Abstract
Study Objective
To assess comorbidities in a community-based cohort of narcolepsy.
Methods
A 2000–2014 community-based narcolepsy cohort was identified in Olmsted County, Minnesota. Records were reviewed by a certified sleep specialist for accuracy of diagnosis and comorbidities were extracted and analyzed. Comorbidities in narcolepsy subjects, both at diagnosis and upon follow up were compared with those from an unaffected, age and sex-matched cohort using conditional logistic regression.
Results
At diagnosis, there was increased association of narcolepsy with anxiety (OR 4.56, 95%CI 1.99–10.44), thyroid disease (3.07, 1.19–7.90), hypertension (2.69, 1.22–5.93), and hyperlipidemia (2.49, 1.05–5.92). At the end of the prolonged observation period of 9.9 years (SD 7.27 years), there was increased association of narcolepsy with peripheral neuropathy (11.21, 1.16–108.11), non-migrainous headache (6.00, 1.73–20.83), glucose intolerance (2.39, 1.05–5.45), and automobile-related trauma (2.43, 1.08–5.45). Persistently increased both at diagnosis and after a prolonged observation period were associations of narcolepsy with obstructive sleep apnea (69.25, 9.26–517.99 decreasing to 13.55, 5.08–36.14), chronic low back pain (5.46, 2.46–12.11 to 2.58, 1.39–4.77), depression (4.88, 2.45–9.73 to 3.79, 2.12–6.79), psychiatric disorders in general (4.73, 2.49–9.01 to 3.40, 1.94–5.98), endocrinopathies (4.15, 1.81–9.56 to 2.45, 1.33–4.49), and obesity (2.27, 1.13–4.56 to 2.07, 1.15–3.7).
Conclusions
In this community-based study of narcolepsy co-morbidities, both at diagnosis and after prolonged follow-up, persistent comorbidities were revealed including obstructive sleep apnea, chronic low back pain, psychiatric disorders in general, endocrinopathies, and obesity. The comprehensive management of narcolepsy requires monitoring for, and managing, these important associated health conditions.
Keywords: Narcolepsy, comorbidity, population surveillance, community based study
1. Introduction
Narcolepsy is a life-long disorder of excessive sleepiness affecting about 1 in ~2000 people, with onset typically in the first through third decades of life.[1] The key feature of cataplexy helps differentiate narcolepsy type 1 (with cataplexy) from type 2 (without cataplexy). The past two decades have seen a rise in public awareness of the disorder, reduced delay in diagnosis,[2] and improved treatment options for narcolepsy, consequently raising hopes for better outcomes. Recent studies, however, describe increased narcolepsy-associated morbidity and mortality.[3–9] Typically, these studies utilized large national[5, 8, 9] or commercial insurance[6] databases of aggregated ICD diagnosis codes and demographics. While shedding more light on mortality and morbidity in narcolepsy, their study designs did not allow for exact verification of coded diagnoses nor of the pathognomonic findings on nocturnal polysomnography (PSG) and multiple sleep latency testing (MSLT). This issue limits the generalizability of these otherwise impotant studies. A need remains therefore for community- or population-based studies of comorbidity and/or mortality that capture all narcolepsy patients within a defined population, with diagnoses being verified by careful review of PSG and MSLT data, and followup documentation by sleep specialists. Further, comorbidities that emerge at the time of narcolepsy diagnosis may differ from those that develop during follow up. Recently, Jennum et al, approached this issue by examining comorbidities present three years prior to and after initial coding of a narcolepsy diagnosis in the Danish Civil Registration System.[8, 9] Here, we utilized the Rochester Epidemiology Project (REP) to examine the comorbidities of narcolepsy in Olmsted County, Minnesota between 2000 and 2014, which additionally allows for accurate study of early and late onset comorbidities via prolonged access to a complete population’s set of medical records.
The REP is a centralized medical records linkage system that stores multi-decade medical and demographic information pertaining to over 95% of the population of Olmsted County, Minnesota. This allows for in-depth, longitudinal study of disease-related trends at the community level.[10] From 2000–2014, the population of Olmsted County grew from ~130,000 to ~150,000. With an amalgamated institutional and community-based ethics review board, the complete medical records for almost the entire population can be analyzed for epidemiological study.[11] Relatively low emigration rates also allow for prolonged periods of observation of cohorts, and permit assessment of incidence trends over time. All patients in Olmsted County receive care at either one or the other of its two medical facilities, Olmsted Medical Center or Mayo Clinic, both of which have had accredited sleep centers and board-certified sleep specialists. This arrangement optimizes the likelihood of capturing all narcolepsy subjects in the cohort.
2. Materials and Methods
2.1. Study participants and design
After approval by the Institutional Review Boards of both Olmsted Medical Center and Mayo Clinic, we utilized the REP database to identify all residents, aged three years and older, assigned with a primary hypersomnia diagnosis code as per the 9th revision of the International Classification of Disease between January 1st, 2000 and December 31st, 2014. These codes were 327.1 (organic hypersomnia), 327.10 (organic hypersomnia, unspecified), 327.11 (idiopathic hypersomnia with long sleep time), 327.12 (idiopathic hypersomnia without long sleep time), 347.xx (narcolepsy, including all subtypes), and 780.54 (hypersomnia, unspecified). To avoid overestimates from inadvertent inclusion of secondary hypersomnia etiologies, patients with medical records only containing the diagnosis code 780.53 (hypersomnia with sleep apnea, unspecified), were excluded. From the pool of gathered subjects, we identified subjects who underwent Multiple Sleep Latency Test (MSLT) studies, as indicated by the Current Procedural Terminology (CPT) code 95805, since this test is used as standerized objective supporting data for diagnosing narcolepsy. The records of all hypersomnia patients with MSLT studies were reviewed manually to identify subjects with narcolepsy, as per the International Classification of Sleep Disorders, Third Edition.[12] Records of all cases of narcolepsy were reviewed by a certified sleep specialist (SK) to confirm accurate diagnosis and categorization into type 1 and type 2. For all cases, the estimated date of symptom onset, date of diagnosis, and details of the sleep evaluation and treatment were recorded. As noted in other studies, in many of our cases, the exact date of symptom onset was difficult to pinpoint as patients’ recollection of symptoms was likely of variable precision [13–16]. As such, while obtaining an accurate list of comorbidities based on date of symptom onset, while epidemiologically ideal, was deemed impractical and date of diagnosis is used as the index date, similar to recent studies.[5, 6, 8, 9] In the presence of sleep apnea, narcolepsy was only diagnosed after full control of sleep disordered breathing. Since the construct of the REP allows for review of the entire medical record of over 95% of the Olmsted County population, no missing data was encountered. Though not the main objective of the study, incidence and prevalence rates of narcolepsy were calculated in order to gain an understanding of the trends in the data.
2.2 Selection of controls and determination of comorbidities in narcolepsy
For each narcolepsy case, four age- and sex-matched controls were identified from the REP database using a matching schema. The controls were randomly chosen among the pool of unaffected residents at the time of the patient’s narcolepsy diagnosis, i.e., the index date for the case-control group. For the narcolepsy subjects, comorbid diagnoses at the time of narcolepsy diagnosis (index date) and at the last documented complete medical visit were abstracted by manual review. A complete medical visit was defined as a patient-physician encounter where a past medical and surgical history was clearly obtained or reviewed and documented in the medical record. A complete list of categories and diagnoses that was abstracted is available in Table S1. Automobile accidents and other trauma requiring medical attention were identified by review of Emergency Department notes in patients’ medical records. Dates and causes of death were also determined by medical record review.
2.3 Analysis of comorbidities associated with narcolepsy
Associations between narcolepsy-related comorbidities were assessed using conditional logistic regression analysis to account for matching. Results are presented as odds ratios and 95% confidence intervals. All tests were two-sided and p-values less than 0.05 were considered statistically significant. Analysis was performed using SAS version 9.4 (SAS Inc., Cary, NC).
2.4 Incidence and prevalence calculation for estimation of generalizability
Incidence rates were calculated using date of new narcolepsy diagnoses between January 1st, 2000 and December 31st, 2014, divided by Olmsted County population counts for each year. Prevalence of narcolepsy was calculated using a cohort with verified Olmsted County residency on January 1st, 2010.
2.5 Narcolepsy-associated mortality
Death occurred in 2/68 patients during our examined time period. As such, our study was underpowered to analyze narcolepsy associated mortality.
3. Results
We identified 3,101 residents in Olmsted County who had been assigned a hypersomnia diagnosis code in the period 2000 to 2014, of which 468 patients underwent MSLT for suspected narcolepsy. A manual review of the sleep history, PSG, and MSLT records established that 68/468 patients met diagnostic criteria for narcolepsy; 28/68 (41.2%) were type 1 (Table 1). The remaining 400/468 patients were diagnosed with hypersomnia related to other health conditions, or were found to not have hypersomnia based on MSLT, for example, symptoms instead represented fatigue. The overall incidence rate of narcolepsy was 2.56 per 100,000 person-years with a female-to-male ratio of 1.5:1. The overall prevalence of narcolepsy on January 1st, 2010 was 32.4 per 100,000.
Table 1.
Age at diagnosis and sex distribution patients with narcolepsy in Olmsted County, Minnesota
| Age Groups | Female | Male | Total | % |
|---|---|---|---|---|
| 0 – 4 | 0 | 0 | 0 | |
| 5 – 14 | 1 (1) | 4 (4) | 5 | 7.4 |
| 15 – 24 | 14 (4) | 5 (3) | 19 | 27.9 |
| 25 – 34 | 12 (6) | 5 (1) | 17 | 25.0 |
| 35 – 44 | 4 (0) | 4 (1) | 8 | 11.8 |
| 45 – 54 | 6 (3) | 7 (2) | 13 | 19.1 |
| 55 – 64 | 2 (1) | 3 (1) | 5 | 7.4 |
| 65 – 74 | 1 (1) | 0 | 1 | 1.5 |
| 75 – 84 | 0 | 0 | 0 | |
| 85+ | 0 | 0 | 0 | |
|
| ||||
| Totals: | 40 (16) | 28 (12) | 68 | 100 |
Numbers in parentheses denote patients with cataplexy
Some health conditions were significantly increased in the narcolepsy group as compared to controls only at the time of the initial diagnosis of narcolepsy (Table 2). They included anxiety disorder (4.56, 1.99–10.44, p = 0.0003), thyroid disease (3.07, 1.19–7.90, p = 0.020), hypertension (2.69, 1.22–5.93, p = 0.014), and hyperlipidemia (2.49, 1.05–5.92, p = 0.039). Conversely, the odds were increased for having one of four disorders at the end of the observation period: peripheral neuropathy (11.21, 1.16–108.11, p = 0.037), non-migrainous headache (6.00, 1.73–20.83, p = 0.005), diabetes or glucose intolerance (2.39, 1.05–5.45, p = 0.039), and automobile-related trauma (2.43, 1.08–5.45, p = 0.031). For four separate comorbidities, the odds were increased both at the time of narcolepsy diagnosis and at the end of the observation period. They included obstructive sleep apnea (at diagnosis: 69.25, 9.26–517.99, p < 0.0001; at end 13.55, 5.08–36.14, p < 0.0001), chronic low back pain (at diagnosis 5.46, 2.46–12.11, p < 0.0001; at end 2.58, 1.39–4.77, p = 0.003), depression (at diagnosis: 4.88, 2.45–9.73, p < 0.0001; at end 3.79, 2.12–6.79, p < 0.0001), and obesity (at diagnosis: 2.27, 1.13–4.56, p = 0.021; at end 2.07, 1.15–3.7, p = 0.015). The proportion of cases and control subjects affected are also shown in Figure 1.
Table 2.
Narcolepsy-associated mortality and comorbidities at the time of diagnosis (pre-observation) and after prolonged observation
| Diagnoses (Pre- and Post-Observation) |
Cases (N=68) % |
Control (N=272) % |
OR | 95% Confidence Intervals | P | |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| Post- | 2.9 | 2.2 | 1.31 | 0.25 | 6.90 | NS |
| Psychiatric Disorders | ||||||
| Pre | 45.6 | 17.3 | 4.73 | 2.49 | 9.01 | <0.0001 |
| Post | 57.4 | 28.7 | 3.40 | 1.94 | 5.98 | <0.0001 |
| Endocrine Disorders | ||||||
| Pre- | 20.6 | 6.6 | 4.15 | 1.81 | 9.56 | 0.001 |
| Post- | 32.4 | 16.2 | 2.45 | 1.33 | 4.49 | 0.004 |
| Obstructive Sleep Apnea | ||||||
| Pre- | 29.4 | 1.8 | 69.25 | 9.26 | 517.99 | <0.0001 |
| Post- | 33.8 | 5.9 | 13.55 | 5.08 | 36.14 | <0.0001 |
| Chronic Low Back Pain | ||||||
| Pre- | 25.0 | 6.3 | 5.46 | 2.46 | 12.11 | <0.0001 |
| Post- | 27.9 | 12.1 | 2.58 | 1.39 | 4.77 | 0.003 |
| Depression | ||||||
| Pre- | 39.7 | 14.7 | 4.88 | 2.45 | 9.73 | <0.0001 |
| Post- | 50 | 21.7 | 3.79 | 2.12 | 6.79 | <0.0001 |
| Obesity | ||||||
| Pre- | 20.6 | 9.9 | 2.27 | 1.13 | 4.56 | 0.021 |
| Post- | 32.4 | 18.4 | 2.07 | 1.15 | 3.70 | 0.015 |
| Anxiety Disorder | ||||||
| Pre- | 20.6 | 5.9 | 4.56 | 1.99 | 10.44 | 0.0003 |
| Post- | 25.0 | 15.1 | 1.91 | 0.99 | 3.69 | NS |
| Thyroid Disease | ||||||
| Pre- | 13.2 | 5.1 | 3.07 | 1.19 | 7.90 | 0.020 |
| Post- | 17.6 | 10.3 | 1.85 | 0.88 | 3.88 | NS |
| Hypertension | ||||||
| Pre- | 20.6 | 10.3 | 2.69 | 1.22 | 5.93 | 0.014 |
| Post- | 25 | 23.5 | 1.13 | 0.55 | 2.32 | NS |
| Hyperlipidemia | ||||||
| Pre- | 17.6 | 9.6 | 2.49 | 1.05 | 5.92 | 0.039 |
| Post- | 27.9 | 24.3 | 1.35 | 0.66 | 2.75 | NS |
| Peripheral Neuropathy | ||||||
| Pre- | 0 | 0.4 | n/a | n/a | n/a | NS |
| Post- | 4.4 | 0.4 | 11.21 | 1.16 | 108.11 | 0.037 |
| Non-migrainous Headache | ||||||
| Pre- | 4.4 | 1.1 | 5.04 | 0.82 | 30.99 | NS |
| Post- | 10.3 | 2.2 | 6.00 | 1.73 | 20.83 | 0.005 |
| Diabetes/Glucose Intolerance | ||||||
| Pre- | 5.9 | 1.8 | 3.10 | 0.83 | 11.55 | NS |
| Post- | 14.7 | 6.6 | 2.39 | 1.05 | 5.45 | 0.039 |
| Automobile-related Trauma | ||||||
| Pre- | 8.8 | 3.3 | 2.96 | 0.98 | 8.94 | NS |
| Post- | 14.7 | 6.6 | 2.43 | 1.08 | 5.45 | 0.031 |
Figure 1. Percentage of patients affected by narcolepsy-associated comorbidities at the time of diagnosis and after prolonged observation.
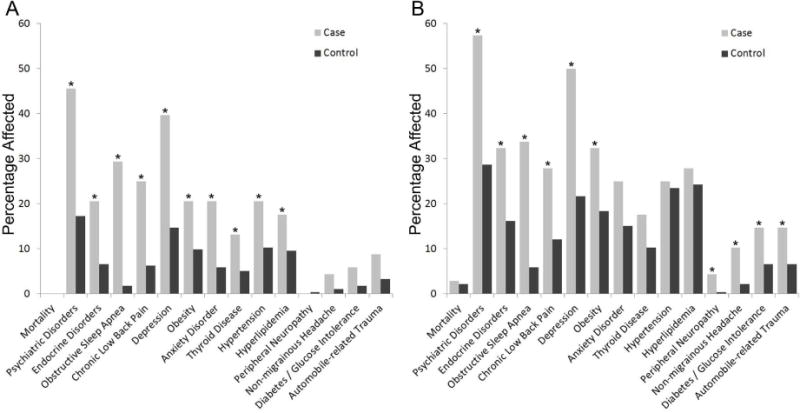
Percentage of patients with narcolepsy (cases) and control cohort affected by individual comorbidities at the time of diagnosis or index date (A), and after prolonged observation (B). Stars indicate significantly elevated odds ratios.
4. Discussion
Using the Rochester Epidemiology Project, this community-based study characterized a near-complete narcolepsy cohort over several years. We found persistent elevations in comorbid obstructive sleep apnea, chronic low back pain, obesity, and depression in our narcolepsy cohort, both at diagnosis and at the end of a prolonged observation period. Obstructive sleep apnea was the most prevalent comorbidity, as was observed in the Danish cohort.[5] A recent cross-sectional study of adults with narcolepsy also found increased prevalence of sleep-disordered breathing in one third of type 1 narcolepsy patients, especially if they were older.[17] The increased sleep apnea prevalence in narcolepsy has practical implications for management, as weight reduction and positive pressure breathing may be indicated for enhancing alertness when OSA is detected. Weight gain in narcolepsy has been well-documented in patients from other geographic regions of the world as well, often in particular association with narcolepsy with cataplexy. [18–20] Our study was able to verify at a community/population level the presence of excessive weight gain at the time of narcolepsy diagnosis. The etiology of this weight gain is likely multifactorial, and may include hypocretin deficiency, decreased energy expenditure, increased sedentary behavior due to sleepiness, and concomittent depression. Persistent weight gain observed at the end of the observation period suggests that the management of narcolepsy remains at less than optimum, and may be due in part to the use of tricyclic medications in some patients. As sleep specialists, we might be prioritizing the pharmacological treatment of sleepiness and cataplexy over a more holistic management of narcolepsy that would include a stronger emphasis on diet and physical exercise. Based on our data, a weight-loss effect from stimulant medication use appears unlikely.
Depression was also highly comorbid with narcolepsy, affecting 39.7% of patients at diagnosis with an increase to 50% after prolonged observation. Depression and narcolepsy likely have a complex, bidirectional relationship,[21] and a histaminergic basis for depression in narcolepsy can be posited.[22, 23] Inocente et. al. have also recently reported depressive feelings in a large cohort of children with narcolepsy.[24] The co-occurrence of narcolepsy and depression also has cautionary management implications, since drugs such as sodium oxybate may exacerbate depression.
The rates of anxiety disorder, thyroid disease, hypertension, and hyperlipidemia were significantly elevated at the time of diagnosis, but all become non-significant after observation due to increasing incidence in the control cohort. The association of narcolepsy with thyroid disease is interesting, as it supports the hypothesis of a co-existing or causal autoimmune disorder,[25] however it should be noted that our sample size may have precluded detection of additional autoimmune conditions with lower prevalence numbers.[26] Separately, while we found a significant increase in automobile-related trauma in our narcolepsy cohort, the absolute proportion of patients affected was low. In most cases, we could not ascertain from the medical record whether the patient was driving or was simply a passenger or victim. A more detailed study of accidents (vehicular or otherwise) is needed in narcolepsy to directly assess this particular comorbidity with significant public safety and qualify of life importance.[27] Additionally, it should be noted that since there is an inevitable yet variable delay between onset of symptoms and time of diagnosis, some of the elevated risk of comorbidities at the time of diagnosis may be directly due to the physiologic effects of narcolepsy. Similarly, a causal relation between several of the comorbidities, e.g., diabetes mellitis leading to peripheral neuropathy, while likely, would require an alternative study design to verify or refute.
The current study is consistent with larger studies finding prevalence typically between 25 and 50 cases per 100,000 person-years.[1] We also found a higher frequency of narcolepsy without cataplexy (type 2) than narcolepsy with cataplexy (type 1). This runs counter to previous epidemiologic studies of narcolepsy,[28] but is consistent with a recent large Wisconsin Sleep Cohort study that similarly analyzed narcolepsy prevalence in the general population [29, 30] and also found a higher frequency of type 2 (0.20%, 95% CI: 0.07–0.58%) compared with type 1 (0.07%, 95% CI: 0.02–0.37%).[30] Taken together, our findings suggest that narcolepsy type 2 may be more frequent in the general population than previously estimated. The exact reasons for the higher proportion of patients with type 2 narcolepsy compared to type 1 in our cohort are not entirely clear. Analysis of the long-term outcomes of large groups of narcolepsy patients with positive MSLT findings without cataplexy may warrant further study to address this finding. It is also possible that this is a changing trend that needs study over a longer period of time and in a larger cohort from a wider geographical region.
While the composition of the Olmsted County population is changing over time, it remains less diverse, more highly educated, and wealthier than the U.S. population at large,[11] which may affect generalizability to other study populations. Furthermore, the on-going management of narcolepsy patients in our accredited sleep centers may have biased the study towards greater likelihood of detecting coexisting obstructive sleep apnea.
The strengths of our study include the capturing of a near-complete cohort of narcolepsy that was also followed for a prolonged period of time (9.9 years). Our study utilized accurate, fully reviewed medical records. Each narcolepsy diagnosis was verified by a sleep medicine specialist on chart review, thus eliminating potential inaccuracies that can occur when narcolepsy cases are gathered from large databases using diagnostic codes. Further, we examined comorbidities separately, both at diagnosis and at the end of such a long period of follow up (9.9 years).
4.1 Conclusions
This study adds significantly to the understanding of comorbidities in narcolepsy, providing a useful comparison to recent large-scale studies that ascertained narcolepsy cases using diagnosis codes.[5, 6, 8, 9] We found significant comorbidities in narcolepsy at diagnosis and upon long-term follow up including obesity, depression, obstructive sleep apnea and thyroid disease that warrant attention for comprehensive management.
Supplementary Material
Highlights.
This population-based study analyzed co-morbidities at diagnosis and after prolonged follow up (mean 9.92 yrs).
At diagnosis, narcolepsy was associated with anxiety, thyroid disease, hypertension, and hyperlipidemia.
Obstructive sleep apnea, low back pain, depression, anxiety, and thyroid disease were persistently elevated, both at diagnosis and after follow up.
Attention to these comorbidities is important in the comprehensive management of patients with narcolepsy
Acknowledgments
We thank Dr. Walter Rocca and Ms. Barbara Abbott for their assistance in utilizing Rochester Epidemiology Project data. No external funding was utilized for the completion of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Drs. Cohen, Mandrekar, St. Louis, Silber, and Kotagal have nothing to disclose.
References
- 1.Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5:37–41. doi: 10.1016/j.sleep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Jennum P, Ibsen R, Petersen ER, Knudsen S, Kjellberg J. Health, social, and economic consequences of narcolepsy: a controlled national study evaluating the societal effect on patients and their partners. Sleep Med. 2012;13:1086–93. doi: 10.1016/j.sleep.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14:488–92. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep. 2013;36:835–40. doi: 10.5665/sleep.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohayon MM, Black J, Lai C, Eller M, Guinta D, Bhattacharyya A. Increased mortality in narcolepsy. Sleep. 2014;37:439–44. doi: 10.5665/sleep.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapella MC, Berger BE, Vern BA, Vispute S, Prasad B, Carley DW. Health-related stigma as a determinant of functioning in young adults with narcolepsy. PLoS One. 2015;10:e0122478. doi: 10.1371/journal.pone.0122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennum P, Pickering L, Thorstensen EW, Ibsen R, Kjellberg J. Morbidity of childhood onset narcolepsy: a controlled national study. Sleep Med. 2017;29:13–7. doi: 10.1016/j.sleep.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Jennum P, Thorstensen EW, Pickering L, Ibsen R, Kjellberg J. Morbidity and mortality of middle-aged and elderly narcoleptics. Sleep Med. 2017;36:23–8. doi: 10.1016/j.sleep.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medicine AAoS. ICSD – International classification of sleep disorders, Third Edition: Diagnostic and coding manual. Third. Westchester, IL: 2014. [Google Scholar]
- 13.Frauscher B, Ehrmann L, Mitterling T, Gabelia D, Gschliesser V, Brandauer E, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the innsbruck narcolepsy cohort. J Clin Sleep Med. 2013;9:805–12. doi: 10.5664/jcsm.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Won C, Mahmoudi M, Qin L, Purvis T, Mathur A, Mohsenin V. The impact of gender on timeliness of narcolepsy diagnosis. J Clin Sleep Med. 2014;10:89–95. doi: 10.5664/jcsm.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15:502–7. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Taddei RN, Werth E, Poryazova R, Baumann CR, Valko PO. Diagnostic delay in narcolepsy type 1: combining the patients’ and the doctors’ perspectives. J Sleep Res. 2016;25:709–15. doi: 10.1111/jsr.12420. [DOI] [PubMed] [Google Scholar]
- 17.Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol. 2013;70:22–6. doi: 10.1159/000348719. [DOI] [PubMed] [Google Scholar]
- 18.Kok SW, Overeem S, Visscher TL, Lammers GJ, Seidell JC, Pijl H, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res. 2003;11:1147–54. doi: 10.1038/oby.2003.156. [DOI] [PubMed] [Google Scholar]
- 19.Aran A, Einen M, Lin L, Plazzi G, Nishino S, Mignot E. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep. 2010;33:1457–64. doi: 10.1093/sleep/33.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poli F, Pizza F, Mignot E, Ferri R, Pagotto U, Taheri S, et al. High prevalence of precocious puberty and obesity in childhood narcolepsy with cataplexy. Sleep. 2013;36:175–81. doi: 10.5665/sleep.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez R, Barateau L, Evangelista E, Dauvilliers Y. Depression and Hypersomnia: A Complex Association. Sleep Med Clin. 2017;12:395–405. doi: 10.1016/j.jsmc.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Shan L, Bao AM, Swaab DF. The human histaminergic system in neuropsychiatric disorders. Trends Neurosci. 2015;38:167–77. doi: 10.1016/j.tins.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Zamarian L, Hogl B, Delazer M, Hingerl K, Gabelia D, Mitterling T, et al. Subjective deficits of attention, cognition and depression in patients with narcolepsy. Sleep Med. 2015;16:45–51. doi: 10.1016/j.sleep.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Inocente CO, Gustin MP, Lavault S, Guignard-Perret A, Raoux A, Christol N, et al. Depressive feelings in children with narcolepsy. Sleep Med. 2014;15:309–14. doi: 10.1016/j.sleep.2013.08.798. [DOI] [PubMed] [Google Scholar]
- 25.Bonvalet M, Ollila HM, Ambati A, Mignot E. Autoimmunity in narcolepsy. Curr Opin Pulm Med. 2017;23:522–9. doi: 10.1097/MCP.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barateau L, Lopez R, Arnulf I, Lecendreux M, Franco P, Drouot X, et al. Comorbidity between central disorders of hypersomnolence and immune-based disorders. Neurology. 2016 doi: 10.1212/WNL.0000000000003432. [DOI] [PubMed] [Google Scholar]
- 27.Gupta R, Pandi-Perumal SR, Almeneessier AS, BaHammam AS. Hypersomnolence and Traffic Safety. Sleep Med Clin. 2017;12:489–99. doi: 10.1016/j.jsmc.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25:197–202. doi: 10.1093/sleep/25.2.197. [DOI] [PubMed] [Google Scholar]
- 29.Mignot E, Lin L, Finn L, Lopes C, Pluff K, Sundstrom ML, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129:1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 30.Goldbart A, Peppard P, Finn L, Ruoff CM, Barnet J, Young T, et al. Narcolepsy and predictors of positive MSLTs in the Wisconsin Sleep Cohort. Sleep. 2014;37:1043–51. doi: 10.5665/sleep.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


