Abstract
Pea (Pisum sativum) and Arabidopsis contain similar, if not identical, blue-light (BL)-responsive systems that alter expression of specific members of the Lhcb (light-harvesting chlorophyll-binding) gene family. In both plants a single, short pulse of low-fluence BL (threshold = 10−1 μmol m−2) causes an increase in the rate of transcription from specific members of the Lhcb gene family in etiolated seedlings. Constructs of the BL-regulated pea Lhcb1*4 promoter (PsLhcb1*4) were created, which altered sequences previously implicated in light responses, deleted the 5′-promoter sequence, or removed the 5′-untranslated region. These constructs were tested for BL induction in transgenic Arabidopsis. The PsLhcb1*4 promoter deletions to −150 bp maintained normal fluence response, time course, and reciprocity characteristics. The 5′- untranslated region contained enhancer elements, but was not necessary for BL induction. The −95 to +2 promoter was capable of responding to BL, whereas sequences from −50 were not. Promoters that lack conserved light-regulatory elements or sequences directly implicated in phytochrome and circadian responses retained BL activity, suggesting that the low-fluence BL response utilizes regions of the promoter independent of those that modulate the phytochrome and circadian responses.
Plants possess multiple photomorphogenic systems designed to sense and respond to light signals. These systems identify specific wavelengths and quantities of light, and tailor programs of gene expression in concert with the developmental state of the plant and prevailing environmental conditions. These photomorphogenic systems include the red/far-red responsive phytochrome systems (Furuya and Schafer, 1996) and several independent BL/UV systems (Young et al., 1992; Kaufman, 1993; Jenkins, 1997).
Genetic and physiological analyses have identified a series of Arabidopsis mutants with defects in perception/transduction of BL signals. The BL mutants include plant lines with defects in the BL photoreceptors CRY1 (Ahmad and Cashmore, 1996), CRY2, (Ahmad et al., 1998), and NPH1 (Liscum and Briggs, 1995; Huala et al., 1997), which are responsible for BL-mediated suppression of hypocotyl elongation and phototrophic curvature, respectively. icx1 mutants exhibit abnormally high transcript levels of flavonoid-biosynthesis genes and increased production of anthocyanins, suggesting that ICX1 may be a negative regulator of the BL/UV systems (Jackson et al., 1995). The persistence of known BL responses, including the induction of Lhcb (light-harvesting chlorophyll-binding) gene transcription by the BLF system (threshold = 10−1 μmol m−2) in pea (Pisum sativum) and Arabidopsis, PsLhcb and AtLhcb, respectively, indicates the presence of additional BL photomorphogenic systems (Gao and Kaufman, 1994).
In pea and Arabidopsis the steady-state level of Lhcb transcript is dependent upon the total fluence of BL received. Excitation of the BLF system increases the rate of transcription of specific members of the PsLhcb1 and AtLhcb1 gene families. This increase in the rate of transcription occurs in response to a single pulse of BL with a threshold of 10−1 μmol m−2. The BL-enhanced rate of transcription continues to increase with increasing fluences of BL, reaching a maximum transcription rate at 104 μmol m−2. The response is immediate, does not require protein synthesis, and probably acts through a heterotrimeric G protein (Warpeha and Kaufman, 1990; Marrs and Kaufman, 1991).
Only specific members of the Lhcb1 gene family are induced by BL. Pea contains at least eight members of this gene family (Alexander et al., 1991). The PsLhcb1*1 and Lhcb1*4 genes are regulated by BL, whereas the Lhcb1*2 and Lhcb1*3 gene are not (White et al., 1995; Tilghman et al., 1997). The Lhcb genes of Arabidopsis are also expressed differentially in response to various light signals. Gao and Kaufman (1994) reported that the AtLhcb1*3 gene is induced by BLF in etiolated seedlings, whereas the AtLhcb1*1 and AtLhcb1*2 genes are not. Sun and Tobin (1990) determined that all three of the AtLhcb1 genes are phytochrome responsive in etiolated Arabidopsis seedlings.
Tilghman et al. (1997) introduced 1.3 kb of the BL-regulated PsLhcb1*4 promoter placed upstream of a GUS reporter gene into Arabidopsis and illustrated that the transgene functioned correctly in response to BL with respect to time course, fluence response, and reciprocity. These results indicated that Arabidopsis is a suitable host with which to study transgenic promoter constructs of the PsLhcb1*4 gene, and demonstrated that the AtLhcb1*3 promoter from –200 bp is inducible by the BLF system.
The −100 to +1 region of the BL-regulated PsLhcb1*4 and the AtLhcb1*3 promoters are more similar to each other than to the comparable region of non-BL-regulated Lhcb promoters (for review, see Arguello-Astorga and Herrera-Estrella, 1998). The −100 to +1 region contains sequence elements implicated in light regulation, including elements that confer responses to phytochrome (Kehoe et al., 1994; Keningsbuch and Tobin, 1995) and circadian induction (Anderson and Kay, 1995). Regions of Lhcb promoters regulated by BL have yet to be identified. The goal of the present study was to utilize transgenic Arabidopsis to determine the region(s) of the pea Lhcb1*4 promoter and/or 5′-UTR necessary for induction of the BLF response.
Our data indicate that truncated versions of the PsLhcb1*4 gene maintain the normal photobiological characteristics of the full-length promoter. The basal response of the BLF system utilizes sequences in the PsLhcb1*4 gene between −95 and +2, and sequences present upstream of −95 or in the 5′-UTR enhance transcription directed by this promoter. Previously defined elements, identified for their roles in phytochrome and circadian regulation, are not necessary for the BL response.
MATERIALS AND METHODS
Plant Material
Arabidopsis ecotype Columbia (Col-0) plants were cultivated in growth rooms at 22°C to 23°C under 90 μmol m−2 white light supplied by cool-white fluorescent bulbs (Econo-W, Philips, Eindhoven, The Netherlands). Seed was collected from dried plants and stored at 4°C.
All planting for photophysiological assays was done under a green safelight (Warpeha and Kaufman, 1989). Seeds were surface-sterilized in 60% (v/v) commercial bleach for 10 min, rinsed three times in at least 10 volumes of water, and then resuspended in 0.5× Murashige-Skoog medium and 0.8% (w/v) low-melting-point agarose. The Murashige and Skoog/agarose/seed suspension was plated onto 50 mL of solidified 0.5× Murashige and Skoog medium containing 0.8% (w/v) agarose in Phytatrays (Sigma), and were then stratified at 4°C for 48 h. Following stratification, the seeds were irradiated with 16 μmol m−2 white light for 10 min to synchronize germination, and were then grown in absolute darkness at 22°C for 6 d.
PsLhcb1*4 Deletion and Mutagenized Constructs
Promoter deletion and replacement constructs were created in the vector pBSK101.3, which contains the GUS (uidA) coding region and the NOS terminator from the pBI101 vector (Jefferson et al., 1987) inserted into the BamHI and EcoRI sites of pBSK(+) (Stratagene). The downstream HindIII site was filled in, and a new HindIII site along with a Pst I site was inserted via a synthetic linker placed upstream of GUS.
Independent promoter constructs representing 5′ promoter deletions of the pea Lhcb1*4 promoter were generated using PCR with Pfu polymerase (Stratagene). Primers were designed to produce promoter fragments extending from −281, −250, −200, −150, −100, −50, or +1 on the upstream end to +64 on the downstream end, and from −281 and −100 on the upstream end to +2 on the downstream end. The primers were designed with PstI and BamHI sites (upstream and downstream, respectively) to allow directional insertion of promoter fragments upstream of the GUS reporter gene in pBSK101.3 vector.
The REP7550 construct contains the PsLhcb1*4 promoter from −281 to +2, wherein the sequence between −75 and −50 has been replaced by a nonsense sequence. The region between −75 and −50 contains several highly conserved sequence elements that have been identified in the promoters of light-regulated genes: a sequence referred to as “G-box like” (Arguello-Astorga and Herrera-Estrella, 1996) and a double-GATA sequence known as the “I box” (Borello et al., 1993). These elements have been previously implicated in phytochrome (Kehoe et al., 1994) and circadian (Anderson and Kay, 1995) regulation of Lhcb1 genes. Both of these conserved sequences exist in the BL-regulated pea and Arabidopsis Lhcb1 promoters between −75 and −50. This region was replaced with a nonsense sequence using PCR-based overlap extension mutagenesis (Ho et al., 1989) utilizing primers containing nonsense sequence between −75 and −50. The mutagenized promoters were placed upstream of GUS in the pBSK101.3 vector into the PstI and BamHI sites.
Promoter/GUS fusions were moved into a modified form of the plant-transformation vector pPZP100 (Hajdukiewicz et al., 1994) called pBL. The pBL vector contains a 35S-Bar cassette conferring basta resistance to transformed plants (Bechtold et al., 1993), which is oriented toward the right border. A reference construct containing a copy of the Arabidopsis 1.3-kb Lhcb1*3 promoter fused to the luciferase reporter is present 3′ of the left border, and was designed for use in future transcription assays. Experimental constructs containing pea Lhcb1*4 promoter/5′-UTR deletions fused to GUS were introduced into unique PstI and XhoI sites in an opposite orientation to and upstream of the 35S::Bar cassette.
Transformation
Arabidopsis plants were transformed by vacuum infiltration (Bechtold et al., 1993). Transgenic plants were selected by resistance to the herbicide Finale (Roussel Uclaf, Montvale, NJ). Confirmation of the presence of the correct experimental promoter constructs was determined by PCR in each independent transformed line using primers that flank the promoter region.
Treatment and Analysis
Plants were grown for 6 d on 0.5× Murashige and Skoog medium in absolute darkness. The BL source was identical to that described in Gao and Kaufman (1994). To assess relative promoter response, a minimum of 10 independent transgenic plant lines for each construct were treated with a single 2-min/47-s pulse of BL with a total fluence of 104 μmol m−2 or with a mock pulse. BLF-mediated induction directed by the truncated PsLhcb1*4 promoters was compared with the induction of the BL-regulated endogenous Arabidopsis Lhcb1 gene. Plants containing the REP7550 constructs were treated with either 102 μmol m−2 BL or 1.8 × 102 μmol m−2 red light, as described previously (Brusslan and Tobin, 1992; Gao and Kaufman, 1994). The −95 to +2 constructs and the BL perception mutants were treated with BL at 102 μmol m−2 or 104 μmol m−2 or with a mock pulse.
Fluence response, time course, and reciprocity experiments were performed on transgenic lines containing the −281 to +64 (with 5′-UTR) promoter, the −281 to +2 promoter (without 5′-UTR), and the −150 to +64 promoter (with 5′-UTR). Fluence-response experiments were performed by treating 6-d-old dark-grown plants with a single pulse of BL at a fluence ranging from 10−1 to 104 μmol m−2, or with a mock pulse. In these experiments tissue was harvested 2 h after termination of the pulse.
Time-course experiments consisted of treating 6-d-old dark-grown plants with a single pulse of BL with a total fluence of 102 μmol m−2. The plants were then harvested at 5, 15, 30, 60, and 120 min after the pulse.
Reciprocity experiments involved treating 6-d-old dark-grown seedlings with a single pulse of BL at 102 μmol m−2 delivered in 5, 20, 100, or 1110 s. Plants were harvested 2 h after the completion of the BL pulse.
The fha1 and nph1 mutants were irradiated with either 102 μmol m−2 or a mock pulse. Plant tissue was harvested 2 h after the completion of the irradiation.
RNA was extracted and used for northern blotting, as previously described (Tilghman et al., 1997), or for slot blotting for quantitative analysis. Transcript accumulation was quantified on a phosphor imager (Molecular Dynamics, Sunnyvale, CA).
RESULTS
REP7550 Response to BL and Phytochrome Induction
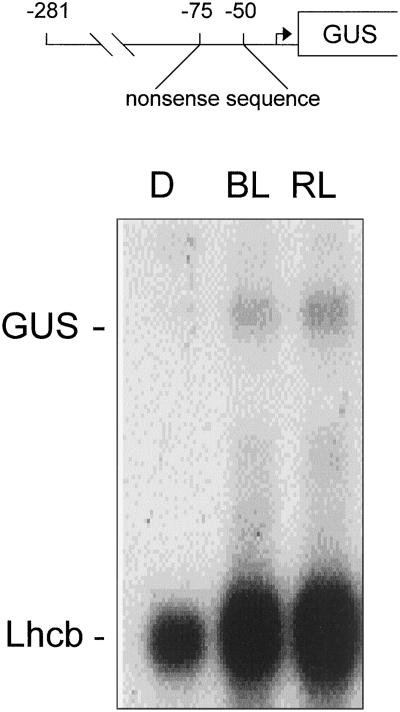
The REP7550 construct represents the pea Lhcb1*4 promoter from −281 to +2 (relative to the site of transcriptional initiation) where the region from −75 to −50 has been replaced with a random sequence. The −75 to −50 substitution eliminates the double-GATA sequence (I box), which has been previously implicated in light regulation (Donald and Cashmore, 1990). The sequence between −49 and +2, which contains the TATA box and the site of initiation, remains unchanged. The sequence between −76 and −281 also remains unchanged. Plants containing this construct are capable of accumulating GUS RNA in response to a single pulse of 102 μmol m−2 BL (Fig. 1). This transgene also maintains its response to phytochrome, as GUS RNA accumulates following a single pulse of red light at 1.8 × 102 μmol m−2.
Figure 1.
The region from −75 to −50 of the PsLhcb1*4 gene is not necessary for phytochrome or BLF regulation. Six-day-old dark-grown transgenic Arabidopsis seedlings containing the mutagenized pea Lhcb1*4 promoter construct REP7550, in which the sequence between −75 and −50 was replaced by a nonsense sequence fused to GUS, were irradiated with a single pulse of either BL or red light (RL) with a total fluence of 102 μmol m−2 or a mock pulse (D). RNA was prepared 2 h after the light treatment and analyzed by northern blotting. The resulting blots were probed simultaneously for GUS and Lhcb RNA, representing expression of the transgene and the endogenous Arabidopsis Lhcb, respectively.
The substitution of the −75 to −50 region failed to eliminate the response to BL, indicating that the sequences necessary for the PsLhcb1*4 gene to respond to excitation of the BLF system are not within this region. A series of constructs with truncated upstream regions was created to help define upstream sequences necessary for the BLF response. PsLhcb1*4 promoter constructs were made extending from −281, −250, −200, −150, and −50 on the upstream end to +64 (where +1 represents the site of transcription initiation and +1 to +64 represents the 5′-UTR) on the downstream end.
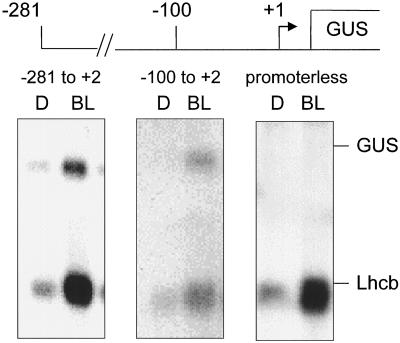
Although we tried on several occasions, we were unable to obtain transgenic lines containing a promoter construct extending from −100 on the upstream end to +64 on the downstream end. In each case we were able to obtain basta-resistant, transformed seedlings; however, the region between −100 and +64 had undergone a rearrangement. This rearrangement was not present in the Agrobacterium tumefaciens strain used to effect the transformation. As a consequence, we eliminated the 5′-UTR (+2 to +64) from the construct. The integrated construct, extending from −100 to +2, did not exhibit these rearrangements.
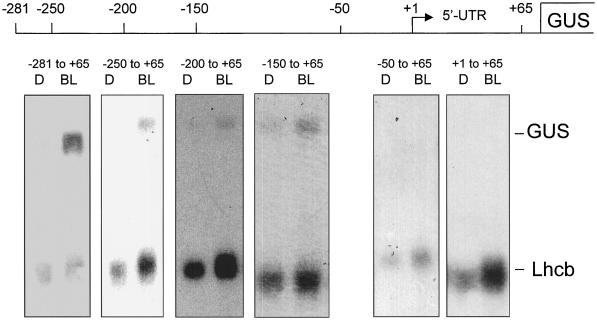
Constructs containing the PsLhcb1*4 upstream sequence extending to −281, −250, −200, and −150 were all capable of responding to low-fluence BL (Fig. 2). The region from −50 to +64 was not capable of initiating a BLF response. Constructs representing the upstream region from −281 or −100 to +2 (i.e. without the 5′-UTR) were also capable of responding to a BL pulse of 104 μmol m−2 (Fig. 3).
Figure 2.
Northern analysis of GUS mRNA accumulation from truncated Lhcb1*4 promoters. Six-day-old dark-grown transgenic Arabidopsis seedlings containing truncated pea Lhcb1*4 promoter constructs fused to GUS were irradiated with a single pulse of BL with a total fluence of 104 μmol m−2 or a mock pulse (D). RNA was prepared 2 h after the light treatment and analyzed by northern blotting. The resulting blots were probed simultaneously for GUS and Lhcb RNA, representing expression of the transgene and the endogenous Arabidopsis Lhcb, respectively. The pea Lhcb1*4 promoters (as indicated on the figure) extend from +65 on the downstream end (where +1 represents the site of transcriptional initiation and +65 represents the A from the ATG) to −281, −200, −150, −50, or −1 on the upstream end.
Figure 3.
Steady-state levels of GUS and Lhcb RNA in response to 104 μmol m−2 BL from constructs lacking the Lhcb 5′-UTR. Six-day-old dark-grown transgenic Arabidopsis seedlings containing truncated pea Lhcb1*4 promoter constructs fused to GUS were irradiated with a single pulse of BL with a total fluence of 104 μmol m−2 or a mock pulse (D). RNA was prepared 2 h after the light treatment and analyzed by northern blotting. The resulting blots were probed simultaneously for GUS and Lhcb RNA, representing expression of the transgene and the endogenous Arabidopsis Lhcb, respectively. The pea Lhcb1*4 promoters (as indicated on the figure) extend from +2 on the downstream end (where +1 represents the site of transcriptional) to either −281 or −100 on the upstream end.
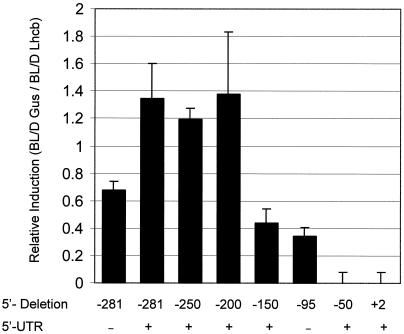
The relative promoter strength of these constructs was measured by comparing the induction of the transgene to the induction of the endogenous BL-responsive AtLhcb gene in the same plant line following BL treatment (Fig. 4). The constructs extending from −281, −250, and −200 exhibited a response to 104 μmol m−2 BL that was identical to that of the endogenous Lhcb1 promoter. The construct extending to −150 directs BL expression at 43% of the level of the constructs containing sequence up to and beyond −200. Based on these data, it is likely that enhancer sequences lie in this −150 to −200 region.
Figure 4.
Relative induction of GUS RNA from Lhcb1*4 truncated promoters. Six-day-old dark-grown Arabidopsis seedlings from each of 10 independent transgenic lines for each truncated pea Lhcb1*4 promoter construct indicated on the figure, were grown separately and irradiated with a single pulse of BL with a total fluence of 104 μmol m−2 or were given no light (D). RNA was prepared 2 h after the light treatment and analyzed by northern blotting. The resulting blots were probed simultaneously for GUS and Lhcb RNA, representing expression of the transgene and the endogenous Arabidopsis Lhcb, respectively. The resulting BL-induced change in GUS RNA in each line was compared with the induction of the endogenous Lhcb gene in that line. The ratio of the two inductions is presented on the figure. Error bars represent se of the mean.
As noted above, sequences within the pea Lhcb1*4 5′-UTR (+1 to +64) are not necessary for the BLF response (Fig. 3). However, removal of this sequence did result in a decrease in the amplitude of the response (Fig. 4), indicating the presence of one or more enhancer elements in the PsLhcb1*4 5′-UTR.
Photophysiological Responses
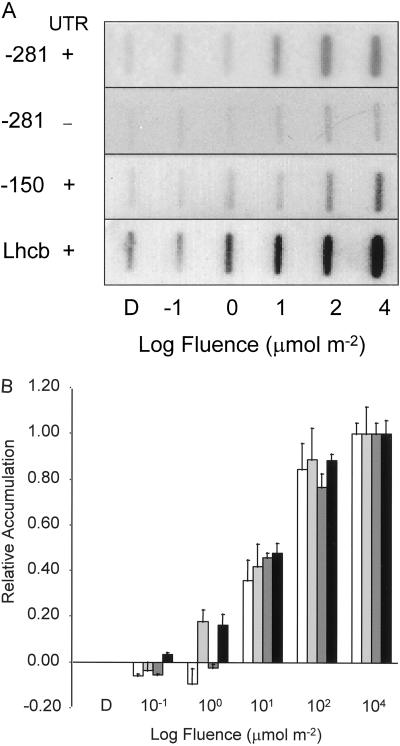
It is important to establish that the photophysiological characteristics of the truncated promoters were identical to the full-length endogenous promoter. Fluence response, time course of accumulation, and reciprocity characteristics were assayed for the −281 promoter with and without the 5′-UTR, as well as for the −150 promoter.
Figure 5A shows the steady-state levels of GUS RNA detected 2 h after a single pulse of BL at various fluences. Data from three independent experiments are presented in Figure 5B. These data illustrate that the promoter deletions to −281 and −150 respond with the same fluence-response characteristics as the endogenous Arabidopsis Lhcb gene, and that the presence or absence of the 5′-UTR does not alter the threshold of the response.
Figure 5.
Fluence response characteristics of transgenic constructs containing the −281 to +2, −281 to +64, and −150 to +64 versions of the pea Lhcb1*4 promoter. A, Six-day-old dark-grown transgenic Arabidopsis containing truncated pea Lhcb1*4 promoter constructs fused to GUS were irradiated with a single pulse of BL with a total fluence of 104 μmol m−2 or were given no light (D). RNA was prepared 2 h after the light treatment and analyzed by slot blotting. The resulting blots were probed for GUS and Lhcb RNA, representing expression of the transgene and the endogenous Arabidopsis Lhcb, respectively. The pea Lhcb1*4 promoters (as indicated on the figure) extend from either −281 or −150 on the upstream end to either +65 (+5′-UTR, where +1 represents the site of transcriptional initiation, +1 to+64 represents the pea Lhcb1*4 5′-UTR and +65 is the A of the ATG start codon) or +2 (−5′-UTR) on the downstream end. B, Compilation of three independent experiments. All data points were plotted relative to dark levels (set to 1%) and 104 μmol m−2 BL levels (set to 100%) and represent the constructs −281 with a 5′-UTR (white bars), −281 without a 5′-UTR (light gray bars), −150 with a 5′-UTR (dark gray bars), and the endogenous Lhcb gene (black bars). All signals were normalized to rRNA. Error bars indicate se of the mean.
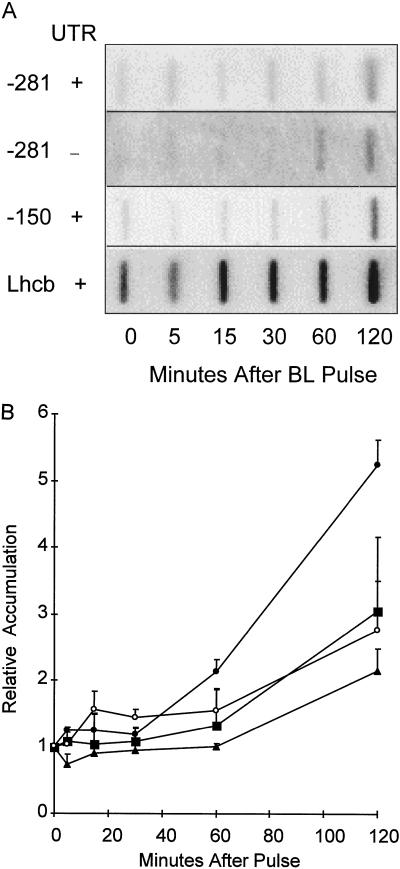
The time course of GUS RNA accumulation from the PsLhcb1*4 promoter deletion constructs was measured following a single 102 μmol m−2 pulse of BL. Transcript was measured at 5, 15, 30, 60, and 120 min following the pulse. Figure 6A shows the response for individual lines tested and Figure 6B shows the compilation of data from three independent experiments. RNA from the −281 promoter constructs began to accumulate between 30 min and 1 h after the pulse, coinciding with the response of the endogenous Lhcb1 genes. This was true regardless of the presence or absence of the 5′-UTR. The −150 deletion became detectable between 1 and 2 h. This apparent late onset was more likely due to the inability to detect the minimal increases from this low-level promoter at 30 min rather than to the lack of a BLF response. Again, the relative time course of the response was similar to that observed with the full-length and endogenous promoters, suggesting that these deletions were responding correctly to stimulation of the BLF system.
Figure 6.
Time course of accumulation of transcript derived from transgenic constructs containing the −281 to +2, −281 to +64, and −150 to +64 versions of the pea Lhcb1*4 promoter in response to 102 μmol m−2 BL. A, Six-day-old dark-grown transgenic Arabidopsis containing truncated pea Lhcb1*4 promoter constructs fused to GUS, were irradiated with a single pulse of BL with a total fluence of 102 μmol m−2. RNA was prepared 0, 5, 15, 30, 60, and 120 min after the light treatment and analyzed by slot blotting. The resulting blots were probed for GUS and Lhcb RNA, representing expression of the transgene and the endogenous Arabidopsis Lhcb, respectively. The pea Lhcb1*4 promoters (as indicated on the figure) extend from either −281 or −150 on the upstream end to either +65 (+5′-UTR, where +1 represents the site of transcriptional initiation, +1 to +64 represents the pea Lhcb1*4 5′-UTR, and +65 is the A of the ATG start codon) or +2 (−5′-UTR) on the downstream end. B, Composite of three independent experiments. Pea Lhcb1*4 promoter constructs extend from −281 with the 5′-UTR (▪), −281 without the 5′-UTR (○), and −150 with the 5′-UTR (▴) were tested and compared with the induction of the endogenous Lhcb genes (•). All data points were plotted relative to dark levels (set to 1) and all signals were normalized to rRNA. Error bars indicate se of the mean.
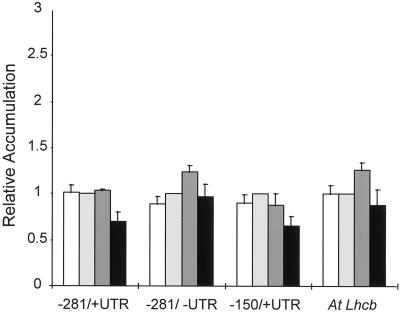
To determine whether the induction from the three representative promoters obeys reciprocity, steady-state levels of GUS RNA were measured following treatment with a single pulse of BL with a total fluence of 102 μmol m−2 delivered over various time intervals ranging from 5 to 1110 s. All three promoters respond by producing RNA levels that were very similar, regardless the length of the irradiation (Fig. 7).
Figure 7.
Reciprocity characteristics for accumulation of transcript derived from transgenic constructs containing the −281 to +2, −281 to +64, and −150 to +64 versions of the pea Lhcb1*4 promoter in response to 102 μmol m−2 BL. Six-day-old dark-grown transgenic Arabidopsis containing truncated pea Lhcb1*4 promoter constructs fused to GUS were irradiated with a single pulse of BL with a total fluence of 102 μmol m−2 delivered in 5 s (white bars), 20 s (light gray bars), 100 s (dark gray bars), or 1110 s (black bars). RNA was prepared 2 h after the light treatment and analyzed by slot blotting. The resulting blots were probed for GUS and Lhcb RNA, representing expression of the transgene and the endogenous Arabidopsis Lhcb, respectively. The pea Lhcb1*4 promoters (as indicated on the figure) extend from either −281 or −150 on the upstream end to either +65 (+5′-UTR, where +1 represents the site of transcriptional initiation, +1 to+64 represents the pea Lhcb1*4 5′-UTR, and +65 is the A of the ATG start codon) or +2 (−5′-UTR) on the downstream end. The results are the average of three independent experiments. All signals were normalized to rRNA. The error bars represent the se of the mean.
Deletion to −95
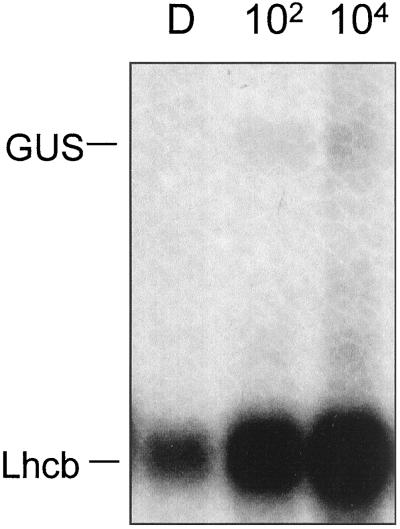
A construct representing the region from −95 to +2 eliminates sequence cognate of the CCA1-binding site (Wang et al., 1997) in the PsLhcb1*4 promoter, as well as additional sequences upstream of +95 that may play an accessory role in BL regulation. The CCA1-binding sequence has been implicated in both the phytochrome and circadian responses of the Arabidopsis Lhcb1*3 gene (Wang and Tobin, 1998). Constructs representing the pea Lhcb1*4 promoter from −95 to +2 maintain a response to 102 μmol m−2 and 104 μmol m−2 BL (Fig. 8).
Figure 8.
BL-induced accumulation of RNA derived from transgenic constructs containing the truncated Lhcb1*4 promoter −95 to +2. Six-day-old dark-grown transgenic Arabidopsis seedlings containing a truncated pea Lhcb1*4 promoter extending from −95 on the upstream end to +2 on the downstream end (where +1 represents the site of transcriptional initiation) fused to GUS were irradiated with a single pulse of BL with a total fluence of 102 μmol m−2 or 104 μmol m−2 or were given no light (D). RNA was prepared 2 h after the light treatment and analyzed by northern blotting. The resulting blots were probed simultaneously for GUS and Lhcb RNA, representing expression of the transgene and the endogenous Arabidopsis Lhcb, respectively.
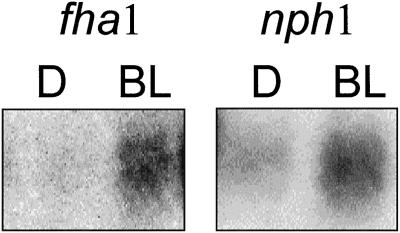
Nph1 and Fha1 Mutants
The Arabidopsis mutants nph1 (Liscum and Briggs, 1995), fha1 (a late-flowering mutant shown to be an allele of cry2) (Guo et al., 1998), and hy4 encode proteins that most likely represent BL photoreceptors. We have previously demonstrated that the hy4 mutation does not affect BLF-mediated induction of the Lhcb1*4 gene (Gao and Kaufman, 1994). However, it is possible that mutations in NPH1 or CRY2 may affect BLF-system-mediated Lhcb expression. These mutant lines were tested for their ability to induce endogenous Lhcb transcripts following irradiation with either 102 μmol m−2 or 104 μmol m−2 of BL. Accumulation of Lhcb transcript was similar to that in the wild type (Fig. 9).
Figure 9.
Induction of endogenous Lhcb RNA in response to BL in recently characterized BL photoreceptor mutants. Six-day-old etiolated seedlings derived from the Arabidopsis mutants nph1 and fha1 were irradiated with 102 μmol m−2 or 104 μmol m−2 BL or were given no light (D). RNA was prepared 2 h after the light treatment and analyzed by northern blotting. The resulting blots were probed for Lhcb RNA.
DISCUSSION
In pea and Arabidopsis the transcription rate of Lhcb1 genes increases in response to a single pulse of BLF (Marrs and Kaufman, 1989; Anderson et al., in review). We previously determined that the BL induction directed by the full-length 1.3-kb pea Lhcb1*4 promoter is normal in transgenic Arabidopsis and occurs independent of phytochrome excitation (Tilghman et al., 1997). Data presented herein indicate that all of the sequences required for a normal BLF response, as characterized by the typical fluence response, time course, and reciprocity, are present within −150 bp of the site of transcription initiation (Figs. 1, 4, and 5). The 5′-UTR is not necessary for the response.
The data presented in Figure 8 indicate that the region between −95 and +2 is also capable of responding to BLF, as transcript accumulated following a single BLF pulse, albeit at 36% of the level of the −281 promoter. These data demonstrate that the CCA1-binding sequence is not necessary for the BLF response. Previous studies have illustrated that the CCA1-binding sequence is required for the response to phytochrome (Wang et al., 1997) and the circadian clock (Wang and Tobin, 1998). The data presented in this study demonstrate that this sequence, which is pivotal in other light responses, is not necessary for the BLF response.
The −95 to +2 region contains several highly conserved elements that have been implicated previously in light responses (Donald and Cashmore, 1990). One of these “light-regulatory elements” is represented by a double-GATA sequence (I box), which is present in many light-inducible promoters (Arguello-Astorga and Herrera-Estrella, 1996) including both BL-regulated pea and Arabidopsis Lhcb promoters. Donald and Cashmore (1990) illustrated that this motif is important for expression from the Arabidopsis rbcS-1A promoter. In the context of the full rbcS 1.7-kb promoter, both I boxes were mutagenized, resulting in a >90% reduction of promoter activity (Donald and Cashmore, 1990). Kehoe et al. (1994) demonstrated that this sequence is required for phytochrome regulation of the cab2 promoter from Lemna gibba. The GATA motif has been described as a target of protein-binding activities in response to light regulation through the circadian clock and phytochrome (Anderson et al., 1994). This region also contains a conserved G-box-like element that has been implicated in light responses (Arguello-Astorga and Herrera-Estrella, 1996). The REP7550 construct, which replaces sequence in the pea Lhcb1*4 promoter between −50 and −75, maintains BL activity, indicating that this region is not necessary for BL activity in the etiolated seedling.
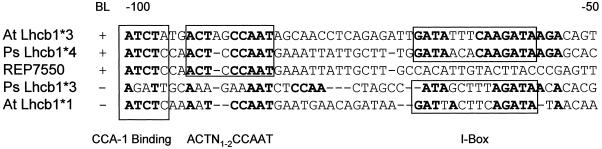
Taken together, deletion analysis and light-regulatory element mutagenesis indicate that sequences necessary for BL induction lie in a 20-bp region between −75 and −95 of the PsLhcb1*4 gene. Comparison of the BL-regulated PsLhcb1 and AtLhcb1 genes reveals identity in sequences at and immediately upstream of the CCAAT sequence. The sequence element CCAAT is highly conserved among light-regulated promoters, and is present in the −100 to −80 region in the BL-responsive Lhcb1 promoters in Arabidopsis, pea, and a number of other species. This element is present in the same position relative to the TATA sequence and is preceded by the sequence ACT in both BL-regulated promoters. The ACT/CCAAT sequence is not present in Lhcb1 genes that do not respond to a single pulse of BLF (Fig. 10). It is possible that the BL response is orchestrated through binding of factors and/or activation of factors into this specific ACT/CCAAT region.
Figure 10.
Comparison of the sequence in the −100 to −50 region of the BL-regulated and non-BL-regulated Lhcb promoters from pea and Arabidopsis. The CCA-1 (Wang and Tobin, 1998), I-box (Donald and Cashmore, 1990), and CCAAT-box domains are indicated on the figure with boxes. Sequence identity to the Arabidopsis Lhcb1*3 promoter are indicated by bold print.
The CCAAT sequence is found in many eukaryotic promoters and is recognized by a set of proteins that can activate transcription. It was recently demonstrated that Arabidopsis contains several homologs to the yeast hap gene family of CCAAT-binding proteins (Edwards et al., 1998). Whereas yeast and vertebrates contain only a single variant of each HAP protein, Arabidopsis contains several different variants of each class. Additionally, while the CCAAT-binding proteins are ubiquitously and constitutively expressed in yeast and vertebrates, the members of the Arabidopsis HAP3 family are expressed in a regulated fashion. Circadian-regulated binding to the CCAAT sequence in the Lhcb1*1 promoter has been described previously (Carre and Kay, 1995), and although this gene contains the CCAAT sequence, it is not BL regulated.
In addition to the enhancer sequences upstream of the site of initiation, enhancers have been identified in the 5′-UTR of many genes. The enhancer motif described as a CT box is present in the 5′-UTR of several plant nuclear genes, including the PsaF and PetH genes in spinach (Bolle et al., 1994) and the HMG2 gene in tomato (Daraselia et al., 1996). The Lhcb1*4 promoters from −281, −100, and −95, which lack the 5′-UTR, still respond to a pulse of BL. However, accumulation of transcript was only 67% of that where the 5′-UTR is present. The 5′-UTR of the PsLhcb1*4 gene does not contain the CT-box sequence.
BLF-mediated Lhcb transcript accumulation is normal in the BL perception mutants nph1 and fha1 (cry2). Both mutants accumulate Lhcb transcript in response to a pulse of BLF, indicating that neither the product of NPH1 nor the product of FHA1 is necessary for the BLF response. It has been shown previously that the BLF response of Lhcb genes is normal in hy4 mutants (Gao and Kaufman, 1994). These results suggest either the presence of an additional BL receptor or receptors that modulate Lhcb expression, or that Lhcb expression may be activated from multiple, redundant photoperception systems. Recent studies in the cry1/cry2 double mutant indicate that the double mutant may have more ranging effects on light-induced phenomena (i.e. suppression of hypocotyl elongation) than either single mutation alone (Ahmad et al., 1998). This synergism between two CRY photoreceptors, and possibly interaction between the CRY and NPH photoreceptors, may have an influence on Lhcb transcript accumulation, and these possibilities will be tested.
This study illustrates that the PsLhcb1*4 promoter relies on the sequence within −95 of the site of transcriptional initiation to initiate the BLF response, which is not dependent upon the double-GATA (I box) motifs common to many light-regulated promoters. Additionally, it is now clear that the BLF response is triggered by activation of transcription within this −95 region and utilizes enhancer sequences upstream of −95 and sequences in the 5′-UTR for high-level expression. The most important aspect of this study illustrates that many of the previously characterized motifs recognized as playing a functional role in phytochrome and/or circadian responses are not required for the BLF response. Ongoing studies will identify the minimal sequences sufficient for inducing the basal BLF response from those now identified in the limited −75 to −95 region of the PsLhcb1*4 promoter.
Acknowledgments
The authors gratefully thank Mary Beth Anderson, John Marsh III, Yevgenia Lapik, and Zhaoming Wang for their useful discussion and critical reading of this manuscript. We also acknowledge Dr. Joseph Kieber and Dr. Keith Woeste for their assistance in the cultivation and transformation of Arabidopsis.
Abbreviations:
- BL
blue light
- BLF
blue low-fluence light
- UTR
untranslated region
Footnotes
This work was supported in part by U.S. Department of Agriculture grant no. 9701418.
LITERATURE CITED
- Ahmad M, Cashmore AR. Seeing blue; the discovery of cryptochrome. Plant Mol Biol. 1996;30:851–861. doi: 10.1007/BF00020798. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR. Cryptochrome blue light photoreceptors of Arabidopsis implicated in phototropism. Nature. 1998;392:720–723. doi: 10.1038/33701. [DOI] [PubMed] [Google Scholar]
- Alexander L, Falconet D, Fristensky BW, White MJ, Watson JC, Roe BA, Thompson WF. Nucleotide sequence of Cab-8, a new type I gene encoding a chlorophyll a/b-binding protein of LHC II in Pisum. Plant Mol Biol 1991. 1991;3:523–526. doi: 10.1007/BF00040649. [DOI] [PubMed] [Google Scholar]
- Anderson SL, Kay SA. Functional dissection of circadian clock and phytochrome regulated transcription of the Arabidopsis CAB2 gene. Proc Natl Acad Sci USA. 1995;92:1500–1504. doi: 10.1073/pnas.92.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SL, Teakle GR, Martino-Catt SJ, Kay SA. Circadian clock and phytochrome-regulated transcription is conferred by a 78bp cis-acting domain of the Arabidopsis CAB2 promoter. Plant J. 1994;6:457–470. doi: 10.1046/j.1365-313x.1994.6040457.x. [DOI] [PubMed] [Google Scholar]
- Arguello-Astorga GR, Herrera-Estrella LR. Ancestral multipartite units in light-responsive plant promoters have structural features correlating with specific phototransduction pathways. Plant Physiol. 1996;112:1151–1166. doi: 10.1104/pp.112.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello-Astorga GR, Herrera-Estrella LR. Evolution of light-regulated plant promoters. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:525–555. doi: 10.1146/annurev.arplant.49.1.525. [DOI] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci. 1993;316:1194–1199. [Google Scholar]
- Bolle C, Sopory S, Lubberstedt T, Herrmann RG, Oelmuller R. Segments encoding 5′-untranslated leaders of genes for thylakoid proteins contain cis-elements essential for transcription. Plant J. 1994;6:513–523. doi: 10.1046/j.1365-313x.1994.6040513.x. [DOI] [PubMed] [Google Scholar]
- Borello U, Ceccarelli E, Giuliano G. Constitutive, light-responsive and circadian clock-responsive factors compete for the different I box elements in plant light-regulated promoters. Plant J. 1993;4:611–619. doi: 10.1046/j.1365-313x.1993.04040611.x. [DOI] [PubMed] [Google Scholar]
- Brusslan JA, Tobin EM. Light-independent developmental regulation of Cab gene expression in Arabidopsis thaliana seedlings. Proc Natl Acad Sci USA. 1992;89:7791–7795. doi: 10.1073/pnas.89.16.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre I, Kay S. Multiple DNA-protein complexes at a circadian-regulated promoter element. Plant Cell. 1995;7:2039–2051. doi: 10.1105/tpc.7.12.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraselia ND, Tarchevskaya S, Narita JO. The promoter for tomato 3-hydroxy-3-methylglutaryl coenzyme A reductase gene 2 has unusual regulatory elements that direct high expression. Plant Physiol. 1996;112:727–733. doi: 10.1104/pp.112.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RGK, Cashmore AR. Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J. 1990;9:1717–1726. doi: 10.1002/j.1460-2075.1990.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Murray JAH, Simon A. Multiple genes encoding the conserved CCAAT-box transcription factor complex are expressed in Arabidopsis. Plant Physiol. 1998;117:1015–1022. doi: 10.1104/pp.117.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M, Schafer E. Photopreception and signaling of induction reactions by different phytochromes. Trends Plant Sci. 1996;1:301–307. [Google Scholar]
- Gao J, Kaufman LS. Blue-light regulation of the Arabidopsis thaliana Cab-1 gene. Plant Physiol. 1994;104:1251–1257. doi: 10.1104/pp.104.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C. Regulation of flowering time by Arabidopsis photoreceptors. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR. Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- Jackson JA, Fuglevand G, Brown BA, Shaw MJ, Jenkins G. Isolation of Arabidopsis mutants altered in the light regulation of chalcone synthase gene expression using a transgenic screening approach. Plant J. 1995;8:369–380. doi: 10.1046/j.1365-313x.1995.08030369.x. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan M. Gus fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G. UV and blue light signal transduction in Arabidopsis. Plant Cell Environ. 1997;20:773–778. doi: 10.1046/j.1365-3040.1997.d01-105.x. [DOI] [PubMed] [Google Scholar]
- Kaufman LS. Transduction of blue light signals. Plant Physiol. 1993;102:333–337. doi: 10.1104/pp.102.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe DM, Degenhardt J, Winicov I, Tobin EM. Two 10-bp regions are critical for phytochrome regulation of a Lemna gibba LHCB gene promoter. Plant Cell. 1994;6:1123–1134. doi: 10.1105/tpc.6.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenigsbuch D, Tobin EM. A region of the Arabidopsis Lhcb1*3 promoter that binds to CA-1 activity is essential for high expression and phytochrome regulation. Plant Physiol. 1995;108:1023–1027. doi: 10.1104/pp.108.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. Mutations in the NPH1 locus disrupt the perception of phototrophic stimuli. Plant Cell. 1995;7:473–485. doi: 10.1105/tpc.7.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs KA, Kaufman LS. Blue-light regulation of transcription for nuclear genes in pea. Proc Natl Acad Sci USA. 1989;86:4492–4495. doi: 10.1073/pnas.86.12.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs KA, Kaufman LS. Rapid transcriptional regulation of the Cab and pEA207 gene families by blue light in the absence of cytoplasmic protein synthesis. Planta. 1991;183:327–333. doi: 10.1007/BF00197729. [DOI] [PubMed] [Google Scholar]
- Sun L, Tobin EM. Phytochrome-regulated expression of genes encoding light-harvesting chlorophyll a/b protein in two long hypocotyl mutations and wild type plants of Arabidopsis thaliana. Photochem Photobiol. 1990;52:51–56. doi: 10.1111/j.1751-1097.1990.tb01754.x. [DOI] [PubMed] [Google Scholar]
- Tilghman JA, Gao J, Anderson MB, Kaufman LS. Correct blue light regulation of pea Lhcb genes in an Arabidopsis background. Plant Mol Biol. 1997;35:293–302. doi: 10.1023/a:1005842503952. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Kenisbuch D, Sun L, Harel E, Ong MS, Tobin EM. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell. 1997;9:491–507. doi: 10.1105/tpc.9.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Tobin EM. Constitutive expression of the circadian clock associated 1 (CCA1) gene disrupts circadian rythms and supresses its own expression. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- Warpeha KMFLS, Kaufman Blue light regulation of epicotyl elongation in Pisum sativum. Plant Physiol. 1989;89:544–548. doi: 10.1104/pp.89.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warpeha KMFLS, Kaufman Two distinct blue light responses regulate the levels of transcripts of specific nuclear-coded genes in pea. Planta. 1990;182:553–558. doi: 10.1007/BF02341031. [DOI] [PubMed] [Google Scholar]
- White MJ, Kaufman LS, Horwitz BA, Briggs WR, Thompson WF. Individual members of the Cab gene family differ widely in fluence response. Plant Physiol. 1995;107:161–165. doi: 10.1104/pp.107.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Liscum E, Hangartner R. Spectral-dependence of light-inhibited hypocotyl elongation in photomorphogenic mutants of Arabidopsis: evidence for a UV-A photosensor. Planta. 1992;188:106–114. doi: 10.1007/BF00198946. [DOI] [PubMed] [Google Scholar]