Abstract
Background
Zinc-finger protein 384 (ZNF384) fusions are an emerging subtype of precursor B-cell acute lymphoblastic leukaemia (pre-B-ALL) and here we further characterised their prevalence, survival outcomes and transcriptome.
Methods
Bone marrow mononuclear cells from 274 BCR-ABL1-negative pre-B-ALL patients were immunophenotyped and transcriptome molecularly characterised. Transcriptomic data was analysed by principal component analysis and gene-set enrichment analysis to identify gene and pathway expression changes.
Results
We exclusively detect E1A-associated protein p300 (EP300)-ZNF384 in 5.7% of BCR-ABL1-negative adolescent/young adult (AYA)/adult pre-B-ALL patients. EP300-ZNF384 patients do not appear to be a high-risk subgroup. Transcriptomic analysis revealed that EP300-ZNF384 samples have a distinct gene expression profile that results in the up-regulation of Janus kinase/signal transducers and activators of transcription (JAK/STAT) and cell adhesion pathways and down-regulation of cell cycle and DNA repair pathways.
Conclusions
Importantly, this report contributes to a better overview of the incidence of EP300-ZNF384 patients and show that they have a distinct gene signature with concurrent up-regulation of JAK-STAT pathway, reduced expression of B-cell regulators and reduced DNA repair capacity.
Subject terms: Cancer genomics, Acute lymphocytic leukaemia
Introduction
Many genomic lesions in precursor B-cell acute lymphoblastic leukaemia (pre-B-ALL) are associated with alterations of cytokine receptors or their signalling pathway mediators, transcription factors or regulators of differentiation.1,2 These lesions have prognostic significance, for example, ETV6-RUNX1 is associated with a relatively favourable outcome compared with poor-risk disease associated with BCR-ABL+ (Philadelphia-positive (Ph+)) or Ph-like ALL. Recently, next-generation sequencing studies have identified novel recurrent pre-B-ALL genomic lesions in genes such as ABL1/2, JAK2, ZNF384, MEF2D and DUX4,3,4 though the incidence and prognostic significance for some of these lesions are yet to be confirmed.
The E1A-associated protein p300 (EP300)-ZNF384 fusion was initially reported as a recurrent fusion in 2015 with an incidence of 1.5% of paediatric B-ALL cases.5 Three additional studies have confirmed low incidence in paediatric populations (Supplementary Table 1), while adolescent and adult patients have a higher frequency.3,6 The EP300 gene encodes a histone acetyltransferase (HAT), which influences transcription through chromatin remodelling and has tumour suppressor activity.7 The 3′ fusion partner zinc-finger protein 384 (ZNF384) encodes a C2H2-type protein that plays a role in transcription and nucleocytoplasmic transport. How EP300-ZNF384 fusion protein expression alters gene transcription to promote leukaemic cell growth and survival is only beginning to emerge.3,6,8
In addition to EP300, multiple 5′ fusion partners of ZNF384 have been reported in lymphoproliferative disorders including TAF15,9,10 ESWR1,9,10 CREBBP,6 TCF3,6,9 ARID1B,9 SYNRG3 and BMP2K,11 implying that ZNF384 is the predominant pathogenic lesion. ZNF384 and the majority of its fusion partners are located within close proximity to the telomeres of their respective chromosome, making the identification of ZNF384 fusions difficult by conventional G-banding.5,9
Given the increasing importance of ZNF384 as a recurrent genetic lesion in pre-B-ALL, we examined its frequency and prognostic significance in our cohort of 274 BCR-ABL1-negative pre-B-ALL patients on which we have transcriptomic sequencing data.
Materials and methods
We studied 274 BCR-ABL1-negative pre-B-ALL patients (152 children, 54 adolescent/young adults (AYA, 16–39 years) and 68 adults) from the patient pool referred to us for Ph-like testing. Informed consent was obtained from each patient and the study was approved by the relevant institutional review board (Royal Adelaide Hospital Ethics Committee) and conducted in accordance with the Declaration of Helsinki. Transcriptomic sequencing data was generated using either Illumina HiSeq 2000 or NextSeq 500 platforms. Fusions and variants were identified (detail in Supplementary methods) and confirmed by reverse transcription polymerase chain reaction and Sanger sequencing. Samples were evaluated for a Ph-like signature by Taqman low-density array as previously described.12
For gene expression analysis, the raw fastq data was aligned by STAR aligner13 (version 2.4.2a) with two-pass method and described in full in the Supplementary methods, and only genes with false discovery rate (FDR) p < 0.05 were considered as statistically significant. Gene-set enrichment analysis (GSEA) was performed using Broad Institute GSEA software version 3.0 and Molecular Signature Database (MSigDB) version 5.2 (details are provided in Supplementary methods).
Five-year survival analysis of outcome data were estimated by Kaplan–Meier and log-rank test was used for significant difference, and we included Ph+ ALL cases for comparison. As part of our standard characterisation, immunophenotypic analysis was also performed for CD10, CD19 and CD34 expression.
Results
Transcriptomic sequencing revealed 7/122 (5.7%) AYA/adult and 2/152 (1.3%) children harboured the EP300-ZNF384 fusion gene (patient details Table 1). One additional paediatric patient harboured a TCF3-ZNF384 fusion. In 7/9 patients the EP300-ZNF384 fusion was detected at diagnosis, and in the one patient where matched diagnosis and relapse samples were available, the fusion was detected in both. In the remaining two patients only the relapse sample was available for study. Patients with EP300-ZNF384 fusions did not express a Ph-like gene signature. Eight of nine patients had identical break points at EP300 exon 6 (22q13) and ZNF384 exon 3 (12p13) (Fig. 1a), with the remaining patient having a ZNF384 breakpoint in exon 2. The resulting truncation of EP300 eliminates the HAT and bromodomain, which reportedly reduces HAT activity and binding of acetylated proteins.8 In contrast to other studies that report multiple upstream ZNF384 fusion partners,3,6,8 we identified predominantly EP300-ZNF384 fusions in our cohort.
Table 1.
Clinical findings and cytogenetic features of pre-B-ALL patients with ZNF384 fusions
| Patient | Sex | Event | Fusion | Age at event | Initial WBC 109/ml | Initial CNS | Treatment | Karyotype | Variants | Outcome | Survival post Dx (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH_A5049 | M | Rel | TCF3-ZNF384 | 11.6 | No data | ALLR3 therapy | 47,XY,der(2)t(2;3)(p13;p21)t(2;7)(q33;q36),der(3)t(;3)(p13;p21),der(7)t(q33;q36),+8[6]/46,XY[18] | Alive | 5.9 | ||
| CH_A1498 | M | Rel | EP300-ZNF384 | 16.6 | No data | ALLR3 therapy | 46,XY,t(2;17)(q13;q11.2),del(6)(?q15q23)[7]/46,XY[13] | RUNX1 E111K | Alive | 17.1 | |
| CH_A2973 | M | Rel | EP300-ZNF384 | 17.1 | 11 | No data | ANZCHOG ALL8 therapy | 46, XY, der (7) del (7) (q?)?inv (7) (q?) [12] /46, XY [7] | FLT3 Y589H | Died | 4.4 |
| AYAI_0004 | M | Dx | EP300-ZNF384 | 16 | No data | Study8 | 46,XY | KRAS T58I | Alive | 6.9 | |
| AYAI_0004 | M | Rel | EP300-ZNF384 | 23 | 2.58 | Clear | Study8 | 45,X,-Y,t(1;12;6)(p36.1;q24.?3;p21),t(2;9)(q11.2;q1?2),del(19)(p13)[14]/46,XY[21] | Alive | ||
| AYAII_0009 | M | Dx | EP300-ZNF384 | 21 | 4.8 | Clear | ALL6 | 45,XY,t(2;11)(p2?1;p15),del(6)(q13q2?3),inc[cp4]/46,XY[32].nuc.ish(TCF3x2)[200],(D4Z1,D10Z1,D17Z1)x2[200],(ABL,BCR)x2[200],(ABL,BCR)x2[200],(MLLx2)[200],(ETV6,RUNX1)x2[200] | NF1 Y1763F FLT3LG R216H | Alive | 3.05 |
| AYAII_0021 | F | Dx | EP300-ZNF384 | 28 | 5.4 | Clear | LALA | t(X;14) | NRAS Q61P PTK2B G414V ETV6 L201P EPOR N487S | Died | 1.81 |
| AYAII_0045 | M | Dx | EP300-ZNF384 | 22 | 77.1 | No data | ALSG | 46,XY | CSFR1 N255I RUNX1 L29S | Died | 11.63 |
| ADI_0002 | M | Dx | EP300-ZNF384 | 42 | No data | 46,XY | PTPN11 G60A | ||||
| ADI_0098 | F | Dx | EP300-ZNF384 | 47 | 7.5 | Clear | Hoeltzer | No data available | SH2B3 L6P EPOR P488S | Died | 0.17 |
| ADI_0159 | F | Dx | EP300-ZNF384 | 43 | 25 | No data | 46,XX,t(13;14)(q14;q22)[11]/46,idem,t(12;22)(p13;q13)[4]/46,XX[45].ish 12p13(ETV6x2)[20],t(12;22)(WCP22+;WCP22−)[2/6].nuc ish(MYC,MLL,IGH)x2[100],(ASSx1,ABL1x1,BCRx2)[90/100] | NRAS G12C | Died | 2.76 |
pre-B-ALL precursor B-cell acute lymphoblastic leukaemia, LALA Leucémies Aiguës Lymphoblastiques de l'Adulte, ZNF384 zinc-finger protein 384, WBC white blood cell, CNS central nervous system, Dx diagnosis, Rel relapse
Fig. 1.
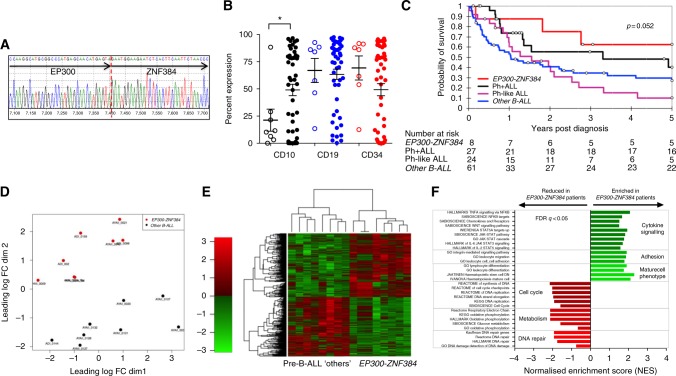
Pre-B-ALL expressing EP300-ZNF384 MNC have reduced CD10 expression and a distinct gene signature, and patients have improved survival. a Sequence of the fusion breakpoint between EP300 (at 22p13.2) and ZNF384 (at 12p13). b Surface expression of CD10, CD19 and CD34 on AYA/adult MNC compared between pre-B-ALL containing EP300-ZNF384 fusion (open circles) to those without detectable EP300-ZNF384 fusions, pre-B-ALL ‘others’ (closed circles) *p = 0.046. Mean ± SEM is shown and analysed by unpaired t-test with two-tail test. c Kaplan–Meier analysis of percent overall survival from diagnosis for patients classified into different subtypes; EP300-ZNF384, n = 8 (red), B-ALL 'other', n = 61 (blue), BCR-ABL1, n = 27 (black) and Ph-like, n = 24 (purple). d Unsupervised clustering using multidimensional scaling (MDS) plots of log-CPM values based on dimensions 1 and 2 reveals differences in gene expression from 8 AYA/adult cases containing the EP300-ZNF384 fusion (red) and 8 AYA/adult pre-B-ALL ‘other’ cases without detectable fusions (black) matched for age, initial white cell count (WCC) and sex (outlined in Supplementary Table 2). The distances that display on the plot correspond to the average (root-mean-square) fold-change in log 2 scale for 500 genes with the most divergent between each pair of samples by default. An interactive MDS plot of this dataset can be found at the Supplementary Figure 1. e Differential gene expression in AYA/adult pre-B-ALL containing EP300-ZNF384 fusion (red, n = 8) compared to those without detectable gene fusions (black, n = 8). Heatmap showing the top 100 genes with significant expression differences based on FDR p < 0.05. An interactive gene expression plot of this data set is available at https://github.com/chungkok/EP300_ZNF384. f Gene set enrichment profiling of transcriptomic sequencing data of EP300-ZNF384 (n = 8) versus pre-B-ALL ‘other’ (n = 8) FDR q < 0.05. Statistical analysis was performed in GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA)
The patients harbouring EP300-ZNF384 fusions had a median age of 24.5 years (range 4.4–47 years). In mononuclear cells (MNCs) of patients with EP300-ZNF384 fusion (n = 8 available), the expression of CD10 was significantly lower when compared with other pre-B-ALL MNC (n = 48) (p = 0.046) (Fig. 1b). Surface expression of CD19 and CD34 was not significantly different between EP300-ZNF384 (n = 7) and other pre-B-ALL groups (p = 0.7882 and p = 0.1916, respectively) (Fig. 1b). Of the five EP300-ZNF384 patients where CD33 expression was available all had high expression.
Outcome data was available from 93 patients in our cohort (8 EP300-ZNF384 patients, 24 Ph-like and 61 other pre-B-ALL). The 5-year survival of our cohort was 28.8%, with an average survival of 2.14 years. The notable poor survival seen in our AYA/adult pre-B-ALL cohort may reflect the inclusion of historical samples. Patients harbouring an EP300-ZNF384 fusion had a 62.5% survival (n = 8) at 5 years. Outcomes for EP300-ZNF384 patients compared favourably to other pre-B-ALL patients (27.2% 5-year survival; n = 61; p = 0.083) and Ph-like ALL patients14 (10.3% 5-year survival, n = 24; p = 0.019) (Fig. 1c), noting that four of the eight EP300-ZNF384 patients were transplanted. For reference, we also included outcomes for 27 Ph+ ALL patients, the majority of whom were treated with tyrosine kinase inhibitor and chemotherapy, which had a 5-year survival of 40.3%.
Using transcriptomic sequencing, a variety of somatic variants were identified in EP300-ZNF384 patients who are known to lead to missense alterations in transcription factors, cytokine receptors and signalling pathways in key cancer driver genes (Table 1). At least one variant was detected in each EP300-ZNF384 case, including RAS mutations in three (33.3%) patients, NRAS G12C and Q61P, and KRAS T58I. Two (22.2%) patients had a RUNX1 mutation, E111K (n = 1) and L29S (n = 1). Mutations were also detected in the FLT3 signalling axis in two patients (22.2%), FLT3 Y589H (n = 1) and FLT3LG R216H (n = 1). Three patients (33.3%) had cytokine receptor mutations including EPOR P488S (n = 1) or N487S (n = 1) and CSF1R N255I (n = 1).
To identify how gene expression is altered when the EP300-ZNF384 is expressed, we then compared the transcriptomic analysis of eight AYA/adult EP300-ZNF384 cases to eight non-EP300-ZNF384 cases matched for age, initial white cell count and sex (patient details Supplementary Table 2). Unsupervised analysis using multidimensional scale (MDS) revealed that the EP300-ZNF384 patient samples have a distinct gene expression profile compared to matched controls (Fig. 1d, Supplementary Figure 1). Gene expression analysis revealed differential expression of 984 genes (FDR p < 0.05) with similar numbers of genes up-regulated (476/984) or down-regulated (508/984). The heatmap (Fig. 1e) shows the differential gene expression based on the top 100 genes that discriminated between the two groups (Supplementary Table 3). Within the EP300-ZNF384 cohort, up-regulation of CLCF1 (p < 0.001), CREB5 (p < 0.001), STGALNAC2 (p < 0.001), CD33 (p < 0.001) and RUNX2 (p < 0.001) was observed and strong down-regulation of OVCH2 (p < 0.001), ARPP21 (p < 0.001), NPY (p < 0.001) and BMP2 (p < 0.001). Expression of B-cell development regulators PAX5 (p = 0.03), IRF4 (p = 0.004) and VPREB1 (p < 0.0001) were also significantly reduced in the EP300-ZNF384 cohort. Altered regulation of myeloid reprogramming genes GATA3, CEBPA or CEBPB previously reported for this subgroup were not observed.6 Of note, the transcription factor, KLF4, which interacts with, and is acetylated by, EP30015 and negatively regulates PI3K signalling, was significantly down-regulated (log FC = −4.69, p < 0.001) in the EP300-ZNF384 patients. KLF4 mediates TP53 action to regulate the G1–S phase transition following DNA damage. TP53 expression was reduced by 1.98 fold in EP300-ZNF384 patients compared to non-EP300-ZNF384 patients (n = 8, p = 0.0141).
GSEA of our transcriptomic data showed that expression of EP300-ZNF384 results in the up-regulation of genes related to Janus kinase/signal transducers and activators of transcription (JAK/STAT) signalling, leukocyte adhesion and differentiation pathways, whereas pathways related to cell cycle, oxidative phosphorylation and DNA repair are reduced (FDR q < 0.05) (Fig. 1f). GSEA analysis also revealed enrichment of genes containing motifs for LMO2 (FDR q = 0.016), RUNX1 (FDR q = 0.014) and GATA1 (FDR q = 0.012), all targets for ZNF384 interaction, and key regulators of haematopoiesis, and reduced expression from genes containing motifs for RB-1 (FDR q = 0.000).
The down-regulation of both TP53 and KLF4 in EP300-ZNF384 samples may facilitate premature S phase entry prior to completion of DNA repair. This together with dampened EP300 acetylation function, a known modulator of TP53, may reduce genomic integrity16 and increase the potential for mutation acquisition in a setting of up-regulated JAK-STAT pathways and thus potentiate leukaemogenesis. EP300-ZNF384 samples have reduced expression of both DNA repair reactome and DNA damage and repair pathways (Fig. 1f), suggesting that EP300-ZNF384 expression contributes to higher genomic instability. Mutations or deletion of TP53 are infrequent in pre-B-ALL but are common in many other tumours, and our transcriptomic data suggest that the down-regulation of DNA repair, relative to non-EP300-ZNF384 pre-B-ALL, is an important feature of this cohort.
Discussion
In contrast to other reports where ZNF384 fusions have multiple 5′ partners, we exclusively detected EP300-ZNF384 fusions in 5.7% of BCR-ABL1-negative AYA/adult pre-B-ALL, making it one of the more prevalent recurrent lesions in this age group. Our EP300-ZNF384 patients cluster in the AYA range and have reduced CD10 surface expression and up-regulated CD33 expression in common with previous reports.3,5 The EP300-ZNF384 subgroup showed improved outcome compared to other pre-B-ALL patients studied, and concurs with a Japanese cohort where ZNF384 fusions have better survival outcomes than Ph-like ALL in AYA.3 This report contributes to a better overview of the incidence of EP300-ZNF384 patients and the prognostic significance of ZNF384 lesions deserves further study and validation. Importantly, we show here that the concurrent reduction in DNA repair capacity and activation of JAK-STAT pathways and B-cell regulators appear to be the hallmark features of the pre-B-ALL subtype expressing EP300-ZNF384.
Electronic supplementary material
Acknowledgements
This work is supported in part by grants from National Health and Medical Research Council (NHMRC) (APP1057746, APP1044884), Australian Genomics Health Alliance (AGHA) Beat Cancer, the Leukaemia Foundation and Bristol-Meyers Squibb Company (to D.L.W.); Channel 7 Children’s Research Foundation (CRF) (to S.L.H.) and Contributing Haematologist Committee (CHC) (to D.Y.). Cytogenetic analysis was performed by Genetics and Molecular Pathology, SA Pathology, Adelaide, SA, Australia.
Author contributions
B.J.M. performed experiments, analysed the data and wrote the manuscript. S.L.H. designed and performed experiments, analysed data, proofread manuscript. C.H.K. performed cluster analysis, gene set enrichment analysis and generated heat map from transcriptomic sequencing data. T.S. performed the experiments and proofread manuscript. J.A. assessed gene transcription levels. D.Y., R.S. and R.B.L. provided patient samples and critically appraised manuscript. T.P.H. critically appraised manuscript. D.L.W. designed experiments, analysed data, proofread manuscript and supervised the project.
Conflict of interest
D.L.W. receives research support from Novartis and BMS, and Honoraria from BMS. T.P.H. and D.Y. receive research support from Novartis, Ariad and BMS, and Honoraria and Advisory role from Novartis. All the other authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary information is available for this paper at 10.1038/s41416-018-0022-0.
References
- 1.Mullighan CG. Genomic characterization of childhood acute lymphoblastic leukemia. Semin. Hematol. 2013;50:314–324. doi: 10.1053/j.seminhematol.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, et al. A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia. 2014;28:2336–2343. doi: 10.1038/leu.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasuda T, et al. Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nat. Genet. 2016;48:569–574. doi: 10.1038/ng.3535. [DOI] [PubMed] [Google Scholar]
- 4.Lilljebjorn H, et al. Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat. Commun. 2016;7:11790. doi: 10.1038/ncomms11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gocho Y, et al. A novel recurrent EP300-ZNF384 gene fusion in B-cell precursor acute lymphoblastic leukemia. Leukemia. 2015;29:2445–2448. doi: 10.1038/leu.2015.111. [DOI] [PubMed] [Google Scholar]
- 6.Liu YF, et al. Genomic profiling of adult and pediatric B-cell acute lymphoblastic leukemia. EbioMedicine. 2016;8:173–183. doi: 10.1016/j.ebiom.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta R, Tiu B, Sakamoto KM. CBP/p300 acetyltransferase activity in hematologic malignancies. Mol. Genet. Metab. 2016;119:37–43. doi: 10.1016/j.ymgme.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Qian M, et al. Whole transcriptome sequencing identified a distinct subtype of acute lymphoblastic leukemia with predominant genomic abnormalities of EP300 and CREBBP. Genome Res. 2017;27:185–195. doi: 10.1101/gr.209163.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shago M, Abla O, Hitzler J, Weitzman S, Abdelhaleem M. Frequency and outcome of pediatric acute lymphoblastic leukemia with ZNF384 gene rearrangements including a novel translocation resulting in an ARID1B/ZNF384 gene fusion. Pediatr. Blood Cancer. 2016;63:1915–1921. doi: 10.1002/pbc.26116. [DOI] [PubMed] [Google Scholar]
- 10.La Starza R, et al. CIZ gene rearrangements in acute leukemia: report of a diagnostic FISH assay and clinical features of nine patients. Leukemia. 2005;19:1696–1699. doi: 10.1038/sj.leu.2403842. [DOI] [PubMed] [Google Scholar]
- 11.Hirabayashi S, et al. ZNF384-related fusion genes consist of a subgroup with a characteristic immunophenotype in childhood B-cell precursor acute lymphoblastic leukemia. Haematologica. 2016;102:118–129. doi: 10.3324/haematol.2016.151035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heatley SL, et al. High prevalence of relapse in children with Philadelphia-like acute lymphoblastic leukemia despite risk-adapted treatment. Haematologica. 2017;102:e409–e493. doi: 10.3324/haematol.2016.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts KG, et al. High frequency and poor outcome of Philadelphia chromosome-like acute lymphoblastic leukemia in adults. J. Clin. Oncol. 2017;35:394–401. doi: 10.1200/JCO.2016.69.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans PM, et al. Kruppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. J. Biol. Chem. 2007;282:33994–34002. doi: 10.1074/jbc.M701847200. [DOI] [PubMed] [Google Scholar]
- 16.Iyer NG, et al. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc. Natl. Acad. Sci. USA. 2004;101:7386–7391. doi: 10.1073/pnas.0401002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.