Abstract
Introduction
Quantitative muscle ultrasound (QUS) in boys with Duchenne Muscular Dystrophy (DMD) shows increased echointensity as muscle is replaced with fat and fibrosis. Studies of quantitative ultrasound in infants/young boys with DMD over time have not been reported.
Methods
We used calibrated muscle backscatter (cMB), a reproducible measure of ultrasound echointensity, to quantify muscle pathology in 5 young boys with DMD (ages 0.5–2.8 years) over 17–29 months. We compared results to repeated assessments of function (n=4) and to muscle ultrasound images from a cross section of 6 male controls (0.6–3.1 years).
Results
cMB in boys with DMD increased (worsened) over time (P<0.001), while function improved. After age 2 years, cMB in most (4 of 5) boys with DMD was higher than in any control.
Conclusions
QUS measures disease progression in young boys with DMD despite functional improvements. QUS could be employed as an outcome measure for serial assessment of young boys with DMD.
Keywords: Ultrasound, Backscatter, Myopathy, Muscle, Children, Duchenne Muscular Dystrophy
Introduction
Duchenne Muscular Dystrophy (DMD) is a common, severe, progressive, X-linked neuromuscular disease caused by mutations in the dystrophin gene. Pathologically, DMD is characterized by replacement of muscle with fat and fibrotic tissue. Progressive weakness results in loss of ambulation in the late first or early second decade, and death ultimately ensues from respiratory impairment or cardiac dysfunction1. Disease progression is typically assessed using measures of function and strength, such as the 6-minute walk test or dynamometry2. These measures require cooperative, ambulatory boys and exclude very young or older non-ambulatory boys from most therapeutic clinical trials. In infants and young boys with DMD, interpretations of functional outcomes over time are also complicated by maturational improvements in strength and function3,4. These absolute gains in function in very young boys with DMD require normalization to typical peer development to detect disease progression. One measure standardized for typical development, the Bayley-III Scales of Infant Development (Bayley-III), can reliably demonstrate both deviations from peer norms and decline over time in young boys with DMD5,6. The Bayley-III, however, is validated only to age 42 months, limiting its applicability in older children. Objective, reliable measures of disease pathology that are appropriate for infants, young boys, and older boys with DMD are needed to improve the assessment and inclusion of these boys in clinical trials.
Quantitative muscle ultrasound (QUS) is a promising tool for measuring muscle pathology in DMD that is well suited for evaluation of infants and older children, as it is painless and can be performed at the bedside. Muscle echointensity increases (muscle appears brighter) as muscle is replaced with fat and fibrosis7–9. The amount of echointensity can be quantified, and the estimated amplitudes of the ultrasound echoes reflected back from the tissue (backscatter) can be determined. Comparison to an external reference allows for calculation of the calibrated muscle backscatter (cMB)10, which can be measured reliably between different ultrasound systems11. QUS can be performed and analyzed reliably by trained evaluators12 and objectively quantifies the presence and degree of muscle pathology in DMD. Prior studies of QUS in DMD have shown that muscle echointensity increases with more advanced disease and age, with worsening strength and function, and across time13–15. Most prior studies of muscle ultrasound in DMD report boys older than age 3 years16–18. QUS over time has not been studied specifically in infants and young boys with DMD. This longitudinal study describes the features of QUS in infants and young boys with DMD compared to repeated measures of motor function and to QUS from normal muscle in a cross section of controls.
Methods
The Washington University Human Resources Protection Office approved this study, and informed consent was obtained by a parent/guardian for all subjects prior to enrollment.
Participants
DMD
Five boys with DMD, ages 0.5–2.8 years, were enrolled from the neuromuscular clinic at Washington University in St. Louis. Each had genetic mutations in the dystrophin gene and clinical features consistent with DMD, such as hypotonia, proximal weakness, high creatine kinase, and positive family history. At the time of enrollment, no boys were taking steroids. One, subject 3, started prednisolone 10mg/kg divided twice weekly, after visit 4 of 5.
Controls
For comparison, we also reviewed ultrasound images of healthy biceps brachii and rectus femoris of unaffected arms/legs of 6 boys, ages 0.6–3.1 years, with unilateral brachial palsy affecting the opposite arm.
Assessments
Functional Assessments
Functional assessments were performed in 4 of the 5 DMD subjects5 concurrent with the ultrasound imaging. Subjects 1–4 with DMD were evaluated using the Bayley-III Scales of Infant Development-Third Edition (Bayley-III) gross motor scaled score (typically developing mean = 10 ± 3), the North Star Ambulatory Assessment Sum score (NSAA, maximum score 34), and the Hammersmith Functional Motor Scale sum score (maximum score 66). All clinical assessments were performed by a trained clinical evaluator (BCM). The Bayley-III is validated in children through age 42 months and was only performed in boys up to that age. Subject 5 did not undergo clinical assessments.
Ultrasound
All images were obtained in the transverse plane using a Philips iu22 ultrasound system and L12-5 linear probe by either CMZ or an ultrasound technician trained by CMZ. All system and imaging settings were held constant for all subjects and visits. Each boy with DMD underwent ultrasound imaging of the right elbow flexors (biceps brachii and brachialis), forearm finger/wrist flexors, rectus femoris, and tibialis anterior muscles. Boys with DMD were seated or held in a parent’s arms. The boy’s arm was held extended and supported by a pillow and knee slightly bent, with the ankle in neutral position. Controls had imaging performed on healthy unilateral biceps brachii and rectus femoris muscle while they were held by parents or were supine with arms and legs extended passively. Muscle grey scale levels (GSLs) were measured by CMZ using QLAB ® from a region of interest of the muscle drawn from the superficial fascia to the deep fascia or bone. Calibrated muscle backscatter decibel levels (cMB) were calculated in 2 steps as in prior studies. First, the measured GSLs were converted to backscatter (decibels) using a previously identified conversion factor (4.54 GSL/dB) for the ultrasound settings used in this study11. Second, muscle backscatter was calibrated to a reference phantom by subtracting the backscatter measured from a 5cm deep image of the phantom10,13. Average cMB was defined as the average cMB of the elbow flexors, forearm flexors, rectus femoris, and tibialis anterior and was calculated for each subject with DMD.
Timing and Frequency of Assessments (Table 1): Subjects 1–4 underwent repeat assessments every 6 months over 2 years as part of their participation in a separate study of outcomes in young boys with DMD5. Subjects 1 and 4 missed a single ultrasound assessment at visit 3 of 5. Subject 2 had an additional initial ultrasound assessment at age 0.8 years without a paired clinical assessment. Subject 5, age 0.5 years at enrollment, had 3 repeat ultrasound assessments at ages 0.5, 1, and 2 years without paired clinical assessments.
Table 1.
| Subject # |
Dystrophin Mutation |
Age at first ultrasound (years) |
Age at first functional assessment (years) |
Number of repeat assessments* |
Months between first and final assessment* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| U | B | N | H | U | B | N | H | ||||
| 1 | Deletion exon 46 | 1.7 | 1.7 | 4 | 4 | 5 | 5 | 24 | 18 | 24 | 24 |
| 2 | Duplication exon 2 | 0.8 | 1.2 | 6 | 5 | 4 | 5 | 29 | 24 | 18 | 24 |
| 3 | Deletion exon 12–44 | 2.9 | 2.9 | 5 | 2 | 5 | 5 | 24 | 6 | 24 | 24 |
| 4 | Deletion exon 46–50 | 1.4 | 1.4 | 4 | 5 | 5 | 5 | 24 | 24 | 24 | 24 |
| 5 | Nonsense exon 70 | 0.5 | n. p. | 3 | n.p. | 17 | n.p. | ||||
performed 6 months apart. U- muscle ultrasound, B- Bayley-III Scales of Infant Development-Third Edition (Bayley-III) gross motor scaled score, N-North Star Ambulatory Assessment Sum score, H- Hammersmith FMS sum score; n.p.: not performed
Statistics
Statistics were performed with PASW® Statistics GradPack 18 and Microsoft Excel® and are reported as median (range) unless otherwise specified. Group comparisons were performed using the Mann-Whitney-U test. Correlation was performed using the Spearman rho. The slope describing the rate of change in average cMB over time was determined for each subject with DMD.
Results
Changes over time in boys with DMD
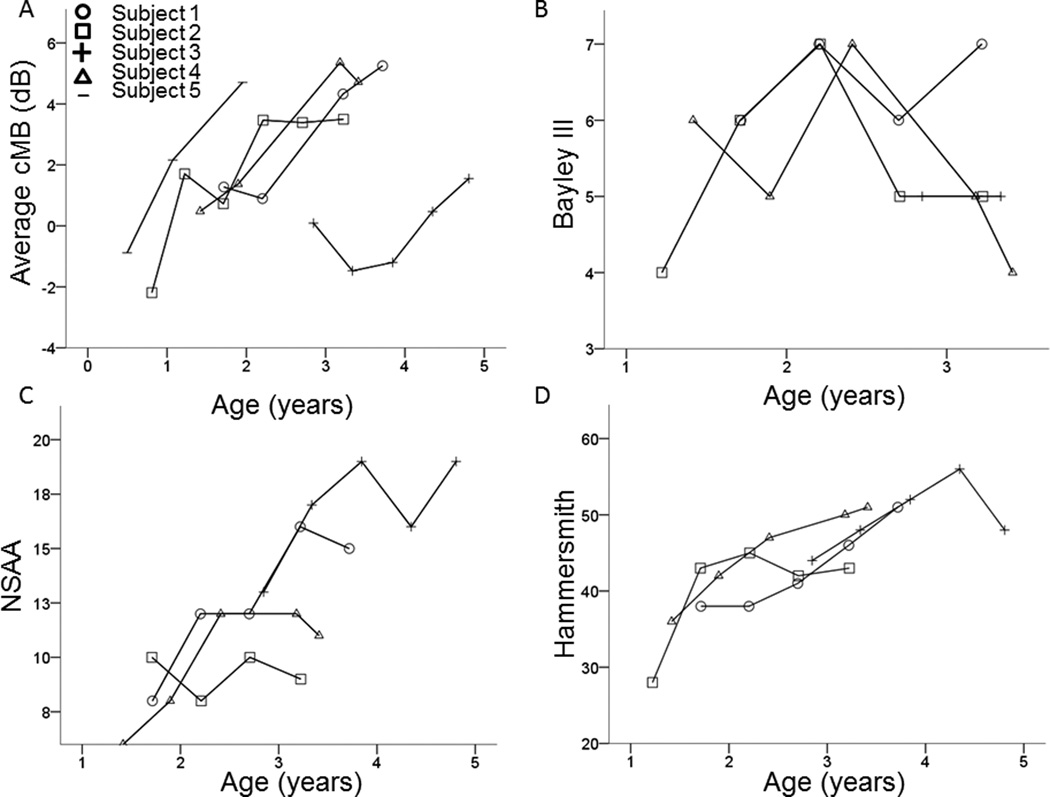
In boys with DMD, average cMB worsened (increased), and function improved over time (Figure 1). cMB increased over time in both proximal and distal muscles (Figure 2). Average cMB was higher [4.0 (1.5 – 6.5) dB] in all 5 boys with DMD at the final visit compared to baseline (Table 2) and increased by 2.3 (1.0 – 3.8) dB/year. In contrast, most boys with DMD showed improved function across time. Function improved in 3 of 4 boys on the NSAA [5.5 (−1 – 7)] and in all 4 boys on the Hammersmith [14 (4 – 15)] at the final visit compared to the baseline (Table 2). All boys showed reduced gross motor function compared to peers at baseline on the Bayley-III (normal 10 ± 3). Gross motor scaled scores on the Bayley-III improved in 2 boys at the final visit compared to baseline (Table 2). Two boys (subjects 1 and 3) became too old (>42 months) for the standardized Bayley III assessment during the study. One boy (subject 2) was too young to participate in the NSAA at the first functional assessment. Subject 3 had lower average cMB and higher function compared to the other boys with DMD at similar ages (Figure 1).
Figure 1.
Average muscle cMB and function over time in boys with Duchenne Muscular Dystrophy: In young boys with Duchenne muscular dystrophy, average cMB worsens (increases) over time (A). The Bayley III gross motor function scaled sum score, which is relative to peer norms, showed reduced function compared to peer norms without a clear trend over time (B). In contrast, the North Star ambulatory assessment (C) and Hammersmith functional motor scale (D), both measures of absolute function, showed improvement over time.
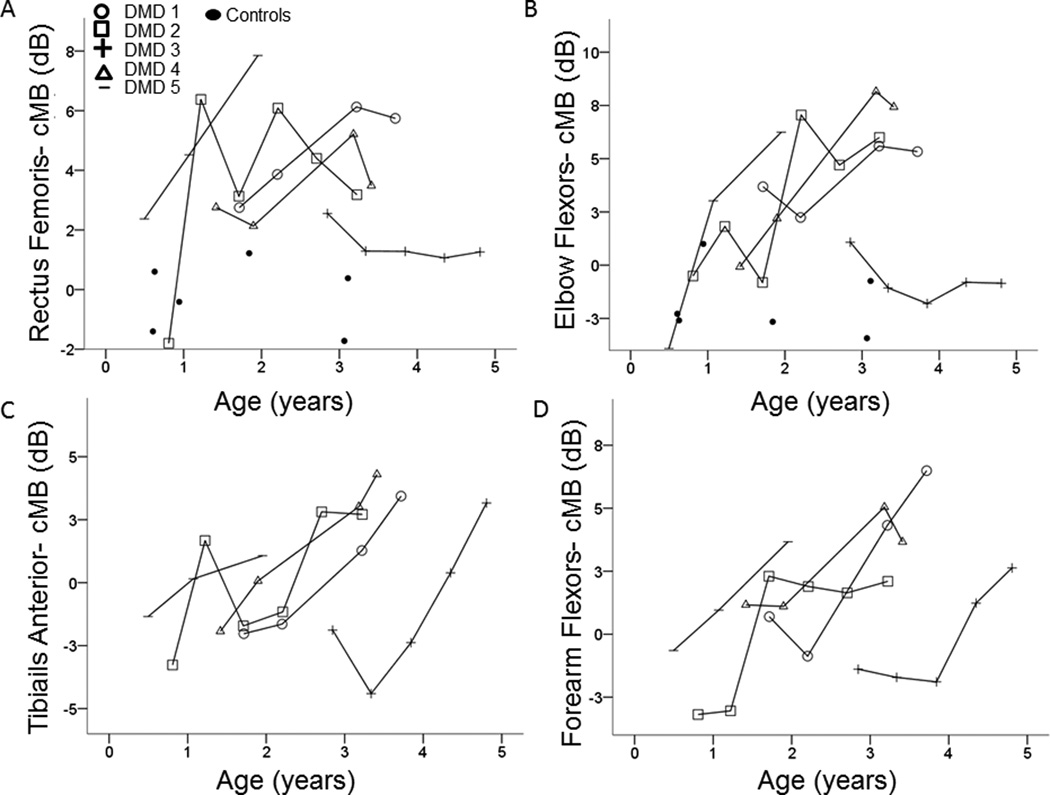
Figure 2.
cMB in boys with DMD is higher than controls at young ages and increases in both proximal and distal muscles over time: cMB in the rectus femoris (A) and elbow flexors (B) is higher in most boys with DMD than in controls, even at very young ages. In boys with DMD, cMB in both proximal (A and B) and distal (C and D) muscles increased over time.
Table 2.
| Subject # |
Average cMB (dB) |
Bayley III- Gross Motor Scaled Score (normal: 10) |
North Star Ambulatory Assessment (maximum: 34) |
Hammersmith Functional Motor Scale- Sum Score (maximum: 66) |
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Baseline | Final | Baseline | Final | |
| 1 | 1.3 | 5.3 | 6 | 7* | 8 | 15 | 38 | 51 |
| 2 | −2.2* | 3.5 | 4 | 5 | 10* | 9 | 28 | 43 |
| 3 | 0.1 | 1.6 | 5 | 5* | 13 | 19 | 44 | 48 |
| 4 | 0.5 | 4.7 | 6 | 4 | 6 | 11 | 36 | 51 |
| 5 | −0.9 | 4.7 | not performed | |||||
due to feasibility related to age, subjects 1 and 3 had the final Bayley score assessed 18 and 6 months after enrollment, respectively, prior to completion of the study, and subject 2 was unable to perform the NSAA due to young age at first assessment. Subject 2 had the first ultrasound (of 6) performed without paired clinical assessments.
Comparison between DMD and Controls
Boys with DMD at enrollment were of similar age to controls [1.4y (0.5–2.9) vs. 1.4y (0.6–3.1), P=0.6]. In controls, cMB of the elbow flexors was −2.4 (−3.4 – −1) and of the rectus femoris was 0 (−1.7 – 1.2) dB. Neither varied with age (rs≤0.1, P≥0.8). In DMD boys, cMB of both the elbow flexors and rectus femoris was similar to controls at baseline (P ≥ 0.1) but higher than controls at the final assessment (P ≤ 0.02, Figure 2). The 2 subjects with DMD imaged before age 1 year (subjects 2 and 5) had normal cMB from the biceps brachii, and 1 (subject 2) also had normal cMB from the rectus femoris. At the final assessment, cMB in boys with DMD (ages 2–4.8 years) was higher than the highest control in 4 of 5 from the elbow flexors and 5 of 5 from the rectus femoris.
Discussion
We show that in the first 4 years of life, boys with DMD already show increased muscle echointensity compared to controls and that it increases over time. Muscle echointensity becomes increasingly abnormal during the first years of life in most boys with DMD. We found that average cMB increased 2.3 (1.0 – 3.8) dB/ year in young boys with DMD, which clearly distinguishes them from healthy infants and children. This increase in muscle echointensity likely reflects increasing levels of fat and fibrosis in the dystrophic muscle with increasing age.
The transition to abnormal appearing muscle detected using ultrasound occurs in the first years of life and suggests a need for early therapeutic intervention in boys with DMD. Both of the infants in our study had normal cMB of the biceps brachii and, in 1, of the rectus femoris prior to age 1 year. These findings are similar to the few previously reported QUS findings of normal or borderline echointensity in 3 infants with DMD, ages 3.5 weeks and 7 and 9 months13,16. It is unlikely that the increased echointensity we measured in cMB in young boys with DMD over time is due to normal aging. A longitudinal study of healthy children found that muscle echointensity remained essentially unchanged (~1% change/year) with age19. Our control subjects also demonstrated no significant increase across age. The increase in cMB we found in DMD boys in this study is higher than predicted in a prior cross sectional study of the elbow flexor muscles of boys and young men with DMD that estimated an increase of 0.8dB/year in cMB. The present study differs in that this study is longitudinal, only 5 very young boys with DMD were included, and older boys and young men were not included.
QUS in DMD detects the worsening muscle pathology over time despite improvements in motor function during early childhood development. We found that in young children with DMD, muscle echointensity worsens, but function improves over this early age period. This differs from boys with DMD who are over age 7 or 8 who typically show worsening of muscle echointensity, strength, and function over time15. Most young boys with DMD in our study showed improving motor function over time when measured using the NSAA or Hammersmith, which are not standardized for typical development. Functional impairment and decline in very young boys with DMD are better demonstrated when outcomes are normalized for typical peer development5,6,20. Unlike functional based measures, muscle echointensity changes very little with age in healthy children19 and therefore does not require maturational normative data to detect disease progression with age.
QUS is feasible in infants and older boys and men with DMD and can be performed regardless of the boy’s age or ability to cooperate13, allowing for serial assessments across time. No functional outcome measure is feasible in DMD across all ages and abilities2,21. In our study, 2 boys became too old for normative assessments using the Bayley-III, and 1 was too young to participate in the NSAA. Similarly, many functional assessments are often not feasible in non-ambulatory boys and men with DMD. Repeated assessments over time using QUS in non-ambulatory boys and men with DMD is feasible15 and requires further study to determine how echointensity changes over time in those with more severe, advanced disease.
Both ultrasound and magnetic resonance imaging (MRI) have been used to quantify progressive muscle pathology in older (ages 3.7 years and older), mostly ambulatory boys with DMD15,22,23. Ultrasound offers some advantage to MRI for visualizing muscle pathology in boys and men with DMD, as it can detect intramuscular fibrosis and can be performed at the bedside. Unlike ultrasound, MRI visualizes both superficial and deep tissues equally and can employ different imaging protocols to preferentially detect specific tissue characertics such as fluid or fat. In facioscapulohumeral muscular dystrophy, QUS measures of echointensity strongly correlated with quantitative MRI measures of muscle fat content24. Additional studies are needed to determine whether QUS, MRI, or functional outcomes best detect a treatment effect in DMD.
In conclusion, this study shows that QUS is feasible in infants and very young boys with DMD and detects the relentless progression of this disease despite the functional gains made during early development. This study, combined with the prior longitudinal study of QUS in older children with DMD15, shows that QUS can be used to objectively measure disease progression from the first years of life through the early teenage years in children with DMD.
Acknowledgments
The study was supported by the Washington University Neuromuscular Research Fund, the National Institute of Health Neurological Sciences Academic Development Award Grant Number K12 NS00169009, and the Muscular Dystrophy Association Duchenne Muscular Dystrophy center grant.
References
- 1.Jeppesen J, Green A, Steffensen BF, Rahbek J. The Duchenne muscular dystrophy population in Denmark, 1977–2001: prevalence, incidence and survival in relation to the introduction of ventilator use. Neuromuscul Disord. 2003;13:804–812. doi: 10.1016/s0960-8966(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 2.Mayhew JE, Florence JM, Mayhew TP, Henricson EK, Leshner RT, McCarter RJ, Escolar DM. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve. 2007;35:36–42. doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- 3.Mazzone E, Vasco G, Sormani MP, Torrente Y, Berardinelli A, Messina S, D'Amico A, Doglio L, Politano L, Cavallaro F, Frosini S, Bello L, Bonfiglio S, Zucchini E, De Sanctis R, Scutifero M, Bianco F, Rossi F, Motta MC, Sacco A, Donati MA, Mongini T, Pini A, Battini R, Pegoraro E, Pane M, Gasperini S, Previtali S, Napolitano S, Martinelli D, Bruno C, Vita G, Comi G, Bertini E, Mercuri E. Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology. 2011;77:250–256. doi: 10.1212/WNL.0b013e318225ab2e. [DOI] [PubMed] [Google Scholar]
- 4.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Miller JP, Province MA. Clinical investigation in Duchenne dystrophy: 2. Determination of the "power" of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 5.Connolly AM, Florence JM, Cradock MM, Malkus EC, Schierbecker JR, Siener CA, Wulf CO, Anand P, Golumbek PT, Zaidman CM, Philip Miller J, Lowes LP, Alfano LN, Viollet-Callendret L, Flanigan KM, Mendell JR, McDonald CM, Goude E, Johnson L, Nicorici A, Karachunski PI, Day JW, Dalton JC, Farber JM, Buser KK, Darras BT, Kang PB, Riley SO, Shriber E, Parad R, Bushby K, Eagle M. Motor and cognitive assessment of infants and young boys with Duchenne Muscular Dystrophy: results from the Muscular Dystrophy Association DMD Clinical Research Network. Neuromuscular disorders : NMD. 2013;23:529–539. doi: 10.1016/j.nmd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly AM, Florence JM, Cradock MM, Eagle M, Flanigan KM, McDonald CM, Karachunski PI, Darras BT, Bushby K, Malkus EC, Golumbek PT, Zaidman CM, Miller JP, Mendell JR. One year outcome of boys with Duchenne muscular dystrophy using the Bayley-III scales of infant and toddler development. Pediatr Neurol. 2014;50:557–563. doi: 10.1016/j.pediatrneurol.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heckmatt J, Rodillo E, Doherty M, Willson K, Leeman S. Quantitative sonography of muscle. J Child Neurol. 1989;4(Suppl):S101–S106. doi: 10.1177/0883073889004001s15. [DOI] [PubMed] [Google Scholar]
- 8.Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, van der Laak JA, Hoogerbrugge PM, van Engelen BG, Verrips A. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009;35:443–446. doi: 10.1016/j.ultrasmedbio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Arts IM, Schelhaas HJ, Verrijp KC, Zwarts MJ, Overeem S, van der Laak JA, Lammens MM, Pillen S. Intramuscular fibrous tissue determines muscle echo intensity in amyotrophic lateral sclerosis. Muscle Nerve. 2012;45:449–450. doi: 10.1002/mus.22254. [DOI] [PubMed] [Google Scholar]
- 10.Zaidman CM, Holland MR, Anderson CC, Pestronk A. Calibrated quantitative ultrasound imaging of skeletal muscle using backscatter analysis. Muscle Nerve. 2008;38:893–898. doi: 10.1002/mus.21052. [DOI] [PubMed] [Google Scholar]
- 11.Zaidman CM, Holland MR, Hughes MS. Quantitative ultrasound of skeletal muscle: reliable measurements of calibrated muscle backscatter from different ultrasound systems. Ultrasound Med Biol. 2012;38:1618–1625. doi: 10.1016/j.ultrasmedbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaidman CM, Wu JS, Wilder S, Darras BT, Rutkove SB. Minimal training is required to reliably perform quantitative ultrasound of muscle. Muscle Nerve. 2014;50:124–128. doi: 10.1002/mus.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidman CM, Connolly AM, Malkus EC, Florence JM, Pestronk A. Quantitative ultrasound using backscatter analysis in Duchenne and Becker muscular dystrophy. Neuromuscular disorders : NMD. 2010;20:805–809. doi: 10.1016/j.nmd.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shklyar I, Geisbush TR, Mijialovic AS, Pasternak A, Darras BT, Wu JS, Rutkove SB, Zaidman CM. Quantitative muscle ultrasound in duchenne muscular dystrophy: A comparison of techniques. Muscle Nerve. 2014 doi: 10.1002/mus.24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen M, van Alfen N, Nijhuis van der Sanden MW, van Dijk JP, Pillen S, de Groot IJ. Quantitative muscle ultrasound is a promising longitudinal follow-up tool in Duchenne muscular dystrophy. Neuromuscular disorders : NMD. 2012;22:306–317. doi: 10.1016/j.nmd.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Pillen S, Verrips A, van Alfen N, Arts IM, Sie LT, Zwarts MJ. Quantitative skeletal muscle ultrasound: diagnostic value in childhood neuromuscular disease. Neuromuscular disorders : NMD. 2007;17:509–516. doi: 10.1016/j.nmd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr. 1982;101:656–660. doi: 10.1016/s0022-3476(82)80286-2. [DOI] [PubMed] [Google Scholar]
- 18.Heckmatt JZ, Pier N, Dubowitz V. Real-time ultrasound imaging of muscles. Muscle Nerve. 1988;11:56–65. doi: 10.1002/mus.880110110. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs J, Jansen M, Janssen H, Raijmann W, Van Alfen N, Pillen S. Quantitative muscle ultrasound and muscle force in healthy children: a 4-year follow-up study. Muscle Nerve. 2013;47:856–863. doi: 10.1002/mus.23690. [DOI] [PubMed] [Google Scholar]
- 20.Henricson E, Abresch R, Han JJ, Nicorici A, Goude Keller E, Elfring G, Reha A, Barth J, McDonald CM. Percent-predicted 6-minute walk distance in duchenne muscular dystrophy to account for maturational influences. PLoS Curr. 2012;4:RRN1297. doi: 10.1371/currents.RRN1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly AM, Malkus EC, Mendell JR, Flanigan KM, Miller JP, Schierbecker JR, Siener CA, Golumbek PT, Zaidman CM, McDonald CM, Johnson L, Nicorici A, Karachunski PI, Day JW, Kelecic JM, Lowes LP, Alfano LN, Darras BT, Kang PB, Quigley J, Pasternak AE, Florence JM. Outcome reliability in non ambulatory boys/men with duchenne muscular dystrophy. Muscle Nerve. 2014 [Google Scholar]
- 22.Willcocks RJ, Arpan IA, Forbes SC, Lott DJ, Senesac CR, Senesac E, Deol J, Triplett WT, Baligand C, Daniels MJ, Sweeney HL, Walter GA, Vandenborne K. Longitudinal measurements of MRI-T2 in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromuscular disorders : NMD. 2014;24:393–401. doi: 10.1016/j.nmd.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollingsworth KG, Garrood P, Eagle M, Bushby K, Straub V. Magnetic resonance imaging in Duchenne muscular dystrophy: longitudinal assessment of natural history over 18 months. Muscle Nerve. 2013;48:586–588. doi: 10.1002/mus.23879. [DOI] [PubMed] [Google Scholar]
- 24.Janssen BH, Pillen S, Voet NB, Heerschap A, van Engelen BG, van Alfen N. Quantitative muscle ultrasound versus quantitative magnetic resonance imaging in facioscapulohumeral dystrophy. Muscle Nerve. 2014;50:968–975. doi: 10.1002/mus.24247. [DOI] [PubMed] [Google Scholar]