Abstract
Non-invasive imaging has played an increasing role in the process of cardiovascular drug development. This review focuses specifically on the use of molecular imaging which has been increasing applied to improve and accelerate certain pre-clinical steps in drug development including the identification of appropriate therapeutic targets, evaluation of on-target and off-target effects of candidate therapies, assessment of dose response, and the evaluation of drug or biologic biodistribution and pharmacodynamics. Unlike the case in cancer medicine, in cardiovascular medicine molecular imaging has not been used as a primary surrogate clinical endpoint for drug approval. However, molecular imaigng has been applied in early clinical trials, particularly in Phase 0 studies, to demonstrate “proof of concept” or to explain variation in treatment effect. Many of these applications where molecular imaging has been used in drug development have involved the “retasking” of technologies that were originally intended as clinical diagnostics. With greater experience and recognition of the rich information provided by in vivo molecular imaging, it is anticipated that it will increasingly be used to address the enormous time and costs associated with bringing a new drug to clinical launch.
Keywords: imaging, drug discovery, molecular imaging
Justification for Changes to the Drug Development Paradigm
Recent breakthroughs in applied molecular biology have potentiated the processes of drug discovery and stepwise drug development to the stage of clinical launch. In particular, drug discovery has been accelerated by the maturation of high-throughput technologies for rapid and automated candidate molecule screening, the pharmaceutical application of “omics” approaches to understand the basis of health and disease-related targets, and the use of “virtual screens” for understanding structure.1,2 Nonetheless, the healthcare industry is currently facing unprecedented obstacles to the development of truly innovative therapeutics. One problem that has become increasingly apparent through media channels is the erosion of trust in pharmaceutical companies based on real or perceived lapses in business ethics, scientific rigor, and scientific reporting. It is unlikely that the scientific community alone will solve these problems.
An equally important challenge is the business model on which drug discovery and both pre-clinical and clinical testing is based. For largely financial reasons, over the last two decades there has been a progressive decline in the proportion of drugs approved that are considered first-in-class, or what the pharmaceutical industry terms “new molecular entities” (NMEs).3–5 The majority of first-in-class therapeutics over the past decade has been in the category of cancer therapeutics or biologics, which have not impacted cardiovascular disease as much as cancer, inflammatory or rheumatologic disease.4,6
Non-invasive imaging has played an increasing role in the process of cardiovascular drug testing. Just a few tangible examples of biologic readouts that have been assessed by conventional imaging in pre-clinical models and humans include the assessment of left ventricular function, pulmonary artery pressure, myocardial ischemia, and arterial morphology (e.g. plaque size by intravascular ultrasound) which have each been used in preclinical and clinical studies to assess drug efficacy or to provide proof-of-mechanism information vital to drug approval. Newer molecular imaging techniques that are able to characterize tissue phenotype have been developed for essentially all forms of non-invasive in vivo imaging.7 Their development is based on the premise that they will improve patient outcomes and healthcare efficiency by providing: (i) earlier diagnosis, (ii) more definitive diagnosis, and (iii) information helpful for selecting the most appropriate therapy. The central theme of this article is focused on how molecular imaging has yet unrealized potential to increase the efficiency of bringing a drug to approval at several different stages of development of new drugs.
Scope of the Problem
Patterns and trends in the activities of the pharmaceutical industry reveal that the development of innovative first-in-class drugs is in decline. In 2016, the United States Food and Drug Administration (FDA) approved only 22 new drugs classified as NMEs or biologics that were approved through the Investigational New Drug (IND) or Biologic License Applications processes.4 Of note, a relatively large proportion (41%) of these new therapeutics were for rare or “orphan” disease applications. None were targeted to cardiovascular disease. Even when considering “same-class” drug approvals, which represent modifications of previously approved drugs, the majority do not necessarily provide any substantial improvement in care. A study examining drugs approved by the European Medicines Agency between 1999 and 2005 found that only around 10% of new drugs had some incremental clinical benefit over existing medications, other than cost or convenience.8
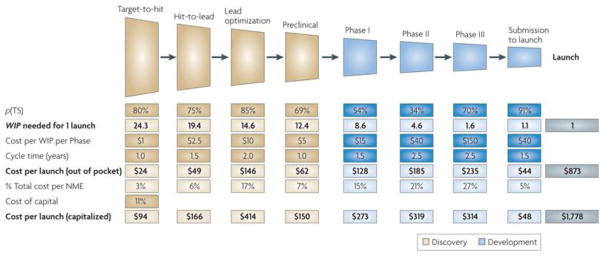
There have been many descriptive models that have been generated to explain the increasing hurdles for pharmaceutical research and development. Understandably, most of these models have been based on industry metrics that do not always take into account basic science efforts to uncover “druggable” processes, which is often performed by academia. One such model is illustrated in Figure 1, which highlights the benchmarks in the process of bringing a drug to market.3 Again, in this model the important and often costly initial process of target identification, which can occur in either the industry or non-industry setting, is not included. A notable feature of this model is that the out-of-pocket expenses from discovery-to-launch approach $0.9 billion for a new drug. Even more concerning is the cost to launch which is over $1.7 billion if one takes into account capitalized costs which were assumed to be 11% in the model but are variable based on industry infrastructure and the cycle time for each development stage. The capitalized cost figures for developing a first-in-class drug have been consistent between models and industry surveys. It is reasonable to assume that the development of NMEs may not be aligned with pharma business pressures to rapidly produce “hits” in order to meet near-term financial expectations of investors.
Figure 1.
Model illustrating research and development costs and time for a new molecular entity (NME) at the different phases of drug development from lead compound “hit” to drug launch. The model is based on both assumptions and data from industry benchmarks which include the probability of transition to the next stage (p[TS]), and the average number of works-in-process (WIP) at each stage needed to culminate in a single successful NME launch. Lighter shade boxes are values that are based on assumed inputs, including capital cost rates which are assumed at 11%. Reproduced with permission.3
From the model in Figure 1, it is clear that there are several opportunities for improving the efficiency of drug discovery and development based on the model descriptors. These include reductions in cycle time, reduced cost per phase, and increased probability of success for transitioning to the next stage, which is heavily influenced by the identification of impactful molecular targets and rapid evaluation of on-target and off-target effects. In an effort to address some of the hurdles, the FDA has implemented a variety of facilitated regulatory pathways such as the Fast Track, Breakthrough Therapy, and Accelerated Approval pathways which expedite the pre- and post-IND phases. These pathways have been estimated to shorten the time for approval by 40–50%.9 The majority of the new FDA drug approvals for 2016 benefited from fast tracking programs.
Molecular imaging has the potential to positively impact the efficiency of drug discovery and development. There are many steps involving both pre-clinical and clinical investigation where molecular imaging has already been used to accelerate new drug investigation (Figure 2). At the current time, application of molecular imaging has been most appreciated in the development process for cancer therapeutics. There is increasing evidence that cardiovascular drug development will be impacted in a similar fashion. In fact, molecular imaging can be particularly impactful in cardiovascular drug development since, unlike in cancer medicine, tissue is not routinely obtained from humans and imaging may be able to provide a “virtual biopsy” of drug effects on the heart or vascular tissues.
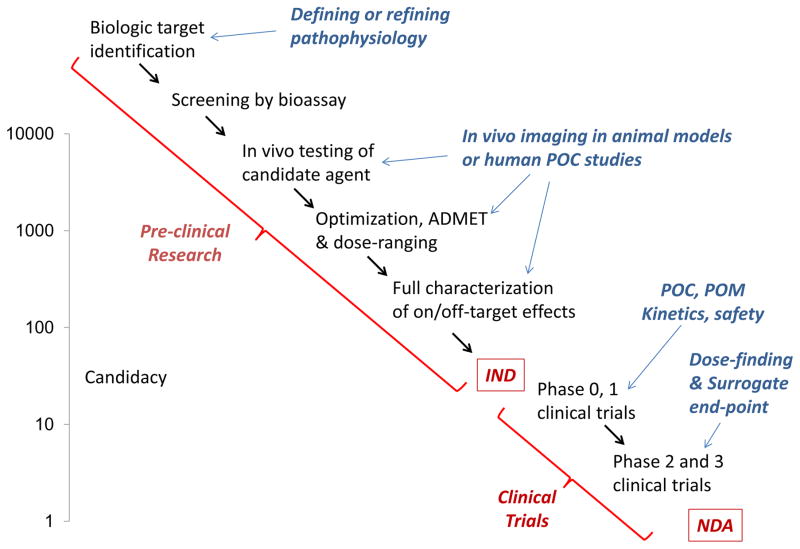
Figure 2.
Schematic illustrating pre-clinical research stages leading to investigational new drug (IND) application and clinical stages leading to new drug application (NDA); and potential roles of molecular imaging for discrete stages. Left y-axis schematically depicts the number of candidate new molecular entities that at screening stage (top) and each subsequent stage to culminate in a single NDA. ADMET = absorption, distribution, metabolism, excretion and toxicity; POC = proof-of-concept; POM = proof-of-mechanism.
Molecular Imaging in Medicine and Science
Methods for assessing specific cellular or molecular processes have been developed for all forms of non-invasive imaging used in cardiovascular medicine and science.7,10,11 These techniques are used to assess disease-related processes such as protein synthesis or trafficking, gene expression, metabolic activity, cell migration, enzymatic activity, and receptor availability. The development of in vivo molecular imaging techniques has been predicated on the ability to provide unique quantitative spatial and temporal information that can be used for a variety of purposes in patients and in pre-clinical models of disease. For drug development, molecular imaging has been used to: (i) identify new “druggable” targets, (ii) evaluate biodistribution and appropriate dosing strategies, (iii) test efficacy and off-target effects, (iv) to select appropriate patient cohorts for initial testing, and (v) to serve as a surrogate endpoint in pre-clinical and clinical studies. The role of molecular imaging is likely to increase given trends in academia and industry to focus more on humans or non-rodent animal models that more clearly resemble humans for the early stages of drug development.12
From a technical standpoint, molecular imaging relies on one of several strategies. A commonly used approach is to engineer contrast agents that are selectively retained by the biologic process of interest. Contrast agents for radionuclide imaging, magnetic resonance imaging (MRI), ultrasound, optical imaging and computed tomography (CT) have all been modified (e.g. conjugation of a targeting ligand) to alter their kinetics and be used for molecular imaging.7,10,11 Targeting moieties used to promote binding to a disease process can exist in a direct linear ratio with the signal-generating contrast agent, which is commonly the case with radionuclide single photon emission tomogaphy (SPECT) or positron emission tomography (PET) agents; or many ligands can be conjugated in a multivalent fashion to the surface of a particle-based contrast such as those used in ultrasound or MRI. A critical factor in the evaluation of the relative value of the different contrast imaging approaches is the biodistribution of the contrast agents (e.g. diffusible versus confinement to the vascular compartment).
Another approach for molecular imaging is to design contrast agents that leverage natural processes for uptake or retention. Examples of this strategy include uptake of 18F-fluorodeoxyglucose (FDG) during PET imaging to detect processes with increased energy expenditure such as advanced atherosclerosis or sarcoidosis, or the detection of inflammation through opsonization and uptake of particle-based contrast agents which are recognized as foreign by immune cells, and may even reveal specific monocyte subtypes in atherosclerosis.13–17 Other forms of molecular imaging rely on the “activation” of contrast agents by the disease-related process of interest. This strategy is most commonly used in optical imaging by development of fluorophores that produce either a change in photon emission or a spectral shift after interaction with a pathogenic pathway such as protease activity or abnormal redox state.18,19 Finally, some forms of molecular imaging do not require exogenous contrast agents but instead are able to detect molecular environment through endogenous signal production, such as MRI-based blood oxygen level dependent (BOLD) imaging,20 or autofluorescence.
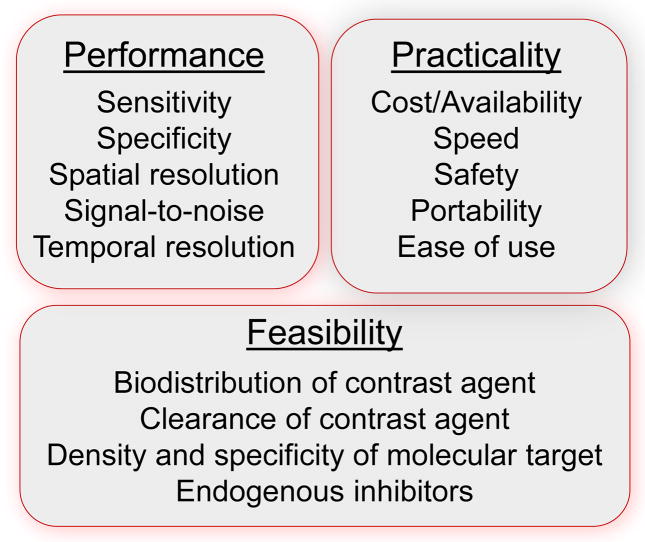
Selection of the most appropriate targeting approach and the most appropriate imaging modality to use within the scope of drug development is based on similar considerations as when applying the technology in the clinical realm (Figure 3). It is worth noting that experience in cancer molecular imaging indicates that an imaging modality used to assess pre-approval drug efficacy is more likely to be adopted by clinicians for patient selection or for assessing therapeutic response. Technical deliberations include need for high sensitivity, spatial resolution, temporal resolution, target specificity, and biodistribution of contrast agent, when applicable, which determines likelihood for accessing the biologic process of interest. Some of the biggest technical concerns are to assure that a signal reflects tissue phenotype rather than primarily reflecting blood flow, vascular permeability, or other variables that can influence tracer uptake. Practical considerations for both academia and pharma include cost, availability, and safety.
Figure 3.
Imaging technology characteristics commonly considered when selecting an approach for molecular imaging in science and in medicine.
Identification of New Targets for Therapy with Imaging
The identification of molecular pathways previously unknown to be involved in the pathophysiology of a disease is often a catalyst for the development of new treatments. Information on newly recognized pathways, proteins or genes allows for the generation of lead candidates for therapy which can be tested by a variety of molecular biology approaches, most commonly in the form of automated high-throughput or focused compound screening processes.1,21 Another approach is that of “rational drug design” or whereby structural proteomics provides knowledge of the detailed structural and biochemical properties of a target molecule (protein, channel, enzyme) in order to design a modifier compound.21 This method has benefited from advances in high resolution techniques for characterizing protein structure by x-ray diffraction crystallography, and the use of computer-based methods for designing small molecule therapeutics and predicting their success through virtual ligand screening.
Non-invasive in vivo molecular imaging has been tasked in the research setting to create new insight into pathobiology in a wide range of disease categories. This application of molecular imaging has helped identify new “druggable” targets. In addition to simply uncovering pathophysiology, the opportunity to temporally evaluate a disease-related process non-invasively can provide critical knowledge of how a drug can be used to its greatest effect, or when it is likely to be of little benefit. Moreover, molecular imaging is often paired with more conventional forms of cardiovascular non-invasive imaging in order to match a molecular phenotype to standard measurements of anatomy, flow, or function.
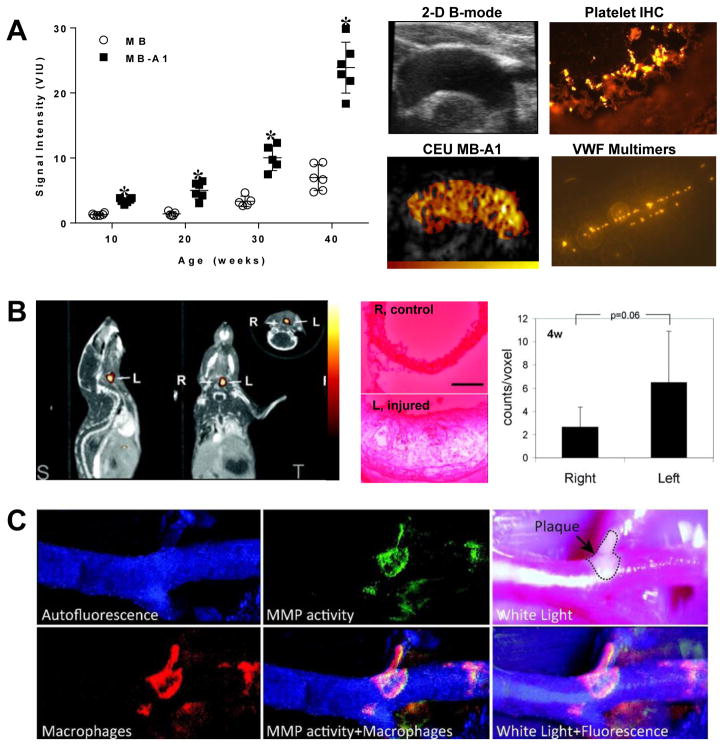
In the field of atherosclerosis biology, most molecular imaging studies have simply demonstrated feasibility of assessing a pathologic process that is already well characterized. Yet, there are examples where molecular imaging has aided in the discovery of a modifiable disease-related process. Molecular imaging of phosphatidylserine expression on the outer leaflet of the cell membrane with annexin-V probes has been used to detect macrophage apoptosis in unstable plaques, but also has been used to demonstrate that this process can occur from non-apoptotic ischemic pathways.22,23 Imaging events that occur at the endothelial-blood interface has been valuable for discovering that abnormal regulation of self-associated Von Willebrand factor multimers at the endothelial surface is largely responsible for platelet-endothelial interactions that play a role in early atherogenesis (Figure 4A).24 Optical and SPECT imaging probes that reveal the release and the enzymatic activity of matrix metalloproteinases (MMPs) and cathepsins have been useful for co-localizing protease activity with macrophages or elastin degradation (Figure 4B and 4C).19,25,26 In vivo PET imaging with the nitroimidazole derivative 18F-fluoromisonidazole which is reduced and retained by cells in hypoxic environments has yielded important information on hypoxic microenvironment in atherosclerosis.27 The folate receptor-β, CX3CR-1 (fractalkine receptor), the mannose receptor, and α5-integrins have all been targeted to examine the roles of specific inflammatory cell types (monocyte subclasses, macrophages) in atherosclerosis and ischemia-related vascular remodeling.28–32 These studies have done more than simply show feasibility of imaging the innate immune response. They have provided support to the notion that altering the balance of monocyte cell type may be a therapeutic target in certain conditions such as atherosclerosis and LV remodeling.33 Even in valve disease, molecular imaging of endothelial cell adhesion molecules such as VCAM-1 has contributed to the notion that progression to aortic stenosis can be modified by altering pro-inflammatory signaling.34 These examples are just a few that highlight the use of targeted imaging probes to reveal new therapeutic targets.
Figure 4.
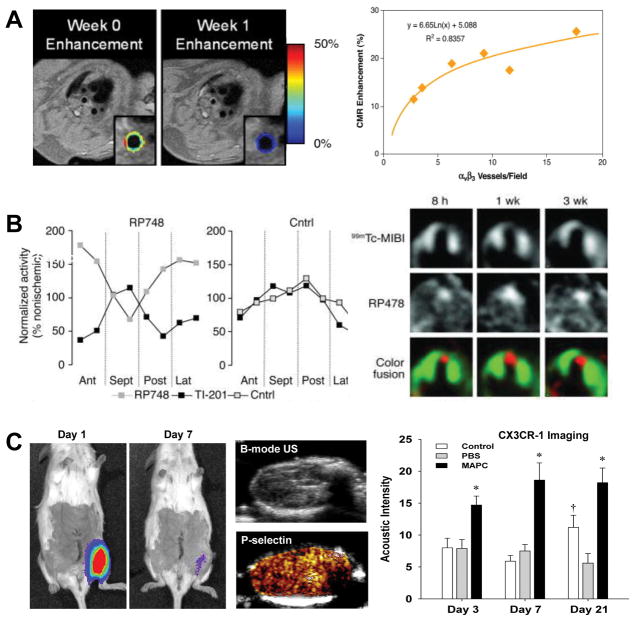
Molecular imaging studies examining pathobiology of atherosclerosis. (A) Ultrasound molecular imaging showing age-dependent increase in signal from platelet-endothelial interactions (MB-A1) in the thoracic aorta of LDL-R−/− and Apobec-1−/− atherosclerotic mice (MB=control agent); and images illustrating the proximal thoracic aorta by B-mode and platelet-targeted contrast-enhanced ultrasound (CEU MB-A1), platelet-endothelial attachment on immunohistochemistry (IHC), and presence of endothelial VWF multimers by ex vivo GPIba-nanobead flow chamber. Modified with permission.24 (B) MicroCT and SPECT fusion molecular imaging images and quantitative data in an apoE−/− mouse 3 weeks after left carotid injury with an 111In-labeled agent targeted to MMPs, and ex vivo zymography showing protease activity of the left carotid. Modified with permission.26 (C) Intravital optical imaging of the carotid artery of an apoE−/− mouse on atherogenic diet illustrating co-localization of signal from an activatable MMP-13 fluorophore, macrophage-targeted fluorescent nanoparticles in a region of plaque. Modified with permission.19
In vivo Testing of Candidate Efficacy
The full characterization of on-target effects and completion of proof-of-mechanism studies are critical steps in drug development. It is a logical assumption that any molecular imaging technique that has been used to uncover modifiable disease-related biology can also be used to non-invasively quantify response to therapy. More commonly, pathophysiology that is not discovered through molecular imaging has also been targeted for evaluation of new therapeutic agents in pre-clinical models and in even in human proof-of-concept trials.
The investment in molecular imaging for pre-clinical in vivo testing of candidate efficacy and off-target effects has been justified in situations where it provides incremental benefit. Conventional forms of non-invasive cardiovascular imaging without recourse to molecular imaging protocols provides information on morphology or function including but not limited to: (i) plaque size, volume, and content in atherosclerosis; (ii) left ventricular volumes, systolic function, diastolic properties, and scar area in heart failure; (iii) myocardial perfusion imaging and metabolism in ischemic heart disease. In this context, the rationale for using molecular imaging is often based on its ability to assess modification of a specific targeted biologic pathway early before a structural or functional outcome in order to select the most appropriate of several candidate agents. Aligning with the concept of “precision medicine”, molecular imaging can be used in diseases that have wide phenotypic variation to predict benefit based on the specific molecular or cellular characteristics or the stage of disease. For example, molecular imaging may be particularly valuable when evaluating potent anti-inflammatory therapies in established atherosclerosis where anatomic imaging is limited, or for selecting only those with high cardiac sympathetic activity in order to test drug/device therapies for ventricular arrhythmias. Imaging data can also provide valuable insight when new therapies fail by demonstrating lack of effect on the intended biologic pathway, thereby avoiding an incorrect assumption that a certain pathway is not a suitable for therapeutic targeting.
In atherosclerosis drug development, probably the most recognized use of molecular imaging that has been applied is 18F-FDG imaging with PET.35 Widespread recognition and acceptance of this technology is based on clinical trials that have used it as an outcome measure for drug efficacy (discussed later) and its documented ability to predict major adverse cardiovascular events.7,10,36 As reviewed elsewhere, molecular imaging with targeted contrast agents has been used to image atherosclerosis and aneurysm formation including inflammation (endothelial adhesion molecule expression, monocyte recruitment, scavenger receptors, phagocytic activity, matrix proteases, etc.), prothrombotic endothelial phenotype (VWF, platelet adhesion, fibrin), oxidative stress (phospholipid or protein oxidized epitopes), lipoprotein accumulation, angiogenesis (integrins and proteases) and matrix content.7,10,11,16,37 For ischemic heart disease and heart failure, molecular imaging of the recruitment of specific inflammatory cell subtypes, matrix remodeling, protease activity, and endothelial and monocyte markers of vascular remodeling have been used to spatially assess tissue ischemia, ventricular remodeling, and endogenous and therapeutic angiogenesis (Figure 5).7,10,32,38–41 For rhythm disorders, imaging of pre- and post-synaptic cardiac sympathetic function with 123I-meta-iodobenzylguanidine activity and 11C-meta-hydroxyephedrine can provide information on susceptibility to life-threatening ventricular arrhythmias.42–44 The practicality of using molecular imaging in pre-clinical drug evaluation or dose ranging has benefited from studies that applied these techniques not only in rodents, but also in more relevant canine or porcine models of post-MI remodeling, and non-human primate models of atherosclerosis.41,45,46
Figure 5.
Molecular imaging of angiogenesis. (A) MRI with color-coded signal enhancement from gadolinium-labeled αvβ3-integrin-targeted nanoparticles showing a treatment-related reduction in plaque angiogenesis at 1 week in the thoracic aorta of an atherosclerotic rabbit model, and correlation between αvβ3-positive vessels on histology with nanoparticle signal (CMR enhancement). Modified with permission.40 (B) Quantitative spatial relationship between perfusion by SPECT (Tl-201 or 99mTc-MIBI) and regions of angiogenesis with an 111In-labeled αvβ3-integrin-targeted SPECT probe (RP748) in a canine model of myocardial infarction illustrating highest area of angiogeneic activity in the infarct area. Modified with permission.41 (C) Imaging of therapeutic angiogenesis with multipotential adult progenitor cells (MAPC) in a murine model of hindlimb ischemia illustrating rapid clearance of cells by optical imaging of luciferase-transfected MAPCs, ultrasound images (B-mode and P-selectin-targeted imaging) of endothelial adhesion molecule expression in a MAPC-treated hindlimb, and quantitative data from CX3CR-1 molecular imaging to detect sustained increased recruitment of pro-angiogenic monocytes in MAPC-treated over control conditions. Modified with permission.32
Illustration that a molecular imaging readout is altered by a therapy already known to be effective in a disease state is often used as a step towards clinical translation of a new diagnostic. These types of studies are also useful when considering whether a molecular imaging technique is suitable for assessing pre-clinical efficacy of a new drug. Statin therapy has been shown in animal models of atherosclerosis, and then eventually in humans to reduce the arterial 18F-FDG signal on PET imaging or signal from uptake of ultra-small superparamagnetic iron oxide (USPIO) nanoparticles (<100 nm in size).13,47 Statins have been used in gene-targeted murine models of atherosclerosis to confirm a therapeutic reduction in the expression of adhesion molecules such as VCAM-1 by targeted MRI, PET, optical and ultrasound molecular imaging.48–50 In heart failure, combinations of clinically-established therapies that modify angiotensin and aldosterone signaling have been used to alter post-MI ventricular remodeling in mice which was correlated with radionuclide imaging of myofibroblast activity.39
The application of molecular imaging to obtain unique information not provided by anatomic or functional imaging regarding the efficacy of new therapies is slowly increasing. In atherosclerotic disease, molecular imaging has provided valuable information for demonstrating efficacy and in some circumstances optimal dosing for investigational agents such as new potent anti-oxidants (e.g. NOX-2 modifiers), HDL-mimetics, and MMP inhibitors.16,40,51 These studies have generally employed molecular imaging in models of advanced disease and examined non-morphologic changes in specific processes such as endothelial cell adhesion molecule expression (selectins, VCAM-1), monocyte recruitment, platelet adhesion, oxidized lipoprotein uptake, and intra-plaque protease activity. The notion that new therapies may be able to prevent atherosclerosis altogether hinges on the ability to identify subjects at exceptionally high risk for accelerated disease at a very early stage. It is unlikely that existing risk assessment paradigms will suffice since they are most commonly used to estimate 10 year risk and are heavily influenced by age, leading to a likely underestimation of lifetime risk in young individuals.52 Molecular imaging has been shown to detect the earliest atherogenic events that occur before histologic evidence for fatty streaks such as endothelial activation, platelet adhesion and oxidized lipid accumulation.24,46,53 Accordingly, these techniques have begun to be used to establish therapeutic benefit of new drugs that prevent atherosclerosis progression.51
Molecular imaging has also been used to provide important proof-of-mechanism information on new therapies that act through vascular remodeling. Targeted imaging of angiogenesis-releated endothelial integrins (αv-integrins), other adhesion molecules, and markers of pro-angiogenic monocytes has been used as an in vivo readout for pro-angiogenic therapies in ischemic heart and limb disease, and for anti-angiogenic therapies intended to reduce plaque neovascularization.32,33,40,54 For pro-angiogenic cell therapy, molecular imaging has been used to assess phenotypic changes that reflect their intended purpose. For example, in vivo optical imaging of a luciferase reporter under the control of a Tie-2 reporter has been used to temporally and spatially assess the transformation to a more “endothelial phenotype” of mesenchymal stem cells injected in mice with myocardial infarction.55 Molecular imaging has been used as an investigative technique for characterizing paracrine effects of cell therapy on host cells such as the activation of a pro-angiogenic innate immune response stimulated by mesenchymal stem cell therapy in limb ischemia (Figure 5C).32
In summary, some of the examples discussed above illustrate how molecular imaging has been used as a readout for the intended or “on-target” drug effects. With the maturation, further validation, and increased penetration of these techniques in research labs, it is highly likely that molecular imaging will play an increasing role in the early evaluation of candidate NMEs.
Biodistribution and Pharmacokinetics
Pharmokinetic and pharmacodynamic profiling is a critical process in understanding drug dosing, toxicity, and likelihood for therapeutic success. Non-invasive imaging has played a role in this process and has been vital in the conduct of many FDA Phase 0 exploratory “microdosing” studies designed to expedite drug approval. In particular, radionuclide imaging with PET and SPECT has been useful for temporally evaluating whole body biodistribution of therapeutic candidates that can be labeled without changing their properties and administered by intravenous route. Another strategy for imaging drug biodistribution is to employ imaging agents that are “activated” or produce signal in the presence of a drug or by a specific interaction of two proteins. This strategy, which has been reviewed elsewhere,56 often involves two separate molecular moieties or a split protein each of which are labeled with an optical reporter system so that in the presence of a therapeutic agent there is a change in confirmation and rearrangement of the reporters to produce a light signal.
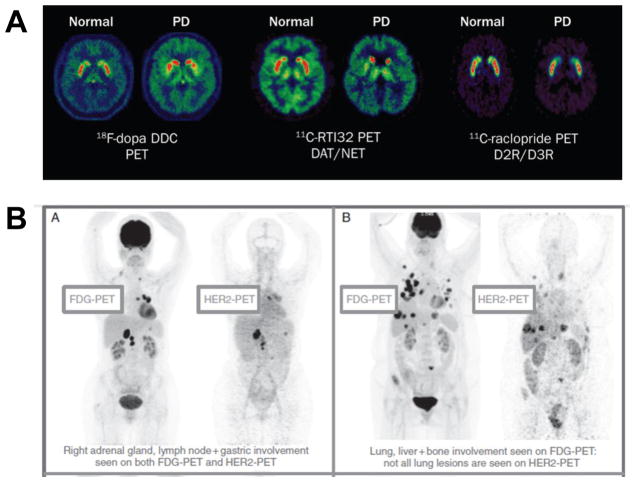
The use of pharmacokinetic imaging of radiolabeled drug candidates has been most impactful in situations where drug uptake at a specific site is desired or when access of drugs to target is in question.57–59 In neurologic diseases, PET and SPECT imaging have been used to evaluate the transport of drugs across the blood-brain barrier, and have been paired with drug-tracers, including 11C-raclopide 123I-ioflupane, to spatially evaluate dopamine transport and receptor occupancy in the striatum in a variety of motion disorders and neurocognitive processes (Figure 6A).57,58 Imaging data on brain receptor occupancy is of critical importance since it differs vastly from blood levels, leading to appropriate dosing strategies which would not have been possible with plasma kinetics alone.58 In cancer medicine, concerns regarding anti-tumor drug delivery, entry, and retention has justified the use of imaging to assess biodistribution in primary and metastatic solid tumors. This application is particularly important for the assessment of macromolecular biologic therapies that are used not only in cancer but other conditions where site-targeted uptake is important. Use of a common agent with diagnostic and therapeutic properties has been quite helpful in understanding not only whether selective cancer targeting has been effective, but also for predicting therapeutic response and explaining variation in response. For example a recent trial in patients with metastatic HER-2-positive breast cancer used 89Zr-labled trastuzamab PET imaging together with FDG PET to document that poor clinical response could be expected in those with low trastuzamab uptake despite positive biopsy results (Figure 6B).59
Figure 6.
Imaging to understand drug biodistribution. (A) PET brain imaging in Parkinson’s disease (PD) to assess dopamine storage (18F-DOPA), transporter function (11C-RT132), and relative dopamine D2 and D3 receptor binding (11C-raclopide). Modified with permission.57 (B) PET imaging of 89Zr-trastuzamab (HER2-PET) illustrating variable uptake of the theapeutic in biopsy-proven HER-2-positive tumor metastases which are all detected by FDG-PET. Reproduced with permission.59
In cardiovascular drug development, we are not aware of any situations where molecular imaging has been critical for understanding pharmacokinetics for drugs that currently approved. However, molecular imaging has the potential to confirm the penetration and retention of drugs targeted to atherosclerotic plaques, thrombus, and ischemic myocardium. In particular major advances have been made in combining a therapeutic and diagnostic agent into a single nanoparticle moiety, commonly referred to as “theranostics” and which have been reviewed elsewhere.60,61 For example gadolinium-labeled nanoparticles loaded with anti-angiogenic compounds such as fumagillin have been used to simultaneously assess the presence of functional plaque neovessels, the selective uptake of the delivery system, and response to therapy.40 Non-invasive imaging with nanoparticle agents that also have direct anti-inflammatory activity has been achieved by targeting macrophage markers and using phototherapy or controlled release of statins, anti-mitogenic compounds, or prostanoids.60,62–64 The premise of the entire field of theranostics is that imaging the biodistribution of a therapeutic agent will enhance the optimization process and accelerate approval.
Another area where molecular imaging has been used extensively to study biodistribution has been to study stem cell therapy. Strategies for labeling and detecting stem cells have been developed for essentially all forms of non-invasive imaging.65 There are substantial practical differences in these techniques with regards to: (a) ability to provide quantitative information on number of cells present, (b) duration of detectability, (c) ability to detect daughter cells after division, (d) toxicity or other effects on stem cell function, and (e) transference of tracer to phagocytic cells involved in stem cell immune clearance. Optical, radionuclide, and MRI imaging have all revealed that the residence time of viable adult mesenchymal and bone marrow-derived stem cells is usually temporary, lasting days to weeks,32,55,65–68 thereby supporting the notion that these cells act primarily through paracrine signaling of endogenous cells. Imaging has also been useful for understanding the failure or unpredictability of cell therapy. For example, PET detection of mesenchymal stem cells has been critical for establishing the concept that the beneficial effects of cell therapy on LV remodeling or angiogenesis are most pronounced when tissue retention of labeled cells is high.69 Molecular imaging has also been useful for evaluating mechanisms for rapid loss of stem cells from the target tissue.66,67 It is hoped that non-invasive imaging of stem cell activity and location will provide insight into the rather small cardiovascular benefits of cell therapy in clinical trials when compared to pre-clinical animal models.
Selection of Patients and Endpoints in Clinical Trials
The use biomarkers as surrogate endpoints in clinical trials has been a topic of lively debate for decades. Both pharma and the regulatory agencies that oversee drug approval have accepted the concept that biomarkers, including imaging biomarkers, have an important role for accelerating and reducing cost of the drug development and approval process. The only biomarker that has been consistently accepted for cardiovascular disease drug approval by the FDA has been serum cholesterol levels.
The application of molecular imaging as a potential biomarker in clinical trials hinges on several requirements. The technique must be quantitative and provide temporal information. High sensitivity for detecting the targeted molecular process is advantageous, although equally important is a complete understanding of how specific the target is to the pathway of interest. For example, αv-integrin-targeted contrast agents have been applied to detect neovascular endothelial phenotype in angiogenesis, but signal from these agents are just as likely to reflect αv-integrin expression present on activated monocytes which are also involved in vascular remodeling.70 For contrast agent-based approaches, a solid understanding of in vivo kinetics is vital. For example, for tracers with high first-pass retention, tissue uptake is not simply dependent on target molecule expression but is also influenced by the relative blood flow. This issue is of critical importance when temporally assessing angiogenesis where changes in perfusion over time must be accounted for when registering tracer intensity.71 Ideally, a targeting approach should not alter the process that is being targeted for drug therapy. For example, there has been demonstration that agents that rely on monocyte/macrophage phagocytosis such as USPIOs may influence monocyte phenotype.72
The most common molecular imaging technique that has been used as an endpoint in cardiovascular clinical trials is 18F-FDG PET to assess atherosclerotic disease activity. This approach has been used to assess the effects of many different inflammation-targeted therapeutics which have been reviewed elsewhere.35 Because of the complexity of 18F-FDG detection in the coronary arteries which are adjacent to the metabolically-active epicardial surface, 18F-sodium fluoride PET has been also for coronary atherosclerosis imaging and could detect early disease based on its ability to detect microcalcification.73 To our knowledge,, though, molecular imaging biomarker has not been used as a primary surrogate outcome measure for drug approval and instead has been used early in clinical experience for for “proof-of-concept”.
The situation is very different in cancer medicine. Clinical trials in breast cancer, gastrointestinal stromal tumors, lung cancer, etc., have definitively shown that changes in primary and metastatic tumor activity on 18F-FDG PET precedes changes in anatomic tumor burden.58 Accordingly, PET imaging has been used as a critical component for the approval of a variety of tyrosine kinase receptor inhibitors and immunotherapy in cancer.
Aside from its use as surrogate endpoint, molecular imaging has the potential to select appropriate cohorts for initial testing of new drugs in clinical trials. As mentioned previously, “high-impact” cohorts could include patients with atherosclerosis who have high inflammatory burden or patients with cardiomyopathy who have high myocardial sympathetic activity for anti-arrhythmic therapy. A barrier to this approach in the pre-NDA phase has been business-related concerns that it could adversely affect return-on-investment by reducing the pool of potential candidates for therapy. However, the use of molecular imaging may be particularly helpful in post-marketing Phase IV clinical studies that are often designed to test drug effectiveness based on dosing or in specific populations of patients, or drug toxicities. There have been examples of this application, such as the use of 18F-FDG PET to compare effectiveness of low and high-dose statin therapy.13
Summary
Instead of providing a comprehensive list of molecular imaging techniques that have been used in cardiovascular science, this review has instead attempted to focus on how this technology can be used in drug development at several stages to accelerate the process and reduce cost. Molecular imaging technologies that have been developed for clinical diagnostics have successfully been re-tasked to understand efficacy of candidate therapeutics in pre-clinical and even in early clinical studies. The lack of history using molecular imaging as a surrogate clinical endpoint for drug approval in cardiovascular medicine has not, however, dampened the prospects of using molecular imaging to make “go or no-go” decisions early in cardiovascular clinical trials or for assessing dosing strategies.
Acknowledgments
The authors are grateful for the helpful comments from Kenneth Krohn, PhD.
SOURCES OF FUNDING
Dr. Lindner is supported by grants R01-HL078610 and R01-HL120046 from the National Institutes of Health (NIH), Bethesda, MD. Dr. Lindner is also supported by grant 14-14NSBRI1-0025 from the National Space Biomedical Research Institute. Dr. Link is supported by the National Cancer Institute (NCI) grant P01CA042045
Footnotes
DISCLOSURES
Dr. Lindner has investigator-initiated grants from GE Healthcare and Pfizer, Inc..
References
- 1.Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, Green DV, Hertzberg RP, Janzen WP, Paslay JW, Schopfer U, Sittampalam GS. Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov. 2011;10:188–195. doi: 10.1038/nrd3368. [DOI] [PubMed] [Google Scholar]
- 3.Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve r&d productivity: The pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 4.Mullard A. 2016 fda drug approvals. Nat Rev Drug Discov. 2017;16:73–76. doi: 10.1038/nrd.2017.14. [DOI] [PubMed] [Google Scholar]
- 5.Naci H, Carter AW, Mossialos E. Why the drug development pipeline is not delivering better medicines. BMJ. 2015;351:h5542. doi: 10.1136/bmj.h5542. [DOI] [PubMed] [Google Scholar]
- 6.Miller KL, Lanthier M. Trends in orphan new molecular entities, 1983–2014: Half were first in class, and rare cancers were the most frequent target. Health Aff. 2016;35:464–470. doi: 10.1377/hlthaff.2015.0921. [DOI] [PubMed] [Google Scholar]
- 7.Nahrendorf M, Sosnovik DE, French BA, Swirski FK, Bengel F, Sadeghi MM, Lindner JR, Wu JC, Kraitchman DL, Fayad ZA, Sinusas AJ. Multimodality cardiovascular molecular imaging, part ii. Circ Cardiovasc Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Luijn JC, Gribnau FW, Leufkens HG. Superior efficacy of new medicines? Eur J Clin Pharmacol. 2010;66:445–448. doi: 10.1007/s00228-010-0808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberti L, Bujar M, Breckenridge A, Hoekman J, McAuslane N, Stolk P, Leufkens H. Fda facilitated regulatory pathways: Visualizing their characteristics, development, and authorization timelines. Front Pharmacol. 2017;8:161. doi: 10.3389/fphar.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindner JR, Sinusas A. Molecular imaging in cardiovascular disease: Which methods, which diseases? J Nucl Cardiol. 2013;20:990–1001. doi: 10.1007/s12350-013-9785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009;29:983–991. doi: 10.1161/ATVBAHA.108.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner JP, Gaskill BN, Weber EM, Ahloy-Dallaire J, Pritchett-Corning KR. Introducing therioepistemology: The study of how knowledge is gained from animal research. Lab Anim. 2017;46:103–113. doi: 10.1038/laban.1224. [DOI] [PubMed] [Google Scholar]
- 13.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR, Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 14.Lindner JR, Coggins MP, Kaul S, Klibanov AL, Brandenburger GH, Ley K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation. 2000;101:668–675. doi: 10.1161/01.cir.101.6.668. [DOI] [PubMed] [Google Scholar]
- 15.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 16.Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111:231–244. doi: 10.1161/CIRCRESAHA.112.268144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuhendler AJ, Pu K, Cui L, Uetrecht JP, Rao J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol. 2014;32:373–380. doi: 10.1038/nbt.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quillard T, Croce K, Jaffer FA, Weissleder R, Libby P. Molecular imaging of macrophage protease activity in cardiovascular inflammation in vivo. Thromb Haemost. 2011;105:828–836. doi: 10.1160/TH10-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich MG, Karamitsos TD. Oxygenation-sensitive cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:43. doi: 10.1186/1532-429X-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lounnas V, Ritschel T, Kelder J, McGuire R, Bywater RP, Foloppe N. Current progress in structure-based rational drug design marks a new mindset in drug discovery. Comput Struct Biotechnol J. 2013;5:e201302011. doi: 10.5936/csbj.201302011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L, Narula J. Noninvasive detection of plaque instability with use of radiolabeled annexin a5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004;350:1472–1473. doi: 10.1056/NEJM200404013501425. [DOI] [PubMed] [Google Scholar]
- 23.Kenis H, Zandbergen HR, Hofstra L, Petrov AD, Dumont EA, Blankenberg FD, Haider N, Bitsch N, Gijbels M, Verjans JW, Narula N, Narula J, Reutelingsperger CP. Annexin a5 uptake in ischemic myocardium: Demonstration of reversible phosphatidylserine externalization and feasibility of radionuclide imaging. J Nucl Med. 2010;51:259–267. doi: 10.2967/jnumed.109.068429. [DOI] [PubMed] [Google Scholar]
- 24.Shim CY, Liu YN, Atkinson T, Xie A, Foster T, Davidson BP, Treible M, Qi Y, Lopez JA, Munday A, Ruggeri Z, Lindner JR. Molecular imaging of platelet-endothelial interactions and endothelial von willebrand factor in early and mid-stage atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002765. doi: 10.1161/CIRCIMAGING.114.002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin k activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Nie L, Razavian M, Ahmed M, Dobrucki LW, Asadi A, Edwards DS, Azure M, Sinusas AJ, Sadeghi MM. Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation. 2008;118:1953–1960. doi: 10.1161/CIRCULATIONAHA.108.789743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49(Suppl 2):129S–148S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 28.Winkel LC, Groen HC, van Thiel BS, Muller C, van der Steen AF, Wentzel JJ, de Jong M, Van der Heiden K. Folate receptor-targeted single-photon emission computed tomography/computed tomography to detect activated macrophages in atherosclerosis: Can it distinguish vulnerable from stable atherosclerotic plaques? Mol Imaging. 2014:13. doi: 10.2310/7290.2013.00061. [DOI] [PubMed] [Google Scholar]
- 29.Jager NA, Westra J, Golestani R, van Dam GM, Low PS, Tio RA, Slart RH, Boersma HH, Bijl M, Zeebregts CJ. Folate receptor-beta imaging using 99mtc-folate to explore distribution of polarized macrophage populations in human atherosclerotic plaque. J Nucl Med. 2014;55:1945–1951. doi: 10.2967/jnumed.114.143180. [DOI] [PubMed] [Google Scholar]
- 30.Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N, Tahara A, Constantinescu CC, Zhou J, Boersma HH, Imaizumi T, Nakano M, Finn A, Fayad Z, Virmani R, Fuster V, Bosca L, Narula J. 2-deoxy-2-[18f]fluoro-d-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- 31.Behm CZ, Kaufmann BA, Carr C, Lankford M, Sanders JM, Rose CE, Kaul S, Lindner JR. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation. 2008;117:2902–2911. doi: 10.1161/CIRCULATIONAHA.107.744037. [DOI] [PubMed] [Google Scholar]
- 32.Ryu JC, Davidson BP, Xie A, Qi Y, Zha D, Belcik JT, Caplan ES, Woda JM, Hedrick CC, Hanna RN, Lehman N, Zhao Y, Ting A, Lindner JR. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation. 2013;127:710–719. doi: 10.1161/CIRCULATIONAHA.112.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swirski FK, Nahrendorf M. Imaging macrophage development and fate in atherosclerosis and myocardial infarction. Immunol Cell Biol. 2013;91:297–303. doi: 10.1038/icb.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 35.Osborn EA, Jaffer FA. Imaging inflammation and neovascularization in atherosclerosis: Clinical and translational molecular and structural imaging targets. Curr Opin Cardiol. 2015;30:671–680. doi: 10.1097/HCO.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, Hoffmann U, Tawakol A. Measurement of arterial activity on routine fdg pet/ct images improves prediction of risk of future cv events. JACC Cardiovasc Imaging. 2013;6:1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Buxton DB. Molecular imaging of aortic aneurysms. Circ Cardiovasc Imaging. 2012;5:392–399. doi: 10.1161/CIRCIMAGING.112.973727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobrucki LW, de Muinck ED, Lindner JR, Sinusas AJ. Approaches to multimodality imaging of angiogenesis. J Nucl Med. 2010;51(Suppl 1):66S–79S. doi: 10.2967/jnumed.109.074963. [DOI] [PubMed] [Google Scholar]
- 39.van den Borne SW, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, Zandbergen HR, Ni Y, Frederik P, Zhou J, Arbo B, Rogstad A, Cuthbertson A, Chettibi S, Reutelingsperger C, Blankesteijn WM, Smits JF, Daemen MJ, Zannad F, Vannan MA, Narula N, Pitt B, Hofstra L, Narula J. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52:2017–2028. doi: 10.1016/j.jacc.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 40.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging. 2008;1:624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, Su H, Edwards DS, Liu S, Harris TD, Madri JA, Zaret BL, Sinusas AJ. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113:1684–1691. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrio I, Cowie MR, Yamazaki J, Udelson J, Camici PG. Cardiac sympathetic imaging with mibg in heart failure. JACC Cardiovasc Imaging. 2010;3:92–100. doi: 10.1016/j.jcmg.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Link JM, Caldwell JH. Diagnostic and prognostic imaging of the cardiac sympathetic nervous system. Nat Clin Pract Cardiovasc Med. 2008;5(Suppl 2):S79–86. doi: 10.1038/ncpcardio1150. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J Investigators A-H. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective admire-hf (adreview myocardial imaging for risk evaluation in heart failure) study. J Am Coll Cardiol. 2010;55:2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Sahul ZH, Mukherjee R, Song J, McAteer J, Stroud RE, Dione DP, Staib L, Papademetris X, Dobrucki LW, Duncan JS, Spinale FG, Sinusas AJ. Targeted imaging of the spatial and temporal variation of matrix metalloproteinase activity in a porcine model of postinfarct remodeling: Relationship to myocardial dysfunction. Circ Cardiovasc Imaging. 2011;4:381–391. doi: 10.1161/CIRCIMAGING.110.961854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chadderdon SM, Belcik JT, Bader L, Kirigiti MA, Peters DM, Kievit P, Grove KL, Lindner JR. Proinflammatory endothelial activation detected by molecular imaging in obese nonhuman primates coincides with onset of insulin resistance and progressively increases with duration of insulin resistance. Circulation. 2014;129:471–478. doi: 10.1161/CIRCULATIONAHA.113.003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, JMUK-I, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, Hayes PD, Varty K, Boyle JR, Gaunt ME, Zalewski A, Gillard JH. The atheroma (atorvastatin therapy: Effects on reduction of macrophage activity) study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Khanicheh E, Mitterhuber M, Xu L, Haeuselmann SP, Kuster GM, Kaufmann BA. Noninvasive ultrasound molecular imaging of the effect of statins on endothelial inflammatory phenotype in early atherosclerosis. PLoS One. 2013;8:e58761. doi: 10.1371/journal.pone.0058761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 50.Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R. 18f-4v for pet-ct imaging of vcam-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2:1213–1222. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Davidson BP, Yue Q, Belcik T, Xie A, Inaba Y, McCarty OJ, Tormoen GW, Zhao Y, Ruggeri ZM, Kaufmann BA, Lindner JR. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with nadph oxidase inhibition. Circ Cardiovasc Imaging. 2013;6:74–82. doi: 10.1161/CIRCIMAGING.112.975193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jorstad HT, Colkesen EB, Boekholdt SM, Tijssen JG, Wareham NJ, Khaw KT, Peters RJ. Estimated 10-year cardiovascular mortality seriously underestimates overall cardiovascular risk. Heart. 2016;102:63–68. doi: 10.1136/heartjnl-2015-307668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsimikas S, Palinski W, Halpern SE, Yeung DW, Curtiss LK, Witztum JL. Radiolabeled mda2, an oxidation-specific, monoclonal antibody, identifies native atherosclerotic lesions in vivo. J Nucl Cardiol. 1999;6:41–53. doi: 10.1016/s1071-3581(99)90064-8. [DOI] [PubMed] [Google Scholar]
- 54.Winter PM, Caruthers SD, Allen JS, Cai K, Williams TA, Lanza GM, Wickline SA. Molecular imaging of angiogenic therapy in peripheral vascular disease with alphanubeta3-integrin-targeted nanoparticles. Magn Reson Med. 2010;64:369–376. doi: 10.1002/mrm.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Najjar A, Zhang S, Rabinovich B, Willerson JT, Gelovani JG, Yeh ET. Molecular imaging of mesenchymal stem cell: Mechanistic insight into cardiac repair after experimental myocardial infarction. Circ Cardiovasc Imaging. 2012;5:94–101. doi: 10.1161/CIRCIMAGING.111.966424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 57.Politis M. Neuroimaging in parkinson disease: From research setting to clinical practice. Nat Rev Neurol. 2014;10:708–722. doi: 10.1038/nrneurol.2014.205. [DOI] [PubMed] [Google Scholar]
- 58.Cunha L, Szigeti K, Mathe D, Metello LF. The role of molecular imaging in modern drug development. Drug Discov Today. 2014;19:936–948. doi: 10.1016/j.drudis.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, Stroobants S, Huizing M, Aftimos P, Tol J, Oyen WJ, Vugts DJ, Hoekstra OS, Schroder CP, Menke-van der Houven van Oordt CW, Guiot T, Brouwers AH, Awada A, de Vries EG, Flamen P. Molecular imaging as a tool to investigate heterogeneity of advanced her2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (t-dm1): The zephir trial. Ann Oncol. 2016;27:619–624. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 60.Tang J, Lobatto ME, Read JC, Mieszawska AJ, Fayad ZA, Mulder WJ. Nanomedical theranostics in cardiovascular disease. Curr Cardiovasc Imaging Rep. 2012;5:19–25. doi: 10.1007/s12410-011-9120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stendahl JC, Sinusas AJ. Nanoparticles for cardiovascular imaging and therapeutic delivery, part 1: Compositions and features. J Nucl Med. 2015;56:1469–1475. doi: 10.2967/jnumed.115.160994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shon SM, Choi Y, Kim JY, Lee DK, Park JY, Schellingerhout D, Kim DE. Photodynamic therapy using a protease-mediated theranostic agent reduces cathepsin-b activity in mouse atheromata in vivo. Arterioscler Thromb Vasc Biol. 2013;33:1360–1365. doi: 10.1161/ATVBAHA.113.301290. [DOI] [PubMed] [Google Scholar]
- 63.Schmitt F, Lagopoulos L, Kauper P, Rossi N, Busso N, Barge J, Wagnieres G, Laue C, Wandrey C, Juillerat-Jeanneret L. Chitosan-based nanogels for selective delivery of photosensitizers to macrophages and improved retention in and therapy of articular joints. J Control Release. 2010;144:242–250. doi: 10.1016/j.jconrel.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, Priem B, Kuan EL, Martel C, Hewing B, Sager H, Nahrendorf M, Randolph GJ, Stroes ES, Fuster V, Fisher EA, Fayad ZA, Mulder WJ. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ransohoff KJ, Wu JC. Advances in cardiovascular molecular imaging for tracking stem cell therapy. Thromb Haemost. 2010;104:13–22. doi: 10.1160/TH09-08-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonios M, Terrovitis J, Chang CY, Engles JM, Higuchi T, Lautamaki R, Yu J, Fox J, Pomper M, Wahl RL, Tsui BM, O’Rourke B, Bengel FM, Marban E, Abraham MR. Myocardial substrate and route of administration determine acute cardiac retention and lung bio-distribution of cardiosphere-derived cells. J Nucl Cardiol. 2011;18:443–450. doi: 10.1007/s12350-011-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gyongyosi M, Hemetsberger R, Wolbank S, Kaun C, Posa A, Marian T, Balkay L, Emri M, Galuska L, Mikecz P, Petrasi Z, Charwat S, Hemetsberger H, Blanco J, Maurer G. Imaging the migration of therapeutically delivered cardiac stem cells. JACC Cardiovasc Imaging. 2010;3:772–775. doi: 10.1016/j.jcmg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 68.Chen IY, Wu JC. Molecular imaging: The key to advancing cardiac stem cell therapy. Trends Cardiovasc Med. 2013;23:201–210. doi: 10.1016/j.tcm.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J, Narsinh KH, Lan F, Wang L, Nguyen PK, Hu S, Lee A, Han L, Gong Y, Huang M, Nag D, Rosenberg J, Chouldechova A, Robbins RC, Wu JC. Early stem cell engraftment predicts late cardiac functional recovery: Preclinical insights from molecular imaging. Circ Cardiovasc Imaging. 2012;5:481–490. doi: 10.1161/CIRCIMAGING.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laitinen I, Saraste A, Weidl E, Poethko T, Weber AW, Nekolla SG, Leppanen P, Yla-Herttuala S, Holzlwimmer G, Walch A, Esposito I, Wester HJ, Knuuti J, Schwaiger M. Evaluation of alphavbeta3 integrin-targeted positron emission tomography tracer 18f-galacto-rgd for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging. 2009;2:331–338. doi: 10.1161/CIRCIMAGING.108.846865. [DOI] [PubMed] [Google Scholar]
- 71.Carr CL, Qi Y, Davidson B, Chadderdon S, Jayaweera AR, Belcik JT, Benner C, Xie A, Lindner JR. Dysregulated selectin expression and monocyte recruitment during ischemia-related vascular remodeling in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011;31:2526–2533. doi: 10.1161/ATVBAHA.111.230177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Settles M, Etzrodt M, Kosanke K, Schiemann M, Zimmermann A, Meier R, Braren R, Huber A, Rummeny EJ, Weissleder R, Swirski FK, Wildgruber M. Different capacity of monocyte subsets to phagocytose iron-oxide nanoparticles. PLoS One. 2011;6:e25197. doi: 10.1371/journal.pone.0025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP. Identifying active vascular microcalcification by (18)f-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. doi: 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]