Abstract.
Vector control programs, particularly in the form of insecticide-treated bed nets (ITNs), are essential for achieving malaria elimination goals. Recent reports of increasing knockdown resistance (kdr) mutation frequencies for Anopheles arabiensis in Western Kenya heightens the concern on the future effectiveness of ITNs in Kenya. We examined resistance in An. arabiensis populations across Kenya through kdr mutations and World Health Organization–recommended bioassays. We detected two kdr alleles, L1014F and L1014S. Kdr mutations were found in five of the 11 study sites, with mutation frequencies ranging from 3% to 63%. In two Western Kenya populations, the kdr L1014F allele frequency was as high as 10%. The L1014S frequency was highest at Chulaimbo at 55%. Notably, the kdr L1014F mutation was found to be associated with pyrethroid resistance at Port Victoria, but kdr mutations were not significantly associated with resistance at Chulaimbo, which had the highest kdr mutation frequency among all sites. This study demonstrated the emerging pyrethroid resistance in An. arabiensis and that pyrethroid resistance may be related to kdr mutations. Resistance monitoring and management are urgently needed for this species in Kenya where resistance is emerging and its abundance is becoming predominant. Kdr mutations may serve as a biomarker for pyrethroid resistance in An. arabiensis.
INTRODUCTION
Despite intensive malaria control efforts, malaria remains a leading cause of morbidity and mortality in Kenya, especially among younger children and pregnant women.1 Vector control programs, particularly in the form of insecticide-treated bed nets (ITNs) are essential for achieving malaria elimination goals2,3 and have coincided with a decrease in malaria-related morbidity rates in Kenya.4 However, increasing insecticide resistance threatens the efficacy of antimalarial interventions.5
Pyrethroids are the only approved insecticide for use in ITNs.6 Its low mammalian toxicity and induction of paralysis using nerve stimulation of dysfunctional sodium channels makes it ideal for ITN usage.5,7 However, a single amino acid change at residue position 1014 in the voltage-gated sodium channel (VGSC) gene of insects has made the insecticide increasingly obsolete. This mutation has been shown to confer knockdown resistance (kdr) by decreasing sodium channel affinity for the insecticide binding site.8 The kdr mutations are found as L1014F (kdr-west) and L1014S (kdr-east) in Anopheles gambiae.9 L1014F refers to a point mutation from leucine to phenylalanine, whereas L1014S represents a mutation from leucine to serine.9,10 Originally, L1014F was found in Western Africa, hence leading to its name kdr-west,11–14 whereas L1014S (kdr-east) was found in Eastern Africa.10,15 However, both mutations are now found throughout Africa and have not been solely concentrated geographically, thus suggesting a shift in kdr mutation frequencies in endemic countries.16–20 In addition, both kdr mutations have been associated with increased susceptibility to Plasmodium falciparum, further heightening malaria risk in areas with high insecticide resistance.21
Mass distribution of ITNs has been followed by a rapid increase in kdr alleles and insecticide resistance in An. gambiae s.s.5 In Kenya, where ITN coverage increased from less than 10% in 200422 to greater than 80% since 2013,23 kdr mutation frequencies in An. gambiae s.s. increased rapidly from 6% in 200115 to near fixation at 98% in 2010.5 In addition to the rise of kdr mutation frequencies in An. gambiae s.s., higher ITN usage has led to a species shift from primarily An. gambiae s.s. to Anopheles arabiensis.2,24–27 As such, the contribution of An. arabiensis to malaria transmission increases in malaria-endemic areas under the current ITN program.
Recently, kdr mutation frequencies in An. arabiensis from Western Kenya have been found to be increasing and were as high as 13% and 39% at certain localities in 2013.6,23 Previously, in 2005, kdr mutation frequencies were not found to exceed 6% at any locality in Western Kenya28 and, moreover, were not detected in 2009.29 Although the evasion of ITNs might explain why the frequency of kdr mutations and physiological insecticide resistance in An. arabiensis has remained relatively low with respect to An. gambiae s.s., we expect an increase in kdr mutations for An. arabiensis to continue. However, we do not expect kdr mutations to increase as rapidly in An. arabiensis as they did in An. gambiae s.s. because of the reduced selection pressure imposed on An. arabiensis which more commonly feed outdoors.
Although ITNs are presently the most cost-effective method of preventing malaria, increased insecticide resistance, and outdoor biting reduce their efficacy and present a major threat to malaria control programs.1 Previous studies have examined the spatial distribution of kdr mutations in various An. arabiensis populations in Africa,5–7,12 but the association between kdr mutations and phenotypic resistance is not well established. Therefore, this study aimed to examine the link between kdr mutations and pyrethroid resistance by comparing the genotypes of phenotypically resistant and susceptible mosquitoes.
MATERIALS AND METHODS
Study design for kdr survey.
Anopheles gambiae s.l. larvae were collected from 11 study sites across Kenya between May 2014 and October 2014 (Figure 1). Not more than five larvae were collected from a given habitat to reduce sampling bias. Sampling bias was tested by comparing mutation frequencies to frequencies when randomly selecting one larva per habitat, and no significant differences were found. Study sites were selected across the diverse geographical regions of Kenya. The major regions were the lowlands surrounding Lake Victoria in Western Kenya (Port Victoria, Homa Bay, Kanyawegi, Chulaimbo, and Miwani), the highlands in Western Kenya (Kamkuywa), the Great Rift Valley in Western Kenya (Kabernet and Marigat), and coastal Kenya (Malindi, Mtwapa, and Gazi).
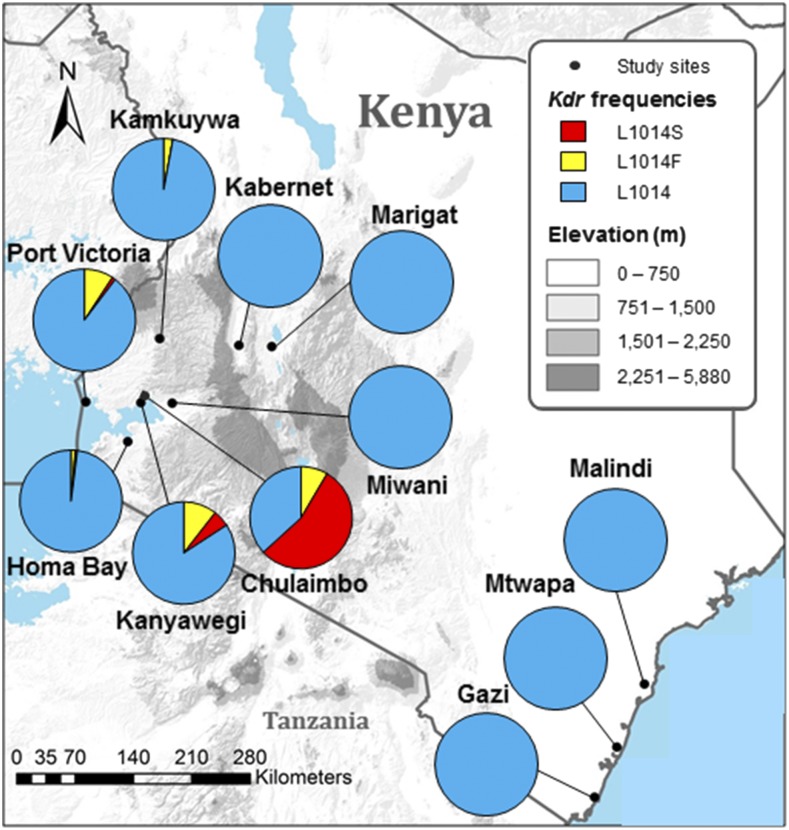
Figure 1.
Knockdown resistance (kdr) allele frequencies in Anopheles arabiensis populations across Kenya, 2014. 1014F mutation prevalences: Kanyawegi (10.5%), Port Victoria (9.2%), Chulaimbo (8.5%), Kamkuywa (2.9%), Homa Bay (1.7%), Kabernet (0.0%), Marigat (0.0%), Miwani (0.0%), Gazi (0.0%), Mtwapa (0.0%), and Malindi (0.0%). 1014S mutation prevalences: Chulaimbo (54.7%), Port Victoria (1.1%), Homa Bay (0.6%), Kanyawegi (5.3%), Kamkuywa (0.0%), Kabernet (0.0%), Marigat (0.0%), Miwani (0.0%), Gazi (0.0%), Mtwapa (0.0%), and Malindi (0.0%). This figure appears in color at www.ajtmh.org.
World Health Organization (WHO) bioassays.
To explore the link between kdr mutations and pyrethroid resistance, we genotyped phenotypically resistant and susceptible An. arabiensis, determined by a standard WHO insecticide susceptibility bioassay.30 Anopheles gambiae s.l. larvae were collected from Port Victoria and Chulaimbo, study sites where kdr mutations in An. arabiensis had previously been detected,22 and reared to adults. Adult female mosquitoes 2–3 days old were aspirated into exposure tubes in batches of 15–20 mosquitoes per tube. Tubes were lined with insecticide (0.05% deltamethrin)-impregnated paper. A subset of tubes was only lined with oil paper to serve as controls. In addition, the Kisumu-susceptible An. gambiae s.s. strain was used as a control. After being held in their respective tubes for 60 minutes, mosquitoes were transferred to a holding tube with 10% sucrose solution and put to standard insectary conditions for 24 hours. These mosquitoes were screened again. If after 24 hours, mosquitoes were knocked down such that they were either dead or unable to fly, they were classified as susceptible.
Procedures.
Genomic DNA was extracted from individual mosquitoes using standard ethanol extraction procedures with phenol:chloroform.31 The final DNA pellet was suspended in 20 μL of 10 mM Tris and 1 mM EDTA buffer. A NanoDrop 1000 Spectrophotometer was used to quantify DNA concentrations, and stock DNA was diluted to an approximate concentration of 1 μg/μL for use in polymerase chain reaction (PCR). Anopheles arabiensis and An. gambiae s.s. were identified within the An. gambiae s.l. complex using a ribosomal DNA PCR assay.32 We genotyped 683 An. arabiensis for kdr alleles: L1014 (wild-type), L1014F (kdr-west), and L1014S (kdr-east) using a Taqman probe assay.33 For detection, the wild-type alleles were labeled with 4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein at the 5′ end and the 1014F and 1014S kdr alleles were labeled with 6-carboxyfluorescein.
Statistical analysis.
For the WHO bioassay, Fischer’s exact tests were performed to make pairwise comparisons for mutation frequencies between resistant and susceptible groups. Odds ratios (ORs) were used to quantify the association between kdr genotype and insecticide-resistant phenotype. Chulaimbo and Port Victoria populations were analyzed separately.
RESULTS
Kdr survey.
A total of 1,425 An. gambiae s.l. specimens were examined (Table 1). Anopheles arabiensis proportions ranged from 12.8% at Chulaimbo to 100% at Miwani, Bogoria, Gazi, Mtwapa, and Malindi (Table 1). Kdr mutations were detected in five An. arabiensis populations: Port Victoria (10.3%), Homa Bay (2.3%), Kamkuywa (2.8%), Kanyawegi (15.8%), and Chulaimbo (63.2%) (Figure 1). The 1014F mutation prevalence was highest at Port Victoria (9.2%), Kanyawegi (10.5%), and Chulaimbo (8.5%), but also observed at Kamkuywa (2.9%) and Homa Bay (1.7%). The 1014S mutation was prevalent at Chulaimbo (54.7%) and detected at low frequencies at Port Victoria (1.1%), Homa Bay (0.6%), and Kanyawegi (5.3%). No mutations were observed in populations outside Western Kenya. The population at Chulaimbo was the only population that significantly deviated from the Hardy–Weinberg equilibrium with regard to kdr alleles (Table 1).
Table 1.
Proportion of Anopheles arabiensis within the Anopheles gambiae s.l. species complex and knockdown resistance genotype frequencies with the Hardy–Weinberg equilibrium parameters for An. arabiensis collected in Kenya, 2014
| Site | Elevation | Number | An. arabiensis (%) | Genotype frequencies (%)* | Hardy–Weinberg equilibrium | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LL | LF | FF | LS | SS | HE† | FIS‡ | ||||
| Port Victoria | 1,139 | 168 | 56.5 | 80.4 | 18.5 | 0.0 | 0.0 | 0.0 | 0.187 | 0.013 |
| Homa Bay | 1,184 | 133 | 68.4 | 95.3 | 3.5 | 0.0 | 1.2 | 0.0 | 0.046 | −0.019 |
| Kamkuywa | 1,487 | 72 | 52.8 | 91.9 | 5.4 | 0.0 | 0.0 | 0.0 | 0.054 | 0.000 |
| Kanyawegi | 1,214 | 129 | 47.3 | 71.1 | 15.8 | 2.6 | 10.5 | 0.0 | 0.028 | 0.050 |
| Chulaimbo | 1,377 | 446 | 12.8 | 26.9 | 17.3 | 0.0 | 0.0 | 55.8 | 0.558 | 0.690§ |
| Miwani | 1,161 | 120 | 100 | 100 | 0.0 | 0.0 | 0.0 | 0.0 | 0.000 | – |
| Marigat | 1,004 | 94 | 100 | 100 | 0.0 | 0.0 | 0.0 | 0.0 | 0.000 | – |
| Kabernet | 1,150 | 101 | 92.1 | 100 | 0.0 | 0.0 | 0.0 | 0.0 | 0.000 | – |
| Gazi | 15 | 30 | 100 | 100 | 0.0 | 0.0 | 0.0 | 0.0 | 0.000 | – |
| Mtwapa | 66 | 44 | 100 | 100 | 0.0 | 0.0 | 0.0 | 0.0 | 0.000 | – |
| Malindi | 14 | 88 | 100 | 100 | 0.0 | 0.0 | 0.0 | 0.0 | 0.000 | – |
L is wild-type at L1014 codon; F is L1014F mutation; S is L1014S mutation.
HE expected heterozygosity.
FIS inbreeding coefficient.
Significant deviation from the Hardy–Weinberg equilibrium.
WHO bioassay.
The control Kisumu-susceptible An. gambiae s.s. strain had a mortality rate of 100%. We observed a mortality rate of 82.8% (95% confidence interval [CI] = [0.792–0.859]) and 73.7% (95% CI = [0.610–0.834]) for An. arabiensis at Port Victoria and Chulaimbo, respectively. Both mortality rates were lower than the WHO 90% threshold for resistance (Figure 2A).
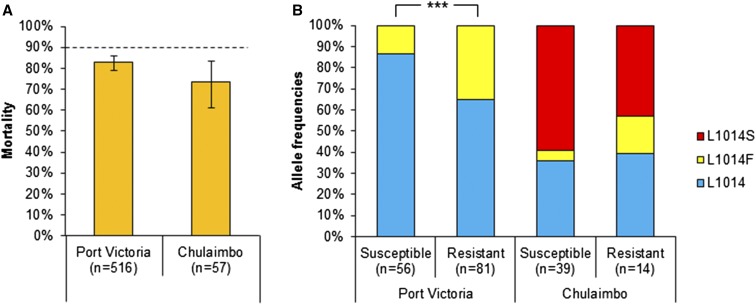
Figure 2.
Mortality rates (A) and frequencies of knockdown resistance alleles of susceptible and resistant groups (B) in Anopheles arabiensis populations in Kenya. The dotted line indicates World Health Organization threshold for confirmed resistance (90%). *** indicates P < 0.001. Error bars indicate 95% confidence interval (CI). Mortality rates at Port Victoria: 82.8% (95% CI = [0.792–0.859]) and Chulaimbo: 73.7% (95% CI = [0.610–0.834]). 1014F mutation prevalences: Port Victoria Susceptible (13.3%), Port Victoria Resistant (35.2%), Chulaimbo Susceptible (5.1%), and Chulaimbo Resistant (17.9%). 1014S mutation prevalences: Port Victoria Susceptible (0.0%), Port Victoria Resistant (0.0%), Chulaimbo Susceptible (59.0%), and Chulaimbo Resistant (42.9%). This figure appears in color at www.ajtmh.org.
A comparison of kdr mutation frequencies between a subset of resistant and susceptible An. arabiensis revealed that deltamethrin-resistant mosquitoes had significantly higher frequencies of the L1014F mutation at Port Victoria (OR = 3.495, 95% CI = [1.809–7.102], P < 0.001, Fischer’s exact test) (Figure 2B), supporting the link between the kdr mutation and pyrethroid resistance. Although both L1014F and L1014S mutations were detected at Chulaimbo, the highest resistant field population, there was no significant difference in allele frequencies between susceptible and resistant groups (P = 0.078; Fischer’s exact test) (Figure 2B). When comparing only the L1014F frequency between groups at Chulaimbo, the difference is marginally significant (OR = 3.957, 95% CI = [0.781–21.713], P = 0.053; Fischer’s exact test) and could be limited by a low sample size in the resistance group (N = 14), whereas there was no significant difference in L1014S frequencies between susceptible and resistant groups (OR = 0.525, 95% CI = [0.197–1.364], P = 0.185, Fischer’s exact test).
DISCUSSION
The observed high proportions of An. arabiensis in this study demonstrate the ongoing species composition shift from predominantly An. gambiae s.s. to An. arabiensis in East Africa.2,24–27 A decline in An. gambiae s.s. relative abundance yet stable population of An. arabiensis has been observed in the lowlands of Kenya in conjunction with an increase in ITN coverage.2,7,23,27 These findings underscore the importance of the role that An. arabiensis are playing in maintaining residual malaria transmission, and as such, will present a major barrier to malaria control and elimination. Understanding An. arabiensis insecticide resistance mechanisms and monitoring for resistance are essential for achieving malaria elimination goals.
The presence of kdr mutations at several sites in Western Kenya indicates the widespread occurrence of kdr mutations among An. arabiensis populations. In particular, the L1014F mutation, first detected in Kenya in 2012,6 was observed in four of the five Western Kenya populations in this study. The emergence of L1014F was also found in neighboring malaria-endemic countries. L1014F has recently been detected in Tanzania in both An. gambiae and An. arabiensis populations.34 Moreover, high frequencies of the L1014F mutation in An. arabiensis have been reported from Ethiopia35–37 and central Sudan.38 A continual increase in this mutation prevalence in Kenya may cause further concern on the future utility of ITNs.
The rise of the L1014F mutation may be particularly concerning, given that this mutation was found to be associated with pyrethroid resistance in An. arabiensis in our Port Victoria study population. Kdr mutations at Chulaimbo were not significantly associated with pyrethroid resistance. This result could be due to the low frequency of L1014F and presence of the L1014S mutation at this site. The prevalence in L1014F mutations was higher in the resistant group at Chulaimbo, but the difference was not statistically significant. In An. gambiae s.s., the L1014S mutation has been found to be more weakly associated with pyrethroid resistance than the L1014F mutation.39 Similarly, the L1014F mutation may also have a stronger association with pyrethroid resistance in An. arabiensis. In Sudan, there was also a significant association found between the 1014F mutation and DDT and pyrethroid resistance in An. arabiensis, but the 1014S mutation was not detected in the populations tested.38 Further studies are needed to investigate the role of the 1014S and 104F mutations in An. arabiensis insecticide resistance. The result also suggests that other mechanisms such as metabolic detoxification or secondary mutations at alternative loci could be involved in pyrethroid resistance in An. arabiensis at Chulaimbo, especially given the high levels of resistance at this site. Metabolic resistance using rapid insecticide detoxification due to the overexpression of P450 enzymes has been found to be a common resistance mechanism for An. arabiensis.35,40–42
Interestingly, kdr mutations were only observed in An. arabiensis specimens from study sites where An. gambiae were also common at proportions exceeding 30%. Stump et al.15 first suggested the possibility that kdr alleles could have been introduced into Kenyan An. arabiensis populations through introgression. Adaptive introgression of kdr alleles has been supported by evidence of consequential contemporary gene flow between An. arabiensis and An. gambiae in East Africa.43,44 This notion is underscored by findings of identical intron sequences in the VGSC between the two species in Kenya.29 Our findings of kdr mutations occurring exclusively in An. arabiensis populations where An. gambiae are common are consistent with the hypothesis that An. arabiensis acquire kdr mutations through introgression with sympatric An. gambiae populations.
Pyrethroid resistance in An. arabiensis has been reported in several countries, including Sudan,38 Ethiopia,35,45 Malawi,46 Tanzania,47 Zanzibar,48,49 and Kenya.7 Despite widespread resistance in major malaria vectors in sub-Saharan Africa, pyrethroids are the only approved insecticide for use in ITNs.6 The findings from this study and Abdalla et al.38 that the L1014F mutation is associated with pyrethroid resistance in An. arabiensis provide evidence on the utility of screening An. arabiensis populations for kdr mutations in informing pyrethroid resistance status and trends. However, that kdr mutations were not associated with resistance at Chulaimbo also highlights the complexity of insecticide resistance and the need for further studies on resistance mechanisms in An. arabiensis.
Kdr mutations could potentially increase and spread rapidly in a pattern like that observed for An. gambiae from 2001 to 2010.5,15 Our results of commonly occurring 1014F mutations associated with pyrethroid resistance in An. arabiensis underscores the importance in searching for alternative methods to pyrethroid-impregnated bed nets for vector control. High levels of resistance in An. gambiae s.s.,5 An. arabiensis behavioral resistance to ITNs,2 an increased proportion of An. arabiensis, and frequent kdr mutations in An. arabiensis from Western Kenya could all contribute to compromised efficacy of ITNs. Therefore, complementary interventions targeting outdoor mosquitoes, such as attractive toxic sugar–baited traps, habitat reduction, and/or biological larvicides, could be important to improving the overall efficacy of antimalarial programs, as well as suppressing pyrethroid resistance. These interventions have been effective for vector control in areas such as Mali,50 Ecuador,51 Peru,51 and Kenya.52
In summary, we found evidence of widespread kdr mutations in Western Kenya and an association between the kdr 1014F mutation and pyrethroid resistance in An. arabiensis. This result is concerning for the effectiveness of ITNs, especially because An. arabiensis is becoming the predominant malaria vector in Kenya and throughout Africa.2 Monitoring for the spread of insecticide resistance in An. arabiensis is critical for resistance management, and consequently, the success of vector control programs.
Acknowledgments:
We thank the technicians and staff from the Kenya Medical Research Institute (KEMRI) at Kisumu for sample collection and undergraduate students for data collection.
REFERENCES
- 1.World Health Organization , 2014. World Malaria Report 2014. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED, 2010. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V, 2011. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27: 91–98. [DOI] [PubMed] [Google Scholar]
- 4.O’Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, Newton CR, Marsh K, 2008. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 372: 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathias DK, et al. 2011. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in western Kenya. Malar J 10: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochomo E, et al. 2015. Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. arabiensis in western Kenya. Parasit Vectors 8: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochomo E, Bayoh MN, Brogdon WG, Gimnig JE, Ouma C, Vulule JM, Walker ED, 2013. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Med Vet Entomol 27: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauron D, Barhanin J, Amichot M, Pralavorio M, Berge JB, Lazdunski M, 1989. Pyrethroid receptor in the insect Na sup+ channel: alteration of its properties in pyrethroid-resistant flies. Biochemistry 28: 1673–1677. [Google Scholar]
- 9.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, Guillet P, Pasteur N, Pauron D, 1998. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol 7: 179–184. [DOI] [PubMed] [Google Scholar]
- 10.Ranson H, Jenson B, Vulule JM, Wang X, Hemingway J, Collins FH, 2000. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol 9: 491–497. [DOI] [PubMed] [Google Scholar]
- 11.Chandre F, Manguin S, Brengues C, Dossou YJ, Darriet F, Diabate A, Carnevale P, Guillet P, 1999. Current distribution of a pyrethroid resistance gene (kdr) in Anopheles gambiae complex from west Africa and further evidence for reproductive isolation of the Mopti form. Parassitologia 41: 319–322. [PubMed] [Google Scholar]
- 12.Awolola TS, Brooke BD, Koekemoer LL, Coetzee M, 2003. Absence of the kdr mutation in the molecular ‘M’form suggests different pyrethroid resistance mechanisms in the malaria vector mosquito Anopheles gambiae s.s. Trop Med Int Health 8: 420–422. [DOI] [PubMed] [Google Scholar]
- 13.Fanello C, Petrarca V, Della Torre A, Santolamazza F, Dolo G, Coulibaly M, Alloueche A, Curtis CF, Toure YT, Coluzzi M, 2003. The pyrethroid knock‐down resistance gene in the Anopheles gambiae complex in Mali and further indication of incipient speciation within An. gambiae s.s. Insect Mol Biol 12: 241–245. [DOI] [PubMed] [Google Scholar]
- 14.Yawson AE, McCall PJ, Wilson MD, Donnelly MJ, 2004. Species abundance and insecticide resistance of Anopheles gambiae in selected areas of Ghana and Burkina Faso. Med Vet Entomol 18: 372–377. [DOI] [PubMed] [Google Scholar]
- 15.Stump AD, Atieli FK, Vulule JM, Besansky NJ, 2004. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg 70: 591–596. [PubMed] [Google Scholar]
- 16.Santolamazza F, et al. 2008. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar J 7: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I, 2007. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar J 6: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balkew M, Gebre-Michael T, Hailu A, 2003. Insecticide susceptibility level of Anopheles arabiensis in two agro-development localities in eastern Ethiopia. Parassitologia 45: 1–3. [PubMed] [Google Scholar]
- 19.Ndjemaï HN, Patchoké S, Atangana J, Etang J, Simard F, Bilong CF, Reimer L, Cornel A, Lanzaro GC, Fondjo E, 2009. The distribution of insecticide resistance in Anopheles gambiae s.l. populations from Cameroon: an update. Trans R Soc Trop Med Hyg 103: 1127–1138. [DOI] [PubMed] [Google Scholar]
- 20.Himeidan YE, Chen H, Chandre F, Donnelly MJ, Yan G, 2007. Permethrin and DDT resistance in the malaria vector Anopheles arabiensis from eastern Sudan. Am J Trop Med Hyg 77: 1066–1068. [PubMed] [Google Scholar]
- 21.Alout H, Yameogo B, Djogbénou LS, Chandre F, Dabiré RK, Corbel V, Cohuet A, 2014. Interplay between Plasmodium infection and resistance to insecticides in vector mosquitoes. J Infect Dis 210: 1464–1470. [DOI] [PubMed] [Google Scholar]
- 22.Noor AM, Amin AA, Akhwale WS, Snow RW, 2007. Increasing coverage and decreasing inequity in insecticide-treated bed net use among rural Kenyan children. PLoS Med 4: e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanjala CL, Mbugi JP, Ototo E, Gesuge M, Afrane YA, Atieli HE, Zhou G, Githeko AK, Yan G, 2015. Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes, western Kenya. Emerg Infect Dis 21: 2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sougoufara S, Harry M, Doucouré S, Sembène PM, Sokhna C, 2016. Shift in species composition in the Anopheles gambiae complex after implementation of long‐lasting insecticidal nets in Dielmo, Senegal. Med Vet Entomol 30: 365–368. [DOI] [PubMed] [Google Scholar]
- 25.Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C, 2013. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors 6: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G, 2015. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J 14: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitau J, Oxborough RM, Tungu PK, Matowo J, Malima RC, Magesa SM, Bruce J, Mosha FW, Rowland MW, 2012. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS One 7: e31481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamau L, Agai D, Matoke D, Wachira L, Gikandi G, Vulule JM, 2008. Status of insecticide susceptibility in Anopheles gambiae sensu lato and Anopheles funestus mosquitoes from western Kenya. J Insect Sci 8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawada H, et al. 2011. Multimodal pyrethroid resistance in malaria vectors, Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus s.s. in western Kenya. PLoS One 6: e22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization (WHO) , 2013. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. Geneva, Switzerland: WHO. [Google Scholar]
- 31.Severson DW, 1997. RFLP analysis of insect genomes. The Molecular Biology of Insect Disease Vectors London, United Kingdom: Springer, 309–320. [Google Scholar]
- 32.Scott JA, Brogdon WG, Collins FH, 1993. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg 49: 520–529. [DOI] [PubMed] [Google Scholar]
- 33.Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, Vontas J, Field LM, 2007. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J 6: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, Kollo D, Malima R, Kafuko J, Mohamed M, Magesa S, 2014. Co‐occurrence and distribution of East (L1014S) and West (L1014F) African knock‐down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Health 19: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fettene M, Olana D, Christian RN, Koekemoer LL, Coetzee M, 2013. Insecticide resistance in Anopheles arabiensis from Ethiopia. Afr Entomol 21: 89–94. [Google Scholar]
- 36.Yewhalaw D, Van Bortel W, Denis L, Coosemans M, Duchateau L, Speybroeck N, 2010. First evidence of high knockdown resistance frequency in Anopheles arabiensis (Diptera: Culicidae) from Ethiopia. Am J Trop Med Hyg 83: 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yewhalaw D, et al. 2011. Multiple insecticide resistance: an impediment to insecticide-based malaria vector control program. PLoS One 6: e16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdalla H, Wilding CS, Nardini L, Pignatelli P, Koekemoer LL, Ranson H, Coetzee M, 2014. Insecticide resistance in Anopheles arabiensis in Sudan: temporal trends and underlying mechanisms. Parasit Vectors 7: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynd A, Weetman D, Barbosa S, Egyir Yawson A, Mitchell S, Pinto J, Hastings I, Donnelly MJ, 2010. Field, genetic, and modeling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Mol Biol Evol 27: 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Githeko AK, Githure JI, Mutunga J, Zhou G, Yan G, 2008. Monooxygenase levels and knockdown resistance (kdr) allele frequencies in Anopheles gambiae and Anopheles arabiensis in Kenya. J Med Entomol 45: 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amenya DA, Naguran R, Lo T, Ranson H, Spillings BL, Wood OR, Brooke BD, Coetzee M, Koekemoer LL, 2008. Over expression of a cytochrome P450 (CYP6P9) in a major African malaria vector, Anopheles funestus, resistant to pyrethroids. Insect Mol Biol 17: 19–25. [DOI] [PubMed] [Google Scholar]
- 42.Cuamba N, Morgan JC, Irving H, Steven A, Wondji CS, 2010. High level of pyrethroid resistance in an Anopheles funestus population of the Chokwe District in Mozambique. PLoS One 5: e11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ, 2013. Insecticide resistance monitoring of field‐collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol 27: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weetman D, Steen K, Rippon EJ, Mawejje HD, Donnelly MJ, Wilding CS, 2014. Contemporary gene flow between wild An. gambiae s.s. and An. arabiensis. Parasit Vectors 7: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asale A, Getachew Y, Hailesilassie W, Speybroeck N, Duchateau L, Yewhalaw D, 2014. Evaluation of the efficacy of DDT indoor residual spraying and long-lasting insecticidal nets against insecticide resistant populations of Anopheles arabiensis Patton (Diptera: Culicidae) from Ethiopia using experimental huts. Parasit Vectors 7: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mzilahowa T, et al. 2016. Increasing insecticide resistance in Anopheles funestus and Anopheles arabiensis in Malawi, 2011–2015. Malar J 15: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, Majambere S, 2014. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar J 13: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haji KA, Khatib BO, Smith S, Ali AS, Devine GJ, Coetzee M, Majambere S, 2013. Challenges for malaria elimination in Zanzibar: pyrethroid resistance in malaria vectors and poor performance of long-lasting insecticide nets. Parasit Vectors 6: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones CM, et al. 2013. The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasit Vectors 6: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y, 2010. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J 9: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kroeger A, Horstick O, Riedl C, Kaiser A, Becker N, 1995. The potential for malaria control with the biological larvicide Bacillus thuringiensis israelensis (Bti) in Peru and Ecuador. Acta Trop 60: 47–57. [DOI] [PubMed] [Google Scholar]
- 52.Fillinger U, Lindsay SW, 2006. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Health 11: 1629–1642. [DOI] [PubMed] [Google Scholar]