Abstract
Medical imaging of the three most common genitourinary (GU) cancers—prostate adenocarcinoma, clear cell renal cell carcinoma (RCC), and urothelial carcinoma of the bladder—has evolved significantly during the past decades. The most commonly used imaging modalities for the diagnosis, staging, and follow-up of GU cancers are computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). Multiplanar multidetector CT and multiparametric MRI (mpMRI) with diffusion-weighted imaging are the main imaging modalities for RCC and urothelial carcinoma, and while mpMRI is rapidly becoming the main imaging tool in the evaluation of prostate adenocarcinoma, biopsy is still required for diagnosis. Functional and molecular imaging using 18-fluorodeoxyglucose (FDG)-PET, and sodium fluoride (NaF)-PET are essential for the diagnosis, and especially follow-up, of metastatic GU tumors. This review will provide an overview of the latest advances in the imaging of these three major GU cancers.
Keywords: Renal cell carcinoma, Bladder urothelial carcinoma, Prostate adenocarcinoma, CT, MRI, PET
1. Introduction
Genitourinary (GU) cancers are some of the most common malignancies. Renal cell carcinoma (RCC) is the most common kidney tumor, with an estimated 62,700 new cases and an estimated 14,240 deaths in 2016 [1, 2] in the United States (US). Urothelial carcinoma of the bladder is more common than RCC, with an estimated 77,000 new cases and 16,390 deaths in 2016 [2] in the US. Adenocarcinoma of the prostate is the second most common cancer in men and the most common GU cancer, with more than 180,000 new cases in the US estimated in 2016 and an estimated 26,120 deaths per year [2].
Computed tomography (CT) is the major imaging modality for RCC and urothelial carcinoma of the bladder. The current recommendation of the National Comprehensive Cancer Network for initial staging of muscle-invasive bladder cancer (MIBC) is CT scan of the chest, abdomen, and pelvis or magnetic resonance imaging (MRI) of the abdomen and pelvis with non-contrast CT scan of the chest.
MRI is helpful in patients allergic to iodinated contrast agents and in patients with renal insufficiency, and may provide more information for the evaluation of locally advanced disease [3, 4]. Advanced MRI techniques are providing better assessment of renal and bladder tumors, while multiparametric MRI (mpMRI) is increasingly being used to diagnose prostate adenocarcinoma.
PET/CT is a powerful noninvasive tool used by major medical centers and many community hospitals for characterizing solid tumors. Fluorodeoxyglucose positron emission tomography (FDG-PET), and sodium fluoride (NaF)-PET are common functional and molecular imaging modalities used for staging and assessing treatment response [3, 5].
In this review, we discuss the latest advances in imaging and their potential impact on the diagnosis and management of RCC, urothelial carcinoma of the bladder, and prostate adenocarcinoma.
2. Renal cell carcinoma
2.1. CT in RCC
CT is the primary modality for the diagnosis, staging, treatment planning, and surveillance of RCC [6-8]. CT can be performed in multiple phases before and after injection of an intravenous (IV) contrast agent. The most reliable phase for the detection of RCC is the nephrographic phase, 70–100 sec after injection of IV contrast [6]. Because papillary carcinoma is hypovascular, the arterial phase of a kidney CT exam can differentiate between clear cell and papillary carcinoma with 95.7% accuracy and a sensitivity and specificity of 98.3%, and 92%, respectively [9]. Clear cell carcinoma, which accounts for 60%–65% of RCCs, appears as a hypervascular mass with a heterogeneous texture due to cystic or necrotic components [10]. Papillary RCC is usually homogeneous, well-defined, and hypovascular [10]. Chromophobic RCC has an intermediate vascularity, is usually well-circumscribed, and measures > 4 cm [10]. Multiphasic multidetector CT scan is useful in differentiating clear cell RCC from other subtypes of RCC and oncocytoma [11].
An important and unique finding in RCC is tumor extension and thrombosis to renal vein and inferior vena cava (IVC) [12]. This has been reported in 4%–10% of all renal neoplasms, having therapeutic and prognostic importance [13, 14]. This invasion can only be limited to the vessel wall. CT is very helpful for venous extension of RCC, best seen in the corticomedullary phase of contrast enhancement [15]. Enhancement of the thrombus will help to differentiate tumor thrombus from bland thrombus [15]. Although CT can be as sensitive as MRI in assessment of tumor thrombus, in some cases it may fail due to extrinsic pressure over the vein or inadequate filling of IVC [16].
Dual-energy CT (DECT) is a new type of CT scanner in which two sets of x-ray beams with two different energy levels are passing through the body, in contrast to traditional CT scans that have an x-ray beam with only one energy level [17-19]. The interaction of these two x-ray beams with body tissues can help in material identification and quantification [18]. These two CT datasets at a low or high polychromatic peak tube energy (kilovoltage, or kV) provide material-specific information that allows for additional characterization of the kidneys and urinary tract [20]. The two varying energy levels can enhance or mute the conspicuity of IV contrast. DECT can be used to interrogate iodine and calcium concentrations and increase the iodine signal to help differentiate pathologic processes and clarify the internal structure of mass lesions [21]. The capability to decompose materials and extract iodine can improve differentiation of lesions such as minimal enhancement in a hypodense kidney tumor (papillary HCC) from a cyst. Quantification of iodine in the lesion can be a biomarker of tumor vascularity [18].
Improving lesion conspicuity can potentially reduce the need for or frequency of follow-up imaging. DECT is helpful in differentiating between clear cell and papillary cell RCC, the latter of which accounts for approximately 15%–20% of all RCCs [6, 21], with an overall accuracy of 95.3% and a sensitivity and specificity of 98.2% and 86.3%, respectively [20]. DECT can also help to assess tumor response, such as changes in tumor vascularity and necrosis [22]. Another clear benefit of DECT is the potential to reduce the radiation dose by applying virtual non-contrast images, negating the need for pre-contrast CT series [23]. This is described in more detail below (CT in urothelial carcinoma).
2.2. MRI in localized invasive RCC: morphologic and functional information
MRI offers a wealth of morphologic and functional information for characterizing and staging of RCCs, which makes it a tool for preoperative staging of RCCs when used with chest CT [24, 25]. Inherent T1 and T2 signal intensity of the mass can be used to differentiate subtypes of RCC. For example, macroscopic fat within angiomyolipomas can easily be differentiated by detecting India ink artifacts surrounding the macroscopic fat-containing areas of the mass on in-and out-of-phase images [26]. Similarly, T2-weighted and post-contrast images can delineate the complexity of cystic or necrotic RCCs. Multiple studies have demonstrated close sensitivity and specificity of CT and MRI in detection of the contrast enhancement and complexity of renal masses. In a study of 69 renal masses, Israel et al. [27] were able to demonstrate similar results for categorization of masses into different Bosniak categories in 81% of CT and MRI exams. In the reminder of the cases, MR images may depict additional septa, thickening of the wall and/or septa, or enhancement, which may lead to an upgraded Bosniak cyst classification and can affect case management. Given the higher accuracy of MRI in depicting the fine details of complex cystic lesions, use of MRI is preferable to CT in cases where upgrading or downgrading the lesion would be consequential.
Diffusion-weighted imaging (DWI) exploits the random motion of water molecules. The extent of tissue cellularity and the presence of an intact cell membrane help determine the impedance of water-molecule diffusion, which can be quantitatively assessed using apparent diffusion coefficient (ADC) maps. The lower the ADC value, the more restricted the diffusion of water molecules in the microenvironment, which can be seen in tissues with a high nucleus-to-cytoplasm ratio or dysfunctional cell membranes such as tumor cells [28]. Several studies have investigated the role of this imaging method in characterizing RCC. A meta-analysis of nine studies of DWI's ability to differentiate benign from malignant renal masses [29] demonstrated the significant difference of ADC, with pooled weighted sensitivity and specificity of 88% and 72%, respectively.
The lack of ionizing radiation and high temporal resolution of MRI are beneficial in designing dynamic contrast-enhanced MRI (DCE-MRI) protocols, which typically have four phases: precontrast, arterial, nephrogenic, and excretory. Important information regarding vascular flow within the tumor can be obtained by acquiring images in different phases of contrast administration, which in turn can be helpful in differentiating benign vs. malignant lesions (Figure 1) [30]. In a study of 152 RCCs, Vargas et al. [31] demonstrated significantly higher enhancement of clear cell carcinomas in all phases compared to papillary and chromophobic lesions.
Figure 1.
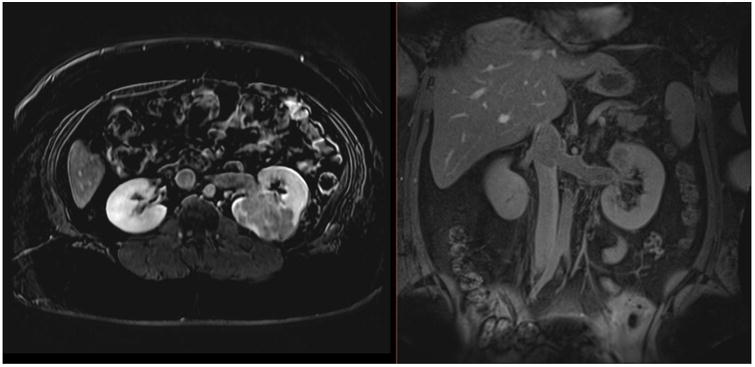
Dynamic contrast-enhanced MRI in a 27-year-old female with a large, enhancing high-grade RCC within the left kidney, with extension into the left renal vein and IVC on axial (A) and coronal (B) delayed post-contrast images. Patient underwent left nephrectomy in addition to IVC thrombectomy.
Extension of RCC into perinephric fat is associated with increased mortality independent of tumor size [32]. Therefore, determining fat involvement is an important prognostic indicator. MRI is superior to CT for detecting RCC involvement of the kidney capsule, perinephric fat, or Gerota's fascia (Figure 2) [33]. Stranding and enhancement of the perinephric fat are reliable signs of extension beyond the capsule [34]. However, early-stage involvement of Gerota's fascia can be difficult to assess [35]. Both multiphasic MRI and CT have sensitivity and specificity of > 90% and 80%, respectively, for detection of renal vein involvement in locally invasive RCCs [16]. Some studies have reported 100% accuracy of multiphase contrast-enhanced MRI for detection of renal vein thrombosis and extension into the IVC [36]. Enhancement of the thrombus is suggestive of tumor growth into the vessel rather than bland thrombosis. Overall, MRI provides a versatile array of morphologic and functional information for accurate pretreatment lesion characterization and local staging for patients with RCC.
Figure 2.
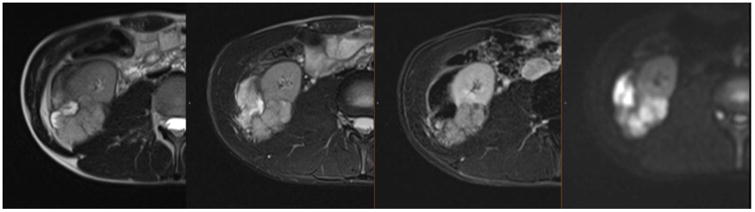
MRI in a 19-year-old male with a large, locally advanced high-grade RCC with papillary features in the setting of hereditary leiomyomatosis and RCC syndrome within the lower pole of the right kidney. The mass demonstrates heterogeneous signal intensity on T2-weighted images (two panels on left) and heterogeneous enhancement on post-contrast image (second from the right) and restricted diffusion on DWI image (right panel). There is extension of the mass into the mesonephric fat beyond the capsule and involvement of Gerota's fascia and right psoas muscle.
2.3. PET/CT for metastatic RCC
PET's utility in evaluating RCC stems primarily from the poor prognosis for patients with advanced disease, as indicated by the presence of metastases. In conjunction with anatomic imaging with CT and MRI, PET offers potentially high levels of visual conspicuity at the most common sites of metastatic disease. In order of frequency, these sites include the lungs, bones, lymph nodes, liver, adrenal glands, and brain [37]. Currently, clinical PET imaging for metastatic RCC is primarily achieved through metabolic imaging with FDG. Table 1 lists selected studies evaluating recurrent or metastatic RCC currently available in PubMed. Reviews, case reports, meta-analyses, and studies with < 10 subjects were excluded from our search. Studies listed calculate sensitivity and specificity but do not evaluate treatment response [38-56].
Table 1.
Selected studies evaluating recurrent or metastatic RCC.
| Author | Year | N | Study type | Instrument | Reference Std | Met Location | Sensitivity | Specificity | PPV | NPV | Acc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Safaei, et al. | 2002 | 36 | Retrospective | PET | Histology, clinical outcome | - | 87 | 100 | - | - | 89 |
| Wu, et al. | 2003 | 18 | Not Reported | PET | Histology, clinical outcome | Bone | 100 | 100 | - | - | - |
| Aide, et al. | 2003 | 53 | Prospective | PET | Histology, clinical outcome | - | 100 | 93 | NA | NA | 94 |
| Chang, et al. | 2003 | 15 | Retrospective | PET | Histology | Lung | 90 | 80 | - | - | 87 |
| Jadvar, et al. | 2003 | 25 | Retrospective | PET | Histology, clinical outcome | - | 87 | 75 | 94 | 33 | 72 |
| Majhail, et al. | 2004 | 24 | Retrospective | PET | Histology | - | 63.6 | 100 | 100 | - | - |
| Kang, et al. | 2004 | 66 | Retrospective | PET | Histology, clinical outcome | Retroperitoneum | 75 | 100 | - | - | - |
| Kang, et al. | 2004 | 66 | Retrospective | PET | Histology, clinical outcome | Lung | 75 | 97 | - | - | - |
| Kang, et al. | 2004 | 66 | Retrospective | PET | Histology, clinical outcome | Bone | 77 | 100 | - | - | - |
| Dilhuydy, et al. | 2007 | 24 | Retrospective | PET | Histology, clinical outcome | - | 75 | 50 | 92.3 | 33.3 | - |
| Park, et al. | 2009 | 63 | Retrospective | PET/CT | Histology, clinical outcome | - | 87 | 83.3 | 77.3 | 92.6 | 85.7 |
| de Llano, et al. | 2010 | 58 | Retrospective | PET | Histology, clinical outcome | - | 80.56 | 86.36 | 90.6 | 73.8 | 58.7 |
| Kumar, et al. | 2010 | 63 | Retrospective | PET/CT | Histology, clinical outcome | - | 90 | 91 | - | - | 90 |
| Nakatani, et al. | 2011 | 23 | Retrospective | PET | Histology, clinical outcome | - | 80 | 67 | 94 | 33 | 78 |
| Bertagna, et al. | 2013 | 68 | Retrospective | PET/CT | Histology, clinical outcome | - | 82 | 100 | 100 | 66.7 | 86.8 |
| Fuccio, et al. | 2014 | 69 | Retrospective | PET/CT | Histology, clinical outcome | - | 90 | 92 | 95 | 85 | 91 |
| Öztürk | 2015 | 132 | Retrospective | PET/CT | Histology, clinical outcome | - | 93.8 | 88.2 | 92.6 | 88.2 | 91.6 |
| Win, Aparici | 2015 | 315 | Retrospective | PET/CT | Histology | - | 100 | 100 | - | - | - |
| Alongi, et al. | 2016 | 104 | Retrospective | PET/CT | Histology, clinical outcome | - | 74 | 80 | 83 | 70 | 84 |
Positron emission tomography (PET), computed tomography (CT), positive predictive value (PPV), negative predictive value (NPV), accuracy (Acc)
RCC can present with limited uptake of FDG, likely due to the dominant incidence of the clear cell subtype of the disease. There is emerging evidence that the rarer papillary variant of RCC may have high avidity for FDG [56, 57]. For the clear cell subtype, there is growing interest in non-FDG molecular imaging agents that may have greater sensitivity and specificity and may potentially add phenotypic information for patient/tumor-specific treatment strategies.
Image acquisition and interpretation of RCC may benefit from more advanced techniques such as FDG-PET/CT. Because of the potentially low levels of FDG uptake in RCC, suspicion must remain high for lesions with mild FDG uptake that are concerning on CT. Additionally, foci of abnormal FDG uptake that may not be clearly identified as suspicious lesions on CT must also be considered important until proven otherwise (Figure 3). Thus, the combination of IV and oral contrast-enhanced CT as part of the CT acquired with PET, or as a separate examination that can be registered to PET, is highly valuable. The spatial combination of sensitivity and specificity of the respective modalities can not only aid in diagnosis and staging of multiple tumor types, but can also help to visually and spatially communicate radiologic findings among the subspecialties involved in patient care. For instance, biopsy and surgical planning with a PET/CT combination (Figure 4) can show the spatial coexistence of complex CT structures and elevated activity [58]. Structures with a large component of nonviable cells, necrosis, and fluid can be approached in favor of regions with viable tumor cells with intact cell membranes, as indicated by higher FDG activity. These regions do not always have a discriminatory appearance on CT. However, a certain level of comfort with FDG-PET is required due to the common confounding occurrence of structures with normal activity, such as urinary elimination in the renal calyces/pelvis, ureters, and bladder, as well as normal FDG activity in the gastrointestinal tract.
Figure 3.
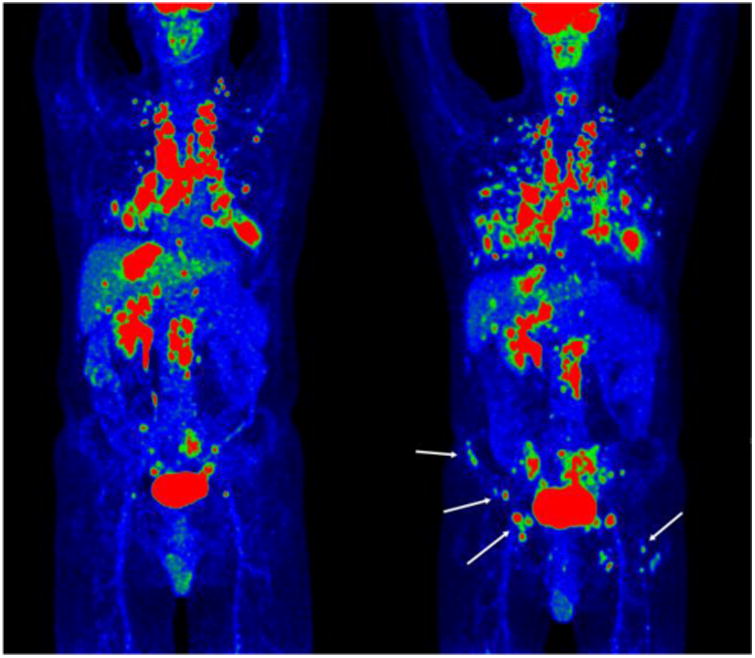
Pre- (left) and post-treatment (right) maximum-intensity FDG-PET projections of the whole body of a patient with RCC. The extent of the new sites of lung metastases are clearly observed, in addition to new sites of osseous metastatic disease (white arrows) in the right iliac wing and left femur, which were not detected on CT.
Figure 4.
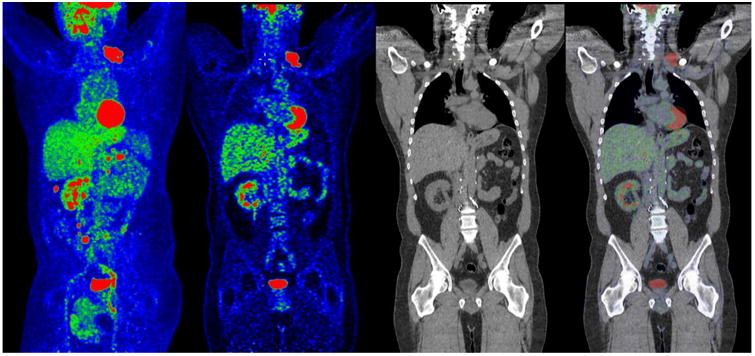
FDG-PET/CT of a patient with RCC. From left to right, maximum-intensity projection PET, coronal PET, CT, and PET/CT fusion are shown. Clearly visualized by PET, the left supraclavicular cluster of nodal metastasis was not detected on CT.
Another application of FDG in RCC is in venous tumor thrombus. Some investigators indicate that high FDG uptake is related to tumor thrombus and provide quantitative cutoffs to help differentiate tumor from bland thrombus [59]. However, prospective trials are currently limited, and we avoid quantitative cutoffs for this purpose because of the known high degree of variability among PET/CT scanners and methods of image reconstruction. The same concerns apply to the use of cutoffs for primary and distant metastatic RCC.
A recent meta-analysis reported an overall sensitivity and specificity for FDG-PET in identifying extrarenal lesions as 79% and 90%, respectively [38]. However, most of the studies included in the analysis were of PET without CT. A multimodality approach might achieve higher sensitivity and specificity. However, the clinical utility of FDG extends beyond traditional measures of diagnostic accuracy. Alongi et al. [51] observed that despite modest sensitivity and specificity for RCC (74% and 80%, respectively), imaging with FDG influenced the therapeutic strategy for a high proportion of patients (43%), and FDG-positive cases resulted in significantly lower five-year overall survival rates compared to FDG-negative cases (69% vs. 19%, respectively).
The high sensitivity of PET, the use of positron-emitting radiotracer agents with various potential tumor targets (glycolysis, hypoxia, amino acids, malignant cell proliferation and amino acid transport, Ab-Ag complex and receptors), and hybrid imaging technologies such as PET-MRI allow us to image malignant lesions more successfully than ever, which results in better patient care and outcomes [60].
3. Urothelial carcinoma of the bladder
3.1. CT scan in urothelial carcinoma
In the last few years, a major advance in cancer imaging has been improved multidetector CT acquisition quality with reduced radiation exposure, thanks to model-based iterative reconstruction and other dose-reduction initiatives. Processing speeds finally allow for real-time noise reduction to make up for reduced exposures. CT can detect UC in the bladder with a sensitivity of 79%–89.7% and specificity of 91%–94.7% [61]. Before patients undergo radical cystectomy, a contrast-enhanced CT or MRI scan is taken to assess local/regional tumor invasion and distant metastases. In MIBC and high-grade non-muscle invasive bladder cancer (NMIBC), CT of the abdomen and pelvis can help to stage and differentiate tumors with transmural spread to the extravesical space from tumors localized to the bladder wall. Furthermore, CT is relatively low-cost, more time-efficient, is often easier and faster to schedule than MRI or PET, and can assess nodal and metastatic stage. Unfortunately, CT is not optimal for accurately determining local extension and early lymph node involvement [5]. Specificity ranges from 68%–100% and does not consistently correlate with pathology because lymph node enlargement can be secondary to other etiologies [5].
Since the development of CT urography (CTU), IV pyelogram is no longer used in the diagnosis and surveillance of localized urothelial carcinoma of the bladder, ureter, and renal pelvis. Instead, a CT scan of the abdomen with and without contrast with delayed/excretory phase images can carefully assess the collecting system, ureters, and bladder, while evaluating the kidney for parenchymal lesions. The specificity and sensitivity of CTU in identifying the source of hematuria range from 83%–99% and 79%–95%, respectively [62-64]. Unfortunately, CTU cannot adequately stage the depth of invasion of the primary bladder tumor because it doesn't have the resolution to distinguish between individual layers of the bladder, nor can it distinguish high-risk MIBC from low-grade NMIBC [65]. In addition, CTU can miss lesions of < 1 cm and carcinoma in situ [66]. When CTU is employed following transurethral resection of bladder tumor (TURBT), inflammatory changes are indistinguishable from tumor extension, reducing the accuracy of local staging to approximately 60% [67]. Also, in patients on surveillance for NMIBC, upper urinary tract surveillance by CTU was inadequate, as only 29% of patients with upper urinary tract disease were diagnosed by routine CTU [68].
Although CTU is an excellent imaging modality for screening for hematuria and surveillance after NMIBC, it is of limited use in staging primary bladder tumors. However, an increase in bladder wall thickness of > 150% is associated with all-cause and urothelial carcinoma mortality [69]. Furthermore, CTU is only 70%–90% accurate in determining lymph node involvement, and false negative rates range from 25%–40%, likely due to CT's inability to distinguish between inflammatory and tumor-positive lymph nodes [68]. Nor is CT useful in detecting small (< 1 cm) lymph nodes with microscopic tumors. Overall, the accuracy of pelvic lymph node staging in localized bladder urothelial carcinoma by CT scan is 78%, with sensitivity and specificity ranging from 30%–53% and 94%–98%, respectively [70]. Thus, while CTU has replaced IV pyelogram for diagnosing and staging of urothelial carcinoma of the bladder, it has limitations (Figure 5).
Figure 5.
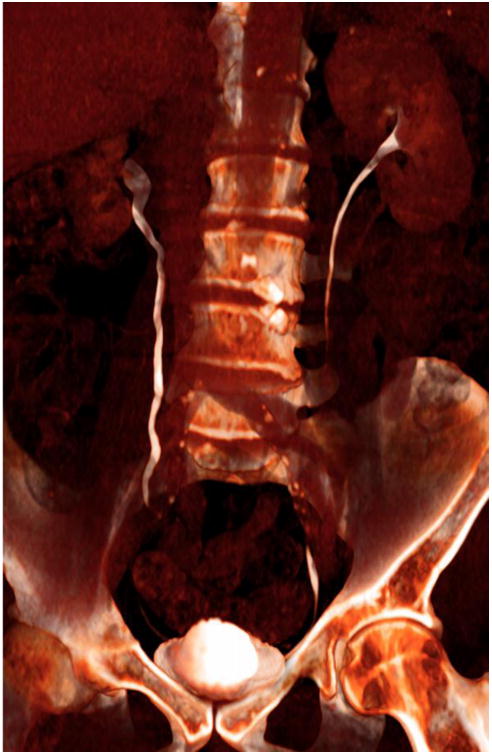
CT urogram multiplanar volume-rendered (MPVR) coronal reformation showing ureters in white, with normal peristalsis, hence incompletely seen the moment this CT was acquired. This was a 10-min delay for a hematuria workup.
DECT is a new imaging approach that is also being investigated in assessing metastatic urothelial carcinoma and RCC, as described above. DECT, which uses two merged CT datasets, provides additional characterization of the urinary tract and abdominal organs. In addition to technological iterative reconstruction solutions to reduced dose, dual energy and virtual non-contrast allow for what is called split-dose CT urogram. The subtraction of IV contrast from CT-acquired data is called virtual non-contrast CT (VNC). In this split-dose CT urogram, 1/3 of the total IV dose is given 8 mins prior to scan; the last 2/3 of IV contrast are injected 2 mins prior to scan. Giving the IV contrast 8 mins prior to scan effectively provides for a delay superimposed on the venous-phase CT images. Then VNC is applied to that dataset to subtract all IV contrast, providing the non-contrast images. This effective triple-phase exam (non-contrast, 2-min, and 8-min delay) is completed in one scan acquisition at 1/3 the radiation dose, since the delay and non-contrast pass are not needed [71]. In addition to the benefits of reduced radiation exposure, iodine mapping and other material characterization post-processing analysis have several benefits.
For patients undergoing treatment for metastatic urothelial carcinoma, there has been a concerted effort to improve tumor quantification with automated volumetric measurements, and to assess tumor necrosis using CT density measurements as a response to therapy (Figure 6) [72]. Many ongoing imaging studies are embedded in clinical trials of antiangiogenic agents, chemotherapy, and immunotherapy with checkpoint inhibition (NCT02788201, NCT01688999 and NCT02496208), with the goal of improving tumor burden and treatment response assessment in metastatic urothelial carcinoma.
Figure 6.
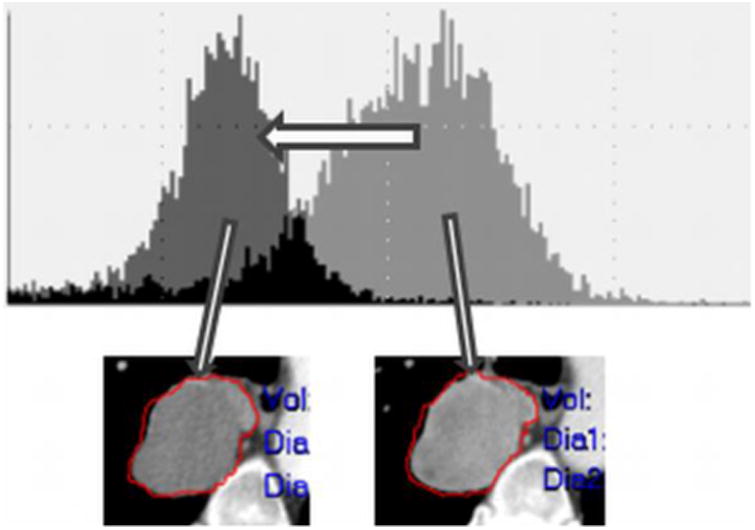
Demonstration of viable tumor volume (VTV) where a metastatic urothelial carcinoma lesion became less dense (less IV contrast enhancement) visually; however, the left shift of the histogram objectively represents likely tumor necrosis from an antiangiogenic treatment.
3.2. MRI in localized muscle-invasive bladder cancer
Routine MRI protocol includes mpMRI sequences, i.e., T1, T2, diffusion-weighted precontrast; T1-weighted gradient echo pre- and contrast-enhanced MRI sequences targeted to the urinary bladder/pelvis; and, when appropriate, a single non-contrast, T1-weighted sequence of the lumbar spine in the axial plane for further evaluation of possible bone metastasis. The mpMRI bladder/pelvis and lumbosacral sequences can be accomplished in a single session without moving the patient [73].
For MIBC and high-grade NMIBC, MRI can detect and stage tumors with relatively high sensitivity and specificity, with the advantage of not having the ionizing radiation associated with CT [5]. Though not commonly used, MRI of the pelvis can stage the bladder tumor after cystoscopic diagnosis. Although TURBT is the preferred, and most accurate, technique for staging NMIBC and MIBC tumors, retrospective analyses have found that TURBT has understaged cases by 42% [74]. MRI, on the other hand, provides extensive soft-tissue resolution. It has even been shown to detect T3 and T4 disease and has proven superior to CT in distinguishing between stages T2a and T2b by identifying bladder-wall invasion [75].
Traditional T1-weighted imaging can identify extravesical fat infiltration, pelvic lymphadenopathy, and suspected bone metastases, but is of limited use in local staging of urothelial carcinoma because these tumors appear similar to the normal detrusor muscle of the bladder wall due to similar signal intensities [76, 77]. On the other hand, T2-weighted imaging can differentiate between tumor edges and the bladder wall because urothelial carcinomas have increased T2-weighted signaling compared to normal detrusor muscle [78].
Triplanar anatomical T1- and T2-weighted sequences are used for local tumor staging of urothelial carcinoma, and have been used to make 3D virtual MR cystoscopies which have detection rates of > 90% [5]. In cases of conventional visualization obscured by significant hematuria, urothelial stricture disease, or lesions within the diverticula, virtual MR cystoscopy can provide an alternative to, and perhaps better visualization than, conventional modalities.
Other forms of MRI, including DWI-MRI and DCE-MRI, are commonly used in RCC and provide functional sequences that can differentiate between tumor and normal detrusor muscle. DWI-MRI can characterize tissue by measuring the Brownian motion of water molecules [79]. In DWI-MRI, malignant tissue has lower apparent diffusion coefficient values than normal tissue. Sensitivity and specificity have been shown to be 80% and 79%, respectively, but when used in combination with anatomic T2-weighted MRI, sensitivity and positive predictive value were 100% in a small cohort (n = 15) of patients. This combination has been shown to provide more accurate staging in patients in clinical stage ≤ T2 [80, 81]. On the other hand, DCE-MRI has been found to be superior to traditional unenhanced T1- and T2-weighted anatomic imaging owing to its ability to distinguish urothelial carcinoma from normal bladder wall. The hypervascularity of tumor tissue leads to earlier and better contrast enhancement compared to normal bladder wall. One study revealed 84% accuracy for DCE-MRI in tumor staging compared to 67% accuracy for unenhanced T1- and T2-weighted imaging [82]. A prospective study investigating the role of DCE-MRI in 122 patients demonstrated an 87.5% sensitivity for differentiation of lymph node-negative organ-confined UC from nonorgan-confined urothelial carcinomaand accuracy of 74%. However, the specificity of MRI findings were mediocre at 47.6% [83].
MR urography (MRU) is used to evaluate the urinary system while avoiding radiation exposure. However, the technology is time-intensive and costly, and requires technical expertise to produce adequate images. As with CTU, the use of MRU is predicated on adequate renal function, because IV gadolinium contrast is required to evaluate the urinary system. In the imaging of bladder tumors, MRU does not offer any improved ability to determine local stage of invasion [84]. However, T1- and T2-weighted imaging might confirm the presence of a bladder tumor. With T1-weighted imaging, a bladder tumor will likely appear as an area of intermediate intensity, while with T2-weighted imaging, a bladder tumor will appear hypointense to the surrounding urine. The Mayo Clinic described a large retrospective analysis of MRU, specifically examining differences between the parenchymal and pyelographic phases [84]. They found that the parenchymal phase was superior for lesion detection in the bladder. Overall accuracy ranged from 78%–87%; sensitivity and specificity ranged from 73%–85% and 75%– 91%, respectively [84]. Recently, a Danish group prospectively compared split-bolus CTU and MRU to flexible cystoscopy, and found that MRU had a slightly superior sensitivity to CTU with similar specificity (sensitivity: 76.9% vs. 61.5%; specificity: 93.4% vs. 94.9%) [85]. Although MRU provides no additional diagnostic information with or without CTU, it can serve as an alternative imaging modality in urothelial carcinoma.
3.3. FDG-PET/CT in metastatic UC
At diagnosis, 10%–15% of patients with urothelial carcinomaare found to already have distant metastatic disease, and 50% of those with true localized disease eventually develop metastasis within two years, even with aggressive therapy [86]. Molecular imaging is critical in accurately staging and evaluating response to treatment, and in developing a therapeutic strategy that avoids unnecessarily aggressive interventions and maximizes quality of life. FDG is the most established radiotracer, but is not widely used in urothelial carcinoma because it is excreted through the urinary tract and bladder. While FDG is not ideal for detecting primary disease, its greatest value is in assessing metastatic lesions and disease recurrence.
FDG-PET/CT has been studied in a few trials, most of which were limited by small patient numbers and lack of histopathologic correlation. Nevertheless, FDG-PET/CT has shown promise in evaluating metastatic disease. A systematic review and meta-analysis in 2012 found only six studies that fit standardized criteria, but pooled data on sensitivity and specificity of FDG-PET/CT for staging or restaging of metastatic disease was 82% and 89%, respectively. Its accuracy for diagnosing metastatic urothelial carcinoma lesions was deemed satisfactory [87].
Early studies have reported superior sensitivity and specificity for FDG-PET/CT in diagnosing urothelial carcinoma metastases. More lesions were detected with FDG-PET/CT than with CT or MRI, which substantially affected clinical management [60]. In evaluating response to therapy in metastatic urothelial carcinoma, Ozturk et al. found FDG-PET/CT and European Organisation for Research and Treatment of Cancer criteria more accurate in determining remission and overall response rate than CT and RECIST criteria [88]. FDG-PET/CT also appears useful in metastatic urothelial carcinoma, with an associated survival advantage in those with a favorable response on imaging after two cycles of first-line chemotherapy (Figure 7) [89].
Figure 7.
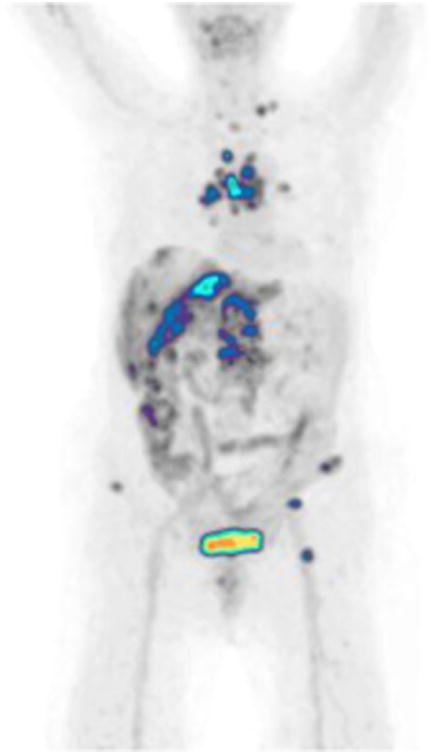
A 62-year-old female with metastatic urothelial carcinoma. Focal abnormal FDG metabolism in supraclavicular, mediastinal, hepatic, retroperitoneal, and osseous metastases.
There are conflicting viewpoints on preoperative staging. Scanning with FDG-PET/CT before radical cystectomy in MIBC has detected metastatic lesions not seen on conventional CT or bone scintigraphy, which significantly correlated with overall survival [90]. Another study demonstrated that FDG-PET/CT was more sensitive than CT at lymph node staging prior to radical cystectomy and had histopathologic validation from lymph node dissection [91]. However, another study asserted that FDG-PET/CT added only a small benefit in detecting lymph node metastasis outside the pelvis compared to CT, with 69% sensitivity and 95% specificity for FDG-PET/CT compared to 41% sensitivity and 98% specificity for CT, which was not considered enough of an advantage to justify the use of FDG PET/CT [92]. A recent study agreed that FDG is no better than CT in detecting metastatic lymph nodes in initial staging of MIBC prior to radical cystectomy [93].
Using FDG-PET/CT for restaging after surgical treatment appears more promising. With a sensitivity of 87.1% and specificity of 89.7%, it outperformed CT, ultrasound, and MRI in changing management and correctly restaging urothelial carcinoma after surgery [94]. Ozturk similarly demonstrated good sensitivity, specificity, positive predictive value, and negative predictive value for FDG-PET/CT in identifying local recurrence and distant metastases in restaging scans after radical cystectomy for MIBC [95].
Despite some disagreement on its value in staging, FDG-PET/CT appears to provide constructive information to guide treatment in this challenging malignancy. The technology is widespread, costs for the scanning procedure are reasonable, and the radiation exposure is acceptable for cancer patients. Imaging metastatic urothelial carcinoma with FDG-PET/CT can efficiently lead to advanced treatment options that may improve patient outcomes and pave the way for innovative therapies.
3.4. PET/MR in metastatic urothelial carcinoma
Both anatomical and nuclear medicine imaging techniques play an important role in the detection of primary and/or local relapse of UC, as well as lymph node and skeletal metastases. Most centers rely on mpMRI for this purpose. The main advantage of MRI is its excellent anatomic resolution, which makes it highly accurate in detecting local recurrence. The advantage of PET/CT is its ability to delineate biochemical or physiologic phenomena, making it easier to differentiate between benign and malignant lesions [96]. MRI-PET, FDA-approved in 2011, simultaneously performs a PET scan and an MRI scan, combining the advantages of PET and MRI, which may be even more beneficial in managing patients with cancer [97].
mpMRI of the bladder and pelvis combined with whole-body or targeted PET imaging may serve as a single staging study, or “one-stop” imaging tool, for patients with intermediate and high-risk urothelial carcinoma(Figure 8). In the PET/CT modality, CT does not provide the excellent soft-tissue contrast that MRI does, yet it gives patients a significant dose of radiation. MRI has the advantage of having no ionizing radiation [98]. Also, PET/CT imaging does not allow for true simultaneous imaging, as does PET/MRI [97]. MRI's anatomical resolution is superior to CT's, while PET provides metabolic and membrane receptor information. In addition to FDG, which is the most commonly used radiotracer in PET/CT, several other radiotracers are available for PET/MRI imaging in the management of urothelial carcinoma (Table 2). Based on preliminary studies, hybrid PET/MRI appears to provide superior sensitivity and specificity for tumor detection and characterization. Further studies are required to determine whether simultaneous acquisition of MRI and PET provide more accurate imaging than subsequent sequencing (Figure 9). In addition, PET/MRI's diagnostic accuracy, influence on therapeutic management, and related economic factors need to be carefully considered and evidenced by larger prospective clinical studies [73]. We found that PET/MRI allowed us to detect malignant lesions more accurately and confidently, potentially allowing physicians to improve treatment planning and disease monitoring (Figure 10) [97].
Figure 8.
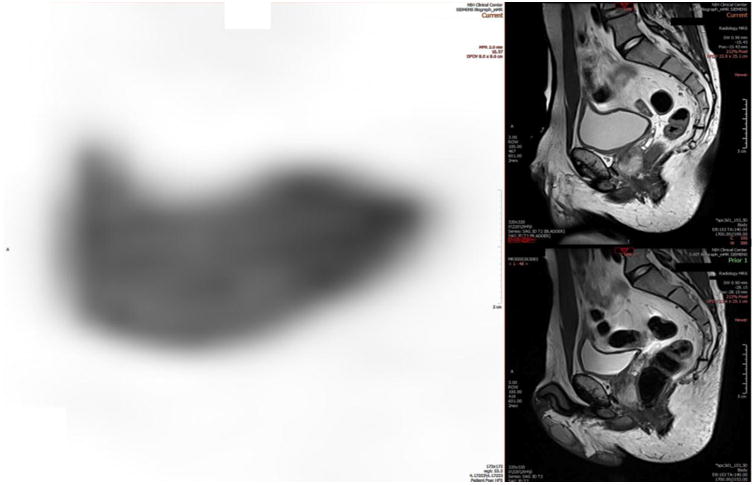
mpMRI of the bladder combined with targeted PET imaging. Moderately distended urinary bladder activity. Adequate image magnification during the interpretation, and diluted radioactive urine by adequate hydration of the patient during and prior to image acquisition are simple factors that improve our ability to properly evaluate the wall of the urinary bladder. A moderately distended urinary bladder is more optimally imaged compared to a non-distended or excessively distended bladder, both of which may hinder accurate visualization of a bladder wall tumor.
Table 2.
Radiotracers used for PET/MRI imaging of urothelial carcinoma.
| Metabolic Tracers | 18F-FDG |
| 11C-choline | |
| 18F-FM choline | |
| 11C-acetate | |
| Amino Acids | 11C-methionine |
| 18F-FLT | |
| Anti-18F-FACBC | |
| Receptor-Specific | 18F-FDHT (androgen receptors) |
| 18F-FMISO (tumor hypoxia, ischemic tumors) | |
| 124I-cG250 (Ab-CaIX- carbonic anhydrase IX clear cell carcinomas) identification of RCC* | |
| Bombesin – neuropeptide, 4 subtype | |
| 18F-DCFPyL | |
| 68Ga-PSMA (HBED-CC) |
Potential agent for local radioimmunotherapy: 131I-cG250 (Ab-CaIX- carbonic anhydrase IX RCC)
fluorine-18-fluorodeoxyglucose (18F-FDG), fluorine-18- Fluoromethylcholine (18F-FM Choline), 3′-deoxy-3′ [(18)F]-fluorothymidine (18F-FLT), anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid (Anti-18F-FACBC), 18F-fluoro-5α-dihydrotestosterone (18F-FDHT), 18fluorine-fluoromisonidazole (18F-FMISO), 24I-labeled chimeric monoclonal antibody G250 (124I-cG250), 18F[fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid (18F-DCFPyL), 68Gallium-labelled ligand of the prostate-specific membrane antigen Glu-NH- CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)] (68Ga-PSMA (HBED-CC)), clear cell renal cell carcinoma (RCC)
Figure 9.
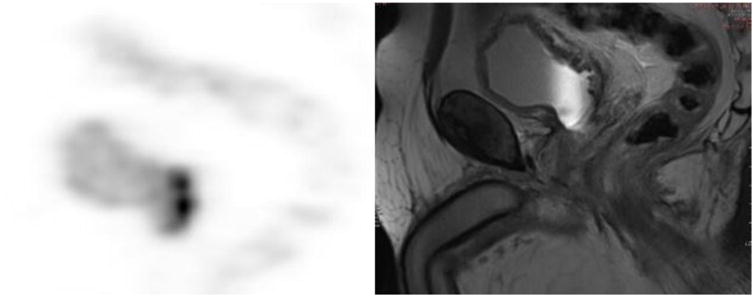
Urothelial carcinoma FDG-PET/MRI. Focal wall thickening and increased FDG activity of the posterior inferior bladder wall in its neck and within the superior portion of the prostate are present.
Figure 10.
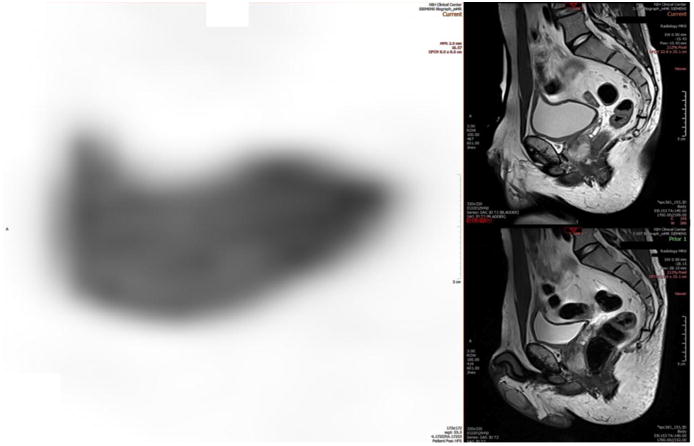
Urothelial carcinoma FDG-PET/MRI. A 59-year-old male with high-grade non-muscle-invasive bladder cancer post-TURBT and intravesical chemotherapy. PET/MRI showed activity at or near the expected segment of intraprostatic urethra and retroperitoneal lymph nodes, upgrading the stage.
In summary, although current evidence shows a good correlation between PET/CT and PET/MRI in lesion detection of most oncologic diseases, including urothelial carcinoma, there are not enough clearly defined prospective studies with enough patients to validate these findings. Initial results are encouraging, particularly the reduction in radiation exposure. However, there is as yet no approved indication by which PET/CT is replaced by PET/MRI. PET/MRI may replace PET/CT for certain indications, such as a need for higher soft tissue resolution. For some tumors, the clear benefits of PET/MRI are its one-stop ease of use, comprehensive tumor staging, and potentially improved lymph node staging in the abdomen and pelvis. An ongoing study is testing PET/MRI in metastatic urothelial carcinoma in addition to conventional CT (NCT02788201).
4. Prostate adenocarcinoma
4.1. CT scan in staging and restaging of prostate adenocarcinoma
CT has a limited role in prostate adenocarcinoma staging, since many patients have metastases predominantly to bone [99]. CT is often used in the initial staging of advanced disease to follow nodal involvement and bone metastasis, and is especially useful in excluding extensive osseous metastasis or evaluating pathologic fractures. Although nodal metastasis on CT is specific, there is low sensitivity [100].
4.2. MRI in localized prostate adenocarcinoma assessment and biopsy
mpMRI of the prostate has been extensively studied in the last two decades. In prostate adenocarcinoma, it was initially used for localization and staging [101]; however, recently it has been increasingly employed to detect intraprostatic lesions for biopsy purposes. This shift in approach is a result of the limitations of transrectal ultrasound (TRUS)-guided systematic biopsies, which could lead to underdiagnoses of clinically significant prostate adenocarcinomas [102]. mpMRI can be employed for cognitive fusion-targeted, in-bore MRI guidance and TRUS/MRI fusion biopsies. The TRUS/MRI fusion method has been extensively studied [103]. In this technique, mpMRI's superior lesion detection is united with TRUS's real-time imaging and intervention capability to accurately guide a biopsy needle within the fused images by electromagnetic tracking or an angle-sensing encoder-loaded mechanical arm [103]. In several large-scale prospective studies, TRUS/MRI fusion-guided biopsy detected more clinically significant prostate adenocarcinomas than TRUS-guided systematic biopsy (Figures 11 and 12) [104-106]. In addition to lesion detection and cancer diagnosis by targeted biopsy approach, mpMRI-guided biopsy can also establish tumor heterogeneity, which can influence patient management and treatment outcomes [107]. Although it is not fully established in clinical routine, mpMRI targeted biopsy can potentially enable sampling of the worst portion of primary prostate adenocarcinoma lesions and aid accurate treatment management [108].
Figure 11.
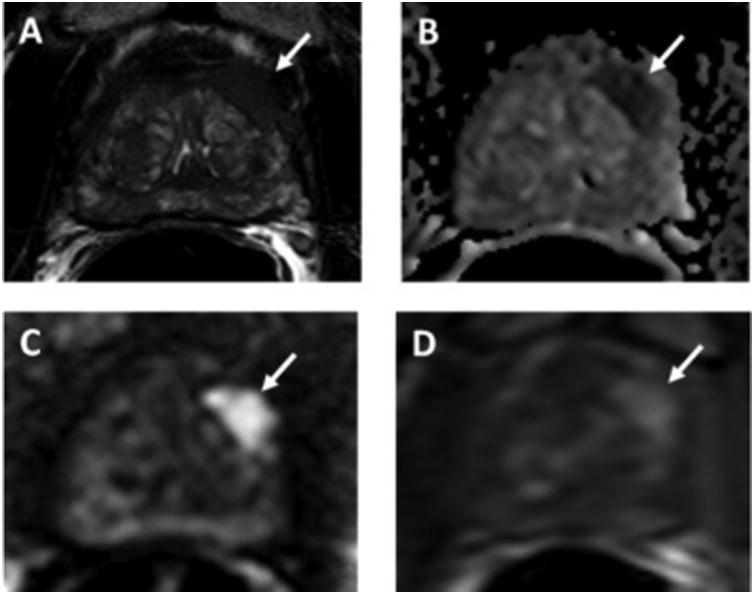
A 68-year-old man with serum PSA of 4.88 ng/mL and history of 2 prior negative TRUS-guided biopsies. Axial T2W MRI shows a hypointense lesion in the left apical anterior transition zone (arrow) with capsular bulge (A). The lesion shows restricted diffusion on ADC maps (B) and b2000 DW-MRI (C) (arrows) and hypervascularity on DCE-MRI (D) (arrow). This PI-RADS 5 lesion underwent TRUS/MRI fusion-guided biopsy, which revealed Gleason 4+5 prostate adenocarcinoma.
Figure 12.
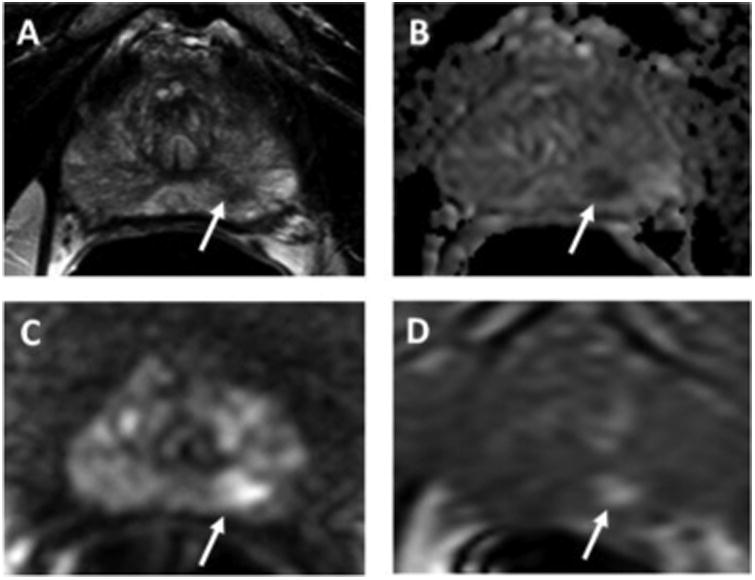
A 72-year-old man with serum PSA of 4.03 ng/mL with no prior biopsy. Axial T2W MRI shows a hypointense lesion in the left apical peripheral zone (arrow) (A). The lesion shows restricted diffusion on ADC maps (B) and b2000 DW-MRI (C) (arrows) and hypervascularity on DCE-MRI (D) (arrow). This PI-RADS 4 lesion underwent TRUS/MRI fusion-guided biopsy, which revealed Gleason 3+4 prostate adenocarcinoma.
Although mpMRI has been reported to accurately detect and stage prostate adenocarcinoma lesions, it still has certain limitations, mainly related to heterogeneity in image acquisition and interpretation and data processing, all of which strongly depend on the radiologist's level of experience. To address this, in early 2015, Prostate Imaging Reporting and Data System (v.2) guidelines were established by the American College of Radiology and the European Society of Urogenital Radiology [109] that aim to standardize all aspects of mpMRI. Initial prospective studies have revealed promising findings on its use in improving prostate adenocarcinoma care [110] and its impact on inter-observer agreement and related cancer detection [111, 112]. A multi-reader study with 34 patients revealed average sensitivity values of 91% and 63% for detecting index and all lesions across all readers, respectively. Index of specific agreement among readers was 93% for the detection of index lesions, 74% for the detection of all lesions, and 85% for scoring index lesions, and 58% for scoring all lesions [111]. However, more prospective studies are needed to better understand this new system. Several research groups are also working on developing new pulse sequences and signal-processing techniques to further improve the detection capability of mpMRI. New research developments include a) motion correction techniques like BLADE, a T2-weighted MRI sequence, b) use of calculated high b value diffusion-weighted images, and c) clinical use of hyperpolarized 13C MR, a new spectroscopic technique for investigating the metabolism of prostate adenocarcinoma [113-115]. These novel imaging techniques require further evaluation in larger studies.
In summary, while mpMRI has had a significant impact on prostate adenocarcinoma care through its use in biopsy guidance, it needs to be standardized and improved for better cancer detection and staging.
5. Nuclear medicine imaging in prostate adenocarcinoma
Prostate adenocarcinoma imaging is an area of vigorous molecular PET research, and could potentially affect hundreds of patients. Detection of tumor and accurate staging is critical in managing appropriate treatment strategies for patients with prostate adenocarcinoma. 18F FDG is not a new tracer but is the most common PET radiotracer commercially available for oncological use. However, its utility in prostate adenocarcinoma is confined to aggressive, poorly differentiated disease (Figure 13) [116, 117]. Several novel imaging agents have been developed in the last decade that show a better ability to identify malignancy. It is beyond the scope of this paper to discuss all available radiotracers under investigation or go into great depth, but we will highlight a few notable agents.
Figure 13.
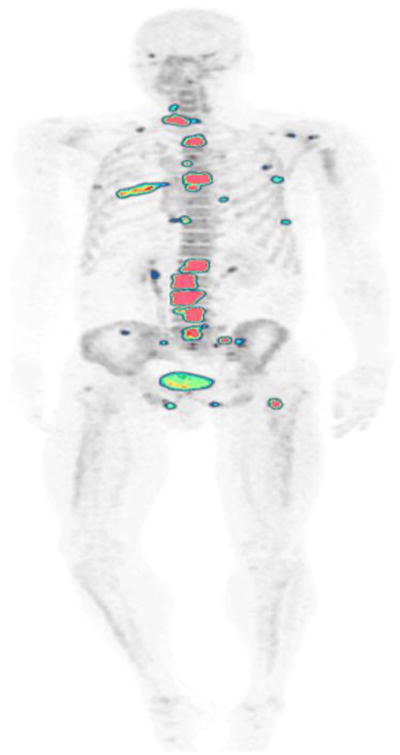
A 53-year-old man with history of prostate adenocarcinoma. NaF-PET/CT demonstrates focal abnormal uptake in multiple bone metastases throughout the skeleton.
11C choline was approved by the FDA in 2012 for evaluating biochemical recurrence in prostate adenocarcinoma patients. The mechanism of action is based on the increased use of choline for the accelerated production of prostate adenocarcinoma cell membranes. Many studies have assessed its value in prostate adenocarcinoma identification, with low sensitivity for primary disease and mixed reviews, demonstrating overall a moderate ability to detect nodal and metastatic tumors after definitive treatment by radical prostatectomy or radiation therapy, and a fair amount of false positive results [118-121]. A fluorinated version has also been developed and is currently available in Europe. Essentially, it is equally effective except for variations in physiologic distribution and a longer half-life radioligand that allows for wider transportation and delivery access.
11C acetate also targets molecular pathways involved in prostate adenocarcinoma cell membrane assembly through upregulated fatty acid synthase, which preferentially converts acetate to fatty acids for incorporation into malignant cell membranes. It also is ineffective in primary disease detection, and is only marginally successful in detecting biochemically recurrent disease [122-124]. Radiolabeled choline and 11C acetate generally perform similarly, but in patients with low PSA values they are not as effective in identifying tumor recurrence [125]. Moreover, with a short (20-min) half-life, 11C acetate's use outside of large research institutions is limited.
18F fluciclovine (also known as 18F FACBC) is the newest agent to gain FDA approval (2016) for biochemical recurrence in prostate adenocarcinoma. An amino acid analog to leucine, it appears to have high uptake in prostate adenocarcinoma cells and performs comparably to 11C acetate and radiolabeled choline in primary, recurrent, and advanced disease [126, 127]. In a recent multicenter trial with almost 600 patients, 18F FACBC identified tumor in only 68% of patients with biochemically recurrent disease [128]. With its recent approval, more experience will be gained to further assess its capabilities in detecting prostate adenocarcinoma in all phases.
Radiotracers under the most aggressive investigation are those that pursue prostate-specific membrane antigen (PSMA), a transmembrane receptor overexpressed on most prostate adenocarcinoma cells. Interestingly, the only other FDA-approved agent for detecting recurrent prostate adenocarcinoma is 111In-capromab pendetide (ProstaScint®), an older radiolabeled PSMA antibody that has fallen out of favor due to poor performance. Next-generation compounds have been much more successful by targeting an external epitope to PSMA, unlike ProstaScint, which has an affinity for an internal epitope. Improved antibodies to PSMA are still being studied, but the small molecule receptor antagonists are rapidly influencing prostate adenocarcinoma imaging. 68Ga-PSMA-HBED-CC PSMA (68Ga PSMA), the most studied tracer in Europe, is slowly gaining attention in the US. Despite its brief history, a recent meta-analysis found that 68Ga PSMA had similar or better sensitivities and specificities for detecting prostate adenocarcinoma than choline compounds [129]. Whereas 11C acetate, radiolabeled choline, and 18F fluciclovine are best used in progressive prostate adenocarcinoma, PSMA tracers appear promising in all stages of the disease and could be helpful in distinguishing challenging benign prostatic tissue from primary tumor [130, 131]. Limited data available for 68Ga PSMA indicate a considerable false negative rate, but low false positive findings [132, 133]. 18F DCFPyL, one of the newest low molecular weight inhibitors to join the field, has also shown similarly encouraging results in detecting primary, recurrent, and metastatic prostate adenocarcinoma and is logistically appealing, with a 110-min half-life and cyclotron radionuclide synthesis [134-136]. Further trials are underway exploring the ability of alpha- and beta-emitter PSMA inhibitors to therapeutically target prostate adenocarcinoma [137, 138].
18F NaF is the oldest and one of the most accurate radiotracers available for detecting bone metastasis. However, there is reluctance to abandon planar bone scintigraphy despite many publications demonstrating the superiority of 18F NaF [139-143]. Imaging prostate adenocarcinoma bone metastasis has traditionally relied on 99Tc bone scintigraphy, which is widely available in most communities but requires a 3- to 4-hour time commitment for patients and has only 47% sensitivity and 88% specificity. Moreover, it is not reliable in detecting early metastasis. If single photon emission computed tomography (SPECT) is added, the sensitivity improves to 82% and specificity to 99%. However, the field of view is limited and requires more scanning time, (usually an extra 45 minutes or more) and is therefore not routinely used [144].
An important distinction regarding 18F NaF is that it detects osteoblastic activity that could be in response to tumor, trauma, or infection and not direct cancer cells; therefore, specificity is low. Especially in the prostate adenocarcinoma patient population, degenerative bone changes due to osteoarthritis can sometimes be difficult to distinguish from malignant disease. These limitations are also found with planar bone scintigraphy. All the tracers previously discussed bind to prostate tumor cells and have the advantage of demonstrating focal uptake in soft tissue as well as bone lesions. Nevertheless, as a marker for bone metastasis, 18F NaF is a potent tool with better image quality and detection rate than bone scintigraphy. Recent research showed that 18F NaF PET/CT findings at baseline and over time correlated with clinical outcomes and survival in advanced prostate adenocarcinoma [145]. Against diffusion-weighted MRI, 18F NaF had better sensitivity but lower specificity for prostate adenocarcinoma metastasis to bone [146]. Research by the National Oncologic PET Registry found that as a staging mechanism for osseous metastasis, 18F NaF scans highly influenced patient management in hundreds of prostate adenocarcinoma cases [147]. Clinicians relied on 18F NaF over other advanced imaging techniques such as CT and MRI, and avoided invasive procedures. Further indications point to 18F NaF's value for monitoring treatment response and prognosis in novel bone-specific therapies, such as 223Ra and dasatinib [148-150]. 18F NaF is an excellent imaging agent for detecting or eliminating bone metastasis in prostate adenocarcinoma. It is emerging as a practical method for evaluating the effects of therapy on bone disease.
6. Conclusion
Multidetector CT, morphologic and functional MRI, and PET/CT can provide valuable information regarding diagnosis, staging, treatment planning, and follow-up of RCC and urothelial carcinoma. mpMRI of the prostate has had a significant impact on prostate adenocarcinoma care, but needs standardization and improved accuracy in detection and staging. PET, with its inherently high sensitivity, and PET/CT functional and molecular imaging are very useful for detecting distant metastases. These and other technologies and approaches, such as the use of positron-emitting radiotracer agents and hybrid imaging technologies such as PET/MRI, enable us to image malignant lesions more successfully than ever, which results in better patient care and outcomes [60]. These newer imaging modalities are increasingly being incorporated into therapeutic clinical trials of GU cancers, which will aid in further determining their clinical impact.
Acknowledgments
This research was supported by the NIH Clinical Center Intramural Research Program
Footnotes
The content is the responsibility of the presenter and does not necessarily represent the official views of the National Institutes of Health
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 3.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–24. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Mueller-Lisse UG, Mueller-Lisse UL. Imaging of advanced renal cell carcinoma. World J Urol. 2010;28:253–61. doi: 10.1007/s00345-010-0557-z. [DOI] [PubMed] [Google Scholar]
- 5.Rais-Bahrami S, Pietryga JA, Nix JW. Contemporary role of advanced imaging for bladder cancer staging. Urologic oncology. 2016;34:124–33. doi: 10.1016/j.urolonc.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Sankineni S, Brown A, Cieciera M, Choyke PL, Turkbey B. Imaging of renal cell carcinoma. Urologic oncology. 2016;34:147–55. doi: 10.1016/j.urolonc.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Sacco E, Pinto F, Totaro A, D'Addessi A, Racioppi M, Gulino G, et al. Imaging of renal cell carcinoma: state of the art and recent advances. Urologia internationalis. 2011;86:125–39. doi: 10.1159/000322724. [DOI] [PubMed] [Google Scholar]
- 8.Leveridge MJ, Bostrom PJ, Koulouris G, Finelli A, Lawrentschuk N. Imaging renal cell carcinoma with ultrasonography, CT and MRI. Nature reviews Urology. 2010;7:311–25. doi: 10.1038/nrurol.2010.63. [DOI] [PubMed] [Google Scholar]
- 9.Ruppert-Kohlmayr AJ, Uggowitzer M, Meissnitzer T, Ruppert G. Differentiation of renal clear cell carcinoma and renal papillary carcinoma using quantitative CT enhancement parameters. AJR American journal of roentgenology. 2004;183:1387–91. doi: 10.2214/ajr.183.5.1831387. [DOI] [PubMed] [Google Scholar]
- 10.Cupido B, Sam M, Winters S, Ahmed B, Seidler M, Huang G, et al. A practical imaging classification for the non-invasive differentiation of renal cell carcinoma into its main subtypes. Abdominal radiology (New York) 2016 Oct 14; doi: 10.1007/s00261-016-0940-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Young JR, Margolis D, Sauk S, Pantuck AJ, Sayre J, Raman SS. Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology. 2013;267:444–53. doi: 10.1148/radiol.13112617. [DOI] [PubMed] [Google Scholar]
- 12.Ciancio G, Soloway M. Resection of the abdominal inferior vena cava for complicated renal cell carcinoma with tumour thrombus. BJU international. 2005;96:815–8. doi: 10.1111/j.1464-410X.2005.05719.x. [DOI] [PubMed] [Google Scholar]
- 13.Mootha RK, Butler R, Laucirica R, Scardino PT, Lerner SP. Renal cell carcinoma with an infrarenal vena caval tumor thrombus. Urology. 1999;54:561. doi: 10.1016/s0090-4295(99)00136-3. [DOI] [PubMed] [Google Scholar]
- 14.Zisman A, Wieder JA, Pantuck AJ, Chao DH, Dorey F, Said JW, et al. Renal cell carcinoma with tumor thrombus extension: biology, role of nephrectomy and response to immunotherapy. The Journal of urology. 2003;169:909–16. doi: 10.1097/01.ju.0000045706.35470.1e. [DOI] [PubMed] [Google Scholar]
- 15.Nouh MA, Inui M, Kakehi Y. Renal Cell Carcinoma with IVC Thrombi; Current Concepts and Future Perspectives. Clinical medicine Oncology. 2008;2:247–56. doi: 10.4137/cmo.s464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallscheidt PJ, Fink C, Haferkamp A, Bock M, Luburic A, Zuna I, et al. Preoperative staging of renal cell carcinoma with inferior vena cava thrombus using multidetector CT and MRI: prospective study with histopathological correlation. Journal of computer assisted tomography. 2005;29:64–8. doi: 10.1097/01.rct.0000146113.56194.6d. [DOI] [PubMed] [Google Scholar]
- 17.Johnson TR. Dual-energy CT: general principles. AJR American journal of roentgenology. 2012;199:S3–8. doi: 10.2214/AJR.12.9116. [DOI] [PubMed] [Google Scholar]
- 18.De Cecco CN, Darnell A, Rengo M, Muscogiuri G, Bellini D, Ayuso C, et al. Dual-energy CT: oncologic applications. AJR American journal of roentgenology. 2012;199:S98–s105. doi: 10.2214/AJR.12.9207. [DOI] [PubMed] [Google Scholar]
- 19.Kaza RK, Platt JF, Cohan RH, Caoili EM, Al-Hawary MM, Wasnik A. Dual-Energy CT with Single- and Dual-Source Scanners: Current Applications in Evaluating the Genitourinary Tract. RadioGraphics. 2012;32:353–69. doi: 10.1148/rg.322115065. [DOI] [PubMed] [Google Scholar]
- 20.Mileto A, Marin D, Alfaro-Cordoba M, Ramirez-Giraldo JC, Eusemann CD, Scribano E, et al. Iodine quantification to distinguish clear cell from papillary renal cell carcinoma at dual-energy multidetector CT: a multireader diagnostic performance study. Radiology. 2014;273:813–20. doi: 10.1148/radiol.14140171. [DOI] [PubMed] [Google Scholar]
- 21.Hartman R, Kawashima A, Takahashi N, Silva A, Vrtiska T, Leng S, et al. Applications of dual-energy CT in urologic imaging: an update. Radiologic clinics of North America. 2012;50:191–205. v. doi: 10.1016/j.rcl.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Cramer TW, Fletcher JG, Paden RG, Boltz TF, 2nd, Stiles WL, Pavlicek W, et al. A primer on the use of dual-energy CT in the evaluation of commonly encountered neoplasms. Abdom Radiol (NY) 2016;41:1618–31. doi: 10.1007/s00261-016-0707-x. [DOI] [PubMed] [Google Scholar]
- 23.Folio LR, Choyke PL. Radiation Exposure Reduction on Multiphase Dual Energy CT Exams: How Virtual Non-Contrast Combined with Iterative Reconstruction Cut Doses in Half. Radiological Society of North America 2014 Scientific Assembly and Annual Meeting; November 30 - December 5, 2014; Chicago IL. [Google Scholar]
- 24.Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v58–v68. doi: 10.1093/annonc/mdw328. [DOI] [PubMed] [Google Scholar]
- 25.Vikram R, Beland MD, Blaufox MD, Moreno CC, Gore JL, Harvin HJ, et al. ACR Appropriateness Criteria Renal Cell Carcinoma Staging. J Am Coll Radiol. 2016;13:518–25. doi: 10.1016/j.jacr.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014;39:588–604. doi: 10.1007/s00261-014-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Israel GM, Hindman N, Bosniak MA. Evaluation of cystic renal masses: comparison of CT and MR imaging by using the Bosniak classification system. Radiology. 2004;231:365–71. doi: 10.1148/radiol.2312031025. [DOI] [PubMed] [Google Scholar]
- 28.Malayeri AA, El Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31:1773–91. doi: 10.1148/rg.316115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Gan Q, Wu Y, Liu R, Liu X, Huang Z, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in differentiating human renal lesions (benignity or malignancy): a meta-analysis. Abdom Radiol (NY) 2016;41:1997–2010. doi: 10.1007/s00261-016-0790-z. [DOI] [PubMed] [Google Scholar]
- 30.Cornelis F, Tricaud E, Lasserre AS, Petitpierre F, Bernhard JC, Le Bras Y, et al. Routinely performed multiparametric magnetic resonance imaging helps to differentiate common subtypes of renal tumours. Eur Radiol. 2014;24:1068–80. doi: 10.1007/s00330-014-3107-z. [DOI] [PubMed] [Google Scholar]
- 31.Vargas HA, Chaim J, Lefkowitz RA, Lakhman Y, Zheng J, Moskowitz CS, et al. Renal cortical tumors: use of multiphasic contrast-enhanced MR imaging to differentiate benign and malignant histologic subtypes. Radiology. 2012;264:779–88. doi: 10.1148/radiol.12110746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqui SA, Frank I, Leibovich BC, Cheville JC, Lohse CM, Zincke H, et al. Impact of tumor size on the predictive ability of the pT3a primary tumor classification for renal cell carcinoma. J Urol. 2007;177:59–62. doi: 10.1016/j.juro.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 33.Kang SK, Chandarana H. Contemporary imaging of the renal mass. Urol Clin North Am. 2012;39:161–70. vi. doi: 10.1016/j.ucl.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Ergen FB, Hussain HK, Caoili EM, Korobkin M, Carlos RC, Weadock WJ, et al. MRI for preoperative staging of renal cell carcinoma using the 1997 TNM classification: comparison with surgical and pathologic staging. AJR American journal of roentgenology. 2004;182:217–25. doi: 10.2214/ajr.182.1.1820217. [DOI] [PubMed] [Google Scholar]
- 35.Dunnick NR. Renal cell carcinoma: staging and surveillance. Abdom Radiol (NY) 2016;41:1079–85. doi: 10.1007/s00261-016-0692-0. [DOI] [PubMed] [Google Scholar]
- 36.Choyke PL, Walther MM, Wagner JR, Rayford W, Lyne JC, Linehan WM. Renal cancer: preoperative evaluation with dual-phase three-dimensional MR angiography. Radiology. 1997;205:767–71. doi: 10.1148/radiology.205.3.9393533. [DOI] [PubMed] [Google Scholar]
- 37.Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973–80. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 38.Wang HY, Ding HJ, Chen JH, Chao CH, Lu YY, Lin WY, et al. Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging. 2012;12:464–74. doi: 10.1102/1470-7330.2012.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu HC, Yen RF, Shen YY, Kao CH, Lin CC, Lee CC. Comparing whole body 18F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphate bone scan to detect bone metastases in patients with renal cell carcinomas - a preliminary report. J Cancer Res Clin Oncol. 2002;128:503–6. doi: 10.1007/s00432-002-0370-1. [DOI] [PubMed] [Google Scholar]
- 40.Aide N, Cappele O, Bottet P, Bensadoun H, Regeasse A, Comoz F, et al. Efficiency of [(18)F]FDG PET in characterising renal cancer and detecting distant metastases: a comparison with CT. Eur J Nucl Med Mol Imaging. 2003;30:1236–45. doi: 10.1007/s00259-003-1211-4. [DOI] [PubMed] [Google Scholar]
- 41.Chang CH, Shiau YC, Shen YY, Kao A, Lin CC, Lee CC. Differentiating solitary pulmonary metastases in patients with renal cell carcinomas by 18F-fluoro-2-deoxyglucose positron emission tomography--a preliminary report. Urologia internationalis. 2003;71:306–9. doi: 10.1159/000072683. [DOI] [PubMed] [Google Scholar]
- 42.Majhail NS, Urbain JL, Albani JM, Kanvinde MH, Rice TW, Novick AC, et al. F-18 fluorodeoxyglucose positron emission tomography in the evaluation of distant metastases from renal cell carcinoma. J Clin Oncol. 2003;21:3995–4000. doi: 10.1200/JCO.2003.04.073. [DOI] [PubMed] [Google Scholar]
- 43.Kang DE, White RL, Jr, Zuger JH, Sasser HC, Teigland CM. Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. J Urol. 2004;171:1806–9. doi: 10.1097/01.ju.0000120241.50061.e4. [DOI] [PubMed] [Google Scholar]
- 44.Dilhuydy MS, Durieux A, Pariente A, de Clermont H, Pasticier G, Monteil J, et al. PET scans for decision-making in metastatic renal cell carcinoma: a single-institution evaluation. Oncology. 2006;70:339–44. doi: 10.1159/000097946. [DOI] [PubMed] [Google Scholar]
- 45.Park JW, Jo MK, Lee HM. Significance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography for the postoperative surveillance of advanced renal cell carcinoma. BJU Int. 2009;103:615–9. doi: 10.1111/j.1464-410X.2008.08150.x. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez Martinez de Llano S, Jimenez-Vicioso A, Mahmood S, Carreras-Delgado JL. Clinical impact of (18)F-FDG PET in management of patients with renal cell carcinoma. Rev Esp Med Nucl. 2010;29:12–9. doi: 10.1016/j.remn.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Kumar R, Shandal V, Shamim SA, Jeph S, Singh H, Malhotra A. Role of FDG PET-CT in recurrent renal cell carcinoma. Nucl Med Commun. 2010;31:844–50. doi: 10.1097/MNM.0b013e32833d6882. [DOI] [PubMed] [Google Scholar]
- 48.Nakatani K, Nakamoto Y, Saga T, Higashi T, Togashi K. The potential clinical value of FDG-PET for recurrent renal cell carcinoma. Eur J Radiol. 2011;79:29–35. doi: 10.1016/j.ejrad.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Ozturk H. Diagnostic role of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in restaging renal cell carcinoma. Minerva Urol Nefrol. 2016;68:263–9. [PubMed] [Google Scholar]
- 50.Win AZ, Aparici CM. Clinical effectiveness of (18)f-fluorodeoxyglucose positron emission tomography/computed tomography in management of renal cell carcinoma: a single institution experience. World journal of nuclear medicine. 2015;14:36–40. doi: 10.4103/1450-1147.150535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alongi P, Picchio M, Zattoni F, Spallino M, Gianolli L, Saladini G, et al. Recurrent renal cell carcinoma: clinical and prognostic value of FDG PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:464–73. doi: 10.1007/s00259-015-3159-6. [DOI] [PubMed] [Google Scholar]
- 52.Safaei A, Figlin R, Hoh CK, Silverman DH, Seltzer M, Phelps ME, et al. The usefulness of F-18 deoxyglucose whole-body positron emission tomography (PET) for re-staging of renal cell cancer. Clin Nephrol. 2002;57:56–62. doi: 10.5414/cnp57056. [DOI] [PubMed] [Google Scholar]
- 53.Podoloff DA, Ball DW, Ben-Josef E, Benson AB, 3rd, Cohen SJ, Coleman RE, et al. NCCN task force: clinical utility of PET in a variety of tumor types. J Natl Compr Canc Netw. 2009;7(Suppl 2):S1–26. doi: 10.6004/jnccn.2009.0075. [DOI] [PubMed] [Google Scholar]
- 54.Jadvar H, Kherbache HM, Pinski JK, Conti PS. Diagnostic role of [F-18]-FDG positron emission tomography in restaging renal cell carcinoma. Clin Nephrol. 2003;60:395–400. doi: 10.5414/cnp60395. [DOI] [PubMed] [Google Scholar]
- 55.Fuccio C, Ceci F, Castellucci P, Spinapolice EG, Palumbo R, D'Ambrosio D, et al. Restaging clear cell renal carcinoma with 18F-FDG PET/CT. Clin Nucl Med. 2014;39:e320–4. doi: 10.1097/RLU.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 56.Bertagna F, Motta F, Bertoli M, Bosio G, Fisogni S, Tardanico R, et al. Role of F18-FDG-PET/CT in restaging patients affected by renal carcinoma. Nucl Med Rev Cent East Eur. 2013;16:3–8. doi: 10.5603/NMR.2013.0002. [DOI] [PubMed] [Google Scholar]
- 57.Shuch B, Stamatakis L, Chen C, Gautam R, Merino M, Choyke PL, et al. Utility of 2-(18F) fluoro-2 deoxy-d-glucose PET/CT in advanced papillary renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(suppl 4) abstr 419. [Google Scholar]
- 58.Kobayashi K, Bhargava P, Raja S, Nasseri F, Al-Balas HA, Smith DD, et al. Image-guided biopsy: what the interventional radiologist needs to know about PET/CT. Radiographics. 2012;32:1483–501. doi: 10.1148/rg.325115159. [DOI] [PubMed] [Google Scholar]
- 59.Hu S, Zhang J, Cheng C, Liu Q, Sun G, Zuo C. The role of 18F-FDG PET/CT in differentiating malignant from benign portal vein thrombosis. Abdom Imaging. 2014;39:1221–7. doi: 10.1007/s00261-014-0170-5. [DOI] [PubMed] [Google Scholar]
- 60.Apolo AB, Riches J, Schoder H, Akin O, Trout A, Milowsky MI, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3973–8. doi: 10.1200/JCO.2010.28.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vikram R, Sandler CM, Ng CS. Imaging and staging of transitional cell carcinoma: part 1, lower urinary tract. AJR American journal of roentgenology. 2009;192:1481–7. doi: 10.2214/AJR.08.1318. [DOI] [PubMed] [Google Scholar]
- 62.Turney BW, Willatt JM, Nixon D, Crew JP, Cowan NC. Computed tomography urography for diagnosing bladder cancer. BJU Int. 2006;98:345–8. doi: 10.1111/j.1464-410X.2006.06216.x. [DOI] [PubMed] [Google Scholar]
- 63.Blick CG, Nazir SA, Mallett S, Turney BW, Onwu NN, Roberts IS, et al. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic. BJU Int. 2012;110:84–94. doi: 10.1111/j.1464-410X.2011.10664.x. [DOI] [PubMed] [Google Scholar]
- 64.Sadow CA, Silverman SG, O'Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an Academic Medical Center. Radiology. 2008;249:195–202. doi: 10.1148/radiol.2491071860. [DOI] [PubMed] [Google Scholar]
- 65.Hugen CM, Duddalwar V, Daneshmand S. Preoperative Imaging for Clinical Staging Prior to Radical Cystectomy. Current urology reports. 2016;17:62. doi: 10.1007/s11934-016-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang LJ, Wong YC, Ng KF, Chuang CK, Lee SY, Wan YL. Tumor characteristics of urothelial carcinoma on multidetector computerized tomography urography. J Urol. 2010;183:2154–60. doi: 10.1016/j.juro.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 67.Hilton S, Jones LP. Recent advances in imaging cancer of the kidney and urinary tract. Surg Oncol Clin N Am. 2014;23:863–910. doi: 10.1016/j.soc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Sternberg IA, Keren Paz GE, Chen LY, Herr HW, Donat SM, Bochner BH, et al. Upper tract imaging surveillance is not effective in diagnosing upper tract recurrence in patients followed for nonmuscle invasive bladder cancer. J Urol. 2013;190:1187–91. doi: 10.1016/j.juro.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 69.Schmid SC, Zahel T, Haller B, Horn T, Metzger I, Holzapfel K, et al. Prognostic value of computed tomography before radical cystectomy in patients with invasive bladder cancer: imaging predicts survival. World journal of urology. 2016;34:569–76. doi: 10.1007/s00345-015-1654-9. [DOI] [PubMed] [Google Scholar]
- 70.Horn T, Zahel T, Adt N, Schmid SC, Heck MM, Thalgott MK, et al. Evaluation of Computed Tomography for Lymph Node Staging in Bladder Cancer Prior to Radical Cystectomy. Urol Int. 2016;96:51–6. doi: 10.1159/000440889. [DOI] [PubMed] [Google Scholar]
- 71.Chen CY, Hsu JS, Jaw TS, Shih MC, Lee LJ, Tsai TH, et al. Split-Bolus Portal Venous Phase Dual-Energy CT Urography: Protocol Design, Image Quality, and Dose Reduction. AJR American journal of roentgenology. 2015;205:W492–501. doi: 10.2214/AJR.14.13687. [DOI] [PubMed] [Google Scholar]
- 72.Folio LR, Turkbey EB, Steinberg SM, Apolo AB. Viable tumor volume: Volume of interest within segmented metastatic lesions, a pilot study of proposed computed tomography response criteria for urothelial cancer. Eur J Radiol. 2015;84:1708–14. doi: 10.1016/j.ejrad.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouchelouche K, Turkbey B, Choyke PL. PET/CT and MRI in Bladder Cancer. J Cancer Sci Ther. 2012;S14 doi: 10.4172/1948-5956.S14-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. European urology. 2007;51:137–49. doi: 10.1016/j.eururo.2006.05.021. discussion 49-51. [DOI] [PubMed] [Google Scholar]
- 75.Verma S, Rajesh A, Prasad SR, Gaitonde K, Lall CG, Mouraviev V, et al. Urinary bladder cancer: role of MR imaging. Radiographics. 2012;32:371–87. doi: 10.1148/rg.322115125. [DOI] [PubMed] [Google Scholar]
- 76.de Haas RJ, Steyvers MJ, Futterer JJ. Multiparametric MRI of the bladder: ready for clinical routine? AJR American journal of roentgenology. 2014;202:1187–95. doi: 10.2214/AJR.13.12294. [DOI] [PubMed] [Google Scholar]
- 77.Ng CS. Radiologic diagnosis and staging of renal and bladder cancer. Seminars in roentgenology. 2006;41:121–38. doi: 10.1053/j.ro.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Tekes A, Kamel IR, Imam K, Chan TY, Schoenberg MP, Bluemke DA. MR imaging features of transitional cell carcinoma of the urinary bladder. AJR American journal of roentgenology. 2003;180:771–7. doi: 10.2214/ajr.180.3.1800771. [DOI] [PubMed] [Google Scholar]
- 79.Cappabianca S, Iaselli F, Reginelli A, D'Andrea A, Urraro F, Grassi R, et al. Value of diffusion-weighted magnetic resonance imaging in the characterization of complex adnexal masses. Tumori. 2013;99:210–7. doi: 10.1177/030089161309900215. [DOI] [PubMed] [Google Scholar]
- 80.Watanabe H, Kanematsu M, Kondo H, Goshima S, Tsuge Y, Onozuka M, et al. Preoperative T staging of urinary bladder cancer: does diffusion-weighted MRI have supplementary value? AJR American journal of roentgenology. 2009;192:1361–6. doi: 10.2214/AJR.08.1430. [DOI] [PubMed] [Google Scholar]
- 81.Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. European radiology. 2007;17:201–4. doi: 10.1007/s00330-006-0281-7. [DOI] [PubMed] [Google Scholar]
- 82.Barentsz JO, Jager GJ, van Vierzen PB, Witjes JA, Strijk SP, Peters H, et al. Staging urinary bladder cancer after transurethral biopsy: value of fast dynamic contrast-enhanced MR imaging. Radiology. 1996;201:185–93. doi: 10.1148/radiology.201.1.8816542. [DOI] [PubMed] [Google Scholar]
- 83.Daneshmand S, Ahmadi H, Huynh LN, Dobos N. Preoperative staging of invasive bladder cancer with dynamic gadolinium-enhanced magnetic resonance imaging: results from a prospective study. Urology. 2012;80:1313–8. doi: 10.1016/j.urology.2012.07.056. [DOI] [PubMed] [Google Scholar]
- 84.Niederhauser BD, Kawashima A, King BF, Takahashi N. Utility of gadolinium-enhanced MR urography in detection of bladder carcinoma. Eur J Radiol. 2013;82:472–7. doi: 10.1016/j.ejrad.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 85.Gandrup KL, Logager VB, Bretlau T, Nordling J, Thomsen HS. Diagnosis of bladder tumours in patients with macroscopic haematuria: a prospective comparison of split-bolus computed tomography urography, magnetic resonance urography and flexible cystoscopy. Scand J Urol. 2015;49:224–9. doi: 10.3109/21681805.2014.981203. [DOI] [PubMed] [Google Scholar]
- 86.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 87.Lu YY, Chen JH, Liang JA, Wang HY, Lin CC, Lin WY, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol. 2012;81:2411–6. doi: 10.1016/j.ejrad.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 88.Ozturk H. Comparing RECIST with EORTC criteria in metastatic bladder cancer. Journal of cancer research and clinical oncology. 2016;142:187–94. doi: 10.1007/s00432-015-2022-2. [DOI] [PubMed] [Google Scholar]
- 89.Giannatempo P, Alessi A, Miceli R, Raggi D, Fare E, Nicolai N, et al. Interim fluorine-18 fluorodeoxyglucose positron emission tomography for early metabolic assessment of therapeutic response to chemotherapy for metastatic transitional cell carcinoma. Clinical genitourinary cancer. 2014;12:433–9. doi: 10.1016/j.clgc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 90.Kibel AS, Dehdashti F, Katz MD, Klim AP, Grubb RL, Humphrey PA, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4314–20. doi: 10.1200/JCO.2008.20.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hitier-Berthault M, Ansquer C, Branchereau J, Renaudin K, Bodere F, Bouchot O, et al. 18 F-fluorodeoxyglucose positron emission tomography-computed tomography for preoperative lymph node staging in patients undergoing radical cystectomy for bladder cancer: a prospective study. Int J Urol. 2013;20:788–96. doi: 10.1111/iju.12045. [DOI] [PubMed] [Google Scholar]
- 92.Goodfellow H, Viney Z, Hughes P, Rankin S, Rottenberg G, Hughes S, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int. 2014;114:389–95. doi: 10.1111/bju.12608. [DOI] [PubMed] [Google Scholar]
- 93.Pichler R, De Zordo T, Fritz J, Kroiss A, Aigner F, Heidegger I, et al. Pelvic Lymph Node Staging by Combined 18F-FDG-PET/CT Imaging in Bladder Cancer Prior to Radical Cystectomy. Clinical genitourinary cancer. 2016 Aug 10; doi: 10.1016/j.clgc.2016.08.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 94.Yang Z, Pan L, Cheng J, Hu S, Xu J, Ye D, et al. Clinical value of whole body fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in the detection of metastatic bladder cancer. Int J Urol. 2012;19:639–44. doi: 10.1111/j.1442-2042.2012.02989.x. [DOI] [PubMed] [Google Scholar]
- 95.Ozturk H, Karapolat I. Efficacy of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography in restaging muscle-invasive bladder cancer following radical cystectomy. Exp Ther Med. 2015;9:717–24. doi: 10.3892/etm.2015.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chandarana H, Heacock L, Rakheja R, DeMello LR, Bonavita J, Block TK, et al. Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology. 2013;268:874–81. doi: 10.1148/radiol.13130620. [DOI] [PubMed] [Google Scholar]
- 97.Civelek A, Apolo A, Agarwal P, Evers R, Bluemke D, Malayeri A. 18F-FDG PET-MRI in the management of muscle invasive bladder cancer: Challenges in imaging and solutions. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57(suppl 2) abstr 1292. [Google Scholar]
- 98.Hirsch FW, Sattler B, Sorge I, Kurch L, Viehweger A, Ritter L, et al. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol. 2013;43:860–75. doi: 10.1007/s00247-012-2570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–95. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 100.Gabriele D, Collura D, Oderda M, Stura I, Fiorito C, Porpiglia F, et al. Is there still a role for computed tomography and bone scintigraphy in prostate cancer staging? An analysis from the EUREKA-1 database. World journal of urology. 2016;34:517–23. doi: 10.1007/s00345-015-1669-2. [DOI] [PubMed] [Google Scholar]
- 101.Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818–24. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Djavan B, Margreiter M. Biopsy standards for detection of prostate cancer. World J Urol. 2007;25:11–7. doi: 10.1007/s00345-007-0151-1. [DOI] [PubMed] [Google Scholar]
- 103.Hong CW, Amalou H, Xu S, Turkbey B, Yan P, Kruecker J, et al. Prostate biopsy for the interventional radiologist. J Vasc Interv Radiol. 2014;25:675–84. doi: 10.1016/j.jvir.2013.12.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016;122:884–92. doi: 10.1002/cncr.29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meng X, Rosenkrantz AB, Mendhiratta N, Fenstermaker M, Huang R, Wysock JS, et al. Relationship Between Prebiopsy Multiparametric Magnetic Resonance Imaging (MRI), Biopsy Indication, and MRI-ultrasound Fusion-targeted Prostate Biopsy Outcomes. Eur Urol. 2016;69:512–7. doi: 10.1016/j.eururo.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313:390–7. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andreoiu M, Cheng L. Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol. 2010;41:781–93. doi: 10.1016/j.humpath.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 108.Mesko S, Marks L, Ragab O, Patel S, Margolis D, Demanes D, et al. Targeted Prostate Biopsy Gleason Score Heterogeneity and Implications for Risk Stratification. Am J Clin Oncol. 2016 Jun 8; doi: 10.1097/COC.0000000000000308. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barentsz JO, Weinreb JC, Verma S, Thoeny HC, Tempany CM, Shtern F, et al. Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur Urol. 2016;69:41–9. doi: 10.1016/j.eururo.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mertan FV, Greer MD, Shih JH, George AK, Kongnyuy M, Muthigi A, et al. Prospective Evaluation of the Prostate Imaging Reporting and Data System Version 2 for Prostate Cancer Detection. J Urol. 2016;196:690–6. doi: 10.1016/j.juro.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 111.Greer MD, Brown A, Shih JH, Summers R, Marko J, Law Y, et al. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: A multireader study. J Magn Reson Imaging. 2016 Jul 8; doi: 10.1002/jmri.25372. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rosenkrantz AB, Ginocchio LA, Cornfeld D, Froemming AT, Gupta RT, Turkbey B, et al. Interobserver Reproducibility of the PI-RADS Version 2 Lexicon: A Multicenter Study of Six Experienced Prostate Radiologists. Radiology. 2016;280:793–804. doi: 10.1148/radiol.2016152542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grant KB, Agarwal HK, Shih JH, Bernardo M, Pang Y, Daar D, et al. Comparison of calculated and acquired high b value diffusion-weighted imaging in prostate cancer. Abdom Imaging. 2015;40:578–86. doi: 10.1007/s00261-014-0246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci Transl Med. 2013;5:198ra08. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenkrantz AB, Bennett GL, Doshi A, Deng FM, Babb JS, Taneja SS. T2-weighted imaging of the prostate: Impact of the BLADE technique on image quality and tumor assessment. Abdom Imaging. 2015;40:552–9. doi: 10.1007/s00261-014-0225-7. [DOI] [PubMed] [Google Scholar]
- 116.Shiiba M, Ishihara K, Kimura G, Kuwako T, Yoshihara H, Sato H, et al. Evaluation of primary prostate cancer using 11C-methionine-PET/CT and 18F-FDG-PET/CT. Annals of Nuclear Medicine. 2012;26:138–45. doi: 10.1007/s12149-011-0551-6. [DOI] [PubMed] [Google Scholar]
- 117.Jadvar H. Is There Use for FDG-PET in Prostate Cancer? Seminars in nuclear medicine. 2016;46:502–6. doi: 10.1053/j.semnuclmed.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. European urology. 2013;64:106–17. doi: 10.1016/j.eururo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 119.Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clinical nuclear medicine. 2013;38:305–14. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 120.Beheshti M, Haim S, Zakavi R, Steinmair M, Waldenberger P, Kunit T, et al. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence: influence of androgen deprivation therapy and correlation with PSA kinetics. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:833–40. doi: 10.2967/jnumed.112.110148. [DOI] [PubMed] [Google Scholar]
- 121.Suardi N, Gandaglia G, Gallina A, Di Trapani E, Scattoni V, Vizziello D, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. European urology. 2015;67:299–309. doi: 10.1016/j.eururo.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 122.Mena E, Turkbey B, Mani H, Adler S, Valera VA, Bernardo M, et al. 11C-Acetate PET/CT in localized prostate cancer: a study with MRI and histopathologic correlation. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:538–45. doi: 10.2967/jnumed.111.096032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mohsen B, Giorgio T, Rasoul ZS, Werner L, Ali GR, Reza DK, et al. Application of C-11-acetate positron-emission tomography (PET) imaging in prostate cancer: systematic review and meta-analysis of the literature. BJU international. 2013;112:1062–72. doi: 10.1111/bju.12279. [DOI] [PubMed] [Google Scholar]
- 124.Leisser A, Pruscha K, Ubl P, Wadsak W, Mayerhofer M, Mitterhauser M, et al. Evaluation of fatty acid synthase in prostate cancer recurrence: SUV of [(11) C] acetate PET as a prognostic marker. The Prostate. 2015;75:1760–7. doi: 10.1002/pros.23061. [DOI] [PubMed] [Google Scholar]
- 125.Vees H, Buchegger F, Albrecht S, Khan H, Husarik D, Zaidi H, et al. 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/mL) after radical prostatectomy. BJU international. 2007;99:1415–20. doi: 10.1111/j.1464-410X.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 126.Turkbey B, Mena E, Shih J, Pinto PA, Merino MJ, Lindenberg ML, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology. 2014;270:849–56. doi: 10.1148/radiol.13130240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ren J, Yuan L, Wen G, Yang J. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: a meta-analysis. Acta radiologica (Stockholm, Sweden : 1987) 2016;57:487–93. doi: 10.1177/0284185115581541. [DOI] [PubMed] [Google Scholar]
- 128.Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H, et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (18F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. The Journal of urology. 2017;197:676–83. doi: 10.1016/j.juro.2016.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. European urology. 2016;70:926–37. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 130.Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. European urology. 2016 doi: 10.1016/j.eururo.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 131.Giesel FL, Sterzing F, Schlemmer HP, Holland-Letz T, Mier W, Rius M, et al. Intra-individual comparison of (68)Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. European journal of nuclear medicine and molecular imaging. 2016;43:1400–6. doi: 10.1007/s00259-016-3346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Budaus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. European urology. 2016;69:393–6. doi: 10.1016/j.eururo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 133.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. European journal of nuclear medicine and molecular imaging. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2016;18:411–9. doi: 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2015;17:565–74. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomacker K, et al. Comparison of [(18)F]DCFPyL and [(68)Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2015;17:575–84. doi: 10.1007/s11307-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:1006–13. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 138.Nonnekens J, Chatalic KL, Molkenboer-Kuenen JD, Beerens CE, Bruchertseifer F, Morgenstern A, et al. 213Bi-Labeled Prostate-Specific Membrane Antigen-Targeting Agents Induce DNA Double-Strand Breaks in Prostate Cancer Xenografts. Cancer biotherapy & radiopharmaceuticals. 2017;32:67–73. doi: 10.1089/cbr.2016.2155. [DOI] [PubMed] [Google Scholar]
- 139.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–97. [PubMed] [Google Scholar]
- 140.Iagaru A, Mittra E, Dick DW, Gambhir SS. Prospective evaluation of (99m)Tc MDP scintigraphy, (18)F NaF PET/CT, and (18)F FDG PET/CT for detection of skeletal metastases. Mol Imaging Biol. 2012;14:252–9. doi: 10.1007/s11307-011-0486-2. [DOI] [PubMed] [Google Scholar]
- 141.Jambor I, Kuisma A, Ramadan S, Huovinen R, Sandell M, Kajander S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016;55:59–67. doi: 10.3109/0284186X.2015.1027411. [DOI] [PubMed] [Google Scholar]
- 142.Schirrmeister H, Guhlmann A, Elsner K, Kotzerke J, Glatting G, Rentschler M, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623–9. [PubMed] [Google Scholar]
- 143.Poulsen MH, Petersen H, Hoilund-Carlsen PF, Jakobsen JS, Gerke O, Karstoft J, et al. Spine metastases in prostate cancer: comparison of technetium-99m-MDP whole-body bone scintigraphy, [(18) F]choline positron emission tomography(PET)/computed tomography (CT) and [(18) F]NaF PET/CT. BJU Int. 2014;114:818–23. doi: 10.1111/bju.12599. [DOI] [PubMed] [Google Scholar]
- 144.Tateishi U, Morita S, Taguri M, Shizukuishi K, Minamimoto R, Kawaguchi M, et al. A meta-analysis of (18)F-Fluoride positron emission tomography for assessment of metastatic bone tumor. Annals of nuclear medicine. 2010;24:523–31. doi: 10.1007/s12149-010-0393-7. [DOI] [PubMed] [Google Scholar]
- 145.Apolo AB, Lindenberg L, Shih JH, Mena E, Kim JW, Park JC, et al. Prospective Study Evaluating Na18F PET/CT in Predicting Clinical Outcomes and Survival in Advanced Prostate Cancer. J Nucl Med. 2016;57:886–92. doi: 10.2967/jnumed.115.166512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mosavi F, Johansson S, Sandberg DT, Turesson I, Sorensen J, Ahlstrom H. Whole-body diffusion-weighted MRI compared with (18)F-NaF PET/CT for detection of bone metastases in patients with high-risk prostate carcinoma. AJR American journal of roentgenology. 2012;199:1114–20. doi: 10.2214/AJR.11.8351. [DOI] [PubMed] [Google Scholar]
- 147.Hillner BE, Siegel BA, Hanna L, Duan F, Shields AF, Coleman RE. Impact of 18F-fluoride PET in patients with known prostate cancer: initial results from the National Oncologic PET Registry. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:574–81. doi: 10.2967/jnumed.113.130005. [DOI] [PubMed] [Google Scholar]
- 148.Yu EY, Duan F, Muzi M, Deng X, Chin BB, Alumkal JJ, et al. Castration-resistant prostate cancer bone metastasis response measured by 18F-fluoride PET after treatment with dasatinib and correlation with progression-free survival: results from American College of Radiology Imaging Network 6687. J Nucl Med. 2015;56:354–60. doi: 10.2967/jnumed.114.146936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic Factors in Patients Treated with 223Ra: The Role of Skeletal Tumor Burden on Baseline 18F-Fluoride PET/CT in Predicting Overall Survival. J Nucl Med. 2015;56:1177–84. doi: 10.2967/jnumed.115.158626. [DOI] [PubMed] [Google Scholar]
- 150.Kairemo K, Joensuu T. Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer-Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT. Diagnostics (Basel) 2015;5:413–27. doi: 10.3390/diagnostics5040413. [DOI] [PMC free article] [PubMed] [Google Scholar]


