Abstract.
Severe congenital malaria associated with Plasmodium vivax is uncommon. In Indonesia, most congenital malaria cases are due to Plasmodium falciparum infections. Most cases of congenital or neonatal malaria in endemic areas are diagnosed from peripheral smear as part of routine sepsis workup. Differentiating congenital and acquired neonatal malaria is very difficult. The case presented in this study describes severe P. vivax malaria with cholestatic jaundice and sepsis-like signs and symptoms in neonates. The mother was asymptomatic and the neonate was successfully treated with intravenous artesunate. Severe P. vivax malaria with cholestatic jaundice in neonates is an uncommon condition that should be included in the differential diagnosis of infants displaying hemolytic anemia, thrombocytopenia, cholestatic jaundice, and hepatosplenomegaly in malaria-endemic zones. Early diagnosis can prevent the use of unnecessary antibiotics and mortality of neonates.
INTRODUCTION
Plasmodium sp. is often transmitted by anopheles mosquitoes, but can also be transmitted from mother to child, causing congenital malaria. The first research by Covell and Bruce-Chwart in the middle of the twentieth century reported congenital malaria incidents of 0.18–0.3% in newborns with malaria-infected mothers during pregnancy in malaria hyperendemic areas, and only 300 sporadic cases were reported in the world according to the literature 50 years after that first report.1–3
Congenital malaria symptoms are not specific and often misdiagnosed as neonatal sepsis.2,4 The symptoms can occur 2–8 weeks after birth, including fever, anorexia, lethargy, anemia, jaundice, and hepatosplenomegaly.1,5–7 Congenital malaria can be caused by Plasmodium falciparum and Plasmodium vivax.7–9 Severe congenital malaria cases associated with P. vivax are uncommon. In Indonesia, most malaria cases are caused by P. falciparum.10 According to the World Health Organization (WHO), liver dysfunction is an uncommon occurrence in malaria.8 Malarial hepatitis is uncommon in P. vivax infections.5,11 Jaundice caused by malaria infection has 0–9% frequency.8
The case presented in this study describes a severe P. vivax malaria infection with intrahepatic cholestatic jaundice found during routine sepsis workups in a neonate.
CASE
A 47-day-old male infant was brought to Prof. Dr. R. D. Kandou General Hospital Manado with jaundice since 7 days of age and high fever since 3 days before admission. The infant was pale and had a swollen stomach. The infant was born at home with a gestational age of 35 weeks and an undocumented birth weight. He was breastfed exclusively since birth. On admission, the infant was febrile (38.3°C), pale, and jaundiced. There was liver and spleen enlargement and no ascites was found.
Laboratory findings showed hemoglobin 4.4 g/dL, hematocrit 13.1%, white blood cell count 8.868 k/mm3, platelet count 95,000/mm3, total bilirubin 24.63 mg/dL, direct bilirubin 18.58 mg/dL, serum glutamic oxaloacetic transaminase (SGOT) 284 U/L, and serum glutamic pyruvic transaminase (SGPT) 154 U/L. Anti-hepatitis A Virus immunoglobulin M (IgM) and immunoglobulin G (IgG), hepatitis B surface antigen, anti-hepatitis C virus antibody, anti-toxoplasma IgM and IgG, and anti-cytomegalovirus IgM and IgG were negative. Serial stool examinations were acholic in appearance. Sepsis workup was completed, and the patient was given a broad-spectrum antibiotic injection.
Blood and urine cultures were negative. Blood smear showed normocytic, normochromic, anisocytosis, and polychromatophilic anemia, and P. vivax (+) trophozoites. Thick and thin blood film examination revealed P. vivax trophozoites with parasite count 800 parasites/μL. Abdominal ultrasound revealed intrahepatic cholestatic hepatocytes (hepatitis malaria). Because of the proven malaria infection, lumbar puncture was not performed.
Although asymptomatic, P. vivax parasitemia was found in the mother from peripheral blood smear. Based on this finding, the infant was diagnosed with severe congenital P. vivax malaria with cholestatic jaundice. Artesunate injection (2.4 mg/kg BW/dose), blood transfusion (10 mL/kg twice), and ursodeoxycholic acid (20 mg per 12 hours orally) were given. Further blood tests showed that hemoglobin increased to 9.9 g/dL. After 7 days of treatment, the condition of the infant improved. Fever, icterus, and hepatosplenomegaly subsided. After three repeated peripheral smear examinations that showed no malarial parasites, the baby was discharged in good clinical condition.
DISCUSSION
Vertical transmission of malaria in a neonate is suspected if symptoms occurred within 7–30 days after birth and asexual parasites are found in the neonate’s umbilical or peripheral blood.4,12–14 This infant started to have jaundice 7 days after birth and fever occurred at the age of 1.5 months. The interval between the birth and the onset of symptoms may be prolonged. This can be explained by the transmission of infection in late pregnancy or during delivery. This can also be caused by transplacentally acquired maternal antibody (IgG). Transfer of protective immunity is, thus, one of the main factors that affects the age of symptom onset. Other literature usually describe the onset of symptoms of congenital malaria typically between 3 and 12 weeks after birth, coinciding with the half-life of maternal IgG antibody in infants, but possibly as late as 21 weeks, based on a review of 82 cases from 1966 to 2005 by Lesko et al.15 Exclusive breastfeeding in these infants might also play a role in limiting the parasite growth through low iron levels and low amino acid and para-benzoic acid levels in breast milk.2 This case is an example of high-risk congenital malaria because the mother was infected with malaria, the socioeconomic level was low, and the mother was primigravida.3,9,12,14,15 His parents used insecticidal nets when the baby was sleeping to prevent mosquito bites. The diagnosis of congenital malaria was not established because neonatal malaria cannot be excluded in this case. Acquired neonatal malaria is due to an infective mosquito bite after birth. Congenital malaria is defined as the presence of Plasmodium asexual stages in a newborn’s cord during the first week of life. The difficulty to differentiate between congenital and acquired P. vivax malaria, especially if it happened after 2 weeks of life, is because of the incubation period of P. vivax malaria.
Severe malaria cases reported to occur during the first few months of life are still rare, but P. falciparum–related cases have been reported in Papua, Indonesia.16 In Papua, congenital P. vivax infections occur in every 1.6 of 1,000 births.2 The mechanisms of transmission of congenital malaria are mother-to-child transmission during birth or pregnancy, direct penetration via chorionic villi , or penetration through premature rupture of membrane.4,9,14,17
Even in older children, the clinical symptoms of severe malaria can be difficult to differentiate from those of severe sepsis.2,9 Malaria parasite tests must be included in routine workups for infants with fever and potential sepsis in malaria-endemic areas. Samples from neonates with severe illness and parasitemia must be taken for blood cultures, and newborns with such symptoms were given antibiotics and antimalarial drugs in some cases.1,2 In this case, the blood culture was negative.
Clinical symptoms of congenital malaria are not specific. The presence of fever, anemia, and splenomegaly might indicate congenital malaria.5,9,11,14 This patient was suffering from fever, severe anemia, thrombocytopenia, icterus, and hepatosplenomegaly. Anemia in P. vivax infection in this case resulted from intravascular and extravascular hemolysis (loss of 34 noninfected cells for one infected cell, lower parasitemia) and impaired red blood cell (RBC) production (dyserythropoiesis and bone marrow insufficiency). Thrombocytopenia occurred because of the bone marrow suppression that often happens in malaria cases.18 According to Poespoprodjo et al.,19 infection with P. vivax was associated with a greater risk of severe anemia (odds ratio [OR] = 2.4; 95% confidence interval [CI]: 1.03–5.91; P = 0.041) and severe thrombocytopenia (OR = 3.3; 95% CI: 1.07–10.6; P = 0.036) than P. falciparum infections.
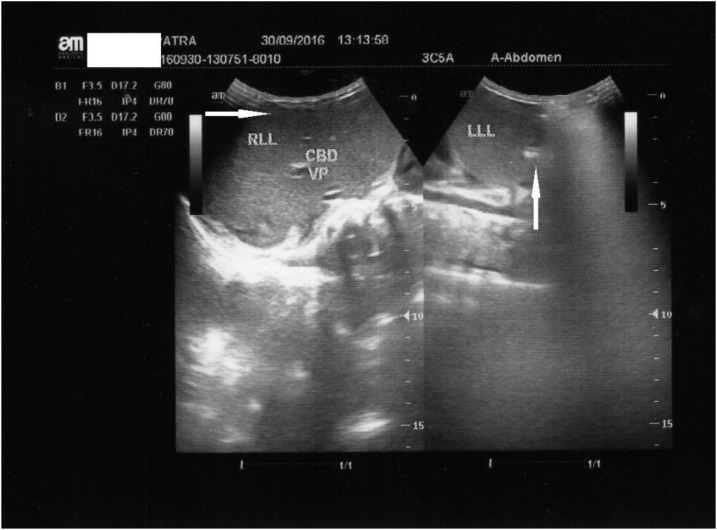
WHO suggested that jaundice is an important manifestation of severe malaria.2,8 There are four pathophysiologies of jaundice: increased breakdown of red cells, disorders of transportation of unconjugated bilirubin, and disorders of uptake, conjugation, and secretion of bilirubin. Jaundice in malaria is usually caused by massive hemolysis (Plasmodium damages erythrocytes). In this patient, jaundice was unusual, more severe, complicated, and had multiple causes. The etiologies were massive hemolysis, malarial hepatitis, and cholestasis. Massive hemolysis caused severe anemia. Malarial hepatitis was observed using abdominal ultrasound (see Figure 1).
Figure 1.
Abdominal ultrasound demonstrated hepatomegaly, decreased echogenicity of the liver, and sharp margins (malarial hepatitis).
The liver is involved in malaria at two stages: during the preerythrocytic cycle and the erythrocytic phase. The first step is linked to the binding of the merozoite circumsporozoite protein-A and the thrombospondin-related adhesive protein to the hepatocytes via heparan sulfate glycosaminoglycans, promoting minimal liver damage. In the erythrocytic phase, jaundice is a common remark and it is directly caused by the infection. Intravascular hemolysis of parasitized and nonparasitized RBCs causes an increase in unconjugated bilirubinemia with mild to moderate jaundice; conjugated hyperbilirubinemia indicates hepatocyte dysfunction. The pathogenesis of hepatic dysfunction is not completely known. Reduction in portal venous flow as a consequence of micro-occlusion of portal venous branches by parasitized erythrocytes; intrahepatic cholestasis due to reticuloendothelial blockage and hepatic microvili dysfunction; suppression of bilirubin excretion due to the effect of parasitemia or endotoxinemia or metabolic acidosis; apoptosis; and oxidative stress are all mechanisms involved in hepatic damage.20
Malarial hepatitis is characterized by a rise in serum bilirubin along with the rise in serum glutamate pyruvate transaminase levels to more than three times the upper limit of normal. This is in the absence of clinical or serological evidence of viral hepatitis.21–23 Laboratory findings of the infant showed a rise in SGOT and SGPT serum levels more than five times the upper limit of normal and other causes of jaundice have been excluded. There was no hepatitis, toxoplasmosis, other (syphilis, varicella-zoster, parvovirus B19), rubella, cytomegalovirus, and herpes infections, human immunodeficiency virus, septicemia, urinary tract infection, or hypothyroidism. Diagnosis of malaria is determined by the identification of organisms by Giemsa staining of thick and thin blood smears.12,14
Cholestasis represents an impairment in bile flow and may be caused by either intrahepatic or extrahepatic disorders. Nonconjugated hyperbilirubinemia is often observed in malaria patients, but conjugated hyperbilirubinemia is rare.8 This suggests a more severe form of malaria and is related to higher complication.8,24 Cholestasis in this patient is shown by increased conjugated bilirubin and acholia in three serial stool examinations.
The medicine of choice for congenital malaria is chloroquine.1 However, there were many chloroquine-resistant cases found in Indonesia, and the recommended treatment of severe malaria is parenteral artesunate.2,25 Antibiotics must be administered when bacteremia cannot be excluded. Researchers from China compared the effectiveness of artesunate and quinine for congenital malaria treatment. Artesunate effectiveness is 92.31% whereas that for quinine is 83.33%. The clearance levels from Plasmodium for artesunate and quinine are 92.31% and 78.57%, respectively. Therefore, artesunate is the first choice for severe congenital malaria treatment.14,25,26 Ursodeoxycholic acid is given to treat cholestatic jaundice.
In our case, Plasmodium sp. was found during the peripheral blood smear test, emphasizing the importance of peripheral blood smear in all cases of neonatal sepsis. Early diagnosis can prevent the use of unnecessary antibiotics and mortality of neonates.9
CONCLUSION
Severe congenital P. vivax malaria should be included in the differential diagnosis of infants displaying signs and symptoms of sepsis with hemolytic anemia, thrombocytopenia, cholestatic jaundice, and hepatosplenomegaly in malaria-endemic areas, even though P. vivax infection in neonates cannot be excluded. Early diagnosis can prevent the use of unnecessary antibiotics and mortality of neonates.
Acknowledgments:
We thank the patient who participated in this study.
REFERENCES
- 1.Raza F, Shariq Y, Iqbal Z, Aghai S, Aziz S, 2017. Congenital malaria in a neonate: a rare condition. ASH KMDC 22: 71–74. [Google Scholar]
- 2.World Health Organization , 2014. Severe malaria. Trop Med Int Health 19 (Suppl 1): 7–131. [DOI] [PubMed] [Google Scholar]
- 3.Pineros-Jimenez JG, Alvarez G, Tobon A, Arboleda M, Carrero S, Blair S, 2011. Congenital malaria in Uraba, Colombia. Malar J 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia R, Rajwaniya D, Agrawal P, 2016. Case Report: Congenital Malaria due to Plasmodium vivax Infection in a Neonate Udaipur, India: Department of Pediatrics, Pacific Medical College and Hospital, Hindawi Publishing Corporation, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagri DR, Mathur P, 2016. P. vivax malaria: a rare cause of cholestatic jaundice in a neonate. Sch J Med Case Rep 4: 315–317. [Google Scholar]
- 6.Thapar RK, Saxena A, Devgan A, 2008. Congenital malaria. Med J Armed Forces India 64: 185–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens SCM, Rutgers MM, de Winter JP, Sloot SC, Jager MM, Mank TG, 2017. Congenital Plasmodium vivax malaria in a non-endemic country; a unique case in the Netherlands. Pediatric Infect Dis 2: 33–35. [Google Scholar]
- 8.Jain A, Kaushik R, Kaushik RM, 2016. Malarial hepatopathy: clinical profile and association with other malarial complications. Acta Trop 159: 95–105. [DOI] [PubMed] [Google Scholar]
- 9.Punta VD, Gulleta M, Matteelli A, Spinoni V, Regazzoli A, Castelli F, 2010. Congenital Plasmodium vivax malaria mimicking neonatal sepsis: a case report. Malar J 9: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elyazar IR, Gething PW, Patil AP, Rogayah H, Kusriastuti R, Wismarini DM, Tarmizi SN, Baird JK, Hay SI, 2011. Plasmodium falciparum malaria endemicity in Indonesia in 2010. PLoS One 6: e21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varma S, Bhatt AS, 2016. Unusual clinical presentation of congenital malaria: cholestasis in newborn. Adv Res Gastroentero Hepatol 1: 1–3. [Google Scholar]
- 12.Naniche D, et al. 2012. Reduction of antimalarial antibodies by HIV infection is associated with increased risk of Plasmodium falciparum cord blood infection. J Infect Dis 205: 568–577. [DOI] [PubMed] [Google Scholar]
- 13.Moya-Alvarez V, Abellana R, Cot M, 2014. Pregnancy-associated malaria in infants: an old problem with present consequences. Malar J 13: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uneke CJ, 2011. Congenital malaria: an overview. Tanzan J Health Res 13: 1–18.24409640 [Google Scholar]
- 15.Lesko CR, Arguin PM, Newman RD, 2007. Congenital malaria in the United States: a review of cases from 1966 to 2005. Arch Pediatr Adolesc Med 161: 1062–1067. [DOI] [PubMed] [Google Scholar]
- 16.Poespoprodjo JR, Hasanuddin A, Fobia W, Sugiarto P, Kenangalem E, Lampah DA, Tjitra E, Price RN, Anstey NM, 2010. Case report: severe congenital malaria acquired in utero. Am J Trop Med Hyg 82: 563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Tao ZY, Fang Q, Wang XM, Zhang H, Stoute JA, Xia H, Cui L, 2012. A case of congenital Plasmodium vivax malaria from a temperate region in Central China. Malar J 11: 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas NM, Anstey NM, Buffet PA, Poespoprodjo JR, Yeo TW, White NJ, Price RN, 2012. The anaemia of Plasmodium vivax malaria. Malar J 11: 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Hasanuddin A, Warikar N, Sugiarto P, Tjitra E, Anstey NM, Price RN, 2009. Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis 48: 1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Autino B, Corbett Y, Castelli F, Taramelli D, 2012. Pathogenesis of malaria in tissues and blood. Mediterr J Hematol Infect Dis 4: e2012061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhalla A, Suri V, Singh V, 2006. Malarial hepatopathy. J Postgrad Med 52: 315–320. [PubMed] [Google Scholar]
- 22.Kochar DK, Singh P, Agarwal P, Kochar SK, Pokharna R, Sareen PK, 2003. Malarial hepatitis. J Assoc Physicians India 51: 1069–1072. [PubMed] [Google Scholar]
- 23.Gathwala G, Dalal P, Gupta M, 2015. Congenital malaria with atypical presentation: a series of three case reports. J Clin Neonatol 4: 206–208. [Google Scholar]
- 24.Feldman AG, Sokol RJ, 2013. Neonatal cholestasis. Neoreviews 14: pii: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization , 2015. Guidelines for the Treatment of Malaria, 3rd edition. Geneva, Switzerland: World Health Organization Global Malaria Programme. [Google Scholar]
- 26.Maestre A, Carmona-Fonseca J, 2014. Immune responses during gestational malaria: a review of the current knowledge and future trend of research. J Infect Dev Ctries 8: 391–402. [DOI] [PubMed] [Google Scholar]