Abstract
Parasitoid wasps sting and inject venom in arthropod hosts, which alters host metabolism and development while keeping the host alive for several days, presumably to induce benefits for the parasitoid young.
Here we investigate the consequences of host envenomation on development and fitness of wasp larvae in the ectoparasitoid Nasonia vitripennis, by comparing wasps reared on live unstung, previously stung, and cold-killed hosts. Developmental arrest and suppression of host response to larvae are major venom effects that occur in both stung and cold-killed hosts, but not unstung hosts; while cold-killed hosts lack venom effects that require a living host. Thus, cold-killed hosts mimic some of the effects of venom, but not others.
Eggs placed on live unstung hosts have significantly higher mortality during development, however successfully developing wasps from these hosts have similar lifetime fecundity to wasps from cold-killed or stung hosts. Therefore, although venom is beneficial, it is not required for wasp survival.
While wasps developing on cold-killed versus stung hosts have similar fitness, multiple generations of rearing on cold-killed hosts results in significant fitness reductions of wasps.
We conclude that the largest benefits of venom are induction of host developmental arrest and suppression of host response to larva (e.g. immune responses), although more subtle benefits may accrue across generations, or under stressful conditions.
Keywords: Nasonia, venom, developmental arrest, ectoparasitoid
Graphical abstract
INTRODUCTION
Parasitoid wasps lay their eggs in or on other arthropods and are the largest group of venomous animals with an estimated 150,000 – 600,000 species (Heraty et al., 2011; Quicke, 1997). They inject venoms into the host to manipulate its development, metabolism, and physiology in ways conducive for the development of the wasp young (Burke & Strand, 2014; De Graaf et al., 2010; Moreau & Asgari, 2015; Mrinalini & Werren, 2017; Poirié et al., 2014). Parasitoids are important agents of arthropod mortality in natural and agricultural ecosystems, and are used extensively in biological control of arthropod pests (Godfray, 1994; Noyes, 2016; Pickens & Miller, 1978; Rivers, 2004). One of the major ecological distinctions within parasitoids is between endoparasitoids, which lay their eggs within their hosts, and ectoparasitoids, which lay their eggs on the surface of their hosts.
Differences in their ecologies are also reflected in their venom effects. The primary effects of endoparasitoid venom are temporary paralysis and immune suppression of the host to protect the wasp’s offspring from encapsulation (Asgari & Rivers, 2011; Burke & Strand, 2014; Mortimer et al., 2013; Parkinson et al., 2002; Vincent et al., 2010), whereas the primary effects of ectoparasitoid venom are developmental arrest and metabolic changes to the host that alters the feeding environment for their developing offspring (Nakamatsu & Tanaka, 2003; Rivers & Denlinger, 1994). While many studies have documented effects of ectoparasitoid venom on the host, the benefits of host envenomation to developing wasp larvae has not been as extensively studied in ectoparasitoids. Previous studies have shown that wasps can develop on dead hosts and that the number of emerged offspring are similar to naturally parasitized hosts in the first generation (Beard, 1964; Legner, 1967; Pickens & Miller, 1978; Rivers & Denlinger, 1995a). However, dead hosts mimic important venom effects, such as developmental arrest and suppression of host response to feeding wasp larva. Therefore, additional experiments are needed to assess the contribution of venom to wasp development and fitness. This study extends the earlier studies by conducting a controlled experiment using egg transfers to compare survival of wasps developing on live unstung, stung, and cold-killed hosts. Egg transfers to living, unstung hosts are an important control for assessing the full consequences of venom that require a developing host capable of an immune response. We also extend prior work by looking at the fitness of the wasps that developed on each host type and assessing the intergenerational consequences for wasps developing on naturally envenomated versus cold-killed hosts.
Nasonia vitripennis (hereafter Nasonia) is a generalist pupal ectoparasitoid wasp that parasitizes several families of flies, including the flesh fly Sarcophaga bullata (Diptera: Sarcophagidae) (Rivers & Denlinger, 1995a). After a suitable host is found, Nasonia will drill through the puparium of the host to inject venom. They then lay their eggs between the outer purparium and the surface of the fly pupa, where their offspring to feed directly on the surface of the pupa as ectoparasites. Ovipositing females also build a tube from the puparium to the pupa in order to feed on host hemolymph (Whiting, 1967). Major effects of Nasonia venom on the host are full developmental arrest (Rivers & Denlinger, 1995b) and the suppression of the host response to the larvae by inhibiting the adhesion and spreading behavior of plasmatocytes and impeding melanization and clotting of the hemolymph (Rivers et al., 2002a; Rivers et al., 2002b). Many other effects have been documented including depression in host respiration (Rivers & Denlinger, 1994), increased gene expression of antimicrobial peptides (Martinson et al., 2014), and increases in free amino acids and polyol sugars (Mrinalini et al., 2014). However, it is not firmly established whether known or other unknown venom effects have nutritional or protective qualities that are essential or beneficial to Nasonia development, as several venom effects are mimicked in the previously tested dead hosts and the fitness of the wasps was never measured.
To test if venom is essential and/or beneficial to Nasonia development we transplanted eggs onto live unstung, previously stung, and cold-killed hosts. The experiment is used to evaluate the consequences of venom on wasp fitness mediated through developmental arrest and suppression of host response to larvae, which occurs in stung and cold-killed but not unstung hosts, versus other venom alterations of the host (including metabolism and gene expression), which occur only in naturally stung, but not cold-killed or unstung hosts. Typically, extensive metabolic and gene expression changes occur within 24 hours post envenomation and naturally stung hosts can remain alive for up to 15 days (Rivers & Denlinger, 1994). Eggs were placed on 1) hosts that were naturally stung by Nasonia (ST) that had the original eggs removed, 2) cold-killed (CK) hosts that have their development and metabolism arrested by death and an absence of a host response to larvae, and 3) Unstung live hosts (UN) that has continued host development and host response to developing Nasonia larvae. Because Nasonia eggs are laid between the host pupa and the pupal casing, they can be easily removed and placed on different hosts (Rivers & Yoder, 1996). To investigate longer-term consequences on wasp fitness, wasps were provided either live hosts for natural parasitization or cold-killed hosts over five successive generations, and then tested for intergenerational fitness effects of the two host types on wasp fitness. Results show that while the largest venom effect on larval fitness is developmental arrest and/or suppression of host responses to wasp larvae, there are also significant accumulating benefits of venom on wasp fitness.
MATERIALS AND METHODS
Host Rearing
All wasps used in this study were maintained on pupae of flesh fly host, Sarcophaga bullata. To ensure high quality host pupae, the rearing of Sarcophaga bullata was conducted by the Werren Lab in a dedicated rearing facility. Fly larvae were reared on fresh cow liver in an airflow hood, while adjusting the density of larvae and amount of food during rearing to avoid overcrowding. These procedures result in hosts of higher quality than those we have previously obtained from biological supply companies, with production of large uniform pupae (gram wet weight ~3 days post pupation of 0.181±0.011SD, coefficient of variation 0.066, n=30). Crowding of parasitoids in hosts was not a factor in these experiments, both due to experimental design and use of large S. bullata hosts. S. bullata pupae are 3.3 times larger in size than C. vomitoria hosts (0.058±0.005SD, n=30), another species routinely used in Nasonia research and cultured in our laboratory. S. bullata pupae that were outliers in shape, color, or texture, were not used in these experiments. Over 80% of S. bullata pupae complete development to adult flies in our stock culture, the remainder typically fail just after pupation but before commencement of metamorphosis. These are easily recognized by “softness” of the puparium, and were discarded prior to experiments. They are also easily recognized upon opening the puparium, and therefore were not used in egg transfer experiments.
Experimental set-up
To obtain Nasonia eggs, 24 hr old Nasonia females (strain AsymCX, n=180) were individually placed in glass tubes with two host pupae. This pretreatment allows females to host feed and increase egg production. To collect Nasonia eggs prior to hatching, the females were then individually placed in tubes with single S. bullata, which had pupated 3 days previously, at 25°C for two hours. The hosts (n=180) were placed in foam plugs that leave only the anterior portion of the puparium exposed for Nasonia stinging and oviposition, for ease of subsequent parasitoid egg removal. After two hours wasps were separated from hosts.
To collect Nasonia eggs, the anterior end of the puparium was removed and saved. All eggs were removed from the surface of each pupa and placed on a polycarbonate membrane on a 1X PBS agar plate. The eggs were then gently mixed and divided into groups of 25 each, which is within the normal range of Nasonia cultch size (Werren, 1983). The groups of eggs were then randomly and equally divided into three experimental treatment groups (ST, CK and UN) each containing 24 hosts. For all treatments, the puparium cap was removed to allow the eggs to be placed directly on the pupa and to assess that all pupae were healthy and viable hosts. Any hosts that were accidentally “nicked” during decapping, which results in host bleeding, were not used in the experiment. All hosts used in the transplant experiment were the same age (within 24 hrs), healthy-looking, and were large enough to easily provide all the nutrition necessary to develop 25 Nasonia larvae. For the ST treatment group, egg groups were placed onto hosts that were stung for egg collection and had all other eggs removed. For the UN treatment group, egg groups were placed onto the anterior end of hosts with caps removed, that were from the same batch as hosts from the other two treatments, but had not been stung or had any interaction with Nasonia. For the CK treatment group, egg groups were placed onto uncapped hosts from the same batch that were killed by placing them at −80°C for 15 min then allowed to equilibrate to room temperature. For all treatments, the puparium cap was then replaced on hosts and secured with two small dots of Elmer’s® glue. Hosts from all treatments were then placed anterior side up in PCR trays inside a plastic container with moist paper towels at 25°C to prevent desiccation of the Nasonia larvae. After the Nasonia had pupated, individual hosts were moved to glass test tubes for the Nasonia to eclose. Two replicates for each treatment described above were performed on different dates (R1 in August 2013 and R2 in September 2013).
The emergence times and percent survival of each tube was recorded. To assess fitness of emerging females from the different treatments, two females from each tube (when available) were transferred every two days to three fresh hosts and their offspring and sex ratio were counted to assess lifetime fecundity (R1 n= CK 48, ST 47, UN 44; R2 n= CK 41, ST 44, UN 26). Three hosts were added to each tube to give the wasp more resources than it could have utilized within 48 hours to protect against variation in host size and quality. The small number of wasps used on unstung host in the second replicate was due to the limited number of wasps emerging from unstung hosts. If no wasps emerged from a tube, two additional wasps were taken from another tube to keep 48 wasps in each treatment, when possible. If the wasps emerged from a tube that did not contain any males, the female was mated with a male from another tube of the same treatment.
Extended generation experiment
We next assessed whether there is a cumulative effect of venom on wasp fitness over successive generations. Two populations were established and maintained for five generations (n=48 for each population). In one, female wasps were provided with live hosts for parasitization (NP for naturally parasitized), and in the other, cold-killed hosts (CK) were provided. Each generation, hosts were randomly divided into the two treatment groups: for the NP treatment, hosts were presented to Nasonia for oviposition and envenomation without any alteration, while CK hosts were killed at −80°C for 15 min then allowed to equilibrate to room temperature prior to wasp exposure for oviposition. For each treatment, individual mated females were placed in a test tube containing three hosts (either NP or CK respectively) and allowed to host feed and oviposit. After two days, the female was transferred once to three new hosts. Offspring typically emerged ~14 days later. Twenty-four hours after wasps emerged, a single female from the first setting of each tube was transferred onto three hosts of its treatment type for the next generation. If females were not available from the first setting, wasps from the second setting were used. If females were not available from both settings, a female from another tube was used to replace the line. This process was repeated for five generations.
Emergence time and sex ratio was recorded at the fifth generation of each treatment. To assess how quickly wasps can recover from host quality differences, the lifetime fecundity of wasps (as described above) from both treatments were determined on both NP and CK hosts (n=40). Lifetime offspring counts and sex ratios were recorded for (i) NP lineage wasps given live previously unstung hosts (ii) CK lineage wasps given cold-killed hosts, (iii), NP lineage wasps given cold-killed hosts and (iv) CK lineage wasps given live previously unstung hosts. Two replicates for each treatment described above were performed on different dates (August and September 2013).
Statistical analyses
All statistical analyses were carried out in R, version 3.1.0. Measures of host fitness (proportion eclosed, lifetime fecundity, sex ratio) were analyzed using logistic regression by generalized linear models (GLM). We fit a binomial distribution (after checking of over-dispersion) to analyze the percent of hosts that had wasps emerge and sex ratios of wasps and we fit a quasibinomial distribution to analyze the proportion of wasps emerged and proportion of females that laid diapause larvae. Wasps that were unmated and produced all male offspring were removed from the sex ratio analysis. We fit a quasipoisson distribution to analyze the total lifetime fecundity of each wasp. Individuals that died before producing any offspring were removed from the analysis. Model comparisons using ANOVA were used to determine if terms (e.g. replicate, final host type) were significant to the models. Terms were retained if their removal significantly reduced the explanatory power of the model. Pairwise comparisons within the models were conducted using General Linear Hypotheses in the multcomp R package. Statistical analyses were also done using non-parametric Wilcoxon rank-sum tests in JMP Pro v 12.1.0 and the results were consistent with results of the GLM analyses.
RESULTS
Fitness of wasps developing on stung, cold-killed, and unstung hosts
We first examined if venom is essential and/or beneficial to Nasonia development by transplanted eggs onto unstung live (UN), stung (ST), and cold-killed (CK), to evaluate different aspects of the venom effects. We found that significantly fewer host settings had wasps emerge from UN hosts (44%) compared to CK (92%) or ST hosts (100%) (χ2 = 56.7, df=2, P < 0.001). Although no wasps emerged from 54% of the UN hosts, only 19% of those hosts successfully developed into flies. This suggests that feeding wasp larvae on UN hosts can increase mortality of the developing fly without the injection of venom, although additional experiments are needed to investigate this further. Although the majority of UN hosts failed to complete development, there is evidence that development did progress further than in the ST hosts, based on increased pigmentation and bristle formation in these hosts. The percent of wasps (out of 25 initial eggs per host) completing development and emerging per host was significantly lower for UN hosts (13% ±2.9 SE) than for CK (47% ±3.4 SE) and ST hosts (45% ±3.2 SE, χ2 = 221.7 df=2, P < 0.001). However, there was not a significant difference between CK and ST treatments in number of emergences (χ2 = 0.297 df=1, P=0.700), which has also been found in previous studies without controlled egg transfers (Beard, 1964; Legner, 1967; Rivers & Denlinger, 1995a). If we restrict the analysis to host settings where at least one wasp emerged, then UN hosts still had a significantly lower percent of wasps completing development per host (31% ±4.2 SE) than CK (52% ±2.9 SE) or ST (45% ±3.1 SE) treatments (χ2 = 25.6 df=2, P < 0.001) (Fig. 1A). In addition to emerging offspring number, the emergence time of wasps from UN hosts was also delayed relative the other two treatments, with peak emergence being on average one day later than for ST and CK hosts (Fig. 1B). The experiment establishes that without developmental arrest and suppression of host response to larva, which is absent in UN but occurs in ST and CK hosts, the developing wasps suffer severe mortality, although some wasps do survive. Thus, it can be concluded that venom induced developmental arrest and suppression of host response to larva greatly enhance parasitoid survival, but are not required for (some) wasps to complete development.
Figure 1.
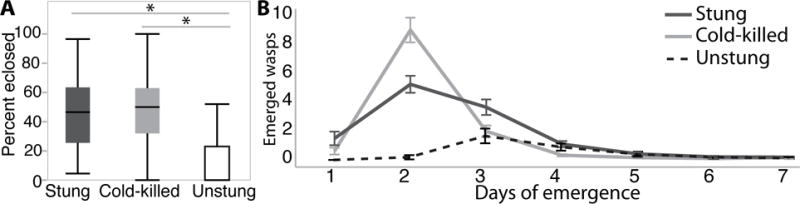
Emergence of wasps developing on stung (dark grey), cold-killed (light grey), and unstung (white) hosts. A) Boxplots of the percent of wasps that eclosed an initial 25 eggs on stung, cold-killed and unstung treatments. Asterisk indicated significant differences in pairwise comparisons B) The number of wasps that emerged per day for stung (dark grey), cold-killed (light grey), and unstung (dashed line) shown with standard error bars.
Wasps raised on UN hosts incur a large fitness cost, with fewer wasps successfully developing. However, the wasps that do emerge tend to have similar fecundity to wasps raised on CK and ST. We found no significant difference in the lifetime fecundity of UN, CK, or ST raised wasps when provided with standard live hosts (averaging 427 ±21 SE, 445 ±20 SE, and 447 ±20 SE offspring, respectively) (F=0.250 df=2, P=0.779) (Fig. 2A). However, the first 48-hour hosting of UN derived wasps did produce fewer offspring (73 ±5.0 SE) than CK (86 ±3.1 SE) or ST (86 ±2.3 SE) derived wasps, although not significantly fewer (Fig. 2B). Adult female wasps routinely host feed and utilize host feeding for egg production; it is therefore possible that the health of females from UN hosts recovered their initial fecundity reduction observed in the first 48-hours by host feeding. Wasps raised on UN hosts did not lay significantly more males (χ2=149.9 df=2, P=0.3912) or diapause larvae (χ2=4.5 df=2, P=0.104) than observed in the other treatments.
Figure 2.
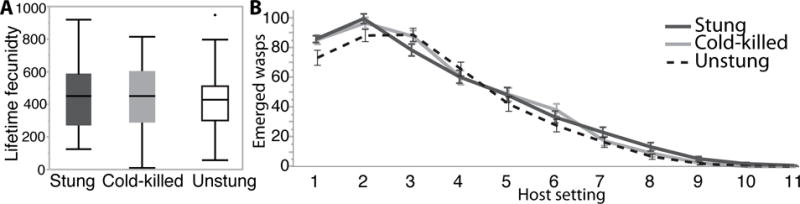
Lifetime fecundity of wasps developing on stung (dark grey), cold-killed (light grey), and unstung live (white) hosts. A) Boxplots of the lifetime fecundity in total number of wasps that eclosed from hosts. B) The number of wasps that eclosed per 48-hour hosting for stung (dark grey), cold-killed (light grey), and unstung (dashed line) shown with standard error bars.
Intergenerational effects of developing on envenomated hosts
In the next set of experiments, we established two independent populations, one in which wasps were provided standard live, non-parasitized host pupae (NP), and the other in which they were provided with recently cold-killed hosts (CK) for five successive generations. Following venom injection, a wide range of physiological changes are induced in the NP hosts, which can remain alive and respiring for up to 15 days post venomization when wasp eggs are removed (Rivers & Denlinger, 1994). Changes include developmental arrest, suppression of the host response to larvae, metabolic changes and alterations in host gene expression (Danneels et al., 2013; Martinson et al., 2014; Mrinalini et al., 2014; Rivers & Denlinger, 1994, 1995a, b). In contrast, the CK hosts cannot show such changes because the host is dead, although development is stopped and the host cannot respond to larval feeding. This experiment therefore allows a test for subtler intergenerational consequences of venom on wasp fitness.
As shown in the first experiment, after one generation there was no significant difference between wasps developing on cold-killed or envenomated hosts in percent emergence, lifetime fecundity, or sex ratio. However, after five generations of development on NP versus CK hosts, significant effects became apparent. By the fifth generation, the CK lineage had significantly fewer wasps emerge after a 48-hour hosting than the NP lineage (68 ±2.2 SE to 86 ±2.3 SE wasps, respectively; F=27.05 df= 1, P <0.001) (Fig. 3), when set on their respective host type (CK or NP). Both groups had a similar sex ratio (82% ±1.1 SE to 86% ±0.6 SE, respectively; χ2=2.5 df=1, P=0.116). Because we did not open hosts to count number of eggs laid, we do not know whether the difference is due to fecundity or egg- adult mortality. However, providing wasps of each lineage with the same or alternate host types (NP or CK) reveals that CK lineage wasps had a significantly lower lifetime fecundity than NP lineage wasps, when both are provided with NP hosts (373.2 ±16 SE vs 453 ±18 SE offspring) (F=16.44 df=1, P <0.001). When both are provided with CK hosts, CK lineage females also had lower fecundity (358 ±14 SE vs 409.1 ±16 SE offspring), but not significantly so (p=0.870) (Fig. 4). Most of the effect occurred in the first 6 days (first 3 settings); on average CK lineage wasps have 21 fewer wasps emerge than ST lineage wasps for the first three settings (Fig. 5).
Figure 3.
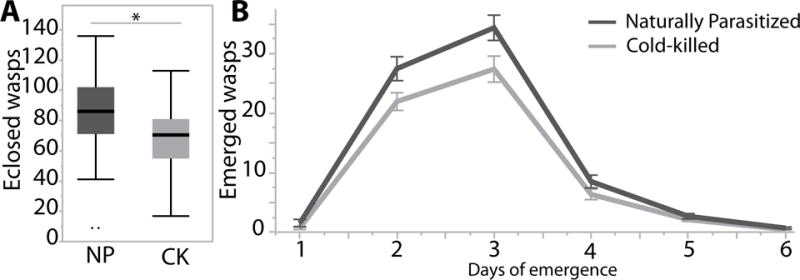
Emergence of wasps raised for five generations on naturally parasitized (dark grey) and cold-killed (light grey) hosts after a 48-hour hosting. A) Boxplots of the total number of wasps that eclosed on naturally parasitized and cold-killed hosts. Asterisk indicated significant differences the pairwise comparison B) The number of wasps that emerged per day shown for naturally parasitized (dark grey) and cold-killed (light grey) hosts with standard error bars.
Figure 4.
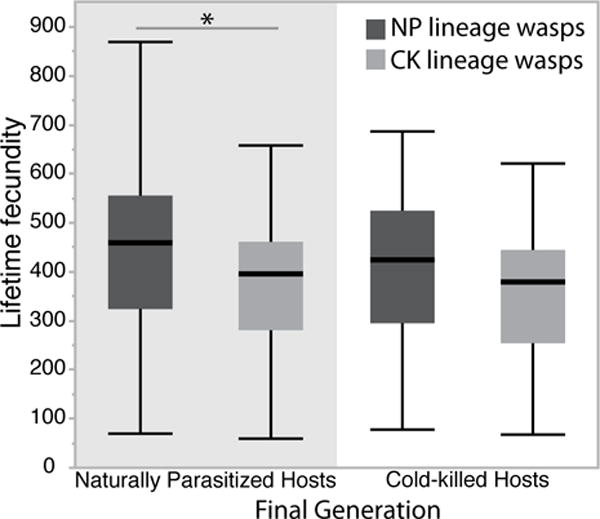
Lifetime fecundity of wasps raised for five generations on naturally parasitized (dark grey) and cold-killed (light grey) hosts shown in boxplots. Fecundity was assessed for each group using a final generation of either naturally parasitized hosts (grey background) or cold-killed hosts (white background). Asterisk indicated significant differences in pairwise comparisons.
Figure 5.
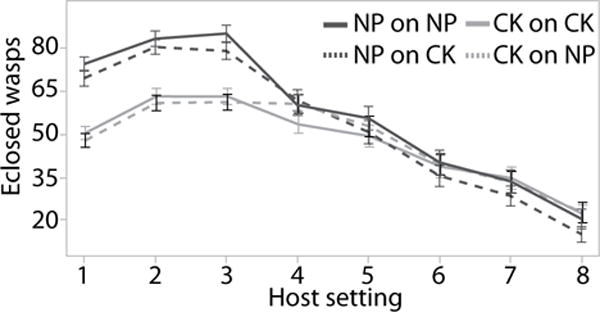
Lifetime fecundity by the number of wasps that emerged per hosting for naturally parasitized (dark grey) and cold-killed (light grey) lineage wasps (listed first) with a final generation of either naturally parasitized hosts or cold-killed hosts (listed second) (e.g. NP on CK is a naturally parasitized lineage wasp raise on cold-killed hosts for the final generation) shown with standard error bars.
DISCUSSION
Nasonia venom consists of nearly a hundred venom proteins, many of which do not share homology with protein sequences outside of the chalcidoid superfamily of parasitoid wasps (De Graaf et al., 2010; Martinson et al., 2017; Werren et al., 2010). Whereas many studies have looked at the effects of these venom proteins on the physiology, gene expression, and metabolism of the host (Danneels et al., 2013; Martinson et al., 2014; Mrinalini et al., 2014; Rivers & Denlinger, 1994, 1995b; Rivers et al., 2002b; Siebert et al., 2015), relatively little work has been done to evaluate the effects of venom injection in the host on developing Nasonia larvae. Earlier studies using cold-killed hosts (Beard, 1964; Legner, 1967; Pickens & Miller, 1978; Rivers & Denlinger, 1995a) had established that ectoparasitoid wasps could successfully develop on dead hosts for a single generation. Thus, by implication, venom effects on living hosts were shown not to be necessary for wasp development. However, cold-killed hosts mimic several key effects of venom, in particular developmental arrest and suppression of host responses to feeding larvae. Examples of the latter could be melanization around the feeding site and activation of other immune responses that could inhibit larval feeding, and possibly compounds that interfere with wasp larval digestion. This study assesses the consequences of these venom effects by comparing egg transfers to live (unstung), stung, and cold-killed hosts and intergenerational consequences of development on cold-killed versus envenomated hosts.
We found that wasps developing on unstung live hosts performed significantly worse than wasps on either stung or cold-killed hosts (Fig. 1), with an approximately 34% overall reduction in egg-to-adult survival. This result shows that Nasonia is capable of fully developing on live hosts without venom (possibly because the saliva of feeding larvae may duplicate some venom effects), although there is significant mortality. Therefore, venom is not essential to Nasonia development. Furthermore, the finding strongly indicates that arresting host development and the suppression of host defenses against feeding larvae are major functions of Nasonia venom.
Interestingly, wasps that do successfully develop in stung, cold-killed, and unstung hosts have very similar lifetime fecundity, indicating that, if they survive, they are not seriously impaired (Fig. 2). This finding would suggest that the venom-induced changes in gene expression and increases of essential metabolites such as free-amino acids, sugars, and lipids (Danneels et al., 2013; Martinson et al., 2014; Mrinalini et al., 2014) are not of central importance, at least under laboratory rearing conditions. However, negative effects on the female wasps may also be rescued through host feeding of these adult females.
We did not test for survival and fecundity under various environmental stressors that present in nature but not in the lab. Stressors (such as during food scarcity, exposure to pathogens, or environmental fluctuations) could reveal additional benefits of venom. Sarcophaga bullata is a flesh fly that pupates in the soil close to putrid meat; a fungal or bacterial infection of the nutrient-rich, immune comprised host would have negative impacts on developing Nasonia larvae. Feeding Nasonia larvae show elevated expression of antimicrobial peptides (Martinson et al., 2017). In addition, venom also induces increased expression of host genes involved for antimicrobial pathways and peptides (Danneels et al., 2013; Martinson et al., 2014). The potential host antimicrobial protection induced by venom could increase the fitness of the developing wasps in the presence of pathogens. If this hypothesis is correct, we would predict that if this experiment was repeated with experimental exposure to the natural microbial community (including pathogens), then the difference between cold-killed and stung hosts would be much more substantial. Nasonia venom has also been shown to increase lipids, sugar metabolism and amino acid biosynthesis in the host that could provide a nutritional benefit to developing larvae (Mrinalini et al., 2014; Rivers & Denlinger, 1994, 1995a, b) additionally venom-induced developmental arrest prevents tissue decay in the host that may reduce its nutritional quality. Nevertheless, it is apparent that females can host feed on CK hosts, because feeding is essential for developing additional eggs, and these females are fecund (Rivers & Denlinger, 1995a). These nutritional benfits could be more significant under stressful circumstances in nature. However, from the results of this study, nutritional changes in the host due to venom effects are not essential to Nasonia development. Finally, sorbitol elevation is a major metabolic change in envenomated hosts (Mrinalini et al., 2014) and is known to be a cryoprotectant (Michaud & Denlinger, 2007). Protection against cold temperatures or freezing could therefore be another advantage of envenomation, particularly for wasp larvae entering overwintering diapause. Experiments are needed to test whether venom provides further benefits in the presence of microbial pathogens or nutritional benefits, and under different environmental conditions such as variation in host quality, food scarcity, or temperature.
Our experiments show accumulative intergenerational benefits on wasp fecundity when wasps developed on envenomated hosts. After five generations, wasps raised on naturally parasitized hosts had higher lifetime fecundity than wasps on cold-killed hosts (approximately 1.27 higher, Fig.s 4 & 5). The causes of these cumulative benefits are not known, but may reflect subtle benefits of venom on development that can accumulate over time. It should be noted that cold-killed hosts are routinely used in mass rearing programs for use of parasitoids in biological control programs, and some studies indicate cold-killed hosts have relatively small effects on wasp fecundity (Tormos et al., 2014). However, our intergenerational experiment suggests that accumulating effects of fitness can be more substantial, possibly warranting further examination of the consequences of cold-killed hosts in biological control.
Thus, we conclude that major benefits of venom are to induce developmental arrest and suppress the host response to the larva; other effects of venom may be significant to the fitness of developing wasps (especially under natural or stressful conditions), but are not essential for parasitoid development. It is noteworthy that envenomated hosts stay alive for at least 15 days post-stinging (although they are typically consumed by wasp larvae within 6–8 days) (Rivers & Denlinger, 1994). Within two days, envenomated hosts undergo extensive metabolic and gene expression changes. It is difficult to imagine that the parasitoid is incapable of producing a venom that could induce immediate death of the host, if developmental arrest and immune suppression were the only phenotypes of importance. Therefore, we may conclude that there is an adaptive advantage of keeping the host alive and altering metabolism and gene expression (e.g. immunity against pathogens). It has been hypothesized that the functions of some venom components may be to ameliorate negative consequences of other venom components that might otherwise prematurely kill the host (Siebert et al., 2015). However, our experiments do not reveal a major advantage under laboratory conditions of keeping the host alive, except for the subtler effects on fecundity revealed in the intergenerational experiments. Further experiments are therefore needed to determine the consequences of venom to wasp survival under more natural conditions, such as stressors (e.g. pathogens, food scarcity) likely to be encountered in nature. This study is one of the first to look at the effect of venom in ectoparasitoids on developing wasp larvae on unstung live hosts and over successive generations and has implications on how to interpret venom composition and host effects going forward.
KEY FINDINGS.
-
-
Nasonia venom is beneficial, but not essential to developing larvae and has implications on how to interpret venom composition and host effects going forward.
-
-
Wasps raised on unstung hosts have higher mortality, but similar lifetime fecundity to wasps on stung or cold-killed hosts. However, after multiple generations on cold-killed hosts, significant fitness reduction appears.
-
-
The major benefits of venom are to induce developmental arrest and suppress the host response to the larva; however other effects of venom may be significant to the fitness of developing wasps under natural or stressful conditions.
Acknowledgments
We thank B. Parker, V. Martinson, and R. Edwards for comments and discussions. We acknowledge A. Dolan, L. Coleman, A. Martin, M. Pagan, T. Vo, M. Cooper, K. Ruiz, B. He, and D. DeLooze for technical assistance, and anonymous reviewers for helpful feedback. This research was supported by the National Institutes of Health (RO1GM098667) and University of Rochester Nathaniel and Helen Wisch Chair to JHW.
Footnotes
The authors declare no competing interests.
Contribution of authors
EOM and JHW contributed to project design and paper writing. EOM contributed to data collection and analysis.
References
- Asgari S, Rivers DB. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annual review of entomology. 2011;56:313–335. doi: 10.1146/annurev-ento-120709-144849. [DOI] [PubMed] [Google Scholar]
- Beard R. Pathogenic stinging of house fly pupae by Nasonia vitripennis (Walker) Journal of Insect Pathology. 1964;6 [Google Scholar]
- Burke GR, Strand MR. Systematic analysis of a wasp parasitism arsenal. Molecular ecology. 2014;23:890–901. doi: 10.1111/mec.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danneels EL, Formesyn EM, Hahn DA, Denlinger DL, Cardoen D, Wenseleers T, Schoofs L, de Graaf DC. Early changes in the pupal transcriptome of the flesh fly Sarcophagha crassipalpis to parasitization by the ectoparasitic wasp, Nasonia vitripennis. Insect Biochemistry and Molecular Biology. 2013;43:1189–1200. doi: 10.1016/j.ibmb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- De Graaf DC, Aerts M, Brunain M, Desjardins CA, Jacobs FJ, Werren JH, Devreese B. Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatic and proteomic studies. Insect Molecular Biology. 2010;19:11–26. doi: 10.1111/j.1365-2583.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray HCJ. Parasitoids: behavioral and evolutionary ecology. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Heraty J, Ronquist F, Carpenter JM, Hawks D, Schulmeister S, Dowling AP, Murray D, Munro J, Wheeler WC, Schiff N. Evolution of the hymenopteran megaradiation. Molecular Phylogenetics and Evolution. 2011;60:73–88. doi: 10.1016/j.ympev.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Legner E. Behavior changes the reproduction of Spalangia cameroni, S. endius, Muscidifurax raptor, and Nasonia vitripennis (Hymenoptera: Pteromalidae) at increasing fly host densities. Annals of the Entomological Society of America. 1967;60:819–826. doi: 10.1093/aesa/60.3.678. [DOI] [PubMed] [Google Scholar]
- Martinson EO, Mrinalini, Kelkar YD, Chang C-H, Werren JH. The evolution of venom by co-option of single copy genes. Current Biology. 2017 doi: 10.1016/j.cub.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson EO, Wheeler D, Wright J, Siebert AL, Werren JH. Nasonia vitripennis venom causes targeted gene expression changes in its fly host. Molecular ecology. 2014;23:5918–5930. doi: 10.1111/mec.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud MR, Denlinger DL. Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): a metabolomic comparison. Journal of Comparative Physiology B. 2007;177:753–763. doi: 10.1007/s00360-007-0172-5. [DOI] [PubMed] [Google Scholar]
- Moreau SJ, Asgari S. Venom proteins from parasitoid wasps and their biological functions. Toxins. 2015;7:2385–2412. doi: 10.3390/toxins7072385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer NT, Goecks J, Kacsoh BZ, Mobley JA, Bowersock GJ, Taylor J, Schlenke TA. Parasitoid wasp venom SERCA regulates Drosophila calcium levels and inhibits cellular immunity. Proceedings of the National Academy of Sciences. 2013;110:9427–9432. doi: 10.1073/pnas.1222351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrinalini, Siebert AL, Wright J, Martinson E, Wheeler D, Werren JH. Parasitoid venom induces metabolic cascades in fly hosts. Metabolomics. 2014:1–17. doi: 10.1007/s11306-014-0697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrinalini, Werren JH. Parasitoid Wasps and Their Venoms. In: Gopalakrishnakone P, Malhotra A, editors. Evolution of Venomous Animals and Their Toxins. Springer; Netherlands: 2017. pp. 187–212. [Google Scholar]
- Nakamatsu Y, Tanaka T. Venom of ectoparasitoid, Euplectrus sp. near plathypenae (Hymenoptera: Eulophidae) regulates the physiological state of Pseudaletia separata (Lepidoptera: Noctuidae) host as a food resource. Journal of Insect Physiology. 2003;49:149–159. doi: 10.1016/s0022-1910(02)00261-5. [DOI] [PubMed] [Google Scholar]
- Noyes J. Universal chalcidoidea database 2015. Natural History Museum; London: 2016. http://www.nhm.ac.uk/research-curation/research/projects/chalcidoids/introduction.html. [Google Scholar]
- Parkinson N, Richards E, Conyers C, Smith I, Edwards J. Analysis of venom constituents from the parasitoid wasp Pimpla hypochondriaca and cloning of a cDNA encoding a venom protein. Insect Biochemistry and Molecular Biology. 2002;32:729–735. doi: 10.1016/s0965-1748(01)00155-2. [DOI] [PubMed] [Google Scholar]
- Pickens L, Miller R. Using frozen host pupae to increase the efficiency of a parasite-release program. Florida Entomologist. 1978:153–158. [Google Scholar]
- Poirié M, Colinet D, Gatti JL. Insights into function and evolution of parasitoid wasp venoms. Current Opinion in Insect Science. 2014;6:52–60. doi: 10.1016/j.cois.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Quicke DL. Parasitic wasps. Chapman & Hall, Ltd; London: 1997. [Google Scholar]
- Rivers D, Ruggiero L, Hayes M. The ectoparasitic wasp Nasonia vitripennis (Walker)(Hymenoptera: Pteromalidae) differentially affects cells mediating the immune response of its flesh fly host, Sarcophaga bullata Parker (Diptera: Sarcophagidae) Journal of Insect Physiology. 2002a;48:1053–1064. doi: 10.1016/s0022-1910(02)00193-2. [DOI] [PubMed] [Google Scholar]
- Rivers D, Yoder JA. Site-specific effects of parasitism on water balance and lipid content of the parasitic wasp Nasonia vitripennis (Hymenoptera: Pteromalidae) European Journal of Entomology. 1996;94:75–82. [Google Scholar]
- Rivers DB. Evaluation of host responses to envenomation as a means to assess ectoparasitic pteromalid wasp’s potential for controlling manure-breeding flies. Biological Control. 2004;30:181–192. [Google Scholar]
- Rivers DB, Denlinger DL. Redirection of metabolism in the flesh fly, Sarcophaga bullata, following envenomation by the ectoparasitoid Nasonia vitripennis and correlation of metabolic effects with the diapause status of the host. Journal of Insect Physiology. 1994;40:207–215. [Google Scholar]
- Rivers DB, Denlinger DL. Fecundity and development of the ectoparasitic wasp Nasonia vitripennis are dependent on host quality. Entomologia Experimentalis et Applicata. 1995a;76:15–24. [Google Scholar]
- Rivers DB, Denlinger DL. Venom-induced alterations in fly lipid metabolism and its impact on larval development of the ectoparasitoid Nasonia vitripennis (Walker)(Hymenoptera: Pteromalidae) Journal of Invertebrate Pathology. 1995b;66:104–110. [Google Scholar]
- Rivers DB, Rocco MM, Frayha AR. Venom from the ectoparasitic wasp Nasonia vitripennis increases Na+ influx and activates phospholipase C and phospholipase A2 dependent signal transduction pathways in cultured insect cells. Toxicon. 2002b;40:9–21. doi: 10.1016/s0041-0101(01)00132-5. [DOI] [PubMed] [Google Scholar]
- Siebert AL, Wheeler D, Werren JH. A new approach for investigating venom function applied to venom calreticulin in a parasitoid wasp. Toxicon. 2015;107:304–316. doi: 10.1016/j.toxicon.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormos J, Sabater-Muñoz B, Asís J, Beitia F. Validation of a methodology for rearing Spalangia cameroni (Hymenoptera: Pteromalidae) on Ceratitis capitata (Diptera: Tephritidae) The Canadian Entomologist. 2014;146:676–683. [Google Scholar]
- Vincent B, Kaeslin M, Roth T, Heller M, Poulain J, Cousserans F, Schaller J, Poirié M, Lanzrein B, Drezen JM. The venom composition of the parasitic wasp Chelonus inanitus resolved by combined expressed sequence tags analysis and proteomic approach. BMC Genomics. 2010;11:693–708. doi: 10.1186/1471-2164-11-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH. Brood size and sex ratio regulation in the parasitic wasp Nasonia vitripennis (Walker)(Hymenoptera: Pteromalidae) Netherlands Journal of Zoology. 1983;34:123–143. [Google Scholar]
- Werren JH, Richards S, Desjardins CA, Niehuis O, Gadau J, Colbourne JK. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;327:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting AR. The biology of the parasitic wasp Mormoniella vitripennis [= Nasonia brevicornis](Walker) The Quarterly Review of Biology. 1967;42:333–406. [Google Scholar]


