Abstract
Significance: Hydrogen sulfide (H2S) at the right concentration is associated with numerous health benefits in experimental organisms, ranging from protection from ischemia/reperfusion injury to life span extension. Given the considerable translation potential, two major strategies have emerged: supplementation of exogenous H2S and modulation of endogenous H2S metabolism.
Recent Advances: Recently, it was reported that hepatic H2S production capacity is increased in two of the best-characterized mammalian models of life span extension, dietary restriction, and hypopituitary dwarfism, leading to new insights into dietary and hormonal regulation of endogenous H2S production together with broader changes in sulfur amino acid (SAA) metabolism with implications for DNA methylation and redox status.
Critical Issues: Here, we discuss the role of dietary SAAs and growth hormone (GH)/thyroid hormone (TH) signaling in regulation of endogenous H2S production largely via repression of H2S generating enzymes cystathionine γ-lyase (CGL) and cystathionine β-synthase (CBS) on the level of gene transcription, as well as reciprocal regulation of GH and TH signaling by H2S itself. We also discuss plasticity of CGL and CBS gene expression in response to environmental stimuli and the potential of the microbiome to impact overall H2S levels.
Future Directions: The relative contribution of increased H2S to health span or lifespan benefits in models of extended longevity remains to be determined, as does the mechanism by which such benefits occur. Nonetheless, our ability to control H2S levels using exogenous H2S donors or by modifying the endogenous H2S production/consumption equilibrium has the potential to improve health and increase “shelf-life” across evolutionary boundaries, including our own. Antioxid. Redox Signal. 28, 1483–1502.
Keywords: : hydrogen sulfide, dietary restriction, aging, growth hormone, thyroid hormone, cystathionine γ-lyase
Introduction
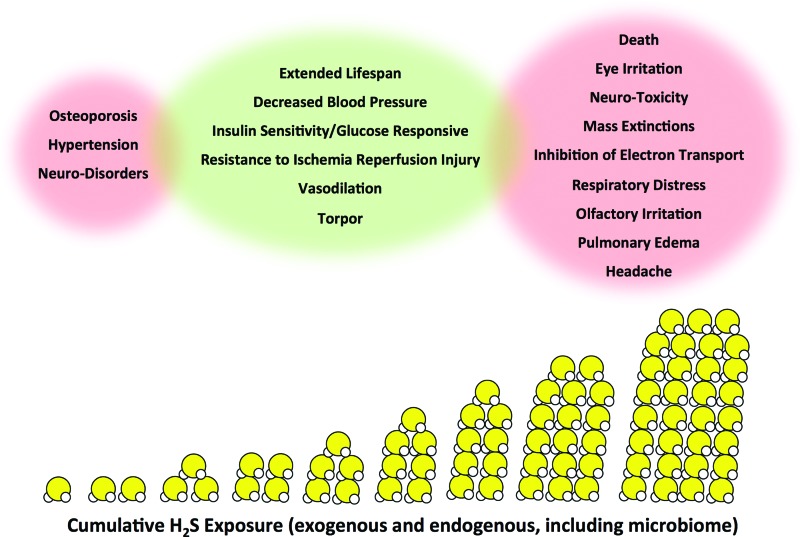
Hydrogen sulfide (H2S) gas elicits a classic U-shaped biological dose/response typical of hormetic compounds (24). This includes detrimental pathological conditions at severely elevated or reduced levels, but beneficial effects at physiological or supraphysiological concentrations (Fig. 1) (82, 181, 189).
FIG. 1.
Biological hormetic dose/response of H2S exposure. H2S, shown in molecular form with one yellow circle (sulfur) and two smaller circles (hydrogen), displays a multiphasic dose/response. Adverse effects and conditions (shown in red) arise from too little or too much H2S exposure. Beneficial effects (shown in green) occur at specific H2S exposure and/or production capacity levels. The potential exists for both negative and positive responses to occur simultaneously at either end of the beneficial H2S exposure range; H2S, hydrogen sulfide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
H2S has historically been associated with toxicity at high levels. Environmental and/or occupational exposure to elevated concentrations of H2S can lead to irritation, respiratory distress, and even death (102). Extreme H2S conditions correlate with mass extinctions throughout geobiological history (92, 94, 123). Toxic levels can also be generated aberrantly in mammals by dysregulation of endogenous H2S metabolism, resulting in a range of pathologies from inflammation (103, 184) to β cell dysfunction and diabetes (167). Mechanistically, H2S toxicity in eukaryotes has been attributed to inhibition of mitochondrial respiration and induction of oxidative stress (31, 79, 86).
Too little H2S also has negative consequences. Mice with genetic defects in endogenous H2S generating enzymes cystathionine γ-lyase (CGL, also known as cystathionase, CTH, or CSE) or cystathionine β-synthase (CBS) are susceptible to hypertension (70, 190), neurodegenerative disorders (127), vascular complications associated with diabetes (167), and symptoms of osteoporosis (110). Because CGL and CBS have functions in addition to H2S production, it is important to note that addition of exogenous H2S corrects many of these problems in rodent knockout models, consistent with a specific role for H2S. In humans, reduced circulating H2S levels correlate with, and may be causally related to, cardiovascular disease (90).
While too much or too little H2S is detrimental, exposure to supraphysiological levels of H2S can be beneficial. For example, in rodents, H2S induces a hypoxia-resistant metabolic state resembling torpor (13), increases vasodilation of blood vessels and lowers blood pressure (197), protects against ischemia/reperfusion injury to multiple organs, including the heart (39) and liver (78), improves insulin sensitivity (188), delays cognitive decline in animal models of Alzheimer's disease (52), and alters many other physiological endpoints with medical relevance (181). In lower organisms including yeast and worms, exogenous H2S extends longevity (65, 114). These benefits of exposure to supraphysiological levels of H2S likely occur through distinct biological mechanisms, including posttranslational modification and functional alteration of cellular targets such as ion channels (110, 118) and growth factor and hormone receptors (171, 196), with many more likely yet to be deciphered.
Given the considerable translational potential of increased H2S in a number of biomedical areas (27, 136, 166), two major strategies have emerged: supplementation of exogenous H2S and modulation of endogenous H2S production. Each approach has unique challenges. Exogenous H2S supplementation is conceptually the most straightforward, with multiple different forms of H2S already available, from the short-acting gas itself (13) or aqueous forms of the H2S salt NaHS (2) to long-acting, slow-release H2S donors (100, 131). Nonetheless, toxicity at high levels and the corrosive and malodorous nature of the gas present challenges (6, 57). H2S is also highly reactive, so that even in orally available, slow-release formulations, targeted delivery to the proper cell type at the optimal dose, once this is fully elucidated, may be challenging to achieve.
The alternate approach to increasing H2S levels is to modulate endogenous production and/or consumption. On the production side, this could include modulation of expression, posttranslational modification, subcellular localization, allosteric regulation, and/or substrate-level control of H2S producing enzymes. Several examples of each of these are known. Acetylcholine and vascular endothelial growth factor both increase H2S production in endothelial cells by activating cognate receptors and increasing calcium/calmodulin-dependent CGL activation (126, 190). In vascular smooth muscle cells, CGL translocates upon endoplasmic reticulum (ER) stress to mitochondria (48), where concentrations of cysteine, a substrate for H2S production, are highest. In the liver, hypoxia increases mitochondrial CBS levels and H2S production by preventing oxygenation of its heme cofactor and thus inhibiting degradation by Lon protease (172). CBS activity is also stimulated by the methionine cycle intermediate S-adenosylmethionine via allosteric regulation (157). Inhibition of phosphodiesterase PDE5 with the drug tadalafil is best known for stimulating nitric oxide production, but also increases H2S production, in part, through cyclic guanosine monophosphate-dependent protein kinase G activation in the heart, which may work to increase CGL transcription via activation of the transcription factor SP1 (145). Tadalafil also activates H2S production in podocytes by increasing CGL protein levels (97). The antidiabetic drug metformin boosts H2S production in several mammalian tissues, although the mechanism remains unknown (185). In conclusion, there is clear evidence that endogenous H2S production is regulated on multiple levels.
On the consumption side, a major mechanism of H2S removal occurs in mitochondria via the sulfide quinone oxidoreductase (SQR), a complex II-like component of the mitochondrial electron transport chain that catalyzes the first step in H2S oxidation to thiosulfate (12, 64, 93). SQR also passes electrons donated by H2S to coenzyme Q, thus promoting proton pumping by the mitochondrial electron transport chain for cellular bioenergetics (48, 74, 93, 111, 117). Like H2S producing enzymes CBS and CGL that are expressed in different cells/tissues (83), SQR displays tissue-specific expression, with high activity in the gut and liver, and low activity in the brain (108). In addition to SQR, sulfur dioxygenase (ethylmalonic encephalopathy 1 protein [ETHE1]) is involved in sulfide oxidation to thiosulfate, which can be readily excreted (64). Because ETHE1 and SQR (as part of the mitochondrial electron transport chain) have direct and indirect oxygen requirements, respectively, H2S consumption and thus steady-state H2S levels can be affected by oxygen tension. Pathophysiological states such as hypoxia can trigger a rapid increase in H2S by modulating both production via CBS stabilization and removal via inhibition of oxygen-dependent detoxification by SQR/ETHE1. Finally, exposure of worms lacking SQR to H2S results in inhibition of protein translation via a mechanism involving phosphorylation and inactivation of the translational initiation factor eIF2α by the amino acid sensing kinase general control nondepressible 2 (GCN2) (68).
While many studies report benefits of exogenous H2S, including ongoing clinical trials of the H2S donor and polysulthionate SG1002 in the context of cardiomyopathy (89), precision manipulation of endogenous H2S levels is currently hampered by our limited knowledge of mechanisms controlling production and consumption, thus representing a major roadblock to progress on this complementary front.
Recently, the finding that hepatic H2S production capacity is increased in two of the best-characterized mammalian models of life span extension, dietary restriction (DR) (65) and hypopituitary dwarfism (66), has led to new insights into regulation of endogenous H2S levels. In this review, we summarize the role of sulfur amino acids (SAA) and endocrine signaling in control of endogenous H2S production, focusing mostly on the production side via regulation of expression of CGL. We discuss the importance of H2S in these longevity models, including the potential role of H2S as an intermediate in nutrient and endocrine/paracrine feedback control. In addition, we demonstrate the plasticity of CBS and CGL gene transcription in response to numerous stimuli, including diet, hormones, drugs, radiation, and disease using the NCBI GEO Profiles database. Finally, we discuss the potential impact of H2S on longevity extension.
Measuring Sulfide Levels
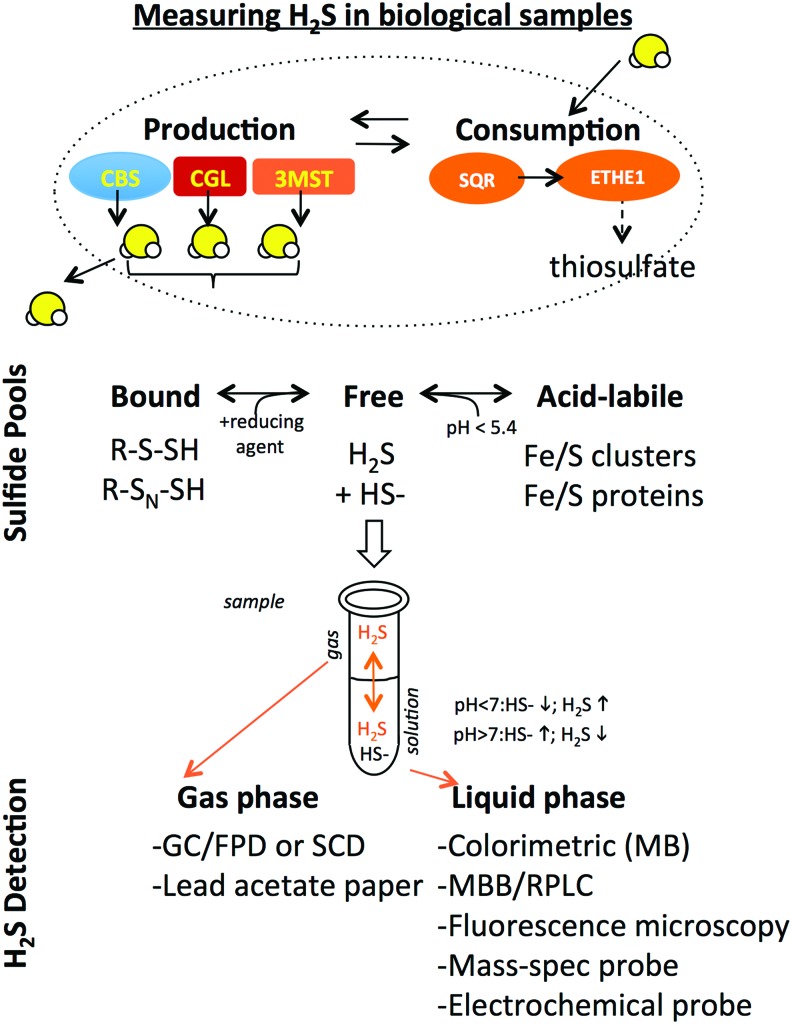
Before discussing the regulation of endogenous H2S production, it is important to consider the techniques used to measure H2S in biological samples and their limitations. The steady-state H2S level present in a cell, organ, or extracellular fluid is a function of enzymatic H2S production and consumption (Fig. 2). At neutral pH, H2S, with a pKa1 of ∼6.9, is in equilibrium with HS- at an ∼1:1 ratio. This is important because H2S, but not HS-, can pass readily through cell membranes into extracellular fluids and evaporates easily from aqueous solution, and thus can be lost (or trapped for later measurement if care is taken) in the gas headspace above a biological sample. It also allows the use of pH to maintain sulfide in solution (HS- at basic pH) or to release it into the gas phase (H2S at mildly acidic pH). The term sulfide is used heretofore when referring to both H2S and HS- species.
FIG. 2.
Measuring H2S in biological samples. Steady-state sulfide levels are determined by the balance of intracellular production and consumption pathways. H2S dissolves readily in aqueous solution, but is also lipophilic and volatile, and can easily cross cell membranes (dotted line) and move between cells or into the gas phase above a sample. Sulfide is present in various pools (bound, acid-labile) that can be distinguished based on their biochemical properties. Bound sulfide consisting of persulfides and polysulfides attached to proteins can be released as free sulfide under reducing conditions, while acid-labile sulfur attached to iron/sulfur clusters present in iron/sulfur proteins can be released under acidic conditions. Free sulfide present in a biological sample equilibrates in liquid and gas phases depending on pH, and can be measured by a number of different techniques as indicated. 3-MST, 3-mercaptopyruvate sulfurtransferase; CBS, cystathione β-synthase; CGL, cystathione-γ-lysase; ETHE1, ethylmalonic encephalopathy 1 protein; GC, gas chromatography; FPD, flame photometric detection; MB, methylene blue method; MBB, monobromobimane; RPLC, reverse-phase liquid chromatography; SCD, sulfur chemiluminescence detection; SQR, sulfide quinone oxidoreductase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Sulfide present in biological samples exists in different pools that can be distinguished based on their biochemical properties. In addition to free sulfide, H2S can exist as sulfane sulfur, a pool that is functionally defined based on the liberation of free H2S in the presence of a reducing agent such as dithiothreitol or tris(2-carboxyethyl) phosphine. Sulfur present in iron/sulfur clusters can also give rise to free H2S under acidic conditions (pH <5.4), as can nonenzymatic reduction of molecular sulfur, however, the contribution of these sources to free H2S pools under physiological conditions remains unclear.
Techniques to measure absolute concentrations of sulfide present in biological samples have been reviewed extensively (61, 71, 119, 122, 170, 181). They can be grouped into two major categories based on whether the sulfide being measured is present in liquid or gas phases. H2S gas trapped/released into the headspace above its source can be measured by gas chromatography followed by flame photometric detection or sulfur chemiluminescence detection (177). Measuring sulfide in the liquid phase often requires prior derivatization to stabilize sulfide, for example, in the methylene blue method in which lead acetate reacts with sulfide to form lead sulfide, and then, in the presence of N,N-dimethyl-p-phenylenediamine under acidic conditions forms methylene blue, which can be detected by absorbance in a spectrophotometer with a lower detection limit of 1 μM (72, 161). Monobromobimane also reacts with and stabilizes sulfide in solution, which can then be detected by its fluorescence after reverse-phase liquid chromatography with a lower detection limit in the nM range (152). More recently, a number of fluorescent dyes have been reported that react specifically with H2S in vitro and in vivo, as well as a mass spectrometry-based probe targeted to mitochondria that can be used in vivo (4). Finally, electrochemical polarographic (122) or amperometric (190) probes can be used in vivo to directly measure changes in steady-state levels over time in response to perturbations such as hypoxia without sulfide derivatization.
Depending on the experimental design, sulfide measurements can report on either maximal production capacity or actual steady-state levels. Measurements of maximal production capacity in the presence of excess substrate and cofactor, typically done in cell-free lysates/extracts, utilize a variety of techniques to measure H2S in liquid or gas phase as described above. Because such measurements generate much more H2S than is present under physiological conditions, they can be performed in relatively high throughput using a variety of detection methods (e.g., lead acetate). They also lend themselves to the use of enzyme inhibitors (e.g., propargylglycine [PAG] for CGL; aminooxyacetic acid for CGL, CBS, or cysteine aminotransferase [CAT]; aspartate for CAT) and substrates that can help dissect the nature of the H2S-producing activity. A major disadvantage is that they do not report on the actual amount of H2S being generated in the sample in vivo. This is typically done in fresh or frozen samples from cells, tissues, or organs in which sulfide generation and consumption pathways are intact, and substrates for sulfide production present at physiological levels. After extraction and derivatization, sulfide is measured using a variety of techniques appropriate for either solution- or gas-based sulfide measurements. This also allows for measurement of different sulfide pools (bound; acid-labile) following appropriate extraction. A limited number of techniques that permit rapid detection of free sulfide in living biological samples (e.g., sulfide amperometric electrodes or polarography in circulating blood) further allow measurement of changes in steady-state free sulfide levels, for example, upon hypoxia. Nonetheless, quantitative assessment of intracellular H2S levels particularly in vivo remains challenging for a host of reasons, including the volatile and highly reactive nature of the molecule and interference from other thiol-containing molecules (121, 122).
DR and Nutrient-Based Regulation of Endogenous Transsulfuration Pathway and H2S Production
DR describes a variety of nutritional interventions in which reduced total calorie or nutrient intake is associated with health benefits, generally including life span extension in experimental organisms. DR also consistently protects against diseases for which age is the prime risk factor, including cancer, neurodegeneration, inflammatory/autoimmune disorders, and cardiovascular disease. DR has pleiotropic beneficial effects on multiple physiological systems, from glucose homeostasis to inflammation to oxidative stress resistance, some or all of which may contribute to its longevity and health effects (46, 160).
Experimental DR regimens vary widely. Classic calorie restriction (CR) regimens in rodents involve enforced restriction of total food intake by 20–60%. CR is also sometimes referred to as intermittent fasting (IF) because animals quickly eat their allotment of food and thus spend the remaining time between meals in a fasted state. Animals on CR/IF regimens are typically fed the same diet as the ad libitum fed controls and thus at a fixed ratio of calories from fat, carbohydrates, and protein, making it difficult to separate the effects of nutrient restriction from those of reduced energy intake. Nonetheless, adding back essential amino acids (EAA) to food restricted flies abrogates longevity benefits (53), likely through effects on nutrient signaling pathways independent of their calorie content.
Other DR regimens that restrict (or dilute) specific nutrients, including protein or EAAs, without periods of enforced food restriction can also extend life span and health span across evolutionary boundaries (99, 159). Restriction of the EAA methionine (methionine restriction [MR]) is one of the best-studied examples in which restriction of an individual EAA can alter physiology and extend longevity. MR consistently extends life span in multiple experimental organisms, including yeast, worms, flies, rodents, and human cells in culture (18, 23, 80, 91, 96, 124, 143). Classical MR regimens in rodents, in which cysteine is absent due to its methionine sparing effects (a better name would thus be “sulfur amino acid restriction”), result in a 42% increase in mean and 44% increase in maximum life span (141), as well as improvements in stress resistance and metabolic fitness (1, 26, 101, 163). Restriction of the EAA tryptophan can also extend life span in rodents (147), while restriction of branched chain amino acids can have metabolic benefits, including improved glucose homeostasis and body composition (43).
Multiple molecular mechanisms have been proposed to account for DR benefits. These nonmutually exclusive pathways include reduced anabolic signal transduction via insulin/insulin-like growth factor-1 (IGF-1) and mechanistic target of rapamycin (mTOR); activation of adaptive redox response pathways; improved metabolic function and nutrient utilization; increased autophagy; and an overall reduction in macromolecular damage from reactive oxygen and nitrogen species (45, 156). Nonetheless, the relative contribution of such molecular, metabolic, and physiological changes to longevity benefits remains poorly understood, as do the relationships between CR, MR, EAA restriction, and other DR regimens.
Recently, we reported that H2S production capacity is increased in multiple experimental models of DR and associated with multiple DR benefits, adding it to the list of potential molecular mechanisms underlying DR action (65, 67). In mice, we found that 1 week of 50% CR increases endogenous hepatic H2S production and improves resistance to the acute multifactorial stress of hepatic ischemia/reperfusion injury. In this model, H2S production and stress resistance correlate with increased CGL expression on the mRNA and protein levels, are prevented by pharmacological inhibition of CGL with the chemical inhibitor PAG or by genetic ablation of CGL, and augmented by CGL overexpression independent of diet. Interestingly, constitutive activation of mTOR in hepatocytes blocks DR-mediated hepatic stress resistance (60) and simultaneously suppresses CGL expression and H2S production capacity in the liver. In worms, flies, and yeast, H2S production capacity is augmented by organism-appropriate DR regimens, including genetic models of reduced food intake in worms (eat 2), MR in flies, and reduced glucose in yeast (65).
While this study was the first to explicitly link augmented endogenous H2S to pleiotropic DR benefits, it is consistent with a number of findings regarding age-related changes in H2S and expression of H2S-producing enzymes in the context of longevity and DR. In fruit flies, life span extension via DR correlates with increased expression and activity of CBS in whole fly homogenates and is blocked by knockdown of CBS or inhibition of CGL with PAG, while either total body or neuron-specific overexpression of CBS is sufficient to extend life span (81). In rats, there is an aging-related decline in CGL and CBS expression and H2S production in multiple tissues, and this is abolished by long-term 10–40% CR from 8 to 38 months of age (135). Similarly, 30% CR for 6 months in rats attenuates renal aging, boosts CGL and CBS expression, and increases H2S production in the kidneys (182). Even short-term DR in rats, in the form of SAA restriction for 1 week, results in increased hepatic CGL expression (155). In mice, SAA metabolic pathways are significantly affected on the transcriptional level by DR in multiple tissues (165). Twenty to forty percent CR results in a dose-dependent increase in hepatic H2S production in both male and female mice of different strain backgrounds, correlating with improved health, although intriguingly, not always with extended longevity (116). Caloric restriction between 0% and 40% for 3 months in male mice increases hepatic CGL expression in a dose-dependent manner (35), while SAA restriction for 5 weeks elevates hepatic CGL and CBS expression (130).
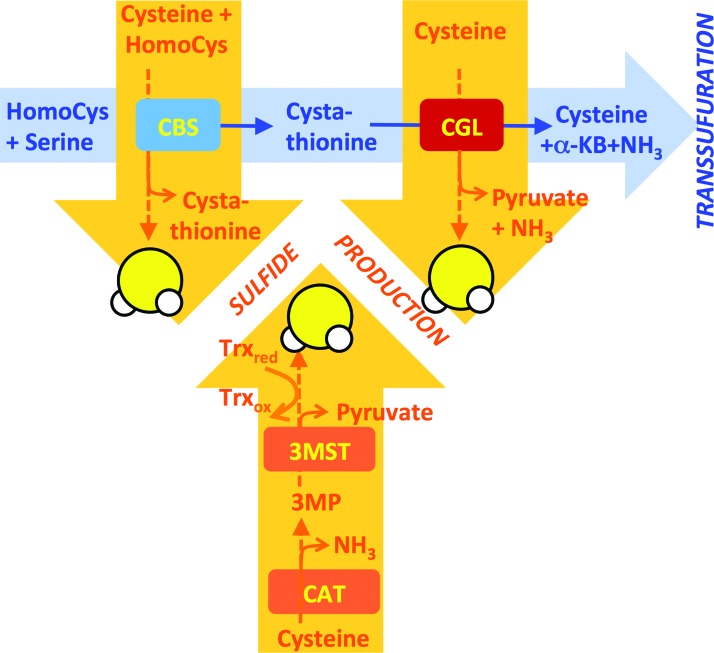
How does diet influence H2S generation, and by what mechanism is H2S increased on DR? One mechanism of increased H2S production is by transcriptional regulation of CBS and CGL. Regulation of these genes is best understood in the context of de novo cysteine biogenesis via the transsulfuration pathway (TSP), the presence of which explains why cysteine is considered a nonEAA. The first step is the condensation of homocysteine with serine to form cystathionine by CBS. Cystathionine is then hydrolyzed to cysteine (and α-ketobutyrate + ammonia) via CGL (Fig. 3). When cysteine levels are low, CGL is upregulated at the mRNA and/or protein level via increased protein expression of activating transcription factor 4 (ATF4) (98, 154, 155), which binds to sequences in the first intron of CGL to activate transcription under stress conditions, including amino acid deprivation (115). In liver-derived cells, cysteine deprivation induces ATF4 expression via the amino acid deprivation sensor GCN2 (98), a kinase that senses uncharged tRNAs and controls translation via phosphorylation of the translation initiation factor eIF2α (50). Cysteine can then be used in protein, glutathione, or taurine biosynthesis. Mouse embryonic fibroblasts lacking ATF4 show little or no CGL transcript or protein (36) and require the addition of cysteine or other antioxidants for growth (58). ATF4 expression is increased on CR or SAA restriction (105, 155), and likely plays a role in increased H2S production, in part, through transcriptional control of the H2S generating enzyme CGL in the liver and potentially other cells/organs (65, 130, 146), although the formal genetic requirement remains to be tested.
FIG. 3.
Transsulfuration and H2S production are separable pathways. Transsulfuration reactions (blue) consist of condensation of homocysteine (HomoCys) derived from the methionine cycle with serine by CBS to form cystathionine. CGL then cleaves cystathionine to form cysteine (Cys) and the by-products α-KB and ammonia. H2S is produced from alternate reactions of CBS and CGL (orange) utilizing homocysteine or cysteine as substrate. H2S is also produced indirectly from cysteine via stepwise deamination to 3MP by cysteine/aspartate amino transferase (CAT) and sulfhydration of 3-meracaptopyruvate sulfur transferase. H2S is released on reduction of sulfhydrated 3-MST, for example, by reduced thioredoxin (Trx). α-KB, α-ketobutyrate; 3MP, 3-methlypyruvate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
It is important to note that while CBS and CGL are both major H2S producers, H2S production is not a by-product of de novo cysteine biogenesis via the TSP. Rather, H2S is produced by a different series of reactions catalyzed by the same proteins with overlapping substrates and products (Fig. 3). CBS produces H2S from homocysteine and cysteine, and is the predominant H2S-producing enzyme in the kidney and brain under normal physiological substrate concentrations (28). In the liver, H2S production by CGL dominates (29), in part, due to CGL protein levels being 60-fold higher than CBS (83). While the catalytic efficiency for H2S production by CGL is higher with homocysteine than cysteine, under normal physiological conditions in the liver, cysteine is the predominant substrate for H2S production (29). Importantly, the catalytic efficiency for cysteine production from cystathionine by CGL is 30 times higher than H2S generation from cysteine (29). In other words, conditions favoring de novo cysteine biogenesis via TSP are different from those favoring H2S production from cysteine, consistent with TSP and H2S production being separable processes. Whether these two processes are mutually exclusive within a given cell, or can occur at the same time in different subcellular compartments, remains to be elucidated.
What is the relationship between TSP and H2S production, and does an increase in one necessarily affect the other? In other words, if DR increases TSP activity to replenish cysteine levels, increased cysteine utilization for H2S generation presents an apparent paradox. In fact, while the substrate preferences for H2S generation by CGL and CBS are described (157), the sources of free cysteine (or homocysteine) for H2S production are not known. Thus, one potential answer to this apparent paradox is that cysteine for protein translation or glutathione biosynthesis produced de novo by the TSP is in a distinct cellular pool or subcellular compartment than free cysteine fueling H2S production. Substrate for H2S generation may instead come from lysosomes via autophagy or via proteolysis, both of which are expected to increase on DR. If cysteine pools are distinct, it may be possible for cysteine generated de novo from TSP to drive glutathione and/or taurine biosynthesis in the same cell at the same time as H2S production is increased. Future studies are required to shed light on metabolic flux through TSP and H2S biosynthetic pathways as a function of diet, including DR.
Contrary to DR, dietary interventions such as high-fat diets (HFD), which can precipitate obesity, metabolic syndrome, and shortened life expectancy, have opposite effects on TSP gene expression and H2S production capacity. For example, mice fed a HFD for up to 16 weeks exhibit reduced CGL expression in the liver, lung, and aortic endothelium, with diminished H2S biosynthesis in liver, kidney, and lung tissues (129). Rats fed HFD for 18 weeks exhibit increased plasma homocysteine concentrations and decreased hepatic enzymatic activities of CBS and CGL (17). Rats fed a Western diet (21% fat) versus control chow (6% fat) for 12 weeks display decreased CGL expression and H2S in aortic tissue, and this correlates with decreased endothelial function and increased reactive oxygen species in this tissue (76).
Exercise appears to reverse some of the negative impacts of HFD feeding on TSP activity and H2S production. For example, in addition to improvements in body weight, hepatic steatosis, and glucose homeostasis, exercise restores CBS and CGL expression in the liver and H2S bioavailability in the plasma and liver in mice fed HFD for 24 weeks (180). Exogenous H2S reduces lipid accumulation, injury, and fibrosis in the kidneys of mice on HFD (186), suggesting that H2S itself may contribute to these benefits.
In addition to effects of macronutrient intake, dietary micronutrients can also affect TSP activity and H2S production. In Dahl rats, a high-salt diet induces hypertension and diminishes renal CBS expression and endogenous H2S content, while supplementation with H2S reverses effects on hypertension and aortic remodeling (70). Chronic intake of the artificial sweetener aspartame for 90 days in mice reduces hepatic TSP metabolites cysteine, S-adenosylmethionine, and S-adenosylhomocysteine, as well as CGL on the mRNA and protein levels (40).
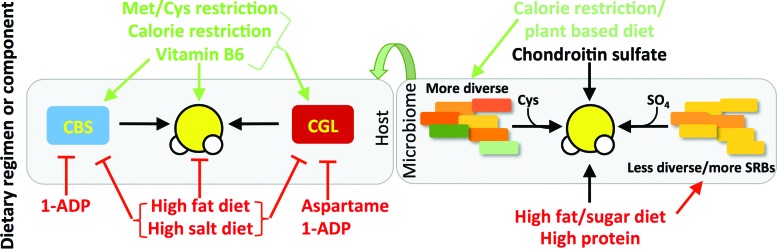
The bioactive form of vitamin B6, pyridoxal phosphate (PLP), is required as a co-factor for CGL and CBS enzymatic activities, with CGL displaying greater sensitivity to PLP deficiency than CBS. As a segment of the U.S. population is considered PLP insufficient (10–12%) or slightly insufficient (10–15%) (54), it brings to light the possibility that dietary PLP, or the lack thereof, can affect human health via control of endogenous H2S production. Interestingly, the metabolite 1-amino D-proline (1ADP), which is found in the common dietary foodstuff flaxseed, is a PLP antagonist, and rats fed a diet with 10 mg 1ADP/kg diet display reduced enzymatic activities of CBS and CGL (113). A summary of dietary effects on TSP and endogenous H2S production is presented in Figure 4.
FIG. 4.
Dietary regimens or ingredients that modulate host and microbiome production of H2S. The effects of different dietary regimens or components on host CBS or CGL activity, or H2S production, are shown in green (stimulatory) and red (inhibitory). Diets in green that increase microbiome diversity (green) also decrease H2S production, while those shown in red either decrease microbiome diversity or increase the potential for H2S generation by sulfate reducing bacteria such as Desulfovibrio (yellow). Inorganic sulfate in the form of common nutraceutical chondroitin sulfate, but also abundant in many plant sources, can increase microbiome H2S production, but does not necessarily perturb gut function. 1ADP, 1-amino D-proline. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Finally, H2S can also be generated on cysteine oxidation via 3-mercaptopyruvate sulfurtransferase (3-MST), which can produce H2S in the presence of a reducing agent from 3-mercaptopyruvate following deamination of cysteine by CAT (153). CAT (but not 3-MST) is a PLP-dependent enzyme like CBS and CGL, however, little is known about dietary regulation of H2S production by this cysteine oxidation pathway.
Although the focus up to this point has been primarily on the interaction of diet and endogenous H2S production in cells, tissues, and organs of eukaryotic cells, bacteria constituting our gut microbiome also produce H2S. Sulfate-reducing bacteria (SRB) such as those from the Desulfovibrio genus use inorganic sulfate as a terminal electron acceptor for respiration, thus generating H2S (25). A wide range of bacteria can also generate H2S via degradation of organic sulfur in the form of cysteine through cysteine desulfhydrase activity present in homologues of eukaryotic CBS, CGL, and CAT/3-MST enzymes. Interestingly, H2S generation by bacteria protects them from antibiotic-induced oxidative damage (150). The presence of high levels of SRB or other H2S producing bacteria such as Fusobacterium is associated with proinflammatory disorders, including inflammatory bowel disease (both ulcerative colitis and Crohn's disease), colorectal cancer, and irritable bowel syndrome (25, 158). Nonetheless, H2S can also protect gut barrier function through modulation of the host inflammatory response in a rodent model of colitis (179), and thus, it remains unclear whether elevated H2S associated with inflammation in the gut is causative of disease or merely an epiphenomenon.
Diet can influence both the composition of the microbiome and its propensity to produce H2S. Individuals with low bacterial richness and diversity have an increased potential for H2S formation from SRB, and are also more likely to be obese and insulin resistant, gain more weight over time, and have increased inflammatory phenotypes (95). Rodents with symptoms of metabolic syndrome induced by HFD also show higher levels of SRB in gut microbiota (194). Mice fed a diet high in fat and simple sugars, as well as the dietary supplement chondroitin sulfate, are associated with increased cecal levels of Desulfovibrio and H2S production, but without compromised gut barrier function (140). Diets high in animal protein are also associated with increased fecal H2S concentrations independent of fat and sugar intake (112). Interestingly, humans on a calorie-restricted (50% fewer calories than their control counterparts), mostly plant-based, diet have greater phylogenic diversity in their fecal microbial populations than controls (55), although the levels of H2S are yet to be established.
Host responses to changes in gut microbiome-derived H2S are also beginning to be characterized. Induction of gut bacterial H2S production via high-protein diet feeding results in an increase in SQR gene expression in host colonocytes (12). Eukaryotic cells, and in particular colonocytes, have the ability to utilize H2S as an electron donor to fuel ATP production via this mitochondrial electron transport chain protein (93). Thus, the increase in SQR in response to augmented bacterial H2S production is indicative of a host adaptive response to either detoxify H2S or utilize it as an inorganic fuel source for ATP production. In gnotobiotic mice lacking a microbiome, H2S levels are reduced in plasma, adipose, and lung tissues, and CGL activity is reduced in many organs (151) consistent with bidirectional effects of microbiome and host on sulfide metabolism (Fig. 4). Future studies should thus consider the effects of dietary macro- and micronutrient composition on both host and microbiome sulfide metabolism and TSP activity.
Long-Lived Hypopituitary Dwarfism and Growth Hormone/Thyroid Hormone Regulation of Endogenous TSP-Dependent H2S Production Capacity
Disruption of the hypothalamic–pituitary axis signaling via spontaneous or induced mutations results in dwarfism, but also extreme longevity, increased stress resistance and improved metabolic fitness in a number of rodent models (10, 149), including genetic deficiency in growth hormone releasing hormone (GHRH) (164) or its receptor (growth hormone releasing hormone receptor, aka little mouse) (42); the anterior pituitary developmental transcription factors Pit1 (Snell dwarf) (42) or Prop1 (Ames dwarf) (19) or the receptor for growth hormone (GHR) (32, 33). In addition, there is a significant inverse correlation in both small and large mammals between thyroid hormone (TH) levels and life expectancy (16). Hence, states that allow for decreased growth hormone (GH) and TH signaling offer prolongevity and antiaging benefits.
In humans, polymorphisms in the thyroid gland receptor for thyroid-stimulating hormone (TSH) resulting in increased TSH but decreased TH production and signaling are associated with extreme longevity in centenarian studies (7, 8, 75, 142). Furthermore, humans with defective GHR signaling (Laron dwarfism), while not apparently longer lived, are resistant to several aging-related diseases, including type 2 diabetes and cancer (56). Conversely, increased TH signaling through active triiodothyronine (T3) binding to the TH receptor (TRs) beta isoform results in DNA damage and premature senescence, two major drivers of aging and aging-related pathologies (193). Likewise, transgenic overexpression of GH in rodents results in large size, hyperinsulinemia, insulin resistance, and decreased life expectancy (11).
What is the mechanistic relationship between longevity extension by DR and hypopituitary dwarfism? Reduced GH/TH signaling leads to CR/MR-like alterations in SAA and energy metabolism without altering food intake (20), while DR alters hypothalamic activation of the pituitary gland (34, 41) and lowers GH (47) and TH signaling (44). This could be due, in part, to a reduction in DR in the adipose-derived hormone leptin, which is implicated in control of hypothalamic thyrotropin-releasing hormone (TRH) levels during fasting via melanocortin and neuropeptide Y signaling (176). Protein restriction decreases GH release from the pituitary and blunts pituitary responsiveness to GHRH (59). Fasting also directly targets the liver and alters the metabolism and bioavailability of TH (77, 187) as well as expression of GHR (162).
Functional interaction studies indicate that benefits of CR or MR on longevity and metabolic fitness are dampened or abrogated entirely in hypopituitary or growth hormone receptor knockout (GHRKO) dwarf mice (5, 14, 15, 22). Conversely, GH supplementation concurrent with DR reverses several of the metabolic effects of DR (30, 51). Together, these data suggest that the mechanisms of DR underlying increased life span and health span work primarily, or at least significantly, through modulation of the hypothalamic–pituitary axis and GH production.
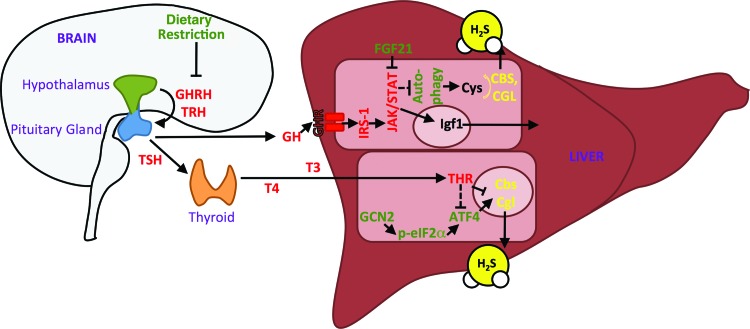
Recently, we identified increased H2S production as another commonality between DR and hypopituitary dwarfism. Hepatic CGL expression and H2S production are elevated in long-lived genetic mouse models of reduced GH and/or TH signaling in vivo and in cultured cells on serum withdrawal, a model of reduced growth factor signaling in vitro (66). Negative regulation of hepatic H2S production by GH and TH is additive and occurs via distinct mechanisms. TH acts primarily via TRs in the liver to repress CBS and CGL gene expression, while GH acts via GHR signaling to control substrate-level H2S production, likely via negative regulation of autophagy. As discussed above, autophagy is a potential source of free cysteine for H2S generation independent of de novo cysteine biogenesis via the TSP. Hepatic H2S production is also increased in long-lived mouse models overexpressing FGF21 (195) or lacking IRS-1 (148). FGF21 regulates both GHR (73) and TH (37) signaling, and is induced on DR (101). IRS-1 is a docking protein that facilitates insulin and IGF-1 signaling, but is also implicated in GHR signaling (106).
Similar to our previous study linking H2S to DR, this was the first study to explicitly associate GH and TH signaling with regulation of endogenous H2S production in the liver. While any causality between health benefits observed in dwarf mice and increased H2S production remains to be established, regulation of H2S production by GH and TH is consistent with a larger body of work linking these hormones to a broader regulation of SAA metabolism, including TSP and glutathione metabolism, as described below.
The connection between GH and TH on SAA metabolism was first observed in hypophysectomized or thyroidectomized/radioiodine ablated rats, in which changes in activities of TSP and transmethylation pathway (TMP) enzymes were noted (62, 63). Similar effects on SAA metabolism are present in long-lived genetic models of GH and TH deficiency despite normal calorie and SAA intake. For example, in Ames dwarf mice, hepatic TSP enzyme activities, as well as those of the TMP involved in methionine-dependent methyl donation and methionine recycling, are significantly increased (173), leading to a decrease in steady-state levels of the universal methyl donor, SAM, and an increase in metabolic flux of methionine through the TSP (20, 174). Increased flux is accompanied by increased expression of TMP and TSP genes, including CBS and CGL in the liver and increased methionine flux through the TSP in the brain and kidney. SAA metabolism is also altered in similar directions in Snell dwarf mice (178). Similar to hypopituitary dwarf mice, GHRKO and growth hormone releasing hormone knockout mice show perturbations in metabolic and gene expression signatures of SAA and glutathione pathways, including increased expression of CBS and CGL (3, 21). Pharmacological inhibition of TH production results in similar changes in hepatic SAA metabolism, CGL expression, and H2S production (66).
Changes in hepatic SAA metabolism described above are coincident with increased expression of TMP and TSP genes, including CBS and CGL, which are likely driven, in part, by ATF4. ATF4 is increased on the protein (but not mRNA) level in multiple genetic and dietary models of longevity (88, 105). While the mechanism in dietary models is likely via GCN2 kinase activation as discussed above, the mechanism in hypopituitary models remains unknown. However, mouse models of subclinical hypothyroidism display increased ER stress (199), a known trigger of ATF4 activation (49). Our recent finding that hepatic ATF4 is also increased specifically in the hypothyroid state (66) suggests a direct or indirect role of TH signaling in ATF4-mediated regulation of CGL. Nonetheless, mechanistic details of ATF4 stabilization under hypothyroid states and direct evidence of a causal role of ATF4 in hypothyroid-induced H2S production remain to be tested in these models.
How could H2S specifically contribute to extended longevity? A variety of nonmutually exclusive possibilities are likely. H2S is best characterized by its ability to posttranslationally modify exposed cysteine or disulfide residues on the surface of target proteins, including membrane receptors, thus altering their structure, stability, and/or function. For example, H2S regulates multiple physiological endpoints via sulfhydration of ATP-dependent potassium channels (KATP channels) present on specific cell types as discussed previously (85, 198). On vascular smooth muscle cells, stimulation of KATP channels results in vasorelaxation (128), while in pancreatic beta cells, KATP stimulation by H2S decreases insulin secretion (192). Thus, increased H2S could contribute to alterations in lipid metabolism and insulin sensitivity observed in these long-lived mouse models via effects on KATP channel activity in these or other cell types.
H2S may also contribute to the reduction of hormones negatively associated with longevity, including IGF-1. Recently, we found that H2S is required for the reduction of circulating IGF-1 and thyroxine (T4) levels found in drug-induced hypothyroidism or upon fasting (66). H2S may also contribute to the decrease in serum T3, T4, blood glucose, and oxygen consumption in rats and chickens fed garlic and garlic-derived polysulfides (134, 168, 169, 175). This role of H2S in the negative regulation of TH and GH action, and thus positive regulation of its own production, is consistent with the ability of NaHS supplementation to increase renal CBS and CGL expression and activity and to improve H2S bioavailability in kidney, plasma, and urine of aged mice (69). Future studies will be required to determine the mechanism by which H2S contributes to the downregulation of these hormones, and whether it involves KATP channel polarization or some other mechanism.
Finally, epigenetic changes could also serve as a link between increased transmethylation and H2S production in dwarf models, with implications for longevity. Treatment of hypopituitary mice with GH early in life abrogates longevity benefits, presumably through epigenetic changes that occur in the presence of GH during this narrow developmental window (125). Intriguingly, hepatic H2S production capacity, which remains high in 18-month-old dwarf mice, is also reduced late in life by early-life GH exposure (66). Whether this is a result of epigenetic changes in TSP genes themselves or some other mechanism, for example, changes in hypothalamic inflammation (144), remains to be seen. A summary of hormonal regulation of endogenous H2S production and the interaction with DR in animals is given in Figure 5.
FIG. 5.
Hormonal control of hepatic H2S production capacity occurs at multiple levels of regulation. Hypothalamic GHRH and thyrotropin-releasing hormone (TRH) induces GH and TSH production/release from the pituitary gland. TSH stimulates the production of T4 in the thyroid gland, which is a precursor to the active form of TH, T3. GH and TH positively regulate each other, and both suppress endogenous H2S production in the liver via independent and additive mechanisms. GH acts via growth hormone receptor (GHR), the adaptor protein IRS-1, and intracellular signal transduction via the JAK2/STAT5 pathway to inhibit H2S production, in part, through substrate-level control via negative regulation of autophagy. T3 binds to THR to negatively regulate CGL and CBS gene expression, as well as the transcriptional activator of CGL expression, ATF4. DR can also increase H2S production through numerous effects, including activation of ATF4 via the amino acid deprivation sensing response via GCN2 and p-eIF2α, as well as by increasing FGF21 expression (green), as well as effects on hypothalamic signals resulting in suppressed production/release and/or signaling of GHRH and TRH. ATF4, activating transcription factor 4; DR, dietary restriction; GCN2, general control nondepressible 2; GH, growth hormone; GHRH, growth hormone releasing hormone; T3, triiodothyronine; T4, thyroxine; TH, thyroid hormone; THR, thyroid hormone receptor; TSH, thyroid-stimulating hormone. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In summary, genetic models of longevity due to perturbations in GH and TH signaling lead to profound alterations in SAA metabolism, including the expression of H2S generating enzymes CGL and CBS, particularly in the liver. Similar changes are observed on DR. An integrated and composite model of the regulation and cross talk between GH, TH, diet, and H2S is presented in Figure 6.
FIG. 6.
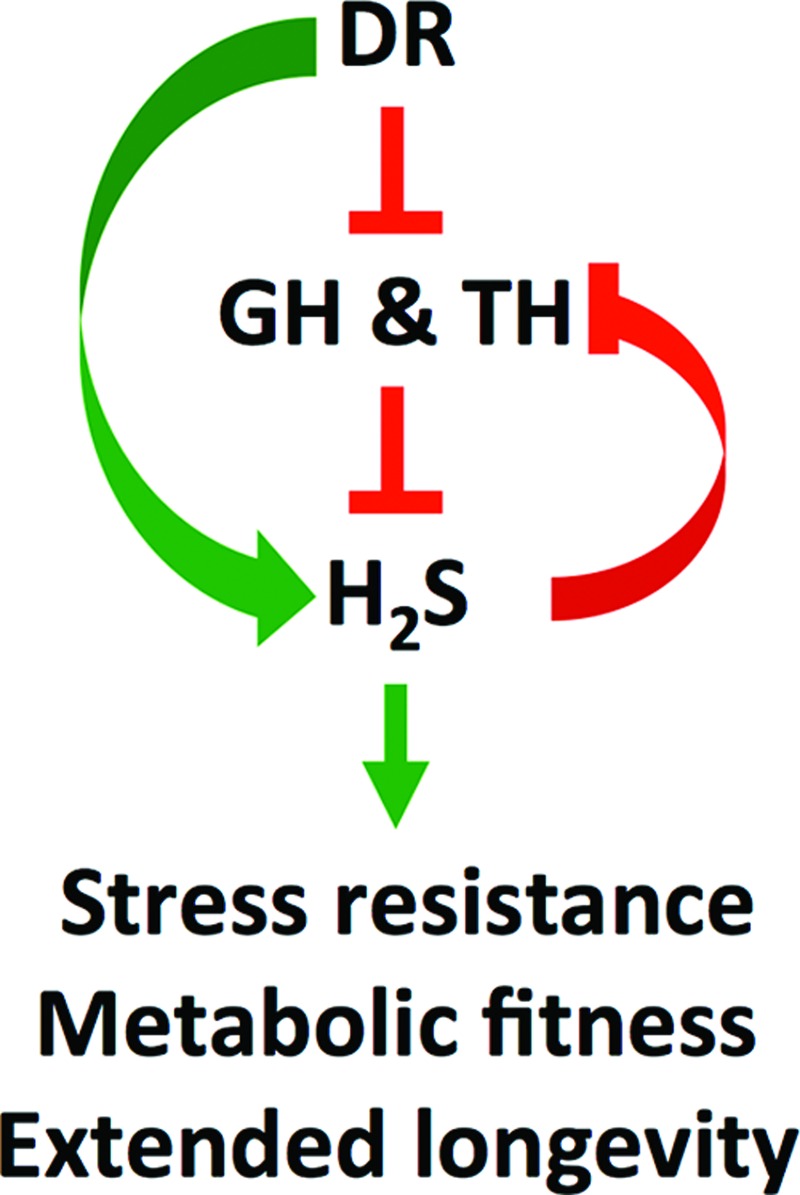
Dietary and hormonal control of H2S in longevity regulation. DR stimulates endogenous H2S production directly through energy and nutrient sensing pathways and/or indirectly through suppression of GH and TH production and signaling. Inhibition of GH and TH signaling is sufficient to activate H2S production pathways, resulting in a positive feedback loop. H2S can act through multiple molecular-, cellular-, and organismal-level mechanisms to increase fitness and longevity. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Plasticity of TSP Gene Expression in Response to Different Stimuli: An Unbiased Approach Using the NCBI GEO Profiles Database
We next used an unbiased approach to identify factors affecting TSP gene expression with the potential to modulate H2S production in human, mouse, and rat tissues and cells using the NCBI Gene Expression Omnibus (GEO Profiles) Database (9, 38). Table 1 summarizes the reported effects of nutrition, hormone activity, drugs, environmental and occupational pollutants, medical treatments, and health-related conditions on CGL and CBS gene expression. A wide range of stimuli affect TSP gene expression. In terms of dietary influences, red meat consumption in humans is associated with decreased CGL expression in the colon of irritable bowel disease patients, but not in control patients (GDS3471/217127_at/CTH). In mice, HFD (GDS4783/10503023/Cth), ketogenic diet (GDS2738/1426243_at/Cth), “fast food diet” (GDS3232/1426243_at/Cth), high-salt diet (GDS1545/D17370_at/Cth), and zinc deficiency (GDS4614/1367838_at/Cth) are all associated with a reduction of CGL expression in various tissues, while fasting increases CGL expression in the liver (GDS3893/9031/Cth). CGL expression in the liver is also subject to circadian control (GDS3084/1367838_at/Cth). Whether or not the circadian-related expression pattern is due to the periodicity of feeding and/or physiological changes during the course of 24 h is yet to be determined.
Table 1.
Plasticity of Transsulfuration Pathway Gene Expression in Response to Dietary, Hormonal, and Other Environmental Cues
| Organism | Tissue | Stimulus or condition | Change in H2S producing enzyme | GeoProfile no. |
|---|---|---|---|---|
| Human | Colon | Red meat consumption | ↓CGL in IBD patients, no change in controls | GDS3897/A_23_P126103/CTH |
| Mouse | Liver | High-fat diet | ↓CGL | GDS4783/10503023/Cth |
| Mouse | White adipose tissue | High-fat diet | ↓CGL | GDS4811/1426243_at/Cth |
| Mouse | Liver | Fasting | ↑CGL | GDS3893/9031/Cth |
| Mouse | Liver | Ketogenic diet | ↓CGL | GDS2738/1426243_at/Cth |
| Rat | Diencephalon | Short-term zinc deficiency | ↓CGL | GDS4614/1367838_at/Cth |
| Rat | Kidney | Salt-induced hypertenstion | ↑CGL in the salt-resistant strain | GDS1545/D17370_at/Cth |
| Mouse | Inguinal adipose tissue | High-saturated fat diet | ↓CGL in high-weight gainer | GDS2319/392857/Cth |
| Mouse | Brain | Chimpanzee diet, human “Fast Food” diet, human “Café” diet | ↓CGL in human “Fast Food” diet | GDS3232/1426243_at/Cth |
| Rat | Liver | Growth hormone addition | ↓CGL | GDS862/8.2.2.19/Cth |
| Human | Visceral adipose tissue | Diabetes | ↓CGL in diabetic patients | GDS3665/105377/CTH |
| Human | Thyroid | Papillary thyroid carcinoma | ↓CGL compared to normal tissue | GDS1665/206085_s_at/CTH |
| Mouse | Hypothalamus | High-fat diet | ↓CGL in high-weight gainers on high-fat diet | GDS2320/392857/Cth |
| Mouse | Liver | Leptin interaction in ob/ob mice | ↓CGL w/Leptin addition | GDS3653/ILMN_2733193/Cth |
| Human | Smooth muscle cell | Serum withdrawal | ↑CGL | GDS3852/217127_at/CTH |
| Mouse | Smooth muscle cell | Retinoic acid | ↓CGL | GDS799/2827/Cth |
| Human | MCF7 breast cancer cells | Estrogen receptor silencing | ↓CBS | GDS4061/212816_s_at/CBS |
| Human | Liver | Alcoholic hepatitis | ↓CBS | GDS4389/212816_s_at/CBS |
| Human | Midbrain | Cocaine | ↑CBS | GDS5047/ILMN_1804735/CBS |
| Human | Bronchial epithelial cells | Cigarette smoke | ↑CGL | GDS3493/217127_at/CTH |
| Rat | Pancreas | Long-term ethanol consumption | ↑CGL | GDS2107/1367838_at/Cth |
| Mouse | Striatum | Morphine | ↑CGL w/Acute, no change w/Chronic | GDS2815/1426243_at/Cth |
| Human | Lymphoblastoid cells | Thapsigargin | ↑CGL | GDS4130/206085_s_at/CTH |
| Mouse | Adipocytes | Metformin | ↑CGL | GDS3808/1426243_at/Cth |
| Human | Lymphoblastic leukemia tumors | Ionizing radiation | ↑CGL in resistant tumors | GDS3471/217127_at/CTH |
| Human | Squamous cell carcinoma | Ionizing radiation | ↑CGL in resistant tumors | GDS3124/217127_at/CTH |
| Human | Prostate adenocarcinoma | Ionizing radiation | ↓CBS | GDS725/38474_at/CBS |
| Mouse | Fibroblasts | Ionizing radiation | ↑CGL in response to IR, ↓CGL in sensitive cells | GDS1445/1426243_at/Cth |
| Mouse | Skin | UV radiation | ↓CGL | GDS2139/1426243_at/Cth |
| Human | Peripheral blood mononuclear cells | Asthma exacerbation | ↓CGL | GDS3615/206085_s_at/CTH |
| Human | Bronchial epithelial cells | Dust mite extract | ↑CGL, ↑CBS | GDS3003/217127_at/CTH |
| Human | Microvascular endothelial cells | Air pollutants | ↑CGL | GDS2889/GI_34328938-A/CTH |
| Rat | Lung | Chronic silica exposure | ↓CGL | GDS4579/1367838_at/Cth |
| Rat | Lung | VALI | ↑CGL in VALI resistant, ↓CGL in VALI sensitive | GDS2709/1367838_at/Cth |
| Mouse | Heart | Particulate matter | ↑CGL | GDS3660/1426243_at/Cth |
| Mouse | Lung | Ozone | ↑CGL | GDS4585/1426243_at/Cth |
| Mouse | Lung | Arsenate | ↑CGL | GDS4914/1426243_at/Cth |
| Rat | Liver | Circadian regulation | Undulations in CGL as a function of time of day | GDS3084/1367838_at/Cth |
| Human | Biceps | Infantile-onset Pompe disease | ↓CGL in Pompe patients | GDS4410/217127_at/CTH |
| Human | Lung | IPF | ↓CGL in early and advanced IPF | GDS4279/206085_s_at/CTH |
| Human | Lung | Interstitial lung diseases | ↓CGL in various interstitial lung diseases | GDS3951/206085_s_at/CTH |
| Human | Kidney | Transplant rejection | ↑CGL in well-functioning transplant | GDS724/32962_at/CTH |
| Human | Prostate tumor | Metastasis | ↑CGL in highly metastatic tumors | GDS2865/217127_at/CTH |
| Mouse | Liver | Hutchinson–Gilford Progeria model | ↑CGL in the progeroid mice | GDS4490/10503023/Cth |
| Mouse | Liver | Glycerol kinase knockout | ↑CGL in the glycerol kinase knockout mice | GDS1555/1426243_at/Cth |
| Human | HCT116 cells | miR-34 overexpression | ↓CGL | GDS2755/217127_at/CTH |
| Mouse | Dermal fibroblasts | Aza-dC mediated demethylation | ↓CGL | GDS2044/1426243_at/Cth |
| Mouse | Brain | SIRT1 deficiency | ↓CGL | GDS4895/1426243_at/Cth |
| Human | iPSC | Transcription factor-induced iPSC | ↑CGL | GDS3842/20849/CTH |
| Mouse | Embryonic neurons | ATF4 deficiency | ↓CGL | GDS3311/1190332/Cth |
| Mouse | Liver | Hepatocellular carcinoma | ↓CGL in the tumor compared to normal tissue | GDS2006/1426243_at/Cth |
| Human | Erythroid cells | Erythroid differentiation in vitro | ↑CGL as differentiation progresses | GDS2431/217127_at/CTH |
| Mouse | Lung epithelial cells | Glutathione (GSH) supplementation | ↓CGL | GDS2875/1426243_at/Cth |
| Human | Lymphoblastoid cells | Starvation-induced autophagy | ↑CGL | GDS1369/206085_s_at/CTH |
| Human | Mammary epithelial cells | Telomerase (hTERT) overexpression | ↑CGL | GDS337/S52028_s_at/CTH |
| Mouse | Embryonic stem cells | Nanog or Oct4 knockdown | ↓CGL with Oct4 knockdown | GDS1824/1426243_at/Cth |
The NCBI GEO Profiles repository was used to extract data regarding the effects of dietary, hormonal, medical, environmental, and genetic stimuli on regulation of CGL (referred as CTH in the data set identifiers) and CBS gene expression. In addition, the biological relevance/endpoint correlating with the change in CGL or CBS gene expression is given in the “Change in H2S Producing Enzyme Column,” when provided in the GEO Profiles repository. For more information on each study, the GEO Profiles number in the far right column can be used to search for more details on the study, the original publications arising from the data set, and the study authors at the GEO Profiles website: www.ncbi.nlm.nih.gov/geoprofiles.
ATF4, activating transcription factor 4; CBS, cystathionine β-synthase; CGL, cystathionine-γ-lyase; iPSC, induced pluripotent stem cells; IPF, idiopathic pulmonary fibrosis; IR, ionizing radiation; VALI, ventilator-associated lung-induced injury.
Endocrine and hormonal influences on CGL and CBS expression are also evident in the GEO Profiles database. In human papillary thyroid carcinoma, CGL expression is decreased compared to adjacent normal tissue (GDS1665/206085_s_at/CTH), while CBS expression is decreased in human MCF7 breast cancer cells on silencing of the estrogen receptor expression (GDS4061/212816_s_at/CBS). Serum/growth factor withdrawal increases CGL expression in human smooth muscle cells, while GH addition to male rats decreases hepatic CGL expression (GDS862/8.2.2.19/Cth). Finally, leptin supplementation (GDS3653/ILMN_2733193/Cth) and retinoic acid treatment (GDS799/2827/Cth) decrease CGL expression in liver and smooth muscle cells, respectively. Future experiments are required to better understand how these changes in TSP gene expression impact H2S production in vivo.
H2S in Longevity Extension
CBS and CGL are multifunctional proteins with important roles in H2S generation, but are also directly responsible for de novo cysteine biogenesis, with indirect effects on protein translation, central one-carbon metabolism, and cellular redox state. Such pleiotropic effects will make it difficult and time-consuming to dissect the specific contribution of endogenous H2S to health and longevity phenotypes in the context of DR or long-lived mutants in rodent model systems. Nonetheless, data are rapidly accumulating in support of the ability of exogenous H2S to slow the aging process and extend longevity across evolutionary boundaries.
Longevity extension by H2S was first shown in the nematode worms Caenorhabditis elegans. Exposure to 50-ppm H2S in air during early development and continuing throughout life extends mean life span by 70% (114). Treatment early in adulthood with the H2S donor molecule and garlic constituent diallyl trisulfide also extends worm longevity in a skn-1-dependent manner (133) as does the slow-releasing H2S donor GYY4137 (137). Deletion of the H2S generating enzyme mpst-1 (3-MST) reduces worm life span, and this can be rescued with exogenous H2S in the form of GYY4137 (138). Increased H2S production is also required for extended longevity on removal of germ cells (183), a distinct model of longevity extension in worms. In the yeast Saccharomyces cerevisiae, the presence of exogenous H2S donors NaHS and GYY4137 during early logarithmic growth extends yeast chronological life span (65).
While the effects of exogenous H2S on rodent longevity are not yet known, there is an aging-dependent decline in endogenous H2S in tissues from wild-type rats (135) and mice (69) that is attenuated by exogenous H2S supplementation (69). Exogenous H2S in the form of injected NaHS or inhaled H2S also prevents neurodegeneration and neurovascular dysfunction in mouse models of Parkinson's induced by intracerebral homocysteine injection or administration of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, respectively (84, 87). On a cellular level, CGL deficiency in mouse embryonic fibroblasts results in premature replicative senescence, a cellular model of aging, and this is rescued by H2S (NaHS) supplementation (191).
H2S can also extend life span and defer senescence in plants. During postharvest of the daylily flower, endogenous H2S production is diminished due to decreased activities of the plant H2S-producing enzymes L- and D- cysteine desulfhydrases, coinciding with senescence of the plant. Exogenous NaHS blocks the decrease in endogenous H2S production, slows senescence, and extends postharvest life of daylilies (109). H2S also alleviates postharvest senescence of broccoli while activating expression of antioxidant defense-related genes (104). Treatment of grapes with a 1 mM NaHS solution decreases rotting and threshing, maintains antioxidant systems, and prevents senescence-related breakdown of chlorophyll and accumulation of carotenoid (120). Kiwi fruit senescence and tissue softening are similarly delayed and antioxidant systems maintained with NaHS treatment in a dose-dependent manner (200).
What are the implications of exogenous H2S exposure on humans? While acute high-dose exposures are lethal, the health effects of chronic low-dose exposure remain inconclusive (107). For example, a series of epidemiological studies on people from Rotorua, New Zealand, with long-term exposure to elevated levels of ambient H2S (average of ∼20 ppb, with a range of 0–64 ppb), found that people in the upper quartiles of H2S exposure had slightly improved psychomotor reaction times and episodic memory (139), without any association with cognitive dysfunction, mood impairment, or peripheral neuropathy (132, 139).
Conclusions
At the right concentration, exogenous H2S has clear health benefits, including longevity extension in experimental model organisms. Increased endogenous H2S production is common to numerous experimental models of extended longevity, including genetic models with shared defects in GH and/or TH signaling (Ames, Snell, GHRKO, FGF21o/e, IRS-1KO), as well as various forms of DR, along with broader changes in SAA metabolism with implications for DNA methylation and redox status. The relative contribution of increased H2S to health span or life span benefits in these models remains to be determined, as does the mechanism by which it occurs. In conclusion, our ability to control H2S levels using exogenous H2S donors or by modifying the endogenous H2S equilibrium has the potential to increase “shelf life” across evolutionary boundaries, including our own.
Abbreviations Used
- 1ADP
1-amino-D-proline
- 3-MST
3-mercaptopyruvate sulfurtransferase
- ATF4
activating transcription factor 4
- CAT
cysteine aminotransferase
- CBS
cystathionine β-synthase
- CGL
cystathionine-γ-lyase
- CR
calorie restriction
- CTH
cystathionase
- DR
dietary restriction
- EAA
essential amino acids
- ER
endoplasmic reticulum
- ETHE1
ethylmalonic encephalopathy 1 protein
- GCN2
general control nondepressible 2
- GH
growth hormone
- GHR
growth hormone receptor
- GHRH
growth hormone releasing hormone
- GHRKO
growth hormone receptor knockout
- H2S
hydrogen sulfide
- HFD
high-fat diet
- IF
intermittent fasting
- IGF-1
insulin-like growth factor-1
- KATP channel
ATP-dependent potassium channel
- MR
methionine restriction
- mTOR
mechanistic target of rapamycin
- PAG
propargylglycine
- PLP
pyridoxal phosphate
- SAA
sulfur amino acids
- SQR
sulfide quinone oxidoreductase
- SRB
sulfate reducing bacteria
- T3
triiodothyronine
- T4
thyroxine
- TH
thyroid hormone
- THR
thyroid hormone receptor
- TMP
transmethylation pathway
- TRH
thyrotropin-releasing hormone
- TSH
thyroid-stimulating hormone
- TSP
transsulfuration pathway
Acknowledgments
This work was supported by the following grants: nos. AG050777 (C.H.); DK090629 and AG036712 (J.R.M.); DK098525 and DK056123 (A.N.H.).
References
- 1.Ables GP, Perrone CE, Orentreich D, and Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One 7: e51357, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves MG, Soares AF, Carvalho RA, and Oliveira PJ. Sodium hydrosulfide improves the protective potential of the cardioplegic histidine buffer solution. Eur J Pharmacol 654: 60–67, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Amador-Noguez D, Yagi K, Venable S, and Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell 3: 423–441, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Arndt S, Baeza-Garza CD, Logan A, Rosa T, Wedmann R, Prime TA, Martin JL, Saeb-Parsy K, Krieg T, Filipovic MR, Hartley RC, and Murphy MP. Assessment of H2S in vivo using the newly developed mitochondria-targeted mass spectrometry probe MitoA. J Biol Chem 292: 7761–7773, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arum O, Bonkowski MS, Rocha JS, and Bartke A. The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell 8: 756–760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asfar P, Calzia E, and Radermacher P. Is pharmacological, H(2)S-induced “suspended animation” feasible in the ICU? Crit Care 18: 215, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atzmon G, Barzilai N, Hollowell JG, Surks MI, and Gabriely I. Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab 94: 1251–1254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atzmon G, Barzilai N, Surks MI, and Gabriely I. Genetic predisposition to elevated serum thyrotropin is associated with exceptional longevity. J Clin Endocrinol Metab 94: 4768–4775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, and Soboleva A. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res 41: D991–D995, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartke A. and Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol 63: 189–225, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Bartke A, Chandrashekar V, Turyn D, Steger RW, Debeljuk L, Winters TA, Mattison JA, Danilovich NA, Croson W, Wernsing DR, and Kopchick JJ. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock-out mice. Proc Soc Exp Biol Med 222: 113–123, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Beaumont M, Andriamihaja M, Lan A, Khodorova N, Audebert M, Blouin JM, Grauso M, Lancha L, Benetti PH, Benamouzig R, Tome D, Bouillaud F, Davila AM, and Blachier F. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: the adaptive response. Free Radic Biol Med 93: 155–164, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Blackstone E, Morrison M, and Roth MB. H2S induces a suspended animation-like state in mice. Science 308: 518, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, and Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One 4: e4567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, and Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci 61: 562–567, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Bowers J, Terrien J, Clerget-Froidevaux MS, Gothié JD, Rozing MP, Westendorp RG, van Heemst D, and Demeneix BA. Thyroid hormone signaling and homeostasis during aging. Endocr Rev 34: 556–589, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Bravo E, Palleschi S, Aspichueta P, Buque X, Rossi B, Cano A, Napolitano M, Ochoa B, and Botham KM. High fat diet-induced non alcoholic fatty liver disease in rats is associated with hyperhomocysteinemia caused by down regulation of the transsulphuration pathway. Lipids Health Dis 10: 60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown-Borg HM. Reduced growth hormone signaling and methionine restriction: interventions that improve metabolic health and extend life span. Ann N Y Acad Sci 1363: 40–49, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown-Borg HM, Borg KE, Meliska CJ, and Bartke A. Dwarf mice and the ageing process. Nature 384: 33, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Brown-Borg HM, Rakoczy S, Wonderlich JA, Armstrong V, and Rojanathammanee L. Altered dietary methionine differentially impacts glutathione and methionine metabolism in long-living growth hormone-deficient Ames dwarf and wild-type mice. Longev Healthspan 3: 10, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown-Borg HM, Rakoczy SG, Sharma S, and Bartke A. Long-living growth hormone receptor knockout mice: potential mechanisms of altered stress resistance. Exp Gerontol 44: 10–19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown-Borg HM, Rakoczy SG, Wonderlich JA, Rojanathammanee L, Kopchick JJ, Armstrong V, and Raasakka D. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell 13: 1019–1027, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene ND, and Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153: 228–239, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrese EJ. and Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal 5: 25–38, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, and Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol 3: 448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caro P, Gomez J, Sanchez I, Naudi A, Ayala V, López-Torres M, Pamplona R, and Barja G. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuvenation Res 12: 421–434, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Chan MV. and Wallace JL. Hydrogen sulfide-based therapeutics and gastrointestinal diseases: translating physiology to treatments. Am J Physiol Gastrointest Liver Physiol 305: G467–G473, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Jhee KH, and Kruger WD. Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J Biol Chem 279: 52082–52086, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, and Banerjee R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601–11612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clemmons DR, Snyder DK, Williams R, and Underwood LE. Growth hormone administration conserves lean body mass during dietary restriction in obese subjects. J Clin Endocrinol Metab 64: 878–883, 1987 [DOI] [PubMed] [Google Scholar]
- 31.Cooper CE. and Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40: 533–539, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Coschigano KT, Clemmons D, Bellush LL, and Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141: 2608–2613, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, and Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144: 3799–3810, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Dacks PA, Moreno CL, Kim ES, Marcellino BK, and Mobbs CV. Role of the hypothalamus in mediating protective effects of dietary restriction during aging. Front Neuroendocrinol 34: 95–106, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derous D, Mitchell SE, Wang L, Green CL, Wang Y, Chen L, Han JJ, Promislow DEL, Lusseau D, Douglas A, and Speakman JR. The effects of graded levels of calorie restriction: XI. Evaluation of the main hypotheses underpinning the life extension effects of CR using the hepatic transcriptome. Aging (Albany NY) 9: 1770–1824, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickhout JG, Carlisle RE, Jerome DE, Mohammed-Ali Z, Jiang H, Yang G, Mani S, Garg SK, Banerjee R, Kaufman RJ, Maclean KN, Wang R, and Austin RC. Integrated stress response modulates cellular redox state via induction of cystathionine γ-lyase: cross-talk between integrated stress response and thiol metabolism. J Biol Chem 287: 7603–7614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domouzoglou EM, Fisher FM, Astapova I, Fox EC, Kharitonenkov A, Flier JS, Hollenberg AN, and Maratos-Flier E. Fibroblast growth factor 21 and thyroid hormone show mutual regulatory dependency but have independent actions in vivo. Endocrinology 155: 2031–2040, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar R, Domrachev M, and Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, and Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finamor I, Perez S, Bressan CA, Brenner CE, Rius-Perez S, Brittes PC, Cheiran G, Rocha MI, da Veiga M, Sastre J, and Pavanato MA. Chronic aspartame intake causes changes in the trans-sulphuration pathway, glutathione depletion and liver damage in mice. Redox Biol 11: 701–707, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flier JS, Harris M, and Hollenberg AN. Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring. J Clin Invest 105: 859–861, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flurkey K, Papaconstantinou J, Miller RA, and Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A 98: 6736–6741, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, Baar EL, Veronese N, Cottrell SE, Fenske RJ, Bertozzi B, Brar HK, Pietka T, Bullock AD, Figenshau RS, Andriole GL, Merrins MJ, Alexander CM, Kimple ME, and Lamming DW. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep 16: 520–530, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontana L, Klein S, Holloszy JO, and Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab 91: 3232–3235, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Fontana L. and Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell 161: 106–118, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fontana L, Partridge L, and Longo VD. Extending healthy life span—from yeast to humans. Science 328: 321–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fontana L, Weiss EP, Villareal DT, Klein S, and Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7: 681–687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu M, Zhang W, Wu L, Yang G, Li H, and Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A 109: 2943–2948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fusakio ME, Willy JA, Wang Y, Mirek ET, Al Baghdadi RJ, Adams CM, Anthony TG, and Wek RC. Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol Biol Cell 27: 1536–1551, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallinetti J, Harputlugil E, and Mitchell JR. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J 449: 1–10, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gesing A, Al-Regaiey KA, Bartke A, and Masternak MM. Growth hormone abolishes beneficial effects of calorie restriction in long-lived Ames dwarf mice. Exp Gerontol 58: 219–229, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giuliani D, Ottani A, Zaffe D, Galantucci M, Strinati F, Lodi R, and Guarini S. Hydrogen sulfide slows down progression of experimental Alzheimer's disease by targeting multiple pathophysiological mechanisms. Neurobiol Learn Mem 104: 82–91, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Grandison RC, Piper MD, and Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462: 1061–1064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregory JF, DeRatt BN, Rios-Avila L, Ralat M, and Stacpoole PW. Vitamin B6 nutritional status and cellular availability of pyridoxal 5′-phosphate govern the function of the transsulfuration pathway's canonical reactions and hydrogen sulfide production via side reactions. Biochimie 126: 21–26, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griffin NW, Ahern PP, Cheng J, Heath AC, Ilkayeva O, Newgard CB, Fontana L, and Gordon JI. Prior dietary practices and connections to a human gut microbial metacommunity alter responses to diet interventions. Cell Host Microbe 21: 84–96, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, and Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3: 70ra13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo W, Cheng ZY, and Zhu YZ. Hydrogen sulfide and translational medicine. Acta Pharmacol Sin 34: 1284–1291, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, and Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Harel Z. and Tannenbaum GS. Dietary protein restriction impairs both spontaneous and growth hormone-releasing factor-stimulated growth hormone release in the rat. Endocrinology 133: 1035–1043, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Harputlugil E, Hine C, Vargas D, Robertson L, Manning BD, and Mitchell JR. The TSC complex is required for the benefits of dietary protein restriction on stress resistance in vivo. Cell Rep 8: 1160–1170, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartle MD. and Pluth MD. A practical guide to working with H2S at the interface of chemistry and biology. Chem Soc Rev 45: 6108–6117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heinonen K. Effects of hypothyroidism and thyroxine substitution on the metabolism of L-methionine, L-cystathionine and taurine in developing rat brain. Acta Endocrinol (Copenh) 80: 487–500, 1975 [DOI] [PubMed] [Google Scholar]
- 63.Heinonen K. Effects of hypo- and hyperthyroidism on the activity of cystathionase in mammalian parenchymatous organs during early development. Experientia 33: 1427–1428, 1977 [DOI] [PubMed] [Google Scholar]
- 64.Hildebrandt TM. and Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275: 3352–3361, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino-Villarreal JH, Mejia P, Ozaki CK, Wang R, Gladyshev VN, Madeo F, Mair WB, and Mitchell JR. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160: 132–144, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hine C, Kim HJ, Zhu Y, Harputlugil E, Longchamp A, Matos MS, Ramadoss P, Bauerle K, Brace L, Asara JM, Ozaki CK, Cheng SY, Singha S, Ahn KH, Kimmelman A, Fisher FM, Pissios P, Withers DJ, Selman C, Wang R, Yen K, Longo VD, Cohen P, Bartke A, Kopchick JJ, Miller R, Hollenberg AN, and Mitchell JR. Hypothalamic-pituitary axis regulates hydrogen sulfide production. Cell Metab 25: 1320–1333 e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hine C. and Mitchell JR. Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp Gerontol 68: 26–32, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horsman JW. and Miller DL. Mitochondrial sulfide quinone oxidoreductase prevents activation of the unfolded protein response in hydrogen sulfide. J Biol Chem 291: 5320–5325, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou CL, Wang MJ, Sun C, Huang Y, Jin S, Mu XP, Chen Y, and Zhu YC. Protective effects of hydrogen sulfide in the ageing kidney. Oxid Med Cell Longev 2016: 7570489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang P, Chen S, Wang Y, Liu J, Yao Q, Huang Y, Li H, Zhu M, Wang S, Li L, Tang C, Tao Y, Yang G, Du J, and Jin H. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide 46: 192–203, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Hughes MN, Centelles MN, and Moore KP. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med 47: 1346–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Ikeda M, Ishima Y, Shibata A, Chuang VTG, Sawa T, Ihara H, Watanabe H, Xian M, Ouchi Y, Shimizu T, Ando H, Ukawa M, Ishida T, Akaike T, Otagiri M, and Maruyama T. Quantitative determination of polysulfide in albumins, plasma proteins and biological fluid samples using a novel combined assays approach. Anal Chim Acta 969: 18–25, 2017 [DOI] [PubMed] [Google Scholar]
- 73.Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, and Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8: 77–83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson MR, Melideo SL, and Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 51: 6804–6815, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Jansen SW, Akintola AA, Roelfsema F, van der Spoel E, Cobbaert CM, Ballieux BE, Egri P, Kvarta-Papp Z, Gereben B, Fekete C, Slagboom PE, van der Grond J, Demeneix BA, Pijl H, Westendorp RG, and van Heemst D. Human longevity is characterised by high thyroid stimulating hormone secretion without altered energy metabolism. Sci Rep 5: 11525, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jenkins TA, Nguyen JC, and Hart JL. Decreased vascular H2S production is associated with vascular oxidative stress in rats fed a high-fat western diet. Naunyn Schmiedebergs Arch Pharmacol 389: 783–790, 2016 [DOI] [PubMed] [Google Scholar]
- 77.Jennings AS, Ferguson DC, and Utiger RD. Regulation of the conversion of thyroxine to triiodothyronine in the perfused rat liver. J Clin Invest 64: 1614–1623, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jha S, Calvert JW, Duranski MR, Ramachandran A, and Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol 295: H801–H806, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang J, Chan A, Ali S, Saha A, Haushalter KJ, Lam WL, Glasheen M, Parker J, Brenner M, Mahon SB, Patel HH, Ambasudhan R, Lipton SA, Pilz RB, and Boss GR. Hydrogen sulfide—mechanisms of toxicity and development of an antidote. Sci Rep 6: 20831, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson JE. and Johnson FB. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS One 9: e97729, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kabil H, Kabil O, Banerjee R, Harshman LG, and Pletcher SD. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc Natl Acad Sci U S A 108: 16831–16836, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kabil O, Motl N, and Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta 1844: 1355–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kabil O, Vitvitsky V, Xie P, and Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 15: 363–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, and Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience 252: 302–319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang M, Hashimoto A, Gade A, and Akbarali HI. Interaction between hydrogen sulfide-induced sulfhydration and tyrosine nitration in the KATP channel complex. Am J Physiol Gastrointest Liver Physiol 308: G532–G539, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, and Lillie LE. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol Appl Pharmacol 103: 482–490, 1990 [DOI] [PubMed] [Google Scholar]
- 87.Kida K, Yamada M, Tokuda K, Marutani E, Kakinohana M, Kaneki M, and Ichinose F. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson's disease. Antioxid Redox Signal 15: 343–352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kilberg MS, Shan J, and Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Sr., Gojon G, Jr., Wang R, Karusula N, Nicholson CK, Calvert JW, and Lefer DJ. H(2)S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127: 1116–1127, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kovacic D, Glavnik N, Marinsek M, Zagozen P, Rovan K, Goslar T, Mars T, and Podbregar M. Total plasma sulfide in congestive heart failure. J Card Fail 18: 541–548, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Kozieł R, Ruckenstuhl C, Albertini E, Neuhaus M, Netzberger C, Bust M, Madeo F, Wiesner RJ, and Jansen-Dürr P. Methionine restriction slows down senescence in human diploid fibroblasts. Aging Cell 13: 1038–1048, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kump L, Pavlov A, and Arthur M. Massive release of hydrogen sulfide to the surface ocean and atmosphere during intervals of oceanic anoxia. Geology 33: 397–400, 2005 [Google Scholar]
- 93.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, and Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta 1797: 1500–1511, 2010 [DOI] [PubMed] [Google Scholar]
- 94.Lamarque JF, Kiehl JT, and Orlando JJ. Role of hydrogen sulfide in a Permian-Triassic boundary ozone collapse. Geophys Res Lett 34: 1–4, 2007 [Google Scholar]
- 95.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Meta HITc, Bork P, Wang J, Ehrlich SD, and Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541–546, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, and Gladyshev VN. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat Commun 5: 3592, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee HJ, Feliers D, Mariappan MM, Sataranatarajan K, Choudhury GG, Gorin Y, and Kasinath BS. Tadalafil integrates nitric oxide-hydrogen sulfide signaling to inhibit high glucose-induced matrix protein synthesis in podocytes. J Biol Chem 290: 12014–12026, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JI, Dominy JE, Sikalidis AK, Hirschberger LL, Wang W, and Stipanuk MH. HepG2/C3A cells respond to cysteine deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol Genomics 33: 218–229, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, and Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci U S A 105: 2498–2503, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, and Deng LW. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One 6: e21077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lees EK, Król E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW, and Delibegovic M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 13: 817–827, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lewis RJ. and Copley GB. Chronic low-level hydrogen sulfide exposure and potential effects on human health: a review of the epidemiological evidence. Crit Rev Toxicol 45: 93–123, 2015 [DOI] [PubMed] [Google Scholar]
- 103.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, and Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19: 1196–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Li SP, Hu KD, Hu LY, Li YH, Jiang AM, Xiao F, Han Y, Liu YS, and Zhang H. Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J Agric Food Chem 62: 1119–1129, 2014 [DOI] [PubMed] [Google Scholar]
- 105.Li W, Li X, and Miller RA. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell 13: 1012–1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang L, Zhou T, Jiang J, Pierce JH, Gustafson TA, and Frank SJ. Insulin receptor substrate-1 enhances growth hormone-induced proliferation. Endocrinology 140: 1972–1983, 1999 [DOI] [PubMed] [Google Scholar]
- 107.Lim E, Mbowe O, Lee AS, and Davis J. Effect of environmental exposure to hydrogen sulfide on central nervous system and respiratory function: a systematic review of human studies. Int J Occup Environ Health 22: 80–90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Linden DR, Furne J, Stoltz GJ, Abdel-Rehim MS, Levitt MD, and Szurszewski JH. Sulphide quinone reductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. Br J Pharmacol 165: 2178–2190, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu D, Xu S, Hu H, Pan J, Li P, and Shen W. Endogenous hydrogen sulfide homeostasis is responsible for the alleviation of senescence of postharvest daylily flower via increasing antioxidant capacity and maintained energy status. J Agric Food Chem 65: 718–726, 2017 [DOI] [PubMed] [Google Scholar]
- 110.Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS, and Shi S. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell 15: 66–78, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luna-Sanchez M, Hidalgo-Gutierrez A, Hildebrandt TM, Chaves-Serrano J, Barriocanal-Casado E, Santos-Fandila A, Romero M, Sayed RK, Duarte J, Prokisch H, Schuelke M, Distelmaier F, Escames G, Acuna-Castroviejo D, and Lopez LC. CoQ deficiency causes disruption of mitochondrial sulfide oxidation, a new pathomechanism associated with this syndrome. EMBO Mol Med 9: 78–95, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magee EA, Richardson CJ, Hughes R, and Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr 72: 1488–1494, 2000 [DOI] [PubMed] [Google Scholar]
- 113.Mayengbam S, Raposo S, Aliani M, and House JD. A Vitamin B-6 antagonist from flaxseed perturbs amino acid metabolism in moderately Vitamin B-6-deficient male rats. J Nutr 146: 14–20, 2016 [DOI] [PubMed] [Google Scholar]
- 114.Miller DL. and Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A 104: 20618–20622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mistry RK, Murray TV, Prysyazhna O, Martin D, Burgoyne JR, Santos C, Eaton P, Shah AM, and Brewer AC. Transcriptional regulation of cystathionine-γ-lyase in endothelial cells by NADPH oxidase 4-dependent signaling. J Biol Chem 291: 1774–1788, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Buron MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, and de Cabo R. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 23: 1093–1112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Módis K, Coletta C, Erdélyi K, Papapetropoulos A, and Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J 27: 601–611, 2013 [DOI] [PubMed] [Google Scholar]
- 118.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, and Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagy P, Palinkas Z, Nagy A, Budai B, Toth I, and Vasas A. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim Biophys Acta 1840: 876–891, 2014 [DOI] [PubMed] [Google Scholar]
- 120.Ni ZJ, Hu KD, Song CB, Ma RH, Li ZR, Zheng JL, Fu LH, Wei ZJ, and Zhang H. Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid Med Cell Longev 2016: 4715651, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 1787: 856–863, 2009 [DOI] [PubMed] [Google Scholar]
- 122.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal 17: 32–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Olson KR. and Straub KD. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology (Bethesda) 31: 60–72, 2016 [DOI] [PubMed] [Google Scholar]
- 124.Orentreich N, Matias JR, DeFelice A, and Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr 123: 269–274, 1993 [DOI] [PubMed] [Google Scholar]
- 125.Panici JA, Harper JM, Miller RA, Bartke A, Spong A, and Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J 24: 5073–5079, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, and Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paul BD, Sbodio JI, Xu R, Vandiver MS, Cha JY, Snowman AM, and Snyder SH. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington's disease. Nature 509: 96–100, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paul BD. and Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012 [DOI] [PubMed] [Google Scholar]
- 129.Peh MT, Anwar AB, Ng DS, Atan MS, Kumar SD, and Moore PK. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide 41: 138–145, 2014 [DOI] [PubMed] [Google Scholar]
- 130.Pettit AP, Jonsson WO, Bargoud AR, Mirek ET, Peelor FF, 3rd, Wang Y, Gettys TW, Kimball SR, Miller BF, Hamilton KL, Wek RC, and Anthony TG. Dietary methionine restriction regulates liver protein synthesis and gene expression independently of eukaryotic initiation factor 2 phosphorylation in mice. J Nutr 147: 1031–1040, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Polhemus DJ, Li Z, Pattillo CB, Gojon G, Giordano T, and Krum H. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc Ther 33: 216–226, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pope K, So YT, Crane J, and Bates MN. Ambient geothermal hydrogen sulfide exposure and peripheral neuropathy. Neurotoxicology 60: 10–15, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Powolny AA, Singh SV, Melov S, Hubbard A, and Fisher AL. The garlic constituent diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Exp Gerontol 46: 441–452, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prasad R, Rose MK, Garg SL, and Puri JP. Thyroid hormone levels in response to dietary supplementation of garlic (Allium Saticum) in chicken. Indian J Poult Sci 45: 97–99, 2010 [Google Scholar]
- 135.Predmore BL, Alendy MJ, Ahmed KI, Leeuwenburgh C, and Julian D. The hydrogen sulfide signaling system: changes during aging and the benefits of caloric restriction. Age (Dordr) 32: 467–481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Predmore BL, Lefer DJ, and Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal 17: 119–140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qabazard B, Ahmed S, Li L, Arlt VM, Moore PK, and Stürzenbaum SR. C. elegans aging is modulated by hydrogen sulfide and the sulfhydrylase/cysteine synthase cysl-2. PLoS One 8: e80135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Kumar SD, Rose P, Tan CH, Dymock BW, Wei F, Swain SC, Halliwell B, Sturzenbaum SR, and Moore PK. Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid Redox Signal 20: 2621–2630, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reed BR, Crane J, Garrett N, Woods DL, and Bates MN. Chronic ambient hydrogen sulfide exposure and cognitive function. Neurotoxicol Teratol 42: 68–76, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, and Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci U S A 110: 13582–13587, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Richie JP, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, and Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 8: 1302–1307, 1994 [DOI] [PubMed] [Google Scholar]
- 142.Rozing MP, Westendorp RG, de Craen AJ, Frölich M, Heijmans BT, Beekman M, Wijsman C, Mooijaart SP, Blauw GJ, Slagboom PE, van Heemst D, and Group LLSL. Low serum free triiodothyronine levels mark familial longevity: the Leiden Longevity Study. J Gerontol A Biol Sci Med Sci 65: 365–368, 2010 [DOI] [PubMed] [Google Scholar]
- 143.Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, Gleixner C, Schmid C, Klug L, Sorgo AG, Eisenberg T, Buttner S, Marino G, Koziel R, Jansen-Durr P, Frohlich KU, Kroemer G, and Madeo F. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet 10: e1004347, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sadagurski M, Landeryou T, Cady G, Kopchick JJ, List EO, Berryman DE, Bartke A, and Miller RA. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell 14: 1045–1054, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, and Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation 120: S31–S36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sbodio JI, Snyder SH, and Paul BD. Transcriptional control of amino acid homeostasis is disrupted in Huntington's disease. Proc Natl Acad Sci U S A 113: 8843–8848, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Segall PE. and Timiras PS. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech Ageing Dev 5: 109–124, 1976 [DOI] [PubMed] [Google Scholar]
- 148.Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, and Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J 22: 807–818, 2008 [DOI] [PubMed] [Google Scholar]
- 149.Selman C. and Withers DJ. Mammalian models of extended healthy lifespan. Philos Trans R Soc Lond B Biol Sci 366: 99–107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shatalin K, Shatalina E, Mironov A, and Nudler E. H2S: a universal defense against antibiotics in bacteria. Science 334: 986–990, 2011 [DOI] [PubMed] [Google Scholar]
- 151.Shen X, Carlstrom M, Borniquel S, Jadert C, Kevil CG, and Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shen X, Peter EA, Bir S, Wang R, and Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med 52: 2276–2283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, and Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009 [DOI] [PubMed] [Google Scholar]
- 154.Sikalidis AK, Lee JI, and Stipanuk MH. Gene expression and integrated stress response in HepG2/C3A cells cultured in amino acid deficient medium. Amino Acids 41: 159–171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sikalidis AK. and Stipanuk MH. Growing rats respond to a sulfur amino acid-deficient diet by phosphorylation of the alpha subunit of eukaryotic initiation factor 2 heterotrimeric complex and induction of adaptive components of the integrated stress response. J Nutr 140: 1080–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126: 987–1002, 2005 [DOI] [PubMed] [Google Scholar]
- 157.Singh S, Padovani D, Leslie RA, Chiku T, and Banerjee R. Relative contributions of cystathionine β-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457–22466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Singh SB. and Lin HC. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms 3: 866–889, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, and Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19: 418–430, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Speakman JR. and Mitchell SE. Caloric restriction. Mol Aspects Med 32: 159–221, 2011 [DOI] [PubMed] [Google Scholar]
- 161.Stipanuk MH. and Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 206: 267–277, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Straus DS. and Takemoto CD. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol Endocrinol 4: 91–100, 1990 [DOI] [PubMed] [Google Scholar]
- 163.Sun L, Sadighi Akha AA, Miller RA, and Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci 64: 711–722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, and Bartke A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife 2: e01098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swindell WR. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genomics 10: 585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007 [DOI] [PubMed] [Google Scholar]
- 167.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 17: 68–80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tahiliani P. and Kar A. The combined effects of Trigonella and Allium extracts in the regulation of hyperthyroidism in rats. Phytomedicine 10: 665–668, 2003 [DOI] [PubMed] [Google Scholar]
- 169.Tahiliani P. and Kar A. Mitigation of thyroxine-induced hyperglycaemia by two plant extracts. Phytother Res 17: 294–296, 2003 [DOI] [PubMed] [Google Scholar]
- 170.Takano Y, Shimamoto K, and Hanaoka K. Chemical tools for the study of hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies. J Clin Biochem Nutr 58: 7–15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, and Zhu YC. VEGFR2 functions as an H2S -targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Teng H, Wu B, Zhao K, Yang G, Wu L, and Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc Natl Acad Sci U S A 110: 12679–12684, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uthus EO. and Brown-Borg HM. Altered methionine metabolism in long living Ames dwarf mice. Exp Gerontol 38: 491–498, 2003 [DOI] [PubMed] [Google Scholar]
- 174.Uthus EO. and Brown-Borg HM. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech Ageing Dev 127: 444–450, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Varmaghany S, Karimi Torshizi MA, Rahimi S, Lotfollahian H, and Hassanzadeh M. The effects of increasing levels of dietary garlic bulb on growth performance, systolic blood pressure, hematology, and ascites syndrome in broiler chickens. Poult Sci 94: 1812–1820, 2015 [DOI] [PubMed] [Google Scholar]
- 176.Vella KR, Ramadoss P, Lam FS, Harris JC, Ye FD, Same PD, O'Neill NF, Maratos-Flier E, and Hollenberg AN. NPY and MC4R signaling regulate thyroid hormone levels during fasting through both central and peripheral pathways. Cell Metab 14: 780–790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vitvitsky V. and Banerjee R. H2S analysis in biological samples using gas chromatography with sulfur chemiluminescence detection. Methods Enzymol 554: 111–123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vitvitsky V, Martinov M, Ataullakhanov F, Miller RA, and Banerjee R. Sulfur-based redox alterations in long-lived Snell dwarf mice. Mech Ageing Dev 134: 321–330, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wallace JL, Vong L, McKnight W, Dicay M, and Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137: 569–578, 578 e1, 2009 [DOI] [PubMed] [Google Scholar]
- 180.Wang B, Zeng J, and Gu Q. Exercise restores bioavailability of hydrogen sulfide and promotes autophagy influx in livers of mice fed with high-fat diet. Can J Physiol Pharmacol 95: 667–674, 2017 [DOI] [PubMed] [Google Scholar]
- 181.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012 [DOI] [PubMed] [Google Scholar]
- 182.Wang WJ, Cai GY, Ning YC, Cui J, Hong Q, Bai XY, Xu XM, Bu R, Sun XF, and Chen XM. Hydrogen sulfide mediates the protection of dietary restriction against renal senescence in aged F344 rats. Sci Rep 6: 30292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wei Y. and Kenyon C. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc Natl Acad Sci U S A 113: E2832–E2841, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Whiteman M. and Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol 4: 13–32, 2011 [DOI] [PubMed] [Google Scholar]
- 185.Wiliński B, Wiliński J, Somogyi E, Piotrowska J, and Opoka W. Metformin raises hydrogen sulfide tissue concentrations in various mouse organs. Pharmacol Rep 65: 737–742, 2013 [DOI] [PubMed] [Google Scholar]
- 186.Wu D, Gao B, Li M, Yao L, Wang S, Chen M, Li H, Ma C, Ji A, and Li Y. Hydrogen sulfide mitigates kidney injury in high fat diet-induced obese mice. Oxid Med Cell Longev 2016: 2715718, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu SY. The effect of fasting on thyroidal T4-5′ monodeiodinating activity in mice. Acta Endocrinol (Copenh) 122: 175–180, 1990 [DOI] [PubMed] [Google Scholar]
- 188.Xue R, Hao DD, Sun JP, Li WW, Zhao MM, Li XH, Chen Y, Zhu JH, Ding YJ, Liu J, and Zhu YC. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid Redox Signal 19: 5–23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang G. Hydrogen sulfide in cell survival: a double-edged sword. Expert Rev Clin Pharmacol 4: 33–47, 2011 [DOI] [PubMed] [Google Scholar]
- 190.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, and Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, and Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18: 1906–1919, 2013 [DOI] [PubMed] [Google Scholar]
- 192.Yang W, Yang G, Jia X, Wu L, and Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol 569: 519–531, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zambrano A, García-Carpizo V, Gallardo ME, Villamuera R, Gómez-Ferrería MA, Pascual A, Buisine N, Sachs LM, Garesse R, and Aranda A. The thyroid hormone receptor β induces DNA damage and premature senescence. J Cell Biol 204: 129–146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y, and Zhao L. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 4: 232–241, 2010 [DOI] [PubMed] [Google Scholar]
- 195.Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, Yu RT, Evans RM, Kliewer SA, and Mangelsdorf DJ. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 1: e00065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao K, Li S, Wu L, Lai C, and Yang G. Hydrogen sulfide represses androgen receptor transactivation by targeting at the second zinc finger module. J Biol Chem 289: 20824–20835, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhao W, Zhang J, Lu Y, and Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhong GZ, Li YB, Liu XL, Guo LS, Chen ML, and Yang XC. Hydrogen sulfide opens the KATP channel on rat atrial and ventricular myocytes. Cardiology 115: 120–126, 2010 [DOI] [PubMed] [Google Scholar]
- 199.Zhou L, Ding S, Li Y, Wang L, Chen W, Bo T, Wu K, Li C, Liu X, Zhao J, Xu C, and Gao L. Endoplasmic reticulum stress may play a pivotal role in lipid metabolic disorders in a novel mouse model of subclinical hypothyroidism. Sci Rep 6: 31381, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhu L, Wang W, Shi J, Zhang W, Shen Y, Du H, and Wu S. Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. J Sci Food Agric 94: 2699–2704, 2014 [DOI] [PubMed] [Google Scholar]