Abstract
Background: Poor sleep quality has previously been shown to be related to insulin resistance in apparently healthy adults. However, it is unclear whether an association between sleep quality and insulin resistance exists among adults with metabolic syndrome (MetS).
Methods: Participants included 347 overweight/obese postmenopausal women without type 2 diabetes (age: 57.5 ± 6.5 years; body mass index [BMI]: 31.7 ± 3.7 kg/m2; 54% with MetS). Sleep quality was assessed with the six-item Medical Outcomes Study Sleep Scale; values were categorized into quartiles. Insulin resistance was calculated from fasting glucose and insulin with the homeostasis model assessment of insulin resistance (HOMA2-IR) method. Analysis of covariance models were used to examine the association between sleep quality and HOMA2-IR after accounting for MetS and covariates (e.g., BMI, cardiorespiratory fitness, and energy intake).
Results: Women with the worst sleep quality had significantly higher HOMA2-IR values than women in all other quartiles (P ≤ 0.05 for each), and women with MetS had significantly higher HOMA2-IR values than women without MetS (P < 0.0001), but the relationship between sleep quality and HOMA2-IR did not differ between those with or without MetS (P = 0.26). Women with MetS in the worst quartile of sleep quality had higher HOMA2-IR values than all other women (P < 0.02). Taking >30 min to fall asleep, frequent restless sleep, and frequent daytime drowsiness were each related to higher HOMA2-IR values (each P < 0.04).
Conclusions: Sleep quality is an important correlate of insulin resistance in postmenopausal women with and without MetS. Intervention studies are needed to determine whether improving sleep improves insulin resistance in populations at elevated cardiometabolic risk.
Keywords: : sleep quality, insulin resistance, metabolic syndrome, postmenopausal
Introduction
Insulin resistance is a key component in the pathogenesis of type 2 diabetes (T2D) and a potent risk factor for cardiovascular disease (CVD).1,2 Insulin resistance is also closely associated with metabolic syndrome (MetS), defined by a cluster of cardiometabolic conditions, including abdominal obesity, hypercholesterolemia, elevated blood pressure, impaired fasting glucose, and/or elevated triglycerides.3 Insulin resistance and MetS independently increase risk for T2D and CVD4,5; among those with MetS, higher levels of insulin resistance are predictive of adverse cardiometabolic outcomes.6,7 Thus, identifying factors associated with insulin resistance—especially in those with high cardiometabolic risk (e.g., MetS)—has significant public health importance.
There is now ample evidence linking poor sleep with insulin resistance.8 Sleep complaints,9 variable sleep timing,10 short or long habitual sleep duration,9,11 and obstructive sleep apnea (OSA)12 have each been associated with insulin resistance in otherwise healthy adults. Moreover, experimental manipulations of sleep quality and duration have reduced insulin sensitivity in young adults.13,14 Accordingly, poor sleep is increasingly recognized as a behavioral risk factor for insulin resistance among otherwise healthy adults.15
Whether poor sleep is associated with insulin resistance in populations with elevated cardiometabolic risk or overt metabolic dysfunction is less clear.16,17 Among adults with T2D, excessively short or long sleep durations have been associated with worse glycemic control,18 of which insulin resistance is a key determinant; a similar (although less consistent) relationship has been observed between sleep quality and glycemic control.18 Additional studies involving adults with T2D have found that sleep debt and OSA are each associated with greater insulin resistance.19,20 Others have found the relationship between sleep and metabolic function to differ according to the presence of T2D.21,22 In contrast, whether poor sleep is associated with worse insulin resistance among adults with MetS is unknown. Identifying whether poor sleep is a risk factor for insulin resistance in those with MetS could lead to targeted sleep interventions for this population, potentially reducing its high risk for adverse cardiometabolic outcomes.23
The possible influence of poor sleep on insulin resistance may be especially applicable to postmenopausal women, among whom poor sleep quality and MetS are each prevalent24,25 and cardiometabolic risk is heightened relative to before the menopausal transition.26,27 The purpose of this study was to examine the association between sleep quality and insulin resistance in a sample of postmenopausal women with a high MetS prevalence. We hypothesized that sleep quality would be associated with insulin resistance and that this relationship would be similar among women with and without MetS.
Methods
The present cross-sectional analyses utilized baseline data from the Dose–Response to Exercise in Women study (DREW; ClinicalTrials.gov identifier: NCT00011193).28,29 A complete description of the study's recruitment and screening procedures is published elsewhere.29 The study was approved annually by the Cooper Institute Institutional Review Board (Dallas, TX), and written informed consent was obtained from all participants before study involvement.
Participants
Participants were women who were aged 45–75 years, postmenopausal, physically inactive (<20 min of exercise on <3 days/week and <8000 steps/day, averaged over 1 week), overweight or obese (body mass index [BMI] of 25–43 kg/m2), and had normal to mildly elevated resting blood pressure (systolic blood pressure of 120–159 mm Hg and diastolic blood pressure ≤99 mm Hg). Exclusion criteria included diagnosed T2D, known CVD, recent hospitalization for mental illness or significant symptoms of depression (score ≥10 on the Center for Epidemiologic Studies-Depression Scale), or any other health condition that would contraindicate exercise participation.
Of 4545 women screened for eligibility, 464 women began the trial. From the initial sample of 464, participants were excluded from these analyses for missing data related to MetS categorization or insulin resistance calculation (n = 88), missing sleep quality data (n = 13), missing covariate data (n = 13), or having glucose or insulin data outside normal physiologic ranges (glucose: <3.5 or >25 mmol/L, insulin: <20 or >400 pmol/L; n = 3), leaving 347 available for analysis. Excluded participants did not differ from those included in analyses on insulin resistance, sleep quality, or any covariates.
Study measures
Sleep quality
Subjective sleep quality was assessed with an abbreviated Medical Outcomes Study (MOS) Sleep Scale,30 in which participants were asked to respond to six sleep-related questions based on the previous 4 weeks. One question addressed the length of time to fall asleep, with five response options ranging from 0–15 min to >60 min. For the remaining five questions (i.e., restless sleep, daytime drowsiness, difficulty falling asleep, difficulty returning to sleep following awakening, and staying awake during the day), participants were asked to indicate the frequency they experienced each symptom on a five-point scale, ranging from none of the time to all of the time.
Item responses were assigned scores using conventional scoring rules.30 Using all six items, a modified sleep problems index (SPI) provided a measure of overall sleep quality (range: 0–100),31 with higher scores indicating worse sleep quality. The MOS Sleep Scale has been used to characterize sleep in healthy adults as well as those with significant sleep disturbance (e.g., insomnia, restless legs syndrome, and chronic pain).30–32
Insulin resistance
Venous blood samples were obtained in the morning following a 12-hr fast. Samples were analyzed for glucose and insulin using a Dimension RXL analyzer (Oxford, CT) in a laboratory that was certified by the College of American Pathologists and met the quality control standards of the CDC Lipid Standardization Program. Insulin resistance was calculated from fasting glucose and insulin based upon the homeostasis model assessment of insulin resistance (HOMA2-IR; v.2.2.2) method.33 Higher HOMA2-IR values indicate higher insulin resistance.34
Metabolic syndrome
MetS was assessed using the harmonized criteria from the 2009 Joint Interim Statement,3 requiring ≥3 of 5 components: waist circumference ≥88 cm, high-density lipoprotein <50 mg/dL, triglycerides ≥150 mg/dL, blood pressure ≥130/85 mmHg or antihypertensive medication use, and plasma glucose ≥100 mg/dL.
Covariates
A variety of health indices, behaviors, and sociodemographic factors were included as covariates due to their possible relationship with insulin resistance and/or sleep. As a measure of cardiorespiratory fitness, the peak rate of oxygen consumption (VO2peak) was assessed with a maximal exercise test on a cycle ergometer and averaged across two assessments conducted on separate days.28 Energy intake, expressed in kcal, was assessed by the Food Intake and Analysis System semiquantitative food frequency questionnaire.35 Height and weight were measured with a calibrated stadiometer and electronic scale, respectively; BMI was calculated as kg/m2.
Age, race, marital status, hormone therapy use, and sleep medication use were self-reported by participants. Race was dichotomized into white and black/other due to the small proportion of races other than white or black (<7%); marital status was dichotomized into currently married and not currently married.
Statistical analyses
Before analyses, HOMA2-IR underwent natural logarithm transformation due to a skewed distribution. For our primary analysis, analysis of covariance (ANCOVA) was used to examine the relationship between sleep quality (SPI) and insulin resistance (HOMA2-IR) following classification of SPI values into quartiles (Q1: SPI ≤12.5; Q2: SPI >12.5–25; Q3: SPI >25–45; and Q4: SPI >45); Q4 represented the quartile of worst sleep quality. MetS status was included as a factor to determine whether sleep quality was related to insulin resistance independent of MetS, and an interaction term was added to determine whether the relationship between sleep quality and insulin resistance differed according to MetS status.
Stratified analyses examined the relationship between sleep quality and insulin resistance in models restricted to those with or without MetS. Additional covariates in these ANCOVA models included age, race, marital status, hormone therapy use, sleep medication use, cardiorespiratory fitness, energy intake, and BMI.
ANCOVA models were also used to examine whether specific sleep complaints were related to insulin resistance following classification of each of the six MOS Sleep Scale item responses according to clinically significant thresholds. Responses for the item assessing time to fall asleep were dichotomized into >30 min or ≤30 min; responses for each of the remaining five items were dichotomized into none/infrequent (none, a little, some of the time) or frequent (most of the time, all of the time). Models examining individual MOS Sleep Scale items included the covariates noted above.
Analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC). All tests were two-tailed, with statistical significance set at P ≤ 0.05.
Results
Table 1 summarizes the participant characteristics. Women were, on average (±standard deviation), 57.5 ± 6.5 years of age and had a BMI of 31.7 ± 3.7 kg/m2. The majority of women were married (92%) and Caucasian (67%); 54% of the sample met criteria for MetS. Women in the second highest quartile of sleep quality had significantly higher cardiorespiratory fitness than all other women, but no other participant characteristics significantly differed across SPI quartiles.
Table 1.
Participant Characteristics
| MOS sleep problems index quartiles | |||||
|---|---|---|---|---|---|
| Characteristic | All (N = 347) | Q1: ≤12.5 (n = 101) | Q2: >12.5–25 (n = 83) | Q3: >25–45 (n = 92) | Q4: >45 (n = 71) |
| Age, years | 57.5 (6.5) | 58.5 (6.5) | 57.1 (6.1) | 57.4 (6.4) | 56.5 (6.8) |
| White race, n (%) | 232 (66.9) | 67 (66.3) | 60 (72.3) | 62 (67.4) | 43 (60.6) |
| Currently married, n (%) | 318 (91.6) | 93 (92.1) | 77 (92.8) | 82 (89.1) | 66 (93.0) |
| Body mass index, kg/m2 | 31.7 (3.7) | 31.9 (3.6) | 31.5 (3.8) | 31.8 (3.8) | 31.6 (3.8) |
| VO2peak, mL/kg/min | 15.4 (2.8) | 14.9 (2.7) | 16.4 (3.2)a | 15.3 (2.4) | 15.3 (2.9) |
| Daily energy intake, kcal | 2306.0 (981.5) | 2216.9 (980.5) | 2357.7 (861.6) | 2367.8 (1085.5) | 2292.1 (983.5) |
| Hormone therapy use, n (%) | 168 (48.4) | 56 (55.4) | 42 (50.6) | 42 (45.7) | 28 (39.4) |
| Sleep medication use, n (%) | 7 (2.0) | 2 (2.0) | 0 (0.0) | 2 (2.2) | 3 (4.2) |
| MetS, n (%) | 188 (54.2) | 55 (54.5) | 43 (51.8) | 51 (55.4) | 39 (54.9) |
Data are expressed as mean (standard deviation) or n (%), as appropriate.
Significantly different from all other quartiles of sleep quality (P < 0.05).
MetS, metabolic syndrome; MOS, Medical Outcomes Study; VO2peak, peak rate of oxygen consumption.
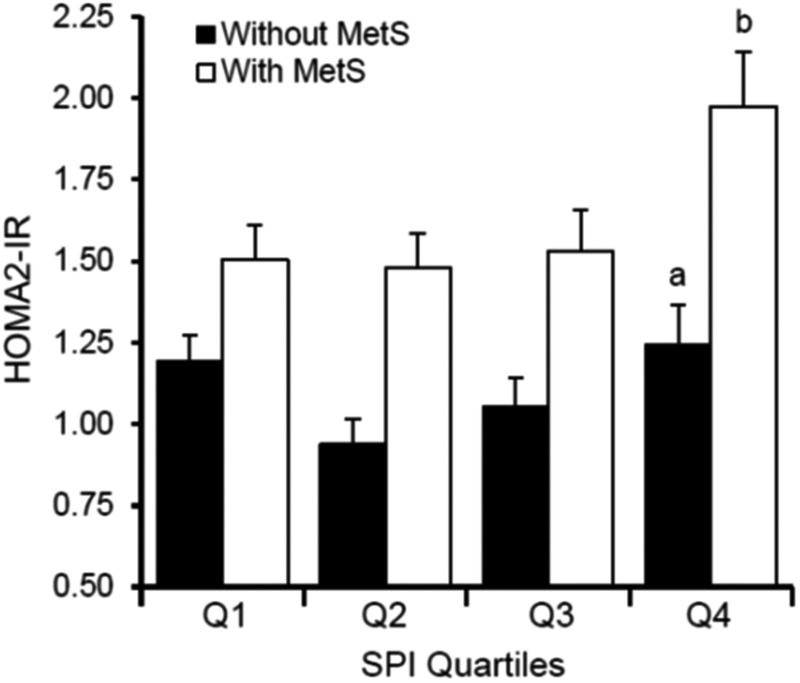
Mean HOMA2-IR value of the sample was 1.37 ± 0.79. As depicted in Fig. 1, HOMA2-IR values differed significantly according to MetS status (F1,331 = 41.60, P < 0.0001) and SPI quartile (F3,331 = 4.32, P < 0.01), but the interaction term was not significant (F3,331 = 1.36, P = 0.26). Women with MetS had significantly higher HOMA2-IR values than women without MetS (P < 0.0001). Women with the worst sleep quality (i.e., Q4) had significantly higher HOMA2-IR values than all other SPI quartiles (quartile 1: P = 0.050; quartile 2: P = 0.002; and quartile 3: P < 0.01), with no other differences in HOMA2-IR values across SPI quartiles. When examining the combination of SPI quartiles and MetS status, women with the worst sleep quality and MetS had significantly higher HOMA2-IR values than any of the other combinations (P < 0.02; Fig. 1).
FIG. 1.
Insulin resistance values across quartiles of sleep quality among women with and without MetS. Data shown are mean (±standard deviation) values before analyses. aSignificantly different from Q2 and Q3 among women without MetS; bSignificantly different from Q1, Q2, and Q3 among women with MetS. HOMA2-IR, homeostasis model assessment of insulin resistance; MetS, metabolic syndrome; SPI, sleep problems index.
In stratified analyses, sleep quality was a significant predictor of HOMA2-IR in women without MetS (n = 159; F3,147 = 2.79, P = 0.04) and with MetS (n = 188; F3,176 = 3.14, P = 0.03). Among those without MetS, women with the worst sleep quality had significantly higher HOMA2-IR values than women in Q2 and Q3 (P < 0.05 for each); among those with MetS, women with the worst sleep quality had significantly higher HOMA2-IR values than all other women (each P < 0.02).
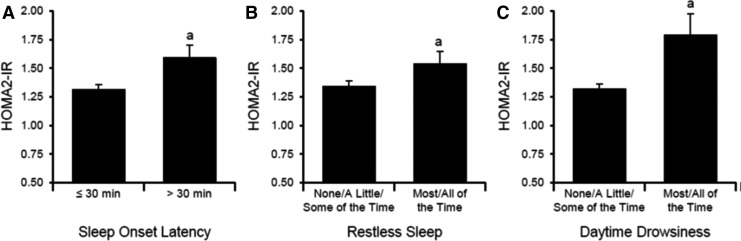
Individual MOS Sleep Scale items that were significantly associated with higher HOMA2-IR values are depicted in Fig. 2. Women who reported taking >30 min to fall asleep (n = 76) had significantly higher HOMA2-IR values than women who reported ≤30 min (F1,337 = 4.58, P < 0.04), and women who reported frequent restless sleep or frequent daytime drowsiness (n = 58 and n = 39, respectively) had higher HOMA2-IR values than women who reported these complaints less frequently (F1,337 = 4.36 [P < 0.04] and F1,337 = 9.98 [P < 0.01], respectively). HOMA2-IR values did not significantly differ for any other individual item (each P > 0.43).
FIG. 2.
Insulin resistance values according to specific sleep symptoms. Data shown are mean (±standard deviation) values before analyses. (A) Sleep onset latency; (B) restless sleep; (C) daytime drowsiness. aSignificantly different from other categories.
Discussion
The present study evaluated the relationship between sleep quality and insulin resistance in a sample of postmenopausal women with a high prevalence of MetS. Poor sleep quality was associated with insulin resistance, and this relationship was similar for women with and without MetS; however, the highest insulin resistance was observed among women with the worst sleep quality and MetS. In addition, taking >30 min to fall asleep, frequent restless sleep, and frequent daytime drowsiness were each significantly related to greater insulin resistance.
Our finding that poor sleep quality was significantly related to insulin resistance is consistent with most previous research on this topic.9,36–40 These results also align with a large body of evidence demonstrating a link between poor sleep quality and the development of T2D,15 of which insulin resistance is a key precursor. We also found that although women with MetS had worse insulin resistance than women without MetS, the relationship between poor sleep quality and insulin resistance was similar between women with and without MetS. Moreover, our results indicate that poor sleep quality has an additive influence on insulin resistance beyond the presence of MetS.
To our knowledge, this is the first study to examine whether the relationship between sleep quality and insulin resistance differed among those with and without MetS. Other cross-sectional studies have found the association between sleep quality and metabolic function to differ according to T2D diagnosis. Engeda et al. found that the relationship between sleep quality and hyperglycemia was more apparent among those with clinically identified prediabetes than those with T2D.21 In contrast, Knutson et al. reported an association between insomnia symptoms and worse insulin resistance, but only among adults with T2D; in fact, among adults without T2D, insomnia symptoms were associated with lower insulin resistance.22 Although our findings are not directly comparable with these studies since none of our participants were diagnosed with T2D, our results do not suggest that the relationship between sleep quality and insulin resistance differs based upon the presence of clustered cardiometabolic risk factors (i.e., MetS).
Because the MOS Sleep Scale is a composite measure of multiple sleep symptoms, we sought to identify which specific items were associated with insulin resistance. Three of the six items were associated with HOMA2-IR: taking >30 min to fall asleep, frequent restless sleep, and frequent daytime drowsiness. Sleep latency exceeding 30 min and restless sleep are complaints commonly attributed to insomnia, which is prevalent among postmenopausal women.41 Arora et al. recently demonstrated that self-reported night-time awakenings, conceptually similar to the measure of restless sleep used in this study, were related to HOMA2-IR through its relationship with BMI in adults with T2D.42 Some studies have found insomnia symptoms to be associated with worse insulin resistance9,40 or increased risk for T2D.15 However, other reports have found no difference in insulin resistance between adults who met diagnostic criteria for insomnia disorder and healthy controls,43–45 while another study reported lower insulin resistance in adults with insomnia-related symptoms in combination with short sleep duration assessed through polysomnography.46 The reason for such discrepant results in the latter study is unclear; however, as that study paired objective sleep disturbance with subjective sleep complaints, this combination could represent a unique sleep phenotype with different pathways contributing to altered metabolic function.47
Frequent or excessive daytime drowsiness is a common OSA symptom, and OSA is often associated with poor sleep quality as a result of the frequent nocturnal arousals following apneic and hypopneic events.48 Moreover, OSA is highly prevalent among overweight and obese postmenopausal women.49 A number of studies have noted an association between OSA and insulin resistance.12 For instance, Nock et al. previously found that a cluster of OSA-related sleep features aggregate with insulin resistance and other cardiometabolic features typically characterized as MetS.50 Moreover, several trials have demonstrated that OSA treatment reduces insulin resistance.12 Thus, the relationship between poor sleep quality and insulin resistance observed in this study may be partly attributable to unmeasured OSA even though we adjusted for BMI, the strongest correlate of OSA among postmenopausal women.49
Although this study was not designed to examine underlying mechanisms, poor sleep quality could contribute to insulin resistance through a variety of pathways, including alterations in daytime autonomic activity, increased inflammation, and persistent activation of the hypothalamic–pituitary–adrenal (HPA) axis.51 Poor sleep quality has been associated with low heart rate variability,13 high levels of inflammatory markers,36 and high cortisol levels.52 In turn, altered autonomic function, chronic inflammation, and HPA axis activation have each been shown to contribute to insulin resistance.53–55 Finally, poor sleep quality may share a common genetic background with metabolic dysfunction. Specifically, a recent study documented a genetic correlation between insomnia symptoms and HOMA-IR and between insomnia symptoms and daytime sleepiness with measures of adiposity.56
Strengths of this study include a sample of postmenopausal women with a high prevalence of poor sleep quality and MetS, which allowed for examination of whether the relationship between sleep quality and insulin resistance differed according to the presence of MetS. We also assessed sleep quality using a validated measure; self-reported sleep quality is an important predictor of numerous health outcomes57 and is unable to be reliably corroborated with objective measures.58 Moreover, analyses accounted for important behavioral and physiological factors (e.g., BMI, cardiorespiratory fitness, and caloric intake), which could confound the relationship between sleep quality and insulin resistance.
However, despite its numerous strengths, limitations of the study should be considered. The abbreviated version of the MOS Sleep Scale used in this study did not assess sleep duration; however, extremely short or long sleep durations have been shown to be associated with insulin resistance.9,11 Although sleep quality and sleep duration are distinct sleep parameters with weak concordance,59 it would have been informative to examine their independent and combined associations with insulin resistance in this sample. Lack of objective OSA assessment is another limitation given the likely high prevalence of OSA in this sample and the known association between OSA and insulin resistance.
An additional limitation is the reliance upon fasting samples to assess insulin resistance. Although HOMA corresponds well to tests of dynamic glucose homeostasis (e.g., euglycemic hyperinsulinemic clamp),34 other techniques are needed to assess peripheral and whole-body insulin resistance. Finally, because of the cross-sectional nature of these analyses, directionality of the association between sleep quality and insulin resistance cannot be established. Although laboratory-based experimental research suggests that poor sleep quality induces metabolic dysfunction,13 it is possible that the relationship may be bidirectional.
In summary, we found that poor sleep quality was associated with insulin resistance in a sample of postmenopausal women, with a similar relationship among women with and without MetS. Notably, women with poor sleep quality and MetS had the highest insulin resistance. Although cross-sectional, these data suggest the possibility of poor sleep quality as a modifiable target to reduce insulin resistance and its associated cardiometabolic risk. Future research should address whether improving sleep quality in women—but especially in women with MetS—leads to reduced cardiometabolic risk.
Acknowledgments
This work was performed at the Cooper Institute (Dallas, Texas). The authors thank the DREW participants and the Cooper Institute staff for their efforts in this study.
Funding Sources
This work was supported by National Institutes of Health grants, no. R01HL66262 (PI: SN Blair) and K23HL118318 (PI: CE Kline), and an American Heart Association Texas Affiliate award 02651404.
Author Disclosure Statement
D.J.B. has served as a paid consultant for BeHealth, Emmi Solutions, and Bayer; developed a CME program supported by CME Institute; and received licensing fees for commercial uses of the Pittsburgh Sleep Quality Index, the Daytime Insomnia Symptoms Scale, and the Consensus Sleep Diary. None of these activities are related to the topic of the article. C.P.E. has consulting relationships with Catapult Health, Naturally Slim, and ACAP Health. C.E.K., M.H.H., and T.S.C. have no competing financial interests.
References
- 1.Reaven GM. Role of insulin resistance in human disease (syndrome X): An expanded definition. Annu Rev Med 1993;44:121–131 [DOI] [PubMed] [Google Scholar]
- 2.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 2011;14:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 4.Gast KB, Tjeerdema N, Stijnen T, et al. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS One 2012;7:e52036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saely CH, Aczel S, Marte T, et al. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J Clin Endocrinol Metab 2005;90:5698–5703 [DOI] [PubMed] [Google Scholar]
- 6.Meigs JB, Rutter MK, Sullivan LM, et al. Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care 2007;30:1219–1225 [DOI] [PubMed] [Google Scholar]
- 7.Ferreira I, Boreham CA, Twisk JW, et al. Clustering of metabolic syndrome risk factors and arterial stiffness in young adults: The Northern Ireland Young Hearts Project. J Hypertens 2007;25:1009–1020 [DOI] [PubMed] [Google Scholar]
- 8.Spiegel K, Knutson K, Leproult R, et al. Sleep loss: A novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol 2005;99:2008–2019 [DOI] [PubMed] [Google Scholar]
- 9.Pyykkonen AJ, Isomaa B, Pesonen AK, et al. Subjective sleep complaints are associated with insulin resistance in individuals without diabetes: The PPP-Botnia Study. Diabetes Care 2012;35:2271–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor BJ, Matthews KA, Hasler BP, et al. Bedtime variability and metabolic health in midlife women: The SWAN Sleep Study. Sleep 2016;39:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu R, Zee PC, Chervin RD, et al. Short sleep duration is associated with insulin resistance independent of adiposity in Chinese adult twins. Sleep Med 2011;12:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015;11:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010;137:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buxton OM, Pavlova M, Reid EW, et al. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anothaisintawee T, Reutrakul S, Van Cauter E, et al. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med Rev 2016;30:11–24 [DOI] [PubMed] [Google Scholar]
- 16.Jain SK, Kahlon G, Morehead L, et al. The effect of sleep apnea and insomnia on blood levels of leptin, insulin resistance, IP-10, and hydrogen sulfide in type 2 diabetic patients. Metab Syndr Relat Disord 2012;10:331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St-Onge MP, Zammit G, Reboussin DM, et al. Associations of sleep disturbance and duration with metabolic risk factors in obese persons with type 2 diabetes: Data from the Sleep AHEAD Study. Nat Sci Sleep 2012;4:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SW, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Med Rev 2017;31:91–101 [DOI] [PubMed] [Google Scholar]
- 19.Arora T, Chen MZ, Cooper AR, et al. The impact of sleep debt on excess adiposity and insulin sensitivity in patients with early type 2 diabetes mellitus. J Clin Sleep Med 2016;12:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronsohn RS, Whitmore H, Van Cauter E, et al. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 2010;181:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engeda J, Mezuk B, Ratliff S, et al. Association between duration and quality of sleep and the risk of pre-diabetes: Evidence from NHANES. Diabet Med 2013;30:676–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutson KL, Van Cauter E, Zee P, et al. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: The Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care 2011;34:1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arora T, Taheri S. Sleep optimization and diabetes control: A review of the literature. Diabetes Ther 2015;6:425–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: A community survey of sleep and the menopausal transition. Menopause 2003;10:19–28 [DOI] [PubMed] [Google Scholar]
- 25.Aguilar M, Bhuket T, Torres S, et al. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015;313:1973–1974 [DOI] [PubMed] [Google Scholar]
- 26.Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol 2009;5:553–558 [DOI] [PubMed] [Google Scholar]
- 27.Atsma F, Bartelink ML, Grobbee DE, et al. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006;13:265–279 [DOI] [PubMed] [Google Scholar]
- 28.Church TS, Earnest CP, Skinner JS, et al. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: A randomized controlled trial. JAMA 2007;297:2081–2091 [DOI] [PubMed] [Google Scholar]
- 29.Morss GM, Jordan AN, Skinner JS, et al. Dose response to exercise in women aged 45–75 year (DREW): Design and rationale. Med Sci Sports Exerc 2004;36:336–344 [DOI] [PubMed] [Google Scholar]
- 30.Hays RD, Stewart AL. Sleep measures. In: Stewart AL, Ware JE., Jr. (eds). Measuring Functioning and Well-being: The Medical Outcomes Study Approach, Durham: Duke University Press; 1992: 235–259 [Google Scholar]
- 31.Hays RD, Martin SA, Sesti AM, et al. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med 2005;6:41–44 [DOI] [PubMed] [Google Scholar]
- 32.Allen RP, Kosinski M, Hill-Zabala CE, et al. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep). Sleep Med 2009;10:531–539 [DOI] [PubMed] [Google Scholar]
- 33.The University of Oxford Centre for Diabetes, Endocrinology and Metabolism: Clinical Trials Unit. HOMA Calculator. Accessed at: www.dtu.ox.ac.uk February 11, 2017
- 34.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 35.Food intake analysis system [computer program]. Version 3.0. Austin: University of Texas, Houston School of Public Health; 1996 [Google Scholar]
- 36.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: Evidence for gender disparity. Brain Behav Immun 2008;22:960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennings JR, Muldoon MF, Hall M, et al. Self-reported sleep quality is associated with the metabolic syndrome. Sleep 2007;30:219–223 [DOI] [PubMed] [Google Scholar]
- 38.Haseli-Mashhadi N, Dadd T, Pan A, et al. Sleep quality in middle-aged and elderly Chinese: Distribution, associated factors and associations with cardio-metabolic risk factors. BMC Public Health 2009;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byberg S, Hansen AL, Christensen DL, et al. Sleep duration and sleep quality are associated differently with alterations of glucose homeostasis. Diabet Med 2012;29:e354–e360 [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto N, Yamanaka G, Ishizawa K, et al. Insomnia increases insulin resistance and insulin secretion in elderly people. J Am Geriatr Soc 2010;58:801–804 [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Wing YK. Sex differences in insomnia: A meta-analysis. Sleep 2006;29:85–93 [DOI] [PubMed] [Google Scholar]
- 42.Arora T, Chen MZ, Omar OM, et al. An investigation of the associations among sleep duration and quality, body mass index and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. Ther Adv Endocrinol Metab 2016;7:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keckeis M, Lattova Z, Maurovich-Horvat E, et al. Impaired glucose tolerance in sleep disorders. PLoS One 2010;5:e9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seelig E, Keller U, Klarhofer M, et al. Neuroendocrine regulation and metabolism of glucose and lipids in primary chronic insomnia: A prospective case-control study. PLoS One 2013;8:e61780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tschepp J, Lauer CJ, Wilde-Frenz J, et al. No impaired glucose tolerance in primary insomnia patients with normal results of polysomnography. Front Neurol 2017;8:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasisht KP, Kessler LE, Booth JN III, et al. Changes in insulin secretion and sensitivity in short sleep insomnia. Sleep 2013;36:955–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vgontzas AN, Fernandez-Mendoza J, Liao D, et al. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med Rev 2013;17:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roure N, Gomez S, Mediano O, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med 2008;9:727–731 [DOI] [PubMed] [Google Scholar]
- 49.Franklin KA, Sahlin C, Stenlund H, et al. Sleep apnoea is a common occurrence in females. Eur Respir J 2013;41:610–615 [DOI] [PubMed] [Google Scholar]
- 50.Nock NL, Li L, Larkin EK, et al. Empirical evidence for “syndrome Z”: A hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep 2009;32:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: Implications for risk and severity of diabetes. Ann NY Acad Sci 2014;1311:151–173 [DOI] [PubMed] [Google Scholar]
- 52.Kumari M, Badrick E, Ferrie J, et al. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab 2009;94:4801–4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Festa A, D'Agostino R, Jr., Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102:42–47 [DOI] [PubMed] [Google Scholar]
- 54.Reynolds RM, Walker BR. Human insulin resistance: The role of glucocorticoids. Diabetes Obes Metab 2003;5:5–12 [DOI] [PubMed] [Google Scholar]
- 55.Liao D, Cai J, Brancati FL, et al. Association of vagal tone with serum insulin, glucose, and diabetes mellitus: The ARIC Study. Diabetes Res Clin Pract 1995;30:211–221 [DOI] [PubMed] [Google Scholar]
- 56.Lane JM, Liang J, Vlasac I, et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat Genet 2017;49:274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buysse DJ. Sleep health: Can we define it? Does it matter? Sleep 2014;37:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan KA, Hardas PP, Redline S, et al. Correlates of sleep quality in midlife and beyond: A machine learning analysis. Sleep Med 2017;34:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med 2008;9:S10–S17 [DOI] [PubMed] [Google Scholar]