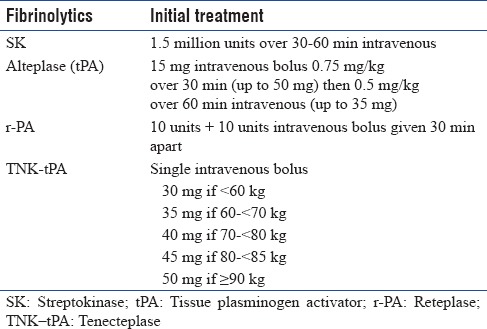
Table 4.
Contra-indications for fibrinolysis
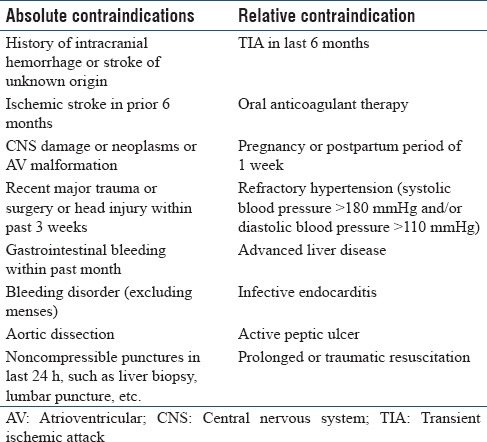
Expert consensus
For better efficacy and patient outcome, tenecteplase should be included in the guidelines as the molecule of choice for thrombolysis which can also bring down the cost in the long run. Pharmacoinvasive therapy is the answer for Indian population with the anticipated delay in PCI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the Medical team of Emcure Pharmaceuticals Ltd., Pune, for the concept development, compilation of data, and manuscript authoring.
REFERENCES
- 1.Ghaffar A, Reddy KS, Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328:807–10. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delhi, India: Ministry of Health and Family Welfare, Government of India; 2005. [Last accessed on 2017 Mar 04]. National Commission on Macroeconomics and Health. Burden of Disease in India. Available from: http://www.who.int/macrohealth/action/NCMH_Burden%20of%20disease(2%20Sep%202005).pdf . [Google Scholar]
- 3.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. Antman EM, Hand M, Armstrong PW, Bates ER, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2008;51:210–47. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2009;120:2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 6.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: The task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–45. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 7.Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology (ESC) Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 8.Dalal JJ, Alexander T, Banerjee PS, Dayasagar V, Lyengar SS, Kerkar PG, et al. 2013 consensus statement for early reperfusion and pharmaco-invasive approach in patients presenting with chest pain diagnosed as STEMI (ST elevation myocardial infarction) in an Indian setting. J Assoc Physicians India. 2014;62:473–83. [PubMed] [Google Scholar]
- 9.Brieger D, Eagle KA, Goodman SG, Steg PG, Budaj A, White K, et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: Insights from the global registry of acute coronary events. Chest. 2004;126:461–9. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 10.Sgarbossa EB, Pinski SL, Barbagelata A, Underwood DA, Gates KB, Topol EJ, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries) investigators. N Engl J Med. 1996;334:481–7. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 11.Widimsky P, Rohác F, Stásek J, Kala P, Rokyta R, Kuzmanov B, et al. Primary angioplasty in acute myocardial infarction with right bundle branch block: Should new onset right bundle branch block be added to future guidelines as an indication for reperfusion therapy? Eur Heart J. 2012;33:86–95. doi: 10.1093/eurheartj/ehr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babuin L, Jaffe AS. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173:1191–202. doi: 10.1503/cmaj.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochman JS, Buller CE, Sleeper LA, Boland J, Dzavik V, Sanborn TA, et al. Cardiogenic shock complicating acute myocardial infarction – Etiologies, management and outcome: A report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36:1063–70. doi: 10.1016/s0735-1097(00)00879-2. [DOI] [PubMed] [Google Scholar]
- 14.Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–7. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 15.Fox KA, Anderson FA, Jr, Dabbous OH, Steg PG, López-Sendón J, Van de Werf F, et al. Intervention in acute coronary syndromes: Do patients undergo intervention on the basis of their risk characteristics? The global registry of acute coronary events (GRACE) Heart. 2007;93:177–82. doi: 10.1136/hrt.2005.084830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KL, Woodlief LH, Topol EJ, Weaver WD, Betriu A, Col J, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I investigators. Circulation. 1995;91:1659–68. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 17.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 18.El-Menyar A, Zubaid M, AlMahmeed W, Sulaiman K, AlNabti A, Singh R, et al. Killip classification in patients with acute coronary syndrome: Insight from a multicenter registry. Am J Emerg Med. 2012;30:97–103. doi: 10.1016/j.ajem.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 20.Xavier D, Pais P, Devereaux PJ, Xie C, Prabhakaran D, Reddy KS, et al. Treatment and outcomes of acute coronary syndromes in India (CREATE): A prospective analysis of registry data. Lancet. 2008;371:1435–42. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 21.Schömig A, Mehilli J, Antoniucci D, Ndrepepa G, Markwardt C, Di Pede F, et al. Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours from symptom onset: A randomized controlled trial. JAMA. 2005;293:2865–72. doi: 10.1001/jama.293.23.2865. [DOI] [PubMed] [Google Scholar]
- 22.Steg PG, Bonnefoy E, Chabaud S, Lapostolle F, Dubien PY, Cristofini P, et al. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: Data from the CAPTIM randomized clinical trial. Circulation. 2003;108:2851–6. doi: 10.1161/01.CIR.0000103122.10021.F2. [DOI] [PubMed] [Google Scholar]
- 23.Pinto DS, Kirtane AJ, Nallamothu BK, Murphy SA, Cohen DJ, Laham RJ, et al. Hospital delays in reperfusion for ST-elevation myocardial infarction: Implications when selecting a reperfusion strategy. Circulation. 2006;114:2019–25. doi: 10.1161/CIRCULATIONAHA.106.638353. [DOI] [PubMed] [Google Scholar]
- 24.Ndrepepa G, Kastrati A, Mehilli J, Antoniucci D, Schömig A. Mechanical reperfusion and long-term mortality in patients with acute myocardial infarction presenting 12 to 48 hours from onset of symptoms. JAMA. 2009;301:487–8. doi: 10.1001/jama.2009.32. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong PW, Gershlick A, Goldstein P, Wilcox R, Danays T, Bluhmki E, et al. The strategic reperfusion early after myocardial infarction (STREAM) study. Am Heart J. 2010;160:30–50. doi: 10.1016/j.ahj.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Victor SM, Subban V, Alexander T, Bahuleyan CG, Srinivas A, Selvamani S, et al. A prospective, observational, multicentre study comparing tenecteplase facilitated PCI versus primary PCI in Indian patients with STEMI (STEPP-AMI) Open Heart. 2014;1:e000133. doi: 10.1136/openhrt-2014-000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander T, Mehta S, Mullasari A, Nallamothu BK. Systems of care for ST-elevation myocardial infarction in India. Heart. 2012;98:15–7. doi: 10.1136/heartjnl-2011-301009. [DOI] [PubMed] [Google Scholar]
- 28.Alexander T, Victor SM, Mullasari AS, Veerasekar G, Subramaniam K, Nallamothu BK, et al. Protocol for a prospective, controlled study of assertive and timely reperfusion for patients with ST-segment elevation myocardial infarction in Tamil Nadu: The TN-STEMI programme. BMJ Open. 2013;3:e003850. doi: 10.1136/bmjopen-2013-003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–82. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 30.Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) Investigators. A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med. 1997;337:1118–23. doi: 10.1056/NEJM199710163371603. [DOI] [PubMed] [Google Scholar]
- 31.Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators. Van De Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: The ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716–22. doi: 10.1016/s0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]
- 32.Žerpytis P, Bilkis V, Katliorius R, Kūgienė R, Žvironaitė V, Katkus R, et al. Treatment of acute STEMI with thrombolysis: Tenecteplase vs.streptokinase. Biomedicina. 2012;22:110–3. [Google Scholar]
- 33.Isezuo S, Subban V, Krishnamoorthy J, Pandurangi UM, Janakiraman E, Kalidoss L, et al. Characteristics, treatment and one-year outcomes of patients with acute coronary syndrome in a tertiary hospital in India. Indian Heart J. 2014;66:156–63. doi: 10.1016/j.ihj.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White DH. the Writing Group on Behalf of the Joint ESC/ACCF/AHA/ WHF Task Force for the Universal Definition of Myocardial Infarction (Expert Consensus Document). Third universal definition of myocardial infarction. Circulation. 2012:126. [Google Scholar]
- 35.Otaal PS, Talwar KK. Limitations of currently available thrombolytic therapy. Indian Heart J. 2009;61:470–5. [PubMed] [Google Scholar]
- 36.Melandri G, Vagnarelli F, Calabrese D, Semprini F, Nanni S, Branzi A, et al. Review of tenecteplase (TNKase) in the treatment of acute myocardial infarction. Vasc Health Risk Manag. 2009;5:249–56. doi: 10.2147/vhrm.s3848. [DOI] [PMC free article] [PubMed] [Google Scholar]


