Abstract
Background
Brain training games (BTG) are believed to play a major role in improving cognitive functions. The current study evaluated if BTG showed positive impact on attention and memory functions compared with baseline visit in healthy subjects.
Material/Methods
The study was carried out from October 2015 until April 2016 in the Department of Physiology, College of Medicine, King Saud University and in King Khalid University Hospital (KKUH), Riyadh, Saudi Arabia.
We enrolled 51 normal healthy subjects to use a computerized cognitive training game (Lumosity) for exercises that target a range of cognitive functions, including attention, processing speed, visual memory, and executive functions for about 15 min per day, at least 7 days per week, for 3 weeks. The control (n=21) group did not perform the training. Both groups took the CANTAB test before and 3 weeks after training for various cognitive functions (flexibility, memory, attention, speed, and problem solving). Serum samples were used to study the brain-derived growth factor (BDNF) and apolipoprotein (Apo) E (APOE) levels.
Results
A significant improvement in Lumosity performance index was observed in the active group compared to the control group by the end of training (p-value 0.001). After the training, a statistically significant difference in most of the CANTAB measures, such as attention-switching task (AST), mean correct latency, AST switching cost, AST mean correct latency (congruent), AST mean correct latency (incongruent), AST mean correct latency (blocks 3 and 5) [non-switching blocks], AST mean correct latency (block 7) [switching block], and MOT mean correct latency (all P=0.000). However, in the control group, significant improvements were not observed. A positive correlation between pattern recognition memory (PRM) and APOE was found and people who had higher ApoE levels had faster response.
Conclusions
An improvement in different cognitive domains was noted, including attention and motor speed. However, this study warrants further research to determine the long-term effect on other cognitive functions and in different groups (e.g., elderly vs. adults).
MeSH Keywords: Attention; Cognition; Memory, Long-Term
Background
During the progress of normal aging, alterations occur in the prefrontal cortex and the medial temporal lobe system, including the hippocampus and the cerebellum [1,2]. These changes are linked to Alzheimer’s disease (AD) and dementia and are linked with impairment in cognitive processes such as short- and long-term memory, speed, and executive functions. Thus, cognitive functions, including attention, memory, and analytical ability, show a functional decrease with age [3–5].
An effective cognitive aging intervention faces 2 challenges: (1) shifting training improvements to untrained tasks and (2) designing interventions that boost compliance. A major research focus is to find new technology to understand aging and delay the process of cognitive decline [2]. Recent studies increasingly use brain training games (BTG) to investigate their impact on cognition and possibility of transfer to untrained tasks [6,7]. Indeed, the term “brain training” has become used to describe cognitively stimulating activities designed to improve mental fitness [8].
Many BTG intervention studies have reported increased performance in cognitive tasks such as speed and accuracy, visuo-motor coordination, attention, memory, working memory, and global cognitive function [9–15]. However, other studies showed no positive effects of BTG on cognitive functions and question the application of such systems as cognitive intervention strategies [16,17]. These findings, although not consistent across all studies, suggest that BTG is an effective intervention tool for cognitive improvement.
In addition to the above, some studies have reported that apolipoprotein E (APOE) and brain-derived neurotropic factor (BDNF) could be play a major role in the development of cognitive functions. While APOE has been associated with risk for late-onset AD, its role the impairment of healthy cognitive function is still unclear [18,19]. The neurotrophin BDNF has the ability to develop and maintain neuronal function and is important for neuronal plasticity. BDNF has been reported to facilitate long-term potentiation of the hippocampus and cortex, which are important processes for memory and learning [19,20].
In the present study, we assessed whether BTG could improve the cognitive abilities (e.g., AST and MOT) compared to baseline in a group of healthy subjects using a broad battery of Cambridge Neuropsychological Test Automated Battery (CANTAB) assessments [21]. In addition, assessments of BDNF and ApoE levels and their correlation with cognitive functions were evaluated. To the best of our knowledge, this is the first study combining BTG and its correlation with APOE ɛ4 and BDNF plasma levels, especially with reference to improvement of cognitive functions.
Material and Methods
Participants
This was a randomized, observational, non-blinded study. All participants were healthy volunteers. After the interview, clinical history-taking and examination were performed. We divided the participants randomly to either the active group or the control group. We included all participants who were willing to complete the 3-week training period. We excluded any participant who could not complete the training or who missed sessions using the BTG in between the training sessions. All subjects knew which group they were in (active vs. control). The study was carried out from October 2015 until April 2016 in the Physiology Department, College of Medicine, King Saud University and in King Khalid University Hospital (KKUH), Riyadh, Saudi Arabia. The research protocol was approved by the Institutional Review Board, College of Medicine and KKUH. The Mini-Mental State Examination (MMSE; Folstein et al., 1975) was used on all subjects [22]. Exclusion criteria were a cognitive impairment (score of <26 on the MMSE) diagnosis of dementia, depression, less than 20/60 vision with or without correction, or current plans to move to another city.
Brain training application (Lumosity)
Lumosity is a brain training application which is available in IOS and Google play. We used this app because it’s easy to access and use. All participants performed the training for a period of 21 days for 10–30 min per day. In each training session, participants played the games Speed Match, Memory Matrix, Rotation Matrix, Face Memory, Money Comb, and Lost In Migration. The control group did not receive training.
Lumosity measured many aspects of cognitive function such as flexibility, memory, attention, speed, and problem solving. The average of these values is known as the Lumosity performance index (LPI). Baseline and follow-up neuropsychological testing were completed by a blinded psychometrician, and the study Principal Investigator was also blinded to group assignments.
A dedicated study technician (remote supervision) supported participants throughout the 3-week study following a protocol for routine weekly contact. Participants were provided with support through phone and email, with the goal of responding to all requests for support within 1 day.
Assessment tasks and procedures
Mini-mental state examination (MMSE)
The MMSE is one of the most widely used tools for quantitative assessment of cognitive function. The test consists of 11 questions assessing various cognitive functions, including 2 questions on orientation, 1 on registration, 1 on memory, 5 on language, 1 on attention and calculation, and 1 on visual construction [23]. The MMSE takes only 5–10 min to administer, which makes it a practical tool for research purposes.
The test has a maximum score of 30, with scores below 23 being indicative of cognitive impairment [24]. The standard MMSE form is in English for English-speaking subjects [23]. For Arabic speakers, a translated version of the MMSE used; this version was introduced in 1999 by a group of Saudi researchers [25].
Blood assays
Venous blood samples were collected from all participants, plasma was separated and stored at −70°C until assayed. Plasma levels of BDNF and APOE were measured by competitive enzyme immunoassay using Human BDNF and APOE ELISA kits following manufacturer’s instructions (Elab Sciences Biotechnology Co., Ltd. China). The detailed description of the procedure is described earlier [26].
Assessment of cognitive functions
Cognitive functions were evaluated by CANTAB to assess executive functions and memory before the participants started using the BTG Lumosity application and after they used it for 3 weeks for the active group. For the control group, evaluations were conducted at baseline and a retest was performed again after 3 weeks. We chose 3 tests from CANTAB: Pattern Recognition Memory (PRM), Motor Screening Task (MOT), and Attention-Switching Task (AST). A detailed description of the CANTAB procedure followed in this study is described elsewhere [27].
Statistical analysis
Data were analyzed using SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). We calculated errors, and numbers of trials and stages completed for the PRM task. The AST measured covers direction errors, proportion of successful stops, and MOT measured response time and the accuracy of pointing. Data was analyzed using the t test and χ2 tests to determine whether cognitive test scores changed within intervention or control groups. Spearman’s rank order correlation test was used to test for any correlation between enhancement of cognitive function after using BTG with the levels of BDNF and APOE. P values less than 0.05 were considered statistically significant.
Results
A total of 72 subjects participated in this study. The active group had 37 (61.42%) male and 14 (23%) female participants, with a mean age of 24.6±7.8 years. The control group consisted of 21 participants with a mean age of 25.4±8.3 years and had 6 females (24.9%). The demographic data of the subjects are summarized in Table 1. The difference in the numbers in the 2 groups at the end of the study was because of a compliance issue with some participants. All subjects who did not complete the 3-week assessment period in the active and control groups and subjects who did not play the game during the study period were excluded from the final data analyses.
Table 1.
Demographic table for active and control group.
| Active | Control | |
|---|---|---|
| Number of subject | 51 | 21 |
| Gender | Male: 37, Female: 14 | Male: 15, Female: 6 |
| Age | 24.6±7.8 | 25.4±8.3 |
| BMI | 25.1±5.27 | 27.4±4.94 |
| BMR | 6947.90±1606.92 | 7820.25±1282 |
| FAT% | 23±9.1 | 23.21±7.33 |
| FAT mass | 16.41±8.58 | 20.1±9.8 |
| FFM | 54.7±13.5 | 62±9.5 |
| TBW | 40.1±9.9 | 45.4±6.97 |
| MMSE | 29.4±0.66 | 29.2±0.63 |
There was significant improvement in performance in Lumosity performance index (LPI) at the end of the training compared to the first day in the active group (p-value 0.001; Figure 1). In the active group, a statistically significant difference was found in the active group after the training in terms of AST mean correct latency (Pre=599.6±164.2, Post=512.2±156.7, P=0.000, Figure 2; Table 2), AST Switching cost (Mean, correct) (Pre=247.6±180.8, Post=173.8±133.4, P=0.000), AST mean correct latency (congruent) (Pre=572.6±158, Post=484.4±144.4, P=0.000, Figure 3; Table 2), AST mean correct latency (incongruent) (Pre=629.3±174.7, Post=541.8±173.2, P=0.000, Figure 4; Table 2), AST mean correct latency (blocks 3,5) [non-switching blocks] (Pre=479.1±116.9, Post=426.6±114.3, P=0.000), AST mean correct latency (block 7) [switching block], (Pre=726.7±244.3, Post=600.4±214.2, P=0.000), and MOT mean correct latency (Pre=933.4±271.3, Post=761.4±209.1, P=0.000, Figure 5; Table 2).There was no significant difference for any of the CANTAB assessments in the control group (Table 2). There was no significant difference in PRM percent correct response and MOT mean error in both groups (Table 2).
Figure 1.
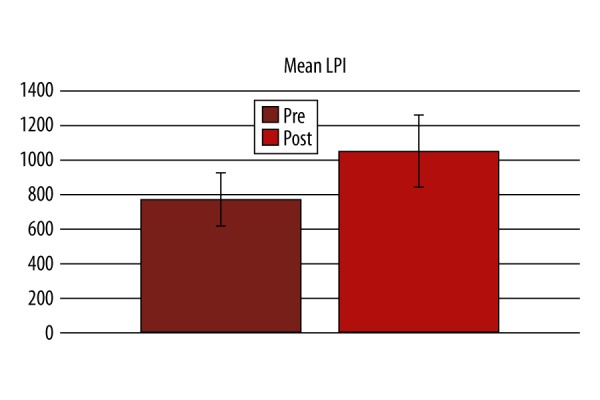
Change in Lumosity performance index (LPI) before and after the training.
Figure 2.
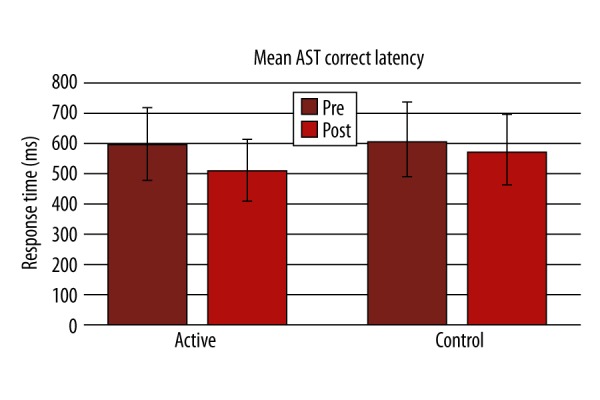
Change in mean attention-switching task correct latency before and after the training between active and control groups.
Table 2.
Cambridge Neuropsychological Test Automated Battery (CANTAB) data for active and control group.
| CANTAB data | ||||
|---|---|---|---|---|
| Active group | Control group | |||
| Pre | Post | Pre | Post | |
| AST Mean correct latency | 599.6±164.2 | 512.2±156.7*** | 615.2±132.9 | 581.3±147.1 |
| AST Congruency cost (Mean, correct) | 58.1±46.7 | 57.3±46.2 | 88.3±56.4 | 81.9±50.1 |
| AST Switching cost (Mean, correct) | 247.6±180.8 | 173.8±133.4*** | 252.3±134.7 | 188±133.6 |
| AST Mean correct latency (congruent) | 572.6±158 | 484.4±144.4*** | 573.5±120.7 | 548±130.3 |
| AST Mean correct latency (incongruent) | 629.3±174.7 | 541.8±173.2*** | 661.8±151.8 | 616.1±168.1 |
| AST Mean correct latency (blocks 3,5) [non-switching blocks] | 479.1±116.9 | 426.6±114.3*** | 487.1±80.3 | 489.2±109.9 |
| AST Mean correct latency (block 7) [switching block] | 726.7±244.3 | 600.4±214.2*** | 739.3±191.6 | 677.3±202.3 |
| AST Percent correct | 94.3±6.1 | 96.4±3.4 | 95.3±4.7 | 97.6±2.3 |
| MOT Mean correct latency | 933.4±271.3 | 761.4±209.1*** | 871.1±167.6 | 782.1±135 |
| MOT mean error | 11.5±3.1 | 11.6±2.96 | 11.3±3.5 | 11.3±3.4 |
| PRM Percent correct | 80.7±9.03 | 89.2±9.3 | 90.6±11.4 | 89.9±8.6 |
Figure 3.
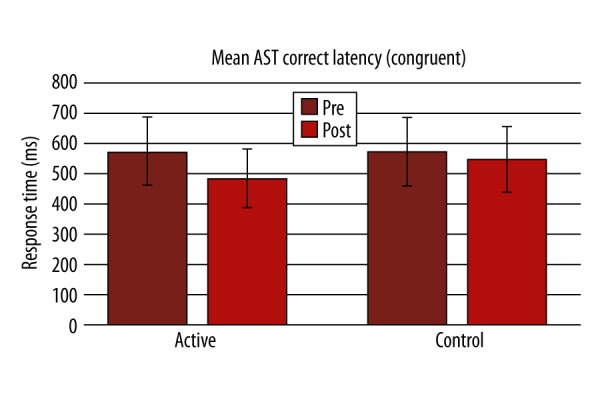
Change in mean attention-switching task correct latency (congruent) before and after the training between active and control groups.
Figure 4.
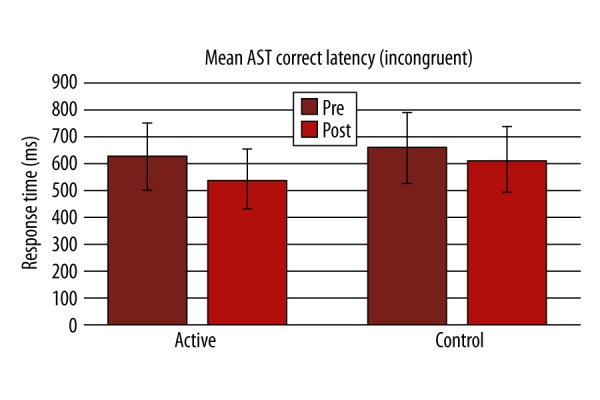
Change in mean attention-switching task correct latency (incongruent) before and after the training between active and control groups.
Figure 5.
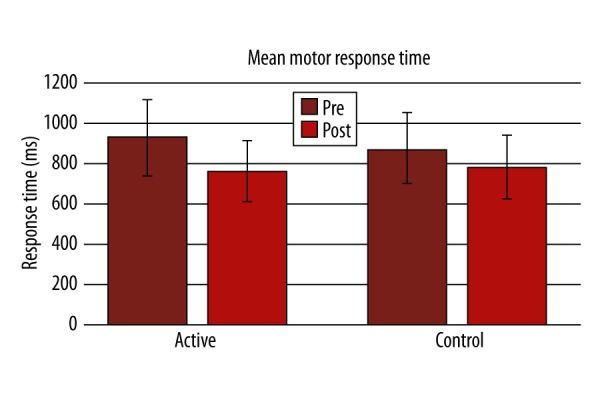
Change in mean motor response time before and after the training between active and control groups.
There was a negative Spearman correlation between MOT and APOE (Figure 6). As the MOT decreased, APOE increased and vice versa. There was a positive Spearman correlation between PRM and APOE (Figure 7). There was no significant difference for BDNF and cognitive marker in both groups.
Figure 6.
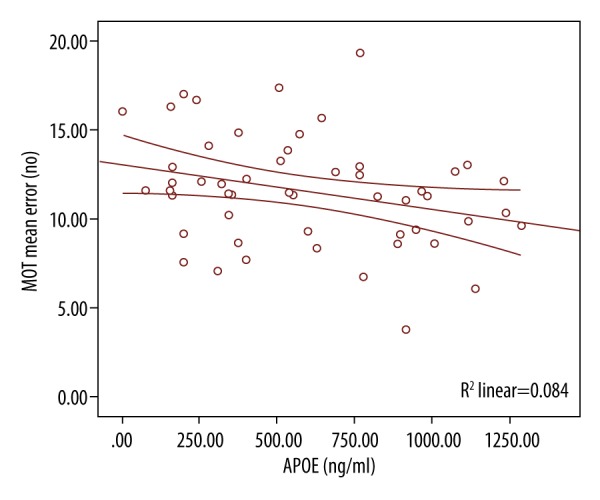
Spearman correlation between MOT and APOE.
Figure 7.
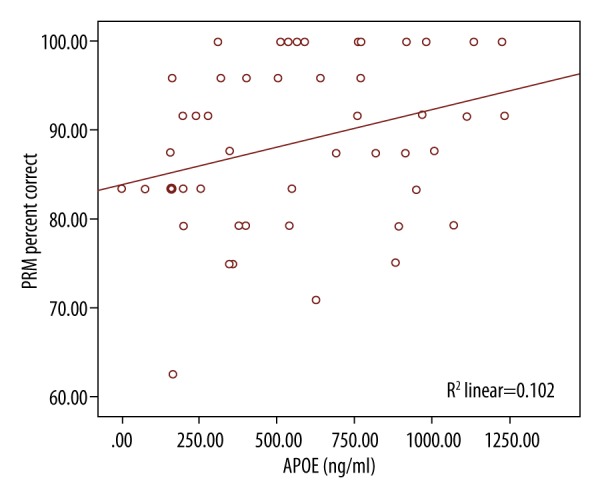
Spearman Correlation between PRM percent correct and APOE.
Discussion
This study was conducted to understand the role of the BTG application Lumosity in improving cognitive function in a randomly selected m population from Saudi Arabia. In addition, assessments of a probable correlation between blood markers APOE and BDNF with cognitive function were performed. The study results showed positive effects of the BTG Lumosity on cognitive functions in young adults. These findings are largely consistent with previous evidence showing improvement in cognitive functions after BTG [12,13]. BTGs were shown to improve executive functions and processing speed in elderly subjects [6]. Moreover, studies showed that BTG can improve the performance of several cognitive functions in the trained group but not in the control group in terms of speed accuracy [3,9], visuo-motor coordination [14], attention [13,14], memory [21], working memory [11], and global cognitive function [12].
Our study produced 2 main findings. Firstly, the trainees improved their game performance across sessions. The improvements in cognitive processes in executive functions, working memory, processing speed, and attention through BTG were closely related to the trained cognitive domains (flexibility, attention, speed). Secondly, the active group performed the AST tasks better than the control group. Our study found a negative correlation between MOT and APOE. As the MOT decreased, APOE increased and vice versa. Also, there was a positive correlation between PRM and APOE. However, there was no significant difference for BDNF and cognitive markers in both groups, although a few animal studies showed brain plasticity to be linked with BDNF [28–30]. Owing to the possible role of APOE and BDNF in modulating cognitive responses, further studies in larger populations may provide more clarity on the exact correlation between cognitive markers and these blood markers.
Although the present study provided interesting results with respect to cognitive improvement after implementation of BTG training, in addition to highlighting a possible association of BDNF and APOE with cognitive markers, it also has a few limitations. Regardless of the nature of remote testing benefits of BTG, there is also uncertainty around consistent administration. The training period of 3 weeks is not enough time to obtain the transfer effect, as BTGs do not change the difficulty of tasks depending on the participant’s performance. Secondly, an adaptive training method, which is more effective than a non-adaptive training for the enhancement of cognitive functions [13,14], was not used for BTG. In addition, further research is needed to explore the use of the same cognitive measures for long-term use of BTG, especially since a few studies have indicated that cognitive training confers long-term benefit in cognitive functions [12,31]. The sample size of our study was small and longer follow-up would be more useful in terms of knowing the long-term effects of BTG.
To get a preliminary understanding of the effect of BTG on cognitive function, we initially performed the study in young populations (the average age of our population was 25 years) and not in older age groups (60 years and above) or diseased patients (Alzheimer and degenerative disorders patients). In addition, the current study analyzed results within the active and passive control group and not between the 2 groups. This study design helped us to identify specific cognitive improvements after the training, whereas the control group showed no substantial improvements after the completion of 3-week training. While a few studies used both an active and a passive control group [12,13,32], some studies used a passive control group [12,31] to compare with the trained group.
Although the effectiveness of BTG will be more clinically relevant in the elderly and diseased patient populations, the elderly may not play the game every day due to aging and technology adaptation, which may affect the study results. However, because the study indicated that BTG influenced cognitive outcomes and longer follow-up, studies in the elderly population with data comparing between active and control groups are needed to confirm the association.
Conclusions
An improvement in various cognitive domains including attention and motor speed was observed after BTG in young adults. A correlation between blood markers such as APOE and BDNF with cognitive function was also established. The study results indicate that playing BTG may also improve cognitive functions and help reduce effects of aging in adults. However, further research is warranted to determine the long-term beneficial effect of BTG on cognitive functions in elderly patient populations. In spite of the deficiencies outlined, this is the first study to evaluate BTG in subjects from Saudi Arabia and provides significant results that pave the way for further clinical studies.
Footnotes
Source of support: Deanship of Scientific Research, King Saud University for the Undergraduate Student’s Research Support Program, project No. USRSP-17–13
References
- 1.Kelley BJ, Petersen RC. Alzheimer’s disease and mild cognitive impairment. Neurol Clin. 2007;25(3):577–609. v. doi: 10.1016/j.ncl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB, Counts SE, Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta. 2016;1862:860–68. doi: 10.1016/j.bbadis.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson EB, Kukull WA, Katzman RL. Cognitive impairment: dementia and Alzheimer’s disease. Annu Rev Public Health. 1992;13:431–49. doi: 10.1146/annurev.pu.13.050192.002243. [DOI] [PubMed] [Google Scholar]
- 4.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 5.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 6.Nouchi R, Taki Y, Takeuchi H, et al. Brain training game improves executive functions and processing speed in the elderly: A randomized controlled trial. PLoS One. 2012;7(1):e29676. doi: 10.1371/journal.pone.0029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anguera JA, Boccanfuso J, Rintoul JL, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen AM, Hampshire A, Grahn JA, et al. Putting brain training to the test. Nature. 2010;465(7299):775–78. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423(6939):534–37. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- 10.McDougall S, House B. Brain training in older adults: Evidence of transfer to memory span performance and pseudo-Matthew effects. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19(1–2):195–221. doi: 10.1080/13825585.2011.640656. [DOI] [PubMed] [Google Scholar]
- 11.Toril P, Reales JM, Ballesteros S. Video game training enhances cognition of older adults: A meta-analytic study. Psychol Aging. 2014;29(3):706–16. doi: 10.1037/a0037507. [DOI] [PubMed] [Google Scholar]
- 12.Janssen G, van Aken L, De Mey H, et al. Decline of executive function in a clinical population: age, psychopathology, and test performance on the Cambridge Neuropsychological Test Automated Battery (CANTAB) Appl Neuropsychol Adult. 2014;21(3):210–19. doi: 10.1080/09084282.2013.793191. [DOI] [PubMed] [Google Scholar]
- 13.Hill EL. Evaluating the theory of executive dysfunction in autism. Dev Rev. 2004;24(2):189–233. [Google Scholar]
- 14.Leckie RL, Weinstein AM, Hodzic JC, Erickson KI. Potential moderators of physical activity on brain health. J Aging Res. 2012;2012:948981. doi: 10.1155/2012/948981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11(8):342–48. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Ackerman PL, Kanfer R, Calderwood C. Use it or lose it? Wii brain exercise practice and reading for domain knowledge. Psychol Aging. 2010;25(4):753–66. doi: 10.1037/a0019277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redick TS, Shipstead Z, Harrison TL, et al. No evidence of intelligence improvement after working memory training: A randomized, placebo-controlled study. J Exp Psychol Gen. 2013;142(2):359–79. doi: 10.1037/a0029082. [DOI] [PubMed] [Google Scholar]
- 18.Robbins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–81. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 19.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Figurov A, Pozzo-Miller LD, Olafsson P, et al. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–9. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 21.Ozonoff S, Cook I, Coon H, et al. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: Evidence from the Collaborative Programs of Excellence in Autism network. J Autism Dev Disord. 2004;34(2):139–50. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PRL. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Inzelberg R, Schechtman E, Abuful A, et al. Education effects on cognitive function in a healthy aged Arab population. Int Psychogeriatr. 2007;19(3):593–603. doi: 10.1017/S1041610206004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurlowicz L, Wallace M. The Mini-Mental State Examination (MMSE) J Gerontol Nurs. 1999;25(5):8–9. doi: 10.3928/0098-9134-19990501-08. [DOI] [PubMed] [Google Scholar]
- 25.Al-Rajeh S, Ogunniyi a, Awada A, et al. Preliminary assessment of an Arabic version of the Mini-Mental state examination. Ann Saudi Med. 1999;19(2):150–52. doi: 10.5144/0256-4947.1999.150. [DOI] [PubMed] [Google Scholar]
- 26.Bashir S, Alghamdi F, Alhussien A, et al. Effect of smoking on cognitive functioning in young Saudi adults. Med Sci Monit Basic Res. 2017;23:31–35. doi: 10.12659/MSMBR.902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahakian BJ, Morris RG, Evenden JL, et al. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111(Pt 3):695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- 28.Lim YY, Villemagne VL, Laws SM, et al. APOE and BDNF polymorphisms moderate amyloid {b-related cognitive decline in preclinical Alzheimer’s disease. Mol Psychiatry. 2015;20(11):1322–28. doi: 10.1038/mp.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woost L, Bazin PL, Taubert M, et al. Physical exercise and spatial training: a longitudinal study of effects on cognition, growth factors, and hippocampal plasticity. Sci Rep. 2018;8(1):4239. doi: 10.1038/s41598-018-19993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–50. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 31.Toril P, Reales JM, Ballesteros S. Video game training enhances cognition of older adults: A meta-analytic study. Psychol Aging. 2014;29(3):706–16. doi: 10.1037/a0037507. [DOI] [PubMed] [Google Scholar]
- 32.Miller DJ, Robertson DP. Educational benefits of using game consoles in a primary classroom: A randomised controlled trial. Br J Educ Technol. 2011;42(5):850–64. [Google Scholar]


