Summary
Background
In a 72-week, randomised controlled trial of obeticholic acid (OCA) in non-alcoholic steatohepatitis (NASH), OCA was superior to placebo in improving serum ALT levels and liver histology. OCA therapy also reduced weight.
Aims
Because weight loss by itself can improve histology, to perform a post hoc analysis of the effects of weight loss and OCA treatment in improving clinical and metabolic features of NASH.
Methods
The analysis was limited to the 200 patients with baseline and end-of-treatment liver biopsies. Weight loss was defined as a relative decline from baseline of 2% or more at treatment end.
Results
Weight loss occurred in 44% (45/102) of OCA and 32% (31/98) of placebo-treated patients (P = 0.08). The NAFLD Activity score (NAS) improved more in those with than without weight loss in both the OCA- (−2.4 vs −1.2, P<0.001) and placebo-treated patients (−1.2 vs −0.5, P = 0.03). ALT levels also improved in those with vs without weight loss in OCA- (−43 vs −34 U/L, P = 0.12) and placebo-treated patients (−29 vs −10 U/L, P = 0.02). However, among those who lost weight, OCA was associated with opposite effects from placebo on changes in alkaline phosphatase (+21 vs −12 U/L, P<0.001), total (+13 vs −14 mg/dL, P = 0.02) and LDL cholesterol (+18 vs −12 mg/dL, P = 0.01), and HbA1c (+0.1 vs −0.4%, P = 0.01).
Conclusions
OCA leads to weight loss in up to 44% of patients with NASH, and OCA therapy and weight loss have additive benefits on serum aminotransferases and histology. However, favourable effects of weight loss on alkaline phosphatase, lipids and blood glucose seen in placebo-treated patients were absent or reversed on OCA treatment. These findings stress the importance of assessing concomitant metabolic effects of new therapies of NASH. Clinical trial number: NCT01265498.
1 INTRODUCTION
Non-alcoholic steatohepatitis (NASH) is one of the more common causes of liver disease in the developed world.1,2 While often clinically silent, with time it can progress to cirrhosis, end-stage liver disease and hepatocellular cancer.3 Indeed, at present, at least 15% of cases of end-stage liver disease undergoing liver transplantation in the United States are attributed to NASH, and this proportion appears to be rising.4 In addition, to its link with liver-related complications, NAFLD is associated with increased all-cause mortality, mostly from cardiovascular causes.5 While its pathogenesis is unknown, NASH is often associated with obesity and metabolic abnormalities such insulin resistance, diabetes, hypertension and dyslipidaemia.4 The cornerstone of management of NASH is optimisation of metabolic risk factors and recommendations on diet and exercise. Currently there are no approved drug therapies specifically targeting NASH but phase 2/3 studies are ongoing.6
Farnesoid X receptor is a nuclear hormone receptor for bile acids, which plays a critical role in bile acid, carbohydrate and lipid metabolism by the liver.7 Pre-clinical studies have demonstrated that activation of FXR improves insulin sensitivity, lowers plasma glucose, free fatty acids, triglycerides and total cholesterol in animal models.8,9 Because of these effects, FXR has been proposed as a target for treatment of NASH.10 Obeticholic acid (OCA), 6-ethylchenodeoxycholic acid, a synthetic variant of the natural bile acid chenodeoxycholic (CDCA), is a selective and potent FXR agonist with a 100-fold greater FXR agonistic activity than CDCA.9 In animal models of NAFLD, OCA decreased hepatic fat and fibrosis11,12 and in a pilot randomised, placebo-controlled trial in patients with type II diabetes, OCA improved insulin sensitivity and was associated with a decrease in serum alanine aminotransferase (ALT) and c-glutamyl transpeptidase levels in those with elevated levels at baseline.13
The Farnesoid X Receptor Ligand Obetacholic Acid in NASH Treatment (FLINT) trial conducted by the NASH clinical research network (NASH-CRN) was a placebo-controlled, randomised clinical trial of OCA (25 mg) vs placebo given once daily for 72 weeks in 283 patients with histologically proven NASH.14 The primary outcome was improvement in liver histology, defined as a decrease in NAFLD Activity Score (NAS) by at least 2 points without worsening of fibrosis, in liver biopsies taken at the end of treatment. At 72 weeks, 45% patients in the OCA group compared to only 21% in the placebo group achieved the pre-defined improvement in liver histology (P = 0.0002). Treatment with OCA improved all features of NAS (steatosis, hepatocellular ballooning, and lobular inflammation) as well as hepatic fibrosis. Histological resolution of NASH, however, occurred in only 19% of OCA-treated vs 13% of placebo recipients, a difference that was not statistically significant. Importantly, OCA therapy was also associated with weight loss, with average weight decreasing by 2.3 kg by week 72 in the OCA group compared to no change (0.0 kg) with placebo.
Since weight loss can improve clinical and metabolic features of NASH, this secondary analysis aim was to evaluate the effects of weight loss and OCA treatment (vs placebo) in improving clinical and metabolic features of NASH.
2 STUDY METHODS
2.1 FLINT trial
The FLINT trial was a prospective, double-blind randomised trial of OCA vs placebo in 283 adult patients with histologically defined non-alcoholic steatohepatitis. The aims, design and primary outcomes of this trial have been published.14 Briefly, subjects underwent a clinical evaluation, including liver biopsy, and those with histological features of NASH and a NAS score of at least 4 (on a scale of 0–8) were randomised to receive either OCA (25 mg) or identical appearing placebo tablets once daily for 72 weeks. All patients were followed with visits and routine blood tests at 12-week intervals and repeat liver biopsy at the end of the 72 weeks of treatment. In this trial, the final 64 patients did not undergo a follow-up liver biopsy because a pre-planned interim analysis showed a highly significant difference in the primary endpoint after completion of therapy in the initial 219 patients. For this reason, therapy was stopped early and subjects still in study were followed up for another 24 weeks on no treatment. The study was conducted between 2011 and 2013 at eight US medical centres and the data were managed by a central Data Coordinating Center (the names of the medical centres, data coordinating centre and principal investigators are provided in the Appendix 1). All patients gave written informed consent and details of the trial were approved by local institutional review boards. In addition, the design, protocol, details and conduct of the trial were monitored by an independent data safety and monitoring board appointed by the funding source, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health.
2.2 Post hoc subgroup analysis
This post hoc subgroup analysis focused on weight change in patients who received OCA or placebo and the association of these changes with changes in histological features, serum enzymes, metabolic factors and adverse events and was limited to patients who underwent repeat liver biopsy at 72 weeks. The changes in body weight between baseline and week 72 were categorised into two strata (1) weight loss (2% or more); (2) no weight loss (<2% loss or weight gain), as done in previous analyses of trials of therapy in NASH.15 In comparing baseline and 72 week liver biopsies, histological improvement was defined as a 2-point or greater decrease in NAS with no worsening of fibrosis. Changes in serum enzymes, lipids and biochemical features of insulin sensitivity in association with weight change in both groups were also evaluated. Finally, changes in LDL cholesterol at 72 weeks by treatment group and weight loss category were evaluated in relation to statin use.
All liver biopsies were scored for features of NAFLD and NASH using a standardised scoring system by the Pathology Committee of the NASH CRN.16 The NAFLD activity score (NAS) comprised the combination of scores given for steatosis (0–3), lobular inflammation (0–3) and ballooning degeneration (0–2). Fibrosis stage ranged from 0 to 4 with stage 3 representing bridging fibrosis and Stage 4 cirrhosis. Details of the scoring system, including its intra- and inter-observer variation, have been described previously.14,16
2.3 Statistical analysis
Comparison of baseline characteristics between patients with vs without 72-week biopsies were assessed using the t test for continuous variables and the chi-square test for categorical variables. Changes from baseline were assessed for the entire study cohort and also between two groups with and without weight loss as defined above. In addition, the interaction of treatment group and weight loss was assessed in a separate model for each outcome variable of interest. Analysis of covariance (ANCOVA) adjusting for the baseline value of the outcome was used to assess group differences and estimate adjusted means. Logistic regression was used to assess group differences in binary outcomes. Statistical analyses were done with SAS version 9.4 (sas Institute, Cary, NC) and Stata release 13 (statacorp, College Station, TX, USA).
The joint effects of OCA and weight loss on 72-week changes from baseline in liver tests, liver histology, lipids, metabolic factors and adverse effects were evaluated, in order to determine whether1 OCA and weight loss had additive effects on these outcomes; or2 OCA and weight loss were not additive (ie, the effects of OCA differed depending on whether or not weight loss had occurred). Case2 is referred to as effect modification or, in statistical modelling, as interaction. The P-value for interaction is used to test whether weight loss and OCA have additive effects with P<0.01 indicating non-additivity.17 Nominal P-values are presented not adjusted for multiple comparisons since the goal of the post hoc analysis was exploratory.18
3 RESULTS
3.1 Study population
Among the 283 patients enrolled in the FLINT trial, 141 were randomised to receive OCA and 142 to placebo. A total of 64 patients did not have an end-of-treatment biopsy based on the findings of the planned interim analysis as detailed in methods. Another eight patients in the OCA group and 11 in the placebo group had already refused or missed the end-of-treatment biopsy. The final cohort for this analysis, therefore, consisted of 102 OCA and 98 placebo patients.
The 200 patients included were predominantly women (66%) and non-Hispanic whites (82%) with an average age of 51 years (Table 1). At baseline, the mean serum ALT value was 82 U/L (range 15–269 U/L) and mean NAS 5.3 (range 2–8). The average body weight was 97 kg (range 61–172 kg) and body mass index (BMI) 35 kg/m2 with 21% of subjects overweight but not obese and 77% obese. Diabetes was present in 54% and 52% were taking anti-diabetic medications. Hyperlipidaemia was common and 48% were taking statins or other lipid-lowering agents at enrolment. OCA and placebo treated groups differed at baseline in mean waist circumference (P = 0.02), insulin (P = 0.001) and HOMA-IR (P = 0.006). Other baseline features were similar in the OCA and placebo groups (Table 1) and were similar to the baseline features of the cohort of 83 patients without follow-up biopsies except for mean steatosis grade of 2.1 in the analysis cohort vs 1.8 in the cohort without follow-up biopsies (P = 0.006) (other data not shown). At baseline, patients with >2% weight loss at 72 weeks had higher mean alkaline phosphatase (P = 0.02), lower HDL (P = 0.03) and lower usage of Vitamin E (P = 0.005) (Table 1).
TABLE 1.
Baseline characteristics of the study population
| Treatment group | Weight loss | Total (n = 200) |
|||||
|---|---|---|---|---|---|---|---|
| Obeticholic acid (n = 102) |
Placebo (n = 98) |
P | ≥2% weight loss (n = 76) |
<2% weight loss (n = 124) |
P | ||
| Demographics | |||||||
| Age (y) | 52 ± 11 | 50 ± 12 | 0.23 | 51 ± 10 | 51 ± 12 | 0.97 | 51 ± 11 |
| Male gender | 31 (30%) | 36 (37%) | 0.37 | 22 (29%) | 45 (36%) | 0.35 | 67 (34%) |
| White race | 87 (85%) | 78 (80%) | 0.35 | 63 (83%) | 102 (82%) | 1.00 | 165 (82%) |
| Hispanic ethnicity | 11 (11%) | 15 (15%) | 0.40 | 6 (8%) | 20 (16%) | 0.09 | 26 (13%) |
| Liver tests | |||||||
| Alanine aminotransferase - U/L | 82 ± 48 | 82 ± 49 | 0.96 | 91 ± 50 | 76 ± 47 | 0.04 | 82 ± 48 |
| Aspartate aminotransferase - U/L | 64 ± 39 | 56 ± 31 | 0.13 | 67 ± 38 | 55 ± 33 | 0.03 | 60 ± 35 |
| Alkaline phosphatase - U/L | 82 ± 28 | 81 ± 25 | 0.90 | 87 ± 26 | 78 ± 26 | 0.02 | 81 ± 27 |
| γ-Glutamyltransferase - U/L | 76 ± 82 | 67 ± 53 | 0.36 | 81 ± 66 | 65 ± 71 | 0.11 | 71 ± 69 |
| Total bilirubin - mg/dL | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.22 | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.49 | 0.6 ± 0.3 |
| Lipids | |||||||
| Cholesterol | — | ||||||
| Total - mg/dL | 190 ± 43 | 190 ± 48 | 0.99 | 191 ± 47 | 190 ± 46 | 0.89 | 190 ± 46 |
| High-density lipoprotein - mg/dL | 43 ± 11 | 45 ± 14 | 0.23 | 41 ± 9 | 45 ± 14 | 0.03 | 43 ± 12 |
| Low-density lipoprotein - mg/dL | 111 ± 38 | 114 ± 41 | 0.63 | 114 ± 41 | 112 ± 39 | 0.80 | 112 ± 40 |
| Triglycerides - mg/dL | 188 ± 108 | 182 ± 177 | 0.79 | 181 ± 92 | 187 ± 171 | 0.73 | 185 ± 146 |
| Metabolic factors | |||||||
| Weight – kg | 100 ± 22 | 95 ± 18 | 0.07 | 99 ± 20 | 97 ± 20 | 0.53 | 97 ± 20 |
| Body mass index - kg/m2 | 35 ± 6 | 34 ± 6 | 0.09 | 35 ± 7 | 34 ± 6 | 0.46 | 34 ± 6 |
| Body mass index classificationf | — | — | 0.31 | — | — | 0.31 | — |
| Underweight | 0 (0%) | 0 (0%) | — | 0 (0%) | 0 (0%) | — | 0 (0%) |
| Normal | 2 (2%) | 2 (2%) | — | 1 (1%) | 3 (2%) | — | 4 (2%) |
| Overweight | 17 (17%) | 25 (26%) | — | 12 (16%) | 30 (24%) | — | 42 (21%) |
| Obese | 82 (81%) | 71 (72%) | — | 63 (83%) | 90 (73%) | — | 153 (77%) |
| Waist circumference – cm | 113 ± 15 | 108 ± 15 | 0.02 | 112 ± 16 | 110 ± 15 | 0.21 | 111 ± 15 |
| Waist to hip ratio | 0.96 ± 0.08 | 0.94 ± 0.08 | 0.19 | 0.96 ± 0.08 | 0.94 ± 0.07 | 0.11 | 0.95 ± 0.08 |
| Fasting serum glucose - mg/dL | 115 ± 33 | 116 ± 43 | 0.97 | 115 ± 31 | 115 ± 42 | 0.98 | 115 ± 38 |
| Insulin - umol/mL | 35 ± 40 | 21 ± 14 | 0.001 | 33 ± 42 | 25 ± 20 | 0.10 | 28 ± 31 |
| HOMA-IRe - mg/dL × umol/mL/405 | 10.4 ± 12.9 | 6.4 ± 6.8 | 0.006 | 9.9 ± 13.92 | 7.5 ± 7.7 | 0.17 | 8.4 ± 10.5 |
| Haemoglobin A1c - % | 6.5 ± 1.1 | 6.4 ± 1.0 | 0.52 | 6.6 ± 1.0 | 6.4 ± 1.1 | 0.09 | 6.5 ± 1.1 |
| Systolic blood pressure - mm Hg | 133 ± 15 | 132 ± 16 | 0.67 | 132 ± 15 | 133 ± 16 | 0.78 | 133 ± 16 |
| Diastolic blood pressure - mm Hg | 76 ± 10 | 78 ± 11 | 0.25 | 77 ± 11 | 77 ± 10 | 0.91 | 77 ± 10 |
| Comorbidities | |||||||
| Hyperlipidaemiaa | 63 (62%) | 62 (63%) | 0.88 | 49 (64%) | 76 (61%) | 0.76 | 125 (62%) |
| Hypertension | 66 (65%) | 57 (58%) | 0.38 | 49 (64%) | 74 (60%) | 0.55 | 123 (62%) |
| Cardiovascular disease | 5 (5%) | 6 (6%) | 0.76 | 6 (8%) | 5 (4%) | 0.34 | 11 (6%) |
| Diabetes | 54 (53%) | 53 (54%) | 0.89 | 47 (62%) | 60 (48%) | 0.08 | 107 (54%) |
| Concomitant medications in the past 6 months | |||||||
| Anti-lipidaemic | 53 (52%) | 43 (44%) | 0.26 | 38 (50%) | 58 (47%) | 0.66 | 96 (48%) |
| Cardiovascular/hypertensive | 70 (69%) | 61 (62%) | 0.37 | 49 (64%) | 82 (66%) | 0.88 | 131 (66%) |
| Anti-diabetic | 50 (49%) | 53 (54%) | 0.48 | 40 (53%) | 63 (51%) | 0.88 | 103 (52%) |
| Metformin | 41 (40%) | 45 (46%) | 0.48 | 35 (46%) | 51 (41%) | 0.56 | 86 (43%) |
| Pioglitazone | 1 (1%) | 5 (5%) | 0.11 | 0 (0%) | 6 (5%) | 0.08 | 6 (3%) |
| Vitamin E | 20 (20%) | 25 (26%) | 0.40 | 9 (12%) | 36 (29%) | 0.005 | 45 (22%) |
| Thiazolidinedione | 2 (2%) | 4 (4%) | 0.44 | 0 (0%) | 6 (5%) | 0.08 | 6 (3%) |
| Aspirin (81 mg) | 29 (28%) | 25 (26%) | 0.75 | 25 (33%) | 29 (23%) | 0.19 | 54 (27%) |
| Liver histology findings | |||||||
| Definite steatohepatitis | 84 (82%) | 78 (80%) | 0.72 | 66 (87%) | 96 (77%) | 0.14 | 162 (81%) |
| Fibrosis - stageb | 1.9 ± 1.0 | 1.8 ± 1.1 | 0.70 | 2.0 ± 1.1 | 1.7 ± 1.0 | 0.04 | 1.8 ± 1.0 |
| NAFLD Activity Scorec | 5.4 ± 1.2 | 5.3 ± 1.3 | 0.43 | 5.4 ± 1.3 | 5.3 ± 1.3 | 0.44 | 5.3 ± 1.3 |
| Hepatocellular ballooning -score | 1.4 ± 0.7 | 1.2 ± 0.7 | 0.32 | 1.5 ± 0.7 | 1.3 ± 0.7 | 0.14 | 1.4 ± 0.7 |
| Steatosis - score | 2.1 ± 0.8 | 2.1 ± 0.8 | 0.89 | 2.1 ± 0.8 | 2.1 ± 0.8 | 0.84 | 2.1 ± 0.8 |
| Lobular inflammation – score | 1.8 ± 0.7 | 1.8 ± 0.7 | 0.79 | 1.8 ± 0.7 | 1.8 ± 0.7 | 0.89 | 1.8 ± 0.7 |
| Portal inflammation - scored | 1.1 ± 0.6 | 1.1 ± 0.6 | 0.43 | 1.1 ± 0.5 | 1.1 ± 0.6 | 0.95 | 1.1 ± 0.6 |
Plus-minus values are means ± SD.
High cholesterol or high triglycerides.
Fibrosis was assessed on a scale of 0 to 4, with higher scores indicating more severe fibrosis.
The Nonalcoholic fatty liver disease activity score (NAS) was assessed on a scale of 0 to 8, with higher scores indicating more severe disease; the components of this measure are steatosis (assessed on a scale of 0 to 3), lobular inflammation (assessed on a scale of 0 to 3), and hepatocellular ballooning (assessed on a scale of 0 to 2).
Portal inflammation was assessed on a scale of 0 to 2 with higher scores indicating more severe inflammation.
Homeostasis model assessment-estimated insulin resistance.
World health organization body mass index classification.
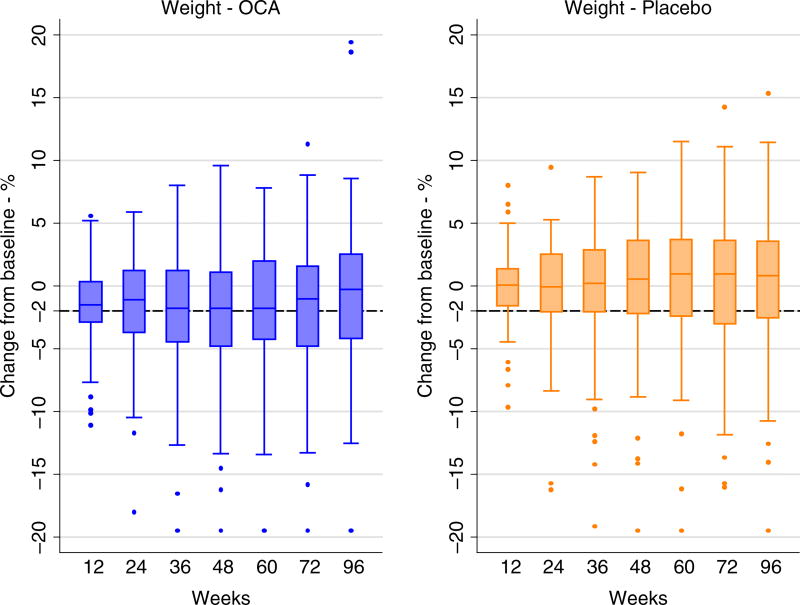
During the 72 weeks of therapy, patients on OCA lost an average of 2.3 kg compared to 0.0 kg in the placebo group. The amount of weight change varied among individuals in both groups and was ≥2% loss in 44% of the OCA compared to 32% in the placebo group (P = 0.08). The distribution of percent change in weight at the 12-week intervals during the course of the FLINT trial in the OCA and placebo groups are shown in Figure 1. As compared to patients on Placebo, a higher proportion of patients on OCA had ≥2% weight loss starting at week 12 and continuing throughout follow-up and during the 24 weeks after stopping treatment.
FIGURE 1.
Weight change in the OCA and Placebo treated patients over the course of the treatment period (0–72 wk) and off treatment follow-up (to 96 wk)
Table 2 shows the joint effects of OCA and weight loss on 72-week changes from baseline in liver tests, liver histology, lipids, metabolic factors and adverse effects.
TABLE 2.
72-wk change from baseline in liver tests, histology and metabolic factors by weight loss within treatment
| 72-wk change from baseline |
OCA group | Placebo group | OCAa Weight loss interaction effect P valued |
||||
|---|---|---|---|---|---|---|---|
| Adjusteda Mean/% | Adjusteda Mean/% | ||||||
| ≥2% Weight loss (N = 45) |
<2% Weight loss (N = 57) |
Weight loss effect in OCA P valueb |
≥2% Weight loss (N = 31) |
<2% Weight loss (N = 67) |
Weight loss effect in Placebo P valuec |
||
| Liver tests | |||||||
| ALT - U/L | −43 | −34 | 0.12 | −29 | −10 | 0.02 | 0.58 |
| AST - U/L | −29 | −23 | 0.15 | −14 | −5 | 0.14 | 0.98 |
| GGT - U/L | −38 | −34 | 0.54 | −26 | 6 | 0.004 | 0.32 |
| Alkaline phosphatase - U/L | 21 | 6 | 0.003 | −12 | −5 | 0.08 | <0.001 |
| Total bilirubin - mg/dL | −0.06 | −0.04 | 0.66 | 0.01 | 0.02 | 0.79 | 0.87 |
| Histology | |||||||
| Histological improvemente - % | 64% | 37% | 0.006 | 32% | 19% | 0.17 | 0.48 |
| Complete resolutionf - % | 22% | 21% | 0.89 | 13% | 13% | 0.94 | 0.89 |
| Complete/partial resolutionf - % | 49% | 37% | 0.22 | 29% | 27% | 0.82 | 0.54 |
| Fibrosis - stage | −0.3 | −0.1 | 0.20 | 0.2 | 0.1 | 0.51 | 0.16 |
| NAFLD activity score | −2.4 | −1.2 | <0.001 | −1.2 | −0.5 | 0.03 | 0.29 |
| Steatosis - score | −1.1 | −0.5 | <0.001 | −0.8 | −0.2 | <0.001 | 0.75 |
| Ballooning - score | −0.6 | −0.3 | 0.07 | −0.2 | −0.1 | 0.49 | 0.51 |
| Lobular inflammation – score | −0.7 | −0.3 | <0.001 | −0.3 | −0.1 | 0.23 | 0.18 |
| Portal inflammation – score | 0.2 | 0.2 | 0.91 | 0.2 | 0.1 | 0.70 | 0.83 |
| Lipids | |||||||
| Total cholesterol - mg/dL | 13 | 2 | 0.12 | −14 | 0 | 0.06 | 0.02 |
| HDL - mg/dL | −1.5 | −0.6 | 0.52 | 3.2 | 0.7 | 0.09 | 0.20 |
| LDL - mg/dL | 18 | 4 | 0.04 | −12 | −3 | 0.14 | 0.01 |
| Triglycerides - mg/dL | −13 | −11 | 0.91 | −25 | 6 | 0.34 | 0.40 |
| Metabolic factors | |||||||
| Weight – kg | −7.1 | 1.2 | <0.001 | −6.3 | 2.8 | <0.001 | 0.90 |
| Body mass index - kg/m2 | −2.2 | 0.7 | <0.001 | −2.0 | 1.0 | <0.001 | 0.85 |
| Waist circumference – cm | −3.5 | 0.2 | 0.006 | −7.0 | 1.9 | <0.001 | 0.01 |
| Waist/hip ratio | 0.002 | −0.001 | 0.77 | 0.006 | −0.014 | 0.06 | 0.22 |
| Haemoglobin A1c - % | 0.1 | 0.1 | 0.67 | −0.4 | 0.2 | <0.001 | 0.01 |
| Systolic blood pressure - mm Hg | −5.6 | −4.0 | 0.57 | −2.3 | −0.9 | 0.66 | 0.98 |
| Diastolic blood pressure - mm Hg | 2.6 | −2.0 | 0.01 | −0.7 | 0.2 | 0.63 | 0.04 |
| Metabolic factors (subjects without diabetes, N = 93) | |||||||
| N | 17 | 31 | — | 12 | 33 | — | — |
| Fasting serum glucose - mg/dL | 8.2 | 3.2 | 0.17 | −13.7 | 3.7 | <0.001 | 0.02 |
| Insulin - umol/mL | 8.6 | 0.5 | 0.11 | −5.4 | 4.4 | 0.002 | 0.73 |
| HOMA-IR - mg/dL × umol/mL/405 | 0.3 | 2.6 | 0.08 | −1.8 | 1.3 | 0.002 | 0.57 |
| Adverse events | |||||||
| Pruritus - % | 27% | 19% | 0.38 | 0% | 7% | NC | NC |
| Nausea/Vomiting/Diarrhoea - % | 11% | 12% | 0.86 | 3% | 12% | 0.20 | 0.30 |
Adjusted for baseline value of outcome.
P-value from test of difference in outcome between ≥2% weight loss vs <2% weight loss in OCA group adjusted for baseline value of outcome.
P-value from test of difference in outcome between ≥2% weight loss vs <2% weight loss in Placebo group adjusted for baseline value of outcome.
P-value from test of difference in outcome between ≥2% weight loss vs <2% weight loss in OCA group vs ≥2% weight loss vs <2% weight loss in Placebo group adjusted for baseline value of outcome.
Decrease in 2 or more points on the NAFLD activity score and no worsening of fibrosis.
Complete resolution defined as no NASH on 72-wk biopsy; Partial resolution defined as borderline or no NASH on 72-wk biopsy.
3.2 Weight change and liver enzymes
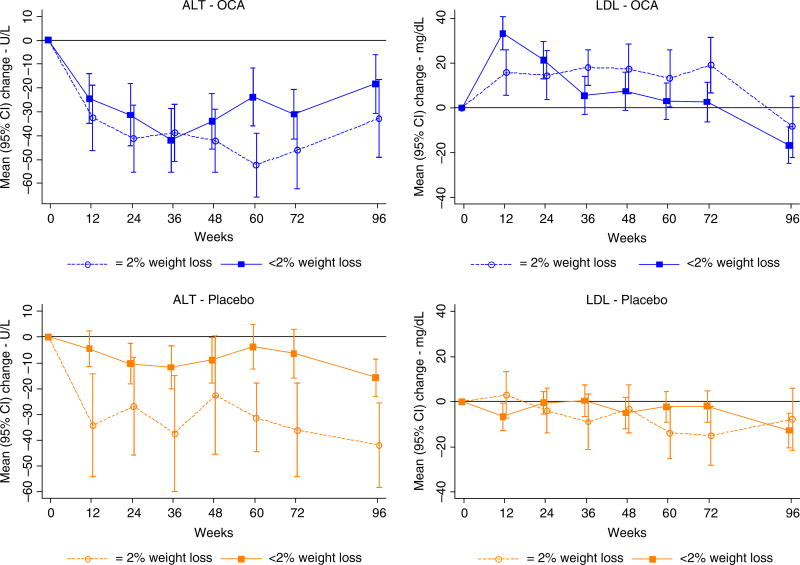
ALT levels decreased in patients receiving OCA and to a somewhat greater degree in those who lost weight (−43 U/L) than in those who did not (−34 U/L) (P = 0.12) (Table 2). Similarly, among patients receiving placebo, decreases in ALT levels were greater in patients with weight loss (−29 U/L) compared to those without (−10 U/L) (P = 0.02). Upon stopping treatment, ALT levels rose in the OCA treated patients but not in those who had received placebo (data not shown). Improvements in AST followed a similar pattern, being greater in patients receiving OCA, but also being more marked in those with weight loss in both groups (Table 2) and upon stopping treatment, AST levels rose in OCA-treated patients but not in those who received placebo (Figure 2). Thus, both OCA and weight loss appeared to improve serum aminotransferase levels, and the effects appeared to be additive.
FIGURE 2.
Change in ALT and LDL cholesterol by treatment (OCA and Placebo treated) and weight change (≥2% vs <2% weight loss) groups during treatment (0–72 wk) and off treatment follow-up (to 96 wk)
Alkaline phosphatase levels demonstrated a different and paradoxical pattern of change, in that they increased with OCA therapy and most markedly in those with weight loss (+21 U/L) compared to those without weight loss (+6 U/L) (Table 2). In contrast, alkaline phosphatase levels decreased in patients on placebo, the degree of change being greater in those who lost weight (−12 U/L) than those who did not (−5 U/L) (interaction P<0.001). The percentage of patients with levels above the upper limit of normal (defined as >115 U/L) at baseline for OCA and placebo were 10% and 9%, respectively, and at the end of treatment for OCA and placebo were 24% and 4%, respectively (data not shown; P<0.0001).
3.3 Weight change and liver histology
The primary outcome, a histological improvement of at least 2 points in the NAS score without worsening of fibrosis was achieved in 49% of OCA treated and 23% of placebo treated patients in this analysis (P<0.001) similar to what was shown in the full cohort.14 However, this outcome was more frequent in patients who lost vs did not lose weight (Table 2); in both the OCA (64% vs 37%, P = 0.006) and placebo-treated patients (32% vs 19%, P = 0.17). With or without weight loss, the proportion achieving this outcome was greater in the OCA than the placebo treated patients. These relationships were also reflected in the total NAS which changed more in the patients who lost vs did not lose weight, both in the OCA treated (−2.4 vs −1.2 points, P<0.001) and the placebo group (−1.2 vs −0.5 points, P = 0.03), with change being greater in the OCA treated patients. Analysis by individual components of the NAS showed a similar pattern of change, being greater among those who lost weight in both treatment groups, but also overall being greater in the OCA group. Changes in fibrosis stage were greater in the OCA treated patients and the greatest change occurred in patients on OCA who lost weight (−0.3 vs −0.1 stages, P = 0.20). Collectively, these results suggested that both OCA treatment and weight loss significantly improved activity scores in NASH and that the effects were additive.
3.4 Weight change and serum glucose and insulin change in nondiabetics
Since evaluation of serum glucose, insulin and HOMA-IR in patients with diabetes is confounded with anti-diabetic treatment, we excluded people with diabetes (n = 107) in the analysis of these factors. In the placebo group, fasting serum glucose levels decreased among those who lost weight, but not in those who did not (−13.7 vs 3.7 mg/dL; P<0.001) (Table 2). A similar pattern was found in the placebo group for insulin levels (−5.4 vs 4.4 umol/mL; P = 0.002) and HOMA-IR (−1.8 vs 1.3 mg/dL × umol/mL/405; P = 0.002) decreasing with weight loss but not without. A similar weight loss effect was seen in the OCA patients; but as compared to those on placebo, OCA patients had significantly higher net effects for changes in fasting serum glucose (+6.8 mg/dL; P = 0.006) (Table S1), insulin (+6.6 umol/mL; P = 0.02) and HOMA-IR (+1.9; P = 0.01). There were no interaction effects; thus indicating additive positive effects of weight loss but negative effects of OCA on changes in serum glucose, insulin and HOMA-IR.
3.5 Weight change and other metabolic effects
Changes in other metabolic factors during therapy often were discrepant between the groups in a pattern that resemble the paradoxical changes in alkaline phosphatase (Table 2). As would be expected, total serum cholesterol levels decreased significantly in the placebo patients who lost weight (−14 mg/dL) compared to those who did not (0 mg/dL). In contrast, total serum cholesterol levels increased significantly in patients taking OCA who lost weight (+13 mg/dL) but less so in patients on therapy in those who did not lose weight (+2 mg/dL) (interaction P = 0.02). LDL cholesterol levels followed a similar paradoxical pattern, with LDL cholesterol increasing (+18 mg/dL) in OCA patients who lost weight, while decreasing in the placebo patients who lost weight (−12 mg/dL) with mean values changing little or not at all in those who did not lose weight in both OCA and placebo groups (interaction P = 0.01). Serial mean serum LDL cholesterol levels in the patients who did and did not lose weight are displayed in Figure 2 separately for the OCA and placebo groups. The LDL cholesterol levels rose within the first 12 weeks of treatment with OCA and then decreased back to baseline in those who did not lose weight, but remained elevated in those on OCA who lost weight, these effects resolving within 24 weeks of stopping treatment. Waist circumference decreased in both OCA (P = 0.006) and placebo groups (P<0.001) among patients who lost weight vs those with no weight loss whereas the waist to hip ratio did not change in the OCA group (P = 0.77) but did in the placebo group (P = 0.06). In placebo patients, mean haemoglobin A1c values decreased by −0.4% in those who lost weight vs increased by +0.2% in those who did not lose weight (Figure 2). In contrast, in OCA patients, the mean haemoglobin A1c levels did not change regardless of weight loss (+0.1% in both groups) (interaction P = 0.01).
Statin therapy for hypercholesterolaemia was permitted and indeed encouraged if present, although the specific therapy was left to the discretion of the primary physician and was not dictated by a FLINT trial protocol. The possible role of statin therapy on changes in LDL cholesterol was assessed by comparing changes in LDL cholesterol levels in the two treatment groups by weight loss stratum and whether statins were used, added or withdrawn during the study (Table 3). In all categories of statin use, LDL cholesterol levels increased more in OCA treated patients who lost weight than in those who did not whereas levels decreased more in placebo recipients who lost weight in comparison to those who did not. This paradoxical effect of change in LDL cholesterol by treatment group and weight loss did not vary by statin use (P = 0.66).
TABLE 3.
Change in LDLa at 72 wk by treatment group, weight loss category at 72 wk and change in statin use at 72 wk
| OCA | Placebo | |||
|---|---|---|---|---|
| 72-wk change in LDL – mg/dL | 72-wk change in LDL – mg/dL | |||
| ≥2% weight loss | <2% weight loss or Gain | ≥2% weight loss | <2% weight loss or gain | |
| Total | ||||
| N | 42 | 53 | 31 | 63 |
| Mean change | 19 | 3 | −15 | −2 |
| No statin use at BL or wk 72 | ||||
| N | 14 | 21 | 12 | 26 |
| Mean change | 14 | 7 | −17 | +2 |
| Statin use at both BL and wk 72 | ||||
| N | 18 | 22 | 11 | 27 |
| Mean change | 27 | 3 | −20 | +2 |
| No statin use at BL; Statin use at wk 72 | ||||
| N | 6 | 8 | 4 | 8 |
| Mean change | −18 | −16 | −57 | −30 |
| Statin use at BL; No statin use at wk 72 | ||||
| N | 4 | 2 | 1 | 2 |
| Mean change | 54 | 32 | −8 | +28 |
P-value = 0.66 from linear regression of change in LDL on interaction of treatment group and weight loss group with statin use group adjusted for baseline LDL.
7 OCA and 4 Placebo patients had missing values for calculated LDL due to high triglycerides.
3.6 Adverse events
Finally, side effects that were more frequent in the OCA than placebo-treated patients (pruritus and gastrointestinal symptoms) but did not differ by weight loss category (Table 2).
4 DISCUSSION
This post hoc analysis showed that the OCA and weight loss had additive effect in improvements of histology and serum aminotransferase levels. The group achieving the most impressive improvements in both histology and reduction in ALT and AST was the OCA-treated cohort with weight loss. Of note, results were similar when weight change was categorised into three groups, with an additional group for 2+ kg weight gain (data not shown). Indeed, the primary outcome of histological improvement without fibrosis progression occurred in 64% of patients on OCA who lost 2% or more during the 72 weeks of treatment compared to 37% of those on OCA who did not lose weight; 32% of placebo recipients who lost weight; and 19% of placebo patients who did not lose weight).
Changes in fibrosis stage were greater in the obeticholic acid treated patients with greatest change in patients who also lost weight suggesting effects of both obeticholic acid treatment and weight loss were additive. These results highlight the important contribution of weight loss to improvements in histology and the importance of having untreated control patients in clinical trials of new therapeutics. More importantly, this study uniquely highlights the potential for additive effects between lifestyle measures and novel therapies, such as obeticholic acid.
Equally striking were the paradoxical and antagonistic effects of OCA and weight loss on lipids and other metabolic factors. Thus, the benefits of weight loss on improving cholesterol levels and haemoglobin A1c seen in placebo patients with weight loss were not seen in patients on OCA with weight loss. This was unexpected. One clue might be the changes in waist circumference and waist/hip ratios seen in the two groups. The decrease in waist/hip ratio that occurred in placebo recipients, who lost weight, was not seen in the OCA recipients with weight loss (interaction P = 0.22), suggesting that weight loss on OCA is Predominantly peripheral fat whereas weight loss occurring in the absence of OCA is largely from the central compartment which is more metabolically active. This hypothesis, however, is based on indirect information and future studies incorporating assessment of changes in fat distribution during OCA therapy should be considered.
Weight loss with OCA therapy has not been reported in treatment studies of patients with primary biliary cholangitis.19 The mechanism underlying this weight loss in patients with NASH is unknown but FXR activation in the intestine may play a role. Intestinally expressed FXR regulates FGF15/19 whose functions include the regulation of lipid and glucose metabolism as well as metabolic rate and satiety hormone levels.8 Differential expression of FXR activation in the intestines of patients with NASH vs other liver diseases may account for absence of weight loss of primary biliary cholangitis patients.19 Again, this is quite speculative and must await more detailed mechanistic studies to elucidate the association between OCA use and weight loss.
It is well established that FXR activation causes reduction in bile acid synthesis by inhibiting the conversion of cholesterol to bile acids which is a major mechanism of cholesterol disposal. Blocking the conversion of cholesterol to bile acids potentially could increase serum cholesterol concentrations, which might explain the changes in serum cholesterol concentrations seen with OCA treatment. Moreover, the FXR agonists may also promote reverse cholesterol transport out of tissues.20,21 Striking in our analysis was the interactive but negative effects of weight loss and OCA on cholesterol levels, with the weight loss group experiencing the greater increase in cholesterol on treatment.
The limitations of this analysis need to be considered. First, the study had a modest sample size, particularly when analysing subgroups of the treatment cohorts and weight categories. In addition, changes in cholesterol, glucose and body weight may well have been due to other unmeasured factors active in this study. Perhaps most importantly, the administration and dose of statins were not standardised and only limited information on statin use was obtained during the trial. Thus, whether a statin was being taken was documented, but changes in dose or the duration of treatment were not. Nevertheless, the consistent pattern of discrepancy between the two treatment groups in response to weight loss was striking and calls for larger prospective studies with more accurate measures of lipids and blood glucose levels and longer duration of follow-up. These analyses also serve as a reminder that NASH is a systemic condition and that treatment of the liver disease cannot be done independent of monitoring and managing other components of the metabolic syndrome.22
In summary, OCA and weight loss have additive beneficial effects on liver enzymes (ALT, AST) and histological features of disease activity in NASH. However, other metabolic beneficial effects of weight loss such as reduction in LDL cholesterol and lowering of blood glucose levels are absent or somewhat reversed on OCA treatment. This is in contrast with the consistent and expected benefit of weight loss achieved in the placebo group with reduction in LDL cholesterol and lowering of blood glucose levels. These findings stress the importance of assessing concomitant metabolic effects and long-term safety of new therapies of NASH.
Supplementary Material
Acknowledgments
Declaration of personal interests: B. Hameed: Research support from Intercept, Gilead, Genfit, Conatus. Advisory: Gilead. N.Terrault: Research support from Gilead, Tobira. Consulting for Echosens. Ryan Gill: Nothing to disclose. N. Chalasani: Research Support from Intercept, Gilead and Galectin. Consulting and Personal fees from AbbVie, DS Biopharma, Lilly, Madrigal, Nusirt, Shire and Tobira. R. Loomba: Research support from Adheron, Galmed, Glaectin, Intercept, Immuron, Oceteta, Genfit, Gilead, GE, Inc, Daiichi, Tobira, Siemens, Promedior, Octeta, KineMed and Adheron. Consulting for Alnylam, Arrowhead, BMS, Boehringer Ingelheim, Celgene, Conatus, Deutrx, Enanta, Figrogen, Galmed, Isis, janseen, Nimbus, Metacrine, Rui Yi, Inc, Shire, Zafgen. Jay H. Hoofnagle and Mark L. Van Natta: Nothing to disclose.
Declaration of funding interests: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713). Additional support is received from the National Center for Advancing Translational Sciences (NCATS) (grants UL1TR000439, UL1TR000436, UL1TR000006, UL1TR000448, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000058). FLINT trial was conducted by the NASH CRN and supported in part by Collaborative Research and Development Agreement (CRADA) between NIDDK and Intercept Pharmaceuticals.
APPENDIX 1 MEMBERS OF THE NON-ALCOHOLIC STEATOHEPATITIS CLINICAL RESEARCH NETWORK
ADULT CLINICAL CENTRES
Cleveland Clinic Foundation, Cleveland, OH: Daniela Allende, MD; Srinivasan Dasarathy, MD; Arthur J. McCullough, MD; Revathi Penumatsa, MPH; Jaividhya Dasarathy, MD.
Columbia University, New York, NY: Joel E. Lavine, MD, PhD.
Duke University Medical Center, Durham, NC: Manal F. Abdelmalek, MD, MPH; Mustafa Bashir, MD; Stephanie Buie; Anna Mae Diehl, MD; Cynthia Guy, MD; Christopher Kigongo, MB, CHB; Mariko Kopping, MS, RD; David Malik; Dawn Piercy, MS, FNP.
Indiana University School of Medicine, Indianapolis, IN: Naga Chalasani, MD; Oscar W. Cummings, MD; Samer Gawrieh, MD; Linda Ragozzino, RN; Kumar Sandrasegaran, MD; Raj Vuppalanchi, MD.
Saint Louis University, St Louis, MO: Elizabeth M. Brunt, MD (2002–2008); Theresa Cattoor, RN; Danielle Carpenter, MD; Janet Freebersyser, RN; Debra King, RN (2004–2015); Jinping Lai, MD (2015–2016); Brent A. Neuschwander-Tetri, MD; Joan Siegner, RN (2004–2015); Susan Stewart, RN (2004–2015); Susan Torretta; Kristina Wriston, RN (2015).
Swedish Medical Center, Seattle, WA: Maria Cardona Gonzalez; Jodie Davila; Manan Jhaveri, MD; Kris V. Kowdley, MD; Nizar Mukhtar, MD; Erik Ness, MD; Michelle Poitevin; Brook Quist; Sherilynn Soo.
University of California San Diego, San Diego, CA: Brandon Ang; Cynthia Behling, MD, PhD; Archana Bhatt; Rohit Loomba, MD, MHSc; Michael S. Middleton, MD, PhD; Claude Sirlin, MD.
University of California San Francisco, San Francisco, CA: Maheen F. Akhter, BS; Nathan M. Bass, MD, PhD (2002–2011); Danielle Brandman, MD, MAS; Ryan Gill, MD, PhD; Bilal Hameed, MD; Jacqueline Maher, MD; Norah Terrault, MD, MPH; Ashley Ungermann, MS.
University of Washington Medical Center, Seattle, WA: Matthew Yeh, MD, PhD.
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN, BSN; Melissa J. Contos, MD; Sherri Kirwin; Velimir AC Luketic, MD; Puneet Puri, MD (2009–2017); Arun J. Sanyal, MD; Jolene Schlosser, RN, BSN; Mohammad S. Siddiqui, MD; Leslie Yost-Schomer, RN.
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD (2008–2015); Kathryn Fowler, MD (2012–2015).
RESOURCE CENTRES
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD.
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Edward C. Doo, MD; Sherry Hall, MS; Jay H. Hoofnagle, MD; Jessica J. Lee, MD, MMSc; Patricia R. Robuck, PhD, MPH (2002–2011); Averell H. Sherker, MD; Rebecca Torrance, RN, MS.
Data Coordinating Center, Johns Hopkins University, Bloomberg School of Public Health, Baltimore, MD: Patricia Belt, BS; Jeanne M. Clark, MD, MPH; John Dodge; Michele Donithan, MHS; Erin Hallinan, MHS; Milana Isaacson, BS; Mariana Lazo, MD, PhD, ScM; Jill Meinert; Laura Miriel, BS; Jacqueline Smith, AA; Michael Smith, BS; Alice Sternberg, ScM; James Tonascia, PhD; Mark L. Van Natta, MHS; Annette Wagoner; Laura A. Wilson, ScM; Goro Yamada, PhD, MHS, MHS, MMS; Katherine Yates, ScM.
Footnotes
AUTHORSHIP
Guarantor of the article: Bilal Hameed, MD.
Author contributions: Bilal Hameed, MD: A,E and G. Norah Terrault, MD: E and G. Ryan M. Gill, MD: G. Rohit Loomba, MD: G. Naga Chalasani, MD: G. Jay H. Hoofnagle, MD: E, G. Mark L. Van Natta: A and C, E, G. All authors approved the final version of the manuscript.
Additional Supporting Information will be found online in the supporting information tab for this article.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162:496–500.e1. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. e641–649; quiz e639–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L. American Association for the Study of Liver D, United States F, Drug. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392–1405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 8.Mencarelli A, Renga B, D’Amore C, et al. Dissociation of intestinal and hepatic activities of FXR and LXRalpha supports metabolic effects of terminal ileum interposition in rodents. Diabetes. 2013;62:3384–3393. doi: 10.2337/db13-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 10.Pellicciari R, Costantino G, Camaioni E, et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 11.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbeke L, Farre R, Trebicka J, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 13.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 14.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoofnagle JH, van Natta ML, Kleiner DE, et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:124–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Cleophas TJ, Zwinderman AH, van Ouwerkerg B, Sobh M. Cardiovascular drug trials: how to examine interaction, and why so. Neth Heart J. 2007;15:61–66. doi: 10.1007/BF03085956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender R, Lange S. Adjusting for multiple testing: when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 19.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 20.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hageman J, Herrema H, Groen AK, Kuipers F. A role of the bile salt receptor FXR in atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1519–1528. doi: 10.1161/ATVBAHA.109.197897. [DOI] [PubMed] [Google Scholar]
- 22.Oh MK, Winn J, Poordad F. Review article: diagnosis and treatment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:503–522. doi: 10.1111/j.1365-2036.2008.03752.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.