Abstract.
In 2013, the under-5 mortality rate in Liberia was 71 deaths per 1,000 live births, with malaria responsible for 22% of those deaths. One of the primary existing control tools, long-lasting insecticide-treated bed nets (LLINs), is thought to be dually effective, acting as a physical barrier but also decreasing the mosquito population in communities. However, there has been little investigation into the protective effects of community-wide bed net use above and beyond the individual level. Using data from the population-representative 2011 Liberia Malaria Indicator Survey, we estimated the association between proportion of a community using LLINs and malaria in children using multi-level logistic regression. To investigate the potential effect measure modification of the relationship by urbanicity, we included an interaction term and calculated stratum-specific prevalence odds ratios (PORs) for rural and urban communities. We calculated a POR of malaria for an absolute 10% increase in community bed net use of 1.13 (95% confidence interval [CI]: 0.91, 1.41) and 0.35 (95% CI: 0.13, 0.92) for rural and urban communities, respectively, indicating a strong, though imprecise, protective effect within urban communities only. Our results indicate that bed net use has an indirect protective effect in urban areas, above and beyond individual use. Little or no such effect of community-wide use is seen in rural areas, likely because of population density factors. Therefore, although all control efforts should be multifaceted, promotion of bed net use in urban areas in particular will likely be a highly effective tool for control.
INTRODUCTION
An infection of the red blood cells by the Plasmodium protozoan, malaria, is spread through a vector, the Anopheles mosquito, and almost 100% of malaria cases in Liberia are due to infection with Plasmodium falciparum.1 As such, the greatest burden of malaria is seen in tropical countries with climates suited to these mosquitos. As of 2013, the under-5 mortality rate in Liberia was 71 deaths per 1,000 live births,1 with malaria responsible for 22% of those deaths, making it the leading cause of years of life lost in Liberia.1 The burden of malaria-related illness and mortality in Liberia was exacerbated during the ’90s and early ’00s by civil conflict throughout the country, which resulted in displacement of large groups of people as well as damaged health services.2,3 More recently, the 2014 Ebola outbreak in West Africa hindered health services for those having malaria with the malaria prevention infrastructure being neglected in favor of Ebola operations.4 Although there are drugs available for treatment of malaria, they can be expensive and difficult to access, especially in resource-poor settings such as Liberia.5 In addition, the emergence of insecticide-resistant mosquitos and drug-resistant malaria has the potential to further hinder control efforts, which focus on preventing transmission from human to mosquito and vice versa.6–10 The combination of these factors heightens the need for highly efficacious preventive, rather than curative, programs.
There are many existing prevention and control methods in place in Liberia and throughout West Africa, supported by both government and nonprofit organizations, including the use of long-lasting insecticide-treated bed nets (LLINs), indoor residual spraying, intermittent prevention treatment in pregnancy, and diagnosis and case management.11,12 LLINs are a well-established tool used throughout malaria-endemic countries to prevent malaria and have been shown to be effective at reducing incidence of infection to those who use them,13,14 although some results have been mixed.15,16 Commonly used insecticides for these nets include permethrin, deltamethrin, and alphacypermethrin, all part of a class of insecticides called pyrethroids. Nets are pretreated with these “long-lasting” insecticides and then distributed by non-governmental organizations to individuals for use while sleeping. These bed nets are thought to be effective in three ways, acting first as a physical barrier preventing mosquitos from infecting those who sleep under them, but also by driving off mosquitos that land on the net through excito-repellency and decreasing the mosquito population in communities through insecticide killing of mosquitos. The latter mechanism should lower the risk of malaria acquisition within some geographic area even to those individuals who do not themselves sleep under a net. As such, the proportion of a community’s use of LLINs, or “community bed net use,” could provide protection above and beyond that provided on the individual level and should be associated with a lowered risk of malaria within that area.
There have been relatively few studies quantifying the efficacy of bed nets in reducing the risk of malaria specifically through its effect on mosquito population size, especially in the community setting. Laboratory assessments of LLINs have found that the insecticide mechanism remains effective for at least 3 years, with regular washing but under otherwise pristine conditions, without the regular wear and tear seen in a community.17 In the field, however, one study found that more than half of nets needed replacement after 2 years based on physical degradation.18 In 2011, Messina et al.19 found a statistically significant, although very small, protective effect of community bed net use on malaria in an analysis exploring several individual and community risk factors. An analysis in Lilongwe, Malawi found similarly small but protective effects of community bed net use on malaria in children.20 In a pooled analysis of several African countries, Larsen et al.21 found that LLIN coverage of greater than 50% within a community was significantly protective, but that lower than that was not unless only looking within households that used LLINs.
Furthermore, there is evidence that the effectiveness of bed net use, both on the individual level and at the community level, may vary by urban versus rural status of communities because of variation in transmission intensity from both vector and human population density. A review of individual use of LLINs found that protective effects were more powerful in areas with low transmission than high transmission.13 A 2011 study in Western Kenya found that LLINs provided protection during the rainy season, a time with high transmission, but not during the dry season.22 The evidence of increasing resistance to insecticides in West Africa, with two recent studies finding reduced efficacy of bed nets due to this spreading resistance, may also affect the level of protection provided by community bed net use.9,10
It is important to consider the effectiveness of this intervention from both mechanisms and on a country-specific basis because of the unique geography, populations, and social characteristics within Liberia, and also because of the shifting insecticide resistance patterns seen within it. Updated and accurate effect estimates will allow policy makers at national and international levels to make informed decisions regarding prevention, control, and elimination strategies. We hypothesize that the proportion of a community using LLINs will provide protection against malaria beyond that provided to the individual using the net and that this relationship will vary by urbanicity. Data from the 2011 Liberia Malaria Indicator Survey (MIS) will allow us to address this gap.
METHODS
Study population.
The data for this study come from the 2011 Liberia MIS, which is a cross-sectional population-based survey conducted by the Demographic and Health Surveys (DHSs) Program. The Liberia MIS is designed to assess the demographic characteristics, health status, and malaria knowledge of women aged 15 years and older, and the prevalence of malaria parasitemia among their children aged 6 months to less than 5 years by conducting the survey in a nationally representative sample of the Liberian population. The 2011 MIS fieldwork was conducted in September 20, 2011, through December 8, 2011, just after the peak of the rainy season, although the climate in Liberia is suitable for malaria transmission year-round in almost the entire country. To determine who would be surveyed for the 2011 study, a two-stage, stratified sampling frame was used based on the National Population and Housing Census conducted in 2008. First, 150 enumeration areas (EAs), out of 7,021 total, were selected from the 15 counties in Liberia using a stratified probability proportional to size, stratified based on county and urbanicity with 15 rural strata and 16 urban strata. Forty percentage of communities came from urban areas and 60% from rural. The second stage of sampling used household listings from each of the 150 selected EAs to identify 30 households within each EA for inclusion.23
All women aged 15–49 and their children aged 6–59 months within each selected household were eligible for inclusion in the study. Women in the study completed a household questionnaire, which included environmental and habitat characteristics regarding water source, sanitation facilities, floor and roofing materials of the home, insecticide treatments, bed net availability and use, any symptoms of illness among children, etc., as well as a women’s questionnaire, which collected information regarding her socioeconomic status, demographic characteristics, pregnancy history, personal illness symptoms, children’s medication use, and personal knowledge of malaria and it’s causes, symptoms, prevention techniques, and treatments. In addition, all children aged less than 5 years living within selected households were offered a malaria rapid diagnostic test (RDT) and submitted a blood sample for further analysis.
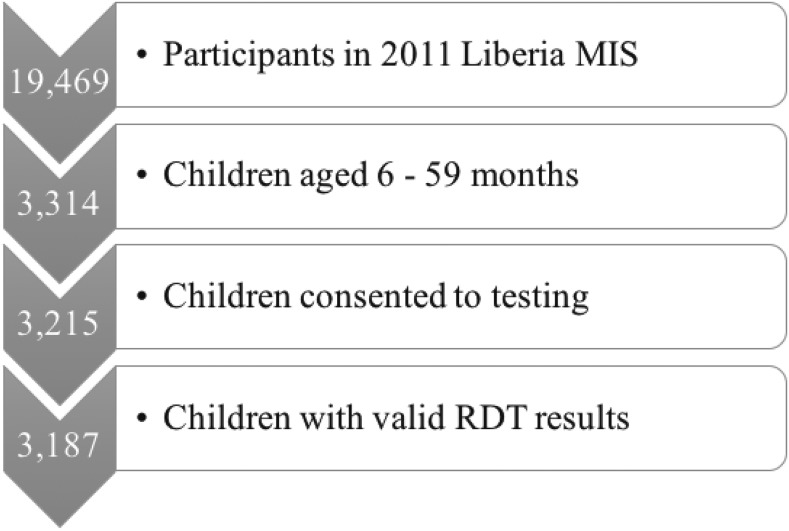
Of the 3,314 children aged 6–59 months who participated in the 2011 MIS, 3,215 were consented to malaria RDT testing, and of those, 3,187 received valid RDT results (see Figure 1). These 3,187 had complete information on all covariates and the exposure and were thus included in our analysis.
Figure 1.
Study population flow chart. This diagram shows the process through which we determined our final analysis dataset and shows the size of the study population at each exclusion point.
Malaria status.
Malaria parasitemia, our outcome, is dichotomized based on the diagnosis of infection with Plasmodium falciparum via the First Response RDT.23 The RDT is administered in the field at the participant’s home and results are provided within 15 minutes. This test detects histidine-rich protein 2 (hrp2), a P. falciparum–specific protein which can be found in the blood of infected individuals.
Individual and community bed net use.
For this analysis, individual bed net use was dichotomized as 1—yes, child slept under a bed net, regardless of treatment status, during the previous night and 0—no, child did not sleep under a bed net during the previous night. Community bed net use was calculated by creating intermediate variables measuring the number of children in each cluster (community population) and the number of children in each cluster who had slept under an LLIN during the previous night. The variable was applied differently to each child depending on whether he or she slept under a bed net during the previous night. If a child had slept under a bed net the previous night, community bed net use was calculated as (number of children who slept under an LLIN during the previous night − 1)/(number of children in that community − 1), whereas if a child had not slept under a bed net the previous night, community bed net use was calculated as (number of children who slept under an LLIN during the previous night)/(number of children in that community).
Covariates.
Sixty-nine of the 150 communities in our study were urban. Twenty-five of these are in the Greater Monrovia geographic region, with the remainder as the capitals of each county and communities near the capitals, with two to six urban communities per county. Age and gender were reported by the mother. Age in months was modeled as a quadratic variable and biological gender as the dichotomous male/female. A wealth index was generated for each household based on household assets and use of services. Assets assessed include electricity, radio, mobile telephone, table and chairs, television, ice box, modes of transportation, etc. The index is scored and normalized to have a mean of 0 and standard deviation of 1. It is then divided into quintiles and coded as a categorized variable ranging from 1 to 5, corresponding to each household’s quintile of wealth within the nation and taking into account urban–rural differences and applied to individuals in the household. Altitude was not included in our adjustment set because of the range of altitudes among communities in Liberia not being wide enough to affect malaria transmission.
Statistical analysis.
All analyses were conducted in SAS 9.3 (SAS Institute, Inc., Cary, NC) and weighted using the children’s sampling weights to account for the 2011 Liberia MIS complex survey design. The analysis was cross-sectional to determine the association between malaria parasitemia and bed net use, both community and individual. Descriptive statistics were used to characterize the study population, overall and within strata of individual bed net use. To quantify the association between proportion of a community using LLINs and malaria in children with appropriate variance estimates, we used multi-level logistic regression with a random intercept and two levels: individual and community. We calculated the relative odds of malaria parasitemia within an individual due to a 1% or 10% change in community LLIN use and the relative odds of malaria parasitemia due to sleeping under a bed net compared with not sleeping under a bed net. To account for potential confounding, we adjusted for age, gender, wealth, and urbanicity in our fully adjusted model. To investigate the potential effect measure modification of the community LLIN use—malaria relationship by community urbanicity—we also built a model that included an interaction term between urbanicity and community bed net use and calculated stratum-specific effect estimates for rural and urban communities.
RESULTS
Sample characteristics.
Of the 3,187 children included in our analysis, 49.0% were female, aged a median of 33 months, and had a median household wealth index of 2. Thirty-nine percentage of the children reported that they had slept under a bed net the previous night and 44.7% tested positive to malaria by RDT. Overall, the median (interquartile range [IQR]) proportion of each community that reported having slept under an LLIN during the previous night was 30.3% (20.2% and 39.4%) and 61.0% of the study population lived in communities that were considered rural compared with 39.0% in urban communities. Children who had slept under a bed net during the previous night were slightly younger, at a median of 30 months old (IQR 27, 46), compared with those who had not slept under a bed net during the previous night, at 34 months (IQR 20, 47), and slightly more female (50.2%) than those not using a net (48.4%). Those using bed nets were slightly wealthier than those who did not use bed nets, with median (IQR) wealth indices of (32,4 and 21,4), respectively. The median (IQR) proportion of the community using LLINs was higher for those who had slept under their own net during the previous night (34.5% [28.3%, 44.1%]) compared with those who had not (28.6% [18.0%, 36.8%]) and a higher proportion of those using bed nets lived in urban communities (42.6%) than those who had not (37.0%). The proportion of children testing positive for malaria by RDT was very similar for both those who had used a net and those who had not, at 44.0% and 45.0%, respectively. The complete demographic characteristics of our study population are described in Table 1.
Table 1.
Characteristics of children < 5 years old, Liberia Malaria Indicator Survey, 2011
| Variable | Overall (N = 3,187) | Bed net + (N = 1,190) | Bed net − (N = 1,997) |
|---|---|---|---|
| Individual | |||
| Age, months (median [IQR]) | 33 (19, 46) | 30 (17, 43) | 34 (20, 47) |
| Gender | |||
| Female, % | 49.0 | 50.2 | 48.4 |
| Male, % | 51.0 | 49.8 | 51.6 |
| Household wealth index (median [IQR]) | 2 (1, 4) | 3 (2, 4) | 2 (1, 4) |
| Bed net/insecticide use | |||
| Slept under net | 35.9 | – | – |
| Did not sleep under net | 64.1 | – | – |
| Community | |||
| % Using LLIN (median [IQR]) | 30.3 (20.2, 39.4) | 34.4 (28.4, 44.1) | 28.6 (18.0, 36.8) |
| Altitude, meters (median [IQR]) | 105 (16, 259) | 105 (15, 259) | 101 (16, 259) |
| Urbanicity | |||
| Rural | 61.0 | 57.4 | 63.0 |
| Urban | 39.0 | 42.6 | 37.0 |
| Malaria, % Rapid diagnostic test positive | 44.7 | 44.0 | 45.0 |
IQR = interquartile range; LLIN = long-lasting insecticide-treated bed nets.
Associations with malaria parasitemia.
Table 2 shows the output from our three statistical models. Model 1 is a crude model including only individual and community bed net use. Model 2 includes individual and community bed net use and is adjusted for age, gender, wealth, and urbanicity. Model 3 includes an interaction term, removing the assumption of constancy of effect across strata of urbanicity and allowing us to calculate stratum-specific estimates of the association between community bed net use and malaria prevalence. Model 3 provided the best fit of all three models, with an Akaike information criterion (AIC) value of 35,719.64 compared with an AIC of 35,720.78 for model 2. Furthermore, we chose model 3 as our final model because of the meaningful interaction between urbanicity and the exposure discussed in the following paragraphs.
Table 2.
Beta coefficients and standard errors (SEs) from multi-level logistic regression, N = 3,187
| Variable | Model 1*† | Model 2*‡ | Model 3§ |
|---|---|---|---|
| Intercept | −0.4758 (0.2891) | −3.1127 (0.3265)‖ | −2.8801 (0.3985)‖ |
| Community bed net use | 0.007508 (0.008573) | 0.01837 (0.008494)‖ | 0.01201 (0.01124) |
| Individual bed net use | −0.005130 (0.02827) | 0.04494 (0.02924) | 0.04261 (0.02927) |
| Gender | – | −0.01305 (0.02700) | −0.01321 (0.02701) |
| Age, months¶ | – | 0.08906 (0.004455)‖ | 0.08907 (0.004456)‖ |
| Age2 | – | −0.00088 (0.000066)‖ | −0.00088 (0.000066)‖ |
| Wealth index | – | 0.9647 (0.08682)‖ | 0.9727 (0.08693)‖ |
| – | 0.9131 (0.08682)‖ | 0.9235 (0.08028)‖ | |
| – | 0.9004 (0.08017)‖ | 0.9104 (0.07306)‖ | |
| – | 0.7596 (0.07296)‖ | 0.7712 (0.06922)‖ | |
| Urbanicity | – | 0.7991 (0.06912)‖ | −1.3139 (0.5740)‖ |
| Urbanicity‖ community bed net use | – | – | 0.01373 (0.01688) |
| Akaike information criterion | 37,307.21 | 35,720.78 | 35,719.64 |
Beta coefficients and SE for each variable in the multi-level logistic regression model. Model 1 includes community and individual bed net use, model 2 includes model 1 variables plus all covariates (gender, age [quadratic], wealth, and urbanicity), and model 3 includes all model 2 variables plus an interaction term between urbanicity and community bed net use.
Weighted by children’s malaria weight.
Unadjusted model.
Fully adjusted model including gender, age (months), wealth, and urbanicity.
Interaction model.
Significant at alpha < 0.05.
Age modeled as a quadratic variable.
From each model, we calculated prevalence odds ratios (PORs) for a 1% and 10% increase in community LLIN use as well as for individual bed net use compared with no individual bed net use. These estimates and their corresponding 95% confidence intervals (CIs) are shown in Table 3. Our crude model indicated that a 10% increase in community bed net use was associated with POR of malaria of 1.08 (95% CI: 0.91, 1.28). From model 2, we calculated a POR of malaria of 1.02 (95% CI: 1.00, 1.04) for a 1% increase in community bed net use and 1.20 (95% CI: 1.02, 1.42) for a 10% increase in community bed net use. However, the stratum-specific PORs from model 3 indicated that whereas rural communities showed roughly the same relationship as we saw with the overall results, the effect of community bed net use was dramatically different among urban communities. For a 1% increase in community bed net use, the POR was 1.01 (95% CI: 0.99, 1.03) for rural communities and 0.28 (95% CI: 0.09, 0.84) for urban communities, indicating a strong, although imprecise, protective effect within urban communities only. For a 10% increase in community bed net use, we calculated PORs of 1.13 (95% CI: 0.91, 1.41) and 0.35 (95% CI: 0.13, 0.92) for rural and urban communities, respectively.
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) for community bed net use and rapid diagnostic test (RDT) results using multi-level logistic regression, N = 3,187
| Model 3—interaction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1—crude | Model 2—fully adjusted | Rural | Urban | |||||
| Prevalence odds ratio (POR) of testing positive for malaria | 95% CI | POR of testing positive for malaria | 95% CI | POR of testing positive for malaria | 95% CI | POR of testing positive for malaria | 95% CI | |
| 1% increase in community bed net use | 1.01 | (0.99, 1.02) | 1.02 | (1.00, 1.04) | 1.01 | (0.99, 1.03) | 0.28 | (0.09, 0.84) |
| 10% increase in community bed net use | 1.08 | (0.91, 1.28) | 1.20 | (1.02, 1.42) | 1.13 | (0.91, 1.41) | 0.35 | (0.13, 0.92) |
| Slept under bed net vs. did not sleep under bed net* | 0.99 | (0.94, 1.05) | 1.05 | (0.99, 1.11) | 1.04 | (0.99, 1.11) | ||
ORs and 95% CIs for each variable in the multi-level logistic regression model, using RDT results as the outcome. Model 1 includes community and individual bed net use, model 2 includes model 1 variables plus all covariates (gender, age [quadratic], wealth, and urbanicity), and model 3 includes all model 2 variables plus an interaction term between urbanicity and community bed net use. Row 3 shows OR (95% CI) for individual bed net use and malaria parasitemia.
Model 3 estimate is for all communities, not stratified by urbanicity.
Individual bed net use was not associated with malaria parasitemia in any model. From all three models, we calculated PORs near the null, with 95% CIs including the null. From model 3, the best fitting model, the odds of parasitemia for those who did sleep under a bed net during the previous night were 1.04 (95% CI: 0.99, 1.11) times the odds for those who did not sleep under a bed net during the previous night.
DISCUSSION
Although we did not see the expected effect of community bed net use on malaria parasitemia across the board, our hypothesis of effect measure modification by urbanicity was supported by the results. We had anticipated a protective effect in both settings with a stronger effect in one (urban) rather than the other (rural), but instead, we saw no protection in rural communities and a strong protective effect in urban communities. Interestingly, we saw no protection provided by individual bed net use in our crude or adjusted models. This is possibly due to physical degradation of the nets over time, leading to holes that would allow mosquitos to still bite those sleeping under the nets. Furthermore, the odds ratios we have calculated for individual bed net use are adjusted for community bed net use, which is a mediator on the individual bed net use and malaria parasitemia causal pathway. This adjustment likely significantly diminishes the association between individual bed net use and malaria parasitemia that should otherwise exist. Also, it is possible that whereas our measurements regarding proportion of the community using bed are relatively accurate, whether a specific individual actually sleeps under the net is subject to greater error. The way the variable is reported, a response to the question of whether the child slept under a bed net during the previous night, does not necessarily provide information on their regular sleeping habits. It may, however, more accurately reflect the proportion of the children using bed nets because the community-level variable does not rely on who specifically used the net.
The variation in the relationship by factors related to urbanicity has been touched on in a few other studies.13,22 These two studies note variation in the relationship between individual bed net use and malaria due to transmission intensity, not specifically urbanicity, but we expect transmission intensity to vary along urban/rural lines. The likely higher density of people within the urban communities could be playing a large role in the increased protection that we see among those communities. Although it then makes sense that we would see a weaker effect in rural communities as compared with urban communities due to factors such as population density, it is possible that we are seeing no protection at all in rural communities because of some threshold effect. Larsen et al.21 detected a threshold effect overall of 50% community LLIN use, although they did not calculate how protective or whether increased coverage above and beyond 50% was incrementally protective. Rural communities specifically had a median (interquartile range [IQR]) proportion sleeping under LLINs of 29.8% (19.8%, 37.7%), whereas urban communities had a median (IQR) of 32.0% (20.5%, 43.5%), which would not meet the threshold observed in the aforementioned study. However, all this is not to say that the denser rural areas would not see a protective effect.
Another possibility to explain lack of protection in rural communities is insecticide resistance. Looking at the bed net use in our population, 96.6% of individuals who did use a bed net used one of the three brands— Olyset, Permanet, and Basf Net—all of which use the pyrethroid class of insecticides, specifically permethrin, deltamethrin, and alphacypermethrin, respectively.24–26 Resistance has been spreading across West Africa7,27–29 and there has been confirmed resistance of the Anopheles gambiae mosquitos to Pyrethroids;30 thus, it would make sense that this spreading resistance would affect the effectiveness of community-wide usage. However, resistance appears widespread throughout Liberia, with a discrepancy between the Greater Monrovia area, where almost half of our urban communities are clustered, and the rest of the country. Therefore, although perhaps insecticide resistance may contribute to the lack of protection in rural communities, it cannot be contributing to the discrepancy of effect between rural and urban communities.
The results from this analysis have implications for the policy surrounding malaria control, prevention, and elimination. Our results indicate that bed nets are particularly effective in urban areas, above and beyond individual use, whereas community-wide use provides no protection beyond the individual in rural areas, likely because of population density factors. Therefore, although all control efforts should be multifaceted, promotion of bed net use in urban areas will likely be a highly effective tool for control, whereas in rural areas, it might be most effective to include bed net use in combination with many other tools that already exist or are in development.
Two major strengths of our study are its generalizability to all children less than 5 years old in Liberia and that it is one of very few in-the-field assessments of effectiveness. Our data source reflects conditions that exist in the communities that are most in need of malaria prevention and reflects what happens with net usage and malaria infection in practice. A 2017 study by Tusting et al.31 using DHS and MIS data from several African countries, that is, in-the-field assessments, found that children living in improved housing were less likely to test positive for malaria, by both RDT and microscopy, when controlling for LLIN use, but found that individually LLIN use resulted in a larger reduction in odds of malaria infection than improved housing. Although the Tusting et al.31 article did not look at community-level bed net use, the results highlight the multifaceted nature of optimal malaria prevention. However, our study also has several limitations. First and foremost, this is a cross-sectional study, so we cannot make inferences regarding causality. The ideal study to investigate protective effects of bed nets would be a longitudinal study comparing malaria transmission before and after introduction, but while maintaining realistic community conditions, we did not have access to such data. More importantly, our results may be subject to reverse causality. Those living in rural communities, areas which tend to have higher transmission of malaria, may experience higher bed net usage because they know they are at a higher risk of malaria infection. Unfortunately, we cannot account for this analytically.
Another limitation is the potential measurement error. Both our exposure and outcome are subject to this and both may therefore bias our results. Bed net use was reported by the mother, and although the researchers noted whether they actually saw a bed net, this reported value may be subject to an interviewer bias in which the mother wants to appear more compliant with prevention recommendations that is accurate. The reported value also does not tell us with perfect accuracy whether the child actually slept under the bed net the previous night (as the question was phrased) or whether the child regularly sleeps under a bed net, which would be more representative of the exposure of interest. Furthermore, we do not know the total population of each community, so we cannot estimate the proportion of community members sleeping under bed nets. Rather, our denominator is just the number of children less than 5 years in each community, which limits our interpretation. Although we know that the effectiveness of the insecticides wanes after several years and we have not taken into account how long ago each net was purchased, we do know that the vast majority of bed nets (83.8%) were obtained within the past 3 years. Another source of measurement error is our outcome. We used the RDT results as our measure of parasitemia, but RDTs do not have perfect sensitivity and specificity. Although the 2011 Liberia MIS has both RDT and microscopy results for malaria diagnosis, we chose to use the RDT results in our analysis. Although many comparisons consider microscopy the gold standard, it does not clearly have better diagnostic accuracy than RDTs. In an analysis of malaria results from the Democratic Republic of the Congo, which considered polymerase chain reaction as the gold standard, microscopy had a higher specificity (95.1%, compared with 86.0% for RDT), but RDTs had a higher sensitivity (71.6%, compared with 61.6% for microscopy).32 Another study found RDT results equally specific but slightly more sensitive than microscopy results.33 Because microscopy results were also available in the data, we repeated our analyses with malaria parasitemia measured by microscopy as the result (see Supplemental Table 1). This analysis showed very similar results with community bed net use for both the overall relationship and stratified rural relationship, and a slightly attenuated association within the urban communities. They also indicated a small but protective effect of individual bed net use, unlike our RDT results. There might be within-cluster variation in the outcome because there is microheterogeneity in malaria risk and therefore prevalence. We, however, do not have within-DHS-cluster spatial data; thus, it is a limitation of the study. Finally, there will be some bias due to unmeasured confounding. Conducting a sensitivity analysis of our results with microscopy results as our outcome could provide insight into bias resulting from this measurement error.
In summary, LLINs had a community protective effect in urban areas of Liberia, but not rural areas. Further research is needed to determine whether this is due to population density, insecticide resistance, or other causes.
Supplementary Material
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.World Health Organization , 2015. Liberia: WHO Statistical Profile, 1–3. Available at: http://www.who.int/gho/countries/lbr.pdf?ua=1. Accessed March 2, 2017.
- 2.Lori JR, Boyle JS, 2015. Force migration: refugee populations. Nurs Outlook 63: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization , 2003. Liberia: Health Situation Analysis Final Report July 2002–November 2003 Available at: http://www.who.int/disasters/repo/11404.pdf. Accessed March 28, 2017.
- 4.Chothia F, 2014. Ebola Drains Already Weak West African Health Systems Available at: http://www.bbc.com/news/world-africa-29324595. Accessed March 9, 2017.
- 5.Mutabingwa TK, 2005. Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop 95: 305–315. [DOI] [PubMed] [Google Scholar]
- 6.Liu N, 2015. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol 60: 537–559. [DOI] [PubMed] [Google Scholar]
- 7.Temu EA, et al. 2012. Pyrethroid resistance in Anopheles gambiae, in Bomi County, Liberia, compromises malaria vector control. PLoS One 7: e44986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agossa FR, Gnanguenon V, Anagonou R, Azondekon R, Aízoun N, Sovi A, Oké-Agbo F, Sèzonlin M, Akogbéto MC, 2015. Impact of insecticide resistance on the effectiveness of pyrethroid-based malaria vectors control tools in Benin: decreased toxicity and repellent effect. PLoS One 10: e0145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.N’Guessan R, Corbel V, Akogbéto M, Rowland M, 2007. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis 13: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toé KH, Jones CM, N’Fale S, Ismail HM, Dabiré RK, Ranson H, 2014. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg Infect Dis 20: 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization , 2008. World Malaria Report 2008 Geneva, Switzerland: World Health Organization, 6–14. ISBN 978 92 4 1564403. [Google Scholar]
- 12.Roll Back Malaria Partnership (WHO) , 2008. The global malaria action plan for a malaria free world. Director 274 Available at: http://www.unhcr.org/4afac5629.pdf. Accessed March 2, 2017. [Google Scholar]
- 13.Lengeler C, 2004. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev CD000363. [DOI] [PubMed] [Google Scholar]
- 14.Agusto FB, Del Valle SY, Blayneh KW, Ngonghala CN, Goncalves MJ, Li N, Zhao R, Gong H, 2013. The impact of bed-net use on malaria prevalence. J Theor Biol 320: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdella YM, Deribew A, Kassahun W, 2009. Does insecticide treated mosquito nets (ITNs) prevent clinical malaria in children aged between 6 and 59 months under program setting? J Community Health 34: 102–112. [DOI] [PubMed] [Google Scholar]
- 16.Louis VR, et al. 2015. An insecticide-treated bed-net campaign and childhood malaria in Burkina Faso. Bull World Health Organ 93: 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention , 2015. Insecticide-Treated Bed Nets Available at: https://www.cdc.gov/malaria/malaria_worldwide/reduction/itn.html. Accessed March 17, 2017.
- 18.Hakizimana E, Cyubahiro B, Rukundo A, Kabayiza A, Mutabazi A, Beach R, Patel R, Tongren JE, Karema C, 2014. Monitoring long-lasting insecticidal net (LLIN) durability to validate net serviceable life assumptions, in Rwanda. Malar J 13: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messina JP, Taylor SM, Meshnick SR, Linke AM, Tshefu AK, Atua B, Mwandagalirwa K, Emch M, 2011. Population, behavioural and environmental drivers of malaria prevalence in the Democratic Republic of Congo. Malar J 10: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escamilla V, et al. 2017. Effects of community-level bed net coverage on malaria morbidity in Lilongwe, Malawi. Malar J 16: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen DA, Hutchinson P, Bennett A, Yukich J, Anglewicz P, Keating J, Eisele TP, 2014. Community coverage with insecticide-treated mosquito nets and observed associations with all-cause child mortality and malaria parasite infections. Am J Trop Med Hyg 91: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atieli HE, Zhou G, Afrane Y, Lee M-C, Mwanzo I, Githeko AK, Yan G, 2011. Insecticide-treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya. Parasit Vectors 4: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Malaria Control Program , 2012. Liberia Malaria Indicator Survey 2011 Available at: https://dhsprogram.com/publications/publication-MIS12-MIS-Final-Reports.cfm. Accessed May 5, 2016.
- 24.Sumitomo Chemical Environmental Health Division , 2017. Olyset Net sumivector.com. Available at: http://sumivector.com/mosquito-nets/olyset-net. Accessed March 14, 2017.
- 25.Vestergaard , 2014. Permanet 2.0 Vestergaard.com. Available at: http://www.vestergaard.com/permanet-2-0. Accessed March 14, 2017.
- 26.Basf , 2017. Interceptor Long-Lasting Insecticidal Nets Basf.com. Available at: https://agriculture.basf.com/en/Pest-Control/Interceptor.html. Accessed March 14, 2017.
- 27.Gnankiné O, Bassolé IHN, Chandre F, Glitho I, Akogbeto M, Dabiré RK, Martin T, 2013. Insecticide resistance in Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) and Anopheles gambiae Giles (Diptera: Culicidae) could compromise the sustainability of malaria vector control strategies in West Africa. Acta Trop 128: 7–17. [DOI] [PubMed] [Google Scholar]
- 28.Hunt RH, Fuseini G, Knowles S, Stiles-Ocran J, Verster R, Kaiser ML, Choi K, Koekemoer LL, Coetzee M, 2011. Insecticide resistance in malaria vector mosquitoes at four localities in Ghana, West Africa. Parasit Vectors 4: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namountougou M, Simard F, Baldet T, Diabaté A, Ouédraogo JB, Martin T, Dabiré RK, 2012. Multiple insecticide resistance in Anopheles gambiae s.l. populations from Burkina Faso, West Africa. PLoS One 7: e48412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vestergaard, KEMRI/CDC, ESRI, IR Mapper , 2016. IR Mapper Available at: www.irmapper.com. Accessed March 12, 2017.
- 31.Tusting LS, Bottomley C, Gibson H, Kleinschmidt I, Tatem AJ, Lindsay SW, Gething PW, 2017. Housing improvements and malaria risk in sub-Saharan Africa: a multi-country analysis of survey data. PLoS Med 14: e1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doctor SM, et al. 2016. Malaria surveillance in the Democratic Republic of the Congo: comparison of microscopy, PCR, and rapid diagnostic test. Diagn Microbiol Infect Dis 85: 16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azikiwe CCA, Ifezulike CC, Siminialayi IM, Amazu LU, Enye JC, Nwakwunite OE, 2012. A comparative laboratory diagnosis of malaria: microscopy versus rapid diagnostic test kits. Asian Pac J Trop Biomed 2: 307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.