Abstract
Muscles are small in spinal muscular atrophy (SMA). It is not known if muscle size changes over time in SMA type 1. We quantified changes over time in muscle size and echointensity during two repeated ultrasound examinations of unilateral proximal (biceps brachii/brachialis and quadriceps) and distal (anterior forearm flexors and tibialis anterior) muscles in three children with SMA type 1. We compared muscle thickness (MT) to body weight-dependent normal reference values. Children were 1, 6, and 11 months old at baseline and had 2, 2 and 4 months between ultrasound examinations, respectively. At baseline, MT was normal for weight in all muscles except an atrophic quadriceps in the oldest child. MT decreased and echointensity increased (worsened) over time. At follow up, MT was below normal for weight in the quadriceps in all three children, in the biceps/brachioradialis in two, and in the anterior forearm in one. Tibialis anterior MT remained normal for weight in all three children. Muscle echointensity increased over time in all muscles and, on average, more than doubled in two children. In children with SMA type 1, muscle atrophies and becomes hyperechoic over time. Quantitative muscle ultrasound measures disease progression in SMA type 1 that warrants additional study in more children.
Keywords: Ultrasound, Backscatter, Spinal Muscular Atrophy, Muscle, Children
1. Introduction
Spinal muscular atrophy (SMA) results from a loss of survival motor neuron 1 (SMN1) genes, has an estimated incidence of up to 1/6000 live births, and is the most common fatal neuromuscular disease of infancy [1]. The loss of survival motor neuron protein results in progressive muscle weakness from degeneration of the α-motor neurons in the anterior horn of the spinal cord and lower brain stem. A truncated survival motor neuron protein, produced by the survival motor neuron 2 (SMN2) gene, partially rescues the phenotype. The type of SMA is defined by the clinical severity and correlates with the number of SMN2 gene copies [2]. Children with SMA type 1 (Werdnig-Hoffmann) are most severely affected and never sit independently, those with type 2 achieve independent sitting, and those with type 3 walk. There are currently therapies in early clinical trials, such as antisense oligonucleotides, designed to lead to more functional SMN2-derived protein for patients with all types of SMA.
Reliable outcome measures that are easy to perform and do not require active patient participation are needed for clinical trials, particularly for the infants with SMA type 1 [3, 4]. Motor unit number estimate (MUNE) and compound muscle action potential (CMAP) testing have been proposed to monitor the degeneration of motor neurons but are painful, require high levels of investigator training to achieve good reliability, and can be tedious to perform. Other techniques such as dual-energy X-ray absorptiometry (DEXA) or MRI have been proposed to monitor muscle size as a biomarker of disease in children with SMA [5–7]. MRI cannot be performed at the bedside and may require sedation. DEXA scanning exposes children to radiation.
Quantitative muscle ultrasound is a simple, painless, and rapid tool for measuring muscle pathology that can be performed at the bedside. Features of muscle measured with ultrasound include size (thickness or cross sectional area) and echointensity. Ultrasound and MRI yield highly correlated measurements of muscle size [8–10]. Both muscle size and echointensity can be quantified reliably using ultrasound by trained examiners [10–12]. Muscle size in children changes with growth and requires normalization for height and/or weight [13]. Muscle echointensity in children does not change with normal growth [13]. Muscle echointensity measured from the grey scale pixel levels in the ultrasound image will vary between different ultrasound systems and set-ups. Calibrated muscle backscatter (cMB) is a measure of echointensity that reduces the inter-system variability in grey scale pixel measurements by estimating the amplitudes of the sound waves scattered back to the ultrasound transducer and referencing values to a common phantom [14, 15]. Both backscatter and grey scale measures of muscle echogenicity have high interrater reliability and similarly detect pathology [16]. cMB provides improved reliability over grey scale measurements for comparing images acquired using different ultrasound systems or configurations [15].
Quantitative muscle ultrasound can measure neuromuscular disease progression even in very young children. For instance, in infants and young children with Duchenne muscular dystrophy, cMB increased 1.0–3.8 dB/year [17]. In children with SMA, muscle thickness is decreased [18] and muscle echointensity is increased and relates to disease severity[19–21]. In SMA type 2 and 3, muscle size does not change over time[5]. It is not known how muscle size or echointensity changes over time in children with the more severe SMA type 1 phenotype. In this study, we show how muscle size and echointensity (cMB) change over time in three infants with SMA type 1.
2. Methods
2.1 Patients
This study was approved by the institutional review board of Washington University, St. Louis and informed consent from parents/guardians was obtained. We evaluated 3 subjects between November 2013 to November 2014 with genetically confirmed SMA type 1. Each had an initial ultrasound examination and then a repeat examination at a subsequent clinic visit. All patients were followed in our neuromuscular clinic by neuromuscular neurologists, and charts were reviewed to determine patient demographics at the time of ultrasound examinations, including age, weight, and height. All 3 children were genetically confirmed SMA type 1 with 0 copies of the SMN1 gene, were severely weak, and did not achieve sitting independently as a motor milestone. Patient 1 had four copies of SMN2 and was able to achieve rolling over once very slowly and raise arms above the head but never achieved sitting. Patients 2 and 3 were more severely weak and had only 2 copies of the SMN2 gene.
2.2 Ultrasound
One investigator (C.M.Z.) obtained all ultrasound images and performed all ultrasound measurements. Ultrasound examinations were performed with a Philips iu22 ultrasound machine imaging system with an L12-5 linear-array probe. System settings were kept constant throughout every study. Ultrasound measurement protocol included 4 unilateral muscles in each child: two proximal arm/leg muscles (biceps brachii/brachialis and quadriceps) and two distal arm/leg muscles (anterior forearm flexors and tibialis anterior). The muscles were measured in the supine position, holding arms and legs extended with muscles relaxed. Arms were supinated, knees extended, and ankle in a neutral position. Transverse ultrasound images of the muscles were obtained at pre-defined anatomical locations that correspond to the maximal muscle diameter at the muscle belly. The biceps brachii/brachialis was measured at two-thirds of the distance from the acromion to the antecubital crease. The forearm flexors were measured at two-fifths of the distance from the antecubital crease to the distal end of the radius. The quadriceps femoris was measured halfway along the line from the anterior superior iliac spine to the superior aspect of the patella. The tibialis anterior was measured at one-quarter of the distance from the inferior aspect of the patella to the lateral malleolus. The transducer was placed perpendicular to the skin to avoid overestimation of muscle thickness, and oblique scanning was avoided by altering the angle of the transducer to achieve the best bone echo and thinnest appearance to the muscle. A liberal amount of transducer gel was applied and minimal pressure of the transducer was exerted on the skin.
2.3 Measurements
Muscle thickness (MT) was measured using the electronic calipers. The thickness of the biceps brachii/brachialis was measured between the upper margin of the humerus and the lower boundary of the ventral fascia of the biceps brachii; the forearm flexors thickness was measured between the interosseous membrane next to the radius and the lower boundary of the ventral fascia of the most ventral flexors; the quadriceps femoris between the upper margin of the femoral bone and the lower boundary of the ventral fascia of the rectus femoris; and the thickness of the tibialis anterior was measured between the interosseous membrane next to the tibia and the lower boundary of the ventral fascia of the tibialis anterior. The rectus femoris had well defined circumferential margins and we therefore also measured the cross sectional area of this muscle using a free form polygon (QLAB ®, Philips Inc.). When muscle echogenicity was very abnormal, identification of the border between fascia and muscle was optimized by dynamic scanning along the length of the muscle prior to measurement.
Muscle grey scale levels were measured using QLAB ® from a region of interest of the muscle drawn from the superficial fascia to the deep fascia or bone. cMB was measured from biceps brachii/brachialis, rectus femoris, anterior forearm muscles, and tibialis anterior. Calibrated muscle backscatter decibel levels (cMB) were calculated from grey scale levels in 2 steps in concordance with a previous study [14]. First, the measured grey scale levels were converted to backscatter (decibels) using a previously identified conversion factor (4.54 grey scale levels/dB) for the ultrasound settings used in this study [15]. Second, muscle backscatter was calibrated to a reference phantom (Model 047; Computerized Imaging Reference Systems, Inc., Norfolk, VA, USA) by subtracting the backscatter measured from the phantom using a rectangular region of interest extending 5cm deep (6.82dB).
Average MT and cMB was defined as the average measurement of the biceps brachii/brachialis, forearm flexors, quadriceps/rectus femoris, and tibialis anterior and was calculated for each subject. The difference in individual and average muscle size measurements between the initial and repeat ultrasound examination was calculated and expressed as a percentage of the baseline. Changes in cMB are reported as the difference between repeated measures. MT was also compared to body weight-dependent muscle-specific reference values [22]. Atrophic muscles were defined as a muscle thickness two standard deviations (<2.5 percentile) below the mean for weight in normals. We report the infant’s weight percentile based on age referenced values for normal children[23]. Weight deceleration over time was defined as a weight percentile for age that crosses two major percentile lines on the growth chart.
3. Results
We studied 3 children aged 11.0, 1.2, and 5.5 months at baseline. The interval between the two ultrasound examinations was 4.1, 1.8, and 1.8 months, respectively. Patient age and weight are summarized in Table 1.
Table 1.
Patient Age and Weight at Time of Ultrasound Assessments
| Patient | Age at ultrasound (months) |
Interval between ultrasound (months) |
Weight (kg) |
Weight Percentile For Age (%) |
|---|---|---|---|---|
| 1 | 11.0 | 4.1 | 7.7 | 15.3 |
| 15.1 | 9.5 | 45.7 | ||
| 2 | 1.2 | 1.8 | 4.8 | 57.6 |
| 3.0 | 6.2 | 39.9 | ||
| 3 | 5.5 | 1.8 | 7.0 | 47.1 |
| 7.3 | 7.4 | 38.1 |
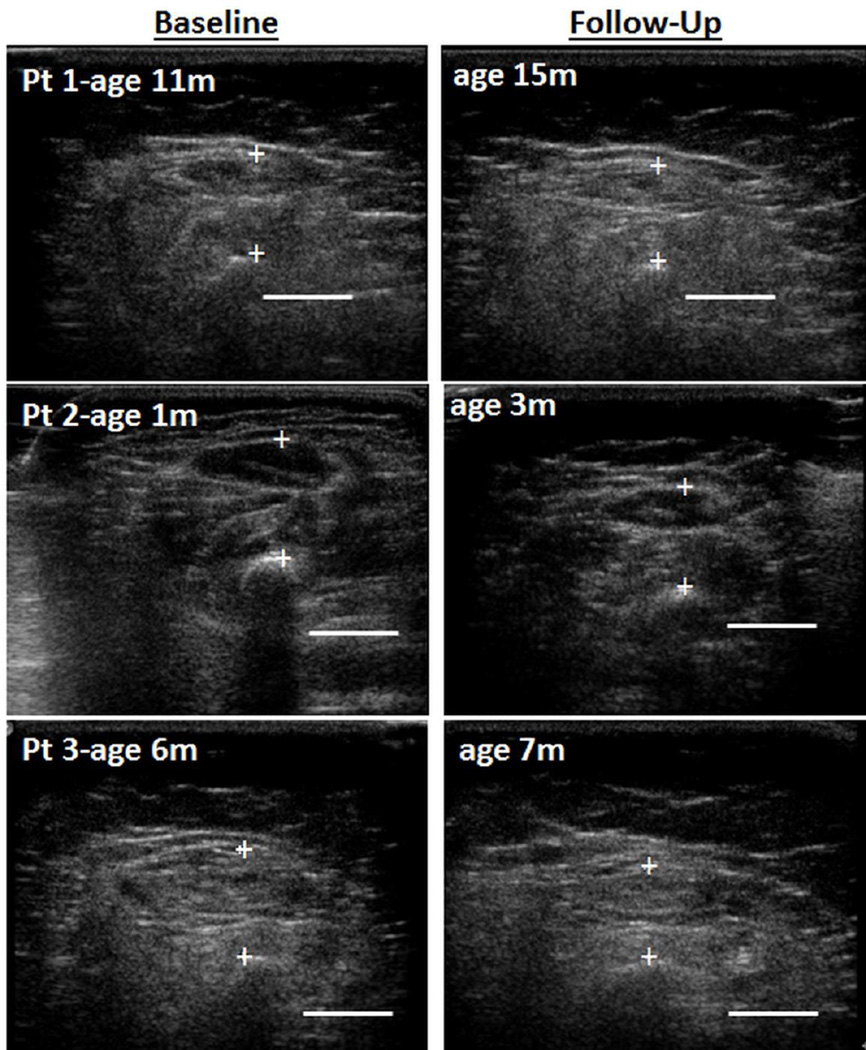
At baseline, only the quadriceps in the oldest child (#1) was atrophic and only the youngest child (#2) had qualitatively normal appearing muscle echointensity (Figure 1). MT decreased over time in most muscles (Table 2). Proximal muscles atrophied over time more than distal muscles. At follow up, MT was below normal for weight in the quadriceps in all three children, in the biceps/brachioradialis in two, and in the anterior forearm in only one. MT in the tibialis anterior remained within normal limits for weight in all three children. Quadriceps and biceps brachii/brachialis thickness decreased as much as 0.3cm. Rectus femoris cross sectional area decreased −26.0%, −25.8%, and −20.9% in children #1, 2, and 3, respectively. The muscle atrophy was not due to weight loss. No patient showed weight deceleration over time.
Figure 1. Ultrasound Images of Quadriceps in Three Children with SMA-1 Over Time.
Legend: Ultrasound cross sectional images of the quadriceps in three patients (Pt) with spinal muscular atrophy (SMA) type 1 shows muscle atrophy and worsening (increased) echointensity over time. Note the relatively normal muscle appearance of the youngest patient (#2, 1 month (m) old) at baseline (left panel) compared to the older patients. Bar = 1cm.
Table 2.
Muscle Thickness Over Time in Children with SMA type 1
| Muscle Thickness (cm) | Muscle Thickness- Change from Baseline (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PT | Age (m) |
BB | FF | Quad | TA | BB | FF | Quad | TA | Average of All Four Muscles |
| 1 | 11.0 | 0.9 | 1.0 | 1.2* | 0.8 | |||||
| 15.1 | 0.6* | 0.9 | 1.2* | 0.7 | −27.5 | −9.2 | 0.0 | −7.5 | −11.4 | |
| 2 | 1.2 | 1.1 | 1.1 | 1.4 | 1.0 | |||||
| 3.0 | 0.8 | 1.1 | 1.1* | 0.9 | −21.2 | +1.9 | −20.6 | −14.9 | −14.1 | |
| 3 | 5.5 | 0.7 | 0.9 | 1.3 | 0.8 | |||||
| 7.3 | 0.6* | 0.8* | 1.1* | 0.8 | −12.7 | −2.2 | −15.6 | −0.8 | −8.6 | |
Legend: PT-patient; M- months; BB -biceps brachii/brachialis; FF-anterior forearm flexors; Quad -quadriceps; TA- tibialis anterior
indicates below the 2.5 percentile for weight-adjusted reference values (normal values given in Supplementary Table 1)
cMB increased over time in all muscles (Table 3). Average cMB increased 3.1 and 4.5 dB in patients 1 and 2. In patient 3, cMB was highest at baseline and remained high over time. cMB increased similarly in proximal and distal muscles.
Table 3.
Muscle Echointensity (cMB) Over Time in SMA I
| Muscle cMB (dB) | Muscle cMB- Difference from Baseline (dB) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PT | Age (m) |
BB | FF | RF | TA | BB | FF | RF | TA | Average of All Four Muscles |
| 1 | 11.0 | 1.0 | 5.2 | 6.1 | 7.2 | |||||
| 15.1 | 4.0 | 7.8 | 9.0 | 11.2 | 3 | 2.6 | 2.9 | 4 | 3.1 | |
| 2 | 1.2 | −4.1 | −1.3 | −1.9 | −3.5 | |||||
| 3.0 | −0.7 | 2.2 | 0.7 | 5.1 | 3.4 | 3.5 | 2.6 | 8.6 | 4.5 | |
| 3 | 5.5 | 6.9 | 9.0 | 7.2 | 11.1 | |||||
| 7.3 | 7.2 | 10.7 | 7.2 | 11.8 | 0.3 | 1.7 | 0 | 0.7 | 0.7 | |
Legend: PT-patient; M- months, cMB- calibrated muscle backscatter; BB-biceps brachii/brachialis; FF- anterior forearm flexors; RF- rectus femoris; TA-tibialis anterior
4. Discussion
This study shows that in SMA type 1, muscle thickness may be within normal limits early in the first year of life and that muscles atrophy rapidly, as much as 28% over 4 months. Proximal muscles atrophied more than distal muscles. Preferential involvement of proximal muscles has been noted in the mouse model of SMA, which shows axon loss first in the motor neurons innervating proximal hind-limb muscles, then in axial muscles, and lastly in the distal hind limb muscles[24]. Few studies have evaluated muscle thickness in children with SMA type 1. One study of 12 patients with SMA type 1 used ultrasound to measure quadriceps muscle and subcutaneous fat thickness. The ratio of muscle to fat was lower in these patients than in healthy children and remained low in two subjects with follow up imaging [18]. Our results also show decreased muscle thickness in children with SMA type 1 and demonstrate a progressive decline in MT over a short time period (2–4 months). Unlike the prior study, we measured thickness of several muscles but not subcutaneous fat because subcutaneous fat is easily compressible and may yield unreliable measurements.
The rapid muscle atrophy seen in this study could be from active loss of motor axons. MUNE and CMAP testing suggest that there is active motor unit loss in the early phases of SMA, followed by a relatively stable chronic phase. The decline in motor unit number is age-dependent and of variable onset [25]. Only the oldest child (11 months old) in our study had an atrophic quadriceps at baseline. All three children in our study developed muscle atrophy over 2–4 months. Disuse and lack of weight-bearing can also contribute to muscle atrophy [26]. Additional studies in more children and with longer follow-up are needed to better characterize the time course and degree of muscle atrophy in children with SMA type 1.
Atrophy in muscle over time was more prominent and rapid in our study of SMA type 1 than seen in a study of children with SMA type 2 and 3. In 11 children and adults aged 6 – 47 years with SMA type 2 and 3, quadriceps muscle volume measured using MRI showed minimal interval change over a 6-month interval [5]. We may have detected more muscle atrophy in our study of infants with SMA type 1 because they were younger or because their phenotype was more severe. The more severe reduction in muscle thickness over time in SMA type 1 compared to SMA type 2 and 3 is similar to the differences in functional decline, which is also more severe in SMA type 1 than type 2 and 3 [27, 28].
Muscle echointensity (cMB) also worsens (increases) rapidly over time in the first year of life in children with SMA type 1. Two children showed large increases in average cMB (3.1–4.5dB) over 2–4 months. This increase is comparable or higher than the change in average cMB over one year in infants and young children with Duchenne Muscle Dystrophy (1.0 – 3.8 dB/year) [17] . The child in our study with the highest cMB at baseline did not show much change over time. This may be because of a ceiling effect. Muscle echointensity is abnormally increased in patients with SMA type 2 and 3 and is highest in those with more severe phenotypes [19, 20]. It is unknown if muscle echointensity increases over time in SMA type 2 or 3.
The rapid change in muscle size and echointensity suggests that these ultrasound measures could be attractive biomarkers for therapeutic trials in SMA 1. The high reliability of quantitative muscle ultrasound supports feasibility for use in multicenter studies. Careful technique will be required to minimize measurement variability in order to detect the changes in muscle thickness (up to 3mm) seen in the three children in this study [13, 29]. Additional longitudinal studies in more children in SMA type 1are warranted to better describe how muscle size and echointensity change with age and over time.
Highlights.
Muscle ultrasound in three children with SMA type 1 showed progressive atrophy and increased echogenicity over time.
Proximal muscles atrophied more than distal muscles.
Muscles ultrasound can appear normal in very young children with SMA type 1.
Quantitative muscle ultrasound may be a useful biomarker in SMA type 1.
Acknowledgments
The study was supported by the Washington University Neuromuscular Research Fund, the National Institute of Health Neurological Sciences Academic Development Award Grant Number K12 NS00169009 and the Institute of Clinical and Translational Sciences UL1 TR000448.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Sproule DM, Kaufmann P. Therapeutic developments in spinal muscular atrophy. Ther Adv Neurol Disord. 2010;3(3):173–185. doi: 10.1177/1756285610369026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012;11(5):443–452. doi: 10.1016/S1474-4422(12)70061-3. [DOI] [PubMed] [Google Scholar]
- 3.Hirtz D, Iannaccone S, Heemskerk J, Gwinn-Hardy K, Moxley R, 3rd, Rowland LP. Challenges and opportunities in clinical trials for spinal muscular atrophy. Neurology. 2005;65(9):1352–1357. doi: 10.1212/01.wnl.0000183282.10946.c7. [DOI] [PubMed] [Google Scholar]
- 4.Zanetta C, Nizzardo M, Simone C, Monguzzi E, Bresolin N, Comi GP, et al. Molecular therapeutic strategies for spinal muscular atrophies: current and future clinical trials. Clin Ther. 2014;36(1):128–140. doi: 10.1016/j.clinthera.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Sproule DM, Montgomery MJ, Punyanitya M, Shen W, Dashnaw S, Montes J, et al. Thigh muscle volume measured by magnetic resonance imaging is stable over a 6-month interval in spinal muscular atrophy. J Child Neurol. 2011;26(10):1252–1259. doi: 10.1177/0883073811405053. [DOI] [PubMed] [Google Scholar]
- 6.Sproule DM, Montes J, Montgomery M, Battista V, Koenigsberger D, Shen W, et al. Increased fat mass and high incidence of overweight despite low body mass index in patients with spinal muscular atrophy. Neuromuscular disorders : NMD. 2009;19(6):391–396. doi: 10.1016/j.nmd.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sproule DM, Punyanitya M, Shen W, Dashnaw S, Martens B, Montgomery M, et al. Muscle volume estimation by magnetic resonance imaging in spinal muscular atrophy. J Child Neurol. 2011;26(3):309–317. doi: 10.1177/0883073810380457. [DOI] [PubMed] [Google Scholar]
- 8.Juul-Kristensen B, Bojsen-Moller F, Holst E, Ekdahl C. Comparison of muscle sizes and moment arms of two rotator cuff muscles measured by ultrasonography and magnetic resonance imaging. Eur J Ultrasound. 2000;11(3):161–173. doi: 10.1016/s0929-8266(00)00084-7. [DOI] [PubMed] [Google Scholar]
- 9.Worsley PR, Kitsell F, Samuel D, Stokes M. Validity of measuring distal vastus medialis muscle using rehabilitative ultrasound imaging versus magnetic resonance imaging. Man Ther. 2014;19(3):259–263. doi: 10.1016/j.math.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91(1):116–118. doi: 10.1007/s00421-003-0961-9. [DOI] [PubMed] [Google Scholar]
- 11.Zaidman CM, Wu JS, Wilder S, Darras BT, Rutkove SB. Minimal training is required to reliably perform quantitative ultrasound of muscle. Muscle Nerve. 2014;50(1):124–128. doi: 10.1002/mus.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillen S, van Keimpema M, Nievelstein RA, Verrips A, van Kruijsbergen-Raijmann W, Zwarts MJ. Skeletal muscle ultrasonography: Visual versus quantitative evaluation. Ultrasound Med Biol. 2006;32(9):1315–1321. doi: 10.1016/j.ultrasmedbio.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs J, Jansen M, Janssen H, Raijmann W, Van Alfen N, Pillen S. Quantitative muscle ultrasound and muscle force in healthy children: a 4-year follow-up study. Muscle Nerve. 2013;47(6):856–863. doi: 10.1002/mus.23690. [DOI] [PubMed] [Google Scholar]
- 14.Zaidman CM, Holland MR, Anderson CC, Pestronk A. Calibrated quantitative ultrasound imaging of skeletal muscle using backscatter analysis. Muscle Nerve. 2008;38(1):893–898. doi: 10.1002/mus.21052. [DOI] [PubMed] [Google Scholar]
- 15.Zaidman CM, Holland MR, Hughes MS. Quantitative ultrasound of skeletal muscle: reliable measurements of calibrated muscle backscatter from different ultrasound systems. Ultrasound Med Biol. 2012;38(9):1618–1625. doi: 10.1016/j.ultrasmedbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shklyar I, Geisbush TR, Mijialovic AS, Pasternak A, Darras BT, Wu JS, et al. Quantitative muscle ultrasound in Duchenne muscular dystrophy: a comparison of techniques. Muscle Nerve. 2015;51(2):207–213. doi: 10.1002/mus.24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidman CM, Malkus EC, Connolly AM. Muscle Ultrasound Quantifies Disease Progression Over Time in Infants and Young Boys with Duchenne Muscular Dystrophy. Muscle Nerve. 2015 doi: 10.1002/mus.24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt R, Voit T. Ultrasound measurement of quadriceps muscle in the first year of life. Normal values and application to spinal muscular atrophy. Neuropediatrics. 1993;24(1):36–42. doi: 10.1055/s-2008-1071510. [DOI] [PubMed] [Google Scholar]
- 19.Wu JS, Darras BT, Rutkove SB. Assessing spinal muscular atrophy with quantitative ultrasound. Neurology. 2010;75(6):526–531. doi: 10.1212/WNL.0b013e3181eccf8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37(6):679–693. doi: 10.1002/mus.21015. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava T, Darras BT, Wu JS, Rutkove SB. Machine learning algorithms to classify spinal muscular atrophy subtypes. Neurology. 2012;79(4):358–364. doi: 10.1212/WNL.0b013e3182604395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholten RR, Pillen S, Verrips A, Zwarts MJ. Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve. 2003;27(6):693–698. doi: 10.1002/mus.10384. [DOI] [PubMed] [Google Scholar]
- 23.WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 24.Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L, et al. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69(3):453–467. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe T, Kawakami Y, Suzuki Y, Gunji A, Fukunaga T. Effects of 20 days bed rest on muscle morphology. J Gravit Physiol. 1997;4(1):S10–S14. [PubMed] [Google Scholar]
- 27.Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–817. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufmann P, McDermott MP, Darras BT, Finkel R, Kang P, Oskoui M, et al. Observational study of spinal muscular atrophy type 2 and 3: functional outcomes over 1 year. Arch Neurol. 2011;68(6):779–786. doi: 10.1001/archneurol.2010.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fivez T, Hendrickx A, Van Herpe T, Vlasselaers D, Desmet L, Van den Berghe G, et al. An Analysis of Reliability and Accuracy of Muscle Thickness Ultrasonography in Critically Ill Children and Adults. JPEN J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115575033. [DOI] [PubMed] [Google Scholar]