Abstract
In 2009, Monfils and colleagues proposed a behavioral procedure that was said to result in a permanent attenuation of a previously established fear memory, thereby precluding a possible return of fear after extinction (Science 2009;324:951-5). By presenting a single retrieval trial one hour before standard extinction training, they found an enduring reduction of fear. The retrieval-extinction procedure holds great clinical potential, particularly for anxiety patients, but the findings are not undisputed, and several conceptual replications have failed to reproduce the effect. These failures have largely been attributed to small procedural differences. This preregistered study is the first endeavor to exactly replicate three key experiments of the original report by Monfils et al. (2009), thereby gauging the robustness of their seminal findings.
Despite adhering to the original procedures as closely as possible, we did not find any evidence for reduced return of fear with the retrieval-extinction procedure relative to regular extinction training, as assessed through spontaneous recovery, reinstatement and renewal. Behavior of animals in the control condition (extinction only) was comparable to that in the original studies and provided an adequate baseline to reveal differences with the retrieval-extinction condition. Our null findings indicate that the effect sizes in the original paper may have been inflated and question the legitimacy of previously proposed moderators of the retrieval-extinction effect. We argue that direct experimental evaluation of purported moderators of the retrieval-extinction effect will be key to shed more light on its nature and prerequisites.
Keywords: Replication, retrieval-extinction, reconsolidation, behavioral updating, post-retrieval amnesia, cued fear conditioning
Introduction
Anxiety disorders and posttraumatic stress disorder are among the most prevalent psychiatric disorders (Kessler et al. 2005), and are hallmarked by substantial disability and poor quality of life (DSM-5). An effective behavioral treatment strategy for these disorders is exposure (Hofmann & Smits 2008, Ougrin 2011), which entails in vivo or imaginal contact with the feared object or situation. An important downside of exposure therapy is the relatively frequent relapse of fear after seemingly successful treatment (Craske & Mystkowski 2006, Loerinc et al. 2015, Yonkers et al. 2003). Extinction learning of conditioned fear memories in the laboratory bears some resemblance to exposure therapy (Myers & Davis 2002, Vervliet, Craske & Hermans 2013, Scheveneels et al. 2016). Likewise, under several circumstances, researchers observe a return of fear after successful extinction learning (Bouton 2002, 2004). This has been interpreted as evidence that extinction does not produce an erasure of the fear memory, but rather involves new learning, resulting in an extinction memory that coexists and competes with the original fear memory. Which memory will be retrieved upon confrontation with the conditioned and subsequently extinguished stimulus depends on the circumstances. The fear memory is, for example, more likely to surface again after the passing of time (spontaneous recovery), after experiencing an aversive event (reinstatement) or outside of the extinction context (renewal). Similarly, even after successful exposure therapy, the original fear memory is not erased, thereby forming an enduring risk for relapse.
In a highly influential paper, Monfils and colleagues proposed a modified extinction procedure that was said to result in a more permanent attenuation of the fear memory, thereby precluding a possible return of fear (Monfils et al. 2009). Using a cued fear conditioning procedure in rats, a tone fear memory was formed and then extinguished again one day later. Various recovery assays, including spontaneous recovery after one month, reinstatement, renewal, and reacquisition tests, provided evidence that the fear memory was retained through extinction. However, in a group of animals that received a single isolated tone presentation one hour before extinction training, there was a significant attenuation of the return of fear. These findings were soon confirmed in human subjects (Schiller et al. 2010) and mice (Clem & Huganir 2010). The recovery-reducing effect of the retrieval-extinction procedure was attributed to the extinction training interfering with reconsolidation of the fear memory after retrieval. It has been argued that consolidated memories can enter a labile state upon retrieval. During this window of instability (< 6 hours), a fear memory storing the association between a conditioned stimulus (CS) and an aversive unconditioned stimulus (US; e.g., shock) may be impaired using certain pharmacological manipulations (Nader et al. 2000, Debiec & LeDoux 2004, Beckers & Kindt 2017) that are assumed to disrupt the reconsolidation of the fear memory into its stable form. Monfils and colleagues proposed that, whereas the administration of amnestic drugs upon memory retrieval can prevent the reconsolidation of a fear memory, extinction training applied within the reconsolidation window can induce updating of the reactivated memory trace, by incorporating non-threatening information about the CS. Their data indeed supported that the retrieval-extinction procedure resulted in a more persistent fear reduction as compared with standard extinction training.
Clearly, these findings have exciting implications regarding the possibility of fear memory modification and hold great clinical potential. However, the fear recovery-reducing effect of the retrieval-extinction procedure is not undisputed and many have questioned whether merely changing the spacing of initial extinction training should result in such a marked loss of the fear memory. Alternative accounts for the superior fear reduction observed after retrieval-extinction relative to regular extinction (e.g., increased variability or spacing of the extinction trials (Rowe & Craske 1998, Urcelay et al. 2009)) suggest that it may reflect enhanced extinction learning, resulting in a stronger or more retrievable extinction memory, rather than the persistent modification of the initial fear memory (Baker, McNally & Richardson 2013, Ponnusamy et al. 2016). The distinction between the reconsolidation-based explanation of Monfils and colleagues and alternative accounts is not trivial, given that the former implies a permanent disruption of the original fear memory, in which case there could never be a return of fear, under any circumstances.
Although Monfils and colleagues have been able to replicate the effect multiple times, independent conceptual replications have met with varying success (see Table 1). To the best of our knowledge, a significant reduction of the return of cued fear in adult rats has not yet been reported outside of the Monfils lab. Already in 2010, an Australian group published a series of experiments that closely followed the original procedures, but failed to replicate the fear recovery-reducing effect, and even found fear augmentation in some cases (Chan et al. 2010). Note that these authors did deviate from the original procedure on some aspects (e.g., a different rat strain was used, animals were handled for three days before the start of experiments, animals were housed in groups of eight, and no experiment assessing the long-term effect on spontaneous recovery was included). In a recent meta-analysis, Kredlow and colleagues (2016) calculated an estimate of the effect size of the retrieval-extinction procedure. Although they reported significant benefits of the retrieval-extinction procedure over regular extinction training in rodent appetitive conditioning and human fear conditioning, this was not the case for rodent fear conditioning. They included 10 fear conditioning reports (involving a total of 34 experiments with 553 rodents) (see Table 1), and found a small, non-significant reduction of the return of fear as compared with standard extinction (Hedges’ g = 0.21). This effect was moderated by the number of animals housed together (i.e., significant effects for animals housed alone) and the delay between the retrieval-extinction procedure and the test for return of fear (i.e., significant effects for spontaneous recovery tests after ≥6 days). Although the meta-analysis was a valuable undertaking, the search for moderators in order to explain the inconsistent results in the literature may be premature, given that we presently have no information regarding the robustness of the initial effect in a direct, independent replication, and given the overall non-significant effect in the existing literature.
Table 1. Previous publications (n = 22) using the retrieval-extinction procedure in rodent fear conditioning studies.
An overview of all published (until March 2017) retrieval-extinction studies and whether they reported significantly superior reduction of fear recovery relative to regular extinction. Adult animals were used for all studies, unless indicated otherwise. For studies that used animals in (late) adolescence, the age (postnatal day, p) at the time of extinction is indicated. aStudy by Monfils and co-workers. bRemote fear memories (≥20-day interval between acquisition and retrieval). cSignificant reduction of fear recovery was found in a reversed control group (Ext-Ret). dNo significant reduction of fear recovery in the re-analysis by Kredlow, Unger & Otto (2016). eNo reduction of fear recovery was found for less recent fear memories (7-day interval between acquisition and retrieval). fDuration of the CS was longer during retrieval and extinction than during acquisition. gA trend (p = .073) toward a reduction of spontaneous recovery was observed.
| Publication | Fear reduction? | Subjects | Conditioned stimulus | Included in Kredlow et al. 2016? |
|---|---|---|---|---|
| Flavell, Barber & Lee 2011 | Yes | Rats | Context | Yes |
| Liu et al. 2014 | Yes | Rats | Context | No |
| Piñeyro et al. 2013 | Yes | Rats | Context | No |
| Rao-Ruiz et al. 2011 | Yes | Mice | Context | Yes |
| Costanzi et al. 2011 | Nob | Mice | Context | No |
| Gräff et al. 2014 | Nob | Mice | Context | No |
| Stafford et al. 2013 | No | Mice | Context | No |
| Auchter et al. 2017a | Yes | Rats | Cue | No |
| Baker, McNally & Richardson 2013 | Yesc | Rats (p34-37) | Cue | Yesd |
| Clem & Huganir 2010 | Yese | Mice | Cue | Yes |
| Jones & Monfils 2016a | Yesb | Rats (p45) | Cue | No |
| Jones, Ringuet & Monfils 2013a | Yes | Rats | Cue | Yes |
| Monfils et al. 2009a | Yes | Rats | Cue | Yes |
| Olshavsky et al. 2013a | Yes | Rats | Cue | Yes |
| Pattwell et al. 2016 | Yes | Mice | Cue | No |
| Chan et al. 2010 | No | Rats | Cue | Yes |
| Chan 2014 | No | Rats | Cue | Yes |
| Flavell, Barber & Lee 2011 | No | Rats | Cue | Yes |
| Gräff et al. 2014 | Nob,f | Mice | Cue | No |
| Ishii et al. 2012 | No | Mice | Cue | Yes |
| Ishii et al. 2015 | No | Mice | Cue | No |
| Ishii et al. 2015 | No | Mice (p28-32) | Cue | No |
| MacPherson et al. 2013 | No | Mice | Cue | No |
| Pattwell et al. 2016 | No | Mice (p30) | Cue | No |
| Ponnusamy et al. 2016 | Noc,g | Rats | Cue | No |
| Xu et al. 2013 | Nof | Mice | Cue | No |
The goal of the present series of three experiments was to try and replicate some of the key findings of Monfils and colleagues, adhering to their reported procedures as strictly as possible. More specifically, we compared the return of fear observed after a retrieval-extinction procedure to the return of fear after regular extinction training, as assessed with spontaneous recovery, reinstatement and renewal tests.
Materials & Methods
Preregistration
This study was registered on the Open Science Framework (OSF) (https://osf.io/aydfs) and the study protocols were previewed by the editor of Neurobiology of Learning and Memory before the start of data collection. Additional details regarding the timing of the behavioral protocols and all raw data can be found on OSF.
Subjects
Male Sprague-Dawley rats (7-8 weeks old, weighing 270 ± 12 g (mean ± SD) three days before the start of the behavioral sessions) (Janvier Labs, Le Genest-Saint-Isle, France) were used for all experiments, which were approved by the KU Leuven animal ethics committee, in accordance with the Belgian Royal Decree of 29/05/2013 and European Directive 2010/63/EU. Animals were individually housed in standard plastic cages and were maintained on a 12h/12h day-night cycle (lights on at 8:00) with food and water available ad libitum. Experiments were carried out between 9:00 and 18:30.
Equipment
Animals were trained and tested in squads of four, in four identical fear conditioning chambers, located in sound-attenuating cubicles (Contextual NIR Video Fear Conditioning System for Rats, Med Associates Inc., St. Albans, VT, USA). Contextual features could be changed in between sessions to create two distinct contexts (Luyten et al. 2016). Context A consisted of a standard chamber, with a standard grid floor, a black triangular insert, illuminated by infrared and white light, and was cleaned and scented with a household cleaning product. Context B consisted of a standard chamber, with a white plastic floor, a white plastic curved back wall insert, infrared light only and was cleaned and scented with another cleaner.
Procedure
In order to replicate the experiments as precisely as possible, we contacted the lead author of the original study (M. Monfils) in advance. She kindly provided many additional details regarding the behavioral procedures. In accordance with the original study, rats were not handled before the start of the behavioral sessions, except for the housing and weighing of the animals. Animals were transported to the lab on a wheeled cart in their homecage just before the start of each behavioral session and returned to the animal facility immediately after each session.
Fear conditioning
On the first day, rats were introduced in context A, and after 10 minutes of acclimation, they received three tone-shock pairings. Each 20-s, 5000-Hz, 80-dB tone (CS) coterminated with a 0.5-s, 0.7-mA footshock (US). The intertrial interval was 180 s on average (range: 100-260 s), and animals remained in the chamber for 180 s after onset of the last tone. Animals were semi-randomly matched into two groups with comparable freezing values to the third tone.
Retrieval-Extinction
Twenty-four hours later, the animals received either a retrieval trial (i.e., one CS) 2 minutes after introduction in the context (Ret group), or were merely exposed to the context for 4 minutes without presentation of a tone (NoRet group). The rats were then returned to the animal facility, and one hour after the end of the retrieval session, brought back to the lab for an extinction training session consisting of 18 or 19 CS presentations (Ret and NoRet group, respectively), starting 5 minutes after introduction in the chamber, with intervals of 180 s (range: 100-260 s). Animals remained in the chamber for 160 s after onset of the last tone. The retrieval and extinction sessions took place in context A for the spontaneous recovery and reinstatement experiments, and in context B for the renewal experiment.
Spontaneous recovery experiment
Twenty-four hours later, rats received a long-term memory (LTM) test consisting of 4 CS presentations in context A, starting 5 minutes after introduction in the chamber, with intervals of 120 s (range: 60-180 s). Animals remained in the chamber for 140 s after onset of the last tone. One month later, the extinction memory was again assessed in a spontaneous recovery test, which also consisted of 4 CS presentations.
Reinstatement experiment
One day after extinction, rats were re-exposed to context A and received 5 unsignaled footshocks. The first US was administered 2 minutes after introduction in the chamber, and the intervals between shocks were 60 s (range: 40-90 s). Animals remained in the chamber for 120 s after onset of the last shock. Twenty-four hours later, the rats were tested for reinstatement, by means of 4 CS presentations in context A.
Renewal experiment
One day after retrieval-extinction in context B, rats received a long-term memory test consisting of 4 CS presentations in context B. Twenty-four hours later, they were tested in the initial fear conditioning context (context A) by means of 4 CS presentations.
Behavioral Scoring
Freezing behavior was scored manually with a stopwatch by two observers blind to the experimental condition (Luyten et al. 2014). Freezing during each CS (i.e., the average of both raters) was expressed as percentage of the total CS duration (20 s). In addition, freezing before the first CS (pre CS freezing) was measured on the reinstatement test.
Sample size calculations
Monfils et al. did not provide effect sizes, but a recent meta-analysis (Kredlow et al. 2016) of the retrieval-extinction effect reported large effect sizes for the spontaneous recovery experiment (Hedges’ g: 1.895), the reinstatement experiment (Hedges’ g: 1.104) and the renewal experiment (Hedges’ g: 1.738) of Monfils et al. Based on this information, we used 8 animals per group in the spontaneous recovery and renewal experiments and 12 animals per group in the reinstatement experiment, yielding a power (G*Power 3.1.9.2, Kiel, Germany) of .84 to obtain a similarly beneficial effect on reinstatement, and of well over .90 to obtain a similarly beneficial effect on spontaneous recovery and renewal of the retrieval-extinction procedure (one-tailed).
Planned statistical analyses
For each experiment, acquisition of cued fear was evaluated with a repeated-measures ANOVA (RM ANOVA), with factors Trial (CS1-3) and Group (Ret, NoRet). Next, two RM ANOVAs were conducted to compare the rate of extinction between groups. The first RM ANOVA compared freezing between groups (factor Group: Ret, NoRet) on all retrieval-extinction trials (factor Trial: CS1-19). A second RM ANOVA compared freezing between groups (factor Group: Ret, NoRet) on the last four extinction trials (factor Trial: CS16-19).
To evaluate the recovery of cued fear in the spontaneous recovery experiment, we conducted a RM ANOVA comparing the average of the last four trials of extinction with the average of the four trials of the long-term memory test, and the average of the four trials of the spontaneous recovery test (factor Session: extinction, long-term memory test, spontaneous recovery test) between groups (factor Group: Ret, NoRet).
To evaluate the recovery of cued fear in the reinstatement experiment, we conducted a RM ANOVA comparing the average of the last four trials of extinction with the average of the four trials of the reinstatement test (factor Session: extinction, reinstatement test) between groups (factor Group: Ret, NoRet).
To evaluate the recovery of cued fear in the renewal experiment, we conducted a RM ANOVA comparing the average of the last four trials of extinction with the average of the four trials of the long-term memory test, and the average of the four trials of the renewal test (factor Session: extinction, long-term memory test, renewal test) between groups (factor Group: Ret, NoRet).
To evaluate the recovery of cued fear in more detail, and to assess group differences and within-session extinction, additional RM ANOVAs (factors Trial and Group) were carried out, including freezing on each tone instead of the average of 4 tones, thereby making optimal use of the information provided by each trial. These analyses were conducted for each test session separately (long-term memory, spontaneous recovery, reinstatement and renewal tests).
The significance level was set at p < .05 for all statistical tests. If Mauchly’s test indicated a violation of the sphericity assumption, a Greenhouse-Geisser correction was applied. To further investigate significant main effects or interactions, Tukey’s posthoc tests were conducted. All analyses were carried out using Statistica 13.1 (Dell Inc, Tulsa, OK, USA) and all graphs were made with GraphPad Prism 7 (GraphPad Software Inc, La Jolla, CA, USA).
Additional statistical analyses (not included in the preregistered analysis plan)
Because of significant within-session extinction on the long-term memory, spontaneous recovery and renewal tests, additional RM ANOVAs (factors Session and Group) were conducted to further evaluate the return of fear, by assessing the change in freezing from the last trial of the long-term memory test to the first trial of the subsequent test.
In addition, following the approach described in the meta-analysis of Kredlow and colleagues, we determined Hedges’ g effect sizes for each experiment (Lakens 2013). More specifically, we calculated the change in freezing from the end of extinction (average of the last four trials) to the test assessing the return of fear (average of the four trials of either the spontaneous recovery test, the reinstatement test or the renewal test), and compared these values between groups.
Results
Experiment 1: Spontaneous recovery
All rats were included in the final analyses (n = 8 per group) (Fig. 1). Note that extra analyses excluding two subjects with less than 5% freezing on the final acquisition trial reached the same conclusions.
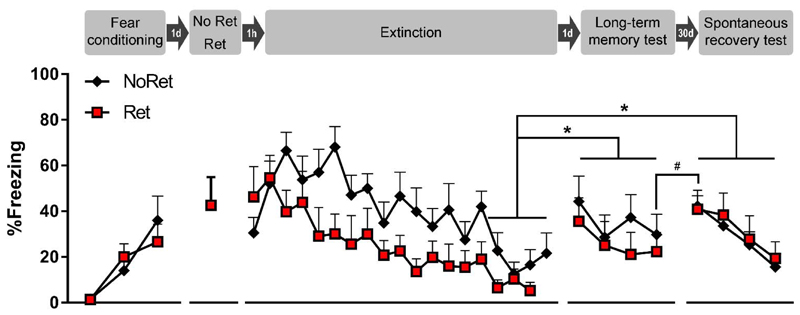
Fig. 1. Spontaneous recovery experiment.
Presentation of an isolated retrieval trial, one hour later followed by an extinction phase, does not prevent spontaneous recovery. All sessions were conducted in context A. Rats were conditioned with 3 tone-shock pairings. Twenty-four hours later, they were exposed to a single retrieval tone (Ret group, n = 8) or context only (NoRet group, n = 8), followed one hour later by an extinction phase consisting of 18 or 19 tones, respectively. Twenty-four hours after extinction training, long-term memory was tested by presenting 4 tones. One month later, spontaneous recovery was evaluated with 4 tones. In contrast to the original Monfils et al. paper, we observed spontaneous recovery already one day after extinction. In addition, trial-by-trial analyses indicate a significant return of fear, in both groups, from the end of the long-term memory test to the start of the spontaneous recovery test one month later. Our data do not support an attenuation of spontaneous recovery in the Ret group as compared with the NoRet group. Freezing during each tone presentation is shown as mean + SEM, # indicates p < .05 and * indicates p < .01. Ret = retrieval, d = day, h = hour.
Cued fear was acquired in both groups, without significant group differences, indicating that matching based upon freezing during the third training tone was effective. Accordingly, the RM ANOVA showed a main effect of Trial (F(2,28) = 12.76, p < .001, η2p = 0.48), but no main effect of Group (F(1,14) = 0.02, p = .88), and no interaction. There was adequate expression of fear on the isolated retrieval trial in the Ret group (42% freezing on average). During the extinction phase, cued fear was extinguished in both groups. To compare the rate of extinction between groups, two RM ANOVAs were conducted. The first RM ANOVA (with Greenhouse-Geisser correction) compared freezing on all retrieval-extinction trials and found a main effect of Trial (F(5.6,78.5) = 8.58, p < .0001, η2p = 0.38), but no main effect of Group (F(1,14) = 2.74, p = .12), and no interaction. In line with the original Monfils et al. paper, we also conducted a second RM ANOVA, comparing freezing between groups on the last four extinction trials. Again, we found no main effect of Group (F(1,14) = 1.43, p =.25), and no main effect of Trial, nor interaction. Taken together, these analyses indicate that there were no significant differences in extinction between both groups.
To evaluate the recovery of cued fear, we conducted a RM ANOVA comparing the average of the last four trials of extinction with the average of the four trials of the long-term memory test, and the average of the four trials of the spontaneous recovery test between groups (see also Fig. S1). We found a main effect of Session (F(2,28) = 10.84, p < .001, η2p = 0.44), but no main effect of Group (F(1,14) = 0.31, p = .59), nor interaction. Tukey’s posthoc tests showed more freezing to the tone during the long-term memory test and spontaneous recovery test than at the end of extinction (both p’s < .01), indicating that there was equivalent recovery of cued fear in both groups, which did not differ significantly between the long-term memory test and spontaneous recovery test.
Additional RM ANOVAs were conducted to further evaluate any group differences and within-session extinction. For the long-term memory test, no group differences were found, but there was a main effect of Trial (F(3,42) = 4.47, p < .01, η2p = 0.24), indicating significant within-session extinction. Tukey’s posthoc tests showed more freezing (p < .05) during the first tone than during the second and fourth trials. For the spontaneous recovery test, no group differences were found either, and there was again a main effect of Trial (F(3,42) = 10.04, p < .0001, η2p = 0.42), indicating significant within-session extinction. Tukey’s posthoc tests found more freezing (p < .05) during the first tone than during the third and fourth. Because of the significant within-session extinction in both test sessions, we conducted an additional RM ANOVA to evaluate the return of fear on the spontaneous recovery test in both groups. We compared freezing during the last trial of the long-term memory test with freezing during the first trial of the spontaneous recovery test, and found a significant effect of Session (F(1,14) = 5.05, p < .05, η2p = 0.27). The extent of returned fear on the spontaneous recovery test did not differ significantly between groups.
Experiment 2: Reinstatement
Two rats met the preregistered exclusion criterion and were excluded from the final analyses (yielding n = 10 in Ret group, n = 12 in NoRet group) (Fig. 2). This exclusion criterion stated that rats showing less than 15% freezing during each of the first 3 tone presentations on the retrieval-extinction day were considered non-learners. Note that extra analyses including these rats or excluding one additional subject with less than 5% freezing during the final acquisition trial reached the same conclusions.
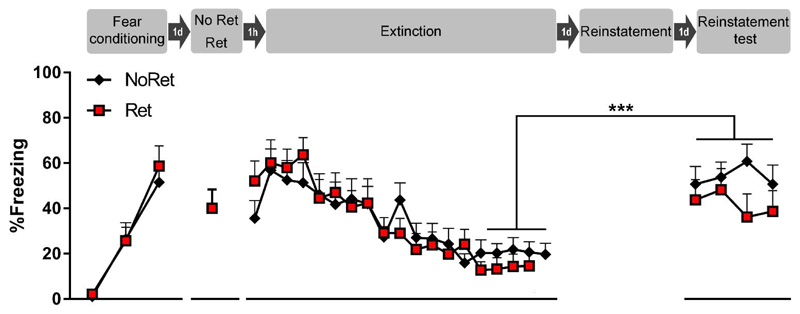
Fig. 2. Reinstatement experiment.
Presentation of an isolated retrieval trial, one hour later followed by an extinction phase, does not prevent reinstatement. All sessions were conducted in context A. Rats were conditioned with 3 tone-shock pairings. Twenty-four hours later, they were exposed to a single retrieval tone (Ret group, n = 10) or context only (NoRet group, n = 12), followed one hour later by an extinction phase consisting of 18 or 19 tones, respectively. Twenty-four hours after extinction training, rats received 5 unsignaled footshocks. One day later, they were tested for reinstatement of cued fear by presenting 4 tones. In line with the original Monfils et al. paper, we observed reinstatement of fear. However, our data do not support a significant attenuation of reinstatement in the Ret group as compared with the NoRet group. Freezing during each tone presentation is shown as mean + SEM and *** indicates p <.0001. Ret = retrieval, d = day, h = hour.
Cued fear was acquired in both groups, without significant group differences, indicating that matching based upon freezing during the third training tone was effective. Accordingly, the RM ANOVA showed a main effect of Trial (F(2,40) = 38.28, p < .0001, η2p = 0.66), but no main effect of Group (F(1,20) = 0.16, p = .69), and no interaction. There was adequate expression of fear on the isolated retrieval trial in the Ret group (40% freezing on average). During the extinction phase, cued fear was extinguished in both groups. To compare the rate of extinction between groups, two RM ANOVAs were conducted. The first RM ANOVA (with Greenhouse-Geisser correction) compared freezing on all retrieval-extinction trials and found a main effect of Trial (F(4.9,98.8) = 12.71, p < .0001, η2p = 0.39), but no main effect of Group (F(1,20) = 0.01, p = .92), and no interaction. In line with the original Monfils et al. paper, we also conducted a second RM ANOVA, comparing freezing between groups on the last four extinction trials. Again, we found no main effect of Group (F(1,20) = 1.96, p =.18), and no main effect of Trial, nor interaction. Taken together, these analyses suggest that there was equivalent extinction in both groups.
During the five minutes preceding the first CS of the reinstatement test, freezing was high in both groups (mean + SEM was 55% + 5% in both groups, unpaired t-test, t(20) = 0.04, p = .97). Notably, pre CS freezing in the original Monfils et al. (2009) paper was surprisingly low (about 1-5%) and may have been reported incorrectly.
To evaluate the recovery of cued fear on the reinstatement test, we conducted a RM ANOVA comparing the average of the last four trials of extinction with the average of the four trials of the reinstatement test between groups (see also Fig. S2). We found a main effect of Session (F(1,20) = 27.94, p < .0001, η2p = 0.58), but no main effect of Group (F(1,20) = 2.23, p = .15), nor interaction, indicating that there was equivalent reinstatement of cued fear in both groups. For the four CSs of the reinstatement test, an additional RM ANOVA (with Greenhouse-Geisser correction) was conducted to evaluate group differences and within-session extinction, none of which were found.
Experiment 3: Renewal
All rats were included in the final analyses (n = 8 per group) (Fig. 3).
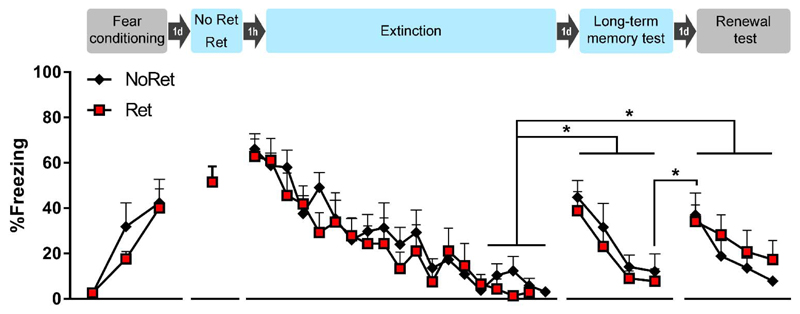
Fig. 3. Renewal experiment.
Presentation of an isolated retrieval trial, one hour later followed by an extinction phase, does not prevent renewal. Rats were conditioned with 3 tone-shock pairings in context A. Twenty-four hours later, in context B, they were exposed to a single retrieval tone (Ret group, n = 8) or context only (NoRet group, n = 8), followed one hour later by an extinction phase consisting of 18 or 19 tones, respectively. Twenty-four hours after extinction training, long-term memory was tested by presenting 4 tones in context B. One day later, renewal was evaluated with 4 tones in context A. In contrast to the original Monfils et al. paper, we observed spontaneous recovery one day after extinction in both groups. In addition, trial-by-trial analyses indicate a significant return of fear, in both groups, from the end of the long-term memory test in context B to the start of the renewal test in context A. Our data do not support an attenuation of spontaneous recovery nor renewal in the Ret group as compared with the NoRet group. Freezing during each tone presentation is shown as mean + SEM and * indicates p < .01. Ret = retrieval, d = day, h = hour.
Cued fear was acquired in both groups, without significant group differences, indicating that matching based upon freezing during the final acquisition trial was effective. Accordingly, the RM ANOVA showed a main effect of Trial (F(2,28) = 24.09, p < .0001, η2p = 0.63), but no main effect of Group (F(1,14) = 0.52, p = .48), and no interaction. There was adequate expression of fear on the isolated retrieval trial in the Ret group (51% freezing on average). During the extinction phase, cued fear was extinguished in both groups. To compare the rate of extinction between groups, two RM ANOVAs were conducted. The first RM ANOVA (with Greenhouse-Geisser correction) compared freezing on all retrieval-extinction trials and found a main effect of Trial (F(5.7,79.9) = 18.94, p < .0001, η2p = 0.57), but no main effect of Group (F(1,14) = 0.04, p = .84), and no interaction. In line with the original Monfils et al. paper, we also conducted a second RM ANOVA, comparing freezing between groups on the last four extinction trials. Again, we found no main effect of Group (F(1,14) = 1.05, p =.32), and no main effect of Trial, nor interaction. Taken together, these analyses suggest that there was equivalent extinction in both groups.
To evaluate the recovery of cued fear, we conducted a RM ANOVA comparing the average of the last four trials of extinction with the average of the four trials of the long-term memory test, and the average of the four trials of the renewal test between groups (see also Fig. S3). We found a main effect of Session (F(2,28) = 10.20, p < .001, η2p = 0.42), but no main effect of Group (F(1,14) = 0.07, p = .79), nor interaction. Tukey’s posthoc tests showed more freezing to the tone during the long-term memory test and renewal test than at the end of extinction (both p’s < .01), indicating that there was equivalent recovery of cued fear in both groups, which did not differ significantly between the long-term memory test and renewal test.
Additional RM ANOVAs were conducted to evaluate group differences and within-session extinction. For the long-term memory test, no group differences were found, but there was a main effect of Trial (F(3,42) = 18.46, p < .0001, η2p = 0.57), indicating significant within-session extinction. Tukey’s posthoc tests showed more freezing (p < .05) during the first tone of this session than during the three following tones. For the renewal test, no group differences were found either, but there was a main effect of Trial (F(3,42) = 7.18, p < .01, with Greenhouse-Geisser correction, η2p = 0.34), again indicating significant within-session extinction. Tukey’s posthoc tests showed more freezing (p < .05) during the first and second tone than during the following trials. Because of the significant within-session extinction in both test sessions, we conducted an additional RM ANOVA to evaluate the return of fear on the renewal test in both groups. We compared freezing during the last trial of the long-term memory test with freezing during the first trial of the renewal test, and found a significant effect of Session (F(1,14) = 12.09, p < .01, η2p = 0.46). The extent of returned fear on the renewal test did not differ significantly between groups.
Effect sizes
When comparing freezing at the end of extinction (last four trials) with the spontaneous recovery test between groups, Hedges’ g was -0.80 (Exp. 1). More specifically, we found more return of fear in the Ret group than in the NoRet group, although this difference was not significant. Comparison of the end of extinction with the reinstatement test between groups resulted in a Hedges’ g effect size of 0.19 (Exp. 2). Here, we found less return of fear in the Ret group than in the NoRet group, but this difference was again not significant and the effect was small. Finally, when comparing the end of extinction with the renewal test between groups, Hedges’ g was -0.60 (Exp. 3). There was again more return of fear in the Ret group than in the NoRet group, without the difference being significant.
Discussion
This study is a direct replication of three key experiments from the influential 2009 Science paper by Monfils and colleagues, in which they introduced a modified extinction procedure that resulted in a more enduring attenuation of the fear memory, by presenting a single tone stimulus one hour before the start of standard extinction training (Monfils et al. 2009). The findings and proposed mechanism are somewhat controversial (Chan et al. 2010, Baker, McNally & Richardson 2013, Ponnusamy et al. 2016), and failed conceptual replications have largely been attributed to (small) procedural differences (Kredlow et al. 2016, Gershman et al. 2017). To date, a direct replication that evaluates the robustness of the initial findings had not yet been published. This was the goal of the current study.
Overall, we did not find any evidence for reduced return of fear with the retrieval-extinction procedure, as assessed with spontaneous recovery, reinstatement and renewal. Behavior of control animals (NoRet group, i.e. extinction only) during training and extinction was similar to that in the Monfils et al. paper. Residual cued fear at the end of extinction was very comparable as well (around 15% freezing), as was the degree of returned fear, thereby providing adequate baseline behavior to reveal differences with the experimental condition (Ret group, retrieval-extinction).
In the spontaneous recovery experiment (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3625935/figure/F1), Monfils and colleagues found negligible spontaneous recovery in both Ret (‘1 hr’ group in the Monfils et al. paper) and NoRet conditions (‘context’ group in the Monfils et al. paper) after 24 hours, and in the Ret group after one month. However, in the NoRet group, there was evidence for a return of fear, and freezing levels almost doubled from the end of extinction (last 4 trials) to the test after one month. In our hands, there was a very comparable return of fear in both groups, already after 24 hours. Although spontaneous recovery occurred at an earlier time point here than in the original paper, this should not have precluded us from finding a retrieval-extinction effect. In fact, in a human fear conditioning study, Schiller, Monfils and colleagues found a complete attenuation of spontaneous recovery one day after extinction training in the Ret condition (Schiller et al. 2010).
In the reinstatement experiment (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3625935/figure/F3), Monfils and colleagues observed a significant return of fear in the NoRet group (a 2.5-fold increase in freezing from the end of extinction to the reinstatement test), but no change in the Ret group. In our hands, both groups showed similar reinstatement of cued fear.
In the renewal experiment (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3625935/figure/F2), Monfils and colleagues found no spontaneous recovery in both groups after 24 hours. One day later, they found a return of fear in the NoRet group, with freezing levels almost doubling from the test in context B to the renewal test in context A. There was no increase in freezing in the Ret group. Here, we found, in line with our first experiment, considerable spontaneous recovery after 24 hours in both groups. When collapsing data over 4 CS presentations, we found no further increase from the 24-hour test in context B to the renewal test in context A. Of note, additional trial-by-trial analyses showed considerable within-session extinction on both days and a significant return of fear in context A in both groups when comparing the last CS in context B with the first CS in context A (a 3.5-fold increase in freezing).
We would like to point out that the experimental protocols and designs, which we adopted from the original study without modification, may not have been optimal to assess return of fear in an unambiguous manner. First, one can argue that the long-term memory test, which takes place one day after extinction training, is not necessary provided that freezing at the end of extinction is equivalent in both groups. Exposure to the context and tones during this test may induce additional extinction and confound fear responses on subsequent spontaneous recovery or renewal tests. Second, the reinstatement procedure with 5 unpredictable shocks may have been overly strong, resulting in considerable context conditioning thereby hampering the interpretation of freezing to the tone. In follow-up studies, Monfils and colleagues have indeed converted to reinstatement procedures with just two shocks (Auchter et al. 2017). In addition, a control condition without reinstatement shocks would be informative to appropriately assess the return of fear. Third, in the absence of additional control conditions in the renewal experiment (e.g. training and extinction in A, then test in B or training in A, extinction in B and then two days of tests in B), we cannot unequivocally state that increased freezing during the renewal test is genuine renewal. It might as well be spontaneous recovery or a combination of both. Nevertheless, any return of fear, be it context-dependent or not, should have been attenuated by the retrieval-extinction procedure, according to the memory updating framework of Monfils and colleagues.
Taken together, we did not reproduce the findings of Monfils and colleagues, even though we copied their procedures as closely as possible. Nevertheless, there were some minor differences in methods which we will enumerate here. Our animals had the same strain, gender, age and body weight, but were purchased from a different supplier (Janvier Labs instead of Hilltop Lab Animals, PA, USA). Also, we used different fear conditioning equipment (MedAssociates instead of Coulbourn Instruments, PA, USA). Finally, there may have been slight differences in the intervals between CS presentations. The intervals were variable, as in the original paper, and we used the same average duration, but we did not have any detailed information about the length of each interval. In our opinion, it is unlikely that these slight procedural differences led to the failure to replicate, especially since our control groups behaved as expected. Thus, despite optimal conditions for replication, including sufficient power based on the effect sizes in the Monfils et al. paper (note that these effect sizes may, however, have been inflated, as a result of the limited sample sizes (typically n = 8 per group), see Button et al. 2013), the use of variable trial intervals (in accordance with Auchter et al. 2017), the use of single-housed animals and inclusion of a long-term spontaneous recovery test (in accordance with Kredlow et al. 2016), we were unable to reproduce the recovery-attenuating effect of the retrieval-extinction procedure and found much lower effect sizes.
Our null findings are, however, in line with the recent meta-analysis by Kredlow and colleagues of fear conditioning experiments with rats or mice, which found an overall non-significant effect of the retrieval-extinction procedure (Kredlow et al. 2016). In search for factors that could explain the inconsistent findings in the literature, the authors examined several potential moderators. The fear-reducing effect was found to be significantly moderated by the number of animals housed together and the delay between the retrieval-extinction procedure and the test for return of fear. Given that, even in a direct replication with optimal conditions, we were unable to reproduce the effect, holding on to these moderators may be premature, and it is unlikely that other failed (conceptual) replications can be fully accounted for by these factors. Rather, the moderators identified in the meta-analysis may be the accidental result of the studies that were included in this analysis. None of the included studies experimentally manipulated these moderators to probe their effect on the retrieval-extinction procedure. Only a few papers, most of which were published after completion of the Kredlow et al. meta-analysis in June 2014, have examined such procedural aspects (see also Table 1). Clem & Huganir (2010) and Gräff and colleagues (2014) compared recent and remote fear memories (≥1 week between acquisition and retrieval), although the latter authors did not include the necessary extinction-only control condition for animals with recent fear memories. Other studies – with contrasting results – have looked at different age groups and some of them found differences regarding the efficacy of the retrieval-extinction procedure (Ishii et al. 2015, Jones & Monfils 2016, Pattwell et al. 2016). A recent study directly compared the variability of the intertrial intervals during extinction training (Auchter et al. 2017) and found that more variability worked remarkably better. While the influence of procedural differences between studies obviously cannot explain our failure of an exact replication of the Monfils et al. paper, we strongly support the experimental assessment of putative moderators as the way forward in identifying true moderators and in resolving the elusive nature of the retrieval-extinction effect. More than a meta-analysis over existing studies, direct experimental assessment of moderators (be they derived from such meta-analysis or from theoretical analysis) has the power to reveal which factors contribute critically to the advantage, if any, of retrieval-extinction over regular extinction training.
Supplementary Material
Highlights.
This is a preregistered, exact replication study of Monfils et al. 2009, Science
They found less return of fear when adding a retrieval trial before extinction
We found no evidence for attenuated fear recovery using retrieval-extinction
This questions the validity of moderators derived from reviews and meta-analyses
Experimental evaluation of purported moderators may be the way forward
Acknowledgments
We would like to thank Kimberly Jones for her assistance with the behavioral experiments and manual freezing scoring. In addition, we are indebted to Marie H. Monfils for providing additional procedural details during the preparation of this work and for constructive feedback on an earlier version of this manuscript.
Funding
This work was supported by ERC Consolidator Grant 648176 (to Tom Beckers).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Washington, DC: 2013. [Google Scholar]
- Auchter A, Cormack LK, Niv Y, Gonzalez-Lima F, Monfils MH. Reconsolidation-extinction interactions in fear memory attenuation: the role of inter-trial interval variability. Frontiers in Behavioral Neuroscience. 2017;11:2. doi: 10.3389/fnbeh.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, McNally GP, Richardson R. Memory retrieval before or after extinction reduces recovery of fear in adolescent rats. Learning & Memory. 2013;20:467–473. doi: 10.1101/lm.031989.113. [DOI] [PubMed] [Google Scholar]
- Beckers T, Kindt M. Memory Reconsolidation Interference as an Emerging Treatment for Emotional Disorders: Strengths, Limitations, Challenges, and Opportunities. Annual Review of Clinical Psychology. 2017;13:99–121. doi: 10.1146/annurev-clinpsy-032816-045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Chan WY. The effects of retrieval-extinction training on the restoration of Pavlovian conditioned fear (Chapter 5) Sydney, Australia: UNSW; 2014. PhD thesis. [Google Scholar]
- Chan WY, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learning & Memory. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzi M, Cannas S, Saraulli D, Rossi-Arnaud C, Cestari V. Extinction after retrieval: effects on the associative and nonassociative components of remote contextual fear memory. Learning & Memory. 2011;18:508–518. doi: 10.1101/lm.2175811. [DOI] [PubMed] [Google Scholar]
- Craske MG, Mystkowski JL. Exposure therapy and extinction: clinical studies. In: Craske MG, Hermans D, Vansteenwegen D, editors. Fear and learning: from basic processes to clinical implications. Washington, DC: American Psychological Association; 2006. [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Flavell CR, Barber DJ, Lee JL. Behavioural memory reconsolidation of food and fear memories. Nature Communications. 2011;2:504. doi: 10.1038/ncomms1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, Monfils MH, Norman KA, Niv Y. The computational nature of memory modification. eLife. 2017;6 doi: 10.7554/eLife.23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, Rei D, Bero AW, Phan TX, Wagner F, Holson E, et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell. 2014;156:261–276. doi: 10.1016/j.cell.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. The Journal of Clinical Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D, Matsuzawa D, Matsuda S, Tomizawa H, Sutoh C, Shimizu E. No erasure effect of retrieval-extinction trial on fear memory in the hippocampus-independent and dependent paradigms. Neuroscience Letters. 2012;523:76–81. doi: 10.1016/j.neulet.2012.06.048. [DOI] [PubMed] [Google Scholar]
- Ishii D, Matsuzawa D, Matsuda S, Tomizawa H, Sutoh C, Shimizu E. An isolated retrieval trial before extinction session does not prevent the return of fear. Behavioural Brain Research. 2015;287:139–145. doi: 10.1016/j.bbr.2015.03.052. [DOI] [PubMed] [Google Scholar]
- Jones CE, Monfils MH. Post-retrieval extinction in adolescence prevents return of juvenile fear. Learning & Memory. 2016;23:567–575. doi: 10.1101/lm.043281.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Ringuet S, Monfils MH. Learned together, extinguished apart: reducing fear to complex stimuli. Learning & Memory. 2013;20:674–685. doi: 10.1101/lm.031740.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kredlow MA, Unger LD, Otto MW. Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychological Bulletin. 2016;142:314–336. doi: 10.1037/bul0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao L, Xue Y, Shi J, Suo L, Luo Y, Chai B, Yang C, Fang Q, Zhang Y, Bao Y, et al. An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biological Psychiatry. 2014;76:895–901. doi: 10.1016/j.biopsych.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG. Response rates for CBT for anxiety disorders: Need for standardized criteria. Clinical Psychology Review. 2015;42:72–82. doi: 10.1016/j.cpr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Luyten L, Schroyens N, Hermans D, Beckers T. Parameter optimization for automated behavior assessment: plug-and-play or trial-and-error? Frontiers in Behavioral Neuroscience. 2014;8:28. doi: 10.3389/fnbeh.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Schroyens N, Luyck K, Fanselow MS, Beckers T. No effect of glucose administration in a novel contextual fear generalization protocol in rats. Translational Psychiatry. 2016;6:e903. doi: 10.1038/tp.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson K, Whittle N, Camp M, Gunduz-Cinar O, Singewald N, Holmes A. Temporal factors in the extinction of fear in inbred mouse strains differing in extinction efficacy. Biology of Mood & Anxiety Disorders. 2013;3:13. doi: 10.1186/2045-5380-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Olshavsky ME, Jones CE, Lee HJ, Monfils MH. Appetitive behavioral traits and stimulus intensity influence maintenance of conditioned fear. Frontiers in Behavioral Neuroscience. 2013;7:179. doi: 10.3389/fnbeh.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougrin D. Efficacy of exposure versus cognitive therapy in anxiety disorders: systematic review and meta-analysis. BMC Psychiatry. 2011;11:200. doi: 10.1186/1471-244X-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, Deisseroth K, et al. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nature Communications. 2016;7 doi: 10.1038/ncomms11475. 11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeyro ME, Ferrer Monti RI, Alfei JM, Bueno AM, Urcelay GP. Memory destabilization is critical for the success of the reactivation-extinction procedure. Learning & Memory. 2013;21:46–54. doi: 10.1101/lm.032714.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R, Zhuravka I, Poulos AM, Shobe J, Merjanian M, Huang J, Wolvek D, O'Neill PK, Fanselow MS. Retrieval and reconsolidation accounts of fear extinction. Frontiers in Behavioral Neuroscience. 2016;10:89. doi: 10.3389/fnbeh.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, Spijker S. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nature Neuroscience. 2011;14:1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Craske MG. Effects of varied-stimulus exposure training on fear reduction and return of fear. Behaviour Research and Therapy. 1998;36:719–734. doi: 10.1016/s0005-7967(97)10017-1. [DOI] [PubMed] [Google Scholar]
- Scheveneels S, Boddez Y, Vervliet B, Hermans D. The validity of laboratory-based treatment research: Bridging the gap between fear extinction and exposure treatment. Behaviour Research and Therapy. 2016;86:87–94. doi: 10.1016/j.brat.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JM, Maughan DK, Ilioi EC, Lattal KM. Exposure to a fearful context during periods of memory plasticity impairs extinction via hyperactivation of frontal-amygdalar circuits. Learning & Memory. 2013;20:156–163. doi: 10.1101/lm.029801.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcelay GP, Wheeler DS, Miller RR. Spacing extinction trials alleviates renewal and spontaneous recovery. Learning & Behavior. 2009;37:60–73. doi: 10.3758/LB.37.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annual Review of Clinical Psychology. 2013;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Kraniotis S, He Q, Marshall JJ, Nomura T, Stauffer SR, Lindsley CW, Conn PJ, Contractor A. Potentiating mGluR5 function with a positive allosteric modulator enhances adaptive learning. Learning & Memory. 2013;20:438–445. doi: 10.1101/lm.031666.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Bruce SE, Dyck IR, Keller MB. Chronicity, relapse, and illness--course of panic disorder, social phobia, and generalized anxiety disorder: findings in men and women from 8 years of follow-up. Depression and Anxiety. 2003;17:173–179. doi: 10.1002/da.10106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.