Abstract
Heavy metals are highly toxic compounds for cells. In this report we demonstrate that the expression of Chlamydomonas reinhardtii thioredoxins (TRX) m and h is induced by heavy metals. Upon exposure of the cells to Cd and Hg, a strong accumulation of both messengers was observed. Western-blot experiments revealed that among these two TRXs, only TRX h polypeptides accumulated in response to the toxic cations. A biochemical analysis indicated that heavy metals inhibit TRX activity, presumably by binding at the level of their active site. Sequence analysis of the C. reinhardtii TRX h promoter revealed the presence of cis-acting elements related to cadmium induction. The origins and purposes of this regulation are discussed. Our data suggest, for the first time to our knowledge, a possible implication of TRXs in defense mechanisms against heavy metals.
Cells have developed defense mechanisms against heavy metals, which are highly toxic compounds. In animals and fungi small Cys-rich proteins called metallothioneins are induced by heavy metals (Thiele, 1992). These proteins can coordinate heavy metals by thiol bonds and confer heavy-metal tolerance on these organisms. Similar proteins and genes have been identified in plants but their function remains unclear (Zenk, 1996; Foley et al., 1997). Moreover, plants contain another class of metal-binding ligands called phytochelatins. Phytochelatins are poly(γ–glutamylcysteinyl)glycines synthesized from GSH and are clearly involved in detoxification processes (for review, see Zenk, 1996). A recent study has shown that TRX-like genes can confer heavy-metal tolerance on Escherichia coli-sensitive strains (Gupta et al., 1997). An increase in TRX expression upon exposure to a heavy metal (Se) has been reported for Bacillus subtilis (Garbisu et al., 1996). This suggested that TRX and TRX-like proteins might be important to repair proteins damaged by heavy metals.
TRXs are small ubiquitous proteins (approximately 12 kD) found in many organisms from bacteria to humans. They are characterized by the highly reactive disulfide of their conserved Cys-X-X-Cys active site (Holmgren, 1985; Eklund et al., 1991; Jacquot et al., 1997a). In plants two isoforms called m and f are located in the chloroplast and are involved in the control of key carbon fixation enzymes by light. Under illumination the photosynthetic electron transfer chain generates reduced Fd, which transfers its electrons to many acceptors, including the TRXs m and f via Fd-TRX reductase. In turn, TRXs are able to reduce several key enzymes of the carbon fixation metabolism, such as Fru-1,6-bisphosphatase and NADP-MDH (Jacquot et al., 1997b; Miginiac-Maslow et al., 1997), which are converted from an inactive to an active form. Another TRX isoform, the h-type, is located in the cytosol and is reduced by NADPH through an NADPH TRX reductase (Florencio et al., 1988). The exact function of the cytoplasmic isoform(s) is still largely unknown. However, several studies have suggested that they could be involved in the response to oxidative stress but also in the cell division cycle (Muller, 1991; Regad et al., 1995; Mouaheb et al., 1998).
In the past few years we have isolated several proteins related to the Fd/TRX system in the unicellular green alga Chlamydomonas reinhardtii. The isolated proteins included Fd (Schmitter et al., 1988), TRX m (Decottignies et al., 1990), and TRX h (Decottignies et al., 1991). The sequencing of these proteins at the amino acid level allowed us to isolate the corresponding cDNAs (Jacquot et al., 1992; Rogers et al., 1992) and genes (Stein et al., 1995a, 1995b).
We report here the detailed analysis of TRX m and TRX h expression in C. reinhardtii, in response to heavy metals. The effects of Cd and Hg have been analyzed at the mRNA and protein levels. We demonstrate that the expression of both genes is regulated by heavy metals at the mRNA level but with distinct kinetics. An increase in protein level was only observed for TRX h. A biochemical analysis of the interaction of TRXs with heavy metals indicates that these cations inactivate TRXs, presumably by binding to their active site. Our data suggest, for the first time to our knowledge, a possible implication of TRXs in defense mechanisms against heavy metals in photosynthetic organisms.
MATERIALS AND METHODS
Strain and Culture Conditions
The Chlamydomonas reinhardtii cell wall-less strain CW15 (137c, mt+, cw15) was obtained from the Chlamydomonas Genetics Center at Duke University (Durham, NC). This strain is widely used since the absence of the cell wall facilitates cell fractionation, RNA extraction, and cell transformation. Cells were grown in a photoautotrophic minimal medium with a final composition per liter of 0.4 g of NH4Cl, 0.1 g of MgSO4.7 H2O, 0.05 g of CaCl2, 0.72 g of KH2PO4, and 0.36 g of K2HPO4 and other components as in high salt medium (Sueoka et al., 1967). The cultures were continuously stirred, bubbled with 5% CO2, and maintained at 28°C. Pregrowth cultures were performed under continuous illumination in Tris-acetate phosphate medium (Gorman and Levine, 1965). Dilutions and other basic procedures were as described previously (Surzycky, 1971). Light intensity was 300 μE m−2 s−1 at the level of the flask culture. Cell growth was performed under continuous illumination and the cultures were transferred to darkness 5 h before analysis.
RNA Isolation
Approximately 30 million cells were collected for each extraction and pelleted by centrifugation (3,000g, 5 min). The pellet was immediately resuspended in 1 mL of TRIzol reagent (GIBCO-BRL). Polysaccharides, membranes, and unlysed cells were eliminated by centrifugation (12,000g, 10 min, 4°C). At this step the supernatant was either stored at −80°C or immediately treated following the manufacturer's protocol. The dried RNA pellet was resuspended in 20 μL of milliQ-treated (Millipore) sterile water.
Northern Analysis
Total RNA was separated on a 1.5% agarose gel containing formaldehyde and blotted onto a positive membrane (Appligene, Illkirch, France) by capillarity in 2× SSC (1× SSC is 0.15 m NaCl and 0.015 m sodium citrate). RNA was covalently fixed onto the membrane by UV-cross linking (UV Stratalinker 1800, Stratagene). The probes were synthesized by random priming (Nonaprimer kit II, Appligene). The constitutive probe is a fragment of the coding region of a G-protein β-subunit-like polypeptide (Schloss, 1990) and was obtained by PCR using the pcf 8-13 plasmid kindly provided by Dr. Karen L. Kindle (Cornell University, Ithaca, NY). The other probes correspond to the 3′-untranslated region of the TRX h and m isoforms (Stein et al., 1995b) and Fd (Stein et al., 1995a) obtained by PCR. Oligonucleotide pairs were as follows: Fd, 5′-GGGCAACGGCTTCGTGCTGACC-3′ and 5′-TTGCAGGTCACCACGGCCATGC-3′; TRX m, 5′-GCCGGCGGGAGTGGGGTTCCCCG-3′ and 5′-GCACCCGCCGACAGCTCCGGACG-3′; and TRX h, 5′-GCAGGGGAGTCAGCAGCTGCGGG-3′ and 5′-CCACTCGCAGCAGCACACCTCCTG-3′.
The membrane blot was hybridized overnight in buffer containing 0.5 m NaHPO4 (pH 7.2), 1 mm Na2EDTA, 7% SDS, and 1% BSA. Hybridization was performed at 62°C for all of the probes. Final washes were performed at 62°C in 40 mm NaHPO4 (pH 7.2), 1 mm Na2EDTA, and 1% SDS. Several exposure times were used to be in the linear range response of the X-Omat film (Kodak). The autoradiograms were quantified by densitometric scans (Masterscan, Scanalytics-CSPI, Billerica, MA). The signals were normalized to the constitutive probe signal. After hybridization the blot was stripped by boiling in 0.01× SSC and SDS 0.5% and then hybridized with another probe. All of the northern experiments reported in this paper were repeated three to four times to confirm the results.
Heavy-Metal and Oxidative Stress Treatments
For heavy-metal treatments the culture was separated into three bottles and transferred to continuous darkness 5 h before the addition of heavy metals. The cultures were supplemented with sterile water (control) or HgCl2 or CdCl2 at final concentrations of 1 μm and 100 μm, respectively. These concentrations correspond to the sublethal dose for each cation. The oxidative stress treatments were performed with cultures supplemented with either H2O2 or diamide at final concentrations of 1 to 2 mm and 5 to 50 μm, respectively.
Protein Extraction and Western Analysis
Total protein was prepared by resuspension of a pellet of 30 million cells in extraction buffer (30 mm Tris-HCl, pH 7.5, and 100 μm PMSF) followed by two rounds of freezing in liquid N2. The extracts were centrifuged at 12,000g for 15 min, and the protein concentration of the supernatant was determined by the Bradford dye-binding assay (Bio-Rad).
Protein samples (30–60 μg) were fractionated by 12% SDS-PAGE and blotted onto a nitrocellulose membrane (Hybond-C extra, Amersham). The blots were blocked with dry powdered milk (5%, w/v) in TBS buffer (50 mm Tris-HCl, pH 7.5, and 0.2 m NaCl) and incubated with antibodies at room temperature. The antibodies used as a first-layer reagent were rabbit polyclonal monospecific anti-TRX h or anti-TRX m IgG. After exposure of the membrane to secondary antibody (goat anti-rabbit IgG conjugated with horseradish peroxidase, Bio-Rad) and thorough washing, the immunoreactive proteins were detected by the color produced upon reaction of horseradish peroxidase with H2O2 and 4-chloro-1-naphtol in TBS buffer. The signals were quantified by densitometric scans (Masterscan).
Biochemical Analysis
Expression and purification of TRX m and TRX h were performed as described previously by Stein et al. (1995b) and Krimm et al. (1998), respectively. The initial concentrations of TRX m and TRX h in 30 mm Tris-HCl, pH 7.9, were 100 μm and 40 μm, respectively. The TRXs were fully oxidized during the purification process, therefore, no oxidation treatment was necessary. Chemical reduction of TRXs was performed in the presence of 10 mm DTT. DTT was removed by ultrafiltrations on a Centricon 10 microconcentrator (Amicon), allowing a dilution of DTT of at least 4000 times with 30 mm Tris-HCl, pH 7.9. The dialyzed samples were supplemented with CdCl2 or HgCl2 (500 μm final concentration). The unreacted metal ions were eliminated by dialysis in the same conditions. TRX activity was estimated as the rate of activation of NADP-MDH after a 10 min incubation with TRX (30 and 15 μm final concentrations for TRX h and TRX m, respectively). Purification of recombinant sorghum NADP-MDH was performed as described previously by Issakidis et al. (1992), and enzyme activation assays were performed according to the method of Stein et al. (1995b). When necessary, EDTA, 2-mercaptoethanol, or DTT was added to the TRX sample before activity measurements at final concentrations of 1, 1, and 10 mm respectively.
RESULTS
Both TRX Messengers Are Induced by Heavy Metals
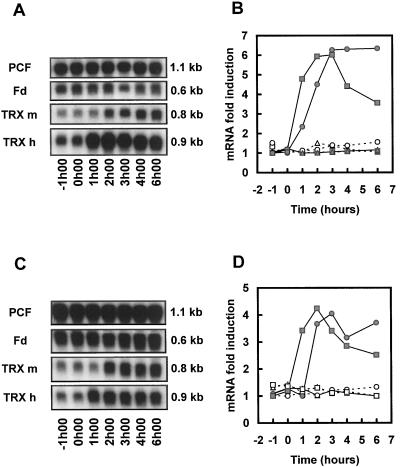
To avoid interference with the light-dependent induction of Fd and TRX gene expression (Lemaire et al., 1999), the heavy-metal treatments were performed on cultures maintained in darkness. The effects of Cd and Hg on the steady-state mRNA levels of Fd, TRX m, and TRX h have been analyzed by northern analysis (Fig. 1). The constitutive probe PCF allowed us to visualize and quantify loading variations. No significant variations in the three messenger levels were observed in the control experiment (data not shown). On the contrary, a strong increase in the level of both TRX messengers was observed when the cells were exposed to Hg or Cd cations (Fig. 1, A and C). The accumulation was more rapid for TRX h mRNA levels, which started increasing within 1 h after the addition of the heavy metal, than for TRX m mRNA levels, which increased after 2 h of treatment. Higher induction levels were observed with Hg (6-fold, Fig. 1B) than with Cd (4-fold, Fig. 1D). TRX h mRNA levels decreased after 3 to 4 h following addition of the heavy metal, whereas TRX m RNA levels showed no significant decrease after 6 h. Fd mRNA levels remained constant throughout the experiment. This point is particularly interesting since Fd and TRX m proteins are involved in the same redox pathway. Moreover, these three messengers are also regulated by light and the circadian clock (Lemaire et al., 1999), whereas heavy metals only induce TRX m and TRX h. These observations indicate that the effect of heavy metals is specific for TRX genes.
Figure 1.
Northern analysis of the variations in TRX m, TRX h, and Fd mRNA levels in response to heavy metals. Cells were maintained in continuous light and transferred to darkness 5 h before treatment. The time of sampling is indicated at the bottom, the hours are relative to the addition of heavy metal (0 h). Autoradiographs of RNA blots from cultures supplemented with HgCl2 (A) or CdCl2 (C); Quantification of the corresponding northern blots (B, HgCl2; D, CdCl2). The final heavy-metal concentrations were 1 μm for HgCl2 and 100 μm for CdCl2. The probes used in each panel are indicated on the left and the sizes of the messengers on the right. PCF corresponds to the constitutive probe signal. White symbols and dashed line, Control culture; gray symbols and continuous line, treated cultures. Triangles, Fd; squares, TRX h; circles, TRX m. The mRNA levels were normalized to the constitutive probe signal. The data are the means from two to four independent experiments.
The Effect on Protein Levels Is Different for Both TRXs
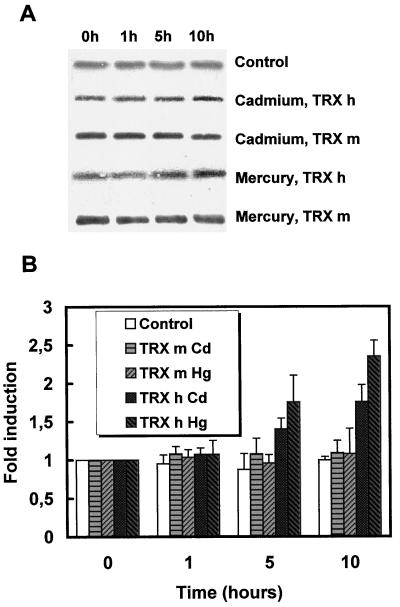
We have further analyzed the effects of heavy metals to check if the mRNA accumulation was followed by an increase in the corresponding protein levels. The western blots performed with polyclonal antibodies specific for the h or m isoform on extracts from control or heavy-metal-treated cells are presented on Figure 2A. A single band corresponding to each TRX was detected. No cross-hybridization was observed using as much as 1 μg of pure TRX (data not shown). The control experiment indicates that the protein levels remained constant in the absence of heavy metal. Upon exposure of cells to Hg, TRX h levels increased within 5 to 10 h after treatment. A more discrete effect of Cd could also be observed. Surprisingly, no increase in TRX m protein level could be observed after heavy-metal treatment. Quantification of protein levels has been performed with a series of three independent western blots (Fig. 2B). The absence of variation for the control experiment was confirmed and indicated that loading variations were relatively limited. TRX m levels did not appear to increase, whereas TRX h levels increased within 5 to 10 h. As previously observed at the mRNA level, Hg induction (2.5-fold) was higher than Cd induction (1.7-fold). Again, the two TRX isoforms appear to be regulated differently.
Figure 2.
Analysis of TRX m and TRX h protein levels in response to heavy metals. A, Western analysis of TRX m and TRX h levels in response to heavy metals. Cells were treated with sterile water (Control), 100 μm CdCl2 (Cadmium), or 1 μm HgCl2 (Mercury). Crude extracts prepared after 0, 1, 5, or 10 h after treatment were analyzed by western blot, as described in Methods. The control corresponds to TRX h in untreated cells. B, Quantification of TRX m and TRX h protein levels in response to heavy metals. Western blots from three independent experiments (performed as in A) were used to quantify the accumulation of TRX m and TRX h after heavy-metal treatment. Quantification was performed by densitometric scans. The average induction factors are presented and the error bars represent sd.
Oxidative Stress Does Not Affect the Expression
The stress induced by heavy metals might be related to an oxidative stress. Heavy metals are known to induce oxidation of amino acids and proteins (Stadtman, 1993). Thus, the induction of TRX messengers by heavy metals could be an indirect effect linked to a modification of the redox state of the cell. When exposed to oxidative stress, living organisms must initiate defense reactions against active oxygen species (Sies, 1991). A TRX-deficient yeast hypersensitive to oxidants can be complemented by two Arabidopsis TRX h isoforms (Mouaheb et al., 1998). This suggests that in plants, TRX h might be implicated in the response to oxidative stress.
We have investigated the effects of H2O2 and diamide on the steady-state mRNA levels of both TRXs. In E. coli these compounds induce two different and independent defense mechanisms based on the OxyR (hydrogen peroxide scavenging) and SoxRS (superoxide scavenging) regulons (Amábile-Cuevas and Demple, 1991; Demple, 1996). In plants similar distinct effects of these two oxidants on gene expression have been reported (Babiychuk et al., 1995). In our study, however, no modification of the mRNA levels corresponding to the two TRXs could be observed after treatment of the cells with the two oxidants (data not shown). This result indicates that the mRNA accumulation observed for both TRXs after heavy-metal treatment is not linked to an indirect effect by way of an oxidative stress response mediated by heavy metals.
Effect of Heavy Metals on TRX Activity in Vitro
Heavy metals are known to bind dithiols with high affinity (Vallee and Ulmer, 1972). Consequently, the active site dithiol of reduced TRXs is a possible target of heavy-metal fixation on the protein. We have analyzed the effect of heavy metals on the activity of TRXs and found that the pretreatment of TRX with Cd, followed by dialysis, totally suppressed its ability to activate NADP-MDH. This ability was restored by the addition of either EDTA or DTT, but not by the addition of 2-mercaptoethanol (Table I). Recovering the activity with EDTA was impossible when the protein was treated with mercury ions (data not shown). This is in agreement with the fact that Hg has a higher affinity for disulfides than for EDTA, whereas the reverse is true for Cd (Vallee and Ulmer, 1972; Matts et al., 1991). These experiments indicate that TRXs are able to bind heavy metals, presumably at the level of their active site dithiol.
Table I.
Effects of Cd binding on THX h's ability to activate NADP-MDH
| DTT-Reduced TRX | Activity | Induced Recovery by Thiol Compounds or EDTA
|
||
|---|---|---|---|---|
| +EDTA | +2-Mercaptoethanol | +DTT | ||
| % (nmol NADPH oxidized/min) | ||||
| Cd treated | 0 (0) | 97 (121.9) | 0 (0) | 104 (183.9) |
| Control | 100 (124.2) | 100 (125.1) | 100 (122.5) | 100 (177.4) |
The percentages correspond to the relative activity measured compared with the control reduced TRX at each step of the analysis. The numbers in parentheses indicate the activity. The samples were dialyzed after reduction to remove DTT and after Cd treatment to remove the cation. The Cd-treated oxidized TRX was fully active in NADP-MDH activation after dialysis and reduction by DTT.
DISCUSSION
In this work we have provided evidence that the expression of TRX h and TRX m is regulated by heavy metals in C. reinhardtii. This regulation can be either direct or indirect. Heavy metals can participate in a metal-catalyzed Fenton-type reaction with superoxide or peroxide molecules to generate highly toxic hydroxyl radicals (Stadtman, 1993). Thus, the observed induction could be a response to this oxidative stress and not to the cation. To verify if heavy metals had a direct or indirect effect we have tested the response of TRX genes to oxidative stress. Treatments of the cells with oxidants generating superoxide radicals or peroxide radicals did not trigger any accumulation of TRX messengers. Thus, the induction of TRX expression by heavy metals does not seem to result indirectly from an oxidative stress. In plants detoxification of heavy metals involves small Cys-rich ligands called phytochelatins. These ligands, synthesized from GSH, are able to bind heavy metals and are stored in the vacuoles (for review, see Zenk, 1996). Upon exposure of the cells to heavy metals, phytochelatins are synthesized and the GSH level drops immediately (Scheller et al., 1987; Rüegsegger et al., 1990). Recent studies suggested that the redox state of GSH, as well as its synthesis, might be linked to TRXs (Muller, 1996; Kojima et al., 1998). Thus, the induction of TRXs by heavy metals might be an indirect effect mediated by the depletion of the GSH pool. The drop in the GSH level can be mimicked by addition of diamide (Giritch et al., 1998), but in our case no accumulation of TRX mRNA was observed when cells were exposed to this oxidant. Consequently, the response of TRX genes to heavy metals does not seem to be an indirect consequence of the synthesis of phytochelatins.
To obtain insight into the regulation of TRX genes by heavy metals we have looked for relevant cis-acting elements in their promoter regions. The cis-acting elements related to the regulation by heavy metals are poorly characterized. One of these elements has been identified in the promoters of parA and pGH2/4, two genes regulated by auxin and Cd (Ellis et al., 1993; Kusaba et al., 1996). This element is related to the as-1 (or ocs) element previously identified in the 35S promoter of cauliflower mosaic virus (Lam et al., 1989). The analysis of the promoters of C. reinhardtii TRXs revealed the presence of a tandemly repeated as-1-related element in the promoter of TRX h, whereas no homologies could be found in the promoter of TRX m (Fig. 3). This result emphasizes the differences between TRX h and TRX m. In fact, both messengers are induced but in a different manner, whereas protein accumulation and relevant cis-acting elements are only observed for TRX h. This suggests that the induction of these TRXs might involve distinct mechanisms and functions. The TRX h response is more rapid than that observed for TRX m. The induction of TRX m mRNA has no effect on the size of the protein pool, suggesting an increase in the protein turnover rate under heavy-metal stress. The biochemical analysis effectively indicates that TRXs are inhibited by heavy metals and consequently TRX degradation might be more important in the presence of the toxic ions. The induction observed for TRX m at the messenger level might, therefore, be a compensation of their inhibition or their increased degradation. Thus, this regulation might be an indirect response operating through a feedback mechanism allowing the TRX pool to be maintained. Whereas the same kind of feedback mechanism could be involved in TRX h regulation, the results obtained for this isoform, as well as the presence of putative cis-acting elements in its promoter, suggest a more direct regulation. The functions of TRX h in plants are still largely unknown. Our data suggest that one of these functions could be linked to the detoxification of heavy metals. However, the observation of a chelation of heavy metals by reduced TRX suggests that it might be much more direct, similar to the heavy-metal chelation by metallothioneins. Further experiments will be necessary to understand the physiological function of TRX regulation by heavy metals.
Figure 3.
Metal-related cis-acting elements in the promoter of TRX h. A, The TRX h promoter contains two tandemly repeated as-1 elements. The numberings are relative to the translation start site and the stars correspond to conserved nucleotides. B, Homologies between as-1-related elements. The as-1-related elements from the TRX h promoter, the parA gene (Kusaba et al., 1996), the 35S promoter of cauliflower mosaic virus (Lam et al., 1989), and the GH2/4 gene (Ellis et al., 1993) are represented. The arrows correspond to the TGACG-related sequences found in all as-1-related elements.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Karen L. Kindle for the gift of the cDNA encoding the constitutive probe. We also thank Anne Trouabal for excellent technical assistance.
Abbreviations:
- NADP-MDH
NADP-malate dehydrogenase
- TRX
thioredoxin
LITERATURE CITED
- Amábile-Cuevas CF, Demple B. Molecular characterization of the sox RS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 1991;19:4479–4484. doi: 10.1093/nar/19.16.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk E, Kushnir S, Belles-Boix E, Van Montagu M, Inzé D. Arabidopsis thaliana NADPH oxidoreductase homologs confer tolerance of yeast toward the thiol-oxidizing drug diamide. J Biol Chem. 1995;270:26224–26231. doi: 10.1074/jbc.270.44.26224. [DOI] [PubMed] [Google Scholar]
- Decottignies P, Schmitter JM, Dutka S, Jacquot JP, Miginiac-Maslow M. Characterization and primary structure of a second thioredoxin from the green alga, Chlamydomonas reinhardtii. Eur J Biochem. 1991;198:505–512. doi: 10.1111/j.1432-1033.1991.tb16043.x. [DOI] [PubMed] [Google Scholar]
- Decottignies P, Schmitter JM, Jacquot JP, Dutka S, Picaud A, Gadal P. Purification, characterization, and complete amino acid sequence of a thioredoxin from a green alga, Chlamydomonas reinhardtii. Arch Biochem Biophys. 1990;280:112–121. doi: 10.1016/0003-9861(90)90525-4. [DOI] [PubMed] [Google Scholar]
- Demple B. Redox signaling and gene control in the Escherichia coli soxRS oxidative stress regulon: a review. Gene. 1996;179:53–57. doi: 10.1016/s0378-1119(96)00329-0. [DOI] [PubMed] [Google Scholar]
- Eklund H, Gleason F, Holmgren A. Structural and functional relations among thioredoxins from different species. Proteins. 1991;11:13–28. doi: 10.1002/prot.340110103. [DOI] [PubMed] [Google Scholar]
- Ellis JG, Tokuhisa JG, Llewellyn DJ, Bouchez D, Singh K, Dennis ES, Peacock WJ. Does the ocs-element occur as a functional component of the promoters of plant genes? Plant J. 1993;4:433–443. doi: 10.1046/j.1365-313x.1993.04030433.x. [DOI] [PubMed] [Google Scholar]
- Florencio FJ, Yee BC, Johnson TC, Buchanan BB. An NADP/thioredoxin system in leaves: purification and characterization of NADP-thioredoxin reductase and thioredoxin h from spinach. Arch Biochem Biophys. 1988;266:496–507. doi: 10.1016/0003-9861(88)90282-2. [DOI] [PubMed] [Google Scholar]
- Foley RC, Liang ZM, Singh KB. Analysis of type 1 metallothionein cDNAs in Vicia faba. Plant Mol Biol. 1997;33:583–591. doi: 10.1023/a:1005790927581. [DOI] [PubMed] [Google Scholar]
- Garbisu C, Ishii T, Leighton T, Buchanan BB. Bacterial reduction of selenite to elemental selenium. Chem Geol. 1996;132:199–204. [Google Scholar]
- Giritch A, Ganal M, Stephan UW, Bäumlein H. Structure, expression and chromosomal localisation of the metallothionein-like gene family of tomato. Plant Mol Biol. 1998;37:701–714. doi: 10.1023/a:1006001701919. [DOI] [PubMed] [Google Scholar]
- Gorman DS, Levine RP. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SD, Wu HC, Rick PD. A Salmonella typhimurium genetic locus which confers copper tolerance on copper-sensitive mutants of Escherichia coli. J Bacteriol. 1997;179:4977–4984. doi: 10.1128/jb.179.16.4977-4984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Issakidis E, Miginiac-Maslow M, Decottignies P, Jacquot JP, Crétin C, Gadal P. Site-directed mutagenesis reveals the involvement of an additional thioredoxin-independent regulatory site in the activation of recombinant sorghum leaf NADP-malate dehydrogenase. J Biol Chem. 1992;267:21577–21583. [PubMed] [Google Scholar]
- Jacquot JP, Lancelin JM, Meyer Y. Thioredoxins: structure and function in plant cells. New Phytol. 1997a;136:543–570. doi: 10.1046/j.1469-8137.1997.00784.x. [DOI] [PubMed] [Google Scholar]
- Jacquot JP, Lopez-Jaramillo J, Miginiac-Maslow M, Lemaire S, Cherfils J, Chueca A, Lopez-Gorge J. Cysteine-153 is required for redox regulation of pea chloroplast fructose-1,6-bisphosphatase. FEBS Lett. 1997b;401:143–147. doi: 10.1016/s0014-5793(96)01459-7. [DOI] [PubMed] [Google Scholar]
- Jacquot JP, Stein M, Hodges M, Miginiac-Maslow M. PCR cloning of a nucleotidic sequence coding for the mature part of Chlamydomonas reinhardtii thioredoxin Ch2. Nucleic Acids Res. 1992;20:617. doi: 10.1093/nar/20.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Matsuki O, Nomura T, Kubodera A, Honda Y, Honda S, Tanooka H, Wakasugi H, Yamaoka Y. Induction of mRNAs for glutathione synthesis-related proteins in mouse liver by low doses of gamma-rays. Biochim Biophys Acta. 1998;1381:312–318. doi: 10.1016/s0304-4165(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Krimm I, Lemaire S, Ruelland E, Miginiac-Maslow M, Jacquot JP, Hirasawa M, Knaff DB, Lancelin JM. The single mutation Trp35→Ala in the 35–40 redox site of Chlamydomonas reinhardtii thioredoxin h affects its biochemical activity and the pH dependence of C36–C39 1H-13C NMR. Eur J Biochem. 1998;255:185–195. doi: 10.1046/j.1432-1327.1998.2550185.x. [DOI] [PubMed] [Google Scholar]
- Kusaba M, Takahashi T, Nagata T. A multiple-stimuli-responsive as-1-related element of parA gene confers responsiveness to cadmium but not to copper. Plant Physiol. 1996;111:1161–1167. doi: 10.1104/pp.111.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E, Benfey PN, Gilmartin PM, Fang RX, Chua NH. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci USA. 1989;86:7890–7894. doi: 10.1073/pnas.86.20.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire S, Stein M, Issakidis-Bourguet E, Keryer E, Benoit V, Gérard-Hirne C, Miginiac-Maslow M, Jacquot JP (1999) The complex regulation of ferredoxin/thioredoxin related genes by light and the circadian clock. Planta (in press) [DOI] [PubMed]
- Matts RL, Schatz JR, Hurst R, Kagen R. Toxic heavy metals ions activate the heme-regulated eukaryotic initiation factor -2α kinase by inhibiting the capacity of hemin-supplemented reticulocyte lysates to reduce disulfide bonds. J Biol Chem. 1991;266:12695–12702. [PubMed] [Google Scholar]
- Miginiac-Maslow M, Issakidis E, Lemaire M, Ruelland E, Jacquot JP, Decottignies P. Light-dependent activation of NADP-malate dehydrogenase: a complex process. Aust J Plant Physiol. 1997;24:529–542. [Google Scholar]
- Mouaheb N, Thomas D, Verdoucq L, Monfort P, Meyer Y. In vivo functional discrimination between plant thioredoxins by heterologous expression in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1998;95:3312–3317. doi: 10.1073/pnas.95.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller EG. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J Biol Chem. 1991;266:9194–9200. [PubMed] [Google Scholar]
- Muller EG. A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth. Mol Biol Cell. 1996;7:1805–1813. doi: 10.1091/mbc.7.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regad F, Hervé C, Marinx O, Bergounioux C, Tremousaygue D, Lescure B. The tef1 box, a ubiquitous cis-acting element involved in the activation of plant genes that are highly expressed in cycling cells. Mol Gen Genet. 1995;248:703–711. doi: 10.1007/BF02191710. [DOI] [PubMed] [Google Scholar]
- Rogers WJ, Hodges M, Decottignies P, Schmitter JM, Gadal P, Jacquot JP. Isolation of a cDNA fragment coding for Chlamydomonas reinhardtii ferredoxin and expression of the recombinant protein in Escherichia coli. FEBS Lett. 1992;310:240–245. doi: 10.1016/0014-5793(92)81340-r. [DOI] [PubMed] [Google Scholar]
- Rüegsegger A, Schmutz D, Brunold C. Regulation of glutathione synthesis by cadmium in Pisum sativum L. Plant Physiol. 1990;93:1579–1584. doi: 10.1104/pp.93.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Huang B, Hatch E, Goldsbrough PB. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987;85:1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss JA. A Chlamydomonas gene encodes a G protein β subunit-like polypeptide. Mol Gen Genet. 1990;221:443–452. doi: 10.1007/BF00259410. [DOI] [PubMed] [Google Scholar]
- Schmitter JM, Jacquot JP, de Lamotte-Guéry F, Beauvallet C, Dutka S, Gadal P, Decottignies P. Purification, properties and complete amino acid sequence of the ferredoxin from a green alga, Chlamydomonas reinhardtii. Eur J Biochem. 1988;172:405–412. doi: 10.1111/j.1432-1033.1988.tb13901.x. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative Stress: Oxidants and Antioxidants. London: Academic Press; 1991. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:787–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- Stein M, Chedozeau B, Jacquot JP. Cloning and sequencing of a ferredoxin gene from Chlamydomonas reinhardtii. Plant Physiol. 1995a;109:721. [Google Scholar]
- Stein M, Jacquot JP, Jeannette E, Decottignies P, Hodges M, Lancelin JM, Mittard V, Schmitter JM, Miginiac-Maslow M. Chlamydomonas reinhardtii thioredoxins: structure of the genes coding for the chloroplastic m and cytosolic h isoforms; expression in Escherichia coli of the recombinant proteins, purification and biochemical properties. Plant Mol Biol. 1995b;28:487–503. doi: 10.1007/BF00020396. [DOI] [PubMed] [Google Scholar]
- Sueoka N, Chiang KS, Kates JR. Deoxyribonucleic acid replication in meiosis of Chlamydomonas reinhardi. J Mol Biol. 1967;25:44–67. doi: 10.1016/0022-2836(67)90278-1. [DOI] [PubMed] [Google Scholar]
- Surzycky S. Synchronously grown cultures of Chlamydomonas reinhardi. Methods Enzymol. 1971;23:67–73. [Google Scholar]
- Thiele DJ. Metal-regulated transcription in eukaryotes. Nucleic Acids Res. 1992;20:1183–1191. doi: 10.1093/nar/20.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee BL, Ulmer DD. Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem. 1972;41:91–128. doi: 10.1146/annurev.bi.41.070172.000515. [DOI] [PubMed] [Google Scholar]
- Zenk MH. Heavy metal detoxification in higher plants: a review. Gene. 1996;179:21–30. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]