Abstract
Objective
To determine whether not waiting for the elimination of direct oral anticoagulants (DOACs) has an effect on the amount of perioperative bleeding in patients who undergo operative treatment of a hip fracture.
Design
Observation, retrospective case–control study.
Setting
A single UK major trauma centre.
Participants
Patients who sustained a hip fracture were identified using the National Hip Fracture Database (NHFD). All those found to be taking a DOAC at the time of fracture were identified (n=63). A matched group not taking a DOAC was also identified from the NHFD (n=62).
Main outcome
Perioperative drop in haemoglobin concentration.
Results
There was no relationship between admission to operation interval and perioperative change in haemoglobin concentration in patients taking DOACs (regression coefficient=−0.06 g/L/hour; 95% CI −0.32–0.20; p=0.64). No relationship was found between the time from admission to operation interval and the probability of transfusion (OR=0.94; 95% CI 0.85 to 1.90; p=0.16) or reoperation (OR=1.04; 95% CI 0.93 to 1.16; p=0.49). One mortality was recorded in the DOAC group within 30 days of admission, and this compared with five in the matched group of patients (p=0.2).
Conclusions
Delaying surgery in patients who sustain a hip fracture who are taking a DOAC drug has not been shown to reduce perioperative bleeding or affect their mortality in this study.
Keywords: hip fractures, anticoagulants, blood transfusion, postoperative complications, perioperative care
Strengths and limitations of this study.
First analysis of the effect of direct oral anticoagulant drugs on patients who have sustained a hip fracture.
Objective outcomes of perioperative change in haemoglobin concentration, transfusion and reoperation.
Context of a representative orthopaedic trauma department may be easily extrapolated to comparable centres.
Possibility of confounding due to lack of specified transfusion criteria.
Underpowered to analyse secondary outcomes of transfusion and reoperation.
Introduction
Direct oral anticoagulants (DOACs) are a group of drugs that have emerged as an alternative to warfarin, with the advantage of not requiring routine drug monitoring.1 In the intervening period their use has expanded to include stroke prophylaxis in atrial fibrillation, venous thromboembolism (VTE) prophylaxis following hip and knee replacement, secondary VTE prophylaxis, and for preventing adverse outcomes following acute management of acute coronary syndromes.2–4 Their uptake has accelerated significantly and they now collectively surpass warfarin as the preferred oral anticoagulant.5 Until the introduction of idarucizumab for dabigatran in 2016, no specific agent has been available for the reversal of the anticoagulant effects of DOACs,6 leaving delay as the only strategy available for reversal of anticoagulation before surgery.
Proximal femoral fractures (hip fractures) are a common occurrence in the UK, with nearly 65 000 episodes recorded in England, Wales and Northern Ireland in 2016, with a 30-day mortality at 6.7% and total costs associated with the injury of over £14 000 per patient within the first year and corresponding to approximately over 1% of the total National Health Service (NHS) budget.7 8
Best practice in the management of hip fractures in the UK is specified in the National Institute for Health and Care Excellence Clinical Guideline 124, ‘Hip fracture: management’.9 Surgery on the day or the day after the injury is recommended by numerous studies, which have associated surgical delay with increased length of stay, major morbidity and an increased mortality of up to 9.4%.10–18 Early surgery (within 36 hours) consequently forms a key requirement for the conditional component of the Best Practice Tariff for fragility hip fracture paid to NHS providers in England.19
The conventional practice in the case of warfarin is to actively reverse anticoagulation and delay surgery until a specified internationalised normalised ratio (INR)threshold has been reached.20–24 The half-lives of the DOACs range between approximately 7 and 15 hours, although this is to a variable degree dependent on renal function.25–29 Given that over a third of patients presenting with hip fracture have renal dysfunction, awaiting washout time is likely to constitute a considerable delay to surgery.30
The safety of anaesthesia and hip fracture surgery in patients taking DOACs has been supported in case reports, but no evidence has yet been published to support the delay of surgery for hip fracture to allow excretion or reversal of DOACs.31 32 Such practice might reduce blood loss, risk of allogeneic blood transfusion, wound complications and reoperation, but may additionally incur a cost through increased time to surgery and subsequent effect on patient outcome. In view of this the unit reporting developed a protocol where patients who were taking DOACs were not delayed for hip fracture surgery. The study reports the results of this protocol.
The aim of this study is to determine whether perioperative change in haemoglobin concentration, probability of receiving allogeneic blood transfusion or reoperation for wound complications in patients who undergo early surgery for hip fracture are affected by treatment with DOACs at time of presentation. Secondarily we aim to determine whether time to surgery has an impact on the same outcomes. We hypothesise that outcomes are not improved by delaying surgery.
Materials and methods
Study design and setting
In an observational, retrospective study design, National Hip Fracture Database (NHFD) records from a single UK major trauma centre encompassing the period January 2015–March 2017 were reviewed. Episodes of hip fracture presenting at this centre are recorded in the NHFD. Patients are managed in close accordance with applicable best practice guidelines (including joint orthopaedic (surgical) and orthogeriatric (medical) care, surgery on the day of or following admission, and multidisciplinary team assessment) and compliance is representative for this type of hospital.9 33
Population
The list of all patients with hip fracture was obtained from NHFD records. Electronic and paper records were reviewed to determine whether patients had been taking a DOAC on admission. Data from consecutive patients were analysed and no exclusion criteria were implemented in order to minimise selection bias. Where a single patient had multiple attendances with hip fracture, only the first was analysed in order to preserve independence of observations.
To reduce confounding, a matched group of patients not taking DOACs was produced, matching on age (within 5 years), sex, operation and American Society of Anesthesiologists (ASA) grade. When multiple matches were available, the earliest episode was used.
Data collection
Demographic, surgical and outcome data were retrieved directly from patient records. Data collected included age; sex; date and time of attendance to the emergency department; DOAC, dose and indication; date and time of surgery; and ASA grade and operation. All orthopaedic trauma operations, blood results and blood transfusions performed at our centre are recorded electronically; these records were interrogated to obtain perioperative haemoglobin change and complication rates.
Statistical analysis
Statistical analysis was performed in R V.3.4.0.34 Relationships between continuous and categorical variables were evaluated using Welch t-tests or Wilcoxon rank-sum tests as determined by assessment of normality using histograms and the Shapiro-Wilk test. Associations between categorical variables were evaluated using the Χ2 test. Linear and logistic regression models were assembled to produce adjusted effect estimates. A 0.05 level of statistical significance was used throughout.
With the primary analysis planned to be the unadjusted linear regression of perioperative change in haemoglobin concentration on admission to operation interval, we calculated that a sample size of 53 would provide 80% power to detect a ‘medium’ Cohen’s f2 >0.15.
Patient and public involvement
This research is based on audit findings relating to the optimisation of anticoagulated patients during the perioperative period, and as a result no patient or public consultation took place.
Results
Group characteristics
There were 1123 cases of hip fracture at this centre during the period January 2015–March 2017. The episodes for 63 patients (5.6% of all episodes) presenting for the first time with proximal femoral fracture (hip fracture) while taking a DOAC were analysed. Group characteristics are shown in table 1.
Table 1.
DOAC group characteristics
| Variable | Mean/Count | Range/% |
| Age | ||
| Years | 85 | 66–100 |
| Sex | ||
| Female | 47 | 75% |
| Male | 16 | 25% |
| Operation | ||
| SHS | 24 | 38% |
| Hemiarthroplasty | 31 | 49% |
| IM nail | 4 | 6% |
| Total hip replacement | 3 | 4% |
| SHS and IM nail (bilateral fractures) | 1 | 2% |
| DOAC | ||
| Apixaban | 14 | 22% |
| Dabigatran | 5 | 8% |
| Rivaroxaban | 44 | 70% |
| Indication | ||
| AF | 57 | 91% |
| Pacemaker | 1 | 2% |
| VTE | 4 | 6% |
| Not identified | 1 | 2% |
AF, atrial fibrillation; DOAC, direct oral anticoagulant; IM, intrameduallry nail; SHS, sliding hip screw; VTE, venous thromboembolism.
A matched group of 62 patients not taking a DOAC or warfarin was identified, which met study matching criteria except in the single case of a 100-year-old male patient undergoing hemiarthroplasty whose closest match was 8 years younger; this case was included in matched analyses. No appropriate match could be identified for the patient undergoing simultaneous sliding hip screw (SHS) and (intramedullary nail) IM nail, which was excluded from matched analyses. Comparison between the DOAC and matched groups is summarised in table 2.
Table 2.
Outcomes in patients taking DOACs and matched patients
| Patients taking DOACs | Matched patients | Difference, OR (95% CI) | P values | ||
| Admission to operation interval | Median (range) | 19 hours (7–64) | 19 hours (3–44) | −0.9 (−2.4 to 3.3) | 0.3* |
| Perioperative change in haemoglobin concentration | Mean (range) | 23 g/L (0–49) | 23 g/L (1–47) | −0.2 (−4.6 to 4.2) | 0.9† |
| Blood transfusion | Count (%) | 11 (18) | 6 (10) | 0.5 (0.1 to 1.6) | 0.3‡ |
| Reoperation for wound complication | Count (%) | 3 (5) | 0 (0) | 0 (0 to 2.4) | 0.2‡ |
| 30-Day mortality | Count (%) | 1 (2) | 5 (8) | 5.0 (0.6 to 236) | 0.2‡ |
*Bias-corrected and accelerated bootstrap CI for difference between paired medians and paired Wilcoxon test.
†Normal CI and paired t-test.
‡Exact McNemar CI and test.
DOACs, direct oral anticoagulants.
Haemoglobin concentration
Perioperative change in haemoglobin concentration in patients taking DOACs was unknown in five patients who underwent transfusion before a formal postoperative haemoglobin measurement. In the remaining 58 patients, the mean haemoglobin drop was 23 g/L (SD=13.5 g/L).
There was no significant relationship between admission to operation interval and haemoglobin drop in unadjusted linear regression analysis (coefficient=−0.06 g/L/hour; 95% CI −0.32–0.20; p=0.64) (figure 1). In multiple regression analysis adjusting for age, sex, operation and ASA grade, the impact of admission to operation interval remained non-significant (table 3).
Figure 1.
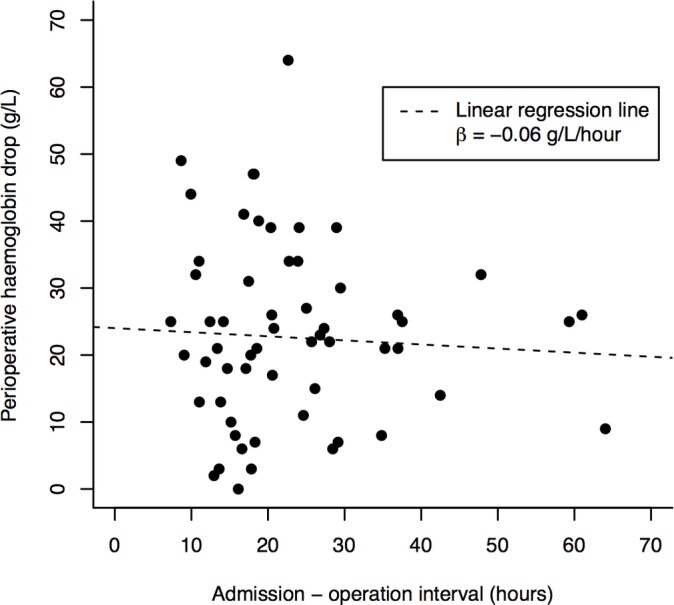
Relationship between admission to operation interval and perioperative change in haemoglobin concentration. The linear regression line is shown.
Table 3.
Adjusted analysis of perioperative change in haemoglobin concentration
| Variable | Coefficient (g/L) | 95% CI | P values |
| Interval | |||
| Hours | −0.12 | −0.40 to 0.16 | 0.38 |
| Age | |||
| Years | −0.01 | −0.55 to 0.53 | 0.97 |
| Sex | |||
| Male | −3.89 | −12.30 to 4.53 | 0.36 |
| ASA grade | 2.46 | −4.76 to 9.69 | 0.50 |
| Operation | |||
| SHS | Reference category | ||
| SHS and IM nail | 43.97 | 17.24 to 70.70 | <0.01 |
| Hemiarthroplasty | 6.21 | −1.08 to 13.51 | 0.09 |
| IM nail | 6.74 | −7.04 to 20.51 | 0.33 |
| Total hip replacement | 17.05 | −0.84 to 34.94 | 0.06 |
When comparing the DOAC and matched groups, there was no significant difference in perioperative change in haemoglobin concentration (paired t-test p=0.92).
DOACs, direct oral anticoagulants; IM, intrameduallry nail; SHS, sliding hip screw.
Blood transfusion
Eleven patients taking DOACs (17.5%; 95% CI 9.4% to 29.5%) received postoperative blood transfusions. There was no significant difference in the admission to operation interval between transfused and not-transfused patients (Wilcoxon rank-sum p=0.29). After adjusting for age, sex, operation and ASA grade in logistic regression analysis, the effect of admission to operation interval on the odds of transfusion remained non-significant (OR=0.94; 95% CI 0.85 to 1.90; p=0.16).
Fifty-seven patients taking DOACs (90%) had a degree of chronic kidney disease (CKD) as classified by the ‘G’ glomerular filtration rate (GFR) component of the Kidney Disease: Improving Global Outcomes (KDIGO) classification system.35 There was a significant trend towards greater transfusion risk in patients taking DOACs with increasing CKD stage on presentation (χ2 p=0.04).
There was a non-significant trend (p=0.3) towards more transfusions in the DOAC than matched group (six transfusions; 9.7%)
Reoperation
Four patients taking DOACs underwent reoperation: three due to wound ooze (4.8%; 95% CI 1.2% to 14.2%) and one due to SHS failure 1 year after the original surgery. Two reoperations for ooze were in patients undergoing SHS, and the remaining patient underwent hemiarthroplasty. There was no significant relationship between admission to operation interval and probability of reoperation for wound ooze (Wilcoxon rank-sum p=0.37) (figure 2). After adjusting for age, sex, operation and ASA grade in logistic regression, the effect of admission to operation interval on the odds of reoperation for wound ooze remained non-significant (OR=1.04; 95% CI 0.93 to 1.16; p=0.49). There was no relationship between CKD and the probability of reoperation for wound ooze (Fisher’s exact test p=0.84). No patients within the matched group underwent reoperation, but the rate of reoperation for wound complication was not significantly different between DOAC and matched groups (McNemar test p=0.25).
Figure 2.

Relationship between admission to operation interval and return to theatre for wound ooze.
Mortality
Only one death within 30 days of admission was recorded among patients taking DOACs (1.6%; 95% CI 0.08% to 9.7%), occurring 8 days after admission. This result was not significantly different from the reported 30-day mortality following hip fractures of 6.7%7 (p=0.17). In the matched group there were five deaths within 30 days of admission; the difference between the groups was not statistically significant (McNemar test p=0.2).
Discussion
The mean perioperative reduction in haemoglobin concentration in patients taking DOACs was 23 g/L, varying significantly between operations and in line with reported values.36–39 There was no difference between these patients and matched controls. This may be because perioperative blood loss is equivalent in the two groups. However, the postoperative haemoglobin measurement used for this test is taken on the first postoperative day; it is possible that additional blood loss in patients taking DOACs is delayed as medication may take as long as five half-lives for >95% elimination.40 Of note, it is thought that the nadir haemoglobin concentration following hip fracture surgery may occur around postoperative day 5.38
Patients taking DOACs who presented to our centre with hip fracture received surgery after a median of 19.4 hours, and the proportion operated within 36 hours was 87.3%. These results represent earlier surgery than generally reported, including the UK national average of 81.3% within 36 hours.11 13–18 41 42
We found no evidence of a relationship between admission to operation interval and perioperative change in haemoglobin concentration in patients taking DOACs. We suggest that the likely reason for this is that the proportion of blood loss avoided by awaiting DOAC washout is small in comparison with the proportion accounted for by the fracture type and choice of surgical intervention, whose impact has been reported previously.43
Of the patients taking DOACs, 17.5% received allogeneic red cell transfusions. Comparison with the literature is complicated by the fact that specified transfusion criteria are not used in our institution. However, this figure falls well within the 11%–45% range reported by a Cochrane review of transfusions in the general hip fracture population with ‘restrictive’ transfusion criteria of postoperative haemoglobin <80 g/L.44–46
We found no evidence of a relationship between admission to operation interval and the probability of transfusion. However, transfusions were relatively rare events and our study is underpowered to detect such a relationship unless it is very large. It is nevertheless reasonable to conclude that our evidence does not support the deliberate delaying of surgery to reduce transfusion risk.
Increasing stage of CKD was associated with increasing probability of transfusion in patients taking DOACs. This is likely to be due to the renal excretion of these drugs, meaning that patients with CKD have greater circulating concentrations of the drugs and consequently are more anticoagulated at the times of fracture and surgery.25–28 Such cases will inevitably be a minority, as DOACs are contraindicated in advanced CKD.
We found that transfusions were slightly more frequent in patients taking DOACs than in matched controls. We hypothesise that, having no specific transfusion criteria, clinicians may have had a lower threshold for transfusion in patients taking DOACs due to a greater perceived risk of blood loss. It was not possible to ascertain precisely why five patients underwent blood transfusion before a formal haemoglobin measurement. However, our institution does make ad hoc use postoperatively of a point-of-care haemoglobin measuring system (HemoCue; Ängelholm, Sweden). We expect that a low point of care testing (POCT) haemoglobin measurement formed the basis for a decision to transfuse before formal measurement, but was not recorded.
Three patients (4.8%) returned to surgery due to wound ooze. We found no evidence of a relationship between the admission to operation interval and probability of reoperation, suggesting that a reduction in risk of wound complication does not justify delaying surgery. This conclusion may be supported by the finding in a larger observational study that surgical delay in fact increases the risk of wound infection.47 Although we found no statistically significant difference in risk of return to theatre between DOAC and matched groups, our study was underpowered to detect this.
It is important to note that reoperation may be a ‘last resort’ response to persistently oozing wounds but is objective, while more subtle indicators such as clinical examination findings and use of negative-pressure dressings are too variable in routine use and documentation to be useful for statistical analysis. Further work on wound complications might therefore employ standardised wound assessment tools such as Additional treatment, the presence of Serous discharge, Erythema, Purulent exudate, and Separation of the deep tissues, the Isolation of bacteria, and the duration of inpatient Stay (ASEPSIS).48
This study benefits from several strengths. The outcomes or perioperative change in haemoglobin concentration, transfusion and reoperation are objective and reliable, and of clinical relevance. The research took place in the context of normal operation of a representative UK hospital, and its findings can therefore be relatively easily extrapolated to comparable centres. Additionally, the admission to operation interval was equal in matched patients taking and not taking DOACs, suggesting that differences in preoperative management were minimal.
The principal weaknesses of the study are its observational design, which allows for the influence of unknown confounders, and the necessarily small sample size. It was therefore underpowered to detect small differences in transfusion and operation rates, and to analyse the impact of CKD on transfusion risk. The lack of specified transfusion criteria also complicates comparison with the literature and means that transfusion results may be confounded by clinician preferences. Furthermore, the timing of preoperative and postoperative haemoglobin measurements is variable. Although all patients had haemoglobin measured on the first postoperative day, the interval between the timing of the sample and both the fracture event and surgery could not be calculated, and this variability is not accounted for. Our groups were matched for pragmatic reasons on a small number of variables. Consequently comparisons between groups were not controlled for some factors of interest, such as exact fracture classification (which may have an effect on blood loss beyond that of the surgical intervention), injury severity (as concurrent injury to other areas of the body may contribute to blood loss) or more detailed comorbidity information (in particular medical conditions and medications that disturb haematopoiesis, haemostasis and inflammation/healing).
Further study should consider the use of multiple centres in order to provide a larger sample. Longer term outcomes such as 3-month and 12 month mortality are of interest. Finally, a prospective design with standardised wound assessment and transfusion criteria would improve the reliability of the data.
In conclusion, we have found no evidence for deliberately delaying surgery in patients taking DOACs who present with hip fractures. In accordance with the established best practice, we recommend that these patients receive definitive surgical treatment at the earliest opportunity.
Supplementary Material
Footnotes
Contributors: BM, HA: study design, data gathering and analysis, creation and editing of manuscript. DS: study design, data gathering and analysis, editing of manuscript. TC: study design, analysis, editing of manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: This study was conducted in line with low risk ethical protocols at our institution as no patient-identifying information was recorded and data were retrieved from existing medical records.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data for this project are owned by the NHS Trust in which the study was conducted.
References
- 1. Rang HP, Dale MM, Ritter JM, et al. Rang and Dale’s pharmacology. 8th edn Edinburgh: Elsevier, Churchill Livingstone, 2016. [Google Scholar]
- 2. National Institute for Health and Care Excellence. Venous thromboembolism: reducing the risk for patients in hospital. Clinical guideline CG92. 2010. https://www.nice.org.uk/guidance/cg92
- 3. National Institute for Health and Clinical Excellence. Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. Clinical guideline (CG172). 2013. https://www.nice.org.uk/guidance/cg172 [PubMed]
- 4. National Institute for Health and Clinical Excellence. Atrial fibrillation: management. Clinical guideline (CG180). 2014. https://www.nice.org.uk/guidance/cg180 [PubMed]
- 5. Loo SY, Dell’Aniello S, Huiart L, et al. Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharmacol 2017;83:2096–106. 10.1111/bcp.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pollack CV, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511–20. 10.1056/NEJMoa1502000 [DOI] [PubMed] [Google Scholar]
- 7. Royal College of Physicians. National hip fracture database annual report 2017. 2017. http://www.nhfd.co.uk/files/2017ReportFiles/NHFD-AnnualReport2017.pdf
- 8. Leal J, Gray AM, Prieto-Alhambra D, et al. Impact of hip fracture on hospital care costs: a population-based study. Osteoporos Int 2016;27:549–58. 10.1007/s00198-015-3277-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Institute for Health and Clinical Excellence. Hip fracture: management. Clinical guideline (CG124). 2011. https://www.nice.org.uk/guidance/cg124 [PubMed]
- 10. Sayers A, Whitehouse MR, Berstock JR, et al. The association between the day of the week of milestones in the care pathway of patients with hip fracture and 30-day mortality: findings from a prospective national registry - The National Hip Fracture Database of England and Wales. BMC Med 2017;15:62 10.1186/s12916-017-0825-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grimes JP, Gregory PM, Noveck H, et al. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med 2002;112:702–9. 10.1016/S0002-9343(02)01119-1 [DOI] [PubMed] [Google Scholar]
- 12. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am 2008;90:1436–42. 10.2106/JBJS.G.00890 [DOI] [PubMed] [Google Scholar]
- 13. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br 2005;87:1123–6. 10.1302/0301-620X.87B8.16357 [DOI] [PubMed] [Google Scholar]
- 14. Moran CG, Wenn RT, Sikand M, et al. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am 2005;87:483–9. 10.2106/JBJS.D.01796 [DOI] [PubMed] [Google Scholar]
- 15. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA 2004;291:1738–43. 10.1001/jama.291.14.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weller I, Wai EK, Jaglal S, et al. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br 2005;87:361–6. 10.1302/0301-620X.87B3.15300 [DOI] [PubMed] [Google Scholar]
- 17. Lefaivre KA, Macadam SA, Davidson DJ, et al. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br 2009;91:922–7. 10.1302/0301-620X.91B7.22446 [DOI] [PubMed] [Google Scholar]
- 18. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma 2006;60:753–7. 10.1097/01.ta.0000214649.53190.2a [DOI] [PubMed] [Google Scholar]
- 19. Department of Health Payment by Results team. Payment by results guidance for 2013-14, 2013. [Google Scholar]
- 20. Al-Rashid M, Parker MJ. Anticoagulation management in hip fracture patients on warfarin. Injury 2005;36:1311–5. 10.1016/j.injury.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 21. Holland P, Hyder N. Warfarin reversal for hip fracture patients. Orthop Proc 2012;94-B:256. [Google Scholar]
- 22. Ahmed I, Khan MA, Nayak V, et al. An evidence-based warfarin management protocol reduces surgical delay in hip fracture patients. J Orthop Traumatol 2014;15:21–7. 10.1007/s10195-013-0274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gleason LJ, Mendelson DA, Kates SL, et al. Anticoagulation management in individuals with hip fracture. J Am Geriatr Soc 2014;62:159–64. 10.1111/jgs.12591 [DOI] [PubMed] [Google Scholar]
- 24. Moores TS, Beaven A, Cattell AE, et al. Preoperative warfarin reversal for early hip fracture surgery. J Orthop Surg 2015;23:33–6. 10.1177/230949901502300108 [DOI] [PubMed] [Google Scholar]
- 25. Stangier J, Rathgen K, Stähle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303. 10.1111/j.1365-2125.2007.02899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gulseth MP, Michaud J, Nutescu EA. Rivaroxaban: an oral direct inhibitor of factor Xa. Am J Health Syst Pharm 2008;65:1520–9. 10.2146/ajhp070624 [DOI] [PubMed] [Google Scholar]
- 27. Shantsila E, Lip GY. Apixaban, an oral, direct inhibitor of activated Factor Xa. Curr Opin Investig Drugs 2008;9:1020–33. [PubMed] [Google Scholar]
- 28. Yin OQ, Tetsuya K, Miller R. Edoxaban population pharmacokinetics and exposure-response analysis in patients with non-valvular atrial fibrillation. Eur J Clin Pharmacol 2014;70:1339–51. 10.1007/s00228-014-1736-4 [DOI] [PubMed] [Google Scholar]
- 29. Blech S, Ebner T, Ludwig-Schwellinger E, et al. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008;36:386–99. 10.1124/dmd.107.019083 [DOI] [PubMed] [Google Scholar]
- 30. White SM, Rashid N, Chakladar A. An analysis of renal dysfunction in 1511 patients with fractured neck of femur: the implications for peri-operative analgesia: Renal dysfunction and fractured neck of femur. Anaesthesia 2009;64:1061–5. 10.1111/j.1365-2044.2009.06012.x [DOI] [PubMed] [Google Scholar]
- 31. Hickel C. Early percutaneous pinning of hip fracture using propofol-ketamine-lidocaine admixture in a geriatric patient receiving dabigatran: a case report. Aana J 2014;82:46–52. [PubMed] [Google Scholar]
- 32. Byron M, Zochert S, Hellwig T, et al. Successful use of laboratory monitoring to facilitate an invasive procedure for a patient treated with dabigatran. Am J Health Syst Pharm 2017;74:461–5. 10.2146/ajhp160168 [DOI] [PubMed] [Google Scholar]
- 33. Royal College of Physicians. NHFD dashboard report 2016: Southmead Hospital. Natl. Hip fract. Database. 2016. http://www.nhfd.co.uk/nhfd/2016db/fry.pdf
- 34. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 35. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 36. Björkelund KB, Hommel A, Thorngren KG, et al. Factors at admission associated with 4 months outcome in elderly patients with hip fracture. Aana J 2009;77:49–58. [PubMed] [Google Scholar]
- 37. Kumar D, Mbako AN, Riddick A, et al. On admission haemoglobin in patients with hip fracture. Injury 2011;42:167–70. 10.1016/j.injury.2010.07.239 [DOI] [PubMed] [Google Scholar]
- 38. Nagra NS, Van Popta D, Whiteside S, et al. Postoperative hemoglobin level in patients with femoral neck fracture. Acta Orthop Traumatol Turc 2016;50:315–22. [DOI] [PubMed] [Google Scholar]
- 39. Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br 2006;88:1053–9. 10.1302/0301-620X.88B8.17534 [DOI] [PubMed] [Google Scholar]
- 40. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis 2016;41:206–32. 10.1007/s11239-015-1310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ 2006;332:947–51. 10.1136/bmj.38790.468519.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Hip Fracture Database (NHFD) annual report 2016. 2016. web1.crownaudit.org/Report2016/NHFD2016Report.pdf
- 43. Wang J, Jiang B, Marshall RJ, et al. Arthroplasty or internal fixation for displaced femoral neck fractures: which is the optimal alternative for elderly patients? A meta-analysis. Int Orthop 2009;33:1179–87. 10.1007/s00264-009-0763-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brunskill SJ, Millette SL, Shokoohi A, et al. The Cochrane Collaboration Red blood cell transfusion for people undergoing hip fracture surgery : Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd, 2015. (accessed 29 Aug 2017). 10.1002/14651858.CD009699.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Foss NB, Kristensen MT, Kehlet H. Anaemia impedes functional mobility after hip fracture surgery. Age Ageing 2008;37:173–8. 10.1093/ageing/afm161 [DOI] [PubMed] [Google Scholar]
- 46. Halm EA, Wang JJ, Boockvar K, et al. Effects of blood transfusion on clinical and functional outcomes in patients with hip fracture. Transfusion 2003;43:1358–65. 10.1046/j.1537-2995.2003.00527.x [DOI] [PubMed] [Google Scholar]
- 47. Cordero J, Maldonado A, Iborra S. Surgical delay as a risk factor for wound infection after a hip fracture. Injury 2016;47(Suppl 3):S56–S60. 10.1016/S0020-1383(16)30607-6 [DOI] [PubMed] [Google Scholar]
- 48. Wilson AP, Treasure T, Sturridge MF, et al. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 1986;1:311–2. 10.1016/S0140-6736(86)90838-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


