Abstract
The discovery of place cells provided fundamental insight into the neural basis by which the hippocampus encodes spatial memories and supports navigation and prompted the development of computational models to explain the emergence of their spatial selectively. Many such works posit that input from entorhinal grid cells is critical to the formation of place fields, a prediction that has received mixed experimental support. Potentially reconciling seemingly conflicting findings is recent work indicating that subpopulations of pyramidal neurons are functionally distinct and may be driven to varying degrees by different inputs. Additionally, new studies have demonstrated that hippocampal principal neurons encode a myriad of features extending beyond current position. Here, we highlight recent evidence for how extensive heterogeneity in connectivity and genetic expression could interact with membrane biophysics to enable place cells to encode a diverse range of stimuli. These recent findings highlight the need for more computational models that integrate these heterogeneous features of hippocampal principal neurons.
Introduction
Decades of research point to a critical role for the hippocampus in supporting declarative memory and spatial navigation [1–3]. The profound memory deficits observed in patient H.M. after bilateral hippocampal resection, combined with subsequent animal and human work, solidified the importance of hippocampal processing in episodic and semantic memory [1,2]. In parallel, a significant leap forward in understanding the neural basis by which the hippocampus supports spatial navigation occurred with the discovery of place cells in multiple regions of the hippocampal formation [4]. Place cells initially appeared to represent an animal’s instantaneous location in an environment, as they were observed to fire in one or few restricted spatial locations that strongly correlated with an animal’s current position. However, consistent with the posited role of the hippocampus in memory, subsequent work has increasingly demonstrated that many place cells also encode features beyond current position such as past and future spatial trajectories [5,6], goal locations and distance to a goal [7••,8••], the position of other animals or objects [9,10], odors [11,12], tactile cues [14], time elapsed [15–17] and the temporal order of items or events [18]. In hippocampal sub-region CA1, the focus of this review, these features are encoded heterogeneously, with different subsets of place cells responding to spatial or non-spatial features, combinations of these features, or different features across different tasks (e.g. [16,19••]). These heterogeneous coding features allow CA1 place cells to represent the broad range of stimuli necessary for building episodic memories of unique events while simultaneously supporting navigation through local environments.
Given the established importance of the hippocampus in memory and navigation, significant experimental and computational efforts have focused on uncovering the mechanisms that generate place cell feature selectivity. Seminal computational models of classic location-modulated CA1 place cells describe how inputs from upstream regions could combine in a feed-forward manner to yield place-specific tuning [20–22]. One cortical region that has been studied extensively in this context is the entorhinal cortex, which provides the primary source of cortical input to the hippocampus. The entorhinal cortex is subdivided into two primary functional regions: the lateral portion (LEC), which encodes non-spatial, contextual features such odor or objects and the medial portion (MEC), which encodes features associated with the location of an animal with respect to its environment and serves as a prime candidate to drive the spatial component of the hippocampal place code [23–26,27•]. Within MEC reside a number of functionally distinct, spatially modulated cell types that include grid cells that fire in periodic spatial locations, border cells that increase their firing rate near environmental boundaries, head direction cells that fire when an animal faces a particular direction and spatial cells with stable non-geometric spatial firing patterns [24–26,27•]. Initially, research focused on the hypothesis that input from grid cells with different phases and spatial scales could sum via a Fourier synthesis mechanism to yield a single downstream place field [20]. As these models often conceptualized CA1 place cells as a relatively homogenous population, it is perhaps not surprising that experimental evidence in support of the grid-to-place model has been mixed [28].
Traditionally, heterogeneity in place cell coding properties has been ascribed to differential connectivity with upstream input regions. For example, the preferential targeting of proximal CA1 by MEC and distal CA1 by LEC is thought to underlie the proximal-distal transition from pure place to more contextual coding and more recent works have shed light on how differences in the coding features of place cells in deep versus superficial CA1 layers might reflect differences in afferent connectivity [29]. Adding potential sources of place cell heterogeneity, however, recent studies have highlighted key roles for single-cell biophysics in gating place cell responses and RNA-sequencing analyses have revealed a greater amount of genetic variability amongst CA1 pyramidal neurons than previously appreciated [30••]. How this diversity in circuit connectivity, biophysics and gene expression interact to contribute to place coding remains incompletely understood. Here, we outline how recent discoveries have shifted the dialogue regarding the mechanisms governing the formation of place fields. We first present a subset of experimental findings with seemingly contradictory findings regarding how MEC grid cell inputs contribute to place cell codes and consider how a closer inspection of input or functional heterogeneity amongst place cells may help reconcile these results. We then more broadly discuss new evidence for how differences in connectivity, biophysical properties and genetic profiles could intersect to yield the heterogeneous nature of hippocampal coding and include proposals for how future work can address these new complexities regarding place cell generation.
Heterogeneity in the functional coding features of inputs can shape place fields
While studies indicate an important role for LEC [31], as well as other brain regions, in driving features of the hippocampal place code, we will focus our initial discussion on how MEC inputs shape CA1 place coding, as recent work has made significant traction in addressing this topic (Figure 1). Shortly after the discovery of grid cells, several computational models proposed that grid cell inputs could give rise to the firing features of place cells in CA1 [20,32–35]. This hypothesis however, has been met with mixed experimental support. Congruent with the hypothesis were the observations that ‘global remapping’, in which place field locations change across environments, occurs in tandem with rotation or translation of the grid pattern [36] and that the increase in place field size along the longitudinal axis of CA1 parallels an increase in the spatial scale of grid cells along the same axis [24,37]. However, early electrolytic and pharmacological manipulations probing the general impact of MEC inputs to CA1 yielded varying results [38–40], potentially due to variability in the extent of MEC impacted by a given manipulation. Thus, recent works have aimed to utilize more temporally precise, reversible manipulations and target specific genetically or functionally defined MEC cell-types. These studies have primarily focused on the role of MEC inputs in determining two cardinal features of the place code: the organization of place maps across environments (i.e. global remapping) and the spatial precision (i.e. field size) of place maps.
Figure 1.
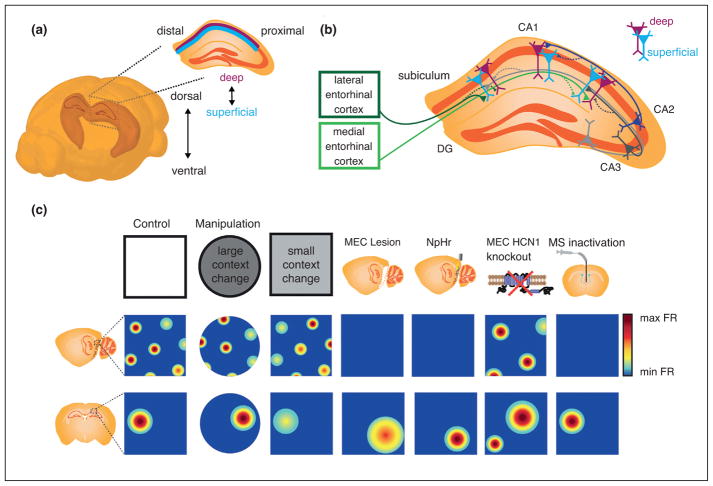
Heterogeneous place cell responses arise, in part, from varying inputs. (a) Schematic of a mouse brain. The shaded area depicts the CA regions of hippocampus, with the inset depicting a coronal section of dorsal hippocampus. The three cardinal axes of CA1 are: dorsal to ventral, proximal (closer to CA2) to distal (closer to subiculum), and deep (closer to stratum oriens) to superficial (closer to stratum radiatum). Images created from the Brain Explorer 2, Allen Brain Atlas and modified from [30••]. (b) Topographical organization of hippocampal inputs has been observed across all axes of hippocampus. Depicted here are a subset of the inputs known to display topographical organization across the radial or proximodistal axes of CA1. Solid lines with larger arrows indicate stronger drive, while dashed lines with smaller arrows indicate weaker drive. CA2 more strongly excites deep CA1, while inputs from LEC and MEC preferentially drive distal, superficial CA1 and proximal, deep CA1, respectively. Input from distal CA3 more strongly drives proximal CA1, and input from proximal CA3 more strongly drives distal CA1 [75]. CA3 input elicits depolarization of superficial CA1 and hyperpolarization of deep CA3 [53•]. Additional inputs display topographical organization across the longitudinal axis (not shown). For example, dorsal and ventral entorhinal cortex (EC) predominately innervate dorsal and ventral CA1, respectively, and via the EC, dorsal hippocampus receives more input from anterior cingulate and retrosplential cortex, while ventral hippocampus receives more input from infralimbic and prelimbic cortices [52]. Modified with permission from [58]. (c) Illustration highlighting experiments in which changes in grid cell representations were associated with altered place cell activity. The top panels depict the experimental condition or type of manipulation. The middle and bottom panels depict the impact on grid and place cell activity, respectively. From left to right: Control recordings in a white rectangular arena; Large changes in environmental context resulted in realignment of the grid pattern and global remapping of place cells [36]; Smaller changes to environmental context altered to varying degrees the firing rate of individual grid nodes and induced rate remapping in place cells [27•]; Lesions of MEC that eliminate grid activity have largely resulted in increased place field size (similar effects were seen following muscimol inactivation of MEC, not shown) [38,40,76]; Optogenetic inhibition of MEC greatly reduced grid cell firing and drove remapping in place cells without impacting field size; MEC-specific knockout of HCN1 channels increased the scale of both grid and place cell representations (particularly amongst place fields located far from environmental boundaries) and decreased place cell stability [50••]; Inactivation of medial septum (MS) largely eliminated the periodicity of grid cell firing patterns, with the impact on place cells including minimal effects on stability and large disruptions in the spatial coding of all place cells save those with fields near boundaries [44,45,49,76].
Confirming that MEC plays a key role in shaping place cell responses, both transient optogenetic inactivation and chemogenetic depolarization of MEC evoke place cell remapping [41•,42]. However, causally linking remapping to changes in the activity of specific MEC cell-types remains a formidable goal as, presently, there are no genetic markers by which to distinguish these functional cell-types. Nonetheless, to more directly examine the role of grid cells in hippocampal remapping, several studies have leveraged the observation that medial septum inactivation disrupts grid activity while minimally affecting other types of spatially modulated cells in MEC [43,44]. In two such works, septal inactivation did not strongly impact previously formed CA1 place fields or prevent the formation of stable place fields in a novel environment, casting doubt on the necessity of grid activity in generating or maintaining established place fields. However, in another study in which rats explored larger spatial environments, septal inactivation resulted in disorganized activity in the majority of place cells, save for a few neurons with fields near environmental boundaries [45]. Potentially reconciling these disparate findings is the possibility that different types of functionally defined MEC cells drive different subpopulations of place cells, and the proportions of these subpopulations sampled or activated could vary across experimental conditions. For example, border cells may provide stronger drive to place cells with fields near environmental boundaries, whereas grid cells could exert a greater influence on place cells far from environmental boundaries, where the animal has access to fewer landmark cues. Such dissociation could explain why the impact of septal inactivation was greater in a large environment in which proximal cues were less readily available. Additional support for the idea comes from the time-course of the development of stable place representations: early during post-natal development, when mature border but not grid activity is expressed [46], place maps provide more accurate information about the edges of an environment; later in development, stable grid cell activity and informative place maps across the entire environment emerge concurrently [47].
In addition to remapping, another feature of the place code is its spatial precision, or place cell field size. Whether MEC, or grid cells specifically, contribute to the size of place fields has been controversial. Fourier synthesis models of grid-to-place transformations predict that removal of small versus large scale grid cell inputs should have opposing effects downstream, increasing or decreasing the size of place fields, respectively [20]. Yet, while many grid-to-place cell models predict that only inactivation of dorsal MEC should increase place scale, increases in place scale appear to occur regardless of where along the dorsal–ventral axis MEC is pharmacologically inactivated [48]. In contrast, neither optogenetic inactivation of MEC nor inactivation of medial septum inactivation affect place scale [44,49]. However, an important consideration is that, at present, nearly all experimental investigations into the impact of grid cell activity on the scale of place representations have involved the inactivation of MEC. Moreover, as noted in the previous paragraph, a growing body of works suggests that distinct subpopulations of place cells may be driven by inputs that convey different functional firing features. The difference in the function coding features of inputs to place cells could underlie the seemingly conflicting reports of how MEC inputs influence both CA1 remapping and the size of place fields. Consistent with this idea, recent work discovered that increasing grid scale through targeted knockdown of HCN1 channels reduced long-term place field stability in a sub-population of CA1 place cells and simultaneously expanded the size of CA1 place fields, with the magnitude of this impact dependent on the distance of a given place field from the nearest environmental boundary [50••]. Thus, it is important to keep in mind that experimental design, such as the availability of proximal or boundary related sensory cues, can have highly variable effects on the coding features of CA1 place cells, and caution should be exercised when drawing conclusions regarding generalizable mechanisms. In addition, consideration should be given to the fact that input from CA1 also influences MEC coding and that this feedback likely plays a critical important role in how MEC inputs then drive CA1 coding [51].
Additional sources of heterogeneity that influence place cells formation and coding features
Many computational models of place field formation have traditionally conceptualized pyramidal cells as a relatively uniform functional population that receives similar types of inputs. Yet, the varied responses of place codes to MEC manipulations, as reviewed above, points to functional heterogeneity in place cells. Some of this heterogeneity can be attributed to heterogeneity in the functional coding features of the inputs place cells receive. In addition, however, a growing body of evidence has revealed that gene expression, morphological characteristics, local and long-range connectivity and intrinsic biophysical properties all vary among hippocampal pyramidal cells, often in a topographically organized manner [8••,30••,52,53•]. Such organization suggests that different sub-regions of hippocampus may play district roles in behavior. Indeed, the graded contributions of place cells to spatial versus emotional or contextual processing along the dorsal–ventral and proximal–distal axes have been the focus of much experimental work (for review see [52,54]). Here, we discuss new evidence for heterogeneous topographies across the radial axis and discuss the implications this may have on features of the place code.
Layer-specific differences in place cell coding features
Determining the layer of recorded place cells can be challenging using conventional electrophysiology, but recent advancements in silicon probe electrodes and optical imaging have successfully confronted this issue [55]. These approaches revealed differences in the firing characteristics of place cells located within deep (closer to stratum oriens) versus superficial (closer to stratum radiatum) layers (Figure 2). For example, deep cells display greater amounts of bursting and are more likely to have place fields [56], whereas superficial cells are more depolarized during sharp wave ripple events [53•]. These differences in firing properties extend to their functional coding features. In a recent study in which head-fixed mice navigated on a cue-enriched treadmill, superficial cells remained more spatially stable over time compared to deep cells [8••]. This long-term instability in deep cell place fields may reflect their unique state-dependent coding features. For instance, when the attentional demands of the task increased, only the spatial stability of deep cells increased, and the stability and precision of deep cells were more strongly modulated by the distance of their place field from the reward zone [8••]. Consistent with these findings, a second study using a similar paradigm reported that superficial cells fired in unique spatial locations across contexts, while deep cells encoded individual landmarks across different contexts [57•]. The ability of deep cells to dynamically encode the environment based on landmark or state-dependent variables may serve an adaptive purpose as the activity of deep, but not superficial, cells has been shown to significantly predict task performance [8••].
Figure 2.
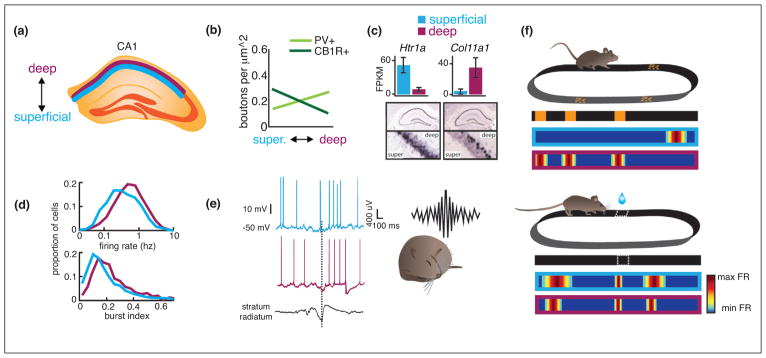
Place cells are a highly heterogeneous cell population. (a) Variation in place cell connectivity, gene expression, coding features and recruitment during behavior is observed across all axes of hippocampus. We focus here on the radial axis. Superficial (blue) and deep (purple) layers of CA1 are depicted in a coronal section of dorsal hippocampus. (b) In addition to differences in long-range inputs (shown in Figure 1b), perisomatic inhibition by different classes of basket cells varies across the radial axis. For example, innervation by parvalbumin positive (PV) interneurons is strongest within deep layers and decreases toward superficial layers. The opposite is true of cholecystokinin (CCK) positive interneurons, which most strongly innervate superficial layers. Data modified from [53•] with permission. (c) While gene expression varies most significantly across the dorsal–ventral axis of hippocampus, gradients are also observed across the radial axis. Shown are two examples of novel genes recently found to preferentially express in superficial (Htr1a) or deep (Col11a1) layers. FPKM: fragments per kilobase of exon per million fragments mapped. Data reproduced with permission from [30••]. (d) Basic firing characteristics including firing rate (top) and burst firing (bottom) differ between pyramidal neurons in superficial and deep layers. Data reproduced with permission from [56]. (e) Superficial and deep pyramidal neurons differentially participate in sharp-wave ripples: pyramidal neurons tend to be depolarized, while deep pyramidal neurons tend to by hyperpolarized. Data modified from [53•] with permission. (f) Superficial and deep pyramidal neurons show unique coding properties and play different roles in behavior. Top: Mice ran on a linear treadmill enriched with local cues. Superficial neurons were more prone to fire in a single, unique location across an environment, whereas a subpopulation of deep neurons encoded specific landmarks across multiple environments. Schematic modified from [55]. Bottom: Deep cells were more strongly impacted by an animal’s behavior on a task in which the mouse navigated to an unmarked location along a treadmill belt to receive reward [8••]. In both deep and superficial layers, place cells with fields located closer to the goal location were more spatially precise than those with fields located far from the goal, but this relationship was stronger amongst deep place cells. In addition, the stability of deep, but not superficial, place cells was increased across days when animals performed the attentional task (data not shown).
What mechanisms could account for the distinct firing properties and functional coding features of deep versus superficial place cells? Differences in afferent connectivity likely contribute: deep CA1 place cells receive stronger excitation from CA2, superficial CA1 place cells receive stronger excitation from CA3 [53•], and the extent of MEC and LEC drive to CA1 varies across both the radial and transverse axes (Figure 1) [58,59]. However, it is also possible that differences in single-cell intrinsic biophysical properties and the genetic expression profiles that underlie such biophysics contribute. For example, a signaling pathway by which activation of cannabinoid type-1 receptors enhances the magnitude of the hyper-polarization activated cation current (Ih) was recently reported to act exclusively in superficial, but not deep pyramidal cells [60]. Ih, in turn, strongly impacts both long-term potentiation and the temporal summation of synaptic inputs [61]. Thus, the differential modulation of Ih through endocannabinoids could render deep versus superficial subsets of cells more sensitive to specific inputs or combinations of inputs.
The contribution of single cell biophysics and intra-hippocampal computation to place responses
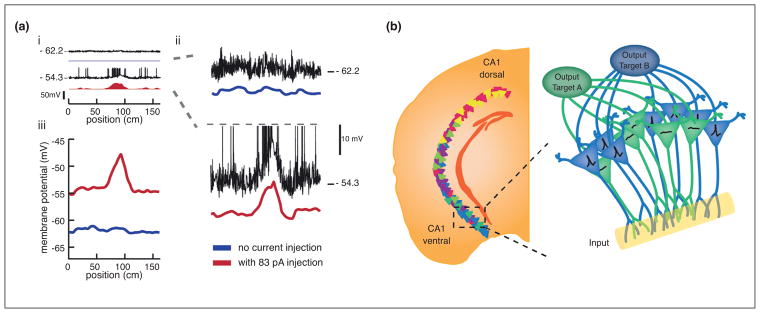
Recent works have also made strides toward identifying the algorithms by which heterogeneous inputs are transformed into place representations. Advances in intracellular recording techniques have made it possible to directly measure the synaptic inputs received by CA1 place cells and the biophysical properties that may gate their responses. In any given environment, only a fraction of principal cells develop place fields, while the majority remain ‘silent’, firing no or very few action potentials. Distinct subsets of principal cells are then active in different environments, enabling place cells to generate non-overlapping population representations of different spatial contexts [62]. What determines which cells are active and which remain silent in a given environment presents a puzzling question. While not entirely understood, recent whole cell recordings of active versus silent cells have begun to provide insight into the mechanistic underpinnings of this coding feature. The subthreshold membrane potential of an active place cell (which represents its net input at the soma) varies in a hill-like fashion as a function of location, while that of a silent cell is essentially flat [13••,63,64]. On its own, this finding could support a model in which simple summation of spatially modulated input is followed by a thresholding processes, driving place cells to either fire in a spatially restricted manner or remain silent. In such a scheme, silent cells would receive either spatially homogenous or weak input. Yet, several observations challenge such a model. Each CA1 pyramidal neuron is thought to receive spatial input at thousands of synapses across its dendritic compartments [65]. Simple summation of these inputs would likely result in multiple membrane potential peaks across space rather than the unimodal hill typically observed, and silent cells would still be expected to have membrane potential peaks, albeit smaller ones that did not reach threshold [64]. Additionally, differences in the intrinsic biophysical properties of active and silent cells appear to predict the initial establishment of a place field. Before exposure to a novel environment, cells that go on to form place fields are more prone to burst in response to current injection and during exploration the firing thresholds of active cells are significantly lower than those of silent cells [63]. Perhaps the strongest argument against the simple summation hypothesis is that injection of uniform positive current can instantly convert silent cells into active place cells that are indistinguishable from typical place cells in their subthreshold membrane potential profiles [64] (Figure 3).
Figure 3.
Heterogeneity in biophysical properties, genetic expression profiles and connectivity interact to shape place responses. (a) ‘Silent’ pyramidal neurons can be converted into place cells by injection of uniform depolarizing current, highlighting a potential role for intrinsic biophysics in gating place responses. (ai) Whole-cell recordings were made from CA1 pyramidal neurons as a rat traversed an oval-shaped track [64]. Linearized position is shown. Top: the membrane potential (black) and firing rate (blue) of silent CA1 pyramidal neuron before injection of positive current. Bottom: the membrane potential (black) and firing rate (red) of the same neuron during injection of positive current. (aii) Closer view of the membrane potential (black) and mean subthreshold membrane potential (color). Spikes have been truncated (gray dashed line). Note the emergence of a hill-shaped ramp in the subthreshold membrane potential during injection of positive current. (aiii) The average membrane potential for all laps before (blue) and during (red) current injection. Note that the membrane potential is relatively flat on laps prior to the current injection, with no indication of where the field will emerge upon depolarization. Figure modified with permission from [64]. (b) Schematic showing the possible interaction between several sources of place cell heterogeneity. Left: Cartoon illustrating genetic variation across the longitudinal axis. The colors of the cells represent pole-specific marker genes in dorsal (warm colors) or ventral (cold colors) CA1. Figure modified with permission from [74]. Inset: within the ventral CA1 pole, pyramidal neurons with similar output projections share similar gene expression patterns. Cartoon depicts a hypothetical scenario in which two different subpopulations are excited to differing degrees upon receiving a shared input. Differences in the genetic profiles of these subpopulations lead to differential expression of ion channels and receptors that renders one subpopulation (blue) more sensitive to the particular input than another subpopulation (green).
This would suggest that the inputs to silent cells are sufficient to drive the formation of a place field and membrane potential hill but only in the presence of additional depolarization [64]. For example, inputs to silent cells may encounter dendritic filtering, which can reflect topographically organized dendritic ion channel expression patterns [66,67], or intrahippocampal gating mechanisms, such as feedback inhibition [68•], that would prevent these inputs from reaching the soma. This gating mechanism, however, could be overcome by strong depolarizing events capable of inducing plasticity. Supporting this idea, the repeated injection of large membrane depolarizations at a consistent track location can artificially induce the formation of a place field in a formerly silent cell [13••]. As many place cells receive excitatory inputs from neural populations that vary their representations with time, such as neurons in CA2 [69], or behavioral state [70•], a subset of CA1 neurons may thus be primed to form place fields in any given environment. The long-term stabilization of the place code for a given environment would then depend on plasticity, which could involve regenerative dendritic spikes or plateau potentials—large calcium-mediated complex spike events [13••,63,71•]. This idea is consistent with newer research indicating that plateau potentials are not necessary for the formation of fields but contribute to their long-term stabilization [72•]. Variability in place cell stability seen, for example, in superficial versus deep layers could then arise from differences in single-cell biophysics that render the conditions for a plateau potential induction more or less favorable.
Heterogeneity in the genetic identity of place cells
In addition to diversity in inputs and biophysics, new work has revealed extensive diversity in the genetic identity of hippocampal pyramidal cells along axes that were previously thought to contain uniform genetic cell populations [30••]. While variability is seen across all axes of hippocampus, that across the dorsal–ventral axis is the largest and, strikingly, comparable in magnitude to that distinguishing different classes of pyramidal neurons (i.e. CA1 and CA3). Differences also emerge when considering the efferent targets of neighboring cells: for example, neurons located within the same dorsal–ventral pole but with projections to different brain regions (i.e. nucleus accumbens or amygdala) exhibit pronounced variation in gene expression [30••]. This is especially intriguing in light of work showing that different classes of interneurons target specific CA1 subpopulations that differ in their outputs [73]. Thus, the long-range output targeting of hippocampal cells presents another feature that may vary together with afferent inputs, local inhibitory circuitry and genetic and biophysical profiles to define unique and possibly independent functional neuronal subpopulations (Figure 3). Interestingly, genetic expression differences do not seem to define discrete cell types but rather emerge as continuous gradients [74]. This could enable cells with otherwise overlapping input and outputs to encode a larger continuum of features. How exactly genetic diversity shapes place cell responses remains an area of ongoing investigation. For example, the levels and distribution patterns of ion channels or receptor expression could vary between cells to differentially alter the excitability of their dendritic segments. This could render some subpopulations more tuned to specific inputs and not others. Moreover, such expression patterns could change across time, motivational state or task demands, altering which features a given pyramidal encodes depending on context. Whether such changes occur and the events that trigger them could be interesting avenues for future research.
Conclusions
While initially conceptualized as a homogenous cell population, CA1 place cells in fact show incredible heterogeneity, allowing them to represent the broad range of stimuli required to encode rich episodic memories and environmental spatial features that create the neural foundation for successful navigation. The last several years have brought important insights into how diversified sets of inputs, biophysics and genetic profiles contribute to the heterogeneity in the place code. Understanding how these features interact to generate features of the place code will be critical for future work. Combined, new data support a model in which subpopulations of pyramidal neurons form distinct networks differentially driven by a complex interaction of their unique biophysical or genetic profiles and heterogeneous inputs. These developments demand that future experimental work consider this heterogeneity when interpreting the results of manipulations to the place cell populations and that computational work attempt to integrate a more heterogeneous perspective when modeling place cells. The continued development of new hypotheses regarding how subpopulations of place cells that share similar input, biophysical, genetic or long-range projection profiles organize within hippocampus, generate unique neural codes and drive behavior will enable a deeper understanding of how hippocampal place cells shape our cognitive experience.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Pychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR. Memory and the hippocampus – a synthesis from findings with rats, monkeys and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 4.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 5.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 6.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Sarel A, Finkelstein A, Las L, Ulanovksy N. Vectorial representation of spatial goals in the hippocampus of bats. Science. 2017;355:176–180. doi: 10.1126/science.aak9589. This study discovered that CA1 pyramidal neurons of the Egyptian fruit bat encode a number of features in addition to the animal’s current location, including a particular distance or angular offset from a goal location. These findings help to explain how place cells could support goal-directed navigation in addition to representing the animal’s current position. [DOI] [PubMed] [Google Scholar]
- 8••.Danielson NB, Zaremba JD, Kaifosh P, Bowler J, Ladow M, Losonczy A. Sublayer-specific coding dynamics during spatial navigation and learning in hippocampal area CA1. Neuron. 2016;91:652–665. doi: 10.1016/j.neuron.2016.06.020. This work is the first to uncover different functional roles during behavior for CA1 cells residing in deep versus superficial layers. During passive navigation, superficial cells were more stable across time, whereas the activity of deep cells was more strongly modulated by goal oriented learning and predictive of task performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omer DB, Maimon SR, Las L, Ulanovsky N. Social place-cells in the bat hippocampus. Science. 2018;359:218–224. doi: 10.1126/science.aao3474. [DOI] [PubMed] [Google Scholar]
- 10.Danjo T, Toyoizumi T, Fujisawa S. Spatial representations of self and other in the hippocampus. Science. 2018;359:213–218. doi: 10.1126/science.aao3898. [DOI] [PubMed] [Google Scholar]
- 11.Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinalhippocampal ensemble activity during associative learning. Nature. 2014;510:143–1447. doi: 10.1038/nature13162. [DOI] [PubMed] [Google Scholar]
- 13••.Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci. 2015;18:1133–1142. doi: 10.1038/nn.4062. This paper demonstrates that the coincident activity of CA3 and entorhinal inputs to CA1 place cells results in large, sustained dendritic events, called plateau potentials. Plateau potentials positively modulated the firing rates of existing place cells, and artificial induction of plateau potentials at any consistent track location was sufficient to drive the formation of a place field in previously silent cells. This is particularly notable as it suggests that place cells receive spatially tuned inputs at all locations, but that specific subsets of these inputs become potentiated to yield a spatially restricted place field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young BJ, Fox GD, Eichenbaum H. Correlates of hippocampal complex-spike cell activity in rats performing a nonspatial radial maze task. J Neurosci. 1994;14:6553–6563. doi: 10.1523/JNEUROSCI.14-11-06553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus BJ, Robinson RJ, 2nd, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “time cells”: time versus path integration. Neuron. 2013;78:1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manns JR, Howard M, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Aronov D, Nevers R, Tank DW. Mapping a non-spatial dimension by the hippocampal-entorhinal circuit. Nature. 2017;543:719–722. doi: 10.1038/nature21692. This groundbreaking study demonstrates that hippocampal and entorhinal neurons can represent position within behaviorally relevant task-related variables that reside outside the spatial domain. In this paper, rats were trained to navigate through a one-dimensional auditory space by pressing a bar to increase the frequency of an auditory tone. Rats would release the bar once the tone reached a learned frequency, which took a variable amount of time. Classically defined grid, border, head direction and place cells encoded different task variables, such as the initial bar press, a specific auditory frequency or the bar release. Importantly, these task-related representations were only clear during active participation in the task. This indicates that entorhinal and hippocampal responses reflect navigation through a behaviorally relevant stimulus space regardless of the coordinate frame of that space. This type of representation may be highly consistent with the proposed role of the parahippocampus in building declarative memories that incorporate multiple coordinate frames. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solstad T, Moser EI, Einevoll GT. From grid cells to place cells: a mathematical model. Hippocampus. 2006;16:1026–1031. doi: 10.1002/hipo.20244. [DOI] [PubMed] [Google Scholar]
- 21.Savelli F, Knierim JJ. Hebbian analysis of the transformation of medial entorhinal grid-cell inputs to hippocampal place fields. J Neurophysiol. 2010;103:3167–3183. doi: 10.1152/jn.00932.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molter C, Yamaguchi Y. Entorhinal theta phase precession sculpts dentate gyrus place fields. Hippocampus. 2008;18:919–930. doi: 10.1002/hipo.20450. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SJ, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, Moser MB, Moser EI. Optogenetic dissection of entorhinal-hippocampal functional connectivity. Science. 2013;340:1232627. doi: 10.1126/science.1232627. [DOI] [PubMed] [Google Scholar]
- 24.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 25.Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- 26.Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 27•.Diehl GW, Hon OJ, Leutgeb S, Leutgeb JK. Grid and nongrid cells in medial entorhinal cortex represent spatial location and environmental features with complementary coding schemes. Neuron. 2017;94:83–92. doi: 10.1016/j.neuron.2017.03.004. This study investigates how spatially modulated cells within the MEC respond to changes in environmental context. By using a within-animal shuffling procedure more commonly used in hippocampal literature, the authors identified a larger number of spatially modulated cells in MEC than previously appreciated. The firing fields of spatially stable, non-grid cells were found to substantially remap in response to contextual changes to the environment. By contrast, under the same conditions grid cell firing fields remained spatially stable but exhibited different firing rates. These complementary coding schemes could provide contextual signals to place cells, and drive the rate remapping that is observed in response to changes in context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bush D, Barry C, Burgess N. What do grid cells contribute to place cell firing? Trends Neurosci. 2014;37:136–145. doi: 10.1016/j.tins.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henriksen EJ, Colgin LL, Barnes CA, Witter MP, Moser MB, Moser EI. Spatial representation along the proximodistal axis of CA1. Neuron. 2010;68:127–137. doi: 10.1016/j.neuron.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N. Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron. 2016;89:351–368. doi: 10.1016/j.neuron.2015.12.013. Using next-generation RNA sequencing, this paper demonstrated that the genetic expression profiles of CA1 pyramidal neurons are far more heterogeneous than previously appreciated. The greatest amount of variability was observed along the dorsal–ventral axis, where the differences neared or matched those observed between different pyramidal cell classes (i.e. CA1 versus CA3). Strikingly, pyramidal neurons could not be well-classified into distinct transcriptionally defined cell types. How such gradients in gene expression contribute to the diverse coding properties of pyramidal neurons will undoubtedly be the topic of much future investigation. [DOI] [PubMed] [Google Scholar]
- 31.Lu L, Leutgeb JK, Tsao A, Henriksen EJ, Leutgeb S, Barnes CA, Witter MP, Moser MB, Moser EI. Impaired hippocampal rate coding after lesions of the lateral entorhinal cortex. Nat Neurosci. 2013;16:1085–1093. doi: 10.1038/nn.3462. [DOI] [PubMed] [Google Scholar]
- 32.Monaco JD, Abbott LF. Modular realignment of entorhinal grid cell activity as a basis for hippocampal remapping. J Neurosci. 2011;31:9414–9425. doi: 10.1523/JNEUROSCI.1433-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S, Frank LM. The structure of networks that produce the transformation from grid cells to place cells. Neuroscience. 2011;197:293–306. doi: 10.1016/j.neuroscience.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuhs MC, Touretzky DS. A spin glass model of path integration in rat medial entorhinal cortex. J Neurosci. 2006;26:4266–4276. doi: 10.1523/JNEUROSCI.4353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 36.Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- 37.Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller VM, Best PJ. Spatial correlates of hippocampal unit activity are altered by lesions of the fornix and entorhinal cortex. Brain Res. 1980;194:311–323. doi: 10.1016/0006-8993(80)91214-7. [DOI] [PubMed] [Google Scholar]
- 39.Van Cauter T, Poucet B, Save E. Unstable CA1 place cell representations in rats with entorhinal cortex lesions. Eur J Neurosci. 2004;29:1933–1946. doi: 10.1111/j.1460-9568.2008.06158.x. [DOI] [PubMed] [Google Scholar]
- 40.Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 41•.Kanter BR, Lykken CM, Avesar D, Weible A, Dickinson J, Dunn B, Borgesius NZ, Roudi Y, Kentros CG. A novel mechanism for the grid-to-place cell transformation revealed by transgenic depolarization of medial entorhinal cortex layer II. Neuron. 2017;93:1480–1492. doi: 10.1016/j.neuron.2017.03.001. This study reports that chemogenetic depolarization of superficial MEC neurons elicits remapping of CA1 place cells. While the locations at which spatially modulated MEC cells fired were unaffected by the manipulation, the firing rates of individual grid nodes were impacted to varying degrees. For a computational model of how rate changes within individual grid nodes could potentially drive place cell remapping see [32]. This paper, along with [20], indicate that the relative firing rates of grid nodes could carry information regarding context, and possibly contribute to the firing locations or rates of place cells. [DOI] [PubMed] [Google Scholar]
- 42.Rueckemann JW, DiMauro AJ, Rangel LM, Han X, Boyden ES, Eichenbaum H. Transient optogenetic inactivation of the medial entorhinal cortex biases the active population of hippocampal neurons. Hippocampus. 2015 doi: 10.1002/hipo.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koenig J, Linder AN, Leutgeb JK, Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science. 2011;332:595. doi: 10.1126/science.1201685. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E. Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci. 2015;18:282–288. doi: 10.1038/nn.3904. [DOI] [PubMed] [Google Scholar]
- 46.Bjerknes TL, Moser EI, Moser MB. Representation of geometric borders in the developing rat. Neuron. 2014;82:71–78. doi: 10.1016/j.neuron.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Muessig L, Hauser J, Wills TJ, Cacucci F. A developmental switch in place cell accuracy coincides with grid cell maturation. Neuron. 2015;86:1167–1173. doi: 10.1016/j.neuron.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ormond J, McNaughton BL. Place field expansion after focal MEC inactivations is consistent with loss of Fourier components and path integrator gain reduction. Proc Natl Acad Sci U S A. 2015;112:4116–4121. doi: 10.1073/pnas.1421963112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandon MP, Koenig J, Leutgeb JK, Leutgeb S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron. 2014;82:789–796. doi: 10.1016/j.neuron.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Mallory CS, Hardcastle K, Bant JS, Giocomo LM. Grid scale drives the scale and long-term stability of place maps. Nat Neurosci. 2018 doi: 10.1038/s41593-017-0055-3. http://dx.doi.org/10.1038/s41593-017-0055-3This study contributes to the longstanding debate as to whether the scale of grid cell representations impacts that of place cell representations. By knocking out HCN1 channels selectively in the MEC of adult mice, the authors increase grid scale without impacting other spatial or velocity signals. The increase in grid scale is accompanied by an increase in the size of CA1 place fields, with the strongest impact apparent in fields located far from environmental boundaries. Their data support a model in which grid cell inputs play a larger role in forming place fields located far from environmental boundaries or landmarks, whereas border cell inputs may contribute to the formation of place fields near environmental boundaries. Additionally, the authors present experimental data and a novel computational model showing that grid scale contributes to the long-term stability of place cell fields. [DOI] [PMC free article] [PubMed]
- 51.Bonnevie T, Fyhn M, Hafting T, Derdikman D, Moser EI, Moser MB. Hippocampal contribution to maintenance of entorhinal grid fields. SFN Neurosci Abstr. 2010:101.4. [Google Scholar]
- 52.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 53•.Valero M, Cid E, Averkin RG, Aquilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominquez D, Bellistri E, de la Prida LM. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci. 2015;18:1281–1290. doi: 10.1038/nn.4074. This study is the first to examine how principal neurons distributed across the radial axis differentially participate in sharp-wave ripples in vivo, which the authors accomplish using sharp and multi-site electrodes. They additionally perform post hoc immunohistochemistry to identify the local inhibitory circuits that could contribute to the differences observed. Their findings add evidence to an emerging picture in which pyramidal neurons located in deep versus superficial layers play distinct functional roles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igarashi KM, Ito HT, Moser EI, Moser MB. Functional diversity along the transverse axis of hippocampal area CA1. FEBS Lett. 2014;588:2470–2476. doi: 10.1016/j.febslet.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Geiller T, Royer S, Choi JS. Segregated cell populations enable distinct parallel encoding within the radial axis of the CA1 pyramidal layer. Exp Neurobiol. 2017;26:1–10. doi: 10.5607/en.2017.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuseki K, Diba K, Pastalkova E, Buzsaki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14:1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Geiller T, Fattahi M, Choi JS, Royer S. Place cells are more strongly tied to landmarks in deep than in superficial CA1. Nat Commun. 2017:8. doi: 10.1038/ncomms14531. http://dx.doi.org/10.1038/ncomms14531Employing multi-site silicon probes, the authors probed the responses of deep and superficial CA1 and CA3 pyramidal neurons as mice traversed a treadmill belt enriched with highly salient tactile cues. They report that within deep layers of dorsal CA1 resides a population of neurons that fire at a consistent distance in relation to particular cues, even across different environments. In contrast, superficial layers were more likely to encode the environment as a whole, firing in a single location and remapping between environments. This study provides further evidence for a functional dissociation between superficial and deep place cells. [DOI] [PMC free article] [PubMed]
- 58.Masurkar AV, Srinivas KV, Brann DH, Warren R, Lowes DC, Siegelbaum SA. Medial and lateral entorhinal cortex differentially excite deep versus superficial CA1 pyramidal neurons. Cell Rep. 2017;18:148–160. doi: 10.1016/j.celrep.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohara K, Pignatelli M, Rovest AJ, Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maroso M, Szabo GG, Kim HK, Alexander A, Bui AD, Lee SH, Lutz B, Soltesz I. Cannabinoid control of learning and memory through HCN channels. Neuron. 2016;89:1059–1073. doi: 10.1016/j.neuron.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- 62.Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epsztein J, Brecht M, Lee AK. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron. 2011;70:109–120. doi: 10.1016/j.neuron.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee D, Lin BJ, Lee AK. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science. 2012;337:849–853. doi: 10.1126/science.1221489. [DOI] [PubMed] [Google Scholar]
- 65.Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- 66.Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaidya SP, Johnston D. Temporal synchrony and gamma-to-theta power conversion in the dendrites of CA1 pyramidal neurons. Nat Neurosci. 2013;16:1812–1820. doi: 10.1038/nn.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Grienberger C, Milstein AD, Bittner KC, Romani S, Magee JC. Inhibitory suppression of heterogeneously tuned excitation enhances spatial coding in CA1 place cells. Nat Neurosci. 2017;20:417–426. doi: 10.1038/nn.4486. This study examined the impact of local inhibition on place cell dynamics by optogenetically reducing the activity of interneurons in stratum oriens and stratum pyramidale while recording the intracellular activity of place cells. Reducing inhibition increased membrane potential depolarization and variance, and in turn, the frequency of action potentials and plateau potentials. Interestingly, similar changes were elicited irrespective of position along the track. Together, their results indicate that place cells receive spatially uniform inhibition that, through suppression of out-of-field excitation, helps to maintain the spatial selectivity of place cells. [DOI] [PubMed] [Google Scholar]
- 69.Mankin EA, Diehl GW, Sparks FT, Leutgeb S, Leutgeb JK. Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron. 2015;85:190–201. doi: 10.1016/j.neuron.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Hardcastle K, Maheswaranathan N, Ganguli S, Giocomo LM. A multiplexed, heterogeneous, and adaptive code for navigation in medial entorhinal cortex. Neuron. 2017;94:375–387. doi: 10.1016/j.neuron.2017.03.025. This paper used an unbiased statistical approach to identify cells within superficial layers of the MEC that encode variables relevant to navigation such as position, heading direction, or running speed. This approach revealed that a larger number of MEC neurons encode these variables than previously reported using tuning-based methods of cell-type identification. In addition, the statistical based approach revealed that neurons can adaptively encode variables, encoding information more accurately at higher running speeds. Many cells showed conjunctive tuning for multiple features, an observation that is mirrored in recent studies of hippocampal pyramidal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Sheffield ME, Dombeck DA. Calcium transient prevalence across the dendritic arbour predicts place field properties. Nature. 2015;517:200–204. doi: 10.1038/nature13871. This study presents the first direct evidence that regenerative dendritic events occur in vivo in place cells. These events occur with spatiotemporal variability and are predictive of both the short and long-term stability of place fields. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Cohen JD, Bolstad M, Lee AK. Experience-dependent shaping of hippocampal CA1 intracellular activity in novel and familiar environments. ELife. 2017:6. doi: 10.7554/eLife.23040. In this work, the authors intracellularly monitored the inputs received by place cells as they formed fields in novel environments. Interestingly, they demonstrate that plasticity-inducing complex spikes are not necessary for the formation of place cells in novel environments, seemingly in contrast to their prominent role in familiar environments [10]. The authors present evidence for a model in which inputs are initially amplified upon exposure to a novel environment, whereas across repeated passes through the environment, these inputs decrease in amplitude but become increasingly more stable within and outside of the place field. Such a model helps to explain the somewhat paradoxical finding that place cell firing rates tend to decrease with familiarity to an environment, while their stability within the environment tends to increase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krook-Magnuson E, Varga C, Lee SH, Soltesz I. New dimensions of interneuronal specialization unmasked by principal cell heterogeneity. Trends Neurosci. 2012;35:175–184. doi: 10.1016/j.tins.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tushev G, Schuman EM. Rethinking functional segregation: gradients of gene expression in area CA1. Neuron. 2016;89:242–243. doi: 10.1016/j.neuron.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Brivanlou IH, Dantzker JLM, Stevens CF, Callaway EM. Topographic specificity of functional connections from hippocampal CA3 to CA1. Proc Natl Acad Sci. 2004;101:2560–2565. doi: 10.1073/pnas.0308577100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlesiger MI, Cannova CC, Boubil BL, Hales JB, Mankin EA, Brandon MP, Leutgeb JK, Leibold C, Leutgeb S. The medial entorhinal cortex is necessary for temproal organization of hippocampal neuronal activity. Nat Neurosci. 2015;18:1123–1132. doi: 10.1038/nn.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]