Abstract
The emerging field of regenerative rehabilitation integrates biological and bioengineering advances in regenerative medicine with rehabilitative sciences. Here we highlight recent stem cell-based examples of the regenerative rehabilitation paradigm to promote tissue repair and regeneration, and we discuss remaining challenges and future directions for the field.
Regenerative medicine, encompassing stem cell therapeutics and tissue engineering, offers a promising approach for the development of complex biological therapies. However, just as donor organ status and host immunological barriers continue to pose major challenges for organ transplantation, donor and host factors also pose a challenge for regenerative medicine. Understanding not only the biochemical but also the biophysical factors that determine the success of regenerative medicine approaches is a critical frontier. Enter mechanotransduction, a tenet of rehabilitative therapies. The application of mechanotransductive principles to regenerative cells, whether endogenous or exogenous, as well as to the host environment in which those cells must function to repair damaged tissues, is creating opportunities and advances. The same is true for electrical fields, temperature gradients, and other mainstays of rehabilitation therapies. The combination of regenerative medicine with rehabilitative principles has the potential to create new approaches for the treatment of tissue injury and disease, leading to the emerging field of regenerative rehabilitation. Although a multitude of restorative biological processes are important for tissue healing, here we focus on the synergy between rehabilitation strategies and regenerative responses mediated by endogenous or transplanted stem cells.
Regenerative Medicine, Bioengineering, and Mechanotransduction
The promise of regenerative medicine has yielded successful stem cell therapies with long-term efficacy, such as the survival and function of hematopoietic stem cells in bone marrow transplantations and epidermal stem cells in skin grafts. In contrast, other stem cell transplantation approaches, such as in the heart or brain, have often resulted in limited functional recovery, and even then recovery is often due to paracrine effects of the transplanted (and mostly dying) cells rather than to the engraftment of stem cells to generate newly differentiated cells in the tissue. Such paracrine effects, leading to tissue remodeling and adaptations, demonstrate that beneficial regenerative responses can occur independent of stem cell engraftment and formation of new parenchymal cells. Nevertheless, technological advances that promote stem cell survival, engraftment, and function in the host environment, which is often unfavorable in diseased or injured tissue, are critical for the success of regenerative therapeutics.
Along these very lines, a major development in regenerative medicine has been the marriage of engineering with biology to enhance therapeutic efficacy. For example, the burgeoning biomaterials field has facilitated the design of synthetic scaffolds that provide transplanted stem cells with a supportive biomechanical environment. Such scaffolds can promote both the survival and proliferation of transplanted stem cells as well as improve cell retention at the site of transplantation. Likewise, the use of bioreactors to prime cells prior to transplantation has become another component in the regenerative medicine toolbox.
The scientific basis for many bioengineering applications to regenerative medicine is the responses of cells and tissues to biophysical stimuli. Extrinsic mechanical cues are transmitted by cytoskeletal structures that, in turn, communicate with cytosolic messengers and/or the nucleus to regulate gene expression and cellular behavior (Figure 1). For instance, stem cells reside in an extracellular matrix whose static biophysical properties (such as stiffness or topology) can have a profound effect on lineage specification (Engler et al., 2006). Dynamic mechanical cues are similarly potent drivers of stem cell responses. During development, shear, tensile, and compressive mechanical signals, which are tightly spatiotemporally regulated, play critical roles in morphogenesis through activation of lineage commitment genes and in the regulation of cell size, shape, and intercalation (Heisenberg and Bellaïche, 2013). Embryonic stem cells differentiated in vitro and exposed to fluid shear stress at a flow rate mimicking that within the dorsal aorta of a developing mouse embryo display increased markers of endothelial and hematopoietic lineages as well as increased hematopoietic colony-forming units when compared to cells exposed to static conditions (Adamo et al., 2009). Throughout adulthood, modulation of tension applied to epidermal stem cells determines the balance between skin wound healing and the formation of fibrosis after traumatic injuries (Wong et al., 2012). Moreover, recapitulating mechanical features of the articular joint microenvironment, including hydrostatic pressure, shear, and compressive forces, promotes stem cell differentiation to chondrocytes. Indeed, every cell in the body, whether intraparenchymal or blood-borne, is subjected to some forms of mechanical stimuli. Likewise, local bioelectrical signals influence cells in a wide variety of some, if not all, tissues. Electrical stimulation promotes axon outgrowth and functional nerve regeneration (Gordon and English, 2016), and endogenous electric currents direct cellular migration for skin wound healing (Zhao et al., 2006). An improved understanding of how biophysical and bioelectric signals are transduced by cells and tissues paves the way for advances in the application of such stimuli to promote functional recovery of tissues.
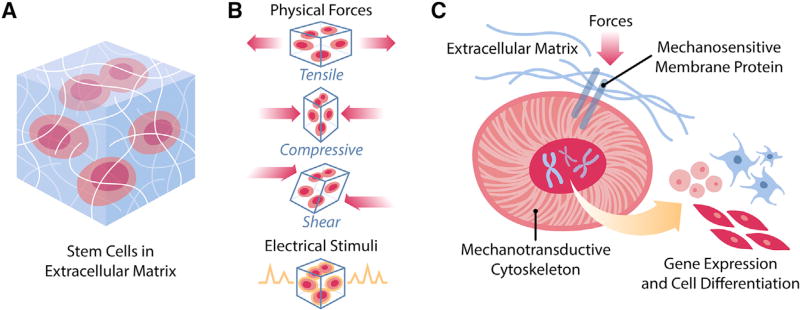
Figure 1. Mechanotransduction of Cells In Vivo.
(A) Cells are embedded in an environment composed of extracellular matrix and local milieu that exert various biophysical pressures.
(B) Among the biophysical pressures are physical stimuli, such as tensile forces, compressive forces, and shear stresses, and electrical stimuli from neural cells and local fields.
(C) In response to biophysical stimulation, cells transduce such signals from the membrane to the nucleus through the cytoskeleton in order to influence gene expression and cell fate.
Rehabilitative Sciences
The concept of biophysical signals influencing stem cell function and tissue regeneration has a direct parallel with the clinical field that integrates physical stimuli and functional outcomes: rehabilitative medicine. Standard approaches comprising the rehabilitation arsenal include exercise prescription as a means of administering tissue loading and conditioning, stretching for the application of tensile forces, electrical stimulation, manual therapies such as joint mobilization and traction, ultrasound, and modulation of tissue temperature by heat or ice. The application of these approaches is based on the clinical evidence that stimuli at a tissue or organismal level can enhance functional outcomes following injury and in the setting of disease. This has been most extensively applied to regeneration in the integumentary, neurologic, neuromuscular, and musculoskeletal systems, primarily because of the obvious mechanical and electrical properties of those tissues. Direct current stimulation has long been utilized to promote stem cell migration and epidermal wound healing. Low-frequency electrical nerve stimulation stimulates targeted innervation and functional recovery in individuals with peripheral nerve injuries. Early initiation of an exercise program is commonly implemented to accelerate regeneration and functional recovery after acute skeletal muscle injury. In some cases, “prehabilitation,” or the implementation of an exercise program prior to an injury such as an elective surgery, may prime the tissue stress response and optimize repair capacity. Furthermore, physical therapeutics have many indirect mediators, such as the secretion of growth factors, the enhancement of vascularization, or the modulation of inflammatory cascades, which have considerable benefit for tissue regeneration.
The potential of rehabilitative strategies to enhance regenerative responses of both endogenous and transplanted cells, by stimulating the cells themselves through mechanotransductive pathways and/or by modifying the physical niche in which the stem cells reside, has led to the inclusion of rehabilitative strategies into the domain of regenerative medicine. This emerging and cross-disciplinary field is increasingly becoming known as “regenerative rehabilitation.”
Regenerative Rehabilitation
The science of regenerative medicine fits naturally into the field of rehabilitation as a medical specialty. Neither is defined by any particular organ system into which traditional medicine is divided. Both have functional restoration as their primary outcomes. As with any emerging field, definitions pose a challenge: overly inclusive definitions lack clarity and overly restrictive definitions limit the scope. Key concepts that are central to the field are that innate regenerative processes can be enhanced by biophysical, electrical, and thermal stimuli; that cell transplantation can be optimized by subjecting cells to stimuli ex vivo and in vivo, such as tensile loading or compression, which enhance their regenerative potential; and that building upon the synergies of physical stimuli and regenerative responses is likely to improve functional outcomes (Figure 2). Based on these premises, a concise definition of regenerative rehabilitation is, “The application of rehabilitation protocols and principles together with regenerative medicine therapeutics toward the goal of optimizing functional recovery through tissue regeneration, remodeling, or repair.”
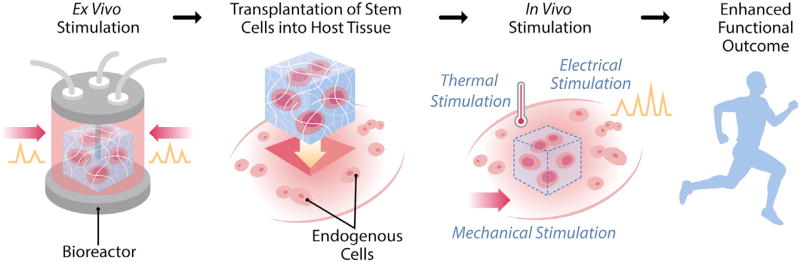
Figure 2. Regenerative Rehabilitation.
An example of how regenerative rehabilitation integrates concepts of mechanotransduction, both ex vivo and in vivo, in a specific therapeutic paradigm, stem cell transplantation, is shown. Stem cells can be manipulated ex vivo, based on biophysical principles, to optimize transplantation efficacy. Both the transplanted cells and the host environment can then be manipulated in vivo by rehabilitative therapies to foster stem cell engraftment, growth, and differentiation. Rehabilitation approaches may include mechanical stimulation (e.g. via exercise and stretching), electrical stimulation (e.g. via transcutaneous electrical nerve stimulation), or thermal stimulation (e.g. via heat or ice). Taken together, these strategies aim to promote enhanced functional outcomes.
A major focus of research in regenerative rehabilitation is to elucidate the complex cell-cell and cell-matrix interactions that are modified by in vivo physical stimuli and to refine those stimuli to achieve optimal functional outcomes in response to regenerative medicine technologies. Examples range from studies of the biophysical properties of stem cell niches to a better understanding of mechanotransductive cascades that dictate stem cell responses. In terms of the stem cell niche, this includes the interaction of donor stem cells with niche matrix components and endogenous cellular constituents. Arguably, the response of these transplanted components to rehabilitative modalities may be as important as the responses of the host tissue itself in determining the efficacy of the regenerative therapeutic strategy.
The addition of rehabilitative strategies to regenerative medicine therapeutics has resulted in notable improvements in both structural and functional outcomes, particularly in experimental models of tissue damage. Below we describe three studies that illustrate the regenerative rehabilitation paradigm.
Skeletal muscle trauma in the form of volumetric muscle loss (VML), a condition of major muscle loss that often results from blast injuries in combat, is a focus of regenerative therapies because of the absence of effective approaches to restore structure or function. The application of stem cell transplantations alone, or physical therapy alone, has resulted in only modest improvements of outcome. However, the addition of physical activity (in the form of forced treadmill running) to stem cell-seeded bioscaffolds resulted in a marked improvement in functional recovery (Quarta et al., 2017). The combined approach resulted not only in improved structure and biomechanics of the new muscle, but also in (and perhaps due to) increased vascularity, maturation of innervation, and a reduction in fibrosis. Clearly, the addition of this rehabilitative strategy greatly augmented the therapeutic benefit of the regenerative medicine approach.
The treatment of nerve injuries via tissue engineering is a promising alternative to the current standard of care, which is the harvesting of a section of nerve from the patient, an approach limited by donor site shortage and morbidity. Nerve guide conduits provide structural support at the site of the defect and encourage axonal growth. The addition of electrical stimuli following implantation of a nerve guide conduit enhances regeneration as evidenced by increased axon diameter and improved functional indices (Song et al., 2016). A better understanding of the mechanisms underlying the additive effect of the combination therapy will aid in the titration of stimulation parameters to maximize the synergy of the two approaches.
Lower limb peripheral arterial disease (PAD) affects one in five individuals over the age of 65. For individuals with PAD, ischemic pain with exertion can have devastating effects on physical function and mobility. In a rat model of limb ischemia, a combination approach of endothelial progenitor cell transplantation and extracorporeal shock wave therapy—which administers mechanical stimulation through high-energy acoustic waves—enhanced angiogenesis and restored blood flow to uninjured control levels (Yeh et al., 2012). Importantly, the beneficial effect of the combination therapy exceeded the benefit of either therapy in isolation.
Regenerative Rehabilitation: Challenges and Future Directions
Whereas examples of success of the regenerative rehabilitation paradigm bode well for the continued expansion of rehabilitative strategies into the broad field of regenerative medicine, challenges and opportunities exist both in terms of the experimental models and in terms of the translation to human regenerative medicine applications. In vitro investigations are paramount for better understanding molecular and cellular responses to biophysical signals. However, the translation of these insights into clinical programs is limited by the fact that reductionist investigations have often failed to capture functional communication and interactions between neighboring tissues and cell systems. Organ-on-a-chip technologies, as well as multi-tissue 3D organoid structures, seek to bridge this gap. The addition of mechanical cues to these model systems will offer an additional layer of insight into cell-cell, cell-fluid, and cell-matrix interactions occurring in vivo. An advantage of these systems over pre-clinical animal models is that they permit the evaluation of the responses of human cells to biophysical stimuli. This becomes particularly relevant in the context of personalized medicine strategies as emerging efforts seek to utilize an individual’s own cells for the creation of organ-on-a-chip devices or 3D organoids. Moreover, although knowledge of mechanotransductive cascades has advanced in the last several decades, our understanding of how sensing mechanisms and cell mechanics are altered under pathological conditions remains an important, yet underexplored, area of investigation. Indeed, pathogenic mechanotransductive signaling may invoke devastating consequences on tissue health, as has been demonstrated in the context of cancer malignancy, where integrin-mediated responses to increased stiffness of the extracellular matrix have been shown to potentiate oncogenic signaling (Levental et al., 2009).
Another caveat is the relevance of experimental rehabilitative strategies to the clinical practice of rehabilitative medicine. For example, physical activity is often modeled in experimental animals by a bout of running, either voluntary or forced. Whereas this activates the neuromuscular system in measurable and reproducible ways, it does not model the kinds of physical therapy regimens prescribed for patients. Forced running is generally accompanied by stress to the animal, thereby imparting potentially confounding systemic effects, while voluntary wheel running lacks the ability to implement targeted and specific doses, a staple of any successful rehabilitation program. The commonly employed loss-of-activity model, hindlimb unloading, is similarly disconnected from clinical scenarios of immobility. It will be important to refine such experimental rehabilitative strategies to focus on those that are predictive of clinical outcomes in patients. Furthermore, the development of preclinical non-invasive or implantable sensors to monitor in vivo tissue responses to physical stimuli can potentially aid the development of clinical rehabilitation and regenerative rehabilitation protocols.
The lack of consensus as to rehabilitative protocols that would define practice guidelines represents another hurdle for regenerative rehabilitation studies. For all physical stimuli that have reportedly led to functional improvements, few have been rigorously characterized at the level of key parameters, such as timing after surgery or injury, frequency, intensity, and duration. All of these may be critical determinants of outcome, ranging from insufficient to efficacious to detrimental. Therefore, researchers face the challenge of considering this range of variables in the design of pre-clinical studies that may be more readily translatable to humans.
Regenerative medicine is certain to become a mainstay of therapy as biologics become a more standard component of the therapeutic armamentarium. The emergence of regenerative rehabilitation as a distinct division of regenerative medicine is likely a harbinger of the continued convergence of complementary technologies to treat disorders for which current medical and surgical treatments are inadequate. Maturation of the rehabilitative sciences described herein, with more mechanistic underpinnings, will further prompt their application to stem cell and regenerative therapeutics.
Acknowledgments
The authors thank Dr. Michael Boninger for valuable feedback on the manuscript and Ms. Molly Thompson for artistic support with the figures. This work was supported by a P2C grant from NIH/NCMRR (HD086843) to T.A.R. and F.A.
References
- Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur. J. Neurosci. 2016;43:336–350. doi: 10.1111/ejn.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta M, Cromie M, Chacon R, Blonigan J, Garcia V, Akimenko I, Hamer M, Paine P, Stok M, Shrager JB, Rando TA. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017;8:15613. doi: 10.1038/ncomms15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Sun B, Liu S, Chen W, Zhang Y, Wang C, Mo X, Che J, Ouyang Y, Yuan W, Fan C. Polymerizing pyrrole coated poly (L-lactic acid-co-ε-caprolactone) (PLCL) conductive nanofibrous conduit combined with electric stimulation for long-range peripheral nerve regeneration. Front. Mol. Neurosci. 2016;9:117. doi: 10.3389/fnmol.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Longaker MT, Gurtner GC. Soft tissue mechanotransduction in wound healing and fibrosis. Semin. Cell Dev. Biol. 2012;23:981–986. doi: 10.1016/j.semcdb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Yeh KH, Sheu JJ, Lin YC, Sun CK, Chang LT, Kao YH, Yen CH, Shao PL, Tsai TH, Chen YL, et al. Benefit of combined extracorporeal shock wave and bone marrow-derived endothelial progenitor cells in protection against critical limb ischemia in rats. Crit. Care Med. 2012;40:169–177. doi: 10.1097/CCM.0b013e31822d74d0. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]