Abstract
Significance: Carbonyl sulfide (COS) is the most prevalent sulfur-containing gas in the Earth's atmosphere, and it plays important roles in the global sulfur cycle. COS has been implicated in origin of life peptide ligation, is the primary energy source for certain bacteria, and has been detected in mammalian systems. Despite this long and intertwined history with terrestrial biology, limited attention has focused on potential roles of COS as a biological mediator.
Recent Advances: Although bacterial COS production is well documented, definitive sources of mammalian COS production have not been confirmed. Enzymatic COS consumption in mammals, however, is well documented and occurs primarily by carbonic anhydrase (CA)-mediated conversion to hydrogen sulfide (H2S). COS has been detected in ex vivo mammalian tissue culture, as well as in exhaled breath as a potential biomarker for different disease pathologies, including cystic fibrosis and organ rejection. Recently, chemical tools for COS delivery have emerged and are poised to advance future investigations into the role of COS in different biological contexts.
Critical Issues: Possible roles of COS as an important biomolecule, gasotransmitter, or sulfide transport intermediate remain to be determined. Key advances in both biological and chemical tools for COS research are needed to further investigate these questions.
Future Directions: Further evaluation of the biological roles of COS and disentangling the chemical biology of COS from that of H2S are needed to further elucidate these interactions. Chemical tools for COS delivery and modulation may provide a first avenue of investigative tools to answer many of these questions. Antioxid. Redox Signal. 28, 1516–1532.
Keywords: : carbonyl sulfide, gasotransmitters, hydrogen sulfide, reactive sulfur species
Introduction
Small gaseous biomolecules, such as nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S), have attracted significant attention due to their important physiological roles as signaling molecules (14, 58, 81, 92, 96, 98, 101, 141, 147, 148, 156, 157). Often referred to as gasotransmitters (146, 149), these gases share several defining characteristics: They are membrane permeable, are generated endogenously by enzymes, and exert action on molecular targets at physiologically relevant concentrations. We note that although commonly referred to as gaseous signaling molecules or gasotransmitters, these small gaseous molecules are solutes rather than gases when they act as signaling agents. In this review, we use the terms gaseous signaling molecules and gasotransmitters to refer to the class of molecules rather than the physical state of the molecules in a biological environment. Highlighting the broad importance of these signaling molecules, gasotransmitter generation and/or metabolism has been implicated in diverse biological processes, including vascular biology, immune functions, metabolism, and stress resistance/response (3, 100, 101, 104, 105, 111, 118, 148, 155). In addition to the primary gasotransmitter criteria, recent and growing evidence supports a complex cross-talk and interconnectivity between NO, CO, and H2S, suggesting that the interactions between these molecules play an important role in gasotransmitter function (6, 10, 28, 29, 42, 154). For example, H2S inhibits CO production through regulation of heme oxygenase 1 (HO-1) (30, 51) and can either stimulate or inhibit different nitric oxide synthase (NOS) isoforms (100, 104, 105, 111, 118). Similarly, both NO and CO inhibit H2S production from heme-containing cystathionine β-synthase (CBS) (110, 112). Complementing regulatory interactions through enzymatic synthesis, NO and H2S also react through different redox pathways to generate reactive sulfur, oxygen, and nitrogen species (RSONS), including thionitrous acid (HSNO), perthionitrite (SSNO−), and nitroxyl (HNO), which further intertwine these gasotransmitters (28, 29, 42, 82, 86).
Despite the significant research on the chemical biology of NO, CO, and H2S, investigations into other potential gasotransmitters, such as sulfur dioxide (SO2), ammonia (NH3), or carbonyl sulfide (COS), remain significantly underdeveloped (Fig. 1) (84, 149). Both SO2 and NH3 are produced enzymatically in mammalian cells and are interconnected with established gasotransmitters, giving credence to the suggestion that they may play significant roles in biology. For example, SO2 can be generated from H2S by NADPH oxidase or from thiosulfate (S2O32–) by thiosulfate sulfurtransferase (52, 90). Alternatively, cysteine oxidation by cysteine dioxygenase (CDO) generates cysteine sulfonate, and subsequent transamination by aspartate aminotransferase (AAT) generates β-sulfinylpyruvate, which spontaneously decomposes to extrude pyruvate and SO2 (123, 129). Although still in its infancy, early investigations into possible biological actions of SO2 suggest roles as a vasorelaxant and in providing protection against myocardial ischemia-reperfusion injury (52). Similarly, NH3 is formed in many pathways, including deamination of amino acids, nucleic acids, nucleotides, and nucleosides; as a byproduct of transsulfuration enzymes, including H2S-producing cystathionine γ-lyase (CSE) and CBS; and from urea recycling (149). Once formed, NH3 may not only provide a viable form of nitrogen for DNA and RNA synthesis but also contribute to acid-base buffering capacity. In addition, NH3 has been demonstrated to increase inducible nitric oxide synthase (iNOS) expression in cultured astrocytes (49) and also to increase iNOS and neuronal nitric oxide synthase (nNOS) expression in animal models of hyperammonemia (131).
FIG. 1.
Structures, space filling models, and electrostatic potential maps of NO, CO, H2S, NH3, SO2, and COS. CO, carbon monoxide; COS, carbonyl sulfide; H2S, hydrogen sulfide; NH3, ammonia; NO, nitric oxide; SO2, sulfur dioxide.
Unlike SO2 or NH3, pathways for enzymatic COS synthesis in mammals have yet to be identified, although COS has been detected in various biological tissues and in exhaled breath, supporting the presence of pathways for endogenous generation (vide infra) (7, 60, 121). Furthermore, COS shares an interconnection with H2S generation through the action of different metalloenzymes, including carbonic anhydrase (CA), which rapidly converts COS to H2S (22, 67, 119). Although historical investigations of terrestrial COS have focused on its atmospheric presence and importance in the global sulfur cycle, contemporary chemical investigations have focused primarily on COS-mediated peptide-bond formation under prebiotic conditions, many of which suggest important roles of COS in origin of life chemical ligation (53, 75). When taken together, the potential role of COS in thermophilic origin of life theories, COS detection in tissues, as well as its cell permeability and moderate water solubility, suggests that COS may play a much more significant role in mammalian chemical biology than initially appreciated. Building on these factors, this review focuses on current knowledge of biological COS formation and consumption, growing evidence that COS may play roles in sulfide transport and disease pathology, emerging chemical tools for investigating COS in biological contexts, and the potential role of COS as a new member of the gasotransmitter family.
Basic Properties
COS (CAS 463-58-1, also referred to as carbon oxysulfide, CO monosulfide, carbon oxide sulfide, OCS) is a colorless and odorless gas in its pure form (41). Initially misidentified as a mixture of carbon dioxide (CO2) and H2S due to its rotten egg smell in an impure state, COS was first characterized by Than in 1867 by the reaction of CO with elemental sulfur vapor in a glowing porcelain tube (Eq. [1]) (8). Although removal of CO impurities was not practical from the initial preparations, experimental modification allowed for generation and purification of COS by acid-mediated hydrolysis of thiocyanate (SCN−) salts (Eq. [2]) (8). This reaction can be used to prepare COS in the laboratory, but the produced gas requires significant purification due to common contamination by gaseous impurities (113). Commercial sources of high-purity COS (generally >97.5%) often contain significant levels of H2S as the main impurity. Although only moderately soluble in water, COS is stable in acidic solution but undergoes base-mediated hydrolysis to generate H2S and CO2 (41). As a moderately lipophilic gas [log Poct(COS) = 0.79 by comparison to log Poct(NO) = 0.70] (57), the dipole moment of COS is more similar to that of NO, CO, and H2S rather than the significantly more polar SO2 and NH3. These properties suggest a sufficient lipid solubility to enable cell membrane permeability and also penetration to the central nervous system. Basic properties of COS, as well as those of H2S, NO, CO, SO2, and NH3 are provided in Table 1.
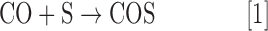 |
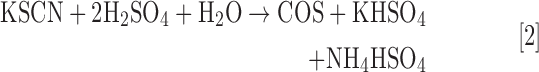 |
Table 1.
Basic Physical Properties of Carbonyl Sulfide and Other Biologically Relevant Gaseous Molecules Receiving Attention as Confirmed or Potential Gasotransmitters
| Physical property | COS | H2S | NO | CO | SO2 | NH3 |
|---|---|---|---|---|---|---|
| Molecular weight (g/mol) | 60.08 | 34.08 | 30.01 | 28.01 | 64.06 | 17.03 |
| Density (g/L) | 2.51 | 1.36 | 1.34 | 1.14 | 2.63 | 0.769 |
| Melting point (°C) | −138.8 | −82 | −164 | −205.0 | −72 | −77.8 |
| Boiling point (°C) | −50.2 | −60 | −152 | −191.5 | −10 | −33.3 |
| Dipole moment (D) | 0.65 | 0.97 | 0 | 0.12 | 1.62 | 1.42 |
| Solubility (H2O, mol/L, 25°C) | 2.0 × 10−2 | 1.1 × 10−1 | 1.9 × 10−3 | 9.9 × 10−4 | 1.5 | 31 |
CO, carbon monoxide; COS, carbonyl sulfide; H2S, hydrogen sulfide; NH3, ammonia; NO, nitric oxide; SO2, sulfur dioxide.
Toxicity and safety
Much like H2S, NO, and CO, COS is a flammable gas that is toxic in high concentrations. COS is a skin, eye, nose, throat, and lung irritant, matching many of the topical toxicity characteristics of H2S. These similarities are likely due to the hydrolysis of COS to form H2S on contact with different mucosal membranes that typically contain CA, thus facilitating H2S generation (vide infra). Similarly, in toxicological investigations of COS, treatment of rats with the CA inhibitor acetazolamide (AAA) reduces measured blood levels of H2S, and also the toxicity of COS, suggesting that at least some of the observed COS toxicity is due to CA-mediated metabolism to H2S (22, 23). Although specific hazardous concentrations of COS are not specified by the US Environmental Protection Agency (EPA), some reports have documented irritation in the upper respiratory tract at concentrations above 20 mg/L, although different toxicity thresholds have also been reported in different investigations (137). Low-to-moderate concentrations are accepted to elicit lachrymatory effects, photophobia, nausea, increased salivation, headache, mental confusion, as well as other characteristics (137). Higher COS concentrations can result in decreased vision, tachycardia, and collapse, and continuous exposure to COS concentrations of 0.1% (v/v) (1000 ppm) can result in death within 2 h due to respiratory paralysis. A limited number of animal studies have investigated acute or chronic COS toxicity, but toxicological investigations of 50 ppm levels of COS in rabbits for a maximum of 7 weeks did not impact the myocardial ultrastructure significantly (54). Similarly, toxicological investigations in rats revealed LC50 of more than 2000 mg/m3 (∼750 ppm), with further investigations classifying COS as a non-carcinogenic, low-toxicity fumigant (150).
Natural sources
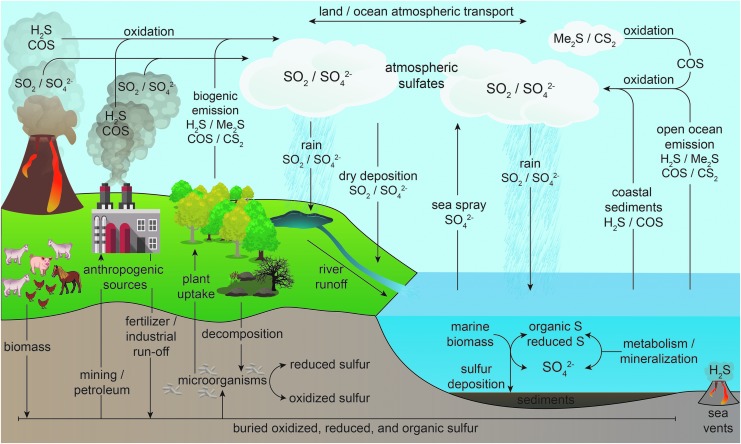
COS is the most prevalent sulfur-containing gas in the Earth's atmosphere and is produced by both biological and chemical pathways (Fig. 2) (46, 137). Primary abiotic COS emissions occur from volcanos, hot springs, and oceans, with biotic sources stemming from soils, trees, marshes, plant roots, manure, microorganisms, and biomass burning (see the Biological Roles of COS section for more detailed information) (151). Up to one half of terrestrial COS is generated from secondary production in the global sulfur cycle through oxidation of atmospheric dimethyl sulfide (Me2S) and carbon disulfide (CS2) (151). Anthropogenic sources of COS, which include aluminum production, coal and automobile fuel burning, and industrial desulfurization, contribute minimally to global COS production, but more recent estimates indicate that industry and manufacturing may produce more COS than has been previously reported (74). Measurement of atmospheric COS levels from Antarctic ice cores have shown that COS levels have risen over the past 350 years, which has been attributed to human sources of industrialization (5). Once produced, COS is more stable in the atmosphere than the common sulfur-containing gases Me2S, H2S, and CS2, which results in a prolonged atmospheric lifetime of about 4 years (68). As a result, COS is generally transported from the troposphere to the stratosphere, where it undergoes photodissociation and oxidation to SO2 and sulfate particles, therefore influencing stratospheric ozone concentrations (137, 139). The long atmospheric lifetime, as well as its formation from CS2 in the atmosphere, makes COS the primary sulfur gas in the Earth's atmosphere, with measured concentrations of 0.5 ppb (151).
FIG. 2.
Simplified overview highlighting the roles of COS in the global sulfur cycle. Primary COS emission sources include volcanoes and hot springs, biomass, and open ocean emission. Approximately one half of atmospheric COS is generated from the oxidation of Me2S and CS2 in the global sulfur cycle. Primary anthropogenic sources include aluminum production, coal and automobile fuel burning, and industrial desulfurization. Once produced, COS has a longer atmospheric lifetime than Me2S, H2S, or CS2 and it is typically transported to the stratosphere, where it undergoes photodissociation and oxidation to SO2 and sulfate particles. COS uptake also occurs in plants, soils, marine algae, and microbes, which often convert COS to CO2. CO2, carbon dioxide; CS2, carbon disulfide; Me2S, dimethyl sulfide.
Although oxidation of COS to sulfate occurs in the atmosphere by hydroxyl radical, the major sink of atmospheric COS is uptake by many plants, soils, marine algae, and microbes, which often convert COS to CO2. This conversion is typically mediated by the ubiquitous enzyme CA as well as by other COS-metabolizing enzymes (vide infra). In addition to COS metabolism by CA, some plants are able to convert COS to CS2, as demonstrated by increased CS2 release after COS absorption by moist soils (88). These observations suggest a link between CS2 and COS not only in the atmosphere but also in soils and vegetation.
Biological Roles of COS
Growing evidence supports the importance of biologically relevant reactive sulfur species, such as persulfides, polysulfides, thiosulfate, or other partially oxidized forms of sulfur-based compounds, but surprisingly, little attention has focused on the most-prevalent sulfur-containing gas in the Earth's atmosphere: COS. Reported investigations into the biological roles of COS remain sporadic, and our understanding of the potential physiological roles and pharmacological roles of COS remain underdeveloped. Despite these limited reports, enzymatic COS production and consumption are well documented, and COS generation in cell culture and in exhaled breath in human subjects suggests a broader role for this important gas in diverse biological processes.
Enzymatic consumption of COS
CAs are a family of ubiquitous metalloenzymes that catalyzes the reversible hydration of CO2 to bicarbonate (HCO3−) (78, 83). CAs comprise five classes found primarily in vertebrates (α-CAs), higher plants and some prokaryotes (β-CAs), archaebacteria (γ-CAs), and diatoms (δ-CAs and ξ-CAs). Although not its natural substrate, CA is also able to readily hydrolyze COS to H2S, providing a broad enzymatic platform for redox-neutral conversion of COS to H2S. The catalytic efficiency (kcat/KM) of CA-mediated COS conversion to H2S is less efficient than the canonical CO2 metabolism (∼8 × 107 M−1 s−1) (66), but it still boasts a high catalytic efficiency of 2.2 × 104 M−1s−1 for bovine CA-II (Table 2) (47). Drawing parallels to CA, other enzymes are also able to catalyze the hydrolysis of COS, even though it is not their natural substrate. For example, CS2 hydrolase, nitrogenase, CO dehydrogenase, and ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) have all been reported to hydrolyze COS (36, 80, 120, 125). Of these enzymes, CS2 hydrolase, initially isolated from acidophilic and thermophilic archaea extremophiles (125), exhibits the greatest catalytic efficiency of enzymatic COS hydrolysis (Table 2). Because the distribution of this enzyme is limited to extreme and sulfur-rich environments, such as sulfotaras, it is unlikely that CS2 hydrolase contributes significantly to global COS consumption. The only known example of an enzyme for which COS is the natural substrate is carbonyl sulfide hydrolase (COSase), which was recently identified, purified from Thiobacillus thioparus strain THI115, and characterized (95, 106). COSase shares both a high sequence homology and a similar Zn(II) active site with the β-CAs and is structurally similar to enzymes in clade D of the β-CA phylogenetic tree (95). Importantly, COSase has a higher efficiency than widely distributed enzymes that are able to catalyze COS hydrolysis, suggesting that it may play an important role in the global consumption of atmospheric COS. Although less efficient than COSase, other metalloenzymes, such as CO dehydrogenase (36), RuBisCO (80), and nitrogenase (120), have been shown to metabolize COS in plants and bacteria with varied levels of catalytic efficiency (Table 2).
Table 2.
Carbonyl Sulfide Degrading Enzymes and Associated Enzyme Kinetic Parameters
| Enzyme | Organism | kcat (s−1) | Km (μM) | kcat/KM (M−1s−1) | References |
|---|---|---|---|---|---|
| CS2 hydrolase | Acidianus sp. strain A1-3 | 1800 | 22 | 8.2 × 107 | (125) |
| COSase | Thiobacillus thioparus strain THI115 | 58 | 60 | 9.6 × 105 | (95) |
| CO dehydrogenase | Rhodospirillum rubrum ATCC11170T | 0.52 | 2.2 | 2.4 × 105 | (36) |
| CA | Bos taurus | 41 | 1.9 × 103 | 2.2 × 104 | (47) |
| RuBisCO | Spinacia oleracea | 3.8 | 1.8 × 103 | 2.2 × 103 | (80) |
| RuBisCO | R. rubrum | 6.3 | 5.6 × 103 | 1.1 × 103 | (80) |
| Nitrogenase | Azotobacter vinelandii | 0.16 | 3.1 × 103 | 5.2 × 101 | (120) |
CA, carbonic anhydrase; COSase, carbonyl sulfide hydrolase; CS2, carbon disulfide; RuBisCO, ribulose-1,5-bisphosphate carboxylase oxygenase.
Modified from Ogawa et al. (95).
Biological production of COS
SCN− is a common organic anion found in plants, mammals, and natural environments. In plants, SCN− is formed from the hydrolysis of glucosinolates, often found in Cruciferae (Brassicaceae) by glucosidases (37). In mammals, SCN− is commonly found in saliva, blood, and milk, and it derives from the ingestion of glucosinolates, often derived from broccoli, cauliflower, and other cruciferous vegetables, as well as from cyanide (CN−) detoxification by the ubiquitous enzyme rhodanese (27). Although mammalian SCN− degradation primarily occurs through peroxidation by myeloperoxidase and lactoperoxidase (153), at a microbial level, a number of chemoorganotrophic bacteria are able to degrade SCN− as a source of nitrogen and sulfur. Furthermore, chemolithoautotrophic sulfur bacteria, such as T. thioparus, have been identified that utilize SCN− as their primary energy source (64). More specifically, the enzyme thiocyanate hydrolase (SCNase), which catalyzes the hydrolysis of SCN− to COS and NH3 (Eq. [3]), was initially isolated and identified in T. thioparus strain THI115 (4). SCNase consists of three subunits (α [19 kDa], β [23 kDa], and γ [32 kDa]), which share a high sequence homology to bacterial nitrile hydratases, and it maintains an unusual distorted square pyramidal low-spin Co(III) active site (63). SCNase has also been identified as the primary enzyme for initiating SCN− hydrolysis in the sulfur-oxidizing bacterium Thiohalophilus thiocyanoxidans (9).
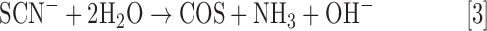 |
In addition to SCNase, COS can also be formed from archaeal CS2 hydrolase, which converts CS2 to H2S and CO2, but it proceeds through intermediate generation of COS (125). CS2 hydrolase, which has been isolated from the hyperthermophilic Archaea Acidianus A1-3 that lives in volcanic solfataras, has a structure that is similar to typical β-CAs, but it does not hydrolyze CO2. Instead, this enzyme has evolved a highly hydrophobic tunnel that serves as a filter by blocking the entrance of CO2 into the active site, which is otherwise identical to that of CA (124). Similarly, in mammalian systems, CS2 can be metabolized to COS by the mixed-function oxidase enzyme system. Liver damage, as well as a measureable decrease in the concentration of cytochrome P450, is observed when rats are treated with CS2 (21, 31, 32). This damage has been attributed to the binding of sulfur species that are released during the hydrolysis of CS2 to COS. Once released, the COS is further metabolized to H2S, most likely by hepatic CA. Alternatively, COS can function as a suicide substrate for cytochrome P450, generating CO2 and sulfur species that react with and inhibit the P450 (31).
Abiotic COS generation
Because much of the COS in the atmosphere derives from open waters, a number of studies have investigated possible mechanisms of COS formation, such as the reaction of carbonyl groups of dissolved organic compounds with thiyl radicals (43). Interestingly, and possibly of more direct biological relevance, is the direct reaction of polysulfides with CO to generate COS (61). Inorganic polysulfides play an important role in the global sulfur cycle and such polysulfides, as well as their organic counterparts, are now understood to be of increasing importance in the biological action of H2S (24, 25, 69, 70, 107). By using inorganic polysulfides and CO, both of which are abundant in aquatic systems, mechanistic investigations revealed that the rate of COS generation had a first-order dependence in both CO and the molar sum of polysulfide species in solution (61). Although further investigations are needed to determine whether the reaction kinetics, pH, and temperature dependence make such mechanisms of COS formation viable under physiological conditions, these observations highlight the interconnected role of COS with CO and polysulfides.
COS detection in mammalian systems
Although the precise mechanisms of COS biosynthesis in eukaryotes remain unknown, two primary pathways for COS genesis have been postulated in investigations of COS in the body: metabolism (or impaired metabolism) of sulfur-containing precursors, and direct generation by cohabitating bacteria. COS has also been detected as a metabolite of different sulfur-containing drugs, supporting that abnormal metabolism of sulfur-containing compounds may, in part, contribute to COS generation. One simple example of such is disulfiram (tetraethylthiuram disulfide), which functions as an acetaldehyde dehydrogenase inhibitor and is commonly used for treatment of chronic alcoholism (57). Reduction of the dithiocarbamate disulfide in disulfuram, followed by partial hydrolysis to form diethylthiocarbamate, may provide a path for COS extrusion, although it is also possible that initial CS2 release generates COS as a metabolic byproduct. In addition, metabolism of the commonly used dithiocarbamate pesticides has also been demonstrated to release COS. Although such systems provide convenient examples of COS in biological contexts, the high concentrations of such sulfur-containing molecules significantly simplify COS detection. By contrast, detection of endogenous COS remains significantly more challenging. These challenges arise primarily from the likely low levels of COS as well as the efficient metabolism of COS by CA to form H2S, which has so far necessitated detection of COS from only gaseous biological samples or from the headspace of samples, where COS can be isolated outside of a CA-rich environment. Despite these challenges, observation of COS in mammalian cell culture, ex vivo tissues, and exhaled breath has provided compelling evidence for the importance of COS in biology and implications for COS involvement in various pathologies.
One strategy for COS detection is to monitor the gas content of the headspace over cell culture or ex vivo tissues. A benefit of this approach is that analysis of the headspace is readily accessible through standard GC-MS techniques or by various spectroscopic techniques (26). In one such example, headspace analysis of porcine coronary artery (PCA) and cardiac muscle tissue in vitro demonstrated COS formation by GC-MS analysis (7). In addition, COS was found to induce arterial dilation. Moreover, stimulation of PCA with acetylcholine and calcium ionophore A23187 resulted in increased COS levels, suggesting that muscarinic acetylcholine receptors (mAChRs), which have already been shown to be involved in the production of CO, NO, and H2S leading to vasorelaxation (13, 44, 55, 91, 94, 158), may also be involved in COS genesis. Although a simple example, these experiments may suggest a promising starting point for future COS investigations.
In addition to the detection of COS in the headspace of tissue culture experiments, COS has also been detected in exhaled breath, providing evidence for the role of COS as a potential gaseous biomarker for various disease states. For example, investigations into the presence of sulfur gases in patients with cystic fibrosis (CF) revealed measurable differences between COS levels in inhaled and exhaled breath (60). As a whole, CF patients had a reduced uptake of atmospheric COS by comparison with healthy patients. Furthermore, CF patients with reduced pulmonary function exhibited greater COS levels in exhaled breath than normal patients, with a strong inverse correlation between COS concentration and all four indices of pulmonary function in CF patients, with no correlation observed in normal patients. Prior research has also demonstrated an inverse correlation between pulmonary function and respiratory bacterial load (99, 114, 115), which, when taken together, provide support for the hypothesis that cohabitating bacteria may play an important role in biological COS generation. Consistent with this hypothesis, recent studies have reported that CA distribution and activity may be altered in CF patients (15, 38, 59), suggesting a lower potential for COS metabolism. One hypothesis, which is consistent with the experimental data, is that impairment of CA function/expression in CF patients may reduce COS metabolism in the lungs, which, when coupled with increased bacterial load and COS production in the respiratory tract, may result in higher COS levels observed in exhaled gas. On the basis of these observations, these studies suggest that exhaled COS may provide a potential non-invasive biomarker for bacterial colonization of the respiratory tract of CF patients.
Furthering the potential role of COS as a biomarker for lung pathologies in exhaled breath, COS has also been investigated as a potential marker of acute rejection (AR) after organ transplant (130). AR after lung allograft is a major risk factor for bronchiolitis obliterans (BO), which is one of the primary causes of death in lung transplant patients. Early detection and diagnosis of AR typically requires routine biopsies, which are invasive and associated with pulmonary complications. In a study investigating the efficacy of non-invasive breath testing for AR monitoring, comparisons between healthy, non-rejection patients and AR patients did not provide significant differences in exhaled ethane, isoprene, acetone, or H2S, all of which are potential organ transplant-related biomarkers. By contrast, exhaled COS levels were demonstrated to provide a biomarker for AR, with elevated COS levels observed for AR patients but not in non-rejection patients. Furthermore, individual patient tracking documented examples of COS levels increasing with worsening AR and decreasing with AR resolution. Although the direct COS origin was not identified, the authors hypothesized that abnormal metabolism of sulfur-containing compounds in AR patients may be responsible for the observed increase in exhaled COS.
In addition to direct lung pathologies, COS levels in exhaled breath have also been characterized in various stages of liver disease (121). When compared with patients with normal liver function, patients with hepatocellular injury (grouped to include: alcoholic cirrhosis, autoimmune cirrhosis, cryptogenic cirrhosis, fulminant hepatitis, hepatitis B, hepatitis C, 1-antitrypsin deficiency, and steatohepatitis) exhibited elevated COS levels in exhaled breath. In addition, COS, but not CS2 or Me2S, was found to correlate with the severity of disease, resulting in increasing COS levels in early-, mid-, and end-stage liver disease, potentially providing a diagnostic tool for early detection. By contrast, patients with bile duct injury diseases (grouped to include: CF, primary biliary cirrhosis, sclerosing cholangitis, and biliary obstruction) exhibited reduced COS levels in exhaled breath. These human studies are consistent with previous observations in isolated rat hepatocytes and liver microsomes, which have been observed to generate COS, possibly from CS2 metabolism or metabolism of other sulfur-containing compounds (23). These data are also consistent with previous experiments where COS has been observed in exhaled breath of rats exposed to CS2, suggesting that the observed COS may be due to metabolism of CS2 or, by analogy, the incomplete metabolism of other sulfur-containing compounds (32).
The examples mentioned earlier not only provide key demonstrations of COS generation from metabolic abnormalities or bacterial colonization associated with disease but also provide compelling evidence for the necessity of further exploration into potential metabolic pathways of COS in biology. Although mammalian COS production from natural sources remains poorly understood, one possible route for COS synthesis is SCN− hydrolysis by SCNase, although the presence of this enzyme has yet to be reported in mammals. Given that COS has been detected in biological samples, particularly in non-diseased tissues, elucidation of possible enzymatic pathways for COS production remains an important area of investigation. Importantly, these studies additionally suggest a key role of bacterial colonization in COS generation in mammalian systems. In the cases of exhaled breath analysis, a distinct interplay between the host, mammalian tissues, and bacterial colonization not only suggests a complex landscape in COS generation but also may provide access to a convenient method of detection for a variety of disease states.
COS Chemistry
The most simple reactions of COS, including hydrolysis, oxidation, reduction, and dissociation, have been known for many years and are the main subject of a thorough COS review published in 1957 (41). Since then, COS chemistry has expanded to address environmental concerns associated with COS contaminants in industrial settings, and catalysts for the low-temperature rapid hydrolysis of COS have been developed. Although the most basic chemical reactions are well researched, more complex and biologically relevant COS-related reactions remain less understood, even though contemporary investigations suggest important biological roles for COS stemming from initial thermophilic origin of life chemistry.
Simple reactions of COS
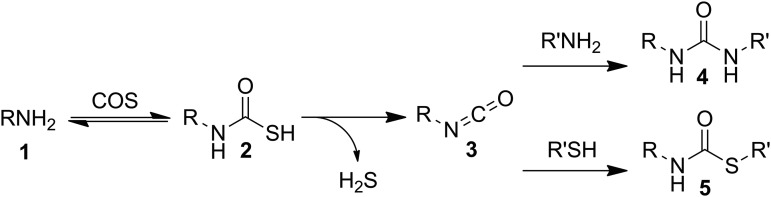
Shortly after the initial synthesis of COS, early reactivity studies demonstrated that COS reacts with primary amines (Fig. 3: 1; bold numbers correspond to bold labels in figures), such as excess aniline, to generate diphenylurea and H2S (8). In such reactions, COS initially reacts with the amine to generate a stable, often isolable monothiocarbamate 2, which, subsequently, extrudes HS− to furnish an electrophilic isocyanate 3. In the presence of excess amine, the resultant isocyanate intermediate is trapped by the amine to generate the urea product 4 (Fig. 3). By tuning the reaction conditions, the generated isocyanate can also react with other nucleophiles, such as thiols, to furnish a thiocarbamate product 5. Although it is challenging to control the reaction conditions and stoichiometries on laboratory-scale syntheses, these reactions are commonly used industrially in the manufacture of ureas and thiocarbamates for use as herbicides and pesticides. Because COS is also known to be a sulfur contaminant in natural gas and hydrocarbon streams, the chemistry detailed earlier can also be utilized for COS removal through the addition of primary amines during the purification process (16).
FIG. 3.
Amines react reversibly with COS to generate thiocarbamates, which can release H2S to afford electrophilic isocyanates. These isocyanates can be trapped in synthetically useful reactions to generate ureas and thiocarbamate esters.
Implications in origins of life and prebiotic bond formation
In addition to the role of COS in laboratory-scale and industrial syntheses, contemporary investigations have focused on the potential role of COS in nascent bond-forming reactions under prebiotic conditions. Perhaps unsurprisingly, the volcanic and geothermal generation of COS, as well as its bond-forming potential with simple nucleophiles, appears consistent with thermophilic origin of life requirements. Much of this work stems from studies by Hirschmann and co-workers in 1971 that focused on peptide formation from 2,5-thiazolidinediones, in which a footnote comments that traces of dipeptides were formed from phenylalanine thiocarbamate—a known reaction product of amines with COS (34).
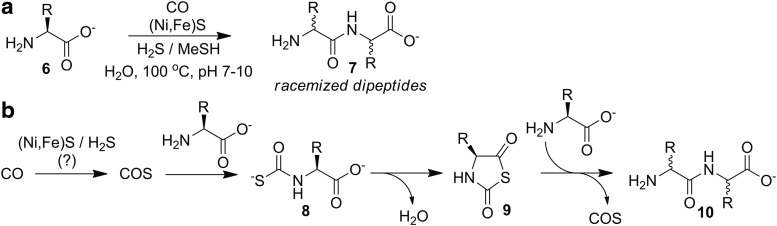
More than 30 years later, more detailed investigations into origin of life peptide synthesis were performed by mimicking volcanic or hydrothermal environments. Using prebiotic building blocks such as CO, nickel and iron sulfides [(Ni,Fe)S], and a reducing atmosphere of H2S or CH3SH at elevated temperatures, amino acids 6 combined successfully to form dipeptides 7 (Fig. 4a). Amino acid chirality was lost, however, likely due to the presence of metal sulfides that may promote racemization of the stereocenter of the anhydride intermediate under the harsh conditions investigated. Trace amounts of COS were detected during the course of the dipeptide-forming reactions, which was consistent with a proposed mechanistic explanation that involved COS as a key intermediate required to generate the thioanhydride 9 before dipeptide 10 formation (Fig. 4b). Supporting the necessity of COS in these reactions, dipeptides were still formed if CO and H2S were replaced with COS, although removal of (Ni,Fe)S abolished dipeptide formation. Highlighting the feasibility of metal-mediated COS formation, a recent report demonstrated the ability of Mo(II) complexes to function as pre-catalysts for the photocatalytic generation of COS from CO and S8 under relatively mild conditions (39).
FIG. 4.
Early examples of COS-related peptide bond formation. (a) Hydrothermal generation of dipeptides in the presence of (Ni,Fe)S, H2S/MeSH, and CO. (b) The proposed mechanism for this conversion generates COS as a key intermediate.
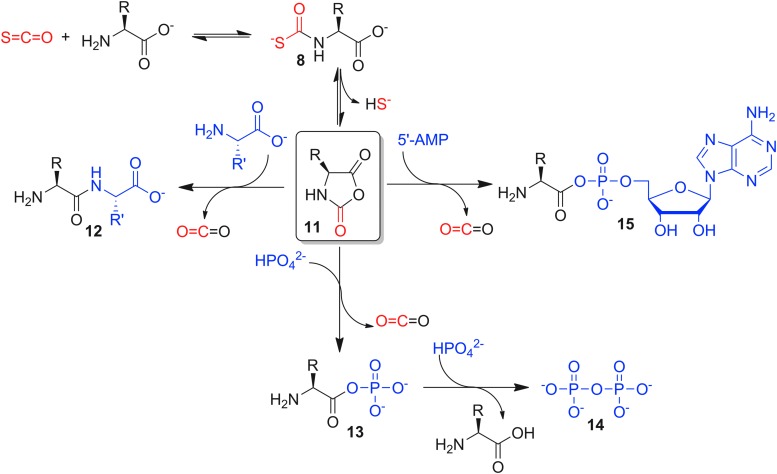
Furthering investigations into the role of COS in primordial amino acid chemistry, an elegant study by Ghadiri and co-workers demonstrated that COS can facilitate the direct formation of small peptides from amino acids in water under mild conditions in the absence of metal sulfides (Fig. 5) (75). Remarkably, even the simplest conditions, such as addition of excess COS gas to an aqueous buffered solution of phenylalanine, resulted in 7% dipeptide formation after 2 days at 25°C. Further investigations revealed quantitative formation of thiocarbamate 8 on addition of COS to a pH 8.9 buffered solution of phenylalanine. Importantly, this intermediate showed good hydrolytic stability, and studies using analytically pure phenylalanine thiocarbamate showed it to be a competent intermediate in the peptide bond formation 12. Such coupling reactions are proposed to occur through formation of a cyclic N-carboxyanhydride (Leuchs' anhydride, 11), which functions as a versatile platform for subsequent reactions with different nucleophiles. Formation of this anhydride, however, requires extrusion of HS− from the thiocarbamate intermediate, which is hindered both by the stability of the thiocarbamate intermediate and by the poor leaving group ability of the hydrosulfide anion. In further optimization of peptide bond formation, significant rate enhancements were observed in the presence of metal ions, oxidizing agents, and electrophilic alkylating agents, suggesting that such species (or Lewis acids, in general) may facilitate decomposition of the monothiocarbamate intermediate and subsequent H2S release.
FIG. 5.
Prebiotic chemistry mediated directly by COS, including formation of peptides, aminoacyl phosphates, and inorganic phosphates. Each pathway proceeds through COS-mediated formation of thiocarbamate 8, followed by sulfide extrusion to generate electrophilic intermediate 11 (Leuchs' anhydride), which functions as a versatile platform for subsequent reactions with different biologically relevant nucleophiles.
In addition to simple peptide-forming reactions, the activation of amino acids by COS has been demonstrated to be a more general pathway to biologically relevant peptide functionalization. For example, under mild aqueous conditions, COS facilitates the formation of aminoacyl phosphates 13 from amino acids and inorganic phosphate. Under identical conditions, no aminoacyl phosphates are observed in the absence of COS. Similarly, when inorganic phosphate is replaced with adenylic acid (5′-AMP), several amino acids produced aminoacyl adenylates 15, which are important for protein biosynthesis. Furthermore, in the presence of Ca(II) and an amino acid, COS was also found to facilitate pyrophosphate 14 formation through intermediate generation of an aminoacyl-phosphate anhydride. Combined with the earlier evidence that COS mediates peptide formation, this work suggests that both prebiotic peptide synthesis and phosphoryl transfer reactions might have relied on a common, COS-activated precursor (76).
Although the atmospheric levels of COS are unlikely to generate suitable concentrations of the required thiocarbamate intermediates to facilitate efficient peptide coupling under global prebiotic conditions, the higher temperatures and COS levels near geothermal locales of COS generation could likely facilitate access to the reaction manifolds that are associated with these important prebiotic bond-forming reactions. More importantly, these studies set the stage for establishing the potential role of COS in biologically relevant reactions and intermediate generation, paving the way for future applications of COS chemistry.
Emerging Tools for COS Investigations
Both chemical and biological tools are needed to expand our understanding of the potential roles of COS in biology. Because direct knockout, overexpression, and blockage of the enzymes that are associated with production of the currently identified gasotransmitters have proved essential in studying the chemical biology of these gases, the definitive identification of COS-producing enzymes or pathways will be equally important. Before such information, downregulation of biological COS will remain a significant challenge. In the interim, one potential strategy to increase COS bioavailability is to shunt pathways that are associated with COS metabolism. For example, because of the wide distribution of CA and its high activity toward COS hydrolysis, CA inhibitors and/or enzymatic knockout could potentially be used to increase COS accumulation. Inhibition of CA by small molecules is a well-researched field (102, 132–136), and a variety of methods are available for strong inhibition of a variety of CA isoforms. Unfortunately, such inhibition studies would also alter the normal CO2/HCO3− equilibrium, and thus normal buffering capacities and cellular pH levels, likely leading to complicating effects. In addition, even small changes in pH would also alter the distribution of H2S and HS−, likely leading to confounding results resulting from further entanglement of COS/H2S/HS− chemical biology.
Before insights gleaned from enzymatic regulation, the development of small-molecule chemical tools for COS research may offer an attractive initial platform for expanding our understanding of COS in biology. Chemical tools for detection and delivery of the canonical gasotransmitters NO, CO, and H2S have been invaluable for investigating the multifaceted roles of these important biological molecules (18, 20, 40, 48, 56, 62, 71, 72, 77, 79, 85, 103, 108, 126, 138, 144, 145, 159), suggesting that similar constructs may find utility for COS investigations. Reaction-based fluorescent probe development for COS is likely to remain a significant challenge based on the inherent reactivity of COS. Because COS is a weaker electrophile than CO2, strategies to intercept COS by nucleophilic trapping are likely to be plagued by unwanted side reactivity, leading to significant selectivity challenges. By contrast to reaction-based detection motifs, small molecules that release COS may offer a more attractive first line of tools for investigating COS in different biological contexts.
General strategies to develop COS donors
Because of the structural similarities between COS and CO2, many decarboxylation reactions can be engineered to release COS rather than CO2 by simple replacement of an oxygen atom with a sulfur atom in the parent scaffold. This basic design concept enables structurally diverse COS donors that provide access to both triggered-/active-release donors, such as those that respond to specific biological or biorthogonal stimuli, and slow-/passive-release donors, including those activated by hydrolysis or a reaction with ubiquitous cellular enzymes and nucleophiles. In addition, easily accessible control compounds are available through synthesis of analogous carbamate compounds, which release CO2 rather than COS. Access to such control compounds is instrumental in differentiating the biological effects of the donor scaffolds themselves from the released COS. Recognizing the potential powerful utility of engineered COS release, our lab was the first to harness such motifs to develop COS-releasing small molecules (127). Since this initial report, we have been delighted that other researchers are using related strategies to broaden the palette of COS-releasing motifs that are available for future biological investigations.
The key breakthrough in our initial design was recognizing that self-immolative benzyl carbamates (Fig. 6a) (17, 73), often used as delivery platforms for prodrugs, fluorophores, and other small molecules (2, 12, 117, 122), could be modified to release COS rather than CO2 by exchanging the canonical carbamate linker with a thiocarbamate (Fig. 6b). In a proof-of-concept demonstration of this approach, we established the utility of on-demand COS extrusion as a strategy to access both analyte replacement fluorescent probes and triggered COS/H2S donors (127). The early motivation of this work was to address a major challenge in reaction-based probes for small-molecule analytes, especially RSONS. Activation of these reporter scaffolds results in analyte consumption, thus perturbing homeostasis. Exploitation of the self-immolative decomposition of thiocarbamates to release COS enabled the generation of the first examples of analyte replacement fluorescent probes, which react with (and consume) H2S to produce a turn-on fluorescent response, concomitant with the release one equivalent of caged H2S in the form of COS, thus providing progress toward analyte homeostasis in reaction-based detection systems (Fig. 6c). By using an H2S-reactive azide trigger, which is reduced to an amine after reduction by H2S (50), we established that the subsequent self-immolative cascade reaction extrudes COS, which is quickly hydrolyzed to H2S by CA. Importantly, this donor motif was found to be stable in whole mouse blood before trigger activation, thus highlighting the biological stability of these platforms. Control experiments on thiocarbamate motifs lacking a latent fluorophore demonstrated that triggered reductive cleavage by tris(2-carboxyethyl)phosphine (TCEP) in mouse blood resulted in COS donation with a nearly 50% efficiency and conversion to H2S by CA. In addition, addition of the CA inhibitor AAA abrogated H2S production in vitro, confirming that H2S release is a result of CA-mediated COS hydrolysis. Although this initial report provided an important contribution toward H2S detection technology, the broader impact is providing a viable and highly tunable COS donating strategy for accessing chemical tools for expanding our understanding of the chemical biology of COS.
FIG. 6.
Initial design of COS-releasing donors. (a) Established strategy of using protected benzylcarbamates to deliver a payload after trigger activation. (b) Translation of this delivery technique by using protected benzylthiocarbamates enables access to COS-releasing motifs. (c) Initial application of caged COS release to develop analyte replacement fluorescent probes for H2S based on azide reduction.
Triggered/active release of COS
Furthering the strategies outlined earlier, simple changes to the general self-immolative thiocarbamate scaffold can provide access to new donor motifs with more specific functions. Notably, the incorporation of protecting groups that selectively respond to specific stimuli and result in on-demand COS release may provide access to highly targeted COS donors with utility in investigating the chemical biology of COS, as well as for site-selective COS/H2S donation. Such triggerable donors can be expected to be useful in therapeutic applications in which COS delivery is targeted to a specific location as well as for studying COS delivery with a high level of temporal control.
Reactive oxygen species triggered COS donors
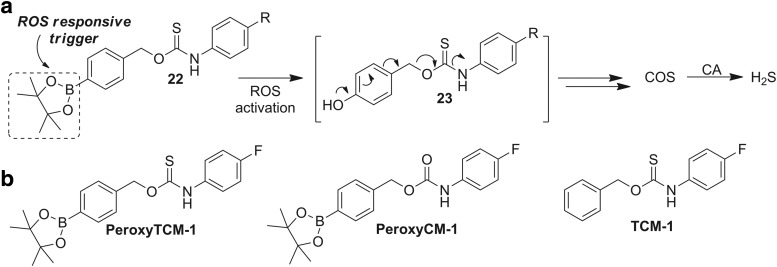
A powerful application of such responsive donors is the judicious choice of triggering analytes that are associated with contexts in which H2S can exert beneficial action. For example, because H2S has been demonstrated to provide protection against increased oxidative stress, a system in which COS release is triggered by reactive oxygen species (ROS) should not only provide access to actively triggered COS donors but also provide a platform with high pharmacological potential. Using an ROS-cleavable aryl boronate as the protecting group (35, 87), we developed a class of COS donors that respond to increased ROS levels (Fig. 7a) (160). Highlighting the responsive nature of this design platform, COS is released from PeroxyTCM-1 in a dose-dependent manner on the addition of H2O2 as well as other ROS and is quickly converted to H2S by CA.
FIG. 7.
COS/H2S donors activated by ROS. (a) Strategy for using ROS-responsive aryl boronates to access ROS-triggered COS/H2S donors. (b) Structure of ROS-triggered COS donor PeroxyTCM-1 and control compounds PeroxyCM-1, which releases CO2, and TCM-1, which lacks the ROS-activated trigger. ROS, reactive oxygen species.
Supporting in vitro investigations, stimulation of endogenous ROS production in Raw 264.7 cells by addition of phorbol 12-myristate 13-acetate (PMA) resulted in COS/H2S release from PeroxyTCM-1, as evidenced by an increase in fluorescence when imaged with the H2S-responsive probe HSN2 (93). Providing early insights into the potential of such donors to impart cellular protection under conditions of increased oxidative stress, PeroxyTCM-1 exhibited a dose-dependent increase in cell viability in HeLa cells treated with exogenous H2O2, which is consistent with ROS protection. Importantly, the use of carbamate control compound PeroxyCM-1 or triggerless TCM-1 (Fig. 7b) did not recapitulate the cytoprotective effects, suggesting that the observed cytoprotection was, indeed, due to the COS/H2S release rather than from the organic scaffold or reaction byproducts. In addition, in vitro investigations also demonstrated that H2O2 can react directly with COS to generate H2S, thus decreasing the need for CA and also highlighting the importance of COS as a potential ROS scavenger.
Bio-orthogonal COS release
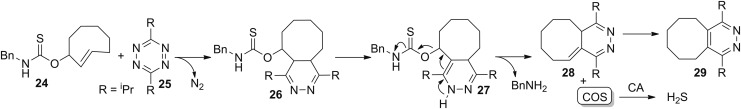
COS donors triggered through bio-orthogonal methods have also been reported. One advantage of this approach is that the donor constructs are stable until exposure to a benign external stimulus and release COS without the need for a detrimental cellular trigger. In addition, bio-orthogonal methods allow for the potential of high spatial and temporal resolution, as evidenced by the utility of such strategies for targeted drug delivery (45, 142). For example, incorporation of a COS-releasing thiocarbamate into the trans-cyclooctene reaction partner 24 of the inverse-electron demand Diels-Alder (IEDDA) click reaction between cyclooctenes and tetrazines 25 (11, 33, 116) enabled access to “click-and-release” COS donors (Fig. 8) (128). The initial cyclooctene-tetrazine click reaction generates a thiocarbamate-functionalized dihydropyridazine 26, which after spontaneous tautomerization 27, deprotonation 28, and rearomatization releases the cyclooctylpyridazine product 29, benzylamine, and COS. Direct COS release was confirmed by GC-MS, and H2S production was observed after incubation with CA. Although preliminary biological compatibility was demonstrated in whole blood and plasma, further biological investigations and applications are needed to establish the fidelity of this platform in more complex contexts and to improve on the efficiency and rate of COS release.
FIG. 8.
Bio-orthogonal COS donors based on the IEDDA click reaction. IEDDA, inverse-electron demand Diels-Alder.
Continuous/passive release of COS
A complementary approach to access caged COS donors is to develop continuous release COS donors, which are activated by ubiquitous cellular nucleophiles or enzymes. Such donors would result in continuous, rather than triggered, COS release, thus increasing basal COS levels in an otherwise normal physiological environment. Similar to well-known hydrolytically activated donors for NO and H2S (1, 65), these compounds are likely to contribute to an important class of tools for investigating COS chemical biology.
Nucleophile activation
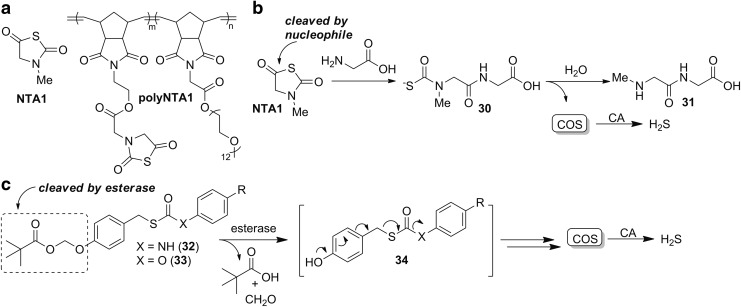
Matson and co-workers recently investigated N-thiocarboxyanhydrides (NTAs, Fig. 9a) as COS-releasing molecules (109). These electrophiles release COS after reaction with nucleophiles, and they are analogous to the thiocarboxyanhydrides proposed by Hirschmann in studies of peptide couplings (9, Fig. 4) (34). In these scaffolds, COS release likely occurs through initial formation of a thiocarbamate intermediate 30 akin to those observed by Ghadiri and co-workers in COS-mediated peptide-forming reactions (Fig. 9b) (75). After preparing small-molecule (NTA1) and polymeric (polyNTA1) derivatives (Fig. 9a), GC-MS experiments confirmed that the NTA derivatives release COS in the presence of mild biological nucleophiles, such as glycine. As further evidence of COS formation, the addition of CA resulted in H2S formation, which was confirmed by using an H2S-responsive electrode. Because alkylthiocarbamates that are similar 30 have been previously used as efficacious CA inhibitors (143), fine tuning of the structure of NTA derivatives and the resultant thiocarbamate intermediates may enable further tuning of rates of COS hydrolysis to H2S. Cell culture investigations demonstrated the ability of NTA1, but not polyNTA1, to promote cell proliferation in brain-derived endothelial cells at levels akin to those observed by treatment with NaSH. As a whole, these continuous-release NTA platforms provide a simple scaffold for further modifications based on the simplicity of the COS-releasing core and also offer the benefit of only releasing innocuous peptide byproducts after COS donation.
FIG. 9.
Continuous-release COS donors. (a) NTA COS-releasing molecules NTA1 and polyNTA1. (b) Proposed mechanism of COS release from NTA-based donors. (c) Esterase-cleaved COS donors and associated mechanism of COS release. NTA, N-thiocarboxyanhydride.
Ubiquitous enzyme activation
Complementing COS donors that function by a reaction with bioavailable nucleophiles, Chakrapani and co-workers recently reported thiocarbamate 32 and thiocarbonate 33 containing COS-donor motifs that are responsive to ubiquitous cellular esterases (19). Leveraging the design strategies outlined in General Strategies to Develop COS Donors and Triggered/Active Release of COS sections, installation of an ester functional group, which is cleaved by intracellular esterases (140), generates a phenolic intermediate 34 that initiates the subsequent self-immolative collapse to extrude COS (Fig. 9c). Esterase-mediated release of COS and subsequent conversion to H2S by CA was confirmed in vitro by using both the methylene blue (MB) assay and an H2S-responsive electrode. COS/H2S release was also confirmed in MCF-7 cells by using the H2S-responsive probe NBD-fluorescein (152). One difference of these platforms from those outlined in General Strategies to Develop COS Donors and Triggered/Active Release of COS sections is the use of an S-alkylthiocarbamate rather than an O-alkylthiocarbamate, as well as investigation into COS/H2S release from thiocarbonates, in addition to thiocarbamates. Preliminary mechanistic investigations suggest that the choice of thiocarbamate versus thiocarbonate may impact the rate-limiting step of COS extrusion, thus providing a pathway for further control and tuning of reaction kinetics and release profiles.
Outstanding questions
Although the recent introduction of a variety of COS donor compounds provides simple ways to introduce exogenous COS into biological samples, the direct CA-mediated metabolism of COS to H2S represents a significant challenge in differentiating the biological actions of COS from those associated with H2S. In addition, because COS metabolism by CA generates CO2/HCO3− in addition to H2S, it is important to consider the total amount of COS metabolized in a system to ensure buffering capacities are not exceeded by these otherwise innocuous products. New insights into the biological roles of COS will likely require thoughtful and careful applications of available H2S and COS donors that are used in concert to investigate specific biomolecular questions. Whether available COS donors function merely as clever sources of biological H2S, or whether the released COS imparts different outcomes in biological contexts remains to be determined. If realized, COS-releasing molecules that provide outcomes distinct from those attributed to available H2S donors will likely play a significant role in assessing and advancing not only the role of COS as a potential gasotransmitter but also its role in potential therapeutic applications that are associated with human health.
Conclusions and Outlook
Key challenges remain in further elucidating the chemical biology of COS, but our current, although limited, understanding of the biological production and consumption of COS suggests that it may play diverse roles. Could COS be poised to be next on the list of established gasotransmitters? This distinction will first require identification of enzymatic COS production in higher organisms and evidence that COS and H2S function independently. It is also possible that COS functions primarily as a source of “caged” H2S that is liberated by CA metabolism. Such a pathway is intriguing because it would provide a source of reduced sulfur that is not ionizable through acid-base equilibria at physiological pH, and that is less susceptible to ambient or enzymatic oxidation through direct action of oxidases or sulfur:quinone oxidoreductase (SQR) (97), thus bypassing interaction with the sulfane-sulfur pool (89). In addition, the neutral state of COS could enable distribution to locales that would otherwise be challenging for H2S/HS− alone. Finally, there is compelling evidence that COS stems from bacterial generation, especially in certain disease pathologies. In these cases, COS could provide a transport mechanism from pathogen to host. Even if eukaryotic COS synthesis is not a major source of endogenous COS, a thorough understanding of the role that COS plays in different diseases will likely be beneficial in early detection and treatment. When viewed more broadly, the absence of well-established metabolic pathways for COS formation in eukaryotic systems paired with the presence of COS-producing pathways from simple and abundant sulfur sources by a variety of bacteria may paint a broader, yet fundamentally underexplored picture of COS functions in sulfur biology and transport.
Abbreviations Used
- AAA
acetazolamide
- AR
acute rejection
- CA
carbonic anhydrase
- CBS
cystathionine β-synthase
- CF
cystic fibrosis
- CS2
carbon disulfide
- CO
carbon monoxide
- CO2
carbon dioxide
- COS
carbonyl sulfide
- COSase
carbonyl sulfide hydrolase
- H2S
hydrogen sulfide
- HCO3−
bicarbonate
- iNOS
inducible nitric oxide synthase
- Me2S
dimethyl sulfide
- NH3
ammonia
- NO
nitric oxide
- NTA
N-thiocarboxyanhydride
- PCA
porcine coronary artery
- ROS
reactive oxygen species
- RSONS
reactive sulfur, oxygen, and nitrogen species
- RuBisCO
ribulose-1,5-bisphosphate carboxylase oxygenase
- SCN−
thiocyanate
- SCNase
thiocyanate hydrolase
- SO2
sulfur dioxide
Acknowledgments
Aspects of work in the authors' lab that focused on H2S/COS delivery are supported, in part, by the NIH (R01GM113030 to M.D.P.), NSF GRFP (DGE-1309047 to A.K.S.), Sloan Foundation (M.D.P.), and Dreyfus Foundation (M.D.P.).
References
- 1.Alexander BE, Coles SJ, Fox BC, Khan TF, Maliszewski J, Perry A, Pitak MB, Whiteman M, and Wood ME. Investigating the generation of hydrogen sulfide from the phosphonamidodithioate slow-release donor GYY4137. MedChemComm 6: 1649–1655, 2015 [Google Scholar]
- 2.Amir RJ, Pessah N, Shamis M, and Shabat D. Self-immolative dendrimers. Angew Chem Int Ed Engl 42: 4494–4499, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Anggard E. Nitric-oxide – Mediator, murderer, and medicine. Lancet 343: 1199–1206, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Arakawa T, Kawano Y, Kataoka S, Katayama Y, Kamiya N, Yohda M, and Odaka M. Structure of thiocyanate hydrolase: A new nitrile hydratase family protein with a novel five-coordinate cobalt(III) center. J Mol Biol 366: 1497–1509, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Aydin M, Williams MB, Tatum C, and Saltzman ES. Carbonyl sulfide in air extracted from a South Pole ice core: A 2000 year record. Atmos Chem Phys 8: 7533–7542, 2008 [Google Scholar]
- 6.Bailey TS, Henthorn HA, and Pluth MD. The intersection of NO and H2S: Persulfides generate NO from nitrite through polysulfide formation. Inorg Chem 55: 12618–12625, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Balazy M, Abu-Yousef IA, Harpp DN, and Park J. Identification of carbonyl sulfide and sulfur dioxide in porcine coronary artery by gas chromatography/mass spectrometry, possible relevance to EDHF. Biochem Biophys Res Commun 311: 728–734, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Beck MT. and Kauffman GB. COS and C3S2—The discovery and chemistry of two important inorganic sulfur-compounds. Polyhedron 4: 775–781, 1985 [Google Scholar]
- 9.Bezsudnova EY, Sorokin DY, Tikhonova TV, and Popov VO. Thiocyanate hydrolase, the primary enzyme initiating thiocyanate degradation in the novel obligately chemolithoautotrophic halophilic sulfur-oxidizing bacterium Thiohalophilus thiocyanoxidans. Biochim Biophys Acta 1774: 1563–1570, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Bianco CL. and Fukuto JM. Examining the reaction of NO and H2S and the possible cross-talk between the two signaling pathways. Proc Natl Acad Sci U S A 112: 10573–10574, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackman ML, Royzen M, and Fox JM. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand diels-alder reactivity. J Am Chem Soc 130: 13518–13519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blencowe CA, Russell AT, Greco F, Hayes W, and Thornthwaite DW. Self-immolative linkers in polymeric delivery systems. Polym Chem 2: 773–790, 2011 [Google Scholar]
- 13.Boehning D. and Snyder SH. Novel neural modulators. Annu Rev Neurosci 26: 105–131, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Bredt DS. and Snyder SH. Nitric-oxide – A physiological messenger molecule. Annu Rev Biochem 63: 175–195, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Briggman JV, Tashian RE, and Spicer SS. Immunohistochemical localization of carbonic-anhydrase-I and carbonic-anhydrase-II in eccrine sweat glands from control subjects and patients with cystic-fibrosis. Am J Pathol 112: 250–257, 1983 [PMC free article] [PubMed] [Google Scholar]
- 16.Bush WV. Process for the selective removal of hydrogen sulphide and carbonyl sulfide from light hydrocarbon gases containing carbon dioxide. US Patent No. 4749555, 1988
- 17.Carl PL, Chakravarty PK, and Katzenellenbogen JA. A novel connector linkage applicable in prodrug design. J Med Chem 24: 479–480, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Chakraborty I, Carrington SJ, and Mascharak PK. Design strategies to improve the sensitivity of photoactive metal carbonyl complexes (photoCORMs) to visible light and their potential as CO-donors to biological targets. Acc Chem Res 47: 2603–2611, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Chauhan P, Bora P, Ravikumar G, Jos S, and Chakrapani H. Esterase activated carbonyl sulfide/hydrogen sulfide (H2S) donors. Org Lett 19: 62–65, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Chen XQ, Tian XZ, Shin I, and Yoon J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem Soc Rev 40: 4783–4804, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Chengelis CP. and Neal RA. Hepatic carbonyl sulfide metabolism. Biochem Biophys Res Commun 90: 993–999, 1979 [DOI] [PubMed] [Google Scholar]
- 22.Chengelis CP. and Neal RA. Studies of carbonyl sulfide toxicity – Metabolism by carbonic-anhydrase. Toxicol Appl Pharmacol 55: 198–202, 1980 [DOI] [PubMed] [Google Scholar]
- 23.Chengelis CP. and Neal RA. Oxidative-metabolism of carbon-disulfide by isolated rat hepatocytes and microsomes. Biochem Pharmacol 36: 363–368, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Chivers T. Ubiquitous Trisulfur Radical Ion S3. Nature 252: 32–33, 1974 [Google Scholar]
- 25.Chivers T. and Elder PJW. Ubiquitous trisulfur radical anion: Fundamentals and applications in materials science, electrochemistry, analytical chemistry and geochemistry. Chem Soc Rev 42: 5996–6005, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Ciaffoni L, Peverall R, and Ritchie GAD. Laser spectroscopy on volatile sulfur compounds: Possibilities for breath analysis. J Breath Res 5: 024002, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Cipollone R, Ascenzi P, and Visca P. Common themes and variations in the rhodanese superfamily. IUBMB Life 59: 51–59, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Cortese-Krott MM, Fernandez BO, Santos JLT, Mergia E, Grman M, Nagy P, Kelm M, Butler A, and Feelisch M. Nitrosopersulfide (SSNO-) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol 2: 234–244, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortese-Krott MM, Kuhnle GGC, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, and Feelisch M. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A 112: E4651–E4660, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Araio E, Shaw N, Millward A, Demaine A, Whiteman M, and Hodgkinson A. Hydrogen sulfide induces heme oxygenase-1 in human kidney cells. Acta Diabetol 51: 155–157, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Dalvi RR, Hunter AL, and Neal RA. Toxicological implications of the mixed-function oxidase catalyzed metabolism of carbon disulfide. Chem Biol Interact 10: 349–361, 1975 [DOI] [PubMed] [Google Scholar]
- 32.Dalvi RR, Poore RE, and Neal RA. Studies of the metabolism of carbon disulfide by rat liver microsomes. Life Sci 14: 1785–1796, 1974 [DOI] [PubMed] [Google Scholar]
- 33.Devaraj NK, Thurber GM, Keliher EJ, Marinelli B, and Weissleder R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc Natl Acad Sci U S A 109: 4762–4767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dewey RS, Schoenewaldt EF, Joshua H, Paleveda WJ, Schwam H, Barkemeyer H, Arison BH, Veber DF, Strachan RG, Milkowski J, Denkewalter RG, and Hirschmann R. Synthesis of peptides in aqueous medium .7. Preparation and use of 2,5-thiazolidinediones in peptide synthesis. J Org Chem 36: 49–59, 1971 [DOI] [PubMed] [Google Scholar]
- 35.Dickinson BC, Huynh C, and Chang CJ. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J Am Chem Soc 132: 5906–5915, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ensign SA. Reactivity of carbon-monoxide dehydrogenase from Rhodospirillum rubrum with carbon-dioxide, carbonyl sulfide, and carbon-disulfide. Biochemistry 34: 5372–5381, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Fahey JW, Zalcmann AT, and Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56: 5–51, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Fanjul M, Salvador C, Alvarez L, Cantet S, and Hollande E. Targeting of carbonic anhydrase IV to plasma membranes is altered in cultured human pancreatic duct cells expressing a mutated (Δf508) CFTR. Eur J Cell Biol 81: 437–447, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Farrell WS, Zavalij PY, and Sita LR. Metal-catalyzed “on-demand” production of carbonyl sulfide from carbon monoxide and elemental sulfur. Angew Chem Int Ed Engl 54: 4269–4273, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Feelisch M. The use of nitric oxide donors in pharmacological studies. Naunyn Schmiedebergs Arch Pharmacol 358: 113–122, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Ferm RJ. The chemistry of carbonyl sulfide. Chem Rev 57: 621–640, 1957 [Google Scholar]
- 42.Filipovic MR, Miljkovic JL, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, and Ivanovic-Burmazovic I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J Am Chem Soc 134: 12016–12027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flock OR, Andreae MO, and Drager M. Environmentally relevant precursors of carbonyl sulfide in aquatic systems. Mar Chem 59: 71–85, 1997 [Google Scholar]
- 44.Furchgott RF. and Zawadzki JV. The obligatory role of endothelial-cells in the relaxation of arterial smooth-muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 45.Gregoritza M. and Brandl FP. The Diels-Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur J Pharm Biopharm 97: 438–453, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Hanst PL, Spiller LL, Watts DM, Spence JW, and Miller MF. Infrared measurement of fluorocarbons, carbon-tetrachloride, carbonyl sulfide, and other atmospheric trace gases. J Air Pollut Control Assoc 25: 1220–1226, 1975 [Google Scholar]
- 47.Haritos VS. and Dojchinov G. Carbonic anhydrase metabolism is a key factor in the toxicity of CO2 and COS but not CS2 toward the flour beetle Tribolium castaneum Coleoptera: Tenebrionidae. Comp Biochem Physiol C Toxicol Pharmacol 140: 139–147, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Hartle MD. and Pluth MD. A practical guide to working with H2S at the interface of chemistry and biology. Chem Soc Rev 45: 6108–6117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haussinger D, Gorg B, Reinehr R, and Schliess F. Protein tyrosine nitration in hyperammonemia and hepatic encephalopathy. Metab Brain Dis 20: 285–294, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Henthorn HA. and Pluth MD. Mechanistic insights into the H2S-mediated reduction of aryl azides commonly used in H2S detection. J Am Chem Soc 137: 15330–15336, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hua W, Chen Q, Gong FQ, Xie CH, Zhou SL, and Gao LC. Cardioprotection of H2S by downregulating iNOS and upregulating HO-1 expression in mice with CVB3-induced myocarditis. Life Sci 93: 949–954, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Huang Y, Tang C, Du J, and Jin H. Endogenous sulfur dioxide: A new member of gasotransmitter family in the cardiovascular system. Oxid Med Cell Longev 2016: 9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huber C. and Wachtershauser G. Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: Implications for the origin of life. Science 281: 670–672, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Hugod C. Myocardial morphology in rabbits exposed to various gas-phase constituents of tobacco-smoke – An ultrastructural-study. Atherosclerosis 40: 181–190, 1981 [DOI] [PubMed] [Google Scholar]
- 55.Ignarro LJ, Buga GM, Wood KS, Byrns RE, and Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric-oxide. Proc Natl Acad Sci U S A 84: 9265–9269, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ignarro LJ, Napoli C, and Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide – An overview. Circ Res 90: 21–28, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Johansson B. Carbonyl sulfide – A copper chelating metabolite of disulfiram. Drug Metab Dispos 17: 351–353, 1989 [PubMed] [Google Scholar]
- 58.Kabil O. and Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 285: 21903–21907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaiser D. and Drack E. Diminished excretion of bicarbonate from single sweat gland of patients with cystic-fibrosis of pancreas. Eur J Clin Invest 4: 261–265, 1974 [DOI] [PubMed] [Google Scholar]
- 60.Kamboures MA, Blake DR, Cooper DM, Newcomb RL, Barker M, Larson JK, Meinardi S, Nussbaum E, and Rowland FS. Breath sulfides and pulmonary function in cystic fibrosis. Proc Natl Acad Sci U S A 102: 15762–15767, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamyshny A, Goifman A, Rizkov D, and Lev O. Formation of carbonyl sulfide by the reaction of carbon monoxide and inorganic polysulfides. Environ Sci Technol 37: 1865–1872, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Kashfi K. and Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol 85: 689–703, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katayama Y, Matsushita Y, Kaneko M, Kondo M, Mizuno T, and Nyunoya H. Cloning of genes coding for the three subunits of thiocyanate hydrolase of Thiobacillus thioparus THI115 and their evolutionary relationships to nitrile hydratase. J Bacteriol 180: 2583–2589, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katayama Y, Narahara Y, Inoue Y, Amano F, Kanagawa T, and Kuraishi H. A Thiocyanate hydrolase of Thiobacillus thioparus – A novel enzyme catalyzing the formation of carbonyl sulfide from thiocyanate. J Biol Chem 267: 9170–9175, 1992 [PubMed] [Google Scholar]
- 65.Keefer LK, Nims RW, Davies KM, and Wink DA. “Nonoates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms. Nitric Oxide 268: 281–293, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Kernohan JC. The activity of bovine carbonic anhydrase in imidazole buffers. Biochim Biophys Acta 81: 346–356, 1964 [Google Scholar]
- 67.Kesselmeier J, Teusch N, and Kuhn U. Controlling variables for the uptake of atmospheric carbonyl sulfide by soil. J Geophys Res Atmos 104: 11577–11584, 1999 [Google Scholar]
- 68.Khalil MAK. and Rasmussen RA. Global sources, lifetimes and mass balances of carbonyl sulfide (OCS) and carbon-disulfide (CS2) in the earths atmosphere. Atmos Environ 18: 1805–1813, 1984 [Google Scholar]
- 69.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem Int 63: 492–497, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Kimura H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid Redox Signal 22: 362–376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, and Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal Chem 70: 2446–2453, 1998 [DOI] [PubMed] [Google Scholar]
- 72.Kumar N, Bhalla V, and Kumar M. Recent developments of fluorescent probes for the detection of gasotransmitters (NO, CO and H2S). Coord Chem Rev 257: 2335–2347, 2013 [Google Scholar]
- 73.Labruere R, Alouane A, Le Saux T, Aujard I, Pelupessy P, Gautier A, Dubruille S, Schmidt F, and Jullien L. “Self-immolative” spacer for uncaging with fluorescence reporting. Angew Chem Int Ed Engl 51: 9344–9347, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Lee CL. and Brimblecombe P. Anthropogenic contributions to global carbonyl sulfide, carbon disulfide and organosulfides fluxes. Earth Sci Rev 160: 1–18, 2016 [Google Scholar]
- 75.Leman L, Orgel L, and Ghadiri MR. Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306: 283–286, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Leman LJ, Orgel LE, and Ghadiri MR. Amino acid dependent formation of phosphate anhydrides in water mediated by carbonyl sulfide. J Am Chem Soc 128: 20–21, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Lin VS. and Chang CJ. Fluorescent probes for sensing and imaging biological hydrogen sulfide. Curr Opin Chem Biol 16: 595–601, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther 74: 1–20, 1997 [DOI] [PubMed] [Google Scholar]
- 79.Lippert AR. Designing reaction-based fluorescent probes for selective hydrogen sulfide detection. J Inorg Biochem 133: 136–142, 2014 [DOI] [PubMed] [Google Scholar]
- 80.Lorimer GH. and Pierce J. Carbonyl sulfide: An alternate substrate for but not an activator of ribulose-1,5-bisphosphate carboxylase. J Biol Chem 264: 2764–2772, 1989 [PubMed] [Google Scholar]
- 81.Maines MD. The heme oxygenase system: A regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517–554, 1997 [DOI] [PubMed] [Google Scholar]
- 82.Marcolongo JP, Morzan UN, Zeida A, Scherlis DA, and Olabe JA. Nitrosodisulfide S2NO- (perthionitrite) is a true intermediate during the “cross-talk” of nitrosyl and sulfide. Phys Chem Chem Phys 18: 30047–30052, 2016 [DOI] [PubMed] [Google Scholar]
- 83.Maren TH. Carbonic anhydrase – Chemistry physiology and inhibition. Physiol Rev 47: 595–781, 1967 [DOI] [PubMed] [Google Scholar]
- 84.Mathew ND, Schlipalius DI, and Ebert PR. Sulfurous gases as biological messengers and toxins: Comparative genetics of their metabolism in model organisms. J Toxicol 2011: 14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McQuade LE. and Lippard SJ. Fluorescent probes to investigate nitric oxide and other reactive nitrogen species in biology. Curr Opin Chem Biol 14: 43–49, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Miljkovic JL, Kenkel I, Ivanovic-Burmazovic I, and Filipovic MR. Generation of HNO and HSNO from nitrite by heme-iron-catalyzed metabolism with H2S. Angew Chem Int Ed Engl 52: 12061–12064, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Miller EW, Albers AE, Pralle A, Isacoff EY, and Chang CJ. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc 127: 16652–16659, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Minami K. and Fukushi S. Volatilization of carbonyl sulfide from paddy soils treated with sulfur-containing substances. Soil Sci Plant Nutr 27: 339–345, 1981 [Google Scholar]
- 89.Mishanina AV, Libiad M, and Banerjee R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat Chem Biol 11: 457–464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitsuhashi H, Yamashita S, Ikeuchi H, Kuroiwa T, Kaneko Y, Hiromura K, Ueki K, and Nojima Y. Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock 24: 529–534, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Moncada S. and Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147: S193–S201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moncada S, Palmer RMJ, and Higgs EA. Nitric-oxide – Physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991 [PubMed] [Google Scholar]
- 93.Montoya LA. and Pluth MD. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem Commun 48: 4767–4769, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mustafa AK, Gadalla MM, and Snyder SH. Signaling by gasotransmitters. Sci Signal 2: re2 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogawa T, Noguchi K, Saito M, Nagahata Y, Kato H, Ohtaki A, Nakayama H, Dohmae N, Matsushita Y, Odaka M, Yohda M, Nyunoya H, and Katayama Y. Carbonyl sulfide hydrolase from Thiobacillus thioparus strain THI115 is one of the beta-carbonic anhydrase family enzymes. J Am Chem Soc 135: 3818–3825, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Olson KR. The therapeutic potential of hydrogen sulfide: Separating hype from hope. Am J Physiol Regul Integr Comp Physiol 301: R297–R312, 2011 [DOI] [PubMed] [Google Scholar]
- 97.Olson KR. Mitochondrial adaptations to utilize hydrogen sulfide for energy and signaling. J Comp Physiol B 182: 881–897, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Olson KR, DeLeon ER, and Liu F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide 41: 11–26, 2014 [DOI] [PubMed] [Google Scholar]
- 99.Ordonez CL, Henig NR, Mayer-Hamblett N, Accurso FJ, Burns JL, Chmiel JF, Daines CL, Gibson RL, McNamara S, Retsch-Bogart GZ, Zeitlin PL, and Aitken ML. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med 168: 1471–1475, 2003 [DOI] [PubMed] [Google Scholar]
- 100.Otterbein LE. and Choi AMK. Heme oxygenase: Colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 279: L1029–L1037, 2000 [DOI] [PubMed] [Google Scholar]
- 101.Pacher P, Beckman JS, and Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pastorekova S, Parkkila S, Pastorek J, and Supuran CT. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 19: 199–229, 2004 [DOI] [PubMed] [Google Scholar]
- 103.Peng B. and Xian M. Fluorescent probes for hydrogen sulfide detection. Asian J Org Chem 3: 914–924, 2014 [Google Scholar]
- 104.Piantadosi CA. Biological chemistry of carbon monoxide. Antioxid Redox Signal 4: 259–270, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Piantadosi CA. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med 45: 562–569, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piazzetta P, Marino T, and Russo N. The working mechanism of the beta-carbonic anhydrase degrading carbonyl sulphide (COSase): A theoretical study. Phys Chem Chem Phys 17: 14843–14848, 2015 [DOI] [PubMed] [Google Scholar]
- 107.Pluth MD, Bailey TS, Hammers MD, Hartle MD, Henthorn HA, and Steiger AK. Natural products containing hydrogen sulfide releasing moieties. Synlett 26: 2633–2643, 2015 [Google Scholar]
- 108.Pluth MD, Tomat E, and Lippard SJ. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Annu Rev Biochem 80: 333–355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Powell CR, Foster JC, Okyere B, Theus MH, and Matson JB. Therapeutic delivery of H2S via COS: Small molecule and polymeric donors with benign byproducts. J Am Chem Soc 138: 13477–13480, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prathapasinghe GA, Siow YL, Xu ZB, and Karmin O. Inhibition of cystathionine-beta-synthase activity during renal ischemia-reperfusion: Role of pH and nitric oxide. Am J Physiol Renal Physiol 295: F912–F922, 2008 [DOI] [PubMed] [Google Scholar]
- 111.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, and Davies KJA. Free radical biology and medicine: It's a gas, man! Am J Physiol Regul Integr Comp Physiol 291: R491–R511, 2006 [DOI] [PubMed] [Google Scholar]
- 112.Puranik M, Weeks CL, Lahaye D, Kabil O, Taoka S, Nielsen SB, Groves JT, Banerjee R, and Spiro TG. Dynamics of carbon monoxide binding to cystathionine beta-synthase. J Biol Chem 281: 13433–13438, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pursglove LA. and Wainwright HW. Colorimetric determination of carbony sulfide in synthesis gas. Anal Chem 26: 1835–1839, 1954 [Google Scholar]
- 114.Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, Schidlow DV, Wilmott RW, Astley SJ, McBurnie MA, Wentz K, and Smith AL. Efficacy of aerosolized tobramycin in patients with cystic-fibrosis. N Engl J Med 328: 1740–1746, 1993 [DOI] [PubMed] [Google Scholar]
- 115.Regelmann WE, Elliott GR, Warwick WJ, and Clawson CC. Reduction of sputum pseudomonas-aeruginosa density by antibiotics improves lung-function in cystic-fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis 141: 914–921, 1990 [DOI] [PubMed] [Google Scholar]
- 116.Rossin R, Verkerk PR, van den Bosch SM, Vulders RCM, Verel I, Lub J, and Robillard MS. In vivo chemistry for pretargeted tumor imaging in live mice. Angew Chem Int Ed Engl 49: 3375–3378, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Roth ME, Green O, Gnaim S, and Shabat D. Dendritic, oligomeric, and polymeric self-immolative molecular amplification. Chem Rev 116: 1309–1352, 2016 [DOI] [PubMed] [Google Scholar]
- 118.Ryter SW. and Otterbein LE. Carbon monoxide in biology and medicine. Bioessays 26: 270–280, 2004 [DOI] [PubMed] [Google Scholar]
- 119.Schenk S, Kesselmeier J, and Anders E. How does the exchange of one oxygen atom with sulfur affect the catalytic cycle of carbonic anhydrase? Chemistry 10: 3091–3105, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Seefeldt LC, Rasche ME, and Ensign SA. Carbonyl sulfide and carbon-dioxide as new substrates, and carbon-disulfide as a new inhibitor, of nitrogenase. Biochemistry 34: 5382–5389, 1995 [DOI] [PubMed] [Google Scholar]
- 121.Sehnert SS, Jiang L, Burdick JF, and Risby TH. Breath biomarkers for detection of human liver diseases: Preliminary study. Biomarkers 7: 174–187, 2002 [DOI] [PubMed] [Google Scholar]
- 122.Shabat D. Self-immolative dendrimers as novel drug delivery platforms. J Polym Sci A Polym Chem 44: 1569–1578, 2006 [Google Scholar]
- 123.Singer TP. and Kearney EB. Intermediary metabolism of L-cysteinesulfinic acid in animal tissues. Arch Biochem Biophys 61: 397–409, 1956 [DOI] [PubMed] [Google Scholar]
- 124.Smeulders MJ, Barends TRM, Pol A, Scherer A, Zandvoort MH, Udvarhelyi A, Khadem AF, Menzel A, Hermans J, Shoeman RL, Wessels H, van den Heuvel LP, Russ L, Schlichting I, Jetten MSM, and den Camp H. Evolution of a new enzyme for carbon disulphide conversion by an acidothermophilic archaeon. Nature 478: 412–416, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Smeulders MJ, Pol A, Venselaar H, Barends TRM, Hermans J, Jetten MSM, and Op den Camp HJM. Bacterial CS2 hydrolases from acidithiobacillus thiooxidans strains are homologous to the archaeal catenane CS2 hydrolase. J Bacteriol 195: 4046–4056, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Song ZJ, Ng MY, Lee ZW, Dai W, Hagen T, Moore PK, Huang D, Deng LW, and Tan CH. Hydrogen sulfide donors in research and drug development. MedChemComm 5: 557–570, 2014 [Google Scholar]
- 127.Steiger AK, Pardue S, Kevil CG, and Pluth MD. Self-immolative thiocarbamates provide access to triggered H2S donors and analyte replacement fluorescent probes. J Am Chem Soc 138: 7256–7259, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steiger AK, Yang Y, Royzen M, and Pluth MD. Bio-orthogonal “click-and-release” donation of caged carbonyl sulfide (COS) and hydrogen sulfide (H2S). Chem Commun 53: 1378–1380, 2017 [DOI] [PubMed] [Google Scholar]
- 129.Stipanuk MH. Metabolism of sulfur-containing amino-acids. Annu Rev Nutr 6: 179–209, 1986 [DOI] [PubMed] [Google Scholar]
- 130.Studer SM, Orens JB, Rosas I, Krishnan JA, Cope KA, Yang S, Conte JV, Becker PB, and Risby TH. Patterns and significance of exhaled-breath biomarkers in lung transplant recipients with acute allograft rejection. J Heart Lung Transplant 20: 1158–1166, 2001 [DOI] [PubMed] [Google Scholar]
- 131.Suarez I, Bodega G, Rubio M, and Fernandez B. Induction of NOS and nitrotyrosine expression in the rat striatum following experimental hepatic encephalopathy. Metab Brain Dis 24: 395–408, 2009 [DOI] [PubMed] [Google Scholar]
- 132.Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 20: 3467–3474, 2010 [DOI] [PubMed] [Google Scholar]
- 133.Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 27: 759–772, 2012 [DOI] [PubMed] [Google Scholar]
- 134.Supuran CT. and Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Pat 10: 575–600, 2000 [Google Scholar]
- 135.Supuran CT. and Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg. Med. Chem 15: 4336–4350, 2007 [DOI] [PubMed] [Google Scholar]
- 136.Supuran CT, Scozzafava A, and Casini A. Carbonic anhydrase inhibitors. Med Res Rev 23: 146–189, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Svoronos PDN. and Bruno TJ. Carbonyl sulfide: A review of its chemistry and properties. Ind Eng Chem Res 41: 5321–5336, 2002 [Google Scholar]
- 138.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007 [DOI] [PubMed] [Google Scholar]
- 139.Taubman SJ. and Kasting JF. Carbonyl sulfide – NO remedy for global warming. Geophys Res Lett 22: 803–805, 1995 [Google Scholar]
- 140.Tsien RY. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 290: 527–528, 1981 [DOI] [PubMed] [Google Scholar]
- 141.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, and Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 142.Versteegen RM, Rossin R, ten Hoeve W, Janssen HM, and Robillard MS. Click to release: Instantaneous doxorubicin elimination upon tetrazine ligation. Angew Chem Int Ed Engl 52: 14112–14116, 2013 [DOI] [PubMed] [Google Scholar]
- 143.Vullo D, Durante M, Di Leva FS, Cosconati S, Masini E, Scozzafava A, Novellino E, Supuran CT, and Carta F. Monothiocarbamates strongly inhibit carbonic anhydrases in vitro and possess intraocular pressure lowering activity in an animal model of glaucoma. J Med Chem 59: 5857–5867, 2016 [DOI] [PubMed] [Google Scholar]
- 144.Wallace JL. and Wang R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov 14: 329–345, 2015 [DOI] [PubMed] [Google Scholar]
- 145.Wang PG, Xian M, Tang XP, Wu XJ, Wen Z, Cai TW, and Janczuk AJ. Nitric oxide donors: Chemical activities and biological applications. Chem Rev 102: 1091–1134, 2002 [DOI] [PubMed] [Google Scholar]
- 146.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 147.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal 5: 493–501, 2003 [DOI] [PubMed] [Google Scholar]
- 148.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012 [DOI] [PubMed] [Google Scholar]
- 149.Wang R. Gasotransmitters: Growing pains and joys. Trends Biochem Sci 39: 227–232, 2014 [DOI] [PubMed] [Google Scholar]
- 150.Wang R, Li X, Cen X, Zeng L, and Tan X. Report on toxicity test of carbonyl sulfide. In: Proceedings of the 7th International Working Conference on Stored Product Protections Chengdu: Sichuan Publishing House of Science and Technology, 1999, Vol. 1, pp. 572–583 [Google Scholar]
- 151.Watts SF. The mass budgets of carbonyl sulfide, dimethyl sulfide, carbon disulfide and hydrogen sulfide. Atmos Environ 34: 761–779, 2000 [Google Scholar]
- 152.Wei C, Zhu Q, Liu WW, Chen WB, Xi Z, and Yi L. NBD-based colorimetric and fluorescent turn-on probes for hydrogen sulfide. Org Biomol Chem 12: 479–485, 2014 [DOI] [PubMed] [Google Scholar]
- 153.Wever R, Kast WM, Kasinoedin JH, and Boelens R. The peroxidation of thiocyanate catalyzed by myeloperoxidase and lactoperoxidase. Biochim Biophys Acta 709: 212–219, 1982 [DOI] [PubMed] [Google Scholar]
- 154.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, and Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun 343: 303–310, 2006 [DOI] [PubMed] [Google Scholar]
- 155.Wink DA. and Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25: 434–456, 1998 [DOI] [PubMed] [Google Scholar]
- 156.Wu LY. and Wang R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev 57: 585–630, 2005 [DOI] [PubMed] [Google Scholar]
- 157.Yuan S, Patel RP, and Kevil CG. Working with nitric oxide and hydrogen sulfide in biological systems. Am J Physiol Lung Cell Mol Physiol 308: L403–L415, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, and Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci U S A 94: 14848–14853, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao Y, Biggs TD, and Xian M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem Commun 50: 11788–11805, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao Y. and Pluth MD. Hydrogen sulfide donors activated by reactive oxygen species. Angew Chem Int Ed Engl 55: 14638–14642, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]