Abstract
Purpose
The aim of this study was to explore intrinsic functional connectivity patterns in patients with herpes zoster (HZ) and postherpetic neuralgia (PHN).
Patients and methods
Thirty-three right-handed HZ patients (13 males; mean age 57.15±9.30 years), 22 right-handed PHN patients (9 males; mean age 66.13±6.77 years), and 28 well-matched healthy controls (HC) (9 males; mean age 54.21±7.72 years) underwent resting-state functional magnetic resonance imaging for intrinsic functional connectivity analyses. Functional connectivity density (FCD) was calculated and compared among the PHN, HZ, and HC groups. In addition, the Pearson correlation coefficient was calculated to compare various clinical indices in the regions with abnormal FCD values.
Results
Compared with the HC, both HZ and PHN patients showed significantly decreased FCD in the precuneus, and patients with HZ displayed significantly increased FCD in the brainstem/limbic lobe/parahippocampalgyrus, whereas patients with PHN displayed significantly increased FCD in the hippocampus (correlation thresholds r=0.25, voxel level of P<0.01 and Gaussian random field theory at a cluster level of P<0.05). However, the FCD was not significantly different between the PHN and HZ patients. Furthermore, the decreased FCD in the precuneus was positively correlated with the visual analog scale score in the PHN group (r=0.672; P=0.001).
Conclusion
Decreased connectivity of the precuneus occurred in both HZ and PHN patients, indicating a disrupted default-mode network. Furthermore, in the HZ group (initial stage of the virus infection), hyperconnectivity was observed in systems involved in pain transmission and interpretation, but hyperconnectivity only occurred in the hippocampus in the PHN group (neuropathic pain stage).
Keywords: functional connectivity density, herpes zoster, postherpetic neuralgia, functional magnetic resonance imaging, resting state, pain
Introduction
Herpes zoster (HZ) is caused by reactivation of the latent varicella zoster virus (VZV) in the spinal or cranial sensory ganglia. It is characterized by a painful erythematous rash in the affected dermatome.1 Individuals infected with VZV who are later diagnosed with HZ are at high risk for progressing to postherpetic neuralgia (PHN). There is no precise consensus on the definition of PHN. Three alternative definitions are usually used by researchers: pain after the rash has healed over;2 persistent pain for more than 1 month after the zoster rash;3,4 persistent pain for more than 3 month after the zoster rash.5 PHN is the most common and persistent complication of chronic neuropathic pain caused by VZV and affects the peripheral and central nervous systems.6–8 The sharp pain experienced by patients with PHN seriously affects their quality of life9 and increases the economic burden on society.10
Recently, several neuroimaging studies11–15 have reported structural and functional alterations in PHN patients, including anatomical damage in the bilateral insula and superior temporal gyrus;11 increased cerebral blood flow in the S1 area, inferior parietal lobule, insula, thalamus, amygdala, and striatum; decreased cerebral blood flow in the frontal cortex;12 and abnormal activation or intrinsic activity in several regions. In task functional magnetic resonance imaging (fMRI), abnormal functional activation was observed in areas involved in affective response, sensory discrimination (the thalamus, primary and secondary somatosensory cortices, insula, and anterior cingulate cortex), and emotion and reward (ventral striatum, amygdala, and orbital frontal cortex).13–15 Unlike task-fMRI, resting-state fMRI (rs-fMRI) focuses on intrinsic functional segregation (local) and integration (distributed) to provide a view of the functional architecture underlying potential neural mechanisms of neuropathic pain.16 Regional homogeneity (ReHo) and the fractional aptitude of low-frequency fluctuation (fALFF) are the most common and widely used methods for characterizing the dynamic properties of the neuronal processing unit. In patients with PHN, ReHo and fALFF show increased activity in the thalamus, cerebellum, limbic system, and frontal lobe and decreased activity in the temporal and parietal lobes.17 Cao et al18 noted differences in local brain activity between HZ and PHN patients using the ReHo and fALFF. In contrast to patients with PHN, patients with HZ showed significantly increased activity in the limbic system, temporal lobe, occipital lobe, and parietal lobe and decreased activity in most of the cerebellum.18 Decreased homotopic connectivity in the dorsolateral prefrontal cortex, the precuneus and posterior cingulate cortex resulted in a decrease in functional integration.16 However, researchers have not determined whether connectivity network disruptions develop in patients with HZ and PHN. Thus, the aim of the current study was to provide a comprehensive view of the effects of HZ and PHN on organizational principles or the connectome of brain function. Functional connectivity density (FCD) is a graph-based and data-driven measurement defined as the number of functional binary network connectivities between each voxel throughout the brain. In contrast to seed-based functional connectivity and independent component analyses, the FCD algorithm has the advantage of exploring the whole-brain connectivity in an unbiased manner,19 as shown in several other studies.20–22
Multiple systems throughout the brain participate in the perception of pain; however, researchers have not determined how the whole brain functionally interacts and integrates during resting states in patients with HZ and PHN. In this study, we sought to evaluate the alterations in whole-brain FCD in patients with HZ and PHN. We hypothesized that patients with PHN and HZ exhibit impaired intrinsic FCD throughout the brain, which was assessed using FCD analyses.
Materials and methods
Participants
All participants were right-handed. Patients were recruited from The First Affiliated Hospital of Nanchang University. Two consulting physicians from the Pain Department made the clinical diagnosis of HZ and PHN based on the International Association for the Study of Pain criteria.23 Consistent with prior literature, all patients with PHN reported persistent pain for more than 30 days following the initial rash caused by HZ or after the resolution of the dermatological symptoms of HZ.3,4 It was a cross-sectional study, not a longitudinal study of the same patients. The HZ or PHN patients underwent MRI scanning after diagnosis and enrolled in the study within 24 hours. All participants underwent an evaluation using the visual analog scale (VAS) for spontaneous pain intensity checking prior to undergoing MRI scans. All patients reported VAS scores ≥5, indicating moderate-to-severe pain (0 indicates no pain and 10 indicates the worst imaginable pain). No antidepressants or antipsychotic drugs were taken before the MRI scans. None of the participants had a history of psychiatric or neurological disorders or any other kind of pain. All age- and gender-matched healthy controls (HC) were also pain-free and did not have any structural abnormalities in the brain or neuropsychiatric disorders. Written informed consent was obtained from each participant or the participant’s guardian prior to data acquisition. The current study was conducted according to the approved guidelines and in compliance with the principles of the Declaration of Helsinki. This study was approved by the Medical Research Ethics Committee and the Institutional Review Board of The First Affiliated Hospital of Nanchang University.
MRI acquisition
All participants underwent MRI scans using a 3.0T Siemens Trio TIM Scanner (Erlangen, Bavaria, Germany) at The First Affiliated Hospital of Nanchang University. The rs-fMRI data were obtained using an echo planar imaging sequence (repetition time [TR] =2,000 ms, echo time [TE] =30 ms, flip angle =90°, matrix =64×64, field of view [FOV] =220×220 mm, 4 mm slice thickness, 30 interleaved axial slices, and 240 time point acquisitions). High-resolution anatomic 3-D T1 (TR =1,900 ms, TE =2.26 ms, flip angle =9°, matrix =240×256, FOV =215×230 mm, slice thickness =1.0 mm, and 176 sagittal slices) images, conventional T1-weighted (TR =250 ms, TE =2.46 ms, slices =19, slice thickness =5 mm, gap =1.5 mm, and FOV =220×220 mm) images, and T2-weighted (TR =4,000 ms, TE =113 ms, slices =19, slice thickness =5 mm, gap =1.5 mm, and FOV =220×220 mm) images were also acquired. For rs-fMRI scans, participants were instructed to close their eyes, remain awake and clear their minds as much as possible. Foam pads were used to reduce head movements, and earplugs were used to minimize scanner noise during the MRI scan. After the scan, subjects were asked whether they had remained awake during the entire scan.
Resting-state date preprocessing
Before preprocessing, conventional T1-weighted and T2-weighted images were reviewed by two senior radiologists in the Department of Radiology of The First Affiliated Hospital of Nanchang University to rule out structural brain lesions. The rs-fMRI images were preprocessed to adjust the time series of images using a toolbox for Data Processing & Analysis of Brain Imaging24 (DPABI 2.1; Chinese Academy of Sciences, Beijing, People’s Republic of China) on MATLAB 7.14.0 (Mathworks, Natick, MA, USA). Pre-processing comprised the following steps: 1) removal of the first 10 functional volumes; 2) slice timing correction; 3) 3-dimensional motion correction; 4) coregistration of each individual’s structural images to the functional images using a linear transformation. The transformed T1 structural images were segmented into white matter, gray matter, and cerebrospinal fluid using a new segment algorithm in DARTEL (diffeomorphic anatomical registration through exponentiated lie algebra);25 5) spatial normalization using the Montreal Neurological Institute template and resampling to 3×3×3 mm voxels; 6) linear detrending and nuisance signal removal (white matter, cerebrospinal fluid, global signal, 6-head motion parameters, 6-head motion parameters at one time point earlier, and the 12 corresponding squared items (Friston 24-parameter model) as covariates) via multiple regressions; and 7) bandpass filtering (ranging from 0.01 to 0.08 Hz) to reduce the effects of low-frequency drift and high-frequency noise. In this study, we excluded four subjects according to the head motion criteria of a maximum spin (x, y, z) of <2.0° and a maximum cardinal direction displacement (x, y, z) of <2.0 mm. The group differences in head motion were evaluated among the patients (HZ, n=33 and PHN, n=22) and the HC subjects (n=28) according to the frame-wise displacement criteria based on the method of Van Dijk et al.26
FCD analysis
FCD was used to evaluate the voxel-wise whole-brain functional connectivity.22 In this study, whole-brain FCD mapping was derived from the binary degree centrality data sets,27 which were calculated using DPABI (http://www.rfmri.org/dpabi). In a binary brain network, FCD is defined as the number of functional connections of a given voxel, as determined using the Pearson correlation coefficients between the time course of each voxel and those of other voxels using a threshold coefficient. To explore whether different thresholds had an impact on the results, five different correlation thresholds (r=0.15, 0.20, 0.25, 0.30, 0.35) were computed in this study.28
Statistical analysis
SPSS 19.0 (SPSS Inc, Chicago, IL, USA) was used to analyze demographic and clinical data. Two-sample t-tests were performed to detect differences in age. The χ2 test was used to compare the gender ratio. The significance level was set to P<0.05.
SPM12 was used to smooth FCD maps with an 8-mm kernel and analyze the smoothed connectivity maps at the group level. Analysis of covariance (ANCOVA) was performed using DPABI toolbox to examine differences among the three groups,19 and Bonferroni’s corrections were used for multiple comparisons, after eliminating the effects of age, gender, and educational level by regression. The significance threshold correction was based on the Gaussian random field theory, with a voxel level of P<0.01 and a cluster level of P<0.05.
FCD values for brain regions that showed significant group differences were extracted and averaged. Correlational analyses were performed to compare the FCD values to the VAS and disease duration in each patient group. Pearson’s correlation coefficients were calculated with a significance level of P<0.05.
Results
Demographic and clinical characteristics
Four patients were excluded due to head movements, and one participant in the HC group was excluded due to a history of mild traumatic brain injury. The final sample included 55 patients (33 HZ and 22 PHN) and 28 HC. The clinical characteristics of the HZ and PHN patients are listed in Table 1. No significant differences in age (P=0.962, 0.223, 0.192 for PHN vs HC, HZ vs HC, and PHN vs HZ, respectively, two-sample t-test) and gender (P=0.522, 0.557, and 0.911 for PHN vs HC, HZ vs HC, and PHNvs HZ, respectively, χ2 test) were observed among the three groups.
Table 1.
Participants’ information
| Clinical information | HZ patients (n=33) | PHN patients (n=22) | HC (n=28) |
|---|---|---|---|
| Age (years, mean ± SD) | 57.15±9.30 | 66.13±6.77 | 54.21±7.72 |
| Gender (male/female) | 13/20 | 9/13 | 9/19 |
| VAS score (mean ± SD) | 6.39±1.17 | 7.36±0.95 | NA |
| Disease duration (days, mean ± SD) | 11.24±6.67 | 60.22±41.24 | NA |
Abbreviations: HC, healthy controls; HZ, herpes zoster; NA, not applicable; PHN, postherpetic neuralgia; VAS, visual analog scale.
FCD results
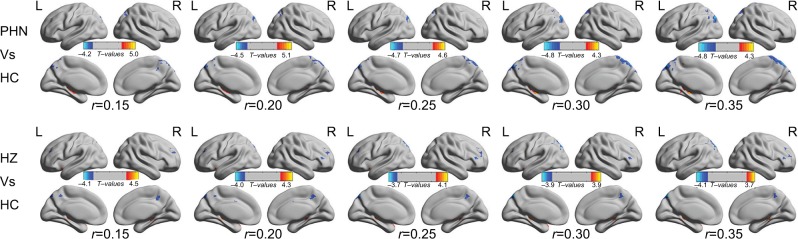
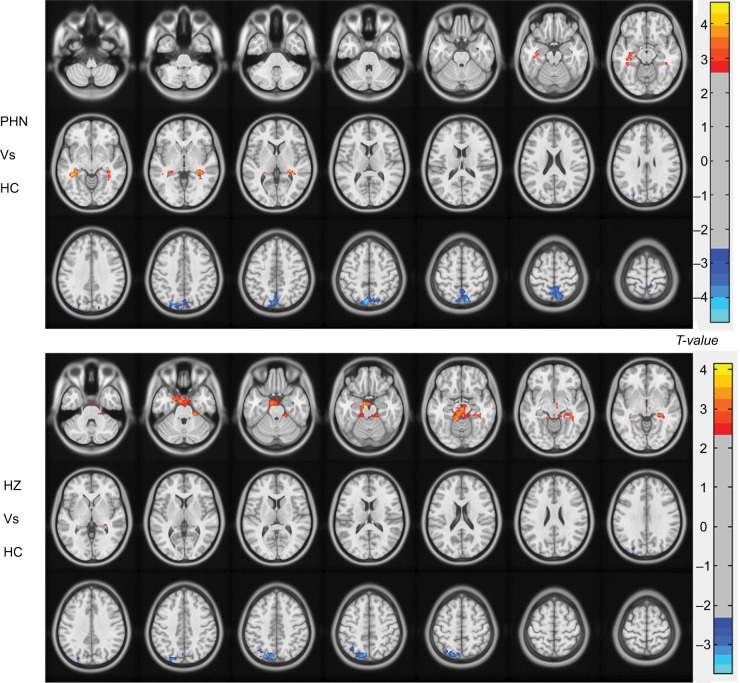
The results of the ANCOVA revealed differences in the FCD maps between the three groups. Using the ANCOVA based on DPABI, we identified three differences in the FCD results: PHN vs HC, HZ vs HC, and PHN vs HZ. Intergroup differences (Figure 1) were also remarkably similar, based on the different correlation thresholds (r=0.15, 0.2, 0.25, 0.3, and 0.35). Therefore, we primarily reported the FCD results in a weighted graph when the correlation threshold was 0.25. Compared to HC, patients with PHN exhibited a significantly decreased FCD in the precuneus and a significantly increased FCD in the bilateral hippocampus (HIP) (Figure 2 and Table 2). Compared to HC, patients with HZ exhibited significantly decreased FCD in the left precuneus and significantly increased FCD in the brainstem, limbic lobe, and parahippocampal gyrus (BM/LL/PHG) (Figure 2 and Table 2). However, the FCD was not significantly different between the PHN and HZ groups.
Figure 1.
Intergroup difference in FCD according to different correlation thresholds.
Notes: Both comparisons of the PHN vs HC and HZ vs HC groups showed remarkably similar alterations in FCD in different brain areas according to different correlation thresholds (r=0.15, 0.2, 0.25, 0.3 and 0.35) (P<0.001, cluster >20 voxels, uncorrected). The hot (cool) color indicates brain areas with significantly increased (decreased) FCD.
Abbreviations: HZ, herpes zoster; PHN, postherpetic neuralgia; HC, healthy control; FCD, functional connectivity density; L(R)H, left (right) hemisphere.
Figure 2.
Between-group differences in FCD (r=0.25) determined by ANCOVA (voxel level of P<0.01, GRF corrected at cluster level of P<0.05).
Abbreviations: HZ, herpes zoster; PHN, postherpetic neuralgia; HC, healthy controls; FCD, functional connectivity density; ANCOVA, analysis of covariance; GRF, Gaussian random field.
Table 2.
Significant differences in FCD among patients with HZ and PHN and HC (r=0.25)
| Condition | L/R | Brain regions | MNI X, Y, Z | Peak | Voxel size |
|---|---|---|---|---|---|
| PHN vs HC | |||||
| L/R | Precuneus | –6, –75, 51 | –4.7079 | 462 | |
| L | HIP | –39, –39, –12 | 4.6489 | 128 | |
| R | HIP | 39, –39, –9 | 4.0002 | 100 | |
| HZ vs HC | |||||
| L | Precuneus | –9, –75, 48 | –3.6394 | 195 | |
| L/R | BM/LL/PHG | –9, –9, –21 | 3.7638 | 504 |
Notes: ANCOVA (the results are based on GRF theory correction, with a voxel level of P<0.01 and a cluster level of P<0.05).
Abbreviations: ANCOVA, analysis of covariance; BM/LL/PHG, brainstem, limbic lobe and parahippocampal gyrus; FCD, functional connectivity density; GRF, Gaussian random field; HC, healthy control; HIP, hippocampus; HZ, herpes zoster; L(R), left (right) hemisphere; MNI, Montreal Neurological Institute; PHN, postherpetic neuralgia.
Correlation between clinical variables and FCD
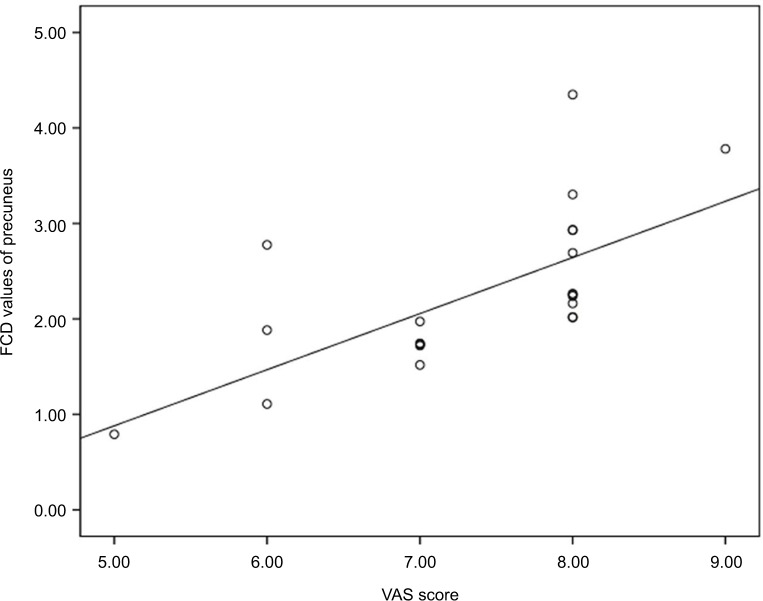
According to the correlation analyses, the FCD value for the precuneus was positively correlated with the VAS score of the PHN group (r=0.672; P=0.001) (Figure 3 and Table 3). However, a significant correlation was not observed between increased FCD and other clinical variables.
Figure 3.
Correlations between abnormal FCD and VAS score in patients with PHN.
Notes: A significant positive correlation was observed between the FCD in the precuneus and the VAS in patients with PHN.
Abbreviations: VAS, visual analog scale; FCD, functional connectivity density; PHN, postherpetic neuralgi.
Table 3.
Correlations between abnormal FCD and clinical variables
| Clinical variables | PHN
|
HZ
|
||
|---|---|---|---|---|
| Precuneus (r/P) | Hippocampus(r/P) | Left precuneus (r/P) | BM/LL/PHG (r/P) | |
| VAS score | 0.672/0.001 | 0.408/0.006 | 0.295/0.096 | 0.133/0.459 |
| Disease duration | 0.213/0.341 | 0.208/0.352 | 0.159/0.377 | 0.021/0.906 |
Abbreviations: BM/LL/PHG, brainstem/limbic lobe/parahippocampal gyrus; HZ, herpes zoster; PHN, postherpetic neuralgia; VAS, visual analog scale; FCD, functional connectivity density.
Discussion
In the current study, we investigated alterations in intrinsic functional connectivity patterns in both HZ and PHN patients using FCD. The FCD was decreased in the precuneus in both HZ and PHN patients. Moreover, patients with PHN showed increased FCD in the HIP, whereas patients with HZ showed increased FCD in the BM/LL/PHG. No significant difference was detected between patients with HZ and patients with PHN. Thus, the impairment in FCD in the precuneus may be relevant to pain-related impairment, and the connectivity of the precuneus may be disrupted in patients with HZ (initial stage of the virus infection). Alterations in functional connectivity in the limbic system (LL and HIP) may also play an important role in the neuropathological mechanism of pain in patients with HZ.
In our study, decreased FCD was detected in the precuneus. The precuneus is the major functional node of the default-mode network. The default-mode netwrok shows the greatest activity at baseline, but its activity is suspended or attenuated during attention-demanding cognitive tasks requiring a high degree of functional connectivity.29,30 The posterior cingulate cortex/precuneus remains highly active at rest and continuously gathers information from both the external and internal milieu, which is important for monitoring sensory information.29 Furthermore, the activity of the precuneus impacts the network connectivity of cortical and subcortical structures involved in processing highly integrated and associative information; the precuneus plays an essential role in the implementation of a wide range of higher-order cognitive functions.31 In this study, we observed decreased FCD in the precuneus in both HZ and PHN patients. Furthermore, a positive correlation between the VAS and the FCD value in the precuneus was observed. Based on these findings, the connectivity in the precuneus may be disrupted in patients with HZ (initial stage of the virus infection), and the impairment of the precuneus may be relevant to the sensation of pain. This impairment might also be related to the unpleasant cognitive experience of patients with HZ.
In addition, we also observed significantly increased FCD in the limbic system and the brainstem of HZ patients. Compared to HC, patients with PHN showed increased FCD in the HIP. Brain regions and functional networks have been shown to be altered during the interval between acute to chronic pain,32,33 and the HIP plays an important role in long-term pain,34–36 which involves pain-related memory and emotions.35,36 As shown in the study by Cardoso Cruz et al,37 the rat HIP is intimately involved in pain memories. This finding may explain why patients with PHN experience recurrent, long-term pain stimuli. We believe that unpleasant emotions are processed in the HIP and sent to the cerebral cortex to be transformed into long-term memory. In parallel, compared to HC, patients with HZ showed significantly increased FCD in the BM/LL/PHG. The brainstem plays major roles in pain processing and the modulation of nociceptive input. It is an important structure in the modulation of both ascending and descending pain pathways. The spinal cord–thalamus pathway participates in pain transmission, the cortex and limbic system are involved in pain interpretation, and the spinal cord–thalamus–cortex–limbic system is a pathway for pain transmission and interpretation.38 In this study, the brainstem and limbic system showed increased FCD, indicating that the BM/LL/PHG, which is involved in the transmission and interpretation of pain, was activated in patients with HZ. The regions involved in pain transmission and interpretation were mainly activated in the initial stage of the virus infection (HZ). The limbic regions of the pain matrix encode emotional aspects of pain perception, and the primary sensory region encodes the intensity of the pain sensation.39,40 Recent pain studies in patients with PHN have also reported functional changes in the limbic regions.12,14 But our results were different from previous touch task-fMRI study13 in PHN, which may indicated the central nervous mechanism of PHN were different between the spontaneous pain and touch-induced pain. rs-fMRI focuses on intrinsic functional connection or correlated spontaneous activity, while task-fMRI main reflects persistent or recurrent patterns of evoked coactivation.
In this study, different altered pattern was detected in HZ and PHN, but no significant difference of FCD was detected between HZ and PHN brains in this study. First, the pain of PHN and HZ is thought to result from damage in a nerve by HZ, but the damage causes nerves in the affected dermatomic area of the skin to send abnormal electrical signals to the brain in PHN. A key factor in the neural plasticity underlying neuropathic pain is altered gene expression in sensory dorsal root ganglia neurons.1 In recently studies, functional and structural change might be correlated with HZ-PHN chronification.41 One of the reasons for the not-significant difference of FCD between HZ and PHN is inhomogeneity functional alteration or alteration of above the correlation thresholds (r=0.25) in those patients, the sample size (patients with PHN), and rigorous statistical methods of FCD comparison.
Several limitations to this study should be considered. First, PHN is associated with depression and anxiety,42,43 which may influence intrinsic brain function. Although no patients with depression and anxiety were enrolled in the current study, we did not specifically perform cognitive and emotional evaluations. Second, the sample size (patients with PHN) was limited and the pain duration (mean 60.2 days) was relatively short. Although monitoring patients from the acute pain stage (HZ) to the chronic stage (PHN) is not easy, we believe it is an effective way to eliminate individual differences that may influence the reliability of the results. Third, FCD was used to compute the long-range and short-range FCD values for each voxel in the brain. Future studies should further explore the differences between long-range FCD and short-range FCD throughout the brain. Finally, patients all have different dermatome involvement. Although no literature show the impact of the distribution of skin lesions on HZ brain, different affected areas may be lead to potentially difference in HZ brain. These need further exploration.
Conclusion
This novel study used voxel-wise FCD to investigate alterations in intrinsic functional connectivity in patients with HZ and PHN. The disrupted connectivity in the precuneus in both HZ and PHN patients reflects changes in the BM/LL/PHG that may mediate the sensation of pain. This pathway was mainly activated in patients with HZ (initial stage of the virus infection), whereas the HIP was activated in patients with PHN (neuropathic pain stage). The HIP may participate in the production of pain memory. These findings expand our understanding of the functional characteristics of HZ and may provide new insights that will improve our understanding of the dysfunctional alterations implicated in the pathophysiology of HZ.
Acknowledgments
The study was supported by grants from the Natural Science Foundation of Jiangxi Province (grant no 2015ZBAB205021) and the Jiangxi Province Education Department Support Program (grant no GJJ160128).
Footnotes
Author contributions
Shunda Hong, Lili Gu, Fuqing Zhou, and Jian Jiang designed the study. Shunda Hong, Lili Gu, Jiaqi Liu, and Muhua Huang acquired the data. Shunda Hong, Fuqing Zhou, Jian Jiang, and Jiaqi Liu processed the neuroimaging data. Jian Jiang performed the statistical analyses. Shunda Hong, Lili Gu, Fuqing Zhou, Jiaqi Liu, Muhua Huang, Jian Jiang, Laichang He, Honghan Gong, and Xianjun Zeng wrote the initial draft. All authors contributed to data analysis, drafting, and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jeon YH. Herpes zoster and postherpetic neuralgia: practical consideration for prevention and treatment. Korean J Pain. 2015;28(3):177–184. doi: 10.3344/kjp.2015.28.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmader K. Postherpetic neuralgia in immunocompetent elderly people. Vaccine. 1998;16(18):1768–1770. doi: 10.1016/s0264-410x(98)00137-6. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137(1):38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 4.Snedecor SJ, Sudharshan L, Cappelleri JC, et al. Systematic review and meta-analysis of pharmacological therapies for pain associated with postherpetic neuralgia and less common neuropathic conditions. Int J Clin Pract. 2014;68(7):900–918. doi: 10.1111/ijcp.12411. [DOI] [PubMed] [Google Scholar]
- 5.Keating GM. Shingles (Herpes Zoster) vaccine (Zostavax(®)): a review in the prevention of Herpes Zoster and Postherpetic Neuralgia. Biodrugs. 2016;30(3):243–254. doi: 10.1007/s40259-016-0180-7. [DOI] [PubMed] [Google Scholar]
- 6.Gan EY, Tian EA, Tey HL. Management of herpes zoster and postherpetic neuralgia. Am J Clin Dermatol. 2013;14(2):77–85. doi: 10.1007/s40257-013-0011-2. [DOI] [PubMed] [Google Scholar]
- 7.Bennett GJ, Watson CP. Herpes zoster and postherpetic neuralgia: past, present and future. Pain Res Manag. 2009;14(4):275–282. doi: 10.1155/2009/380384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oaklander AL. The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain. 2001;92(1):139–145. doi: 10.1016/s0304-3959(00)00481-4. [DOI] [PubMed] [Google Scholar]
- 9.Pickering G, Gavazzi G, Gaillat J, Paccalin M, Bloch K, Bouhassira D. Is herpes zoster an additional complication in old age alongside comorbidity and multiple medications? Results of the post hoc analysis of the 12-month longitudinal prospective observational ARIZONA cohort study. BMJ Open. 2016;6:e009689. doi: 10.1136/bmjopen-2015-009689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friesen KJ, Falk J, Alessi-Severini S, Chateau D, Bugden S. Price of pain: population-based cohort burden of disease analysis of medication cost of herpes zoster and postherpetic neuralgia. J Pain Res. 2016;9:543. doi: 10.2147/JPR.S107944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu B. Microstructural abnormalities in gray matter of patients with postherpetic neuralgia: a diffusional kurtosis imaging study. Pain Physician. 2016;19(4):E601–E611. [PubMed] [Google Scholar]
- 12.Liu J, Hao Y, Du M, et al. Quantitative cerebral blood flow mapping and functional connectivity of postherpetic neuralgia pain: a perfusion fMRI study. Pain. 2013;154(1):110–118. doi: 10.1016/j.pain.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Geha PY, Baliki MN, Wang X, Harden RN, Paice JA, Apkarian AV. Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain. 2008;138(3):641–656. doi: 10.1016/j.pain.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Liu J, Li L, et al. A study on small-world brain functional networks altered by postherpetic neuralgia. Magn Reson Imaging. 2014;32(4):359–365. doi: 10.1016/j.mri.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128(1):88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jian J, Gu L, Dan B, et al. Altered homotopic connectivity in postherpetic neuralgia: a resting state fMRI study. J Pain Res. 2016;9:877–886. doi: 10.2147/JPR.S117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao S, Song G, Zhang Y, et al. Abnormal local brain activity beyond the pain matrix in postherpetic neuralgia patients: a resting-state functional MRI study. Pain Physician. 2017;20(2):E303. [PubMed] [Google Scholar]
- 18.Cao S, Li Y, Deng W, et al. Local brain activity differences between herpes zoster and postherpetic neuralgia patients: a resting-state functional MRI study. Pain Physician. 2017;20(5):E687. [PubMed] [Google Scholar]
- 19.Beucke JC, Sepulcre J, Talukdar T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70(6):619–629. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 20.Zuo XN, Ehmke R, Mennes M, et al. Network centrality in the human functional connectome. Cereb Cortex. 2012;22(8):1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
- 21.Tomasi D, Volkow ND. Functional connectivity density mapping. Proc Natl Acad Sci USA. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Lan L, Yi S, et al. Abnormal intrinsic functional hubs in severe male obstructive sleep apnea: evidence from a voxel-wise degree centrality analysis. PLoS One. 2016;11(10):e0164031. doi: 10.1371/journal.pone.0164031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: International Association for the Study of Pain; 1994. [Google Scholar]
- 24.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14(3):339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 25.Goto M, Abe O, Aoki S, et al. Diffeomorphic anatomical registration through exponentiated lie algebra provides reduced effect of scanner for cortex volumetry with atlas-based method in healthy subjects. Neuroradiology. 2013;55(7):869–875. doi: 10.1007/s00234-013-1193-2. [DOI] [PubMed] [Google Scholar]
- 26.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai F, Lei G, Gong H, et al. Network centrality of resting-state fMRI in primary angle-closure glaucoma before and after surgery. PLoS One. 2015;10(10):e0141389. doi: 10.1371/journal.pone.0141389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 31.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain J Neurol. 2006;129(Pt 3):564. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 32.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136:2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gondo M, Moriguchi Y, Kodama N, et al. Daily physical complaints and hippocampal function: an fMRI study of pain modulation by anxiety. Neuroimage. 2012;63:1011–1019. doi: 10.1016/j.neuroimage.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Ploghaus A, Narain C, Beckmann CF, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. Neuroscience. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang S, Kroll A, Lipinski SJ, et al. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 2009;29:823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardoso-Cruz H, Lima D, Galhardo V. Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J Neurosci. 2013;33:2465–2480. doi: 10.1523/JNEUROSCI.5197-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krarup C. An update on electrophysiological studies in neuropathy. Curr Opin Neurol. 2003;16(5):603–612. doi: 10.1097/01.wco.0000093104.34793.94. [DOI] [PubMed] [Google Scholar]
- 39.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 40.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 41.Cao S, Qin B, Zhang Y, et al. Herpes zoster chronification to postherpetic neuralgia induces brain activity and grey matter volume change. Am J Transl Res. 2018;10(1):184–199. [PMC free article] [PubMed] [Google Scholar]
- 42.Sah DW, Ossipo MH, Porreca F. Neurotrophic factors as novel therapeutics for neuropathic pain. Nat Rev Drug Discov. 2003;2:460–472. doi: 10.1038/nrd1107. [DOI] [PubMed] [Google Scholar]
- 43.Denkinger MD, Lukas A, Nikolaus T, Peter R, Franke S, AS Group Multisite pain, pain frequency and pain severity are associated with depression in older adults: results from the ActiFE Ulm study. Age Ageing. 2014;43:510–514. doi: 10.1093/ageing/afu013. [DOI] [PubMed] [Google Scholar]