Abstract
Aim
To assess the relative effects of different types of exercise and other non-pharmaceutical interventions on cancer-related fatigue (CRF) in patients during and after cancer treatment.
Design
Systematic review and indirect-comparisons meta-analysis.
Data sources
Articles were searched in PubMed, Cochrane CENTRAL and published meta-analyses.
Eligibility criteria for selecting studies
Randomised studies published up to January 2017 evaluating different types of exercise or other non-pharmaceutical interventions to reduce CRF in any cancer type during or after treatment.
Study appraisal and synthesis
Risk of bias assessment with PEDro criteria and random effects Bayesian network meta-analysis.
Results
We included 245 studies. Comparing the treatments with usual care during cancer treatment, relaxation exercise was the highest ranked intervention with a standardisedmean difference (SMD) of −0.77 (95% Credible Interval (CrI) −1.22 to −0.31), while massage (−0.78; −1.55 to −0.01), cognitive–behavioural therapy combined with physical activity (combined CBT, −0.72; −1.34 to −0.09), combined aerobic and resistance training (−0.67; −1.01 to −0.34), resistance training (−0.53; −1.02 to −0.03), aerobic (−0.53; −0.80 to −0.26) and yoga (−0.51; −1.01 to 0.00) all had moderate-to-large SMDs. After cancer treatment, yoga showed the highest effect (−0.68; −0.93 to −0.43). Combined aerobic and resistance training (−0.50; −0.66 to −0.34), combined CBT (−0.45; −0.70 to −0.21), Tai-Chi (−0.45; −0.84 to −0.06), CBT (−0.42; −0.58 to −0.25), resistance training (−0.35; −0.62 to −0.08) and aerobic (−0.33; −0.51 to −0.16) showed all small-to-moderate SMDs.
Conclusions
Patients can choose among different effective types of exercise and non-pharmaceutical interventions to reduce CRF.
Keywords: cancer related fatigue, non-pharmaceutical interventions, exercise, network meta-analysis, indirect comparison meta-analysis.
Introduction
The National Comprehensive Cancer Network (NCCN)1 defines cancer-related fatigue (CRF) as ‘a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning'. CRF is one of the most common and distressing symptoms of cancer and cancer treatment2 that can be prevalent before treatment onset and usually increases during therapy.3 4 Compared with the fatigue experienced by healthy individuals, CRF is more severe, more distressing and less likely to be relieved by rest.1 Overall, the prevalence of CRF has been estimated between 25% and 99%, depending on the patient population, type of treatment received and method of assessment.5 CRF can persist for up to 5 years after completion of treatment or even longer6–8 and is associated with significant impairment in overall quality of life during and after treatment.9 Besides, in a large longitudinal study of patients with breast cancer, CRF predicted decreased recurrence-free survival and overall survival.2
Although the aetiology of CRF is still unknown, some possible biological mechanisms have been postulated, such as proinflammatory cytokines, hypothalamus–pituitary–adrenal axis deregulation,10 circadian rhythm desynchronisation, skeletal muscle wasting and genetic deregulation.1 11 Bower5 described the following risk factors for CRF: genetic risk factors (eg, single nucleotide polymorphisms), psychological (eg, depression) and behavioural risk factors (eg, physical inactivity). Some of these factors might be targeted by exercise. For example, skeletal muscle wasting may be avoided or reversed by resistance exercise.
A broad variety of non-pharmacological interventions are used against CRF and many trials, systematic reviews and meta-analyses have been published.12–44 The Oncology Nursing Society ‘Putting Evidence into Practice’ tool on CRF proposed exercise and physical activity as a first-line intervention for CRF.45 Furthermore, this guideline states that apart from erythropoiesis-stimulating agents (Erythropoietin) and low-dose dexamethasone for patients with advanced cancer (both drugs with potential serious adverse events), there is to date not enough scientific evidence to support the effectiveness of pharmacological agents and nutritional supplements to reduce CRF in patients with cancer. Similar recommendations have been made by the NCCN guidelines for CRF.1 Taken together, for most cancer types there is evidence to support exercise or other non-pharmaceutical treatments as effective options to reduce CRF during and after treatment. However, it is still unclear which of these anti-CRF interventions yields the largest treatment effect, as there is no systematic review comparing the effects of different non-pharmaceutical interventions on CRF. An indirect-comparisons meta-analysis, using direct evidence (interventions compared within one trial) and indirect evidence (comparing interventions across different trials), could inform clinicians and scientists on the ranking of the effectiveness of those interventions. Indirect-comparisons meta-analysis is an upcoming statistical method already used in other medical fields.46
The aim of the present indirect-comparisons (network) meta-analysis was to assess the relative effects of different types of exercise and other non-pharmaceutical interventions on CRF in patients with cancer during and after cancer treatment. In this paper, we focused on exercise-related non-pharmacological interventions, such as physical activity, aerobic and resistance training, and relaxation, while nutritional interventions were excluded.
Methods
This study is reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.47 An a priori written protocol exists but was not registered.
Design
We conducted a systematic review and an indirect-comparisons (network) meta-analysis.
Database and search strategy
The digital databases PubMed, Cochrane Database of Systematic Reviews and Cochrane Controlled Clinical Trials (CENTRAL) were searched, from inception to 4 January 2017. We developed a search strategy for each database (see online supplementary appendix 1), without language restriction. The Cochrane highly sensitive filter for clinical trials was used.48 In addition, JT and RH retrieved reviews and meta-analyses on exercise for CRF and screened references of selected articles for additional topic-related publications.
bjsports-2016-096422supp001.pdf (26KB, pdf)
Inclusion criteria for study selection
Published randomised or quasi-randomised trials evaluating the effect of all kind of exercise or other non-pharmaceutical interventions such as cognitive–behavioural or relaxation interventions on CRF or vitality in patients with CRF during or after active cancer treatments were eligible for inclusion. All cancer types were included, because we assumed that CRF is a general cancer problem and that the working mechanisms and effects of the different interventions targeting CRF would be similar across all cancer diagnoses.
Exclusion criteria
We excluded trials comparing drugs or nutritional supplementations, acupuncture, electroacupuncture, acupressure, moxibustion or healing without touching the patients (eg, Reiki or healing over the phone), as well as expressive writing intervention because we focused on exercise and conventional physiotherapy-related interventions (eg, movement therapy, relaxation or massage). Studies with an intervention duration of <3 weeks as well as interventions aiming to improve sleep quality were also excluded.
Classification in ‘during’ or ‘after’ cancer treatment
Studies including patients receiving chemotherapy or radiotherapy as the initial cancer treatment or as treatment in the presence of metastasis or cancer recurrence were classified as ‘during’, while those studies including patients currently not on chemotherapy or radiotherapy were defined as ‘after’. Studies including both types of patients were classified according to the majority of patients. Studies including patients receiving androgen suppression therapy without chemotherapy or radiotherapy were defined as ‘after’.
Study selection
Two reviewers (RH, JT) independently screened titles and abstracts for eligibility. In case of disagreement, consensus was reached through discussion. At this stage, studies were excluded only if the available information in the title or the abstract made it clear that the article was not eligible. Full texts were retrieved for the other articles and read independently by these two reviewers. A consensus meeting was held to discuss disagreements.
Assessment of risk of bias
Risk of bias (RoB) was assessed using the ratings published in the PEDro database. We used items of random sequence generation, allocation concealment, baseline differences between groups, blinding of participants, blinding of therapists and blinding of assessors, incomplete outcome data and intention-to-treat analysis. If the study was not scored in the PEDro database, one reviewer (RH or JT) assessed RoB using the same PEDro rating scale criteria. RoB was provided only with the intention to give a detailed overview of the characteristics of the included studies (ie, RoB was not used to exclude studies). Furthermore, we assessed the risk for publication bias with the comparisons of the active interventions against the control groups with funnel plots and Egger’s tests,49 which regresses the effect estimates on their standard errors, weighted by the inverse of the variance.
Data extraction
Study characteristics were extracted into a spreadsheet by one of three reviewers (RH, JT, LNB). Data for the calculation of effect sizes for fatigue or vitality from the first time point after the end of the period of the intervention under assessment in the specific study were extracted by one reviewer (RH) and controlled by a second reviewer (JT). Change values were extracted whenever mean and SD of the changes were available or when the available data allowed their calculation. If change values were not available or could not be calculated, post-treatment values for mean and SD were extracted. If SD were missing, different methods were used to estimate the SD, such as using p values, CIs or extracting data from figures. If only median and IQRs were presented, means were estimated by the median while SDs were calculated by dividing the IQR by 1.35.48 The effect sizes were converted by reversing signs for means so that higher values always indicated more fatigue.50 The corresponding authors were contacted in case of missing data or unclear reporting. If in a given study fatigue was assessed by more than one questionnaire, we selected the questionnaire presented as primary outcome or as the first result for fatigue.
Summary measures
Because fatigue was assessed with different questionnaires, we used standardised mean difference (SMD) as the effect size measure. The SMD is an effect size without metric and is calculated as the difference in mean outcome between two groups, divided by the pooled SD of the measure. For the interpretation of the SMD, following benchmarking anchors were used: 0.2 indicates a small effect size, 0.5 a moderate effect size and 0.8 a large effect size.51
Data synthesis
Bayesian network meta-analysis (random effects models) was used to compare the relative effectiveness of the different interventions under investigation. Bayesian Inference Using Gibbs Sampling (BUGS) was performed with Just Another Gibbs Sampler (JAGS). JAGS is designed to work with the R language and environment for statistical computation.52 From within R, we used the gemtc53 and the rjags package.54
To rank the interventions, the probability of each intervention being the most effective, the second most effective, etc was calculated. Rankograms were established and the surface under the cumulative ranking curve (SUCRA55) was calculated for each intervention. The surface under the cumulative ranking curve represents an inversely scaled average rank of the intervention, scaled such that it is 1 if the intervention always ranks first and 0 if the intervention always ranks last. The SUCRA of a specific intervention can be interpreted as the average proportion of interventions worse than the intervention under consideration.
The differences of the SMD between the direct and indirect comparisons were selected to evaluate the fundamental assumption of consistency, that is, that direct and indirect evidence are compatible or that all studies are exchangeable. Heterogeneity was assessed using Higgins I2 for each pairwise comparison.
Non-informative uniform distribution of effect sizes and precision were chosen for all parameters of the model to enable the data to dominate the prior information in the final results. Four Markov chains were run simultaneously with different initial values, which were set arbitrarily. The convergence to a stable solution was checked visually with the Brooks-Gelman-Rubin method. The first 50 000 samples were discarded and the posterior summaries were based on 500 000 simulations. The posterior distributions of the SMDs are presented with the medians and 95% Crls (95% CrI), representing a range of values in which the SMD lies with a probability of 95%.
Results of all analyses (during and after cancer treatment and sensitivity analyses for effects of small-study samples) are presented in forest plots comparing all interventions with control as well as in matrices showing effect estimates of all comparisons.
Comparison of consistency and inconsistency models
The deviance information criteria (DIC) of the consistency model and the unrelated means model (inconsistency) were compared, and the model with the lower DIC value was selected.
To further explore the inconsistency between the direct and indirect evidence, we ran an ensemble of node-splitting models.56
Sensitivity analyses
Because small studies tend to have larger effects compared with larger studies, we conducted a sensitivity analysis excluding studies with less than 25 patients per intervention arm.57
Results
Study selection and characteristics
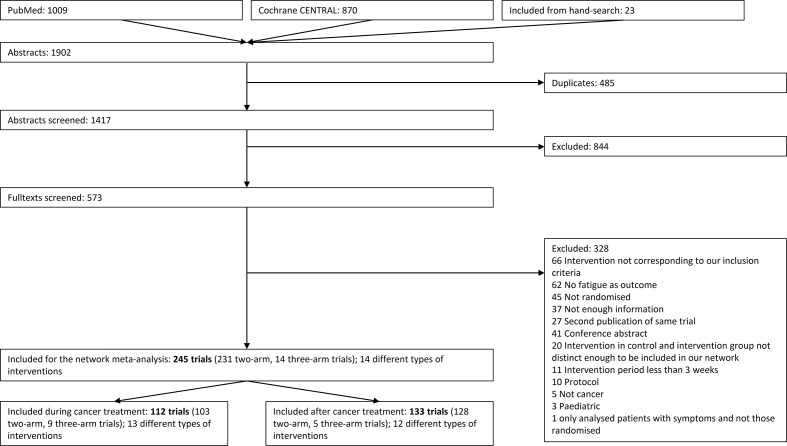
The systematic search in the databases and the hand search yielded 1471 articles, and we screened 573 full texts and finally included 245 for further qualitative and quantitative analyses (see figure 1). Characteristics of the included studies are shown in the online supplementary appendix 1 and supplementary appendix 2. The date of publication ranged from 1989 to 2017. Of the included articles, 112 articles reported on patients during cancer treatment and 133 articles after the treatment. Five studies included patients both during and after treatment and were classified according to the higher proportion of patients (three studies classified during, and two after). The duration of the interventions ranged from 3 to 52 weeks and the follow-up from 0 to 12 weeks after the end of the intervention. Most of the studies reported on women with breast cancer (n=126; 51%), followed by studies reporting on mixed patient groups (n=52; 21%; 79% of these were breast cancer), prostate cancer (18; 7%), haematopoietic stem cell transplantation (10; 4%) and colorectal cancer (7, 3%).
Figure 1.
Flow chart of the study selection process.
bjsports-2016-096422supp002.pdf (88.6KB, pdf)
RoB of included trials
Over all comparisons, the random sequence generation was adequate in most trials (97%); allocation concealment was problematic in 61% of the trials. Baseline characteristics were not balanced in 17% of the studies; patients and outcome assessor could not be blinded in most trials (99%), therapists were never blinded. More than 15% of drop-outs were present in 36% of the trials and only 51% of the trials reported an intention-to-treat analysis. Supplementary appendix 2 shows the assessment of the RoB in the included trials separate for each direct comparison, separated for during and after cancer treatment.
There is some evidence for small-study effects, as assessed with the Egger’s test in the pairwise meta-analysis for the comparisons aerobic versus control, combined resistance and aerobic training versus control, combined cognitive –behavioural therapy (CBT) versus control and relaxation versus control. One reason for the presence of these small-study effects might be publication bias.
Indirect-comparisons meta-analysis for exercise and non-pharmaceutical interventions during cancer treatment
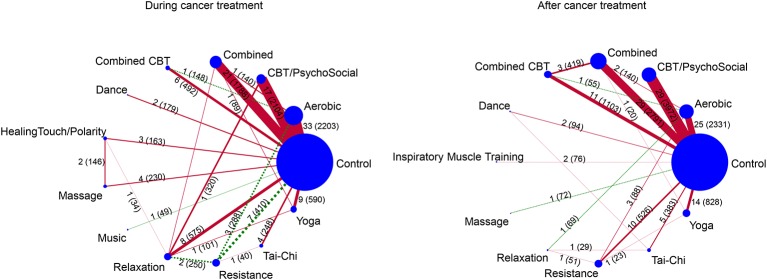
The network consisted of 103 studies with two arms and 9 studies with three arms reporting on 13 different interventions (34 arms on aerobic, 23 combined aerobic and resistance training, 18 psychosocial and CBT (CBT psychosocial), 12 relaxation (including stretching, meditation, etc), 10 yoga, 10 resistance training, 6 multimodal trainings (exercise and psychosocial combined, combined CBT), four massage, three healing-touch, two dance and one music therapy; 93 arms were control groups). The number of direct comparisons and patients per comparison are shown in figure 2.
Figure 2.
Plots of the two networks (during and after cancer treatment). The size of the circles corresponds to the number of patients within the groups, the width of the lines between the treatment circles indicates the statistical precision of the comparison (ie, inverse of the variance). The numbers on or besides the lines between the treatments indicate the number of trials comparing the two treatments and the numbers in parentheses indicate the number of patients in the given comparison. Red coloured lines indicate that more than 50% of the trials had no allocation concealment or more than 15% drop-outs; green dashed lines indicate that 50% or more of the trials had correct allocation concealment and less than 15% drop-outs. CBT, cognitive–behavioural therapy.
Comparing the deviance criteria for the consistency and inconsistency models (unrelated means model) revealed that the consistency model was to be preferred (DICconsistency=452.7, DICinconsistency=457.413).
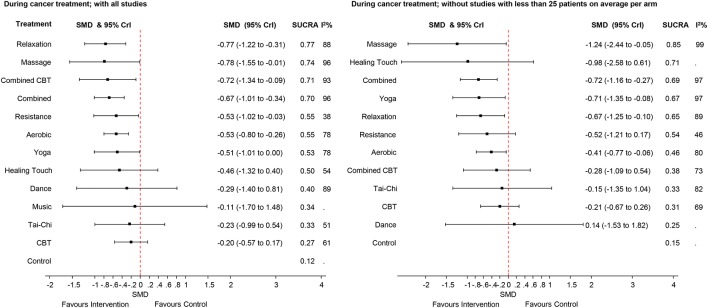
The effect sizes for the differences between all interventions are presented in the online supplementary appendix 5 and supplementary appendix 6. Figure 3 presents the findings of the indirect-comparisons meta-analysis as effect sizes and their 95% CrI for the different types of exercise and non-pharmaceutical interventions on CRF during cancer treatment compared with usual care (control group).
Figure 3.
Forest plots for the comparisons between the active interventions and the control intervention (usual care) for the studies performed during cancer treatment. The left side of the panel shows all studies. The right side shows only the studies with more than 25 patients per arm (on average). CBT, cognitive–behavioural therapy; SMD, standardised mean difference; SUCRA, surface under the cumulative ranking curve; 95% Crl, 95% Crl.
bjsports-2016-096422supp005.pdf (62.3KB, pdf)
bjsports-2016-096422supp006.pdf (59.8KB, pdf)
Relaxation was the best ranked intervention with an SMD of −0.77 (95% CrI −1.22 to −0.31) for the reduction of fatigue. Moderate-to-large effect sizes were observed for massage (SMD −0.78 (−1.55 to −0.01), CBT combined with physical activity (combined CBT, SMD −0.72 with 95% CrI −1.34 to −0.09), combined aerobic and resistance training (SMD −0.67 (−1.01 to −0.34), resistance training (SMD −0.53 with 95% CrI −1.02 to −0.03), aerobic (SMD −0.53 (−0.80 to −0.26) and yoga (SMD −0.51 (−1.01 to 0.00), compared with usual care. The other interventions had credible intervals including a zero effect. The observed statistical heterogeneity (I2) ranged from 38% to 96% in the pairwise comparisons (figure 3).
The SUCRA indicated that relaxation was the best ranked intervention with a SUCRA of 0.77. The control intervention (usual care, ie, no exercise) had the lowest SUCRA (0.12).
In the node-splitting model, no comparison with statistical significant inconsistency was observed, and the overall heterogeneity was not reduced (88%), indicating that inconsistency did not explain the heterogeneity. However, power to detect inconsistency is low given the high heterogeneity.
Sensitivity analysis by excluding small studies with less than 25 patients per arm on average
In the sensitivity analyses to test the effect of smaller sample size on the overall weighted mean in the ‘during’ studies, the effect sizes were smaller for most of the comparisons; for example, the effect size for the combined CBT interventions was reduced from −0.72 to −0.28 when excluding smaller studies, with credible intervals now including the zero effect.
The effect size of massage, combined aerobic and resistance training increased.
The exclusion of small studies did not yield in a reduction of the heterogeneity.
Indirect-comparisons meta-analysis for exercise and non-pharmaceutical interventions after cancer treatment
The network consisted of 12 interventions. The majority of trials included a non-exercise group (eg, waiting list, usual care). The most applied intervention was combined aerobic and resistance training (n=32), followed by aerobic training (n=30) and psychosocial /CBT (n=29).
Comparing the deviance criteria for the consistency and inconsistency models (unrelated means model) revealed that the consistency model was to be preferred (DICconsistency=506.3, DICinconsistency=509.3).
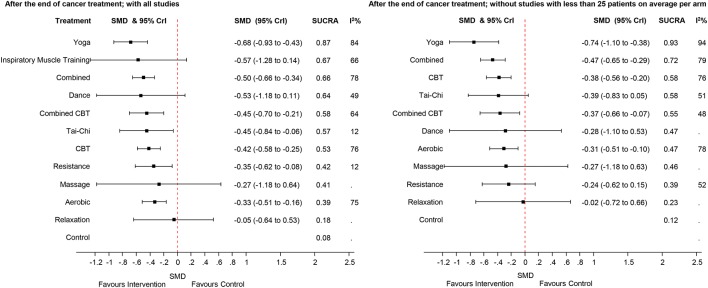
The effect sizes for the differences between all interventions are presented in the online supplementary appendix 7 (all studies) and supplementary appendix 8 (excluding studies with less than 25 patients per arm on average). The left part of figure 4 presents the findings of the indirect-comparisons meta-analysis as effect sizes and their 95% CrI for the different intervention after cancer treatment compared with usual care.
Figure 4.
Forest plots for the comparisons between the active interventions and the control intervention (usual care) for the studies performed after cancer treatment. The left side of the panel shows all studies. The right side shows only the studies with more than 25 patients per arm (on average). CBT, cognitive–behavioural therapy; SMD, standardised mean difference; SUCRA, surface under the cumulative ranking curve; 95%Crl, 95% Crl.
bjsports-2016-096422supp007.pdf (172.1KB, pdf)
bjsports-2016-096422supp008.pdf (57.1KB, pdf)
Yoga showed a moderate-to-large effect size (SMD=−0.68, 95% CrI −0.93 to −0.43) compared with usual care. Combined aerobic and resistance training showed a moderate effect size with an SMD of −0.50 (95% CrI −0.66 to −0.34). Inspiratory muscle training (IMT) and dance therapy also showed moderate effect sizes (IMT: −0.57; dance: −0.53) compared with usual care, but with a credible interval including a zero effect size (IMT: −1.27 to 0.13; dance: −1.17 to 0.11)). Small-to-moderate effect sizes were found for combined CBT (SMD −0.45, 95% CrI −0.70 to −0.21), Tai-Chi (SMD −0.45, 95% CrI −0.84 to −0.06), CBT (SMD −0.42, 95% CrI −0.58 to −0.25), resistance (SMD=−0.35, 95% CrI −0.62 to −0.08) and aerobic training (SMD=−0.33, 95% Crl −0.51 to −0.16). The other interventions had credible intervals including a zero effect size. The observed statistical heterogeneity (I2) ranged from 12% to 84% in the pairwise comparisons (figure 3).
The SUCRA indicated that yoga was the highest ranked intervention with a SUCRA of 0.87. The control intervention (ie, no exercise) had the lowest SUCRA (0.08).
The node-splitting model showed no statistical significant inconsistency between direct and indirect comparisons.
In the sensitivity analyses to test the effect of smaller sample size on the overall weighted mean in the ‘after treatment’ studies, the effect size of dance therapy decreased from −0.53 to −0.28, but in both analyses the corresponding 95% CrI included the zero effect size. Inspiratory muscle training studies disappeared in the sensitivity analysis. The exclusion of small studies did not yield in a reduction of the heterogeneity.
Discussion
This indirect-comparisons meta-analysis, including 245 studies evaluating the effects of exercise and other non-pharmaceutical interventions on CRF, found strong evidence that several interventions reduce CRF substantially more than usual care, as was shown by the moderate-to-high effect sizes, either during or after cancer treatment.
The ranking of the effectiveness of the different types of interventions, which was possible due to the Bayesian approach, may help healthcare professionals (eg, oncologists, nurses, physiotherapists, family practitioners) and patients with cancer in their shared clinical decision making process. For example, the preferences of the patient, contraindications, the availability and the costs of the interventions may influence their decision. Our ranked interventions help patients and practitioners prioritise evidence-based interventions during and after treatment. Healthcare professionals may offer their patients a variety of exercises or other non-pharmaceutical interventions, such as relaxation, yoga, CBT combined with physical activity or resistance or aerobic training, to tackle CRF. Since different interventions showed moderate-to-high effects, patients should choose among these a modality that is most convenient for him or her. For example, if a patient with cancer does not want to lift weights to protect against CRF, he or she might choose to walk, cycle or do yoga instead.
Another important finding of this study was that relaxation seems to be an effective intervention during cancer-related treatment but becomes less important after cancer treatment. This indicates that a strategy to tackle CRF efficiently during cancer treatment should include relaxation sessions besides the personalised exercise or other non-pharmaceutical interventions. After cancer treatment, however, time invested in relaxation sessions is less effective and more time should be spent with physical activity enhancing interventions. Yoga, on the other hand, was beneficial both during and after cancer treatment. The same applies for aerobic, resistance and combined aerobic–resistance training, be it on a somewhat lower effect size level.
Study strengths and limitations
The strengths of this indirect-comparisons meta-analysis were the inclusion of a comprehensive set of types of interventions, the large number of included studies, the statistical method and the stringent methodological approach.
A major limitation of this study was the difficulty to classify the interventions described in the included studies. First, there were different kinds of intervention combinations. For example, relaxation exercises were often embedded in CBT.58 Second, because specific information on training intensity or the extent of exercises was lacking in most of the studies, it was not possible to analyse the effect of high versus low training intensity or high versus low exercise volume. Third, we are well aware that the control groups in our network meta-analysis are very heterogeneous and that this may strongly increase the heterogeneity of the results.
The fact that only two digital databases were searched may be another limitation of this review. However, we are confident that we did not miss important trials, as we included all relevant studies also presented in other previously published meta-analyses.
Only the effect sizes at the first time point after the end of the interventions were extracted and not those at the later follow-up time points. This can also be criticised; however, we believe that exercise interventions, just as drugs (eg, for high blood pressure), are the most effective during their application period only. Therefore, the effect sizes at the end of the exercise or other non-pharmaceutical intervention period were considered as the most relevant. If a study presented more than one questionnaire for the outcome fatigue, data of only one were extracted. Methods for the inclusion of more than one fatigue questionnaire have been described elsewhere (multivariate meta-analysis). However, these methods are still in development and require the estimation of the correlations between the results yielded by the different fatigue questionnaires, which are most often not reported.
A further limitation might be that the descriptive data were only extracted by one reviewer and that the RoB was extracted from the PEDro database. Also, that RoB of those studies not evaluated in the PEDro database was assessed by only one reviewer.
Because of the moderate-to-high RoB, the large number of studies with small sample sizes, the risk for publication bias, the small number of studies for some pairwise comparisons, the moderate-to-high clinical and statistical heterogeneity and the low statistical precision, the effect sizes and the ranking of the interventions should not be considered as conclusive. Furthermore, the lack of detailed descriptions of the exercise modalities hampered a planned metaregression on the effect of training intensity or the extent of exercises on the observed effect sizes.
In this study, all cancer diagnoses were analysed together. This may have resulted in an increased heterogeneity and might have led to confounding. However, it seems plausible to assume that the effects of the modalities targeting CRF are similar across diagnoses and other reviews also decided to mix different cancer diagnoses.59
For some of the included interventions, only few and very small trials were found. Some of them showed no effect (eg, Tai-Chi ‘during’ studies) but with large credible intervals which were still compatible with a moderate to large beneficial effect on fatigue. In the ‘after’ studies, Tai-Chi showed a moderate effect size on CRF. Perhaps Tai-Chi is a too strenuous exercise modality for patients during active cancer treatment. If this is the reason for the non-effect during cancer treatment, it might be possible to adapt the intensity of the Tai-chi classes. This is supported by the fact that yoga showed a large effect during and after cancer treatment. To clarify these questions, larger studies are needed for Tai-chi interventions.
Comparison with other studies
There are several meta-analyses evaluating the effect of exercise and other non-pharmaceutical interventions on CRF12–44 and most of them are in favour of exercise or non-pharmaceutical interventions as compared with usual care (control). The conclusions of this study are based on similar sets of published studies as used in the other meta-analyses. This indirect-comparisons meta-analysis allowed for additional conclusions, such as the ranking of the different exercise and non-pharmaceutical interventions. The NCCN guidelines on CRF60 and the ‘Putting Evidence into practice guidelines’ of the US Oncology Nursing Society45 differentiate between active (during) treatment and post-treatment (after) phase and recommend for both phases exercise activities, such as walking, stretching and cycling to manage CRF. NCCN guidelines also recommend endurance and resistance training. The results of this review are in line with previous reports but in addition, they suggest that during cancer treatment relaxation exercises may be the first choice to manage CRF and that yoga and combined CBT also might have a large effect size on CRF. For the management of CRF after cancer treatment, the results suggest that yoga might be most effective but that combined aerobic and resistance training, CBT, combined CBT and Tai-Chi all showed moderate effect sizes.
Different working mechanisms may explain the effectiveness of the interventions included in this study. Active exercises (eg, resistance training, aerobic training, dance, yoga, etc) may counteract the decreased level of activity during or after cancer treatment, and hence improve physical capacity. Furthermore, higher physical activity levels may have a beneficial effect on mental health.61 Psychosocial interventions aim at reducing fatigue through an improved coping with the stressful situation and activity management.42 Relaxation techniques, massage, music therapy or yoga may also help to reduce stress or anxiety levels and decrease fatigue via the adrenal–pituitary axis.33 62
Implications for practice
These findings may have important clinical implications. They suggest that the effectiveness of the different types of non-pharmaceutical interventions varies depending on the cancer treatment status of the patients. During cancer treatment, relaxation training seems to have the largest effect. In addition, health professionals should consider physical activity such as yoga, combinations of exercise and psychosocial interventions, resistance training or endurance training as beneficial interventions.
After the cancer treatment, relaxation seems no longer the best choice for reducing CRF. Hence, in this stage the health professionals should propose more physical activity enhancing interventions to their clients.
Unfortunately, the results of this study do not allow for a more detailed specification of the exercise modalities such as training intensity or exercise volume. Health professionals might consult the ranking of the interventions presented in this study, when planning an optimal, individually adapted exercise programme to reduce CRF. Because some of the interventions included in this analysis showed quite similar effects and SUCRA values (see ranking in figures 3 and 4), health professionals now have a choice between different interventions and can for example take into account the individual patient’s preferences.
Surveys reported that 25%–50% of oncologists already discuss the possibility of initiating exercise therapy during and after cancer treatment with their patients.63–65 We hope that the present review may contribute to increase this number. Furthermore, our results emphasise that patients can really choose from a list of effective exercise or non-pharmaceutical alternatives to reduce CRF. This may increase adherence to the intervention. However, fatigue itself might be a barrier to exercise among patients with cancer and survivors.66 Thus, appropriate strategies should be tailored to the individual needs and abilities of patients and re-evaluated regularly.
Our study supports the importance of offering high-quality continuous education for physicians and other health professionals (such as the American College of Sports Medicine initiative). For example, physiotherapists working with patients with cancer should be aware that depending on the treatment status, interventions such as relaxation exercises or yoga may be even more effective in such patients to prevent or reduce CRF as compared with traditional aerobic or strength exercise. While the latter interventions are part of a physiotherapy educational programme, the first are probably not. Hence, continued training programmes on CBT, relaxation therapy, yoga, Tai-Chi or dance therapy are needed to enhance the health literacy and the intervention options of physiotherapists working in the field of oncology, and collaborations with practitioners who have those skills should be considered. This will allow healthcare professionals to provide evidence-based, individualised, safe and effective exercise programmes.
Implications for research
We agree with the ‘no one-size-fits-all remedy exists’ statement of Tomlinson and colleagues.28 This review provides indications on what type of exercise might be better suited to improve CRF in patients but it does not allow to suggest clear cut exercise modalities. The current knowledge on exercise modalities such as training intensity, exercise volume, resting intervals, training frequency, etc is not yet optimal. The influence of exercise on the inflammation–immunity axis is very complex. Too much exercise may be detrimental for the inflammatory and immune reaction process while an optimal exercise dosage may positively regulate the inflammation–immunity system. There is but limited knowledge on the complex interactions between the patient, exercise regulation of the inflammation–immune axis and tumour biology.67
Future studies might evaluate the different modalities and how these modalities should be adapted to the patients’ individual situations or preferences. For example, different resistance training intensities and volumes should be compared in large randomised trials.
Further important research topics are adverse events, patient preferences, exercise adherence and economic evaluations such as cost-effectiveness and cost–utility studies. Finally, methodological research is needed to evaluate which questionnaires are best suited to assess CRF and to decide on the best tools, which might increase comparability across studies.
Conclusion
Although exercise and non-pharmaceutical therapies have been shown to be effective in reducing CRF, there was a lack of comparison between different types of exercises. Evidence from this indirect-comparisons meta-analysis indicated that during cancer treatment, relaxation, massage, CBT combined with physical activity, aerobic and resistance training (alone or combined), as well as yoga, all showed similar moderate-to-large effect sizes. After cancer treatment, the importance of relaxation seems to decrease while yoga might be the best option. Combined aerobic and resistance training, CBT, combined CBT and Tai-Chi showed all moderate effect sizes. This enables the patient and the healthcare professional to choose out of a variety of evidence-based alternatives according to patients’ preference and abilities to tackle CRF.
What is already known?
There is evidence for the effectiveness of different non-pharmaceutical interventions for cancer-related fatigue (CRF) in patients during and after cancer treatment.
The relative effects and the ranking of the different non-pharmaceutical interventions are not known.
What are the new findings?
During cancer treatment, relaxation, massage, cognitive–behavioural therapy (CBT) combined with physical activity, aerobic and resistance training (alone or combined), and yoga were able to reduce CRF, showing moderate-to-large effect sizes.
After cancer treatment, yoga showed a large effect size for the reduction of CRF, while combined aerobic and resistance training, CBT alone or combined with physical activity, Tai-Chi as well as aerobic or resistance training alone all showed moderate effect sizes.
How might it impact on clinical practice in the near future?
During cancer treatment, relaxation exercises, massage, cognitive-behavioural therapy (CBT) combined with physical activity, aerobic and resistance training (alone or combined), and yoga might be the first choice to manage CRF.
After cancer treatment, the clinician might encourage more physical activity enhancing interventions.
Patient and healthcare professionals can now choose out of a variety of evidence-based non-pharmaceutical interventions according to patients’ preferences and abilities to tackle CRF.
bjsports-2016-096422supp003.pdf (393.4KB, pdf)
bjsports-2016-096422supp004.pdf (683.6KB, pdf)
Acknowledgments
We would like to thank Tamara Soom and Corinne Biel for their help in retrieving articles.
Footnotes
Contributors: RH and JT had the idea for the study. RH, JT and LNB searched and screened the studies and extracted the data. AM, RH and JT contributed to statistical analysis. RH and JT wrote the first draft of the manuscript. ME, MLV, AM, RK, RH and JT revised the manuscript. All authors contributed to the design of the study, contributed to the interpretation and discussion of the data and results, read and agreed on the final version.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. NCCN NCCN. Cancer-Related Fatigue : Network N, NCCN clinical practice guidelines in oncology (NCCN guidelines). Version 1. 2016 ed: NCCN National Comprehensive Cancer Network, 2016:1–56. [Google Scholar]
- 2. Lawrence DP, Kupelnick B, Miller K, et al. . Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr 2004;32:40–50. 10.1093/jncimonographs/lgh027 [DOI] [PubMed] [Google Scholar]
- 3. Hickok JT, Roscoe JA, Morrow GR, et al. . Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer 2005;104:1772–8. 10.1002/cncr.21364 [DOI] [PubMed] [Google Scholar]
- 4. Phillips SM, McAuley E. Physical activity and fatigue in breast cancer survivors: a panel model examining the role of self-efficacy and depression. Cancer Epidemiol Biomarkers Prev 2013;22:773–81. 10.1158/1055-9965.EPI-12-0983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bower JE. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bower JE, Ganz PA, Desmond KA, et al. . Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 2000;18:743–53. 10.1200/JCO.2000.18.4.743 [DOI] [PubMed] [Google Scholar]
- 7. Cella D, Davis K, Breitbart W, et al. ; Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 2001;19:3385–91. 10.1200/JCO.2001.19.14.3385 [DOI] [PubMed] [Google Scholar]
- 8. Minton O, Stone P, Richardson A, et al. . Drug therapy for the management of cancer related fatigue. Cochrane Database Syst Rev 2008;1:CD006704. [DOI] [PubMed] [Google Scholar]
- 9. Curt GA. The impact of fatigue on patients with cancer: overview of FATIGUE 1 and 2. Oncologist 2000;5(Suppl 2):9–12. 10.1634/theoncologist.5-suppl_2-9 [DOI] [PubMed] [Google Scholar]
- 10. Eickmeyer SM, Gamble GL, Shahpar S, et al. . The role and efficacy of exercise in persons with cancer. Pm R 2012;4:874–81. 10.1016/j.pmrj.2012.09.588 [DOI] [PubMed] [Google Scholar]
- 11. Saligan LN, Olson K, Filler K, et al. ; Multinational Association of Supportive Care in Cancer Fatigue Study Group-Biomarker Working Group. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer 2015;23:2461–78. 10.1007/s00520-015-2763-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised multimodal exercise interventions on cancer-related fatigue: systematic review and meta-analysis of randomized controlled trials. Biomed Res Int 2015;2015:1–13. 10.1155/2015/328636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Supervised exercise reduces cancer-related fatigue: a systematic review. J Physiother 2015;61:3–9. 10.1016/j.jphys.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 14. Tian L, Lu HJ, Lin L, et al. . Effects of aerobic exercise on cancer-related fatigue: a meta-analysis of randomized controlled trials. Support Care Cancer 2016;24:969–83. 10.1007/s00520-015-2953-9 [DOI] [PubMed] [Google Scholar]
- 15. Cramer H, Lauche R, Klose P, et al. . A systematic review and meta-analysis of exercise interventions for colorectal cancer patients. Eur J Cancer Care 2014;23:3–14. 10.1111/ecc.12093 [DOI] [PubMed] [Google Scholar]
- 16. Zhang J, Yang KH, Tian JH, et al. . Effects of yoga on psychologic function and quality of life in women with breast cancer: a meta-analysis of randomized controlled trials. J Altern Complement Med 2012;18:994–1002. 10.1089/acm.2011.0514 [DOI] [PubMed] [Google Scholar]
- 17. Fong DY, Ho JW, Hui BP, et al. . Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ 2012;344:e70 10.1136/bmj.e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitch MI. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Evid Based Nurs 2012;15:108–9. 10.1136/ebnurs-2012-100688 [DOI] [PubMed] [Google Scholar]
- 19. Buffart LM, van Uffelen JG, et al. , Riphagen II. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2012;12:559 10.1186/1471-2407-12-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oh HS, Seo WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs 2011;8:191–201. 10.1111/j.1741-6787.2011.00214.x [DOI] [PubMed] [Google Scholar]
- 21. Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, et al. . The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol 2010;22:208–21. 10.1016/j.clon.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 22. Jacobsen PB, Donovan KA, Vadaparampil ST, et al. . Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol 2007;26:660–7. 10.1037/0278-6133.26.6.660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du S, Hu L, Dong J, et al. . Patient education programs for cancer-related fatigue: a systematic review. Patient Educ Couns 2015;98:1308–19. 10.1016/j.pec.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 24. Hasenoehrl T, Keilani M, Sedghi Komanadj T, et al. . The effects of resistance exercise on physical performance and health-related quality of life in prostate cancer patients: a systematic review. Support Care Cancer 2015;23:2479–97. 10.1007/s00520-015-2782-x [DOI] [PubMed] [Google Scholar]
- 25. Bradt J, Goodill SW, Dileo C. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev 2011;10:CD007103 10.1002/14651858.CD007103.pub2 [DOI] [PubMed] [Google Scholar]
- 26. Bradt J, Dileo C, Grocke D, et al. . Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev 2011;8:CD006911 10.1002/14651858.CD006911.pub2 [DOI] [PubMed] [Google Scholar]
- 27. Bergenthal N, Will A, Streckmann F, et al. . Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev 2014;11:CD009075 10.1002/14651858.CD009075.pub2 [DOI] [PubMed] [Google Scholar]
- 28. Tomlinson D, Diorio C, Beyene J, et al. . Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil 2014;93:675–86. 10.1097/PHM.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 29. Galvão DA, Taaffe DR, Spry N, et al. . Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis 2007;10:340–6. 10.1038/sj.pcan.4500975 [DOI] [PubMed] [Google Scholar]
- 30. Baumann FT, Zopf EM, Bloch W. Clinical exercise interventions in prostate cancer patients--a systematic review of randomized controlled trials. Support Care Cancer 2012;20:221–33. 10.1007/s00520-011-1271-0 [DOI] [PubMed] [Google Scholar]
- 31. Zou LY, Yang L, He XL, Xl H, et al. . Effects of aerobic exercise on cancer-related fatigue in breast cancer patients receiving chemotherapy: a meta-analysis. Tumour Biol 2014;35:5659–67. 10.1007/s13277-014-1749-8 [DOI] [PubMed] [Google Scholar]
- 32. Shennan C, Payne S, Fenlon D. What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psychooncology 2011;20:681–97. 10.1002/pon.1819 [DOI] [PubMed] [Google Scholar]
- 33. Tsai HF, Chen YR, Chung MH, et al. . Effectiveness of music intervention in ameliorating cancer patients' anxiety, depression, pain, and fatigue: a meta-analysis. Cancer Nurs 2014;37:E35–E50. 10.1097/NCC.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 34. Mishra SI, Scherer RW, Snyder C, et al. . Exercise interventions on health-related quality of life for people with cancer during active treatment. Clin Otolaryngol 2012;37:390–2. 10.1111/coa.12015 [DOI] [PubMed] [Google Scholar]
- 35. Mishra SI, Scherer RW, Snyder C, et al. . The effectiveness of exercise interventions for improving health-related quality of life from diagnosis through active cancer treatment. Oncol Nurs Forum 2015;42:E33–E53. 10.1188/15.ONF.E33-E53 [DOI] [PubMed] [Google Scholar]
- 36. Mishra SI, Scherer RW, Snyder C, et al. . Are exercise programs effective for improving health-related quality of life among cancer survivors? A systematic review and meta-analysis. Oncol Nurs Forum 2014;41:E326–E342. 10.1188/14.ONF.E326-E342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mishra SI, Scherer RW, Geigle PM, et al. . Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev 2012;8:CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carayol M, Delpierre C, Bernard P, et al. . Population-, intervention- and methodology-related characteristics of clinical trials impact exercise efficacy during adjuvant therapy for breast cancer: a meta-regression analysis. Psychooncology 2015;24:737–47. 10.1002/pon.3727 [DOI] [PubMed] [Google Scholar]
- 39. Carayol M, Bernard P, Boiché J, et al. . Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: what is the optimal dose needed? Ann Oncol 2013;24:291–300. 10.1093/annonc/mds342 [DOI] [PubMed] [Google Scholar]
- 40. Pan Y, Yang K, Shi X, et al. . Tai Chi Chuan exercise for patients with breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2015;2015:1–15. 10.1155/2015/535237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan YQ, Yang KH, Wang YL, et al. . Massage interventions and treatment-related side effects of breast cancer: a systematic review and meta-analysis. Int J Clin Oncol 2014;19:829–41. 10.1007/s10147-013-0635-5 [DOI] [PubMed] [Google Scholar]
- 42. Goedendorp MM, Gielissen MF, Verhagen CA, et al. . Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009;1:CD006953 10.1002/14651858.CD006953.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown JC, Huedo-Medina TB, Pescatello LS, et al. . Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–33. 10.1158/1055-9965.EPI-10-0988 [DOI] [PubMed] [Google Scholar]
- 44. Bourke L, Smith D, Steed L, et al. . Exercise for men with prostate cancer: a systematic review and Meta-analysis. Eur Urol 2016;69:693–703. 10.1016/j.eururo.2015.10.047 [DOI] [PubMed] [Google Scholar]
- 45. Mitchell SA, Hoffman AJ, Clark JC, et al. . Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs 2014;(18 Suppl):38–58. 10.1188/14.CJON.S3.38-58 [DOI] [PubMed] [Google Scholar]
- 46. Uthman OA, van der Windt DA, Jordan JL, et al. . Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. Br J Sports Med 2014;48:1579 10.1136/bjsports-2014-5555rep [DOI] [PubMed] [Google Scholar]
- 47. Hutton B, Salanti G, Caldwell DM, et al. . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 48. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, 2011. [Google Scholar]
- 49. Egger M, Davey Smith G, Schneider M, et al. . Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borenstein M, Hedges LV, Higgins JPT, et al. . Introduction to Meta-Analysis. Chichester, West Sussex: John Wiley & Sons Ltd, 2009. [Google Scholar]
- 51. Cohen J. A power primer. Psychol Bull 1992;112:155–9. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 52. R Foundation for Statistical Computing. R: a language and environment for statistical computing [program], 2015. [Google Scholar]
- 53. gemtc. Network Meta-Analysis using bayesian methods. 2015. R-package [program]. 0.7-1 version.
- 54. rjags. Bayesian graphical models using MCMC, 2015. R package [program]. 4-4 version. [Google Scholar]
- 55. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 56. van Valkenhoef G, Dias S, Ades AE, et al. . Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods 2016;7:80–93. 10.1002/jrsm.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dechartres A, Trinquart L, Boutron I, et al. . Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304 10.1136/bmj.f2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vargas S, Antoni MH, Carver CS, et al. . Sleep quality and fatigue after a stress management intervention for women with early-stage breast cancer in southern Florida. Int J Behav Med 2014;21:971–81. 10.1007/s12529-013-9374-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised multimodal exercise interventions on Cancer-Related fatigue: systematic review and Meta-Analysis of randomized controlled trials. Biomed Res Int 2015;2015 10.1155/2015/328636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. NCCN NCCN. Cancer-Related Fatigue : Network N, NCCN clinical practice guidelines in oncology (NCCN guidelines: NCCN National Comprehensive Cancer Network, 2015:1–45. [Google Scholar]
- 61. Sharma A, Madaan V, Petty FD. Exercise for mental health. Prim Care Companion J Clin Psychiatry 2006;8:106 10.4088/PCC.v08n0208a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev 2012;17:21–35. [PubMed] [Google Scholar]
- 63. Jones LW, Courneya KS, Peddle C, et al. . Oncologists' opinions towards recommending exercise to patients with cancer: a Canadian national survey. Support Care Cancer 2005;13:929–37. 10.1007/s00520-005-0805-8 [DOI] [PubMed] [Google Scholar]
- 64. Jones LW, Courneya KS. Exercise discussions during cancer treatment consultations. Cancer Pract 2002;10:66–74. 10.1046/j.1523-5394.2002.102004.x [DOI] [PubMed] [Google Scholar]
- 65. Jones LW, Courneya KS. Exercise counseling and programming preferences of cancer survivors. Cancer Pract 2002;10:208–15. 10.1046/j.1523-5394.2002.104003.x [DOI] [PubMed] [Google Scholar]
- 66. Courneya KS, Segal RJ, Reid RD, et al. . Three independent factors predicted adherence in a randomized controlled trial of resistance exercise training among prostate cancer survivors. J Clin Epidemiol 2004;57:571–9. 10.1016/j.jclinepi.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 67. Koelwyn GJ, Wennerberg E, Demaria S, et al. . Exercise in regulation of Inflammation-Immune Axis function in Cancer initiation and progression. Oncology 2015;29. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2016-096422supp001.pdf (26KB, pdf)
bjsports-2016-096422supp002.pdf (88.6KB, pdf)
bjsports-2016-096422supp005.pdf (62.3KB, pdf)
bjsports-2016-096422supp006.pdf (59.8KB, pdf)
bjsports-2016-096422supp007.pdf (172.1KB, pdf)
bjsports-2016-096422supp008.pdf (57.1KB, pdf)
bjsports-2016-096422supp003.pdf (393.4KB, pdf)
bjsports-2016-096422supp004.pdf (683.6KB, pdf)