Abstract
Background
Suitable and scalable in vitro culture conditions for parasite maintenance are needed to foster drug research for loiasis, one of the neglected tropical diseases which has attracted only limited attention over recent years, despite having important public health impacts. The present work aims to develop adequate in vitro culture systems for drug screening against both microfilariae (mf) and infective third-stage larvae (L3) of Loa loa.
Methods
In vitro culture conditions were evaluated by varying three basic culture media: Roswell Park Memorial Institute (RPMI-1640), Dulbecco’s modified Eagle’s medium (DMEM) and Iscove’s modified Dulbecco’s medium (IMDM); four sera/proteins: newborn calf serum (NCS), foetal bovine serum (FBS), bovine serum albumin (BSA) and the lipid-enriched BSA (AlbuMax® II, ALB); and co-culture with the Monkey Kidney Epithelial Cell line (LLC-MK2) as a feeder layer. The various culture systems were tested on both mf and L3, using survival (% motile), motility (T90 = mean duration (days) at which at least 90% of parasites were fully active) and moulting rates of L3 as the major criteria. The general linear model regression analysis was performed to assess the contribution of each variable on the viability of Loa loa L3 and microfilarie. All statistical tests were performed at 95% confidence interval.
Results
Of the three different media tested, DMEM and IMDM were the most suitable sustaining the maintenance of both L. loa L3 and mf. IMDM alone could sustain L3 for more than 5 days (T90 = 6.5 ± 1.1 day). Serum supplements and LLC-MK2 co-cultures significantly improved the survival of parasites in DMEM and IMDM. In co-cultures with LLC-MK2 cells, L. loa mf were maintained in each of the three basic media (T90 of 16.4–19.5 days) without any serum supplement. The most effective culture systems promoting significant moulting rate of L3 into L4 (at least 25%) with substantial maintenance time were: DMEM + BSA, DMEM + NCS, DMEM-AlbuMax®II, DMEM + FBS all in co-culture with LLC-MK2, and IMDM + BSA (1.5%), DMEM + FBS (10%) and DMEM + NCS (5%) without feeder cells. DMEM + 1% BSA in co-culture scored the highest moulting rate of 57 of 81 (70.37%). The factors that promoted L. loa mf viability included feeder cells (β = 0.490), both IMDM (β = 0.256) and DMEM (β = 0.198) media and the protein supplements NCS (β = 0.052) and FBS (β = 0.022); while for L. loa L3, in addition to feeder cells (β = 0.259) and both IMDM (β = 0.401) and DMEM (β = 0.385) media, the protein supplements BSA (β = 0.029) were found important in maintaining the worm motility.
Conclusions
The findings from this work display a range of culture requirements for the maintenance of Loa loa stages, which are suitable for developing an effective platform for drug screening.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2852-2) contains supplementary material, which is available to authorized users.
Keywords: Loa loa, L3 larvae, Microfilariae, In vitro culture system, Viability, Moulting
Background
Loiasis is a parasitic disease caused by the filarial nematode Loa loa that is transmitted through the bite of an infected Chrysops fly. Loiasis is endemic in the rainforest areas of West and Central Africa [1]. The common clinical signs of loiasis are the subconjunctival migration of the adult worm, reported for the first time by Mongin in 1770 [2], Calabar Swelling, pruritis, oedemas and arthralgia. Interest in this filarial species, which has long been considered to be less pathogenic than related species [3], came from several reports in Cameroon indicating that high microfilaraemia of L. loa is associated with severe and sometimes fatal encephalopathic reactions in patients who had taken ivermectin for onchocerciasis treatment [4–7]. Loiasis is a neglected tropical disease (NTD) which has attracted only limited attention in drug research and development. Apart from surgical removal of adult worms moving under the skin or across the eye that can be done to relieve anxiety, only two medications have so far been employed for clinical cases since the last century, namely diethylcarbamazine (DEC) and albendazole. The latter is sometimes used in patients who are not cured with multiple DEC treatments. Several cases of brain inflammation, coma and death have been reported in people with heavy infections when they are treated with DEC [8, 9]. The risk of side effects has limited the deployment of mass drug administration of ivermectin in areas where the L. loa prevalence exceeds 20% [10–12], impeding the goals stated by the African Programme for Onchocerciasis Control (APOC) in areas of co-endemicity. Progress in drug research and development for loiasis requires suitable screening systems both at in vitro and in vivo levels. Though innovations in filarial animal models have recently been achieved [13, 14], in vitro maintenance systems of the different stages of L. loa have not been established. The present study aimed to design suitable in vitro culture systems for drug screening against both infective larvae (L3) and microfilariae (mf) of L. loa.
Methods
Isolation and purification of L. loa L3
Loa loa L3 were obtained from dissected Chrysops flies that had previously fed on a consented microfilaremic individual at Ediki Forest (South West region, Cameroon). Engorged Chrysops were kept in captivity for 12 days, to allow development to the infective stage (L3). The flies were fed daily with 15% sucrose solution soaked in cotton wool. After 12 days of rearing, the flies were dissected in Petri dishes containing RPMI 1640 medium (Sigma-Aldrich, St Louis, USA). The head, thorax and abdomen were separated and teased apart in three different Petri dishes. Fly tissues were incubated for 20 min to allow L3 larvae to migrate out. A sterile pipette was used to pick the larvae and pooled in a shallow convex glass dish [15]. The worms were transferred into 15 ml centrifuge tubes (Corning, Kennebunk-ME, USA) for purification. Only L3 harvested from the head (where more mature larvae are expected to be found) were used in this study. The remaining larvae were frozen to be used in other studies (immunology and molecular biology). The L3 were washed using a Percoll® (GE Healthcare, Pharmacia, Uppsala, Sweden) technique. The L3 suspension concentrated in less than 1 ml RPMI was slowly layered on the surface of a 15 ml tube containing stock iso-osmotic Percoll® and centrifuged (Humax 14k human, Germany) at 800× rpm for 10 min. The process was repeated to remove microbial contaminants. At the end, the L3 were washed twice with RPMI-1640 by centrifugation at 1500× rpm for 10 min to remove Percoll® remnant.
Isolation and purification of L. loa mf
Loa loa mf were obtained from baboons (Papio anubis) experimentally infected with human strain of L. loa reared in Kumba Medical Research Station (South West region, Cameroon). Peripheral blood samples of hypermicrofilaraemic baboons were collected as described in the previous reports [13]. Microfilaraemic loads were determined microscopically on thick films. Calibrated thick blood smears were prepared by spreading a 50 μl venous blood sample from a 75 μl non-heparinised capillary tube, onto a clean slide over an area of 1.5 × 2.5 cm [16]. After drying, films were dehemoglobinized and stained with Giemsa. The Percoll® density centrifugation method previously described [17] was used to purify mf from infected blood samples.
In vitro culture of parasites
Four supplements were used at 3 concentrations each: fetal bovine serum (Lonza) and newborn calf serum (Sigma-Aldrich, Berlin, Germany) at 15%, 10% and 5%; bovine serum albumin (Sigma-Aldrich, Berlin, Germany) and AlbuMax® II (Gibco Life Technologies, Cergy-Pontoise, France) at 1.5%, 1% and 0.5%. Three basic media were used: RPMI-1640 and IMDM (Sigma-Aldrich, St Louis, USA) and DMEM (Gibco Life Technologies, Cergy-Pontoise, France). Ciprofloxacin (5 μg/ml) was used as antibiotic and fluconazole (10 μg/ml) as antifungal. Flat bottom culture plates (48-well) with lids (Corning, Kennebunk, ME, USA) were loaded as follows: 800 μl of the different media with a range of 20–30 microfilariae or 10–15 larvae per well. Cultures were carried out in triplicates.
Monkey kidney cell co-culture
Monkey kidney epithelial cells (LLC-MK2) (ATCC, USA) were cultured in flasks at 37 °C in a CO2 incubator (Sheldon Mfg. Inch, Cornelius, OR, USA) at 5% CO2 until the cell layer became fully confluent. For new inoculations and other cell manipulations, trypsin was used to detach cells from the walls of the flasks. Cells were then dislodged with trypsin solution (25%) containing EDTA, the mixture was kept at 37 °C for less than 1 h. The cell suspension was centrifuged at 1,500 rpm for 10 min, the supernatant was discarded, and the pellet re-suspended and diluted to 105 cells/ml in complete culture medium. Aliquots (100 μl) of cell suspensions were plated into a 48-well culture plate and kept in the incubator for cells to become fully confluent.
Assessment of parasite viability
The viability of the parasites was assessed daily, by visual inspection (by two individuals) under an inverted microscope until they die. Their motility was scored on a 4-point scale [18, 19]: 0, no movement or immotile; 1, intermittent shaking of head and tail; 2, sluggish (shaking of the whole worm on a spot); 3, vigorous movement (shaking of the whole worm and migration from one spot to was considered).
Data processing and analysis
Three different batches of L3 larvae and microfilariae were used for each culture system. For each batch of parasites, 4 replicate wells were used per system. Raw data collected daily on record sheets were entered into a template designed on Microsoft Excel 2007. Three variables were defined and computed to assess the viability of the parasites (mean motility and mean mortality, T90).
Motility variable was computed based on the scoring system described above, and using the following formula.
where Si is the score of point scale i and Ni is the total number of worms at a point scale i.
The variable T90 was defined as the duration at which 90% of the worms were still fully active (score 3 above) in the well. This variable was set as one of the major indicators of the suitability of the culture system, with relevance to drug screening for loiasis. From values obtained after testing each system on three batches of parasites, T90 values were expressed as mean ± standard deviation.
The Kruskal-Wallis test was used to assess the global significant differences between the distribution of the median T90 across media and supplements, and the pairwise multiple comparisons of the ranked data was performed using the Pairwise Multiple Comparisons of Mean Rank (PCMR) package in R version 3.1.4. Mann-Whitney U-test was used to compare the the medians of the T90 between the cell free and cell containing culture. Statistical tests were interpreted using a 5% significance level. It was considered that a valid appreciation of the effect of any drug could be possible only in a system where at least 90% of parasites motility were sustained till the end of the experiment.
Factors that promoted parasite survival were identified using the multiple linear regression. The general linear model (GLM) was built using the hierarchical stepwise method. A total of 5 blocks were achieved with the 5 factors (incubation time, presence of feeder cells, basic medium, serum/protein, protein concentration) and those that contributed significantly to the improvement of the model were identified based on the F-statistics and the adjusted R-square (Additional file 1: Table S1). The incubation time was treated as a metric factor. Dichotomous variables such as the presence of monkey kidney cells were coded using binary figures. For each nominal or ordinal factor (Basic culture media, protein or protein concentration), sets of dummy variables were created and compared to one of the categories defined as reference. While RPMI-1640 was used as a reference against DMEM and IMDM, the four sera (Albumax II, BSA, FBS and NCS) were compared to the serum free culture (No serum). The three concentrations of each serum (0.5%, 1% and 1.5% for Albumax II and BSA; 5%, 10% and 15% for FBS and NCS) were labelled using the three ordinal levels: low, medium and high concentration; they were also compared to the serum-free culture. Interaction factors were created between explanatory variables and added to the models. The prediction of the motility by the protein concentration was poor, and there was a non-statistical difference between different concentrations of protein as will be discussed latter. Based on experimental observations, interactions were expected between the other three variables. Therefore, two ways and three ways interaction terms were created between those three experimental parameters: the presence of feeder cells, the culture medium used in reference to RPMI1640 and the type of protein supplement.
The passage of the L. loa larvae from the third (L3) to the fourth (L4) stages was further considered the second target product profile in assessing the suitability of the culture systems tested. For each of the 78 culture systems designed and evaluated, the moulting rate (percentage of moulted worms) as well as the timeframe was computed.
Results
Evaluation of the effect of each basic culture medium on the viability of L. loa L3 and mf
The T90 values of the parasite motility in different basic media were evaluated and results are presented in Fig. 1 and the statistical report in Table 1. The values of T90 ranged from 3.0 day to 6.5 days for L3. In the absence of protein supplement and feeder cells, there were significant difference between the T90 value of the 3 media vis a vis the L3 (χ2 = 38.793, df = 2, P < 0.0001) and mfs (χ2 = 38.793, df = 2, P < 0.0001). Pairwise comparison indicated that L3 survived longer particularly in IMDM compared to DMEM (P < 0.0001) and RPMI (P < 0.0001), whereas mf hardly exceeded 3 days survival, irrespective of the culture medium tested in absence of supplements. However, mf survival time was significantly higher in DMEM than in RPMI (P < 0.0001) or IMDM (P = 0.0010).
Fig. 1.

Effect of each basic medium on the mean values of T90 of L. loa L3 (a) and microfilarae (b). Microfilariae and infective larvae of Loa loa were cultured with three different media (RPMI, DMEM and IMDM) without neither serum supplement, nor feeder cells. Pairwise multiple comparisons: Dunn’s post-hoc test for multiple comparisons of independent samples. The P-values indicated here are those that were found significant when comparing two basic media. Number of observations: n = 12. Abbreviations: DMEM, Dulbecco’s modified Eagle’s medium; IMDM, Iscove’s modified Dulbecco’s medium; RPMI, Roosevelt Memorial Park Institute; L3, third-stage infective larva; T90, mean duration at which 90% of parasites were fully active
Table 1.
Statistical report on the effect of serum/protein concentration in various culture systems with regards to the mean values of T90 of L. loa microfilariae and L3
| Parasite | Feeder layer | Medium | Protein | Kruskal-Wallis χ2 | df | P-value |
|---|---|---|---|---|---|---|
| Loa loa mf | No | DMEM | Albumax | 4.2585 | 3 | 0.2349 |
| BSA | 9.1681 | 3 | 0.0271 | |||
| FBS | 9.6542 | 3 | 0.0218 | |||
| NCS | 9.5639 | 3 | 0.0227 | |||
| IMDM | Albumax | 16.1960 | 3 | 0.0010 | ||
| BSA | 22.8860 | 3 | < 0.0001 | |||
| FBS | 19.1060 | 3 | 0.0003 | |||
| NCS | 23.1030 | 3 | < 0.0001 | |||
| RPMI | Albumax | 20.5860 | 3 | 0.0001 | ||
| BSA | 17.1480 | 3 | 0.0007 | |||
| FBS | 7.5795 | 3 | 0.0556 | |||
| NCS | 11.3590 | 3 | 0.0099 | |||
| LLC-MK2 | DMEM | Albumax | 6.9561 | 3 | 0.0733 | |
| BSA | 6.1987 | 3 | 0.1023 | |||
| FBS | 4.9629 | 3 | 0.1745 | |||
| NCS | 6.1198 | 3 | 0.1059 | |||
| IMDM | Albumax | 0.7759 | 3 | 0.8552 | ||
| BSA | 1.7466 | 3 | 0.6266 | |||
| FBS | 3.0427 | 3 | 0.3851 | |||
| NCS | 0.8119 | 3 | 0.8466 | |||
| RPMI | Albumax | 16.8520 | 3 | 0.0008 | ||
| BSA | 14.6670 | 3 | 0.0021 | |||
| FBS | 0.3286 | 3 | 0.9546 | |||
| NCS | 2.3611 | 3 | 0.5009 | |||
| Loa loa L3 | No | DMEM | Albumax | 7.0250 | 3 | 0.0711 |
| BSA | 8.3441 | 3 | 0.0394 | |||
| FBS | 8.1322 | 3 | 0.0434 | |||
| NCS | 6.0725 | 3 | 0.1081 | |||
| IMDM | Albumax | 1.7999 | 3 | 0.6150 | ||
| BSA | 1.8722 | 3 | 0.5993 | |||
| FBS | 3.8436 | 3 | 0.2789 | |||
| NCS | 9.6063 | 3 | 0.0222 | |||
| RPMI | Albumax | 8.4292 | 3 | 0.0379 | ||
| BSA | 4.2135 | 3 | 0.2393 | |||
| FBS | 6.5292 | 3 | 0.0885 | |||
| NCS | 1.4905 | 3 | 0.6845 | |||
| LLC-MK2 | DMEM | Albumax | 1.8177 | 3 | 0.6111 | |
| BSA | 1.3618 | 3 | 0.7145 | |||
| FBS | 5.8987 | 3 | 0.1166 | |||
| NCS | 1.6916 | 3 | 0.6388 | |||
| IMDM | Albumax | 16.0890 | 3 | 0.0011 | ||
| BSA | 5.2627 | 3 | 0.1535 | |||
| FBS | 2.1496 | 3 | 0.5419 | |||
| NCS | 4.9566 | 3 | 0.1750 | |||
| RPMI | Albumax | 6.2386 | 3 | 0.1006 | ||
| BSA | 5.5548 | 3 | 0.1354 | |||
| FBS | 4.0227 | 3 | 0.2590 | |||
| NCS | 5.6006 | 3 | 0.1327 |
Evaluation of the effect of serum/protein supplementation on the viability of L. loa larvae and microfilariae in culture
Figure 2 and Fig. 3 show the effect of serum/protein supplements in various basic media with regards to the mean values of T90 of L. loa L3 and mf, respectively.
Fig. 2.

Effect of serum/protein supplements in various basic media with regards to the mean values of T90 of L. loa L3. Infective larvae of Loa loa were cultured with three different media (RPMI, DMEM and IMDM) with each of the four serum/protein supplements [Albumax (a), FBS (b), BSA (c) and NCS (d)] without feeder cells. Pairwise multiple comparisons: Dunn’s post-hoc test for multiple comparisons of independent samples. The P-values indicated here are those that were found significant when comparing two concentrations of the serum/protein. Number of observations: n = 12. Abbreviations: DMEM, Dulbecco’s modified Eagle’s medium; IMDM, Iscove’s modified Dulbecco’s medium; RPMI, Roosevelt Memorial Park Institute; BSA, bovine serum albumin; FBS, fetal bovine serum; NCS, newborn calve serum; L3, third-stage infective larva; T90, mean duration at which 90% of parasites were fully active
Fig. 3.

Effect of serum/protein supplements in various basic media with regards to the mean values of T90 of L. loa microfilariae. Microfilariae of Loa loa were cultured with three different media (RPMI, DMEM and IMDM) with each of the four serum/protein supplements [Albumax (a), FBS (b), BSA (c) and NCS (d)] without feeder cells. Pairwise multiple comparisons: Dunn’s post-hoc test for multiple comparisons of independent samples. The P-values indicated here are those that were found significant when comparing two concentrations of the serum/protein. Number of observations: n = 12. Abbreviations: DMEM, Dulbecco’s modified Eagle’s medium; IMDM, Iscove’s modified Dulbecco’s medium; RPMI, Roosevelt Memorial Park Institute; BSA, bovine serum albumin; FBS, fetal bovine serum; NCS, newborn calve serum; T90: mean duration at which 90% of parasites were fully active
Effect of serum/protein on the viability of L. loa larvae
AlbuMax® II
Generally, the supplementation of DMEM and IMDM with AlbuMax® II promoted the viability of larvae, increasing T90 by 1.5–2.5-fold. RPMI supplemented with AlbuMax® II inhibited the larvae viability (T90 < 1 day, Fig. 2a).
Fetal bovine serum (FBS)
This serum could support the viability of larvae for up to 8.7 days when IMDM was supplemented with 10% FBS. T90 > 5 days was observed in all concentrations of FBS except in the RPMI supplement medium (Fig. 2b).
Bovine serum albumin (BSA)
All media supplemented with BSA also improved larvae viability with T90 ≥ 5 days, except for DMEM supplemented with 1% BSA and all concentrations of BSA with RPMI (Fig. 2c).
Newborn calf serum (NCS)
Considering media supplemented with NCS, only DMEM with 5% NCS, IMDM with 10/15% NCS had T90 > 5 days (Fig. 2d). Considering the 5-day cut-off point for drug screening, up to twenty culture media formulated based on the two basic culture media (DMEM and IMDM) and the four serum/protein supplements can be exploited for Loa L3 in priority: DMEM with 0.5% AlbuMax® II (T90 = 5.5 ± 1.9), 1% AlbuMax® II (T90 = 6.6 ± 2.4), 1.5% AlbuMax® II (T90 = 6 ± 1.8), 0.5% BSA (T90 = 7.2 ± 0.3), 1.5% BSA (T90 = 5 ± 1.9), 5% FBS (T90 = 6.3 ± 2), 10% FBS (T90 = 7.8 ± 2.2), 5% FBS (T90 = 7.3 ± 2.7), 5% NCS (T90 = 6.4 ± 2.5), and IMDM with 0.5% AlbuMax® II (T90 = 8.5 ± 3.8), 1% AlbuMax® II (T90 = 8 ± 2.5), 1.5% AlbuMax® II (T90 = 6.8 ± 2.5), 0.5% BSA (T90 = 6.7 ± 1.2), 1% BSA (T90 = 6.2 ± 1.5), 1.5% BSA (T90 = 7 ± 2.2), 5% FBS (T90 = 7.1 ± 3), 10% FBS (T90 = 8.7 ± 2.6), 15% FBS (T90 = 7.7 ± 3.1), 10% NCS (T90 = 7.1 ± 2.4), 15% NCS (T90 = 8.1 ± 2.9).
Effect of serum/protein on the viability of L. loa microfilariae
AlbuMax® II
All concentrations of AlbuMax® II supplement improve the viability of mf regardless the basic medium except for RPMI. Although the improvement was noticeable, all T90 were less than 5 days as shown in Fig. 3a.
Fetal bovine serum (FBS)
All concentrations of fetal bovine serum improved parasite viability but T90 > 5 days was reported only with FBS supplemented DMEM and IMDM (Fig. 3b).
Bovine serum albumin (BSA)
Generally, BSA boosted the microfilariae viability. The T90 values above 5 days were reported with all concentrations of BSA supplemented DMEM and IMDM + 0.5% BSA (Fig. 3c).
Newborn calf serum (NCS)
With respect to media supplemented with NCS, only RPMI supplemented with 10% NCS could not sustain the L. loa mf for up to T90 = 5 days. Generally, NCS supplementation improved the L. loa mf viability by up to 4-fold as compared to basic medium without protein supplement (DMEM with 10%NCS, T90 = 12 ± 1 days and DMEM only, T90 = 3 ± 0.4 days), respectively (Fig. 3d).
With respect to Loa mf, none of the media supplemented with AlbuMax® II irrespective of the protein concentration could improve microfilaria viability for up to T90 ≥ 5. Nevertheless, up to eighteen culture media formulations based on all three basic culture media and the four serum/protein supplements had interesting T90 values. These are DMEM with 0.5% BSA (T90 = 7. 3 ± 1.8), 1% BSA (T90 = 6.2 ± 1.4), 1.5% BSA (T90 = 6.7 ± 1.7), 5% FBS (T90 = 6 ± 3.1), 10% FBS (T90 = 7.5 ± 2.9), 15% FBS (T90 = 7.7 ± 1.7), 5% NCS (T90 = 9.3 ± 4.4), 10% NCS (T90 = 12 ± 1), 15% NCS (T90 = 9.6 ± 2.5); IMDM with 0.5% BSA (T90 = 7.4 ± 3.1), 5% FBS (T90 = 11.2 ± 2.6), 10% FBS (T90 = 10.2 ± 1.9), 15% FBS (T90 = 11.5 ± 3.3), 5% NCS (T90 = 6.1 ± 1.2), 10% NCS (T90 = 6.8 ± 1.2), 15% NCS (T90 = 7.5 ± 1.4) and RPMI with 5% NCS (T90 = 5.4 ± 4.9), 15% NCS (T90 = 5.1 ± 3.2).
Assessment of the importance of monkey kidney cells as feeder layer
The findings on the effect of the monkey kidney epithelial cells as feeder on the survival of the L. loa L3 and mf are summarized in Fig. 4 and Fig. 5, respectively and the summary of the statistical report is presented in Table 2. Co-culture of L3 or mf with LLC-MK2 cells significantly improved the longevity of parasites culture with each basic media in the absence of serum/protein supplements. With respect to Loa L3, only DMEM with LLC-MK2 (No serum/protein supplement) enhanced viability by 3-fold (9.8 ± 2.7), as compared to DMEM without serum (T90 of 3 ± 1). It was not the case regarding IMDM with LLC-MK2 (T90 = 5.8 ± 2.4) and RPMI with LLC-MK2 (T90 = 5.2 ± 2). When combining feeder cells with serum/protein supplements, the parasite survival and motility were even more increased, with highest T90 per basic culture medium varying from 10.0 (5% FBS in IMDM) to 17.8 days (5% NCS in DMEM).
Fig. 4.

Effect of monkey kidney cells in various serum/protein supplemented basic media with regards to the mean values of T90 (days) of L. loa L3. The black arrows indicate the T90 values in absence of the feeder cells. Infective larvae of Loa loa were cultured with three different media (RPMI, DMEM and IMDM) with each of the four serum/protein supplements [Albumax (a), FBS (b), BSA (c) and NCS (d)] and (LLC-MK2) feeder cells. For each medium and each concentration of the serum/protein, culture with monkey kidney cells was compared to serum free culture (Mann-Whitney U-test). Number of observations: n = 12. Abbreviations: DMEM, Dulbecco’s modified Eagle’s medium; IMDM, Iscove’s modified Dulbecco’s medium; RPMI, Roosevelt Memorial Park Institute; BSA, bovine serum albumin; FBS, fetal bovine serum; NCS, newborn calve serum; L3, third-stage infective larva; T90, mean duration at which 90% of parasites were fully active; ¥: significantly different from the equivalent cell free culture
Fig. 5.

Effect of monkey kidney cells in various serum/protein supplemented basic media with regards to the mean values of T90 of L. loa microfilariae. Microfilariae of Loa loa were cultured with three different media (RPMI, DMEM and IMDM) with each of the four serum/protein supplements [Albumax (a), FBS (b), BSA (c) and NCS (d)] and (LLC-MK2) feeder cells. For each medium and each concentration of the serum/protein, culture with monkey kidney cells was compared to serum free culture (Mann-Whitney U-test). Number of observations: n = 12. Abbreviations: DMEM, Dulbecco’s modified Eagle’s medium; IMDM, Iscove’s modified Dulbecco’s medium; RPMI, Roosevelt Memorial Park Institute; BSA, bovine serum albumin; FBS, fetal bovine serum; NCS, newborn calve serum; T90, mean duration at which 90% of parasites were fully active; ¥, significantly different from the equivalent cell free culture
Table 2.
Summary statistics on the effect of the addition of monkey kidney cells in various serum/protein supplemented basic media with regards to the mean values of T90 of L. loa L3 and microfilariae
| Medium | Serum/ Protein | Concentration (%) | Loa loa L3 | Loa loa mf | ||
|---|---|---|---|---|---|---|
| Mann-Whitney U | P-value | Mann-Whitney U | P-value | |||
| DMEM | No serum | 32 | 0.0084 | 24 | 0.0139 | |
| Albumax | 0.5 | 16 | 0.0452 | 12 | 0.0471 | |
| 1 | 16 | 0.0476 | 35 | 0.0025 | ||
| 1.5 | 21 | 0.0467 | 16 | 0.0286 | ||
| BSA | 0.5 | 15 | 0.0167 | 12 | 0.0471 | |
| 1 | 19 | 0.0171 | 48 | 0.0006 | ||
| 1.5 | 14 | 0.0462 | 32 | 0.0040 | ||
| FBS | 5 | 6 | 0.5462 | 15 | 0.0357 | |
| 10 | 15 | 0.0167 | 24 | 0.0095 | ||
| 15 | 12 | 0.0448 | 35 | 0.0025 | ||
| NCS | 5 | 15 | 0.0167 | 6 | 0.0200 | |
| 10 | 22 | 0.0381 | 20 | 0.0159 | ||
| 15 | 24 | 0.0095 | 20 | 0.0158 | ||
| IMDM | No serum | 25 | 0.4945 | 56 | 0.0003 | |
| Albumax | 0.5 | 20.5 | 0.7479 | 54 | 0.0004 | |
| 1 | 36 | 0.0137 | 80 | 0.0004 | ||
| 1.5 | 21.5 | 0.9999 | 70 | 0.0001 | ||
| BSA | 0.5 | 14.5 | 0.6303 | 36 | 0.0069 | |
| 1 | 32 | 0.0345 | 96 | 0.0002 | ||
| 1.5 | 25 | 0.9497 | 96 | 0.0002 | ||
| FBS | 5 | 26 | 0.024 | 18 | 0.0257 | |
| 10 | 12 | 0.1419 | 91 | 0.0003 | ||
| 15 | 11 | 0.1079 | 72 | 0.0490 | ||
| NCS | 5 | 31 | 0.0411 | 54 | 0.0004 | |
| 10 | 38 | 0.0813 | 96 | < 0.0001 | ||
| 15 | 31 | 0.0414 | 96 | < 0.0001 | ||
| RPMI | No serum | 26 | 0.0109 | 98 | 0.0003 | |
| Albumax | 0.5 | 14 | 0.2413 | 32.5 | 0.3170 | |
| 1 | 20 | 0.1087 | 83 | 0.0685 | ||
| 1.5 | 10 | 0.7483 | 86 | 0.1764 | ||
| BSA | 0.5 | 6 | 0.9999 | 40 | 0.002 | |
| 1 | 9 | 0.4000 | 77 | < 0.0001 | ||
| 1.5 | 17 | 0.0111 | 77 | 0.0006 | ||
| FBS | 5 | 15 | 0.3571 | 35 | 0.0057 | |
| 10 | 16 | 0.2857 | 56 | < 0.0001 | ||
| 15 | 16 | 0.1905 | 91 | 0.0004 | ||
| NCS | 5 | 6.5 | 0.9999 | 29 | 0.0282 | |
| 10 | 9 | 0.4000 | 88 | 0.0003 | ||
| 15 | 13 | 0.2000 | 88 | 0.0003 | ||
Co-culture of microfilariae with feeder cells increased T90 values up to 7-fold (22.5 ± 2.7 days). All serum/protein supplemented systems in co-culture were suitable (T90 significantly greater than 5 days) with DMEM and IMDM; but RPMI supplemented with AlbuMax® II remained sub-standard despite the presence of LLC-MK2 feeder layer. Microfilariae co-cultured on LLC-MK2 in basic culture media alone T90 values (DMEM T90 = 19.5 ± 2.8; IMDM T90 = 19.5 ± 5.2; and RPMI T90 = 16.4 ± 1.7) were not statistically different from their best protein/serum supplemented counterpart (0.5% BSA supplemented DMEM on LLC-MK2 T90 = 22.5 ± 2.7; 5% NCS supplemented IMDM on LLC-MK2 T90 = 20.3 ± 4.9; and 1% BSA supplemented RPMI on LLC-MK2 T90 = 20.8 ± 1.6). The ranking of the observed T90 values of the various tested systems are summarised in Additional file 2: Table S2.
Effect of the different culture systems on the moulting from L3 to the fourth-stage larvae of L. loa
Moulting was observed following L. loa L3 culture, but its occurrence varied widely with culture conditions with values up to 70.37% (in 1% BSA supplemented DMEM in co-culture with LLC-MK2). Variation in moulting rate are presented in Figs. 6, 7 and 8, Additional file 3: Table S3, with an illustration in Fig. 9. The proportion of moulting recorded with RPMI was below 25%. This contrasted with DMEM and IMDM where significant moulting rates were noted, both in cell-free and co-culture of L3 with LLC-MK2. Apart from the isolated case of IMDM with up to 27.27% (24 moulted worms out of a total of 88), serum/protein supplementation was found indispensable for the transition from L3 to L4 in vitro. The higher moulting rates were generally observed with DMEM, compared to IMDM. The highest moulting rate was observed in protein and feeder layer supplemented DMEM as illustrated in Fig. 6. Loa loa L3 moulting began on day 9 and observation was continued until day 29, supplementation with BSA scored highest moulting rates, with 57.32, 70.37 and 58.62% larvae moulted in 0.5, 1 and 1.5% BSA supplement, respectively.
Fig. 6.

Effects of serum/protein supplements at different concentrations added to DMEM basic medium with or without feeder layer on the moulting L. loa infective larvae in culture. Number of observations: n = 12. Abbreviation: DMEM: Dulbecco’s modified Eagle’s medium
Fig. 7.
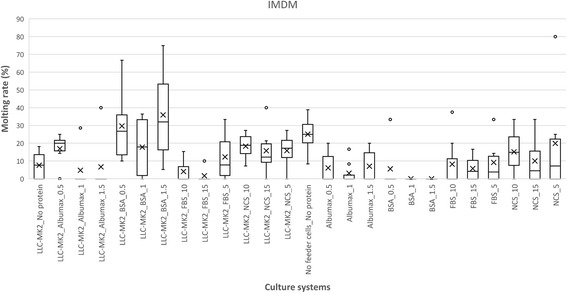
Effects of serum/protein supplements at different concentrations added to IMDM basic medium with or without feeder layer on the moulting L. loa infective larvae in culture. Number of observations: n = 12. Abbreviation: IMDM: Iscove’s modified Dulbecco’s medium
Fig. 8.
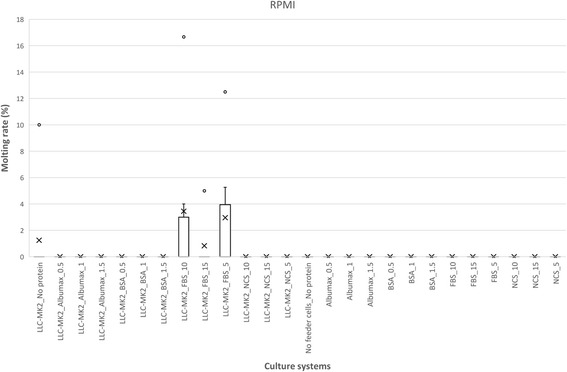
Effects of serum/protein supplements at different concentrations added to RPMI basic medium with or without feeder layer on the moulting L. loa infective larvae in culture. Number of observations: n = 12. Abbreviation: RPMI: Roosevelt Memorial Park Institute
Fig. 9.
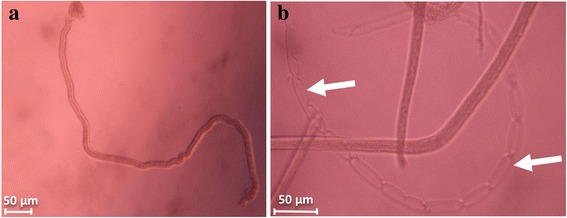
Image of moulted worms observed under inverted microscope. a Fully moulted Loa L4 larva. b Cast cuticule from moulted Loa L3 (arrows)
Linear regression analysis of different factors that influence the in vitro maintenance of L. loa microfilariae and L3
Bivariate analysis indicated strong association between motility and incubation time (Spearman’s rho = -0.674, P < 0.001). This was the first to be introduced in the single linear regression analysis (R2 = 0.452), and the remaining variables were successively added to construct the final GLM. Before interaction terms were introduced in the models, the important factors that contributed to the improvement of worm motility were identified separately based on their standardized coefficient (Fig. 10). For Loa loa mf, these factors included feeder cells (β = 0.490), both IMDM (β = 0.256) and DMEM (β = 0.198) media and the protein supplements NCS (β = 0.052) and FBS (β = 0.022); for Loa loa L3, in addition to feeder cells (β = 0.259) and both IMDM (β = 0.401) and DMEM (β = 0.385) media, the proteins supplement BSA (β = 0.029) were found important for the maintenance of the worm motility.
Fig. 10.

Graphical representation of the standardized coefficients of the main effects of different factors on the predicted Loa loa microfilariae and L3 motility. Adjusted R2: L. loa mf = 0.709; L. loa L3 = 0.716
From the built models, the important variables required to meet the threshold of T90 are as followed and classified with respect to their importance. Feeder cells were found as the most important. In combination to DMEM (DMEM-LLCMK2), the unstandardized coefficient decreased although the interaction was the best among others. In addition, the combination of three variables (feeder layer-basic culture medium-protein/serum supplement) was not mandatory as the interaction LLCMK2-Albumax instead weakened the model with a negative coefficient.
The model was diagnosed by assessing the assumptions of normal distribution and homoscedasticity. The histogram of the residuals (errors) in the model was used to check if they are normally distributed (Additional file 4: Figure S1). Although not perfect, the frequency distribution of the residuals has a shape close to that of the normal Gauss curve, indicating evidence of normal distribution. Additionally, P-P plot was used for further check (Additional file 5: Figure S2). Here, the expected and observed cumulative probabilities were closed suggesting that the assumption of normal distribution of the residual was far to be not violated. The scatterplot of standardized residuals against standardized predicted values were used to assess the assumption of homoscedasticity (Additional file 6: Figure S3). The variance of residuals were random distributed indicating that the assumption of homoscedasticity was likely to be safe.
Discussion
Drug discovery research for L. loa has so far attracted only very limited attention compared to other filarial diseases. Repurposing attempts have been conducted with existing drugs with only limited success. The effect of several antimalarial drugs (quinine, chloroquine, amodiaquine and artesunate) was investigated on loiasis in a randomized, placebo-controlled approach in central Cameroon [20]. This study recorded no significant change in parasite loads in any of the treatment groups. Another study tested different intermittent doses of albendazole on Loa loa microfilaraemia, the reduction in mf load obtained was insufficient to prevent the risk of severe adverse reactions during ivermectin mass drug administration in loiasis co-endemic areas [21]. These observations demonstrated that repurposing of existing antiparasitic therapies may not be a suitable approach to develop drugs with satisfactory therapeutic window. The traditional approach starting from standard in vitro discovery through preclinical and clinical testing necessitates in vitro maintenance of L. loa stages for a minimum duration required for drug screening. Herein we designed and tested the effect of varying 78 culture conditions on both L3 and mf viability starting with three basic culture media, four serum/protein supplements and one feeder cell. In general, L. loa L3 survived for longer periods than mf in the different basic culture media with neither protein nor feeder cell supplementation. For the maintenance of L3, IMDM exhibited the best performance, whereas on microfilariae, DMEM had the highest T90 though only three-day survival. Considering a minimum cut-off point of five-day maintenance of 90% highly active larvae or microfilariae for in vitro drug screening on L. loa microfilariae and infective larvae, IMDM was the only basic medium that could be employed as such without any need of protein supplement/feeder layer. However, none of the media would be suitable for in vitro investigations requiring longer periods of incubation.
Four different serum/protein supplements were therefore applied at increasing concentrations to the three basic culture media in order to improve their nutritional potencies for both L3 and mf. The results obtained were highly diverse. In RPMI, serum/protein supplementation rather caused drop in T90 which was more pronounced for AlbuMax® II followed by FBS, NCS and BSA, in both L3 and mf. Consequently, none of the formulations based on RPMI supplemented with serum/protein (without feeder layer) was successful in keeping mf alive and active for more than five days. Mengome et al. [22] recently reported on screening of 12 methanolic extracts of nine traditional plant remedies employed in Gabon, on L. loa mf maintained in vitro using modified Eagle’s medium supplemented with 10% foetal calf serum with five-day incubation time. Our findings showing an average T90 value of 7.5 ± 2.9 days and thus corroborate the data reported by these authors, confirming the suitability of the culture system employed.
A drastic increase in the survival time and viability of the L. loa mf was obtained with addition of LLC-MK2 cells as feeder layer, except for AlbuMax® II in RPMI. The T90 values were extended for all culture formulations (except AlbuMax® II in RPMI), even exceeding 20 days.
The hypothesis that LLC-MK2 cells alone can be enough to sustain the viability of the L. loa parasites in vitro was tested. In all the three basic culture media, RPMI, DMEM and IMDM, without serum/protein supplement, parasites could still survive 15 days and beyond. Toback et al. [23] reported that monkey kidney cells during their growth in vitro produce growth factors such as the epidermal growth factor (EGF), interleukin growth factor (IGF) and transforming growth factor (TGF-β). Thus, these factors are possibly supportive of L. loa larval growth and/or survival, which facilitate their in vitro maintenance.
Culture systems capable of sustaining moulting of infective larvae present an additional advantage for in vitro investigations on the parasite, including both physiological studies and the exploration of drug targets. In addition to the survival of parasites, the effect of the different culture systems on the moulting of L3 was further examined. Maintenance and moulting of filariae larvae using different culture systems have been reported for Onchocerca spp. [24, 25], Wuchereria bancrofti [26–29] and Brugia malayi [30, 31]. The present study is the first attempt to optimise such systems for L. loa larvae.
Of the total of 659 moulted worms observed, the distribution with respect to each medium was as followed: DMEM (70%) followed by IMDM (29%) and finally RPMI (1%). The proportion of moulting observed in DMEM + LLC-MK2 varied with the nature of the serum/protein supplement. 62.44% (138/221) in BSA, 30.15% (79/262) in NCS, 24.52% (64/261) in AlbuMax® II and 22.38% (45/201) in FBS. The moulting rate in BSA was statistically different (P < 0.001) from the three other sera. With BSA supplementation, 1% proved to be optimum with a 70.37% moulting rate, compared with 1.5% and 0.5% with rates of 58.62% and 57.32%, respectively. Although the role of albumin in the promotion of L. loa L3 moulting is still to be elucidated, Ishima et al. [32] reported on some beneficial effects from the interaction of albumin with other biological factors such as insulin, epidermal growth factor in the in vitro culture of mammalian cells, suggesting that this combination of BSA and LLC-MK2 as supplements provided optimal in vitro conditions for L. loa L3 moulting. Smith et al. [33] have developed a serum-free in vitro system for Brugia malayi third infective larvae, by supplementing RPMI 1640 with either arachidonic, linoleic or linolenic acids and this supported consistent and reproducible moulting to the fourth larval stage in the presence of a basidiomycetous yeast, Rhodotorula minuta. In serum-free cultures lacking R. minuta, L3 larvae survive for upward of two weeks, but did not moult. Smith & Rajan [34] subsequently used this system to study the effect of tetracycline on three different species of filarial nematodes, Brugia malayi, Brugia pahangi and Dirofilaria immitis.
In summary, IMDM and DMEM were the two basic media found to be more suitable to culture L. loa L3 and mf, respectively, for short incubation time (for up to 7 days); DMEM + 5% NCS and IMDM + 10% FBS are suitable to culture both L. loa mf and L3 for relatively long incubation time (for up to two weeks); DMEM + LLC-MK2 was suitable to culture L. loa mf for long incubation time while DMEM + 1% BSA + LLC-MK2 provided optimal moulting conditions for L. loa L3.
Conclusions
This study has demonstrated the effects of protein supplemented basic media in association with or without monkey kidney cells on the survivorship of L. loa microfilariae and on the survival and moulting of L. loa infective larvae. The findings from this work provide a range of culture requirements for the maintenance of L. loa, which are suitable for developing an effective platform for drug screening.
Additional files
Table S1. Summary of the contribution of the main effects of various variables in the model. (DOCX 26 kb)
Table S2. Ranking of the various experimental systems. (DOCX 26 kb)
Table S3. Moulting rate (%) of L. loa L3 in different in vitro culture systems. (DOCX 17 kb)
Figure S1. Motility standardized residual histogram of the motility. (DOCX 41 kb)
Figure S2. Gaussian regression P-P plot of predicted motility. (DOCX 27 kb)
Figure S3. Scatterplot of standardized residuals against standardized predicted values. (DOCX 29 kb)
Acknowledgements
The authors thank Mr Bernard Synkalbe and Mr Bruno Oben for their technical support.
Funding
This work was supported by a Bill and Melinda Gates Foundation Global Health Grand Challenges Explorations Grant awarded to JDT, SW and MJT (OPP10867).
Abbreviations
- APOC
African Programme for Onchocerciasis Control
- BSA
bovine serum albumin
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
foetal bovine serum
- IMDM
Iscove’s modified Dulbecco’s medium
- LLC-MK2
Lewis lung carcinoma Monkey Kidney cell line 2
- NCS
newborn calf serum
- NTDs
neglected tropical diseases
- RPMI
Roswell Park Memorial Institute
- T90
mean duration (days) at which at least 90% of worms were fully active
Authors’ contributions
ZD, FFF, NVTG and AJN contributed to the design of the study, carried out in vitro culture, data analysis and wrote the manuscript. JAOK, CNWP, PE and DTB produced parasitic materials and edited the manuscript. FRD, MJT and JDT assisted in study design and edited the manuscript. SW conceived and designed the study, and oversaw laboratory activities, data analysis and edited the manuscript. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Baboons (Papio anubis) used as parasite reservoir were handled according to international legislation and guidelines of the Cameroon National Veterinary Laboratory (LANVET, Ministry of Livestock, Fisheries and Animal Industry). The study design as well as different protocols was approved by the REFOTDE Institutional Animal Ethics Committee (RIAEC), with an ethical clearance obtained from this board, and the Cameroon National Ethics Committee (Ministry of Public Health). Handling of the animals and the investigations carried out were done strictly according to the international guidelines of rearing animals and using them in medical research under the official authorisation of the Ministry of Scientific Research in Cameroon (Research permit N° 028/MINRESI/B00/C00//C10/C12/2007). The manipulations of the animals were done strictly according to the Animal Welfare Legislation and Policies, complied with the Animals (Scientific Procedures) Act 1986 (ASPA) and its associated codes of practice on animal housing and care [35]. Informed consent was obtained the human participant. Previous works that used the same procedures are found here [13, 15].
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2852-2) contains supplementary material, which is available to authorized users.
Contributor Information
Denis Zofou, Email: zofden@yahoo.com.
Fanny Fri Fombad, Email: ffffombad@gmail.com.
Narcisse V. T. Gandjui, Email: gvictornarcisse@yahoo.com
Abdel Jelil Njouendou, Email: abdeljelile@yahoo.fr.
Arnaud Jonas Kengne-Ouafo, Email: arnaudkengne@yahoo.com.
Patrick W. Chounna Ndongmo, Email: ndongmopatrick@yahoo.com
Fabrice R. Datchoua-Poutcheu, Email: fabriceroberto@yahoo.fr
Peter A. Enyong, Email: enyongap@gmail.com
Dizzle Tayong Bita, Email: b.tayong@yahoo.com.
Mark J. Taylor, Email: mark.taylor@lstmed.ac.uk
Joseph D. Turner, Email: joseph.turner@lstmed.ac.uk
Samuel Wanji, Email: swanji@yahoo.fr.
References
- 1.Padgett JJ, Jacobsen KH. Loiasis: African eye worm. Trans R Soc Trop Med Hyg. 2008;102:983–989. doi: 10.1016/j.trstmh.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Mongin A. Observations sur un ver trouvé sous la conjonctive à Maribarou, île Saint-Dominique. J Med Chir Pharm Paris. 1770;32:338–339. [Google Scholar]
- 3.Pinder M. Loa loa - a neglected filaria. Parasitol Today. 1988;4:279–284. doi: 10.1016/0169-4758(88)90019-1. [DOI] [PubMed] [Google Scholar]
- 4.Poitevin R. Encéphalite filarienne à Loa loa. A propos d’un cas survenu après prise orale d’ivermectine. Thèse de Docorat en Medecine (MD). Université Paris XI. France. 1996;
- 5.Chippaux JP, Boussinesq M, Gardon J, Gardon-Wendel N, Ernould JC. Severe adverse reaction risks during mass treatment with ivermectin in loiasis-endemic areas. Parasitol Today. 1996;12:448–450. doi: 10.1016/0169-4758(96)40006-0. [DOI] [PubMed] [Google Scholar]
- 6.Boussinesq M, Gardon J, Gardon-Wendel N, Kamgno J, Ngoumou P, Chippaux J-P. Three probable cases of Loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am J Trop Med Hyg. 1998;58:461–469. doi: 10.4269/ajtmh.1998.58.461. [DOI] [PubMed] [Google Scholar]
- 7.Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350(9070):18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- 8.Lukiana T, Mandina M, Situakibanza NH, Mbula MM, Lepira BF, Odio WT, et al. A possible case of spontaneous Loa loa encephalopathy associated with a glomerulopathy. Filaria J. 2006;5:6. doi: 10.1186/1475-2883-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carme B, Boulesteix J, Boutes H, Puruehnce MF. Five cases of encephalitis during treatment of loiasis with diethylcarbamazine. Am J Trop Med Hyg. 1991;44:684–690. doi: 10.4269/ajtmh.1991.44.684. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Diseases Control and Prevention (CDC). Parasites - Loiasis. http://www.cdc.gov/parasites/loiasis/treatment.html. Accessed Jan 2016.
- 11.Boussinesq M, Gardon J, Kamgno J, Pion S, Gardon-Wendel N, Chippaux J-P. Relationships between the prevalence and intensity of Loa loa infection in the Central Province of Cameroon. Ann Trop Med Parasitol. 2001;95:495–507. doi: 10.1080/00034983.2001.11813662. [DOI] [PubMed] [Google Scholar]
- 12.Boussinesq M. Loiasis. Ann Trop Med Parasitol. 2006;100:715–731. [DOI] [PubMed]
- 13.Wanji S, Eyong EE, Tendongfor N, Ngwa C, Esuka E, Kengne-Ouafo A, et al. Parasitological, hematological and biochemical characteristics of a model of hyper-microfilariaemic loiasis (Loa loa) in the baboon (Papio anubis). PLoS Negl Trop Dis. 2015;9:e0004202. [DOI] [PMC free article] [PubMed]
- 14.Halliday A, Guimaraes AF, Tyrer HE, Metuge HM, Patrick CN, Arnaud KO, et al. A murine macrofilaricide pre-clinical screening model for onchocerciasis and lymphatic filariasis. Parasit Vectors. 2014;7:472. doi: 10.1186/s13071-014-0472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tendongfor N, Wanji S, Ngwa JC, Esum ME, Specht S, Enyong P, et al. The human parasite Loa loa in cytokine and cytokine receptor gene knock out BALB/c mice: survival, development and localization. Parasit Vectors. 2012;5:43. doi: 10.1186/1756-3305-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wanji S, Eyong E-EJ, Tendongfor N, Ngwa CJ, Esuka EN, Kengne-Ouafo AJ, et al. Ivermectin treatment of Loa loa hyper-microfilaraemic baboons (Papio anubis): assessment of microfilarial load reduction, haematological and biochemical parameters and histopathological changes following treatment. PLoS Negl Trop Dis. 2017;11:e0005576. doi: 10.1371/journal.pntd.0005576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hoegaerden M, Ivanoff B. A rapid, simple method for isolation of viable microfilariae. Am J Trop Med Hyg. 1986;35:148–151. doi: 10.4269/ajtmh.1986.35.148. [DOI] [PubMed] [Google Scholar]
- 18.O'Neill M, Geary JF, Agnew DW, Mackenzie CD, Geary TG. In vitro flubendazole-induced damage to vital tissues in adult females of the filarial nematode Brugia malayi. Int J Parasitol Drugs Drug Resist. 2015;5:135–40. [DOI] [PMC free article] [PubMed]
- 19.Njouendou AJ, Ritter M, Ndongmo WPC, Kien CA, Narcisse GTV, Fombad FF, et al. Successful long-term maintenance of Mansonella perstans in an in vitro culture system. Parasit Vectors. 2017;10:563. doi: 10.1186/s13071-017-2515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamgno J, Djomo PN, Pion SD, Thylefors B, Boussinesq M. A controlled trial to assess the effect of quinine, chloroquine, amodiaquine, and artesunate on Loa loa microfilaremia. Am J Trop Med Hyg. 2010;82:379–385. doi: 10.4269/ajtmh.2010.09-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamgno J, Nguipdop-Djomo P, Gounoue R, Tejiokem M, Kuesel AC. Effect of two or six doses 800 mg of albendazole every two months on Loa loa microfilaraemia: a double blind, randomized, placebo-controlled trial. PLoS Negl Trop Dis. 2016;10:e0004492. doi: 10.1371/journal.pntd.0004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mengome LE, Akue JP, Souza A, Feuya Tchoua GR, Nsi Emvo E. In vitro activities of plant extracts on human Loa loa isolates and cytotoxicity for eukaryotic cells. Parasitol Res. 2010;107:643–50. [DOI] [PubMed]
- 23.Toback FG, Walsh-Reitz MM, Mendley SR, Kartha S. Kidney epithelial cells release growth factors in response to extracellular signals. Pediatr Nephrol. 1990;4:363–371. doi: 10.1007/BF00862521. [DOI] [PubMed] [Google Scholar]
- 24.Lok JB, Pollack RJ, Cupp EW, Bernardo MJ, Donnelly JJ, Albiez EJ. Development of Onchocerca lienalis and O. volvulus from the third to fourth larval stage in vitro. Tropenmed Parasitol. 1984;35:209–11. [PubMed]
- 25.Townson S, Tagboto SK. In vitro cultivation and development of Onchocerca volvulus and Onchocerca lienalis microfilariae. Am J Trop Med Hyg. 1996;54:32–7. [DOI] [PubMed]
- 26.Franke ED, Riberu W, Wiady I. In vitro cultivation of third-stage larvae of Wuchereria bancrofti to the fourth stage. Am J Trop Med Hyg. 1987;37:370–5. [DOI] [PubMed]
- 27.Neves JM, Nardi NB, Andrade L, Dreyer G. In vitro differentiation of Wuchereria bancrofti (Filariidae). Braz J Med Biol Res. 1991;24:1011–6. [PubMed]
- 28.Zaraspe G, Cross JH. Attempt to culture Wuchereria bancrofti in vitro. Southeast Asian J Trop Med Public Health. 1986;17:579–581. [PubMed] [Google Scholar]
- 29.Zheng H, Sahai BM, Kilgannon P, Fotedar A, Green DR. Specific inhibition of cell-surface T-cell receptor expression by antisense oligodeoxynucleotides and its effect on the production of an antigen-specific regulatory T-cell factor. Proc Natl Acad Sci USA. 1989;86:3758–3762. doi: 10.1073/pnas.86.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falcone FH, Schlaak M, Haas H. In vitro cultivation of Brugia malayi, a parasitic nematode that causes human lymphatic filariasis. Altex. 1995;12:179–87. [PubMed]
- 31.Mak J, Lim P, Sim B, Liew L. Brugia malayi and B. pahangi: cultivation in vitro of infective larvae to the fourth and fifth stages. Exp Parasitol. 1983;55:243–248. doi: 10.1016/0014-4894(83)90018-8. [DOI] [PubMed] [Google Scholar]
- 32.Ishima Y, Akaike T, Kragh-Hansen U, Hiroyama S, Sawa T, Suenaga A, et al. S-nitrosylated human serum albumin-mediated cytoprotective activity is enhanced by fatty acid binding. J Biol Chem. 2008;283:34966–34975. doi: 10.1074/jbc.M807009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith HL, Paciorkowski N, Babu S, Rajan TV. Development of a serum-free system for the in vitro cultivation of Brugia malayi infective-stage larvae. Exp Parasitol. 2000;95:253–264. doi: 10.1006/expr.2000.4531. [DOI] [PubMed] [Google Scholar]
- 34.Smith HL, Rajan TV. Tetracycline inhibits development of the infective-stage larvae of filarial nematodes in vitro. Exp Parasitol. 2000;95:265–270. doi: 10.1006/expr.2000.4525. [DOI] [PubMed] [Google Scholar]
- 35.Hollands C. The animals (scientific procedures) act 1986. Lancet. 1986;328(8497):32–33. doi: 10.1016/S0140-6736(86)92571-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of the contribution of the main effects of various variables in the model. (DOCX 26 kb)
Table S2. Ranking of the various experimental systems. (DOCX 26 kb)
Table S3. Moulting rate (%) of L. loa L3 in different in vitro culture systems. (DOCX 17 kb)
Figure S1. Motility standardized residual histogram of the motility. (DOCX 41 kb)
Figure S2. Gaussian regression P-P plot of predicted motility. (DOCX 27 kb)
Figure S3. Scatterplot of standardized residuals against standardized predicted values. (DOCX 29 kb)


