Abstract
Purpose of Review
Asthma is a chronic airway disease that affects more than 300 million people worldwide. Current treatment focuses on symptomatic relief by temporally dampening inflammation and relaxing the airway. Novel combative strategies against asthma and hopefully a cure are yet to be developed. The goal of this review is to summarize recent literature on neurotrophins (NTs) in experimental models and clinical settings of asthma research.
Recent Findings
We highlight studies of early phases of asthma that collectively reveal a profound impact of elevated NT levels following initial detrimental insults on long-term airway dysfunction.
Summary
We hope this review will foster insights into the complex interaction between NTs, nerves, immune cells, and airway structural cells during a critical time window of development and disease susceptibility. Future studies are required to better understand the role of NTs in asthma pathophysiology and to evaluate whether NTs and their receptors may serve as new drug targets.
Keywords: Neurotrophins, Asthma, Nerve growth factor, Neurotrophin 4, Brain-derived neurotrophic factor
Introduction
Asthma is a chronic inflammatory disease of the airway. The salient features of asthma include bronchial smooth muscle thickening, reversible airway hyperreactivity, aberrant matrix deposition, and mucus overproduction; all of which contribute to the blockade of airflow into the lung [1]. Asthma is strongly associated with detrimental exposures to allergen, respiratory virus, cigarette smoke, ozone, and pollutants in early life. A majority of asthmatics progress from wheezing in early childhood to chronic airway dysfunction [2]. Due to technical difficulties of studying the airway in young children, mechanisms that connect early life events to long-term abnormalities in the structure and function of the lung remain poorly understood. Further complexity of asthma is reflected by different inflammatory signatures among the patient population, which implies the heterogeneity of the underlying pathophysiology [1]. Collectively, these factors pose great challenges for clinical intervention of asthma. Current treatment strategies only aim to alleviate the symptoms rather than eradicating pathogenic changes and as such, have no beneficial effect on the progression of asthma. Notably, up to 55% of asthmatic patients still experience poorly controlled symptoms [3–5]. Additional setbacks arise from a considerable concern of the side effects in pediatric patients from long-term usage of the drugs for asthma [6]. Effective treatment of asthma is clearly an unmet medical need.
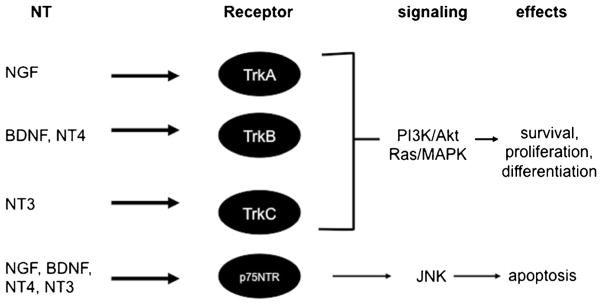
Neurotrophins (NTs) were initially discovered as essential trophic factors for the survival and axon outgrowth of neurons during the development of the central and peripheral nervous systems [7]. Since then, many studies have implicated nerves and NTs in the pathophysiology of asthma and other allergic diseases. The family of NTs consists of nerve growth factor (NGF), brain-derived neurotrophic factor (BNDF), neurotrophin 3 (NT3), and neurotrophin 4 (NT4). All four NTs are expressed as precursors that undergo N-terminal cleavage of pro-domains to generate the mature proteins. The pro-domain of NTs affects protein folding and the interaction with the receptor. NTs bind to their respective high-affinity tropomyosin receptor kinase (Trk) receptors with relatively high fidelity: TrkA for NGF, TrkB for BDNF and NT4, and TrkC for NT3 [8] (Fig. 1). In addition, all four NTs bind to a low-affinity p75 neurotrophin receptor (p75NTR) [9, 10]. Notably, Trks are tyrosine kinase receptors that trigger the activation of the Ras/MAPK and PI3K/Akt signaling pathways upon NT binding. Trks play essential roles in the survival, proliferation, and differentiation of the nervous system. In contrast, p75NTR belongs to a family of transmembrane molecules that include receptors for the tumor necrosis factor family. NT signaling through p75NTR induces cell apoptosis through the intracellular JNK pathway [8]. The biological roles of p75NTR remain elusive. As p75NTR can interact with the Trk receptors and modulate the high-affinity binding of NTs to the Trk receptors, the function of p75NTR may be context-dependent.
Fig. 1.
NTs and their receptors. NTs bind to respective high-affinity Trk receptors with relative specificity that triggers the activation of the PI3K/Akt and Ras/MAPK pathways and in turn, induces cell survival, proliferation, and differentiation. All four NTs also bind to a low-affinity p75NTR to induce apoptosis through the JNK pathway
Elevated levels of NTs have been found to be associated with asthma. Airway epithelium, smooth muscle cells, and immune cells are the likely source of elevated levels of NTs. Notably, in addition to Trk-expressing nerves, non-neuronal cell types in the lung have also been reported to express the Trk receptors. For example, TrkA was found in macrophages, basophils, mast cells, eosinophils, and memory B cells [11]. TrkB has also been reported in primary human airway smooth muscle cells in culture [12]. Therefore, NTs may contribute to asthma pathophysiology through two modes of action. The direct mode is mediated by NT signaling in Trk-expressing cells (Fig. 2). Alternatively, NTs indirectly modulate allergic inflammation and airway dysfunction through the role of NTs in airway innervation and nerve-derived neurotransmitters, such as acetylcholine. Related to the indirect role, the lung is innervated by a combination of sensory, sympathetic, and parasympathetic nerves that regulate coughing, breathing, airway smooth muscle tone, and mucus overproduction [13]. Most nerves that innervate the airway originate from neurons whose cell bodies are located outside the lung. NTs expressed by airway smooth muscle (ASM) and pulmonary neuroendocrine cells (PNECs) serve as essential target-derived trophic factors to orchestrate the process of innervation. In experimental models and clinical studies, increased NT expression and the genetic polymorphism of NTs may deregulate the two modes of action and contribute to the susceptibility and severity of asthma. Details regarding the source of NTs and the cell types that express the Trk receptors are provided by respective sections in the body of this review.
Fig. 2.
The expression of NTs and the Trk receptors in the lung. Airway epithelium, airway smooth muscle cells, and immune cells express NTs, including NGF, BDNF, and NT4. Nerves innervating the airway smooth muscle and pulmonary neuroendocrine cells (PNECs) express p75NTR and TrkA/B receptors. In addition, a variety of immune cells including macrophages, T cells, B cells, eosinophils, and mast cells express the TrkA receptor, which promote their survival, cytokine secretion, degranulation, and enhanced phagocytosis. TrkB expression is reported in primary human airway smooth muscle cells in culture
This review will summarize recent findings of NTs in asthma with a special emphasis on the role of NTs in airway development and early phases of the disease in children. We apologize for omitting many original papers on this topic due to word limits. For readers who are interested in NTs in a broad spectrum of allergic diseases, we recommend a very recent review by S. Manti et al. that provides an extensive summary of neuroimmune interactions [14].
Nerve Growth Factor (NGF) and Its Receptor TrkA
NGF was identified as a trophic factor necessary for survival and differentiation of developing sympathetic and sensory neurons [15]. However, whether NGF is required for airway innervation is unknown. Due to early postnatal lethality of mice deficient in NGF and its high-affinity receptor TrkA, functional studies of NGF and TrkA in allergic inflammation resort to in vitro culture models, a transgenic model that over-expresses NGF in the airway epithelium, and loss-of-function approaches, using neutralizing antibodies and siRNA against NGF in vivo.
TrkA and NGF are expressed in many cell types, thus potentiating their roles in regulating many aspects of asthma (Fig. 2). Multiple reports have shown TrkA expression in B cells, activated Tcells, macrophages, and granulocytes [11]. In addition, NGF expression was found in airway epithelium, B cells, T cells, eosinophils, and mast cells [16–21]. NGF treatment of eosinophils in culture triggered the activation of the TrkA receptor and promoted IL-4 secretion [20, 22]. NGF also promoted chemotaxis, mast cell degranulation, and enhanced neutrophil and macrophage phagocytosis [23, 24]. Furthermore, NGF promoted cell survival of multiple immune cells, such as eosinophils, mast cells, neutrophils, B cells, and monocytes, likely by inhibiting apoptosis through the regulation of anti-apoptotic proteins Bcl-2 and Bcl-xl [25, 26]. Interestingly, Hu and colleagues reported an age-dependent decline of NGF, P75NTR, and TrkA expression [27]. In their study, RSV infection upregulated the level of NGF to a greater extent in weanlings compared to adult rats.
Previous studies in rodent models provide evidence for the interaction between NGF and allergic inflammation. For example, exposure to sidestream tobacco smoke in early life caused an increase in NGF expression in concurrence with high levels of NGF in BALF [28]. Notably, this phenotype was only apparent in mice that were exposed to tobacco smoke as pups, while the same exposure had no effect on NGF levels in weanlings. Infection by respiratory syncytial virus (RSV) or allergen exposure also doubled the levels of NGF in lungs of rodent models [27]. In addition, NGF over-expression in airway epithelium driven by the CC10 promoter caused airway hyperinnervation by sensory nerves, increased bronchoconstriction to capsaicin stimulation, and worsened allergic inflammation following allergen exposure [29, 30]. In contrast, mice treated with a neutralizing NGF antibody during allergen exposure showed a reduction in the number of eosinophils, levels of type 2 cytokines, and airway bronchoconstriction [29]. Consistently, in vivo inhibition of NGF via siRNA administration diminished bronchoconstriction, airway innervation density, and immune cell infiltration following allergen exposure [31••]. The effect of NGF in these rodent models of asthma was at least in part mediated by neurotransmitters, such as serotonin and substance P [29, 30, 32–34].
Clinical studies have reported that increased levels of NGF are associated with asthma. Asthmatic patients have significantly elevated NGF levels in the serum compared to non-allergic controls [35]. In addition, children infected with RSV have elevated levels of NGF in cell fractions of the BALF compared to uninfected children [36]. More recently, genome wide association studies (GWAS) identified that NGF serum levels were affected by an epistatic interaction between variants of NGF rs6330 and TrkA rs6334 in asthmatics [37]. However, whether NGF levels have any impact on the age of onset, severity, and treatment outcomes of asthmatic patients remains to be investigated.
Brain Derived Neurotrophic Factor (BDNF) and the TrkB Receptor
BDNF is required for airway innervation during embryogenesis [38]. In mouse embryonic lung, BDNF is expressed by ASM progenitors as early as E12.5 and serves as a target-derived trophic factor for the innervating nerves that express TrkB. Mice deficient in BDNF had significant defects in branching axons and 50% reduction in ASM innervation density compared to WTcontrols [39]. BDNF expression has also been described in airway epithelium and immune cells, such as T cells, macrophages, and mast cells, while TrkB expression has been detected on CD45+ lymphocytes, mast cells, alveolar type II cells, and eosinophils [11, 40••, 41]. Notably, while others showed TrkB receptor in cultured human ASM cells [12], TrkB lineage tracing in mice found no evidence of TrkB expression in ASM cells. The discrepancy may be caused by differences between species or by culture conditions [40••]. In the mouse model of allergic inflammation, BDNF levels were elevated in BALF and in activated macrophages [11]. Unfortunately, BDNF−/− and TrkB−/− mice fail to thrive postnatally with severe defects in central and peripheral nervous systems. The early mortality of these mutant mice limits functional studies in vivo aiming to understand the role of BDNF and TrkB in allergic inflammation in the airway.
The BDNF gene has 9 different promoters and can produce 17 known splice variants and 3 protein isoforms: pre-BDNF, pro-BDNF, and mature BDNF [42, 43]. While mature BDNF can support cell survival through TrkB signaling, the physiological function of pro-BDNF remains to be identified [44, 45]. In addition to the complexity of BDNF transcripts and post-transcriptional modifications, in vitro studies have uncovered additional regulatory mechanisms of BDNF expression and function in ASM. ASM cells in culture express a variety of TRPC channels, which modulate calcium levels in response to airway inflammation. TNFα treatment in primary ASM cells enhanced BDNF release in a TRPC3-dependent mechanism [46]. In human ASM, BDNF secretion could be triggered through a hypoxic-cAMP-dependent mechanism [47]. Furthermore, treatment of human airway epithelial cells with IL-13 increased BDNF Vlb splice variant [48•]. These findings suggest that type 2 cytokines may regulate BDNF levels in asthma.
Patients with moderate and severe asthma had higher serum levels of BDNF compared to mild asthmatic children [37, 49]. In asthmatic adults provoked with allergen, BDNF levels were elevated significantly in the BALF [49]. In addition, Watanabe et al. correlated asthma severity to elevated BDNF levels, where severe asthmatics had higher levels of mature BDNF isoforms compared to healthy controls [48•]. Furthermore, a previous study identified that the Val66Met polymorphism in the BDNF gene was protective against asthma in children [50••]. The Val66Met BDNF variant causes reduced secretion of BDNF from neurons [51]. In contrast, another BDNF SNP (rs7124442) was found to be associated with disease severity in children measured by increased exhaled nitric oxide, a clinical marker of asthma [52].
Neurotrophin 3 (NT3) and the TrkC Receptor
There are few studies of NT3 in the lung. Mice deficient in NT3 have a 50% reduction in sympathetic innervation [53, 54]. However, whether NT3 plays a role in sympathetic innervation of the lung is unknown. In a mouse model of allergic asthma, NT3 treatment caused a switch of non-cholinergic innervation to cholinergic innervation [55]. Clinical reports show that NT3 levels were higher in BALF and serum from asthmatic patients compared to nonasthmatics [56].
Neurotrophin 4 (NT4) and the TrkB Receptor
NT4 binds to the same high-affinity TrkB receptor as BDNF. NT4 levels in mouse lungs peaked at postnatal day 14. In contrast, the BDNF levels were higher in the fetal lung than postnatal lungs [57••]. In addition, compared to BDNF−/− and TrkB−/− mice that die early postnatally, NT4−/− mice mature into adulthood, breed normally, and exhibit no obvious behavior defects. These findings indicate that BDNF and NT4 play distinct roles that may be explained at least in part by the difference in temporal expression. NT4 was expressed by ASM, PNECs, and mast cells in postnatal lungs of mouse, nonhuman primates, and humans [40••, 57••, 58••] (Fig. 3). NT4 is required for the innervation of the lung, as NT4−/−mice have significantly less nerve density in ASM and selective reduction of PNEC innervation by purinergic nerves [40••, 57••].
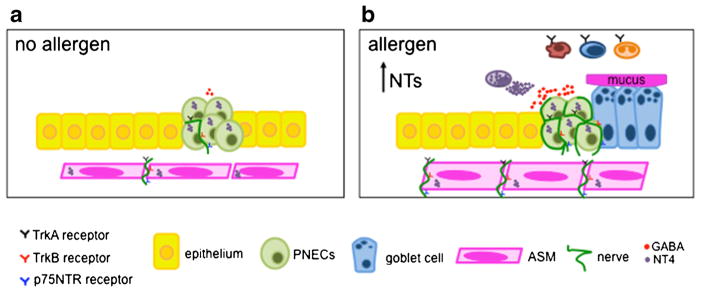
Fig. 3.
A model of deregulated NT function following early life allergen exposure. a During postnatal development, ASM and PNECs express NT4, while the innervating nerves express TrkB, thereby establishing a functional connection. b In a neonatal mouse model of allergic inflammation, the levels of NTs are increased. Under this condition, NTs may directly signal in the recruited Trk receptor-expressing immune cells to regulate the survival, cytokine secretion, degranulation, and enhanced phagocytosis. Specifically, NT4 produced by mast cells mediates airway hyperinnervation following early life allergen exposure and indirectly induces airway hyperreactivity and mucus overproduction through nerve-derived neurotransmitters
NT4 from mast cells has no effect on NT4-dependent innervation during normal postnatal development. However, early life allergen exposure increases the number of mast cells, triggers mast cell degranulation to release NT4, which thereby elevates the levels of NT4 to increase airway innervation [58••] (Fig. 3). Airway hyperinnervation following allergen exposure in mice was associated with persistent airway hyper-reactivity and mucus overproduction into adulthood without additional allergen challenges. In contrast, allergen exposure in adult mice had no effect on NT levels and elicited short-term airway dysfunction. These findings emphasize early life as a critical time window for the susceptibility to asthma. Notably, NT4−/− mice were protected from early life allergen-induced airway hyperinnervation, hypercontractility, and mucus overproduction, while having no change in airway inflammation [57••, 59]. Lineage tracing of TrkB in mice showed highly selective TrkB expression in nerves (Fig. 3). Therefore, elevated NT4 levels following early life allergen exposure likely through an indirect mode of action by causing airway dysfunction through aberrant neural innervation [58••]. Functional rescue assays in NT4−/− mice identified that the hypercontractile phenotype of ASM is caused by cholinergic hyperinnervation, while deregulated neural control of GABA secretion from PNECs is involved in mucus overproduction following early life allergen exposure [40••, 59]. Consistent with the findings in the rodent model of early life allergen exposure, children with asthma have elevated serum levels of NT4 [56].
P75NTR
There is a paucity of literature on the low-affinity NT receptor p75NTR. In the lung, p75NTR was expressed in large bundles of nerve fibers along the airway [60]. These nerve fibers are detected as early as embryonic day 11 and extend into the trachea and lung [61]. P75NTR expression has also been detected on human basophils and B cells [25, 62]. Characterization of p75NTR knockout mice has identified a reduction in sensory innervation but no change in sympathetic innervation in the lung [60]. Mice deficient in p75NTR were protected against capsaicin-induced AHR and ozone-induced neutrophilia, which might be attributed to reduced substance P levels from sensory nerves, compared to controls [60, 63]. Given that p75NTR can form heterodimers with Trk receptors, p75NTR may regulate inflammation via direct binding to NTs or indirectly by affecting Trk signaling [10, 64].
Conclusion
NTs contribute to asthma pathophysiology through direct and indirect interactions with immune cells, ASM cells, airway epithelium, and nerves (Fig. 2). While NTs and the Trk receptor have been shown to be expressed by a variety of immune cells, the expression and precise roles in airway physiology and diseases warrant further investigation.
The lung continues to grow after birth and during post-natal development; structural cells in the lung, nervous system, and the immune system undergo dramatic changes in configuration and functional maturation. Compared to adult, mature lungs, exposure to a variety of environmental insults preferentially affects NT levels and airway innervation in early life in rodent models. These observations indicate that NT-mediated mechanisms may be particularly relevant to the early phase of asthma in children. In a nonhuman primate model of asthma, exposure to ozone and house dust mite allergen in infancy causes hyperinnervation of airway epithelium [65]. Therefore, it is possible that elevated NT levels following detrimental exposures in the childhood may disrupt airway innervation and the communication with immune cells and airway epithelium, which in turn causes prolonged airway dysfunction and increased disease susceptibility.
To fully understand the role of NTs in normal and diseased lungs during the critical time window of airway development, genetic tools that enable temporally and tissue-specific inhibition of NT signaling in rodent models of asthma need to be developed. A transgenic mouse line in which the Trk signaling activity can be reversibly blocked by a small molecule, 1NMPP1, may be employed for asthma-related studies [66]. In addition, whether genetic polymorphisms and transcriptional and post-transcriptional regulation of NTs affect the function of NTs in airway development and asthma warrants future investigation. To bring mechanistic findings of NTs from animal models to the bedside, translational research of childhood asthma is crucial to establish disease relevance. Lastly, fundamental knowledge regarding the complex neural network in human lungs requires joint efforts from basic researchers and clinicians. We expect that these studies will provide insights into the role of NTs in the pathogenesis of asthma and lead to the discovery of novel treatment strategies and ultimately, a cure.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors. All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25. doi: 10.1038/nm.2678. https://doi.org/10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 2.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372(9643):1058–64. doi: 10.1016/S0140-6736(08)61447-6. https://doi.org/10.1016/s0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauthier M, Ray A, Wenzel SE. Evolving concepts of asthma. Am J Respir Crit Care Med. 2015;192(6):660–8. doi: 10.1164/rccm.201504-0763PP. https://doi.org/10.1164/rccm.201504-0763PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181(8):788–96. doi: 10.1164/rccm.200909-1448OC. https://doi.org/10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 5.Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–71. doi: 10.1164/rccm.200701-085OC. https://doi.org/10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 6.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115(2):233–42. doi: 10.1016/j.jaci.2004.11.014. https://doi.org/10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14(23):2919–37. doi: 10.1101/gad.841400. https://doi.org/10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 8.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. https://doi.org/10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 9.Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25(11):1386–403. doi: 10.1002/neu.480251107. https://doi.org/10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 10.Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 1999;18(3):616–22. doi: 10.1093/emboj/18.3.616. https://doi.org/10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun A, Lommatzsch M, Mannsfeldt A, Neuhaus-Steinmetz U, Fischer A, Schnoy N, et al. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am J Respir Cell Mol Biol. 1999;21(4):537–46. doi: 10.1165/ajrcmb.21.4.3670. https://doi.org/10.1165/ajrcmb.21.4.3670. [DOI] [PubMed] [Google Scholar]
- 12.Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L447–56. doi: 10.1152/ajplung.00501.2005. https://doi.org/10.1152/ajplung.00501.2005. [DOI] [PubMed] [Google Scholar]
- 13.Aven L, Ai X. Mechanisms of respiratory innervation during embryonic development. Organ. 2013;9(3):194–8. doi: 10.4161/org.24842. https://doi.org/10.4161/org.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manti S, Brown P, Perez MK, Piedimonte G. The role of neurotrophins in inflammation and allergy. Vitam Horm. 2017;104:313–41. doi: 10.1016/bs.vh.2016.10.010. https://doi.org/10.1016/bs.vh.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237(4819):1154–62. doi: 10.1126/science.3306916. https://doi.org/10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 16.Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci U S A. 1993;90(23):10984–8. doi: 10.1073/pnas.90.23.10984. https://doi.org/10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallbook F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27(9):2295–301. doi: 10.1002/eji.1830270925. https://doi.org/10.1002/eji.1830270925. [DOI] [PubMed] [Google Scholar]
- 18.Ehrhard PB, Ganter U, Stalder A, Bauer J, Otten U. Expression of functional trk protooncogene in human monocytes. Proc Natl Acad Sci U S A. 1993;90(12):5423–7. doi: 10.1073/pnas.90.12.5423. https://doi.org/10.1073/pnas.90.12.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noga O, Englmann C, Hanf G, Grutzkau A, Guhl S, Kunkel G. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin Exp Allergy. 2002;32(9):134854. doi: 10.1046/j.1365-2745.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- 20.Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol. 2006;117(4):787–94. doi: 10.1016/j.jaci.2005.12.1339. https://doi.org/10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa H, Azuma M, Uehara H, Takahashi T, Nishioka Y, Sone S, et al. Nerve growth factor derived from bronchial epithelium after chronic mite antigen exposure contributes to airway hyperresponsiveness by inducing hyperinnervation, and is inhibited by in vivo siRNA. Clin Exp Allergy. 2012;42(3):460–70. doi: 10.1111/j.1365-2222.2011.03918.x. https://doi.org/10.1111/j.1365-2222.2011.03918.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamada A, Watanabe N, Ohtomo H, Matsuda H. Nerve growth factor enhances survival and cytotoxic activity of human eosinophils. Br J Haematol. 1996;93(2):299–302. doi: 10.1046/j.1365-2141.1996.5151055.x. https://doi.org/10.1046/j.1365-2141.1996.5151055.x. [DOI] [PubMed] [Google Scholar]
- 23.Horigome K, Pryor JC, Bullock ED, Johnson EM., Jr Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J Biol Chem. 1993;268(20):14881–7. [PubMed] [Google Scholar]
- 24.Bischoff SC, Dahinden CA. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79(10):2662–9. [PubMed] [Google Scholar]
- 25.Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, et al. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85(3):345–56. doi: 10.1016/s0092-8674(00)81113-7. https://doi.org/10.1016/S0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto K, Okada T, Kannan Y, Ushio H, Matsumoto M, Matsuda H. Nerve growth factor prevents apoptosis of rat peritoneal mast cells through the trk proto-oncogene receptor. Blood. 1995;86(12):4638–44. [PubMed] [Google Scholar]
- 27.Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L494–502. doi: 10.1152/ajplung.00414.2001. https://doi.org/10.1152/ajplung.00414.2001. [DOI] [PubMed] [Google Scholar]
- 28.Wu ZX, Hunter DD, Batchelor TP, Dey RD. Side-stream tobacco smoke-induced airway hyperresponsiveness in early postnatal period is involved nerve growth factor. Respir Physiol Neurobiol. 2016;223:1–8. doi: 10.1016/j.resp.2015.11.009. https://doi.org/10.1016/j.resp.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Path G, Braun A, Meents N, Kerzel S, Quarcoo D, Raap U, et al. Augmentation of allergic early-phase reaction by nerve growth factor. Am J Respir Crit Care Med. 2002;166(6):818–26. doi: 10.1164/rccm.200202-134OC. https://doi.org/10.1164/rccm.200202-134OC. [DOI] [PubMed] [Google Scholar]
- 30.Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol. 1998;18(2):149–57. doi: 10.1165/ajrcmb.18.2.2803m. https://doi.org/10.1165/ajrcmb.18.2.2803m. [DOI] [PubMed] [Google Scholar]
- 31••.Chen YL, Huang HY, Lee CC, Chiang BL. Small interfering RNA targeting nerve growth factor alleviates allergic airway hyperresponsiveness. Mol Ther Nucleic Acids. 2014;3:e158. doi: 10.1038/mtna.2014.11. https://doi.org/10.1038/mtna.2014.11. siRNA targeting of NGF alleviated OVA induced eosinophilic inflammation and airway hyperresponsiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piedimonte G, Rodriguez MM, King KA, McLean S, Jiang X. Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs. Am J Phys. 1999;277(4 Pt 1):L831–40. doi: 10.1152/ajplung.1999.277.4.L831. [DOI] [PubMed] [Google Scholar]
- 33.Piedimonte G. Neural mechanisms of respiratory syncytial virus-induced inflammation and prevention of respiratory syncytial virus sequelae. Am J Respir Crit Care Med. 2001;163(3 Pt 2):S18–21. doi: 10.1164/ajrccm.163.supplement_1.2011113. https://doi.org/10.1164/ajrccm.163.supplement_1.2011113. [DOI] [PubMed] [Google Scholar]
- 34.Graham RM, Friedman M, Hoyle GW. Sensory nerves promote ozone-induced lung inflammation in mice. Am J Respir Crit Care Med. 2001;164(2):307–13. doi: 10.1164/ajrccm.164.2.2007115. https://doi.org/10.1164/ajrccm.164.2.2007115. [DOI] [PubMed] [Google Scholar]
- 35.Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini L, Manni L, et al. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci U S A. 1996;93(20):10955–60. doi: 10.1073/pnas.93.20.10955. https://doi.org/10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, et al. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172(2):233–7. doi: 10.1164/rccm.200412-1693OC. https://doi.org/10.1164/rccm.200412-1693OC. [DOI] [PubMed] [Google Scholar]
- 37.Szczepankiewicz A, Rachel M, Sobkowiak P, Kycler Z, Wojsyk-Banaszak I, Schoneich N, et al. Neurotrophin serum concentrations and polymorphisms of neurotrophins and their receptors in children with asthma. Respir Med. 2013;107(1):30–6. doi: 10.1016/j.rmed.2012.09.024. https://doi.org/10.1016/j.rmed.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Langsdorf A, Radzikinas K, Kroten A, Jain S, Ai X. Neural crest cell origin and signals for intrinsic neurogenesis in the mammalian respiratory tract. Am J Respir Cell Mol Biol. 2011;44(3):293–301. doi: 10.1165/rcmb.2009-0462OC. https://doi.org/10.1165/rcmb.2009-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, et al. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci. 2011;31(43):15407–15. doi: 10.1523/JNEUROSCI.2745-11.2011. https://doi.org/10.1523/jneurosci.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Barrios J, Patel KR, Aven L, Achey R, Minns MS, Lee Y, et al. Early life allergen-induced mucus overproduction requires augmented neural stimulation of pulmonary neuroendocrine cell secretion. FASEB J. 2017;31(9):4117–28. doi: 10.1096/fj.201700115R. https://doi.org/10.1096/fj.201700115R. First paper to show a nerve-derived mechanism regulates mucus overproduction in an early life mouse model of asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam SY, Tsai M, Yamaguchi M, Yano K, Butterfield JH, Galli SJ. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997;90(5):1807–20. [PubMed] [Google Scholar]
- 42.Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90(3):397–406. doi: 10.1016/j.ygeno.2007.05.004. https://doi.org/10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakash YS, Martin RJ. Brain-derived neurotrophic factor in the airways. Pharmacol Ther. 2014;143(1):74–86. doi: 10.1016/j.pharmthera.2014.02.006. https://doi.org/10.1016/j.pharmthera.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(5695):487–91. doi: 10.1126/science.1100135. https://doi.org/10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 45.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98(6):3555–60. doi: 10.1073/pnas.061020198. https://doi.org/10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vohra PK, Thompson MA, Sathish V, Kiel A, Jerde C, Pabelick CM, et al. TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Biochim Biophys Acta. 2013;1833(12):2953–60. doi: 10.1016/j.bbamcr.2013.07.019. https://doi.org/10.1016/j.bbamcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aravamudan B, Thompson MA, Pabelick CM, Prakash YS. Mechanisms of BDNF regulation in asthmatic airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2016;311(2):L270–9. doi: 10.1152/ajplung.00414.2015. https://doi.org/10.1152/ajplung.00414.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Watanabe T, Fajt ML, Trudeau JB, Voraphani N, Hu H, Zhou X, et al. Brain-derived neurotrophic factor expression in asthma. Association with severity and type 2 inflammatory processes. Am J Respir Cell Mol Biol. 2015;53(6):844–52. doi: 10.1165/rcmb.2015-0015OC. https://doi.org/10.1165/rcmb.2015-0015OC. BDNF protein is increased in sputum and epithelial cells in patients with airway responsiveness and asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virchow JC, Julius P, Lommatzsch M, Luttmann W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med. 1998;158(6):2002–5. doi: 10.1164/ajrccm.158.6.9803023. https://doi.org/10.1164/ajrccm.158.6.9803023. [DOI] [PubMed] [Google Scholar]
- 50••.Jesenak M, Babusikova E, Evinova A, Banovcin P, Dobrota D. Val66Met polymorphism in the BDNF gene in children with bronchial asthma. Pediatr Pulmonol. 2015;50(7):631–7. doi: 10.1002/ppul.23065. https://doi.org/10.1002/ppul.23065. First paper showing a BDNF polymorphism in asthmatic children that is protective. [DOI] [PubMed] [Google Scholar]
- 51.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. https://doi.org/10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 52.Szczepankiewicz A, Sobkowiak P, Rachel M, Breborowicz A, Schoneich N, Bruce K, et al. Multilocus analysis of candidate genes involved in neurogenic inflammation in pediatric asthma and related phenotypes: a case-control study. J Asthma: Off J Assoc Care Asthma. 2012;49(4):329–35. doi: 10.3109/02770903.2012.669442. https://doi.org/10.3109/02770903.2012.669442. [DOI] [PubMed] [Google Scholar]
- 53.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368(6467):147–50. doi: 10.1038/368147a0. https://doi.org/10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 54.Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77(4):503–12. doi: 10.1016/0092-8674(94)90213-5. https://doi.org/10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 55.Pan J, Rhode HK, Undem BJ, Myers AC. Neurotransmitters in airway parasympathetic neurons altered by neurotrophin-3 and repeated allergen challenge. Am J Respir Cell Mol Biol. 2010;43(4):452–7. doi: 10.1165/rcmb.2009-0130OC. https://doi.org/10.1165/rcmb.2009-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szczepankiewicz A, Rachel M, Sobkowiak P, Kycler Z, Wojsyk-Banaszak I, Schoneich N, et al. Serum neurotrophin-3 and neurotrophin-4 levels are associated with asthma severity in children. Eur Respir J. 2012;39(4):1035–7. doi: 10.1183/09031936.00136611. https://doi.org/10.1183/09031936.00136611. [DOI] [PubMed] [Google Scholar]
- 57••.Aven L, Paez-Cortez J, Achey R, Krishnan R, Ram-Mohan S, Cruikshank WW, et al. An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyper-reactivity. FASEB J. 2014;28(2):897–907. doi: 10.1096/fj.13-238212. https://doi.org/10.1096/fj.13-238212. First paper to show early life allergen exposure elevated levels of NT4/TrkB signaling to cause persistent airway hyperreactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Patel KR, Aven L, Shao F, Krishnamoorthy N, Duvall MG, Levy BD, et al. Mast cell-derived neurotrophin 4 mediates allergen-induced airway hyperinnervation in early life. Mucosal Immunol. 2016;9(6):1466–76. doi: 10.1038/mi.2016.11. https://doi.org/10.1038/mi.2016.11. First paper to show that elevated levels of NT4 come from recruited mast cells under early life allergen exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel KR, Bai Y, Trieu KG, Barrios J, Ai X. Targeting acetylcholine receptor M3 prevents the progression of airway hyperreactivity in a mouse model of childhood asthma. FASEB J. 2017;31(10):4335–46. doi: 10.1096/fj.201700186R. https://doi.org/10.1096/fj.201700186R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerzel S, Path G, Nockher WA, Quarcoo D, Raap U, Groneberg DA, et al. Pan-neurotrophin receptor p75 contributes to neuronal hyperreactivity and airway inflammation in a murine model of experimental asthma. Am J Respir Cell Mol Biol. 2003;28(2):170–8. doi: 10.1165/rcmb.4811. https://doi.org/10.1165/rcmb.4811. [DOI] [PubMed] [Google Scholar]
- 61.Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn: Off publ Ame Assoc Anat. 2001;221(1):48–60. doi: 10.1002/dvdy.1124. https://doi.org/10.1002/dvdy.1124. [DOI] [PubMed] [Google Scholar]
- 62.Burgi B, Otten UH, Ochensberger B, Rihs S, Heese K, Ehrhard PB, et al. Basophil priming by neurotrophic factors. Activation through the trk receptor. J Immunol. 1996;157(12):5582–8. [PubMed] [Google Scholar]
- 63.Nassenstein C, Kammertoens T, Veres TZ, Uckert W, Spies E, Fuchs B, et al. Neuroimmune crosstalk in asthma: dual role of the neurotrophin receptor p75NTR. J Allergy Clin Immunol. 2007;120(5):1089–96. doi: 10.1016/j.jaci.2007.07.007. https://doi.org/10.1016/j.jaci.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Dobrowsky RT, Carter BD. Coupling of the p75 neurotrophin receptor to sphingolipid signaling. Ann N Y Acad Sci. 1998;845:32–45. doi: 10.1111/j.1749-6632.1998.tb09660.x. [DOI] [PubMed] [Google Scholar]
- 65.Larson SD, Schelegle ES, Walby WF, Gershwin LJ, Fanuccihi MV, Evans MJ, et al. Postnatal remodeling of the neural components of the epithelial-mesenchymal trophic unit in the proximal airways of infant rhesus monkeys exposed to ozone and allergen. Toxicol Appl Pharmacol. 2004;194(3):211–20. doi: 10.1016/j.taap.2003.09.025. https://doi.org/10.1016/j.taap.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46(1):13–21. doi: 10.1016/j.neuron.2005.03.009. https://doi.org/10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]