Abstract
Increased genetic testing in personalized medicine presents unique challenges for couples, including managing disease risk and potential discrimination as a couple. This study investigated couples' conflicts and support gaps as they coped with perceived genetic discrimination. We also explored the degree to which communal coping was beneficial in reducing support gaps, and ultimately stress. Dyadic analysis of married adults (N = 266, 133 couples), in which one person had the genetic risk for serious illness, showed that perceived discrimination predicted more frequent conflicts about AATD-related treatment, privacy boundaries, and finances, which, in turn, predicted wider gaps in emotion and esteem support, and greater stress for both spouses. Communal coping predicted lower support gaps for both partners and marginally lower stress.
Keywords: conflict, communal coping, personalized medicine, discrimination, social support, genetics, AATD, stress
Investigating Married Adults' Communal Coping with Genetic Health Risk and Perceived Discrimination
The advent of personalized medicine, or the use of a patient's environmental and genomic information in his or her health care, provided the promise of early prevention and tailored treatment (Feero, Guttmacher, & Collins, 2010). Genetic testing is becoming more complex and less expensive (sequencing whole genomes for less than $1,000), bringing it into many clinical settings (Jameson & Longo, 2015). Personalized medicine includes genetic testing of asymptomatic persons, referred to as predictive genetic testing, as well as testing symptomatic persons to diagnose a genetic cause for their existing disease, referred to as diagnostic genetic testing (Korf & Rehm, 2013; Ormond & Cho, 2014). Predictive testing has been used for a variety of chronic causes of morbidity and mortality, including cancer, heart disease, chronic obstructive pulmonary disease (COPD; Rahaghi et al., 2012), diabetes, asthma, and obesity (Chung, 2007). Predictive testing may reveal a wide range of risks, including being pre-symptomatic for a future disease (e.g., single-gene disorders like Huntington's disease), having an increased risk relative to the general population (e.g., inherited cancer), or being an asymptomatic carrier of a disease that could affect one's children (e.g., cystic fibrosis). As genetic medicine grows, so does concern about the potential use of genetic information for discrimination (Billings et al., 1992; Bombard et al., 2012; Freedman et al., 2003; Klitzman, 2009, 2010; Lowstuter et al., 2008; Otlowski, Taylor, & Bombard, 2012).
Despite its advantages for diagnosis and treatment, personalized medicine can contribute to stressful life circumstances for families, in which family members cope with the risk for future disease or question possible causes for current disease, as well as worry about experiencing discrimination. In this study, we focused on married partners' coping with Alpha-1 antitrypsin deficiency (AATD), an inherited, monogenic disorder associated with adult-onset lung and liver disease (Zuo, Pannell, Zhou, & Chuang, 2016). Although testing for common chronic health conditions has increased, few studies have investigated coping with such information (McBride, Koehly, Sanderson, & Kaphingst, 2010). Genetic health information may be considered as a shared problem requiring joint action among family members (McBride et al., 2010), especially if one is worried about discrimination that could be associated with it. We considered family adaptation in the context of spouses because marriage is a central relationship for middle-aged and older adults with serious health conditions (Revenson & Lepore, 2012). The study included couples in which one spouse had genes associated with AATD and the other did not. We framed our investigation within the theoretical model of communal coping (TMCC; Afifi, Hutchinson, & Krouse, 2006), because of its focus on adaptation as a social process that might lead to positive or negative outcomes depending on how it unfolds.
AATD
AATD exemplifies the complications of genomic medicine. It is associated with the defective production of alpha-1 antitrypsin, which protects lung tissue from damage (e.g., exposure to toxins in the air) and performs other positive functions for the body. Low levels of antitrypsin predispose people to COPD and liver disease, which can lead to early death (Klitzman, 2009). The diseases typically occur in later adulthood (Zuo et al., 2016) and have significant health burdens. For example, COPD can lead to difficulty walking and needing to use special equipment (e.g., oxygen tanks) to manage symptoms (Wheaton, Cunningham, Ford, & Croft, 2015).
The prognosis for AATD varies widely: Some people with genes associated with severe AATD may never have symptoms, while those with genes associated mild AATD can develop serious symptoms through environmental exposure (e.g., pollutants) or health behaviors (e.g., smoking; Tanash, Nilsson, Nilsson, & Piitulainen, 2010). This environmental influence makes AATD a compelling target for predictive testing, so people can modify their behaviors to limit disease. Unfortunately, AATD is also a salient case for discrimination.
Genetic Discrimination
As early as 1992, Billings and colleagues documented cases of discrimination based on genetic risk. They warned of a new vulnerable group of “asymptomatic ill” (p. 479) who had the potential to experience disease and to experience discrimination based on that potential. Genetic discrimination was defined as differential treatment of asymptomatic people or their family members on the basis of their real or presumed genetic characteristics (Billings et al., 1992; Bombard et al., 2012). Until the 2008 Genetic Information Nondiscrimination Act (GINA) was passed, insurance companies were not banned from using family histories of inherited disease and genetic tests to determine health insurance coverage (Otlowski et al., 2012). Unfortunately, GINA covered only health insurance—it did not cover life or disability insurance (Clayton, 2015). Furthermore, GINA did not extend to discrimination once someone was symptomatic (Clayton, 2015) and did not prohibit employers from using genetic information for hiring or promotion decisions (Otlowski et al., 2012).
The potential use of genetic information for discrimination is a reported worry among the general public (Hall et al., 2005), asymptomatic (Bombard et al., 2012) and symptomatic adults with genetic health risks (Klitzman, 2009, 2010), and U.S. physicians (Freedman et al., 2003; Lowstuter et al., 2008). People with the genetic risk for AATD report both expectations for and experiences with job and insurance discrimination against themselves and their children (e.g., Klitzman, 2009, 2010). In fact, one case of discrimination involving AATD was included in the congressional testimony supporting the creation of GINA (Feldman, 2012; Jones & Sarata, 2008) and another is the only instance in which the Equal Employment Opportunity Commission ruled that discrimination occurred on the basis of genetic health risk (Silvers & Stein, 2002).
Research shows a gap between perceived and experienced discrimination: Two to three times as many people believe that genetic discrimination occurs than report it (Otlowski et al., 2012). One explanation for this gap is that the extent of actual genetic discrimination has been exaggerated (e.g., Hook, 1992). An alternative explanation is that people proactively avoid discrimination (Bombard et al., 2012). For example, people with genetic risk for Huntington's disease, breast cancer, or AATD report avoiding testing, increasing privacy rules around results, keeping quiet about family history, getting married, and avoiding changing jobs to prevent discrimination (Bombard et al., 2012; Klitzman, 2010).
Perceived genetic discrimination could be viewed as an individual or family problem to be resolved by one or more people (Klitzman, 2010). Coping together with a spouse may leave married adults feeling less stressed overall, or it may increase stress. In the next section, we describe the theory framing this study's investigation into how couples cope with having a genetic risk for AATD and discuss the potential for communal coping to play a beneficial role in the process.
Theoretical Model of Communal Coping (TMCC)
People cope with stressful life circumstances through their interactions with others, especially close others (Afifi et al., 2006). Among couples facing chronic health conditions, a supportive intimate relationship protects against the effects of stress (Stanton, Revenson, & Tennen, 2007). Support from medical professionals and broader networks can also assist coping with health conditions; however, people typically desire and receive greater support from close relational partners (High & Steuber, 2014). Support from intimate partners can take on even greater significance in the case of unfamiliar or stigmatized conditions, including AATD, because active strategies to guard privacy and avoid discrimination might pre-empt support from other sources and because the condition is poorly understood by the broader community. Community and institutional supports are less effective than close others in assisting families when norms and expectations fail to offer a clear blueprint for understanding and addressing support needs (McCubbin, 1979).
Couples engage in communal coping when they assume joint ownership of problems and manage stress interactively (Afifi et al., 2006). TMCC, like other models of interpersonal coping (e.g., Berg & Upchurch, 2007), views the coping process as involving both an appraisal of the stressor as well as actions for addressing it as shared or not (see also Lyons, Mickelson, Sullivan, & Coyne, 1998). Different variations in appraisal and actions result in four forms of coping (T. D. Afifi et al., 2006). Individual coping manifests as a person appraises the stressor as personal and attempts to cope with it alone (Afifi et al., 2006). To illustrate, although both married adults may anticipate discrimination, they might appraise the stressor as belonging solely to the person with the genetic health risk, so that the affected individual copes without help from his or her partner. The second form, referred to as parallelism and protective buffering, occurs when people appraise the stressor as shared, but they work independently to manage it (parallelism) or attempt to shield the other from it (protective buffering). The third form of coping, referred to as directed support, involves appraising the stressor as personal, but treating the responsibility as shared. In contrast to the first two, the third form involves conversation about the stressor in which people ask partners to help (support seeking), transfer stress to their partner as they talk about it (contagion), or attempt to increase the partner's obligation for the stress. Conversation is even more integral to the fourth form, communal coping (Afifi et al., 2015). In this case, couples regard an illness directly experienced by one spouse as co-owned and work proactively together to manage stressors associated with the condition (e.g., Kowal, Johnson, & Lee, 2003).
Conflict and support gaps
In the formative study generating TMCC, Afifi et al. (2006) noted that communal coping sometimes evoked conflicts as families engaged in joint problem solving and confronted the family stressor directly in conversation. Perceived genetic discrimination could be a source of conflict because it raises questions about who is impacted (e.g., by family insurance coverage) and who has the responsibility to manage it. Genetic discrimination is discussed often in the context of losing employment and access to insurance, which affect finances (Klitzman, 2010; Otlowski et al., 2012). Financial pressures are one catalyst for couple conflict and can contribute to marital distress over time, if not managed through effective problem-solving (Conger, Rueter, & Elder, 1999). In addition, worries about genetic discrimination focus on the misuse of personal information (Otlowski et al., 2012), which might generate disagreements about privacy boundaries as couples weigh information's potential to promote their own and their family's health against the costs of potential discrimination. Thus, we expect that stronger perceived genetic discrimination is associated with more conflicts about AATD-related treatment, privacy boundaries, and finances.
Even though conflict is natural and can be beneficial, it can also lead to negative outcomes, such as chronic stress (Rodriguez & Margolin, 2013) and problematic interpersonal communication. Married adults are less likely to provide support to each other after experiencing conflicts (Minnotte, Stevens, Minnotte, & Kiger, 2007), and may respond, instead, with more blame and negative emotional displays during conversation (e.g., Green, Schaefer, MacDermid & Weiss, 2011). Thus, conflict about AATD may increase gaps in support.
Support gaps (e.g., Xu & Burleson, 2001), also called support adequacy (e.g., High & Steuber, 2014), refer to discrepancies between support desired from someone, such as a spouse, and the support received from that person. Although there are multiple forms of supportive communication (Xu & Burleson, 2001), this study focuses on emotional support (i.e., expressing affection for the support seeker) and esteem support (i.e., promoting the support seeker's self-worth). Emotional support is one of the most desired and received forms of support, and yet, married adults report desiring more emotional support from their spouses than they receive (High & Steuber, 2014). Esteem support is especially important in the present context because genetic discrimination threatens self-worth. Partners are the greatest source of esteem and emotional support for married adults facing a challenging health condition (High & Steuber, 2014).
Communal coping has the potential to reduce support gaps, thereby leaving people less stressed (Gamarel & Revenson, 2015). Because the problem and actions to address it are co-owned within communal coping, both partners are better positioned to anticipate and provide desired support. In Afifi et al.'s (2006) seminal article on TMCC, they described families engaging in communal coping as confronting stressors directly, even having conflicts about them, but framing their engagement as a loving, strong team. The descriptions included expressions of affection and esteem. Such descriptions align with the communication of effective support groups, in which members with similar challenges seek information, validate personal experience, express emotions, and develop new identities (e.g., Cline, 1999).
The above comments suggest that communal coping might promote overall resilience and reduce stress levels for couples because it leaves both partners feeling better supported, despite experiencing conflicts and other pressures. Prominent theories of coping and social support suggest that people show resilience in the face of external stressors when they have adequate resources (internal and external) to allow adaptation to and recovery from adversity (Hobfoll, 1989; McCubbin, 1979). Internal resources include effective coping strategies (McCubbin, 1979) and emotional reserves that accrue from approaching problems and life generally with a communal orientation (Afifi, Merrill, & Davis, 2016). In this sense, communal coping represents a general pattern of relating that can help couples process and absorb pressures associated with genetic risk and discrimination without feeling overwhelmed.
The Present Study
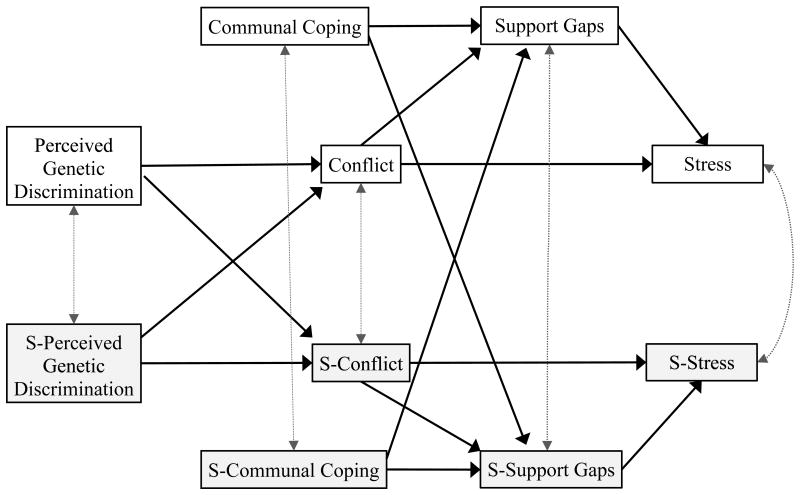
We model two ways that communal coping might benefit couples: 1) by reducing support gaps and promoting overall resilience, independent of other factors contributing to stress, or 2) by moderating effects of perceived discrimination and couple conflict. Figure 1 displays the hypothesized overall model, reflecting the direct influence of communal coping in reducing support gaps and stress. Perceived genetic discrimination is investigated as a stressor for married adults that may lead to more frequent conflicts about AATD-related issues (treatment, privacy boundaries, and finances), which, in turn, might predict wider gaps in emotion and esteem support. We expect that more frequent conflicts and larger support gaps leave married adults feeling more stressed in general. Communal coping is typically considered a beneficial coping style that reduces support gaps, thereby reducing stress. The model shows the direct influence of communal coping on support gaps and stress in a dyadic format that explicitly considers the two members of the couple: the partner with the genetic health risk and his or her spouse. Dyadic analysis is important in the context of stress and coping because family members can hold different perspectives on responsibility for stressors and appropriate coping strategies (Afifi et al., 2006). The dyadic model identifies similarities within the couple on key variables and provides additional insight into TMCC's proposition that family members' coping and stress are interdependent.
Figure 1.
Model in which perceived genetic discrimination affects support gaps as it leads to more conflicts about the genetic health condition. Intraclass correlations appear as dotted, double-headed grey arrows. Genetic registry members are represented in the top half of the model and their spouses are represented in the lower half.
Although not reflected in the model, we also test communal coping as a moderator of the effects of perceived genetic discrimination on stress through conflict and support gaps. Afifi et al. (2016) suggest that because communal coping builds relational and emotional reserves, people appraise relationally stressful situations from a broader mindset, experience less stress, and communicate in ways that preserve the relationship and maintain benevolence toward the partner. This suggests that communal coping could moderate impacts of perceived discrimination on couple conflict, support gaps, and stress. In a similar vein, Afifi, Felix, and Afifi (2012) found that communal coping with spouses and other close relational partners moderated the effects of uncertainty on psychological distress during a natural disaster.
Considering Time and Circumstances
Afifi et al. (2006) argued that time and situational elements may influence whether couples engage in communal coping. In the current study, for example, it is unclear whether healthy people seeking predictive testing versus sick persons learning of a potential genetic basis for their symptoms have different likelihoods of engaging in communal coping. We investigated couples' communal coping in relation to situational factors associated with AATD and genetic testing, including the length of the couple's relationship, the time since diagnosis, disease progression, and circumstances around testing in the following research question:
RQ. Are relational length, time since diagnosis, disease progression, or testing circumstances associated with the couple's level of communal coping?
Methods
Participants and Procedures
Participants were recruited through the Alpha-1 Research Registry at the Medical University of South Carolina (MUSC) in May, 2015. The study was approved by institutional review boards at MUSC and the authors' university. The registry includes members who have received genetic testing for AATD and were identified as having a version of the SERPHINA1 gene associated with AATD. Our sample was drawn from those who provided e-mail addresses and were willing to be contacted for research. Invitations were e-mailed to registered members with any version of the genetic characteristics associated with AATD (i.e., mild and severe deficiency) who were 21 years of age or older (N = 2,288). The e-mail indicated that the study was designed to understand experiences of people living with AATD and included a link to an online survey. Members were asked to share the survey link and their registry identification code with their spouses and to ask them to complete the survey. A total of 282 married members completed the survey, and 154 spouses completed it as well. After matching members and spouses by the member's identification code, the final sample included 133 matched couples (N = 266 participants).
Of the 266 participants, most were White (96%), reflecting the biology of AATD (de Serres, Blanco, & Fernandez-Bustillo, 2010). Half were male and half were female (2 participants did not answer), and 98% of the couples were heterosexual. Fifty-nine percent of members had a severe deficiency (n = 79), and the rest had a mild deficiency (41%, n = 54). The distribution varied by gender. Fifty percent of the women had the severe versions, while 78% of the men had the severe versions, χ2 (1, 131) = 6.52, p < .05, Cramer's V = .22. Fifty-eight percent (n = 155) were employed and 26% (n = 70) retired. Average age was 53 years (SD = 13.23, Median = 53, Range = 56). Average relational length was 24 years (SD = 15.02, Median = 22, Range = 58.92). Members received their genetic test results either in the past six months (9%, n = 12), the past year (6%, n = 8), the past 1-5 years (39%, n = 52), the past 5 to 10 years (17%, n = 22), or more than 10 years ago (29%, n = 39).
Measures
A confirmatory factor analysis of the measurement model (communal coping, perceived genetic discrimination, actual support, desired support, spousal talk, conflict, and stress) was estimated with maximum likelihood. All factors were allowed to covary but error terms were not. We used a combination rule of RMSEA ≤ .06 and SRMR ≤ .09 to assess model fit (Hu & Bentler, 1999). A multiple-groups analysis was run in AMOS (Version 24) using the entire dataset, comparing members and spouses. The goodness-of-fit statistics for the unconstrained model (SRMR = .06, RMSEA = .05, 90% CI [.04, .05]) and models constraining measurement weights (SRMR = .06, RMSEA = .05, 90% CI [.04, .05]), structural covariances (SRMR = .07, RMSEA = .05, 90% CI [.04, .05]), and measurement residuals (SRMR = .08, RMSEA = .05, 90% CI [.04, .05]) were below the cutoff values. Items showed factor loadings of .70 and higher. SPSS (Version 24) was used to create and analyze composite scores. The composite scores showed minimal skewness and kurtosis (<|1.8|) and were not transformed.
Communal coping
Three items (adapted from Afifi et al., 2015) were used to assess communal coping: We address problems as a team. My partner and I talk about how we will get through the experience together. We engage in activities or events together to cope. Participants were instructed to think about how they and their partner handle stressful or difficult aspects of AATD before responding to the items. Responses, marked on 5-point scales (1 = strongly disagree, 5 = strongly agree), were averaged into one score (α = .88 for members, α = .92 for spouses), with higher scores indicating more communal coping.
Perceived genetic discrimination
Four items (adapted from the discrimination section of Link, Cullen, Struening, Shrout, & Dohrenwend's [1989] discrimination-devaluation scale) were used to assess perceived discrimination based on a genetic health condition (e.g., Most employers would pass over the application of a person with a genetic health risk in favor of someone else. Most people would not want their children to marry someone identified with a genetic mutation.). Responses, measured on 5-point scales (1 = strongly disagree, 5 = strongly agree), were averaged into one score (α = .84 for members, α = .89 for spouses).
Support gaps
Five items adapted from the emotional and esteem subscales of Xu and Burleson's (2001) spousal support measure were used to assess support gaps. Items included telling you that he/she loves you and feels close to you, expressing understanding of a situation that is bothering you, showing some physical affection (hugs, hand-holding, shoulder patting, etc.) to comfort you when you are upset, providing you with hope or confidence, and telling you that you are still a good person even when you have a problem. Participants were instructed to reflect on conversations with their partner about AATD and to estimate how often they received the different types of reactions from him or her. Responses, marked on 5-point scales (1 = never, 5 = all the time), were averaged into one score (α = .95 for members, α = .97 for spouses). After completing those items, participants were asked to rate how much they desired the different reactions from their spouse, using the same scales (α = .95 for members, α = .98 for spouses). Support gaps were calculated as the difference between participants' desired and received support. Support gaps included positive scores (desiring more than one received) and negative scores (receiving more than desired).
Spousal talk
For descriptive purposes, nine items assessed how frequently couples engaged in conversations about Alpha-1 (e.g., I have talked with my spouse about how the Alpha-1 results make me feel.). Responses, marked on 5-point scales (1 = never, 5 = frequently), were averaged into one score (α = .89 for members, α = .93 for spouses).
Conflict
Three items assessed the frequency of arguments or disagreements about AATD (How often have you and your spouse had arguments or disagreements about financial costs associated with AATD? … about aspects of AATD-related treatment? … about deciding who to tell and who not to tell about the AATD test results?). Responses, measured on 5-point scales (1 = never, 5 = all the time), were averaged into one score (α = .70 for members, α = .75 for spouses).
Stress
Five items (adapted from Cohen, Kamarck, and Mermelstein's [1983] perceived stress scale) were used to assess the extent to which people appraise their life as stressful in the past month (e.g., In the past month, I felt difficulties were piling up so high that I could not overcome them). This measure has been associated with physical stress responses (e.g., Cohen, Kessler, & Gordon, 1995). Responses, measured on 5-point scales (1 = never, 5 = frequently), were averaged into one score (α = .92 for members, α = .92 for spouses).
Results
Descriptive Statistics and Preliminary Analyses
Registered members ranged in their disease progression, including being asymptomatic (59%), or having one (13%), two (19%), three (5%), or four (4%) indicators of disease progression. Notably, members with severe and mild deficiency both reported the other signs of disease progression, which aligned with the variable prognosis of AATD (e.g., Tanash et al., 2010). The married adults reported talking with their spouses about AATD on a regular basis (M = 3.02, SD = 0.88 for members, and M = 2.96, SD = 0.99 for spouses).
Forty-three percent of the registered members were involved in predictive testing (i.e., healthy when tested). The other 57% were sick when they were tested and identified with the genetic risk for AATD (i.e., diagnostic testing). More than half (59%) reported having severe deficiency, the rest (41%) reported having mild deficiency. The test results were associated with testing situations: 75% of those with severe deficiency received diagnostic testing, while 69% of those with mild deficiency had predictive testing, χ2 (1, 131) = 24.45, p < .05, Cramer's V = .43.
Key variables
The descriptive statistics for the key variables appear in Table 1. Almost half (44%) of the members and spouses (43%) expressed some level of perceived discrimination. Over half of the members (59%) and spouses (56%) reported never having conflicts about financial costs, treatment or disclosures related to AATD. More than a third of the members (37%) and nearly a third of spouses (29%) reported desiring more emotional and esteem support from their partner than they received. Most of the participants (79% of members and 76% of spouses) reported some level of communal coping. Pair-wise t tests were used to assess whether members and their spouses differed in their beliefs, reported conflicts, support gaps, or coping styles. None of the differences were statistically significant. Registered members and spouses reported similar levels of beliefs, conflicts, support gaps, and communal coping style.
Table 1. Descriptive Statistics and Differences Between AATD Members and Their Spouses (N = 133 Couples).
| Member | Spouse | ||||
|---|---|---|---|---|---|
|
| |||||
| M | SD | M | SD | t | |
| 1. Perceived Genetic Discrimination | 2.75 | 0.84 | 2.57 | 0.95 | 1.86 |
| 2. Conflict | 1.32 | 0.50 | 1.40 | 0.61 | -1.68 |
| 3. Support gaps | 0.13 | 0.89 | 0.05 | 0.98 | 0.75 |
| 4. Stress | 2.54 | 0.88 | 2.38 | 0.84 | 1.72 |
| 5. Communal Coping | 3.84 | 0.92 | 3.83 | 1.01 | 0.11 |
Note. Paired-sample t tests, two-tailed.
Age and sex
In an exploratory analysis, we investigated whether age or sex was associated with the key variables. Age was related to two variables, but these effects varied between members and spouses. Age was positively related to communal coping for spouses, r(131) = .20, p < .05, but not for members, r(131) = .00, ns. In contrast, age was negatively associated with stress for members, r(131) = -.23, p < .01, but not spouses, r(131) = -.14, ns. Sex differences appeared for members and spouses. For members, men perceived greater discrimination (M = 2.95, SD = 0.82) than did women (M = 2.61, SD = 0.84), t(131) = 2.29, p < .05, r = .20. Male members also reported more conflicts (M = 1.47, SD = 0.62) than did female members (M = 1.22, SD = 0.36), t(131) = 2.94, p < .01, r = .25. Conversely, male spouses reported fewer conflicts (M = 1.28, SD = 0.54) than did female spouses (M = 1.59, SD = 0.66), t(131) = -2.91, p<.01, r = -.20. Last, male members reported less stress (M = 2.34, SD = 0.89) than did female members (M = 2.67, SD = 0.86), t(131) = -2.07, p < .05, r = -.18.
Zero-order, intraclass correlations for the key variables were .21 (support gaps), .23 (stress), .26 (perceived genetic discrimination), .39 (communal coping), and .46 (conflict) (all p < .05). The results suggest that partners have the least consistency in their reported support gaps and the most consistency in reported conflicts.
Structural Model
The dyadic data were treated as distinguishable (Griffin & Gonzalez, 1995), in that each couple included a member of the genetic registry and a non-registered spouse. Hypotheses were examined with an actor-partner model with the composite variables using path analysis (Gonzalez & Griffin, 2012) in AMOS (Version 24). The parameters were estimated using bootstrapping procedures (2,000 bootstrap samples) and bias-corrected confidence intervals (Hayes & Scharkow, 2013).
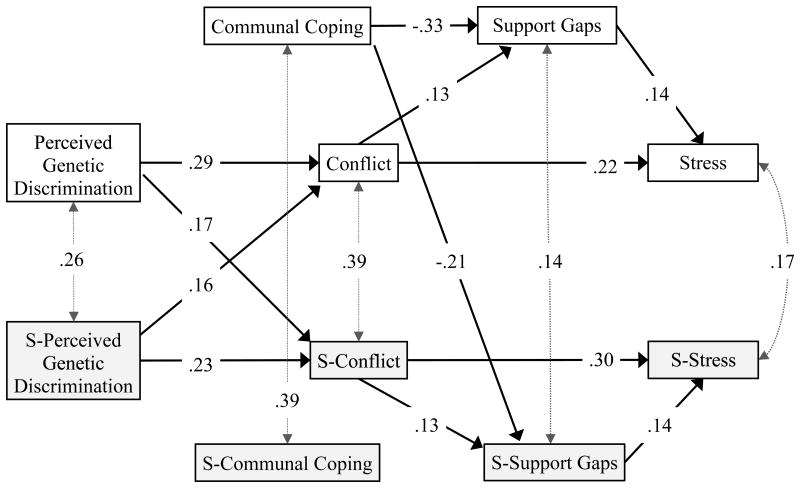
The hypothesized model fit the data well (Hu & Bentler, 1999): χ2 (26, N = 133 couples) = 25.90, p = .47, SRMR = .05, RMSEA = .00, 90% CI [.00, .07]. Two of the hypothesized paths were not statistically significant: spouses' communal coping was not associated with their own reports of support gaps (unstandardized b = -0.12, SE = 0.09, p = .27) or the members' reports of support gaps (unstandardized b = 0.06, SE = 0.08, p = .47). For parsimony, these two paths were removed at the same time and the model was re-estimated (Kline, 2015). The fit for the final model remained strong: χ2(28, N = 133 couples) = 28.86, p = 0.43, SRMR = .05, RMSEA = .02, 90% CI [.00, .07] (see Figure 2 for path estimates). The squared multiple correlations for the predicted variables were all statistically significant, including members' conflict (R2 = .13, p<.001), members' support gaps (R2 = .13, p<.001), members' stress (R2 = .07, p<.01), spouses' conflict (R2 = .10, p<.001), spouses' support gaps (R2 = .06, p<.001), and spouses' stress (R2 = .11, p<.01).
Figure 2.
Parameters shown on the paths are standardized regression weights; intraclass correlations appear on the dotted, grey, double-headed arrows. The goodness of fit indices for the revised model are χ2(28, N = 133 couples) = 28.86, p = .43, SRMR = .05, RMSEA = .02, 90% CI [.00, .07]. All of the path estimates were statistically significant at p < .05, one-tailed. The intraclass coefficients were statistically significant at p < .05, one-tailed. “S-” designates the spouse's variables.
As predicted, stronger perceptions of genetic discrimination were associated with more frequent conflicts about aspects of AATD-related treatment, deciding who to tell and not tell about the genetic test results, and financial costs associated with AATD. The effects were intrapersonal and interpersonal. Intrapersonally, members' perceptions of genetic discrimination were positively associated with their own reports of more frequent conflict about AATD (standardized b = .29, 95% CI [.13, .44], p<.05). Spouse's perceptions were also associated with their own reports of conflict (standardized b = .23, 95% CI [.04, .39], p<.05). Interpersonally, members' perceptions of genetic discrimination positively predicted their spouse's report of more frequent conflict (standardized b = .17, 95% CI [.00, .33], p<.05). Spouses' perceived discrimination also positively predicted members' reports of conflict (standardized b = .16, 95% CI [.01, .30], p<.05). The overlapping confidence intervals indicated that intrapersonal and interpersonal effects of perceived discrimination on conflict were similar for members and spouses.
As predicted, reports of more frequent conflicts about aspects of AATD were positively related to larger support gaps for members (standardized b = .13, 95% CI [.02, .28], p<.05) and for spouses (standardized b = .13, 95% CI [.00, .26], p<.05). The overlapping confidence intervals showed that the intrapersonal effect for conflict on support gaps was similar for members and spouses.
As predicted, reports of more frequent conflicts about aspects of AATD were positively related to greater stress for members (standardized b = .22, 95% CI [.03, .39], p<.05) and for spouses (standardized b = .30, 95% CI [.10, .47], p<.05). Larger support gaps were also related to greater stress for members (standardized b = .14, 95% CI [.01, .29], p<.05) and for spouses (standardized b = .14, 95% CI [.01, .31], p<.05). As shown by the overlapping confidence intervals, the intrapersonal effect for conflict and support gaps on stress was similar for members and spouses.
The results showed that members' communal coping was negatively associated with their own support gaps (standardized b = -.33, 95% CI [-.48,-.15], p<.05) and their spouse's support gaps (standardized b = -.21, 95% CI [-.33,-.03], p<.05). Spouses' communal coping was not associated with their own or their member's support gaps.
Mediation
To assess conflict and support gaps as mediators, we evaluated whether the indirect effects were statistically significant (Preacher, 2015), with bias-corrected confidence intervals (Hayes & Scharkow, 2013) using bootstrapping procedures (2,000 bootstrap samples). With respect to intrapersonal effects, the standardized indirect effect between perceived genetic discrimination and support gaps was .04 for members (95% CI [-.01, .10], p= .08) and .03 for spouses (95% CI [.00, .08], p <.05). The results showed that conflict served as a mediator of the intrapersonal effects of perceived discrimination on support gaps for spouses, and a nonsignificant trend for members. The standardized indirect effect between perceived discrimination and stress was .07 for members (95% CI [.00, .16], p <.05) and .07 for spouses (95% CI [.01, .16], p <.05). The results showed that conflict and support gaps served as mediators of the intrapersonal effects of perceived discrimination on stress. The standardized indirect effect between conflict and stress was .02 for members (95% CI [.00, .06], p= .09) and .02 for spouses (95% CI [.00, .06], p= .07). The results showed a nonsignificant trend for the expected mediating role of support gaps. Last, the standardized indirect effect between members' communal coping and stress was -.05 (95% CI [-.11, .00], p= .06), again indicating a nonsignificant trend for the expected mediating role of support gaps.
As for the interpersonal effects, the standardized indirect effect between members' perceived genetic discrimination and their spouse's support gaps was .02 (95% CI [.00, .07], p= .06). The standardized indirect effect between spouses' perceived discrimination and member's support gaps was .02 (95% CI [.00, .07], p= .07). The results showed nonsignificant trends for the expected interpersonal mediating roles of the actors' perceived discrimination on their partner's support gaps (e.g., the member's perceived discrimination and his or her spouse's support gaps. The standardized indirect effect between members' perceived discrimination and their spouse's stress was .05 (95% CI [.00, .15], p <.05). The standardized indirect effect between spouses' perceived discrimination and their member's stress was .04 (95% CI [.00, .11], p <.05). The results showed that conflict and support gaps served as mediators of the interpersonal effects of perceived discrimination on stress. The standardized indirect effect between members' communal coping and their spouse's stress was -.03 (95% CI [-.08, .01], p=.06). Similar to intrapersonal effects, this finding indicated a nonsignificant trend for the expected mediating role of support gaps.
Moderation: Communal Coping Couples
To examine whether and how communal coping moderates the impacts of perceived discrimination on couple conflict, support gaps, and stress, we categorized couples into two groups: high and low communal coping couples. To categorize couples, we combined the individual responses (i.e., the member's and the spouse's scores) into one score for each couple. By combining partners' scores, we index their overall level of communal coping as a couple, and their combined reservoir of relational and emotional resources for managing stressful situations. Next, couples were divided into high and low communal coping groups based on the median of their combined scores for communal coping. Sixty-eight couples were categorized as high communal coping couples (M = 4.46, SD = 0.35), while sixty-five couples were categorized as low communal coping (M = 3.19, SD = 0.61). There was a statistically significant difference in communal coping between the two groups, t(131) = 14.75, p < .001.
Table 2 shows the means, standard deviations, and inter-correlations among perceived genetic discrimination, conflict, support gaps, and stress for couples categorized with high and low communal coping. Moderation was tested in two ways. First, a post-hoc analysis of the structural model was conducted with a multi-group analysis, comparing couples with high versus low communal coping. The model with constrained parameter weights was no different from the unconstrained model, χ2diff (df = 12) = 12.60, p = .40, which suggests that the model applies across groups. Second, we evaluated the equality of correlations between couples high and low in communal coping with the Fisher r to z transformation (Kenny, Kashy, & Cook, 2006). There was only one difference: members' perceived genetic discrimination was associated with their spouses reporting more stress in couples with high communal coping (.27), but the interpersonal effect did not hold for those with low communal coping ( -.08), z = 2.01, p <.05. Of note, the intraclass correlation for stress differed between groups: stress levels were similar among couples with high communal coping (.40), but not among couples with low communal coping (.06), z = 0.02, p <.05, suggesting that communal coping might lead to greater shared experience in terms of stress levels. However, findings provided minimal support for communal coping as a moderator.
Table 2. Intercorrelations Among Variables for AATD Members and Their Spouses (N = 133 couples) with High or Low Couple-levels of Communal Coping.
| M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|---|
| High (n =68) | |||||||||
|
| |||||||||
| 1. P Genetic Discrim | 2.72 | 0.82 | |||||||
| 2. Conflict | 1.29 | 0.40 | .23 | ||||||
| 3. Support gaps | -0.04 | 0.73 | -.04 | .02 | |||||
| 4. Stress | 2.45 | 0.88 | .26* | .21 | .16 | ||||
|
| |||||||||
| 5. S P Genetic Discrim | 2.55 | 0.99 | .25* | .22 | .00 | .09 | |||
| 6. S Conflict | 1.40 | 0.56 | .20 | .33** | .11 | .15 | .37** | ||
| 7. S Support gaps | -0.19 | 0.71 | .02 | .08 | .16 | .09 | .04 | .05 | |
| 8. S Stress | 2.29 | 0.79 | .27a* | .20 | -.06 | .40 a** | .20 | .33** | .11 |
|
| |||||||||
| Low (n = 65) | |||||||||
|
| |||||||||
| 1. P Genetic Discrim | 2.77 | 0.87 | |||||||
| 2. Conflict | 1.34 | 0.58 | .41** | ||||||
| 3. Support gaps | 0.31 | 1.00 | .12 | .19 | |||||
| 4. Stress | 2.62 | 0.88 | .37** | .29* | .15 | ||||
|
| |||||||||
| 5. S P Genetic Discrim | 2.59 | 0.92 | .27* | .26* | -.09 | .22 | |||
| 6. S Conflict | 1.41 | 0.66 | .25* | .54** | .03 | .20 | .18 | ||
| 7. S Support gaps | 0.31 | 1.15 | .09 | .04 | .17 | .03 | -.01 | .18 | |
| 8. S Stress | 2.47 | 0.89 | -.08a | .24 | .06 | .06 a | -.05 | .32* | .18 |
Notes. Data was in the dyad structure (1 dyad per row) for this analysis. The cells shadowed in grey are the intraclass correlations. “S” denotes the spouse's scores. Correlations were compared between groups with Fisher's r to z transformation (Kenny, Kashy, & Cook, 2006). Correlations with the subscript a differ between groups at p<.05, two-tailed.
Independent t tests were used to compare the mean levels between groups. The only difference was in reports of support gaps. In low communal-coping couples, registry members reported larger support gaps (desiring more support than they received) than in high communal-coping couples, t(131) = 2.33, p<.05, r = .20, as did their spouses, t(131) = 3.04, p<.05, r = .26. These findings supported the overall effect of communal coping on couple's support provision.
Time, Circumstances, and Communal Coping
RQ1 explored how relational length, time since diagnosis, disease progression, and circumstances around testing were associated with the couple's level of communal coping. Correlations showed that couples' communal coping was positively associated with disease progression, r(131) = .24, p<.05, but not with relational length, r(131) = .03, ns, or time since diagnosis, r(131) = .08, ns. Testing circumstances predicted couples' communal coping: communal coping was higher if the member received diagnostic genetic testing (i.e., diagnosing an existing symptom; M = 4.01, SD = 0.73) versus predictive testing (i.e., identifying genetic predictors for an asymptomatic person; M = 3.61, SD = 0.85), t(131) = 2.96, p<.01, r = .25.
In a post hoc analysis, we explored whether situational variables were associated with couples' average scores for other key variables. Time since diagnosis and relational length were not associated with any of the key variables. Disease progression was positively associated with couples' perceptions of genetic discrimination, r(131) = .17, p<.05. Testing circumstance was associated with conflict. Couples reported more frequent AATD-related conflicts when the member had diagnostic (M = 1.45, SD = 0.52) versus predictive testing (M = 1.24, SD = 0.37), t(131) = 2.56, p<.05, r = .22.
Discussion
This study, framed by TMCC (Afifi et al., 2006), investigated coping with perceived genetic discrimination in couples in which one spouse had genetic characteristics associated with AATD. The potential for genetic discrimination adds to already stressful life circumstances for tested individuals and their partners, who may feel pressure to guard test results and proactively avoid discrimination while simultaneously managing disease risk, progression, and treatment. Since couples often guard the privacy of test results to avoid perceived discrimination (Bombard et al., 2012; Klitzman, 2010), AATD and similar disorders can leave couples more isolated in addressing problems, thereby increasing the importance of interpersonal coping and mutual support among intimate partners. The overall model tested in this study assumes that perceived genetic discrimination is a relational stressor for tested individuals and their partners, which can lead to more conflicts about how to manage AATD and widen gaps between desired and experienced emotional and esteem support.
Results mostly supported the presumed impacts of perceived discrimination for both partners. Stronger perceived genetic discrimination was associated with more conflicts about AATD-related treatment, privacy boundaries, and finances. The effects were interpersonal and intrapersonal. More conflicts were associated with more stress, both directly and indirectly through larger gaps in desired and experienced emotion and esteem support. Communal coping did not moderate these impacts but did have an overall effect on support gaps, such that both partners felt better supported when they treated the genetic disorder as a shared problem that they managed together as a couple. The one surprising finding was that only members' communal coping led to smaller gaps. In contrast, spouses' reported communal coping was not associated with their own or their partner's support gaps.
Coping with Perceived Genetic Discrimination
Almost half of the sample (44% of members and 43% of spouses) believed that genetic discrimination may occur by employers and insurance companies. These levels are similar to those reported for other genetic health conditions (Bombard et al., 2012) and by the public at large (Hall et al., 2005). Couples' average level of perceived discrimination did not relate to their time together, time since testing, or disease progression; however, male registry members perceived greater discrimination than female members. This finding may reflect socioeconomic differences in our sample. While male and female members were equally likely to be employed (46% and 56%, respectively), more male members (30%) versus female members (16%) had their names on the insurance policy covering them and their spouse. The costs of discrimination on livelihood and insurance may have been most salient to male members.
Conflicted Coping
Overall, the couples reported fighting infrequently about AATD. However, the presence of conflict, even infrequent, was associated with members and spouses reporting more stress, which resonated with existing research (Rodriguez & Margolin, 2013). The formative study associated with TMCC (Afifi et al., 2006) also noted the presence of conflicts as families engaged in problem-solving together. One reason is that the conversation may shift from communal coping to some form of directive support in which one person wants another to change their appraisal of the problem or their responsibility to address it (Afifi et al., 2006). For example, members concerned about perceived genetic discrimination may be motivated to seek help from their spouse on how to resolve it or to ask the spouse to assume responsibility for working through it (e.g., if their spouse holds the insurance policy in their name). Spouses may attempt to address their concerns about potential discrimination on their own, but then learn about the members' perceived discrimination as they communicate about how to address it. Communication models of health disclosures (e.g., Greene, 2009) typically put the person with the health condition as the one primarily responsible for making decisions about privacy rules. Confidants occupy a supporting role in enforcing the discloser's privacy rules (Petronio, 2002). However, genetic discrimination affects families as a whole, because they share insurance coverage, economic circumstances, and inherited health conditions. Thus, perceived genetic discrimination may be a salient example in which appraisals of the stressor as my/your/our problem, as well as the responsibility to address it as shared or not, represent difficult issues that sometimes elicit conflict.
Communal Coping with Genetic Health Conditions
Many of the couples in this study agreed that they handle stressful or difficult aspects of AATD as a team, with average scores above 3.8 on a 5-point scale for members and spouses. The relatively high level of communal coping reported by couples was similar to couples' reports in other studies of TMCC (e.g., Afifi et al., 2015).
Communal coping was beneficial for couples. We modeled the influence of communal coping in two ways: first, as a pattern of relating that lowers support gaps and improves overall resilience, independent of other factors; and second, as a moderator of effects of perceived discrimination and couple conflict. The results supported the overall influence of communal coping on living with AATD. Communal coping predicted lower support gaps and marginally lower stress for both partners. The dyadic analysis showed that the effect of communal coping on support gaps was driven by the members' reports, suggesting that the appraisals of registered members influenced support experienced by both partners. Perhaps when registry members see the disorder as a shared problem that they address with their spouses, this promotes responsiveness to the mutual support needs of both partners.
Although communal coping moderated effects of external stressors in a different research context (e.g., Afifi et al., 2012), we found little support for communal coping as a moderator of the effects of perceived discrimination on conflict, support gaps, and stress. We found that in high communal coping couples (but not in low communal coping couples) the intraclass correlation for stress was quite strong (.40) and that members' perceived discrimination was positively associated with spouses' stress. These findings suggest that among high communal coping couples, communal coping may lead to greater shared stress as spouses absorb members' worries about anticipated discrimination. In contrast, high communal coping couples had smaller support gaps than low communal coping couples. It is possible that in the context of serious chronic health conditions with genetic risk factors that achieving strong levels of communal coping as a couple allows them to reduce support gaps, but it may also facilitate emotional contagion. These results demonstrate that while communal coping can have positive impacts on marital relationships, it is not risk free. Future research should investigate potential contagion effects and its implications for marital functioning.
Demographic and situational factors
We explored whether demographic or situational factors influenced couples' communal coping. Communal coping seemed achievable regardless of time. Couples had been together from 1 month to over 50 years and had learned about the genetic test results from within the past month to more than 10 years ago. Couples' level of communal coping was not associated with relational length or time since diagnosis. Gender also was not related to communal coping, which conflicts with expectations for gender differences in communal coping (Lewis et al., 2006) but aligns with previous studies (Afifi et al., 2015).
Testing circumstances did influence communal coping. Couples had higher communal coping when the genetic characteristics were found through diagnostic versus predictive testing. Research on genetic testing has suggested that genetic testing's assistance in making a diagnosis can provide an ending to the frustrating and expensive “diagnostic odyssey” (Korf & Rehm, 2013, p. 1512; Klitzman, 2009), an increased feeling of personal control (Smith & Pollin, 2009), and a prelude to more effective treatment (Klitzman, 2009; Korf & Rehm, 2013). The presentation of AATD-related symptoms mimics other conditions like asthma, which can lead to a five to eight year lag time between onset of symptoms and AATD testing (Stoller et al., 2005). Diagnostic testing may be associated with seeing the health threat as more serious and as being solvable, which may motivate couples toward communal coping (Lewis et al., 2006). It is also possible that people who received diagnostic testing were more dependent on their spouse due to their poor health. This might place the stressor at the forefront of the relationship thereby serving as a catalyst for communal coping.
Diagnostic testing is interesting because it was associated with more communal coping and more conflicts. A diagnostic test provides a reason for illness, but spouses may have different perspectives on how genes influence health and such differences can foster conflict (see also Afifi et al., 2015). For this sample, 75% of those receiving diagnostic testing had severe deficiency. Spouses may have differed in their perceptions of how well a genetic-based health condition could be prevented, which actions to enact and how quickly, and who is responsible for taking action. Married adults differ in the extent to which they believe that lifestyle choices can shape whether genes determine future health (Parrott, Smith, Hong, & Worthington, 2015). Moreover, one protective action for AATD is to avoid environmental toxins, such as quitting smoking and avoiding air pollution (Klitzman, 2009), which can require major lifestyle changes that evoke conflict.
Practical Implications
The results of this study suggest that couples need guidance on how to have constructive, supportive conversations. Even though spouses, on average, reported talking about AATD on a regular basis, some couples reported arguing about AATD and many members and spouses reported getting less support than they desired from their partner. As seen in other studies of couples (Fergus & Skerrett, 2015; Gamarel & Revenson, 2015), smaller support gaps were associated with less stress. More communal coping resulted in smaller support gaps. New interventions will be needed, since current interventions have focused on individual coping with genetic risk information (McBride et al., 2010). The intraclass coefficient between partners on communal coping, while statistically significant, was small (.39). This finding provides an interesting conundrum for communal coping: do couples' perceptions of communal coping need to be in sync to be productive? Interventions to promote effective communal coping may not need to ensure that married adults have synchronized perceptions of communal coping at any given moment to be productive (Fergus & Skerrett, 2015). Couples may need to become strong and flexible in their coping styles to be most resilient to stress (Fergus & Skerrett, 2015; Revenson & Lepore, 2012). In addition, people's perceptions of communal coping, especially as a global measure toward a complex genetic health condition, may fluctuate from time to time.
Limitations and Future Research
Although the present study benefits from including married adults with a documented genetic-based condition, recruiting from a research registry may result in selection bias. People who are willing to stay involved in research might differ from those who are not willing, thus limiting our ability to generalize these findings to other married couples in which one spouse has AATD. People with genetic characteristics of mild deficiency may also be less likely to be involved in registries than those with severe deficiency. Indeed, more participants reported genetic characteristics associated with severe than mild deficiency.
The small sample in this study also limited statistical power. Consequently, findings should be treated cautiously until they have been replicated. Due to the cross-sectional design, the study did not capture the dynamic nature of conversation or stress, which is a key feature of coping (Afifi et al., 2006) and limited our ability to make causal claims. Ultimately, we were interested in effects of discrimination, conflict, and coping on stress, and this causal order is reflected in the model. However, overall stress could also increase couple conflict and impact coping styles. Future longitudinal studies should probe the extent to which stress is an outcome or cause of couple communication and coping styles.
While this study provided insights into marital coping with genetic health conditions, it did not assess family-wide coping. The findings on communal coping for spouses may not generalize to other family pairs, such as parent and child. Whether and how young children should receive genetic tests and learn of results is an ongoing debate (McBride et al., 2010; Tercyak, 2009). Like other stressors, such as some aspects of divorce (Afifi et al., 2006) and economic hardship (Afifi et al., 2015), children may need to be protected from the responsibility for coping with genetic health risks.
Conclusion
Advances in genomic science and declining costs for genomic testing may lead to a day when every person's genome is sequenced and used in health care, even though the clinical utility has yet to be firmly established (Korf & Rehm, 2013; Ormond & Cho, 2014). Families may find themselves coping with an array of relevant and irrelevant genetic test results for current and/or future disease (Korf & Rehm, 2013; Ormond & Cho, 2014), along with concerns about whether others will use the results to discriminate against them (Klitzman, 2010). This study offers insights into how communal coping with a genetic health risk may be associated with more or less stress by highlighting the roles of conflict and support gaps, and the benefits and costs of communal coping. This model may extend to other major health issues without connections (yet) to genetic characteristics, particularly those encumbered by the potential for social rejection.
Acknowledgments
Authors' Note: Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number R21HG007111 and by the National Institute on Drug Abuse under Award Number P50-DA010075-16. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are grateful to the members of the Alpha-1 Research Registry and their spouses for sharing their thoughts with us. We thank Roxanne Parrott, Anne Merrill, Mary Poss, Amber Worthington, and Amanda Applegate for their feedback on an earlier version of this paper.
Contributor Information
Rachel A. Smith, The Pennsylvania State University
Alan Sillars, University of Montana.
Xun Zhu, The Pennsylvania State University.
References
- Afifi TD, Davis S, Merrill AF, Coveleski S, Denes A, Afifi W. In the wake of the Great Recession: Economic uncertainty, communication, and biological stress responses in families. Human Communication Research. 2015;41:268–302. doi: 10.1111/hcre.12048. [DOI] [Google Scholar]
- Afifi TD, Hutchinson S, Krouse S. Toward a theoretical model of communal coping in postdivorce families and other naturally occurring groups. Communication Theory. 2006;16:378–406. doi: 10.1111/j.1468-2885.2006.00275.x. [DOI] [Google Scholar]
- Afifi TD, Merrill AF, Davis S. The theory of resilience and relational load. Personal Relationships. 2016;23:663–683. doi:101111/pere.12159. [Google Scholar]
- Afifi WA, Felix ED, Afifi TD. The impact of uncertainty and communal coping on mental health following natural disasters. Anxiety, Stress, & Coping. 2012;25:329–347. doi: 10.1080/10615806.2011.603048. [DOI] [PubMed] [Google Scholar]
- Amos (Version 24.0) [Computer Program] Chicago, IL: IBM SPSS; [Google Scholar]
- Berg CA, Upchurch R. A developmental-contextual model of couples coping with chronic illness across the life span. Psychological Bulletin. 2007;133:920–954. doi: 10.1037/0033-2909.133.6.920. doi:0.1037/0033-2909.133.6.920. [DOI] [PubMed] [Google Scholar]
- Billings PR, Kohn MA, de Cuevas M, Beckwith J, Alper JS, Natowicz MR. Discrimination as a consequence of genetic testing. American Journal of Human Genetics. 1992;50:476–482. [PMC free article] [PubMed] [Google Scholar]
- Bombard Y, Palin J, Friedman JM, Veenstra G, Creighton S, Bottorff JL, Canadian Respond-HD Collaborative Research Group Beyond the patient: The broader impact of genetic discrimination among individuals at risk of Huntington's disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2012;159:217–226. doi: 10.1002/ajmg.b.32016. [DOI] [PubMed] [Google Scholar]
- Chung WK. Implementation of genetics to personalize medicine. Gender Medicine. 2007;4:248–65. doi: 10.1016/S1550-8579(07)80044-1. [DOI] [PubMed] [Google Scholar]
- Clayton EW. Why the Americans with Disabilities Act matters for genetics. The Journal of the American Medical Association. 2015;313:2225–2226. doi: 10.1001/jama.2015.3419. [DOI] [PubMed] [Google Scholar]
- Cline RJW. Communication in support groups. In: Frey LR, Gouran DS, Poole MS, editors. The handbook of group communication theory and research. Thousand Oaks, CA: Sage; 1999. pp. 516–538. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:386–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU. Strategies for measuring stress in psychiatric and physical disorders. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress. New York: Oxford University Press; 1995. pp. 3–28. [Google Scholar]
- Conger RD, Rueter MA, Elder GH. Couple resilience to economic pressure. Journal of Personality and Social Psychology. 1999;76:54–71. doi: 10.1037/0022-3514.76.1.54. [DOI] [PubMed] [Google Scholar]
- de Serres FJ, Blanco I, Fernandez-Bustillo E. Ethnic differences in alpha-1 antitrypsin deficiency in the United States of America. Therapeutic Advances in Respiratory Disease. 2010;4:63–70. doi: 10.1177/1753465810365158. [DOI] [PubMed] [Google Scholar]
- Feldman EA. The Genetic Information Nondiscrimination Act (GINA): Public policy and medical practice in the age of personalized medicine. Journal of Genetic Internal Medicine. 2012;27:743–746. doi: 10.1007/s11606-012-1988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feero WG, Guttmacher AE, Collins FS. Genomic medicine –An updated primer. The New England Journal of Medicine. 2010;362:2001–2011. doi: 10.1056/NEJMra0907175. [DOI] [PubMed] [Google Scholar]
- Fergus K, Skerrett T. Resilient couple coping revisited: Building relationship muscle. In: Skerrett K, Fergus K, editors. Couple resilience: Emerging perspectives. Dordrecht, Netherlands: Springer; 2015. pp. 199–210. [Google Scholar]
- Freedman AN, Wideroff L, Olson L, Davis W, Klabunde C, Srinath KP, Ballard-Barbash R. U.S. physicians' attitudes toward genetic testing for cancer susceptibility. American Journal of Medical Genetics. 2003;120A:63–71. doi: 10.1002/ajmg.a.10192. [DOI] [PubMed] [Google Scholar]
- Gamarel K, Revenson T. Dyadic adaptation to chronic illness: The importance of considering context in understanding couples' resilience. In: Skerrett K, Fergus K, editors. Couple resilience: Emerging perspectives. Dordrecht, Netherlands: Springer; 2015. pp. 83–106. [Google Scholar]
- Gonzalez R, Griffin D. Dyadic data analysis. In: Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA handbook of research methods in psychology, Vol 3: Data analysis and research publication. Washington, DC: American Psychological Association; 2012. pp. 439–450. [DOI] [Google Scholar]
- Green SG, Schaefer RAB, MacDermid SM, Weiss HM. Partner reactions to work-to-family conflict: Cognitive appraisal and indirect crossover in couples. Journal of Management. 2011;37:744–769. doi: 10.1177/0149206309349307. [DOI] [Google Scholar]
- Greene K. An integrated model of health disclosure decision-making. In: Afifi T, Afifi W, editors. Uncertainty and information regulation in interpersonal contexts: Theories and applications. New York: Routledge; 2009. pp. 226–253. [Google Scholar]
- Griffin D, Gonzalez R. The correlational analysis of dyad-level data: Models for the exchangeable case. Psychological Bulletin. 1995;118:430–439. doi: 10.1037/0033-2909.118.3.430. [DOI] [Google Scholar]
- Hall MA, McEwen JE, Barton JC, Walker AP, Howe EG, Reiss JA, Thomson EJ. Concerns in a primary care population about genetic discrimination by insurers. Genetic Medicine. 2005;7:311–316. doi: 10.1097/01.GIM.0000162874.58370.C0. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychological Science. 2013;24:1918–1927. doi: 10.1177/0956797613480187. [DOI] [PubMed] [Google Scholar]
- High AC, Steuber KR. An examination of support (in)adequacy: Types, sources, and consequences of social support among infertile women. Communication Monographs. 2014;81:157–178. doi: 10.1080/03637751.2013.878868. [DOI] [Google Scholar]
- Hobfoll SE. Conservation of resources: A new attempt at conceptualizing stress. American Psychologist. 1989;44:513–524. doi: 10.1037/0003-066X.44.3.513. [DOI] [PubMed] [Google Scholar]
- Hook EB. Muddling genetic discrimination. American Journal of Human Genetics. 1992;51:899–901. [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Jameson JL, Longo DL. Precision medicine – Personalized, problematic, and promising. The New England Journal of Medicine. 2015;372:2229–2234. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- Jones NL, Sarata AK. Report for Congress Genetic information: Legal issues relating to discrimination and privacy. 2008 Retrieved from http://biotech.law.lsu.edu/crs/RL30006_20080310.pdf.
- Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. New York, NY: The Guilford Press; 2006. [Google Scholar]
- Kline RB. Methodology in the social sciences: Principles and practice of structural equation modeling. Fourth. New York, NY: Guilford Press; 2015. [Google Scholar]
- Klitzman R. The impacts of social contexts in testing for Alpha-1 Antitrypsin deficiency: The roles of physicians and others. Genetic Testing and Molecular Biomarkers. 2009;13:269–276. doi: 10.1089/gtmb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. Views of discrimination among individuals confronting genetic disease. Journal of Genetic Counseling. 2010;19:68–83. doi: 10.1007/s10897-009-9262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf BR, Rehm HL. New approaches to molecular diagnosis. The Journal of the American Medical Association. 2013;309:1511–1521. doi: 10.1001/jama.2013.3239. [DOI] [PubMed] [Google Scholar]
- Kowal J, Johnson SM, Lee A. Chronic illness in couples: A case for emotional focused therapy. Journal of Marital and Family Therapy. 2003;29:299–319. doi: 10.1111/j.1752-0606.2003.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM. Understanding health behavior change among couples: An interdependence and communal coping approach. Social Science & Medicine. 2006;62:1369–1380. doi: 10.1016/j.socscimed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Link BG, Cullen FT, Struening EL, Shrout PE, Dohrenwend BP. A modified labeling theory approach to mental disorders: An empirical assessment. American Sociological Review. 1989;54:400–423. doi: 10.2307/2095613. [DOI] [Google Scholar]
- Lowstuter KJ, Sand S, Blazer KR, MacDonald DJ, Banks KC, Lee CA, Weitzel JN. Influence of genetic discrimination perceptions and knowledge on cancer genetics referral practice among clinicians. Genetics in Medicine. 2008;10:691–698. doi: 10.1097/GIM.0b013e3181837246. [DOI] [PubMed] [Google Scholar]
- Lyons RF, Mickelson K, Sullivan JL, Coyne JC. Coping as a communal process. Journal of Social and Personal Relationships. 1998;15:579–607. doi: 10.1177/0265407598155001. [DOI] [Google Scholar]
- McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The behavioral response to personalized genetic information: Will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annual Review of Public Health. 2010;31:89–103. doi: 10.1146/annurev.publhealth.012809.103532. [DOI] [PubMed] [Google Scholar]
- McCubbin H. Integrating coping behavior in family stress theory. Journal of Marriage and Family. 1979;41:237–244. doi: 10.2307/351693. [DOI] [Google Scholar]
- Minnotte KL, Stevens DP, Minnotte MC, Kiger G. Emotion-work performance among dual-earner couples: Testing four theoretical perspectives. Journal of Family Issues. 2007;28:773–793. doi: 10.1177/0192513X07299676. [DOI] [Google Scholar]
- Ormond KE, Cho MK. Translating personalized medicine using new genetic technologies in clinical practice: the ethical issues. Personalized Medicine. 2014;11:211–222. doi: 10.2217/pme.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otlowski M, Taylor S, Bombard Y. Genetic discrimination: International perspectives. Annual Review of Genomics and Human Genetics. 2012;13:433–454. doi: 10.1146/annurev-genom-090711-163800. [DOI] [PubMed] [Google Scholar]
- Parrott RL, Smith RA, Hong SJ, Worthington A. Congruence-incongruence patterns in Alpha-1 Antitrypsin Deficiency couples' genetic determinist beliefs and perceived control over genes: Implications for clinical and public health genomic communication. Journal of Genetic Counseling. 2015;24:532–540. doi: 10.1007/s10897-014-9786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronio S. Boundaries of privacy: Dialectics of disclosure. Albany, NY: State University of New York Press; 2002. [Google Scholar]
- Rahaghi FF, Sandhaus RA, Strange C, Hogarth DK, Eden E, Stocks JM, Stoller JK. The prevalence of Alpha-1 antitrypsin deficiency among patients found to have airflow obstruction. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2012;9:352–358. doi: 10.3109/15412555.2012.669433. [DOI] [PubMed] [Google Scholar]
- Revenson TA, Lepore SJ. Coping in social context. In: Baum A, Revenson TA, Singer JE, editors. Handbook of health psychology. 2nd. New York, NY: Psychology Press; 2012. pp. 193–217. [Google Scholar]
- Rodriguez AJ, Margolin G. Wives and husbands' cortisol reactivity to proximal and distal dimensions of couple conflict. Family Processes. 2013;52:555–569. doi: 10.1111/famp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers A, Stein MA. An equality paradigm for preventing genetic discrimination. Vanderbilt Law Review. 2002;55:1341–1395. [PubMed] [Google Scholar]
- Smith AC, Pollin TI. Patient education. In: Uhlmann WR, Schuette JL, Yasher B, editors. A guide to genetic counseling. 2nd. Hoboken, NJ: Wiley-Blackwell; 2009. pp. 177–205. [Google Scholar]
- Stanton AL, Revenson TA, Tennen H. Health psychology: Psychological adjustment to chronic disease. Annual Review of Psychology. 2007;58:565–592. doi: 10.1146/annurev.psych.58.110405.085615. [DOI] [PubMed] [Google Scholar]
- Stoller JK, Sandhaus RA, Turino G, Dickson R, Rodgers K, Strange C. Delay in diagnosis of alpha-1 antitrypsin deficiency: A continuing problem. Chest. 2005;128:1989–94. doi: 10.1378/chest.128.4.1989. [DOI] [PubMed] [Google Scholar]
- Tanash HA, Nilsson PM, Nilsson JA, Piitulainen E. Survival in severe alpha-1 antitrypsin deficiency (PiZZ) Respiratory Research. 2010;11:44. doi: 10.1186/1465-9921-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercyak KP. Introduction to the special issue: Psychological aspects of genomics and child health. Journal of Pediatric Psychology. 2009;34:589–595. doi: 10.1093/jpepsy/jsn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton AG, Cunningham TJ, Ford ES, Croft JB. Employment and activity limitations among adults with chronic obstructive pulmonary disease - United States, 2013. Morbidity and Mortality Weekly Report (MMWR) 2015;64:289–95. [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Burleson BR. Effects of sex, culture, and support type on perceptions of spousal social support: An assessment of the “support gap” hypothesis in early marriage. Human Communication Research. 2001;27:535–566. doi: 10.1111/j.1468-2958.2001.tb00792.x. [DOI] [Google Scholar]
- Zuo L, Pannell BK, Zhou T, Chuang C. Historical role of alpha-1-antitrypsin deficiency in respiratory and hepatic complications. Gene. 2016;589:118–122. doi: 10.1016/j.gene.2016.01.004. [DOI] [PubMed] [Google Scholar]