Abstract.
Undernourished children in low-income contexts often suffer from environmental enteric disorder—damage to the intestines probably caused by chronic exposure to bacterial pathogens from feces. We aimed to identify strategies for reducing infants and young children’s (IYC) exposure to human and animal feces in rural farming families by conducting direct observation of 30 caregiver–infant dyads for 143 hours and recording water, sanitation, and hygiene (WASH)–related behaviors to identify possible pathways of fecal–oral transmission of bacteria among IYC in rural Zambia. In addition to mouthing visibly dirty hands, toys, sibling’s body parts, and food, 14 IYC actively ingested 6.1 ± 2.5 (mean ± standard deviation [SD]) pieces of soil and stones and one ingested animal feces 6.0 ± 0 times in the span of 5 hours. Ninety-three percent (21 of 30) of mothers reported observing the index-child eating soil and 17% (5 of 30) of mothers reported observing the index-child eating chicken feces. Adult and child handwashing was uncommon, and even though 70% (28 of 30) of households had access to a latrine, human feces were found in 67% of homestead yards. Most animals present in the household were un-corralled, and the highest observable counts of feces came from chickens, pigs, and cattle. To protect IYC in low-income communities from the exploratory ingestion of feces and soil, Baby WASH interventions will need to interrupt fecal–oral microbial transmission vectors specific to IYC with a focus on feasibility, caregiver practices, and local perceptions of risk.
INTRODUCTION
Global stunting prevalence has been slowly decreasing since 2008, but stunting impacts an estimated 26% of children below the age of 5 years (165 million) as of 2011.1 Stunting in childhood increases the risk for reduced developmental potential, poor health outcomes, and reduced economic productivity.2–4 Childhood stunting is a multifaceted problem and, in the African and Asian contexts that share much of the burden of childhood stunting, robust dietary interventions have reduced the average child-height deficit by only about one-third.5,6 Exposure at an early age to poor water, sanitation, and hygiene (WASH) conditions could place infants and young children (IYC) at an increased risk of stunting, and an analysis of trends in Demographic and Health Surveys data suggests that open defecation accounts for 54% of international variation in child height.7 Proposed as a major pathway to stunting in low-income contexts, environmental enteric dysfunction (EED) is a subclinical damage to the gut from chronic oral exposure to fecal bacteria that results in poor nutrient absorption, systemic immune system stimulation, and poor growth.6,8 Research in the Gambia showed that 43% of linear growth failure could be explained by increased intestinal permeability, a measure of EED.9 In a study of Bangladeshi children, children living in cleaner households—defined by adequate sanitation, hygienic washing conditions, and water uncontaminated by Escherichia coli (E. coli)—had fewer markers of EED and were less stunted than children living in a contaminated households.10 In another study in Bangladesh, the practice of IYC ingestion of soil was associated with EED and stunting.11
WASH interventions that interrupt the pathways between fecal contamination and oral ingestion of contaminated soil and objects might prevent EED, especially when the interventions target IYC under 24 months of age.6,12,13 However, typical WASH interventions target oral contact with contaminated hands, drinking water, soil, utensils, food, and flies, and could miss addressing key fecal–oral vectors of pathogens common among IYC during exploratory mouthing behaviors.12,14 IYC in rural, low-income contexts are frequently placed on the ground in spaces with dirt contaminated with feces from free-range livestock and are especially at risk of ingesting dirt and feces during their routine exploratory mouthing behaviors.10,15 IYC mouthing behaviors increase the burden of fecal microbe ingestion and the risk of developing EED, a contributor to stunting. These behaviors and associated risk might also explain the link between livestock ownership and childhood stunting despite the positive nutritional aspects of animal source foods.16
Baby WASH interventions developed to interrupt the unique fecal–oral route of microbial transmission between contaminated soil and IYC mouthing behaviors in early developmental stages are recommended to protect IYC under 24 months.12,17 One of the key tenets of Baby WASH is protecting IYC from fecal contamination in the home environment with interventions that are safe, feasible, and locally acceptable.18 Although a handful of interventions have been carefully designed to interrupt this infant-specific fecal–oral route of microbial transmission, little is known about physical, social, and caregiver contexts across countries.17 Exposure to fecal bacteria among IYC in low-income countries is likely different across cultural contexts, household environments, and children’s developmental stages.7,19
Although studies in Peru, Bangladesh, and Zimbabwe have quantified IYC ingestion of soil and microbial ingestion risks for very young children, the hand-to-mouth behaviors of children in many other at-risk countries have yet to be investigated.11,14,20 Little is known about caregiver beliefs, caregiver practices, and the pattern of livestock presence in the household throughout the day as they relate to infant mouthing behaviors and Baby WASH. Zambia, with a stunting rate of 40% in children under 5, is one of the African countries with the highest burden of undernutrition for young children and stands to benefit from improved interventions to prevent fecal microbial ingestion, EED, and stunting.21 A qualitative study of community-led total sanitation (CLTS) in Zambia suggested that CLTS is effective for improving sanitation beliefs and behaviors, but lacked observation of WASH behaviors after CLTS.22
In this study, we observed potential fecal–oral pathways of microbial transmission among IYC in rural Zambia to inform future WASH interventions targeted toward toddling IYC. We observed IYC in their home environments to document infant mouthing behaviors and development, investigated caregiver practices and beliefs about Baby WASH and IYC ingestion of soil, and identified livestock patterns in the household in rural Zambia, a country in which infant-focused fecal–oral microbial transmission vectors have not been assessed.
MATERIALS AND METHODS
Study setting and population.
The study was conducted in collaboration with CARE USA’s Nutrition at the Center (N@C) program in rural Zambia. N@C is a 5-year intervention designed to improve nutritional outcomes for mothers and children (age 0–24 months) through interventions in maternal, infant, and young child nutrition and health, WASH, food security, and women’s empowerment. The study took place in six rural villages participating in the N@C program in the Lundazi District of Zambia, close to the Malawi border. The main tribes in the Lundazi District are Tumbuka, Chewa, and Ngoni. The language most commonly spoken is Tumbuka, which is the main language of only 2.5% of Zambia’s population.23,24 The Tumbuka are a patrilineal tribe in which community roles are defined by gender and polygamy is widely practiced. Although the legal age of marriage in Zambia is 18, early marriages in the Lundazi district are common.25 The district town of Lundazi is isolated from the nearest large town, Chipata, by 170 km of paved road. The rural landscape is mostly cleared of trees for subsistence farming and cattle raising. The region is prone to flooding and food insecurity, especially in January, the month of highest rainfall.23 In general, villages consist of one area with multiple homesteads and support structures and a secondary area of fields with crops.
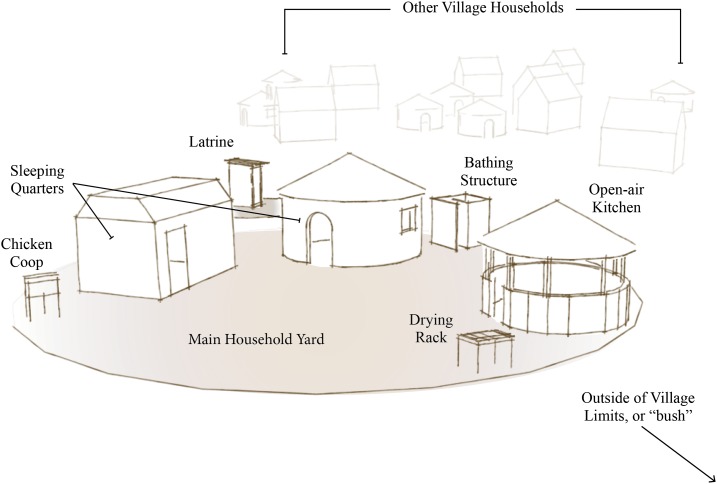
Thirty households from six villages with IYC between 3 and 24 months old were purposively selected from four health center catchment areas. Households consisted of two or more traditional mud- or brick-walled houses with grass-thatched roofs as well as a few auxiliary shade or storage structures constructed of wood and reeds. The immediate household yard and kitchen area were open yards with bare, loose sandy soil without a fence to separate one household from another (Figure 1).
Figure 1.
Typical household structure layout, Eastern Province, Zambia. This figure appears in color at www.ajtmh.org.
Measures.
Caregiver–infant observation.
Following the methods of Ngure et al.,15 we observed infant and caregiver behavior with a semistructured data collection tool to record mouthing episodes, caregivers’ handwashing behaviors, washing of infant’s hands, and WASH technologies. Observation visits were conducted on any day of the week, excluding Sunday. Researchers observed all objects that were mouthed by the target-child, whether the object was visibly dirty, and the frequency of object-mouth episodes. Mouthing was defined as putting any item or fingers in to a target-child’s mouth, regardless of ingestion. Repeat mouthing episodes of the same object were also observed. Researchers observed and recorded the mother’s handwashing behavior during handwashing opportunities, defined as after adult toilet use, contact with animal feces, after diaper changes, after sweeping, in preparation to feed the infant, in preparation to handle food, and in preparation to eat. Researchers observed and recorded any infant diaper change and the first five infant handwashing episodes observed were recorded along with triggering events (e.g., infant crawling on dirt, before feeding episodes, after diaper changes, etc.).
The second researcher also used a pretested, structured observation tool to conduct spot checks on the cleanliness of the caregivers’ and IYC’s hands and determined whether the household had a handwashing station and functional latrine, and whether there was evidence of their recent use.
Caregiver behavior questionnaire.
A qualitative questionnaire was used to conduct interviews with the mother regarding water, hygiene, and sanitation practices in addition to beliefs about IYC ingestion of soil. The questionnaire followed the standard caregiver questionnaire modules for water access, hygiene and sanitation access and behaviors, household characteristics, and demographics in the sanitation hygiene infant nutrition efficacy (SHINE) trial with additional measures of infant development structured around the Multiple Indicator Cluster survey’s questionnaire for children under five.26,27
Livestock observation.
We created a research tool to quantify the free-range livestock present in the household. At three time points—in the morning upon arrival, at noon, and in the afternoon at the end of the observation session—researchers conducted spot checks and recorded the number of corralled and roaming animals and the location and type of animal or human feces throughout the household and yard.
Procedures.
On the first visit to each household, research staff introduced the study and obtained oral informed consent to participate in the study from the index-child’s caregiver. All respondent caregivers were the index-child’s mother. The informed consent was read to the mother in Tumbuka. The Tumbuka version of the consent form was translated and back-translated by the field research staff under the supervision of the field supervisor before the study. The Institutional Review Board of Cornell University (Ref. No. 1405004690) and the University of Zambia Biomedical Research Ethics Committee (Ref. No. 013-11-13) approved this study.
Research visits were conducted between 7:30 am and 3:00 pm in each of the 30 households on all days of the week except Sunday. In the morning, researchers first observed the relative cleanliness and characteristics of the household. One researcher was tasked to follow the index-child and record episodes of mouthing behaviors. A second researcher observed WASH behaviors of the primary caregiver and conducted spot checks and recorded the number of corralled and roaming animals at three time points throughout the observation and interview process. This researcher also counted the number and location of animal and human feces present in the household at the same three time points.
In the afternoon, after completing the infant observation, the researchers used a pretested, structured questionnaire to record mothers’ self-reported household demographic information, hygiene practices, water access, and livestock ownership. Researchers also asked open-ended questions on caregivers’ beliefs about IYC eating soil and animal feces. To maintain the quality of data, the field supervisor conducted random spot checks with each research staff pair throughout the observation process and the researchers conducted debriefing sessions at the end of each day after household visits. Researchers double-checked and cross-checked questionnaires and the recording of key events and behaviors to maintain consistency in data collection. After all infant observations, data were analyzed to identify the key potential vectors, defined as 1) objects mouthed most frequently and 2) objects that were ever mouthed and were most visibly dirty.14
RESULTS
Household characteristics.
We observed 30 caregiver–infant dyads for a total of 143 hours during the household and infant observation. Each household was observed for a median of 5 hours and observation time ranged from 2 to 7 hours per household because of heavy seasonal rains limiting the outdoor observation in some households. Household characteristics are shown in Table 1.
Table 1.
Maternal and household characteristics (N = 30) Lundazi area, Eastern Province, Zambia
| Characteristics | No. (%) or mean ± standard deviation |
|---|---|
| Mother’s age, years | 27.7 ± 9.0 |
| Did not disclose | 7 (23%) |
| Marital status | |
| Married | 28 (93%) |
| Single | 2 (7%) |
| Education level | |
| Did not complete primary | 21 (70%) |
| Completed primary | 7 (23%) |
| Secondary | 2 (7%) |
| No. children per household | |
| Total | 2.9 ± 1.8 |
| 5–18 years old | 1.5 ± 1.5 |
| 2–5 years old | 0.3 ± 0.5 |
| < 2 years old | 1.0 ± 0.3 |
| Household size | 5.3 ± 2.1 |
| Dirt or cow-dung floor | 26 (87%) |
The area in which the index-child often played was swept in 13 households (43%) upon arrival. In the 2 weeks before observation, six households reported that there was an entire day in which water was unavailable from the primary water source. Additional household hygiene characteristics are shown in Table 2.
Table 2.
Household hygiene characteristics
| No. (%) or mean ± standard deviation | |
|---|---|
| Latrine ownership | |
| Own | 11 (37%) |
| Shared | 10 (33%) |
| Do not use/do not have | 9 (30%) |
| Handwashing (HW) | |
| HW station | 12 (57%) |
| Water at HW station | 8 (67%) |
| Soap at HW station | 0 (0%) |
| Wet ground at HW station | 7 (58%) |
| Primary water sources | |
| Borehole (protected) | 11 (37%) |
| Protected well | 5 (17%) |
| Unprotected well | 6 (20%) |
| River/stream | 8 (27%) |
| Communal water source | 29 (97%) |
| Estimated time to get to water source (minutes) | 20.6 ± 28.7 |
| Household observations (number of HH, upon arrival) | |
| Spilled food or drink visible on kitchen floor upon arrival | 21 (70%) |
| Human feces observed in household compound | 21 (70%) |
| Poultry feces observed in household compound | 9 (30%) |
Animals and feces observed in the household.
Most animals present in the household area throughout the day were free-range (Table 3). Excluding feces observed in animal enclosures, chicken feces were the most prevalent type of feces. Feces were observed throughout the household compound, and chicken feces were observed near the index-child in 17.2% of households. Human feces were found in five different locations in and around the household yard of multiple households. Additional locations and types of animal feces are summarized in Table 4.
Table 3.
Un-corralled animals observed in household
| Animal type | Observation time of day | |||||
|---|---|---|---|---|---|---|
| Morning | Noon | Afternoon | ||||
| No. HH (%) | Median no. of animals (range) | No. HH (%) | Median no. of animals (range) | No. HH (%) | Median no. of animals (range) | |
| Chicken | 21 (70) | 5 (1−28) | 23 (77) | 3 (1–41) | 22 (73) | 7 (1–40) |
| Dog | 16 (53) | 2 (1–2) | 13 (43) | 2 (1–16) | 15 (50) | 2 (1–2) |
| Cow | 12 (40) | 5 (1–26) | 3 (10) | 19 (1–30) | 4 (13) | 6 (1–26) |
| Pig | 10 (33) | 3 (1–16) | 10 (33) | 4 (1−16) | 9 (30) | 2 (1–16) |
| Dove or pigeon | 6 (20) | 8 (1–20) | 7 (23) | 5 (1–20) | 8 (27) | 7 (2–36) |
| Goat | 5 (17) | 5 (2–20) | 4 (13) | 4 (4–5) | 4 (13) | 7 (4–50) |
| Duck | 5 (17) | 4 (2–5) | 3 (10) | 2 (2–6) | 3 (10) | 2 (1–2) |
| Cat | 2 (7) | 1 (1) | 3 (10) | 1 (1) | 1 (3) | 1 (1) |
| Sheep | 2 (7) | 4 (2–6) | 0 (0) | 0 | 1 (3) | 4 (4) |
| Guinea fowl | 1 (3) | 5 (5) | 0 (0) | 0 | 0 (0) | 0 |
Animals corralled at morning observation: 2 HH (6 sheep, 10 goats). Animals corralled at noon observation: 0 HH. Animals corralled at afternoon observation: 1 HH (1 pigeon).
Table 4.
Feces observed in household environment
| No. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | Chicken | Pig | Cattle | Human | Goat | Duck | Dog/cat | Dove/pigeon | Sheep |
| Near infant’s location | 5 (17.2%) | – | – | 1 (3.4%) | – | – | – | 1 (3.4%) | 1 (3.4%) |
| Toilet | 6 (20.7%) | 5 (16.7%) | 3 (10.3%) | 4 (13.8%) | 1 (3.4%) | – | – | – | – |
| Bathing area | 7 (24.1%) | 1 (3.4%) | – | 1 (3.4%) | 1 (3.4%) | – | – | – | – |
| Kitchen | 11 (37.9%) | 1 (3.4%) | 1 (3.4%) | 1 (3.4%) | – | 2 (6.9%) | – | – | – |
| Laundry area | 4 (13.8%) | – | – | – | – | – | – | – | – |
| Inside main house | 2 (6.9%) | 1 (3.4%) | 1 (3.4%) | – | – | – | – | – | – |
| Sleeping area | 9 (31%) | 1 (3.6%) | – | – | – | – | – | – | |
| Household yard | 19 (65.5%) | 3 (10.3%) | 5 (17.2%) | – | 3 (10.3%) | 2 (6.9%) | 1 (3.4%) | 4 (13.8%) | 1 (3.4%) |
| Bush surrounding household compound | 8 (27.6%) | 4 (13.8%) | 6 (20.7%) | 8 (27.6%) | 3 (10.3%) | – | 6 (20.7%) | – | 1 (3.4%) |
N = 29, one observation booklet missing.
During 34 observed diaper changes, infant feces were tossed in the household yard after 14 (41%) diaper changes and left unattended for over 30 minutes on seven occasions (21%). Feces were disposed properly in the latrine, in the garbage pit, or buried on 11 (33%) diaper change events.
Fecal–oral microbial transmission vectors for IYC.
IYC were free to crawl on bare soil where poultry and other animals were free to roam. We observed 12 IYC crawling, 6 IYC walking on their own, and 4 IYC running on their own. Caregivers reported that the age at which the index-child first ate soil/feces ranged between 4 and 11 months old, with a mean age of 6.3 ± 1.9 months.
The most common mouthing location was the household yard, followed by inside of housing structures, the kitchen, and outside of the boundaries of the household yard (i.e., the exterior yard). Additional information on mouthing location is displayed in Table 5.
Table 5.
Location of fecal–oral microbial transmission vectors for infants and young children
| Location | No. of households (%) | Vector-mouth episodes* mean ± standard deviation |
|---|---|---|
| Household yard | 29 (97%) | 14.0 ± 5.5 |
| Inside house | 13 (43%) | 2.3 ± 4.3 |
| Kitchen | 8 (27%) | 0.8 ± 2.4 |
| Outside of household compound boundaries | 3 (10%) | 0.4 ± 1.4 |
Mouthing episodes per infant; excluding repeat mouthing incidents.
Objects of infant mouthing behaviors.
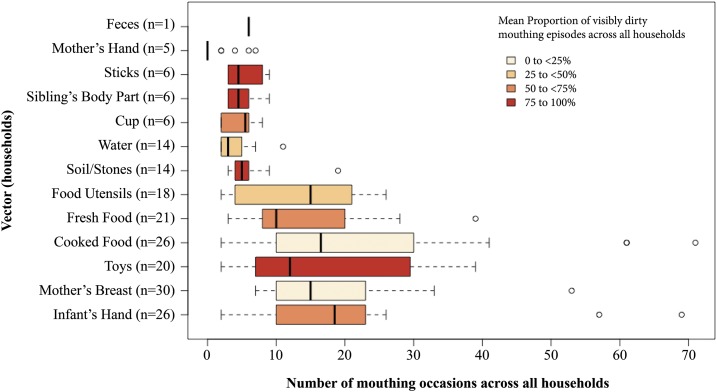
We observed IYC frequently mouthing the mother’s breast, the index-child hand, home-cooked food, fresh fruit, toys (e.g., plastic containers and dolls), food utensils, soil/stones, water, cups, sibling body parts, and sticks. Infants’ hands, toys, home-cooked food, and soil/stones were the most frequently mouthed potential fecal–oral microbial transmission vectors. Including repeated mouthing of the same object, we observed a mean of 93.1 ± 28.8 mouthing episodes per infant and, on average, 46.8% (SD ± 22.9) of those mouthing episodes were visibly dirty.
We identified the index-child’s hands, sibling’s hands, arms, or legs, sticks, fresh food, cups, and feces of any kind as major fecal–oral microbial transmission vectors, defined as a mean of > 50% visibly dirty mouthing observations of objects across all households. Across all households, the mean percentage of mouthing episodes with visibly dirty index-child hands was 73.7% (SD ± 28.8). We observed IYC mouthing index-child hands, toys, and home-cooked food in greatest number of households.
In addition to visibly dirty hands, toys, sibling’s hands, arms, or legs, and food, we observed 14 IYC actively ingest a median of 5 (range 3–19) pieces of soil and stones and one infant ingested animal feces six times in the span of 5 hours in the household. This ingestion of animal feces was the singular incident observed in the 143 hours of observation across all households. A visual summary of mouthing behaviors can be seen in Figure 2.
Figure 2.
Infants and young children mouthing vectors, Eastern Province, Zambia. This figure appears in color at www.ajtmh.org.
Practices of care and caregiver attitudes.
When asked about observing their infant eating soil or feces, 28 caregivers (93%) reported that they had seen the index-child eat soil. Ten of these caregivers reported that their infant had eaten soil within the past month, and seven caregivers reported that the index-child eats soil either every day or once per week. Five (17%) caregivers had seen the index-child eat chicken feces, although only one caregiver reported seeing the index-child eating chicken feces within the past month. Two (7%) caregivers saw the index-child eat other animal feces (defined as any animal feces that did not belong to chickens), but neither mother reported seeing the index-child eat animal feces in the past month.
Most mothers reported that eating soil, chicken feces, or other animal feces causes illness/diarrhea, stomachache, and/or worms (Table 6). Of the five caregivers who said that eating soil “helps close anterior fontanel,” three caregivers additionally reported that soil causes worms, one mother reported that soil eating causes constipation, and another said IYC eating soil will get malaria.
Table 6.
Mothers’ perceptions of outcomes from children eating soil and animal feces
| Belief | Baby eating soil No. (%) | Baby eating chicken feces No. HH (%) | Baby eating other animal feces No. HH (%) |
|---|---|---|---|
| Helps baby’s immunity | – | 1 (3%) | – |
| Helps baby’s gut/intestines | 5 (17%) | – | – |
| Other: closes anterior of fontanel | 5 (17%) | – | – |
| Makes baby grow poorly | 7 (23%) | 9 (30%) | 10 (33%) |
| Makes baby’s brain develop poorly | 3 (10%) | – | 1 (3%) |
| Causes stomachache | 18 (60%) | 17 (57%) | 17 (57%) |
| Causes diarrhea/illness | 12 (40%) | 19 (63%) | 22 (73%) |
| Causes worms | 18 (60%) | 10 (33%) | 14 (47%) |
| Causes baby to lose teeth | – | 1 (3%) | 1 (3%) |
| Do not know | 5 (17%) | 8 (27%) | 9 (30%) |
| Other: causes malaria | 1 (3%) | 4 (13%) | 6 (20%) |
| Other: causes constipation | 4 (13%) | – | 1 (3%) |
| Other: causes vomiting | 1 (3%) | 3 (10%) | 1 (3%) |
| Other: causes cough | – | 2 (7%) | 3 (10%) |
| Other: causes malaria | – | – | 1 (3%) |
When asked how caregivers could keep IYC from eating soil and/or animal feces, all caregivers reported various strategies for keeping infants away from soil and animal feces. The most common strategies were placing the child on a play mat, putting the child on the back, and removing the animal feces from the area.
Handwashing practices.
Researchers observed 455 handwashing opportunities but only 231 opportunities were followed by handwashing. A summary of these opportunities and practices is displayed in Table 7. Caregiver handwashing with soap was uncommon, and only 26 of 65 observed fecal-contact events resulted in caregiver handwashing. Researchers observed caregivers using soap during only five of all fecal-contact handwashing events. Fourteen caregivers (48%) reported using dirty dishwater to wash their hands, and researchers observed caregivers using clean, running water in 22% of handwashing events.
Table 7.
Mothers’ hand washing (HW) practices and opportunities
| Key events | Opportunities total | Any HW | Used soap | Used running water | Air dried |
|---|---|---|---|---|---|
| No. (% out of opportunities) | |||||
| After adult toilet | 14 | 7 (50) | 1 (7) | 1 (7) | 6 (43) |
| After contact with animal feces | 28 | 11 (39) | 3 (11) | 5 (18) | 11 (39) |
| After diaper change | 23 | 8 (35) | 1 (4) | 0 | 1 (4) |
| Fecal contact subtotal | 65 | 26 (40) | 5 (8) | 6 (9) | 18 (28) |
| After sweeping | 12 | 3 (25) | 0 | 2 (17) | 2 (17) |
| Before feeding infant | 35 | 21 (60) | 0 | 8 (38) | 21 (60) |
| Before handling food | 42 | 21 (50) | 0 | 14 (33) | 21 (50) |
| Before eating | 26 | 17 (65) | 0 | 9 (35) | 17 (65) |
| Other | 210 | 117 (56) | 16 (8) | 56 (27) | 100 (48) |
| Key events total | 455 | 231 (51) | 26 (6) | 101 (22) | 197 (43) |
Most observed infant handwashing events were not triggered by a specific event. Handwashing events for IYC were few, with a median of one handwashing event occurring per index-child during the time of observation (range 0–5).
DISCUSSION
In this observational study, IYC in rural Zambia were frequently exposed to fecal microbes through several pathways of transmission through their daily and developmentally appropriate crawling and mouthing behaviors. Motor development milestones, the social and physical environment, and caregiver practices and attitudes all create a context in which it is common for IYC to ingest soil and mouth dirty objects. This context includes the animal and human feces observed in households, the ingestion of soil and mouthing of visibly dirty objects by IYC, and infrequent handwashing practices by caregivers. As Ngure et al.14 demonstrated, IYC in Zimbabwe could consume up to 4,240 E. coli just by consuming soil in the kitchen yard during the dry season; this study suggests that this cohort of rural Zambian IYC are also at risk of high fecal–oral microbial transmission.
Animal and human feces are common in rural households observed in this study.
Animal feces.
We observed a wide range of animals—from cattle to dogs to pigeons—and animal feces in the household area. In corroboration with findings in Zimbabwe and peri-urban Peru, poultry and their feces were the most common.14,20 In this study, poultry feces were the most numerous in areas near IYC and in common household areas. Notably, caregivers reported that less livestock, specifically chickens, were present in the homestead because of the seasonally dependent and bi-yearly Newcastle disease die-off. The seasonal lack of chickens—a high risk vector for fecal–oral microbial transmission in other rural and peri-urban contexts—in conjunction with dry topsoil in the household could indicate that the risk of fecal–oral microbial transmission varies for Zambian IYC at different times of the year.14,20
Although feces of large livestock were observed in the bush and within the household area, poultry feces were the only type of animal feces identified close to the infant. For this reason, small livestock feces may present a bigger risk to the infant’s physical environment than larger livestock. As feces were observed from animals both in the household yard and in the nearby bush, the potential to track in fecal contamination into the household is high in this rural context.
Human feces.
Regardless of the presence of a latrine, human feces were visible in the open in more than half of the households observed. Human feces were most often observed in the bush surrounding the household but were also observed in the household yard and, in some cases, adjacent to the latrine. Open defecation near the latrine could be from either adults or children in the household, as the observation methods used in this study did not allow for a delineation of adult or child feces. Although we observed clear pathways from the household to latrines, the observations were conducted in the dry season during which there is a clear path to all structures before the tall grasses begin to grow. Recent research from rural Zambia noted that being observed entering a latrine was embarrassing and that sharing a toilet facility with in-laws or members of the opposite sex was considered taboo.22 In this context, our observations suggest that open defecation is still practiced in the households regardless of latrine access, and additional WASH behavior change education is needed to complement latrine building.
Infant feces were often tossed in the yard or left unattended after most of the observed diaper changes. The observed behavior is consistent with a larger study of how parents self-report their disposal of children’s feces in rural Zambia.22 The disposal of infant and child feces is critical; child feces carry more infectious pathogens than adult feces but are considered less disgusting than feces from adults.22 Previous WASH literature has emphasized the importance of the consequences of “spillover effects” of poor WASH practices from other households.19 In this close-community context, open defecation in one household has consequences for the children of neighboring households.
Ingestion of soil and chicken feces could account for a high load of bacterial ingestion in the Zambia context.
Most caregivers reported observing the index-child eating soil and a minority reported seeing their infant eat chicken feces. When combined with observation of IYC ingesting soil and chicken feces, caregivers’ self-reports point to a large proportion of IYC ingesting soil and animal feces. Caregivers first observed the index-child eating soil or feces when the index-child was around 6 months old, which corresponds with the age at which IYC are frequently able to sit by themselves and are more often placed on the ground in lieu of being carried the caregiver’s back. There did not appear to be any clear patterns of caregivers’ understandings of the effects of IYC eating soil or feces, with responses ranging from “causing Ebola or malaria” to “caus[ing] the baby to lose teeth” to “clos[ing] anterior fontanel.” These responses align with Zimbabwean caregivers’ perceived outcomes from IYC ingestion of soil, especially the belief from a few Zambian caregivers that eating soil or feces could help close the anterior fontanel.17
The lack of knowledge of the impact of IYC ingestion of soil has the potential to be a large barrier for the uptake and continued use of any WASH behaviors or technologies designed to protect IYC. As IYC begin to sit up, crawl, and eat soil within a wide age, Baby WASH education modules would benefit from framing the risks of soil ingestion around IYC’s incidence of crawling rather than a specific IYC age range. Developmental motor milestones could be a simple and straightforward indicator to caregivers of when they should be extra vigilant about their IYC eating soil and feces.
Despite some of the caregivers’ beliefs about the negative effects of soil and animal feces ingestion, caregivers did report many strategies for keeping IYC from ingesting soil and feces, including putting the child on the back, using a mat on the ground to separate the child from the soil, and cleaning up the feces in the yard. These positive strategies that mothers’ volunteered could form the basis for positive messaging for behavior change and intervention design.
Although the community setting of rural Zambian villages may hinder household WASH efforts because one household’s poor WASH practices could spill over to other households, the village setting also presents opportunities for interventions that involve multiple caregivers. In this way, Baby WASH interventions in this context could benefit from social or caregiving support between mothers who live very close by.
Mouthing behaviors place IYC at risk for fecal–oral microbial transmission.
Soiled infant’s and sibling’s hands are a frequent potential vector for transmission of fecal microbes. Siblings were observed as visibly dirty in > 75% of occasions in which the index-child mouthed their hands or arms. As siblings of IYC are often playmates and caregivers, future studies characterizing fecal–oral transmission routes of microbial ingestion should further characterize sibling WASH behaviors and IYC–sibling interactions. This study also highlights the index-children’s hands as major possible fecal–oral microbial transmission vectors, confirming findings in rural Zimbabwe and emphasizing the need for interventions to interrupt the soiled index-child hands to mouth fecal–oral microbial transmission route.14
Caregiver and IYC handwashing practices are not fully used.
Handwashing with soap was observed less among caregivers and handwashing for IYC was infrequent. Typical WASH technology such as latrines and handwashing stations were observed at many of the households but were not apparently connected with behaviors at the household level, suggesting that their use is not yet a social norm. This finding is corroborated by prior studies across multiple country contexts of caregiver handwashing practices.28 Handwashing events for IYC were few and, consistent with findings in rural Zimbabwe, most were not triggered by a specific event.14 Few caregivers reported that they would use soap to wash their hands after defecation, suggesting that there is remarkably little social desirability to wash hands. Notably, the use of dirty dishwater to wash hands further suggests that water scarcity may be an additional barrier to handwashing practices.
Summary.
We highlight parallels between the kind of free-roaming animals, the frequency and density of the animal feces within the household area, and the IYC mouthing events and type of vectors in each of these locations. Although this study is limited by observations only during a specific season and does not collect samples to directly assess E. coli contamination in the rural Zambian environment, we provide rich contextual data and information on rural Zambia, and further corroborate observational findings by Ngure et al.14 in rural Zimbabwe.
Our findings are consistent research in Zimbabwe and other country contexts.12,14,15,29 WASH technologies such as latrines and handwashing stations are available but underused in these communities. This study demonstrates that existing evidence-based WASH interventions in rural Zambia have not and will not effectively interrupt the fecal–oral route of microbial transmission for IYC. For example, interventions primarily focused on handwashing and human feces disposal rather than animal feces disposal in the household yard as it could miss IYC’s direct fecal–oral route of microbial transmission when IYC are placed on the ground. Unfortunately, handwashing interventions for IYC would be both difficult for caregivers to monitor and difficult for caregivers to practice given the many random triggers for IYC handwashing. Furthermore, IYC handwashing could also create more opportunities for soiled IYC hands if not closely followed by proper drying of hands as wet hands collect more soil. Educating rural communities on existing WASH practices is a necessary complement to efforts to provide a clean environment for young children, but is not sufficient to adequately protect IYC from fecal–oral microbial transmission.12 Interventions that break IYC’s direct contact with soil and soiled objects in the household environment are necessary in conjunction with sanitation and hygiene interventions as they currently exist.
New interventions and programs have been developed to address these environmental health risks that potentially diminish the benefits achievable for childhood health and growth from improved dietary interventions. For example, clean mats to place IYC on, protective play enclosures, and methods to assist caregivers with the disposal of animal feces in the household yard provide opportunities to directly break fecal–oral microbial transmission for IYC specifically. The SHINE trial in Zimbabwe is using a play yard and play mat to create clear separation of the infant from the frequently contaminated soil with integrated education and behavior-change modules.17 This visual marker of a “clean space” for IYC could help mothers reduce the workload needed to keep the entire house clean. Integrated interventions, focused on the infant’s and toddler’s environment, are promising not just in Zimbabwe but also in the current context of rural Zambia, where free-range livestock from multiple households presents even more risk for crawling children. Our findings of the household context of IYC mouthing behaviors and soil ingestion can guide future, integrated interventions to improve domestic hygiene with country-specific practices and guidelines.
Acknowledgments:
We thank the Sall Family Foundation for their support of Nutrition at the Center; Dr. Francis Ngure, the Community-Engaged Nutrition Intervention Research (CENTIR) Group (Division of Nutritional Sciences, Cornell University); the Zvitambo SHINE team for their guidance throughout this pilot study; our field research team for infant observation and data collection; and the study participants.
REFERENCES
- 1.Black MM, Dewey KG, 2014. Promoting equity through integrated early child development and nutrition interventions. Ann N Y Acad Sci 1308: 1–10. [DOI] [PubMed] [Google Scholar]
- 2.Caulfield LE, de Onis M, Blössner M, Black RE, 2004. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 80: 193–198. [DOI] [PubMed] [Google Scholar]
- 3.Hoddinott J, Alderman H, Behrman JR, Haddad L, Horton S, 2013. The economic rationale for investing in stunting reduction. Matern Child Nutr 9 (Suppl 2): 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker SP, et al. 2011. Inequality in early childhood: risk and protective factors for early child development. Lancet 378: 1325–1328. [DOI] [PubMed] [Google Scholar]
- 5.Dewey KG, Adu-Afarwuah S, 2008. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr 4 (Suppl 1): 24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphrey JH, 2009. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374: 1032–1035. [DOI] [PubMed] [Google Scholar]
- 7.Spears D, 2013. How Much International Variation in Child Height Can Sanitation Explain? World Bank Policy Research Working Paper No. 6351. Available at : https://ssrn.com/abstract=2212559. Accessed October 15, 2016.
- 8.Solomons NW, 2003. Environmental contamination and chronic inflammation influence human growth potential. J Nutr 133: 1237. [DOI] [PubMed] [Google Scholar]
- 9.Lunn PG, Northrop-Clewes CA, Downes RM, 1991. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338: 907–910. [DOI] [PubMed] [Google Scholar]
- 10.Lin A, et al. 2013. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am J Trop Med Hyg 89: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George CM, et al. 2015. Geophagy is associated with environmental enteropathy and stunting in children in rural Bangladesh. Am J Trop Med Hyg 92: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngure FM, Reid BM, Humphrey JH, Mbuya MN, Pelto G, Stoltzfus RJ, 2014. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Ann N Y Acad Sci 1308: 118–128. [DOI] [PubMed] [Google Scholar]
- 13.Dangour AD, Watson L, Cumming O, Boisson S, Che Y, Velleman Y, Cavill S, Allen E, Uauy R, 2013. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev 8: CD009382. [DOI] [PubMed] [Google Scholar]
- 14.Ngure FM, et al. 2013. Formative research on hygiene behaviors and geophagy among infants and young children and implications of exposure to fecal bacteria. Am J Trop Med Hyg 89: 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngure FM, Humphrey JH, Menon P, Stoltzfus R, 2013. Environmental Hygiene, Food Safety and Growth in Less than Five Year Old Children in Zimbabwe and Ethiopia. Ithaca, NY: Division of Nutritional Sciences, Cornell University. [Google Scholar]
- 16.Mosites EM, 2015. The influence of livestock ownership and health on the nutritional status of children in Eastern Africa. PhD dissertation, Department of Epidemiology, University of Washington, Seattle, WA.
- 17.Mbuya MN, et al. Sanitation Hygiene Infant Nutrition Efficacy Trial Team , 2015. Design of an intervention to minimize ingestion of fecal microbes by young children in rural Zimbabwe. Clin Infect Dis 61 (Suppl 7): S703–S709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mbuya MN, Humphrey JH, 2016. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: an opportunity for stunting reduction in developing countries. Matern Child Nutr 12 (Suppl 1): 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hathi P, Haque S, Pant L, Coffey D, Spears D, 2017. Place and child health: the interaction of population density and sanitation in developing countries. Demography 54: 337–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquis GS, Ventura G, Gilman RH, Porras E, Miranda E, Carbajal L, Pentafiel M, 1990. Fecal contamination of shanty town toddlers in households with non-corralled poultry, Lima, Peru. Am J Public Health 80: 146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zambian Central Statistical Office , Zambian Ministry of Head , 2014. Zambia Demographic and Health Survey 2013–14. Lusaka, Zambia, and Baltimore, MD: Central Statistical Office, Ministry of Health, and ICF International. [Google Scholar]
- 22.Lawrence JJ, Yeboah-Antwi K, Biemba G, Ram PK, Osbert N, Sabin LL, Hamer DH, 2016. Beliefs, behaviors, and perceptions of community-led total sanitation and their relation to improved sanitation in rural Zambia. Am J Trop Med Hyg 94: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orcutt M, 2013. Maternal mortality in eastern Zambia: accessing health care for delivery and obstetric emergencies. World Transp Policy Pract 19: 9–28. [Google Scholar]
- 24.Marten L, Kula NC, 2008. Zambia: ‘One Zambia, one nation, many languages’. Simpson A, ed. Language and National Identity in Africa. Oxford, United Kingdom: Oxford University Press, 291–313.
- 25.Mushibwe CP, 2014. What are the Effects of Cultural Traditions on the Education of Women? (The Study of the Tumbuka People of Zambia). Hamburg, Germany: Anchor Academic Publishing.
- 26.Humphrey JH, et al. Sanitation Hygiene Infant Nutrition Efficacy Trial Team , 2015. The sanitation hygiene infant nutrition efficacy (SHINE) trial: rationale, design, and methods. Clin Infect Dis 61 (Suppl 7): S685–S702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNICEF , 2015. Multiple Indicator Cluster Surveys (MICS). Series of Country Surveys. New York, NY: UNICEF. [Google Scholar]
- 28.Curtis VA, Danquah LO, Aunger RV, 2009. Planned, motivated and habitual hygiene behavior: an eleven country review. Health Educ Res 24: 655–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teunis PF, Reese HE, Null C, Yakubu H, Moe CL, 2016. Quantifying contact with the environment: behaviors of young children in Accra, Ghana. Am J Trop Med Hyg 94: 920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]