Abstract
Biochar (BC) amendments may be suitable to increase the ecosystems resistance to drought due to their positive effects on soil water retention and availability. We investigated the effect of BC in situ ageing on water availability and microbial parameters of a grassland soil. We used soil containing 13C labeled BC and determined its water holding capacity, microbial biomass and activity during a 3 months incubation under optimum and drought conditions. Our incubation experiment comprised three treatments: soil without BC (Control), soil containing aged BC (BCaged) and soil containing fresh BC (BCfresh), under optimum soil water (pF 1.8) and drought conditions (pF 3.5). Under optimum water as well as drought conditions, soils containing BC showed higher soil organic carbon (SOC) mineralization as compared to control soil. Moreover, BC effects on the soil water regime increase upon in situ aging. Native SOC mineralization increased most for soils containing BCaged. The BCaged led to improved C use under drought as compared to the other treatments. We conclude that BC addition to soils can ameliorate their water regime, especially under drought conditions. This beneficial effect of BC increases upon its aging, which also improved native substrate availability.
Introduction
A major challenge of climate change is the increasing frequency of extreme hydrological events such as droughts (IPCC 2007), which will have strong impacts on terrestrial ecosystems and the biogeochemical carbon (C) cycle1. In particular, droughts can affect the quantity and quality of organic matter (OM) retained in soil, as soil moisture is one of the most important factors driving microbial processes. Recently, addition of biochar (BC) to soil was suggested to ameliorate water retention under drought conditions2. Biochar is a highly aromatic material produced by thermal degradation of organic materials with limited or no air supply and is distinguished from charcoal by its use as a soil amendment3. Its addition to soil was found to alter the soil’s physical structure and air capacity4 and to be beneficial for water holding capacity (WHC), water retention, and plant available water5,6. Biochar (BC) is proposed as beneficial for soil improvement but the usage competition of organic material as feedstock and economical aspects are mayor points of criticism for agricultural scale usage as soil amendment7. Moreover, the aging of BC and the interpretation of its effects on soil physical and microbial parameters suffer from a strongly varying composition and characteristics of the BC. To overcome these common critical issues, we used a highly homogeneous BC, produced at industrial scale – a waste product from heat production.
In mineral soils, drought reduces the microbial activity8 due to physiological stress and limited substrate supply to microbial cells9. Microorganisms can react to this physiological stress by physiological changes or by a shift of their communities towards microbes with higher water stress resistance10. Highly stressed microbes will then use substrate for maintenance and not for growth11, which ultimately affects their metabolic efficiency.
Microbial activity response to drought was found to be dependent on soil organic matter quality12 and soil properties13. Biochar addition, depending on BC feedstock14 and production conditions15–17, may strongly affect hydraulic properties18 and thereby alter microbial activity under drought conditions.
The degradation of BC is assumed to be mostly microbial induced, but Zimmerman19 and Cheng, et al.20 showed that abiotic BC-C release due to processes such as chemical oxidation, photooxidation, or solubilisation can significantly contribute to the C release. Aging after field exposure can significantly alter physico-chemical parameters of the BC21 and therefore most probably its effects on soil parameters22,23. Biochar addition to soil may also change microbial community composition24. An indicator used to determine the microbial use of carbon is the metabolic quotient (qCO2), considering the respiration rate CO2-C per unit microbial biomass C25. The qCO2 was found to decrease by 13% after BC amendment compared to the control indicating improved soil biophysical conditions26. While the microbial biomass responded with growth, the total soil CO2 production remained unchanged after BC amendment.
Biochar properties strongly changed as a consequence of short term field exposure of less than 6 months. In particular, increasing surface area and changes in chemical properties were observed27–29, recently discussed as caused by organic coatings30,31. This resulted in increasing wettability of the BC over time, and hence, a better availability for microbial degradation32. Artificial weathering induced through chemical and/or physical treatments increased carbonyl and carboxylic functional groups as well as the biological stability of the residual BC33,34.
We investigated the effects of physico-chemical changes occurring in BC amended soils with time of field exposure on the response of microbial parameters to drought stress. We hypothesized that BC aging impacts soil hydraulic properties and affects microbial activity under drought conditions. We set up an incubation experiment with soil containing 13C labeled BC (1) added to soil after production (BCfresh) and (2) sampled after 3 years of field exposure (BCaged). The 13C labeling allowed us to monitor mineralization of native SOC in addition to total SOC. Our specific objectives were to quantify the effect of fresh and aged BC (i) on soil hydraulic properties ii) microbial biomass, its activity and metabolic quotient and iii) native SOC mineralization. The soils were incubated under two different water potentials to investigate the impact of drought on these parameters.
Material and Methods
Study site
The soil used in this study was sampled from a BC field experiment located at the site of the long-term field experiment “SOERE ACBB” managed by INRA (National Institute of Agricultural Research) in Lusignan (46°25″12.91″N; 0°07″29.35″ E), France. Mean annual rainfall is 800 mm and the mean annual temperature is 11 °C. The soil is classified as a Dystric Cambisol35 with loamy texture (11% sand, 72% silt and 17% clay), a bulk density of 1.4 g cm−3 and a pH of 6.0 (control soils) and 6.4 (biochar plots). It was considered that total C concentrations represent organic carbon (OC) because we did not find any evidence of inorganic C contribution to our soils using acid treatment. The OC and total N content was 1.4% and 0.16%, respectively36. Before 2012, the field was a temporary grassland (C3 plants) and is now cultivated with Festuca arundinacea and Dactylis glomerata. The field experiment comprised 8 plots of a size of 16 m²: 4 BC amended plots and 4 control plots. A single addition of 3 kg m−2 13C labeled BC to the upper 10 cm took place in May 2012 by a rotary hoeing. After three years, BC amendment led to pH increase from 6.0 (control) to 6.4 (BC). An aliquot of the BC used for the field experiment was stored in the laboratory for three years for reasons of comparison.
Biochar production and parameters
The BC was produced by gasification of maize (Zea mays L.) (C4 plant) silage (~10 mm) for 40 min at 1200 °C (heating rate 26–40 °C/min) in a commercial reactor (©A.G.T. – Advanced Gasification Technology s.r.l., Cremona, Italy). The shape and size of the pellets did not change during gasification. General parameters of the gasification BC used for the experiment are listed in Table 1.
Table 1.
General parameters of the biochar.
| Parameter | Unit | |
|---|---|---|
| pH (0.01 M CaCl2, 1:2.5 w-vol) | 10.1 ± 0.2 | |
| Salinity | mS cm−1 | 9.6 |
| Ash (550 °C) | % DM | 10.3 ± 1.7 |
| Carbon | % | 69.5 ± 1.3 |
| Nitrogen | % | 1.6 ± 0.1 |
| C/N | 41 | |
| H/C | 0.4 | |
| O/C | 2.9 | |
| δ13C | ‰ | −13.7 ± 0.1 |
Sampling and Pre-treatment
For the incubation experiment disturbed soil samples were randomly taken from the top 10 cm from the eight soil plots in September 2015. Disturbed samples were stored in plastic bags and transported to the laboratory. We did not sieve the soils to retain BC pellets >2 mm. Plant residues and roots were manually removed. In addition, we collected three undisturbed (100 cm³ sampling rings) soil samples from each of the eight plots for determining soil water retention curves of control soil and BC amended soils.
Incubation Experimental Design
The experimental design is presented in Fig. 1. Incubations were carried out in triplicates with three different treatments: control soil from the field experiment, BC containing soil from the field experiment sampled three years after the amendment (BCaged) and control soil from the field experiment amended with fresh BC (BCfresh). BCfresh was the same BC as used for the field experiment but stored dry and dark for three years in the laboratory. The BC amounts added were chosen in accordance with the amount of BC per amount soil C in the field, which had been determined before using the C content and δ13C ratio of BC amended soils from the field experiment.
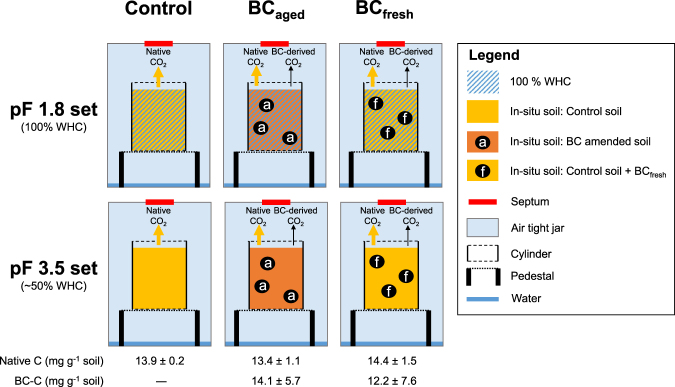
Figure 1.
Experimental set-up: Each amendment and set had three replicates, analyzed at five time points (n = 30). Control soil was soil from the field experiment without BC addition. BCaged was disturbed BC amended soil of the field experiment 3 years after field exposure. BCfresh was control soil from the field experiment mixed with fresh BC.
We filled 160 g of the sample into plasic cylinders and compressed it to a bulk density of 1.4 g cm−³ (according to the in-situ bulk density). Afterwards, these samples were drained in pressure plate extractors at pF 1.8 and 3.5 to set up the experimental water conditions, representing a water holding capacity (WHC) of 100% and about 50%, respectivly.
The cylinders were then placed in 1 L glass jars and 10 ml distilled water were added to the jars to maintain soil humidity. In total the experiment consisted of 90 jars. All jars were flushed for 20 min with moistened CO2 free-air to remove CO2 from the jars atmosphere and capped with air-tight lids. The jars were incubated in the dark at a constant temperature of 20 °C to minimize abiotic degradation by photooxidation. Decomposition was measured by monitoring the CO2 release at days 2, 7, 15, 28 and 90 using a MICROGC (Agilent, Santa Clara, USA). Therefore gas was sampled in the headspace with a syringe and injected in a GC analyser to determine CO2 concentration. Another gas sample was injected into the GC/IRMS system (isotopic ratio mass spectrometer; Microgas) to measure the 13C isotope signature of the CO2. This allowed to distinguish C4-BC mineralisation from native C3-SOC mineralisation using the isotopic mass balance. After gas measurement, the jars were flushed and sealed as discribed above. At the measuring days whole sample sets were removed to determine water content, microbial biomass and contents of C and N. To avoid anaerobic conditions, we monitored the CO2 and flushed the jars at least every 14 days with synthetic air throughout the incubation period.
The C and N concentrations of bulk soils and fresh BC were measured by dry combustion with an elemental CNS analyser (elementar vario MAX CUBE, Hanau, Germany). The δ13C signature of the pure fresh BC was δ13C = −13.7‰ and of the C3-SOM δ13C = −27.3‰. As reported before37, there was no change in the δ13C from fresh to weathered BC.
Microbial biomass
Extraction of microbial biomass was carried out after modification of the chloroform fumigation extraction method38. Each sample was divided into two sub-samples of 10 g: a non-fumigated reference sample and a sample fumigated with chloroform. The fumigated samples were incubated under ethanol-free chloroform (CHCl3) vapor in a desiccator for 16 h, followed by 6 vacuum-purge cycles to remove the CHCl3. Both sets were extracted with 40 ml of 0.03 M K2SO4, shaken in an overhead shaker for 30 min and centrifuged for 10 min with 10,000 RPM. The supernatants were removed, filtered, frozen to −20 °C and freeze dried. Organic C and N content as well as δ13C signature of the K2SO4-extractable C were analyzed using an isotope ratio mass spectrometer (Delta V Advantage Thermo Fisher Scientific, Bremen, Germany) coupled with an elemental analyzer (Flash 2000, Thermo Fisher Scientific, Bremen, Germany). All δ13C values were expressed relative to the Pee Dee Belemnite (PDB) international isotope standard.
Calculations and statistics
The proportion of native C in the microbial biomass (bC3) was calculated by the two component stable isotopic mixing model approach (IMM) after Balesdent and Balabane39:
| 1 |
where δC4 is the δ13C isotope signature of the pure BC and δC3 of the native SOC of the control soil. δCmixture is the δ13C signal of the soil sample extracts. The same model was used to partition the BC-C contribution to the CO2 efflux. For the determination of respired BC-C, the BC-C proportion were multiplied by accumulated CO2–C. Correspondingly, remaining BC-C in the soil was calculated by subtracting mineralized BC-C from total BC-C added at the beginning of the experiment. The extent of the priming effect of the biochars on native SOC mineralization were calculated as the difference between the CO2 efflux from SOC in the control compared to the native CO2 efflux of native SOC from soil-biochar mixtures, estimated with the two-component isotopic mixing model.
Microbial biomass was calculated by dividing the measured OC concentrations per g dry soil by the factor kEC = 0.45. The extractable C of the non-fumigated set were used as approximated values for salt-extractable organic carbon (DOC).
The microbial biomass based metabolic quotient (qCO2) was calculated to evaluate the microbial C use efficiency and the substrate availability. A high metabolic quotient may indicate a low efficiency of C mineralization and a higher substrate availability40. The qCO2 was calculated by using the equation
| 2 |
where CO2-C is the cumulative respired CO2-C in mg kg−1 soil h−1 and Cmic the corresponding microbial biomass C in g kg−1 soil.
By using the software SHIPFIT2.041, we fitted the water retention characteristics to the data by using the unimodal Kosugi retention function42 given by:
| 3 |
where hm (L) is the pressure head corresponding to the median pore radius, σ (–) is the standard deviation of the log-transformed pore-size distribution density function, and erfc() is the complementary error function. For unsaturated soils h (L) is defined as positive.
For statistical analyses we used the software RStudio, version 3.3.1 for Windows43. Significant differences between the amendments and control were tested with a one-way analysis of variance (ANOVA).
Results
While the water retention curves of undisturbed field samples showed little differences for plots with and without BC, in the incubation experiment, volumetric water contents of BC containing soils at pF 3.5 were significantly increased compared to the control (Fig. 2).
Figure 2.
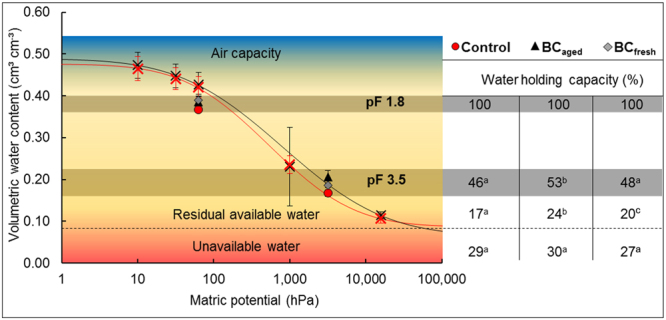
Volumetric water contents of the undisturbed soil samples (red (control) and the black crosses (BCaged) (n = 12) fitted with the model of Kosugi42 (lines)). The water holding capacities (WHC) of the pF 1.8 and 3.5 sets (n = 30) were determined after the incubation. Data within one row with different letters are significantly different (p < 0.05).
Moreover, after incubation, BCaged showed higher volumetric water contents compared to BCfresh at pF 3.5. Air capacity as well as plant unavailable water (>pF 4.2) was not affected by the amendments. However, the presence of BCaged increased remaining plant available water under drought conditions (pF 3.5 to 4.2) compared to control soil and soils containing BCfresh.
Carbon and N contents are given in Table 2. Following BC addition (aged or fresh), the SOC contents doubled, whereas SON contents increased only by about 16%. Consequently, C to N ratio increased from 9.8 for control soil to 16.5 and 15.7 for soils containing BCaged and BCfresh, respectively.
Table 2.
Mean C contents, N contents and C to N ratios of bulk soils (n = 18) from 0 days to 90 days of incubation with standard deviations.
| pF value | C content | N content | C to N ratio | |
|---|---|---|---|---|
| mg g−1 | mg g−1 | |||
| Control | 1.8 | 13.88a ± 0.41 | 1.44a ± 0.03 | 9.62a ± 0.17 |
| 3.5 | 14.01a ± 0.33 | 1.41a ± 0.03 | 9.91a ± 0.03 | |
| BCaged | 1.8 | 28.27b ± 4.36 | 1.65b ± 0.08 | 16.83b ± 1.54 |
| 3.5 | 26.87b ± 2.29 | 1.65b ± 0.04 | 16.09b ± 0.92 | |
| BCfresh | 1.8 | 27.52b ± 5.82 | 1.69b ± 0.09 | 15.90b ± 0.84 |
| 3.5 | 26.38b ± 3.21 | 1.69b ± 0.06 | 15.55b ± 0.59 |
Different letters within one column mark significant different values (p < 0.05).
The cumulative total C mineralization, native SOC and BC-C emissions during the 90 days of incubation are presented in Fig. 3a–c.
Figure 3.
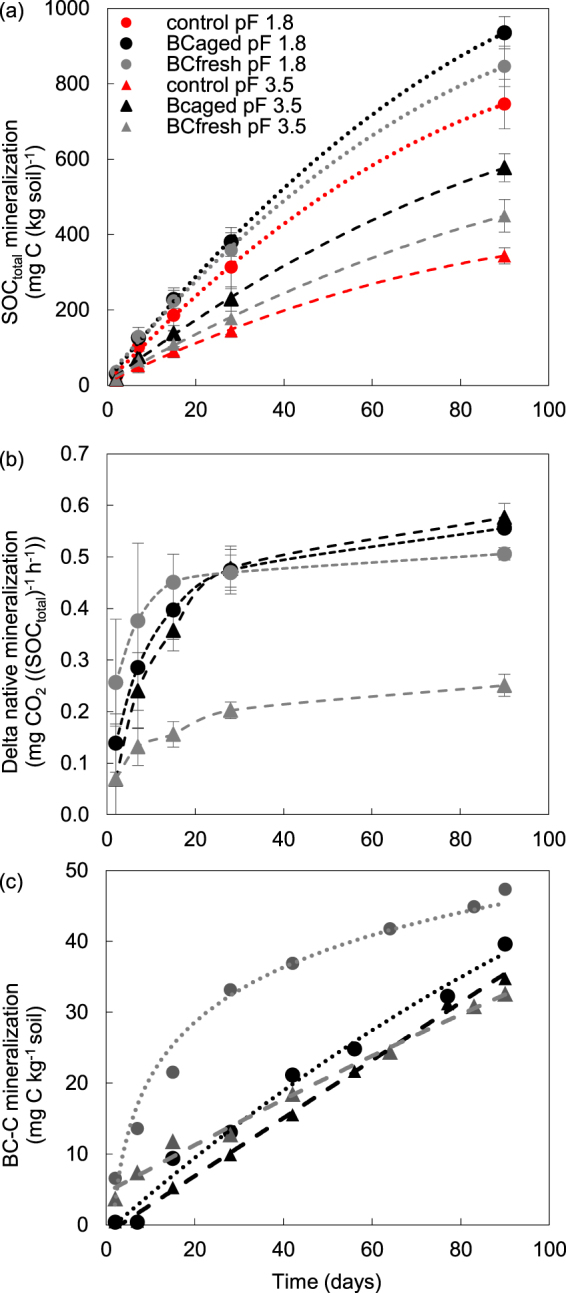
(a–c) (a) Cumulative CO2 emissions, (b) additional native mineralization (mg per kg SOC) in BC amended soil and (c) cumulative BC-C mineralization (mg per kg) during 90 days of the experiment. The vertical bars represent the standard deviation (n = 12).
Our results show two separated groups with regard to the OC mineralization according to the soil water potential (Fig. 3a). The total respired C at pF 1.8 was higher than at pF 3.5 for all three treatments. Under optimum water conditions (pF 1.8), the highest C mineralization was observed for BCaged (935.5 mg C kg−1 soil) followed by BCfresh (846.5 mg C kg−1 soil) and control (734.0 mg C kg−1 soil). Under drought conditions (pF 3.5), these values decreased by 54% for the control soil and by 38% and 47% and BC amended soils (BCaged and BCfresh, respectively).
Both amendments increased native SOC mineralization (Fig. 3b). The highest native SOC losses were found at optimum water conditions for BCaged (896.3 mg C g−1 soil). This corresponds to a positive priming effect leading to 21% increased native SOC mineralization with regards to the control. The addition of BCfresh induced a lower priming effect corresponding to 8% increase of native SOC mineralization as compared to the control. Under drought conditions, the increase of native SOC mineralization was even higher, with BCaged leading to 59% and BCfresh to 22% more native C loss compared to the control.
The BC-C contributions to the respired CO2-C were higher under drought than under optimum conditions (Fig. 3c). Biochar derived C in BCaged soils accounted for 3.5% (pF 1.8) and 5.9% (pF 3.5) and in BCfresh soils for 5.6% (pF 1.8) and 8.8% (pF 3.5) of the total mineralized C, over the 90 days of incubation period. However, low amounts of BC-C were mineralized during the 90 days of incubation. Less than 0.5% of BC-C was respired. Higher BC-C proportions were respired in soils containing BCfresh (0.3% of BC-C) than in soils containing BCaged (0.2% of BC-C). Despite a ‘BC-C flush’ at pF 1.8 after BCfresh addition at the beginning of the experiment (Fig. 3c), the mineralization of both BC types differed only marginal after 90 days.
Under optimum water conditions, microbial biomass ranged between 155.4 ± 12.4 mg kg−1 soil to 171.2 ± 13.7 mg kg−1 soil at the beginning of the experiment. After 90 days of incubation, microbial biomass decreased in all treatments and ranged between 98.2 ± 6.2 mg kg−1 soil and 121.7 ± 3.3 mg kg−1 soil. Similar values were recorded for drought conditions (Table 3).
Table 3.
Microbial biomass C (MBC) of all treatments during the 90 days of incubation. Asterisks indicate significant differences (p < 0.05).
| Set | Unit | Treatment | Incubation time (days) | |||||
|---|---|---|---|---|---|---|---|---|
| 2 | 7 | 15 | 28 | 90 | ||||
| pF 1.8 | MBC | mg C (kg soil)−1 | Control | 158* | 142,9 ± 7.9 | 149 ± 4.2 | 108,4 ± 4.0 | 98,2 ± 6.2 |
| BCaged | 171,2 ± 13.7 | 162 ± 1.0 | 154,7 ± 33.8 | 168,7 ± 34.0 | 111,2 ± 11.4 | |||
| BCfresh | 155,4 ± 12.4 | 156,5 ± 16.6 | 160,9 ± 4.3 | 155,1 ± 3.7 | 121,7 ± 3.3 | |||
| Proportion BC-C | % | BCaged | −8,1 ± 0.4* | −7,1 ± 1.8* | −0,4 ± 1.4 | −1 ± 1.0* | −1,4 ± 1.6* | |
| BCfresh | −4,6 ± 0.6* | −2,3 ± 1.5* | −0,7 ± 0.4 | 2,9 ± 1.0* | 3,4 ± 1.7* | |||
| pF 3.5 | MBC | mg C (kg soil)−1 | Control | 130,3 ± 9.2 | 138,6 ± 3.1 | 137,2 ± 12.3 | 136,1 ± 16.4 | 118,3 ± 13.9 |
| BCaged | 137 ± 9.1 | 157,7 ± 37.4 | 145,7 ± 16.8 | 157 ± 42.1 | 132,8 ± 1.5 | |||
| BCfresh | 151,7 ± 0.8 | 162,6 ± 5.5 | 153,1 ± 5.8 | 156,7 ± 4.2 | 162,2 ± 18.7 | |||
| Proportion BC-C | % | BCaged | −4,4 ± 1.2* | −5,8 ± 8.9 | −0,8 ± 1.8 | −2 ± 1.7 | −4,3 ± 1.1 | |
| BCfresh | 1,2 ± 2.7* | 0,2 ± 0.9 | 0,5 ± 1.4 | 2,5 ± 0.7 | −1,2 ± 2.5 | |||
| pF 1.8 | MBC | mg C (kg SOC)−1 | Control | 10,9* | 10,4 ± 0.3 | 11 ± 0.4 | 7,5 ± 0.4 | 7,3 ± 0.1 |
| BCaged | 5,7 ± 2.2 | 7 ± 3.3 | 6,4 ± 1.4 | 6,7 ± 1.4 | 3,6 ± 1.2 | |||
| BCfresh | 6,2 ± 3.7 | 4,4 ± 1.3 | 6,2 ± 1.1 | 7,4 ± 2.4 | 5,3 ± 1.5 | |||
| pF 3.5 | mg C (kg SOC)−1 | Control | 9,2 ± 0.6 | 9,9 ± 0.2 | 10,1 ± 0.5 | 9,5 ± 1.0 | 8,5 ± 0.6 | |
| BCaged | 4,8 ± 1.6 | 6,4 ± 3.5 | 5,5 ± 1.0 | 6,3 ± 3.1 | 5,3 ± 1.2 | |||
| BCfresh | 4,6 ± 1.1 | 6 ± 0.9 | 5,8 ± 0.1 | 7,5 ± 0.7 | 5,9 ± 1.2 | |||
*No replicates.
Between the start and the end of the experiment, the microbial biomass of BC amended soils decreased by 35% and 38% at pF 1.8 (aged and fresh), whereas it remained rather constant at pF 3.5. The δ13C abundance in the fumigation extracts from control, BCaged and BCfresh were very similar resulting in high uncertainties for the calculation of the BC-C proportion (Table 3).
Within the first week of the incubation experiment, microbial biomass from all samples showed a depletion of 13C to differing degrees (up to 8.1 ± 0.4% for BCaged at pF 1.8). This effect leveled out with progressing time but microbial biomass in the treatments BCaged remained depleted in 13C at both water potentials compared to the control after 90 days of incubation. BCfresh showed 3.4 ± 1.7% incorporation of BC derived C into microbial biomass under optimum conditions, whereas under drought, BC-C was not incorporated into the soil microbial biomass.
The metabolic quotient (qCO2) of the control soils decreased during incubation from 3.56 to 2.95 mg CO2 -C g−1 biomass C h−1 under optimum water conditions and from 2.24 to 1.14 mg CO2 -C g−1 biomass C h−1 under drought conditions (Fig. 4).
Figure 4.
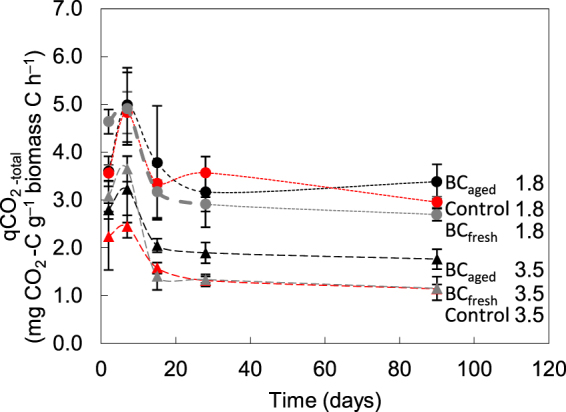
Metabolic quotient (qCO2) during the incubation experiment. Every data point represents a mean value of three replicates with standard deviation.
Due to the CO2 flush and consistent microbial biomass C in the first week, the qCO2 increased in all treatments. In drought-affected soils, only BCaged addition had an effect on qCO2. The qCO2 of soils containing BCfresh remained similar to the qCO2 of the control (1.15 and 1.14 mg CO2 -C g−1 biomass C h−1, respectively) after 90 days of incubation. In contrast, BCaged showed a significantly higher qCO2 (1.76 mg CO2 -C g−1 biomass C h−1).
Discussion
Biochar effects on water conditions
We found a positive effect of BC on the water holding capacity, which was more pronounced in the BCaged than in the BCfresh amended soil (Fig. 2). BCaged treatments showed at the end of the incubation experiment an increased volumetric water content under drought conditions compared to the other treatments. This additional water can be assigned to plant available water because the unavailable water (>pF 4.2) was not affected by BC addition (Fig. 2). We assume that fragmentation and mechanical stresses of freeze-thaw cycles during field exposure form new cracks and fractures and thus increase the pore connectivity of BCaged compared to BCfresh particles22. The additionally retained plant available water could reduce water stress and hence retard drought effects to plants44.
Biochar effects on microbial activity
As found in previous studies e.g.8,13, total mineralization decreased with decreasing water contents in all treatments (Fig. 3a). Nevertheless, SOC mineralization decreased less in BC containing soils and was strongly dependent on the nature of BC (fresh or aged; Fig. 3a). In agreement with the literature, under optimum water conditions, we found a flush of SOC mineralization at the beginning of the experiment for treatments with BCfresh (pF 1.8). This flush was observed in many studies after addition of fresh BC to soil45–48, and may be related to mineralization of labile C compounds of the BC and stimulation of native SOC mineralization49, e.g.50. It may indicate an immediate adaption of microorganisms to BCfresh usage51 but is most likely a short-term effect on SOM e.g.52. Responsible for this fast response might be so-called ‘r-strategist’ microbes, which are adapted to respond quickly to newly available C sources, which may be present in BC in form of volatile organic matter53,54. These organisms re-mineralize soil nutrients and co-metabolize more refractory OM in the process (Kuzyakov et al., 2000; Kuzyakov, 2010).
The absence of a mineralization flush in the beginning of the experiment for BCaged treatments may be explained by depletion of labile C compounds leaving behind a recalcitrant BC residue4,49,55. After degradation of labile BC components, it was even found that BC lowered native SOC mineralization below the level of the control samples, likely by toxic compounds of the BC or mineral adsorptive protection56,57. Higher native C mineralization in treatments with BCaged as compared to BCfresh may be explained by sorption of potential inhibitors. Extracellular reactions could consequently increase the breakdown of native SOM58. Increased drought intensified the effect of BC aging on native SOC mineralization (Fig. 3b).
Microbial biomass growth and incorporation of Biochar-C
Microorganisms benefit from improved water supply during droughts due to the presence of BC as indicated by increased microbial biomass and microbial activity as compared to the control (Tables 2 and 3). The values for incorporation of BC-C into microbial biomass presented here reflect the maximum possible values as chloroform can contribute to a dissolution of BC and extraction with K2SO4 may overestimate BC-C incorporation into microbial biomass52,59. For all amendments, we found a general trend of decreasing microbial biomass at pF 1.8 (22% in BCfresh and 38% in the control), whereas it remained rather constant at pF 3.5 (−7% in BCfresh and 9% in the control) during the 90 days of incubation (Table 3). The dynamics of microbial biomass at pF 1.8 may be related to a fast consumption of C, followed by a depletion of easy available substrate. In contrast, at pF 3.5 the development of microbial biomass suffers from water stress as indicated by stagnating values.
We found no significant differences in microbial biomass between the three treatments under optimal water conditions (Table 3). However, the BCfresh addition affected the dynamics of microbial biomass during the experiment, maintaining higher total biomass amounts as compared to soils containing BCaged or control soils. Zhou, et al.26 found in BC amended soils an overall moderate increase in microbial biomass by 26%, which tended to decrease with increasing duration of the experiment. The increase in microbial biomass may be explained by microbial use of the labile or extractable carbon pool60 of the BC, and the decreasing effect with time to its exhaustion52,61. The porous structure of BC can be a suitable habitat for microbes (Lehmann et al., 2014), offering favorable microsites and protection from predators62. This aspect, however, tend to play a minor role, as microsites and therefore microbial biomass should increase with aging. The BCaged, however, showed no significant effects on microbial biomass compared to the control.
The effect of BC properties are underlined by Ameloot, et al.63 and Durenkamp, et al.64, who found even lower microbial biomass in BC amended soils than in the control and related this to BC production conditions or/and feedstocks. Wood derived BC, as used in their studies may be unfavorable for microbial colonization56,65.
Under drought conditions, we observed stable microbial biomass in the control soil during the 90 days incubation period. In BCaged soils, microbial biomass tended to increase only during the first 28 days, whereas BCfresh addition augmented microbial biomass by 37% compared to the control. These observations may be explained by more favorable water conditions in BC containing soils, combined with easily decomposable compounds in the case of BCfresh addition.
In contrast to the significant BC-C mineralization (Table 2, Fig. 3a), the incorporation into microbial biomass is rather low (Table 3). Incorporation of BC-C into microbial biomass strongly varies between fresh and aged BC. Incorporation of BCfresh into the microbial biomass (Table 3) demonstrates that labile BC-C was utilized not only as energy source but also as a C substrate by microorganisms52,56,66. Microorganisms tended to incorporate BCfresh at pF 1.8, at similar amounts as observed by other authors (e.g. Kuzyakov, et al.52 (1.5 to 2.6%). In contrast, BCaged seems to be preferentially mineralized, as the 13C content of the microbial biomass was depleted (Table 3).
Additionally, the BC-C proportion to OC released at pF 3.5 significantly increased compared to pF 1.8 (Table 2, Fig. 3c). The low but continuous proportions of BC-C released throughout the experiment in BCaged treatments (Fig. 3c) indicate a persistent microbial use of BC-C. In contrast, Ameloot, et al.63 found that wood derived BC was not used as substrate for microorganisms after 1 to 4 years of field exposure.
Effects of Biochar on metabolic efficiency
The qCO2 or the specific respiration rate is used to evaluate the metabolic efficiency of the soil microbial biomass. In this experiment the measured qCO2-values of 1.14–4.99 mg CO2 -C g−1 biomass C h−1 are in a comparable range to other disturbed arable soils67. We found the highest qCO2-values of 3.56–4.64 mg CO2 -C g−1 biomass C h−1 (pF 1.8) and 2.24–3.08 mg CO2 -C g−1 biomass C h−1 (pF 3.5) at the beginning of the experiment. Thereafter, the values decreased slightly in all treatments until the end of the incubation (Fig. 4). Only at day 7 of incubation, the qCO2 increased by 6–39% (pF 1.8) and 9–19% (pF 3.5) due to the CO2 flush. Decreasing qCO2 in our experiment can be explained by relatively constant mineralization (per h) and decreasing microbial biomass (Fig. 3a and Table 3) in contrast to many other studies, where respiration remained constant but microbial biomass increased26. High qCO2 values imply relatively ‘large’ C losses (through respiration) and less C converted to biomass, ultimately reducing the potential for long-term C sequestration in organo-mineral complexes68,69. However, as changes in mineralization were not in parallel to decreases in microbial biomass, this could suggest microbial community shifts. We cannot exclude changes in microbial community composition, which additionally would affect the qCO2 because distinct microbial groups are able to decompose and assimilate C compounds at different rates depending on their composition70. These have been evidenced with some taxa-specific community changes in the works by Farrell, et al.53,71,Chen, et al.72 and Gomez, et al.73. Gomez, et al.73 additionally found that as BC amendments became larger, the decrease of microbial biomass with time was alleviated. This suggests that the BC confers buffering on the microbial community. Another explanation for microbial biomass decrease at constant mineralization could be recycling of the dead microbial biomass as a labile C source. The high qCO2 of all treatments at pF 1.8 could further indicate nutrient gain by overflow respiration or C excretion (‘luxury consumption’, ‘waste metabolism’74, ‘N-mining’)75. This was also found for nutrient-limited conditions across a wide range of soil and litter types e.g. for N75.
Whereas all treatments at pF 1.8 show similar qCO2-values, water stress induced significant BC effects. At pF 3.5, a low qCO2 of 1.14 mg CO2 -C g−1 biomass C h−1 and 1.15 mg CO2 -C g−1 biomass C h−1 in control and BCfresh samples could be ascribed to shifts from growth to maintenance respiration or preparation for dormancy stages as water stress is an important constraint for microorganisms. This is explained by limited substrate supply due to slow diffusion rates along the increasingly tortuous paths of thin water films or a change in the physiology of microbes as they adjust to more desiccating conditions10. For example, intracellular solutes are accumulated, which affect microbial growth biochemically because of high costs for osmoregulation76. Highly stressed microbes will then use substrate for maintenance energy requirements and not for growth11.
In contrast, the qCO2-values in soils containing BCaged differ from the other two treatments, showing a qCO2-value of 1.76 mg CO2 -C g−1 biomass C h−1 under drought conditions. In general, higher qCO2-values suggest improved biophysical conditions for microbial activity. We assume that control soil and soil containing BCfresh were strongly affected by water stress as reflected by stagnating microbial biomass and lower C-mineralization than in BCaged samples. This suggests that higher available water in the latter treatment might have led to improved conditions and microbes remaining longer metabolically active with retardation of their dormant stage.
Conclusion
We investigated water content, microbial biomass and activity under contrasting water conditions in temperate grassland soils containing similar amounts of fresh and aged BC produced by gasification. We conclude that aging of BC significantly increased plant available water in drought-affected soils. Both BC amendments led to considerable increases in SOC mineralization despite water stress, with BCaged showing the greatest effects. Moreover, the BCfresh-treatment maintained microbial biomass, whereas the BCaged treatment showed significantly increased qCO2 values. While representing only a small fraction of the C mineralized, BC seems to be a constantly available C source. We thus conclude that BC addition to soil is beneficial for microbial biomass and activity under drought and that these effects are increasing with time after field exposure. Our results have further implications. Particularly, the results presented in this study support the assumption that BC amendment may be a viable means of mitigating current and future water shortages in drought-affected soils under climate change, with major positive effects for available water for plant growth and microbial activity.
Acknowledgements
Supported by Deutsche Forschungsgemeinschaft (DFG) through the TUM International Graduate School of Science and Engineering (IGSSE) and the TUM funding programme Open Access Publishing. The authors are grateful to Xavier Charrier for the setup and maintenance of the field experiment. The field experiment was funded by the European Commission under the framework of the FP7 project “Eurochar”. Christophe Naisse is acknowledged for his help in providing the information about the experiment. Furthermore, we would like to thank Valérie Pouteau and Christophe Labat for the support in the laboratory and Florian Schmalzl for the support during the sampling campaign.
Author Contributions
L.P. planned and executed the experiments, collected and evaluated the data and wrote the manuscript; C.W.M. planned the experiments, supervised analyses and worked on the manuscript; I.K.K. planned the experiment and worked on the manuscript; M.v.L. analysed the data and worked on the manuscript; C.G. executed analyses and worked on the manuscript; C.R. planned the experiment, supervised the experiments and worked on the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ciais, P. et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature437, 529–533, http://www.nature.com/nature/journal/v437/n7058/suppinfo/nature03972_S1.html (2005). [DOI] [PubMed]
- 2.Liang C, et al. Biochar alters the resistance and resilience to drought in a tropical soil. Environmental Research Letters. 2014;9:064013. doi: 10.1088/1748-9326/9/6/064013. [DOI] [Google Scholar]
- 3.Lehmann, J. & Joseph, S. Biochar for environmental management: science, technology and implementation. (Routledge, 2015).
- 4.Paetsch, L. et al. A multi-technique approach to assess the fate of biochar in soil and to quantify its effect on soil organic matter composition. Organic Geochemistry (2017).
- 5.Laird DA, et al. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma. 2010;158:443–449. doi: 10.1016/j.geoderma.2010.05.013. [DOI] [Google Scholar]
- 6.Mulcahy D, Mulcahy D, Dietz D. Biochar soil amendment increases tomato seedling resistance to drought in sandy soils. Journal of arid environments. 2013;88:222–225. doi: 10.1016/j.jaridenv.2012.07.012. [DOI] [Google Scholar]
- 7.Lal, R. Biochar and Soil Carbon Sequestration. In: M. Guo, G. He, S.M. Uchimiya (Eds.), Agricultural and Environmental Applications of Biochar: Advances and Barriers. SSSA Special Publications, pp. 175–197 (2016).
- 8.Manzoni S, Schimel JP, Porporato A. Responses of soil microbial communities to water stress: results from a meta‐analysis. Ecology. 2012;93:930–938. doi: 10.1890/11-0026.1. [DOI] [PubMed] [Google Scholar]
- 9.Skopp J, Jawson M, Doran J. Steady-state aerobic microbial activity as a function of soil water content. Soil Science Society of America Journal. 1990;54:1619–1625. doi: 10.2136/sssaj1990.03615995005400060018x. [DOI] [Google Scholar]
- 10.Stark JM, Firestone MK. Mechanisms for soil moisture effects on activity of nitrifying bacteria. Applied and environmental microbiology. 1995;61:218–221. doi: 10.1128/aem.61.1.218-221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herron PM, Stark JM, Holt C, Hooker T, Cardon ZG. Microbial growth efficiencies across a soil moisture gradient assessed using 13C-acetic acid vapor and 15N-ammonia gas. Soil Biology and Biochemistry. 2009;41:1262–1269. doi: 10.1016/j.soilbio.2009.03.010. [DOI] [Google Scholar]
- 12.Sanaullah M, Chabbi A, Rumpel C, Kuzyakov Y. Carbon allocation in grassland communities under drought stress followed by 14C pulse labeling. Soil Biology and Biochemistry. 2012;55:132–139. doi: 10.1016/j.soilbio.2012.06.004. [DOI] [Google Scholar]
- 13.Moyano FE, et al. The moisture response of soil heterotrophic respiration: interaction with soil properties. Biogeosciences. 2012;9:1173–1182. doi: 10.5194/bg-9-1173-2012. [DOI] [Google Scholar]
- 14.Spokas KA, Reicosky DC. Impacts of sixteen different biochars on soil greenhouse gas production. Ann. Environ. Sci. 2009;3:4. doi: 10.1016/j.chemosphere.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad M, et al. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource Technology. 2012;118:536–544. doi: 10.1016/j.biortech.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Al-Wabel MI, Al-Omran A, El-Naggar AH, Nadeem M, Usman ARA. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresource Technology. 2013;131:374–379. doi: 10.1016/j.biortech.2012.12.165. [DOI] [PubMed] [Google Scholar]
- 17.Kloss S, et al. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. Journal of Environmental Quality. 2012;41:990–1000. doi: 10.2134/jeq2011.0070. [DOI] [PubMed] [Google Scholar]
- 18.Glaser B, Lehmann J, Zech W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal – a review. Biol Fertil Soils. 2002;35:219–230. doi: 10.1007/s00374-002-0466-4. [DOI] [Google Scholar]
- 19.Zimmerman AR. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar) Environmental Science & Technology. 2010;44:1295–1301. doi: 10.1021/es903140c. [DOI] [PubMed] [Google Scholar]
- 20.Cheng C-H, Lehmann J, Thies JE, Burton SD, Engelhard MH. Oxidation of black carbon by biotic and abiotic processes. Organic Geochemistry. 2006;37:1477–1488. doi: 10.1016/j.orggeochem.2006.06.022. [DOI] [Google Scholar]
- 21.Sorrenti G, Masiello CA, Dugan B, Toselli M. Biochar physico-chemical properties as affected by environmental exposure. Science of the total Environment. 2016;563:237–246. doi: 10.1016/j.scitotenv.2016.03.245. [DOI] [PubMed] [Google Scholar]
- 22.Spokas KA, et al. Physical disintegration of biochar: An overlooked process. Environmental Science & Technology Letters. 2014;1:326–332. doi: 10.1021/ez500199t. [DOI] [Google Scholar]
- 23.Ouyang L, Wang F, Tang J, Yu L, Zhang R. Effects of biochar amendment on soil aggregates and hydraulic properties. Journal of soil science and plant nutrition. 2013;13:991–1002. [Google Scholar]
- 24.Jenkins JR, et al. Biochar alters the soil microbiome and soil function: results of next-generation amplicon sequencing across Europe. GCB Bioenergy. 2017;9:591–612. doi: 10.1111/gcbb.12371. [DOI] [Google Scholar]
- 25.Anderson T-H, Domsch KH. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as ph, on the microbial biomass of forest soils. Soil Biology and Biochemistry. 1993;25:393–395. doi: 10.1016/0038-0717(93)90140-7. [DOI] [Google Scholar]
- 26.Zhou H, et al. Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: A Meta-analysis. Agriculture, Ecosystems & Environment. 2017;239:80–89. doi: 10.1016/j.agee.2017.01.006. [DOI] [Google Scholar]
- 27.Heitkötter J, Marschner B. Interactive effects of biochar ageing in soils related to feedstock, pyrolysis temperature, and historic charcoal production. Geoderma. 2015;245–246:56–64. doi: 10.1016/j.geoderma.2015.01.012. [DOI] [Google Scholar]
- 28.LeCroy C, Masiello CA, Rudgers JA, Hockaday WC, Silberg JJ. Nitrogen, biochar, and mycorrhizae: alteration of the symbiosis and oxidation of the char surface. Soil Biology and Biochemistry. 2013;58:248–254. doi: 10.1016/j.soilbio.2012.11.023. [DOI] [Google Scholar]
- 29.Cheng C-H, Lehmann J, Engelhard MH. Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochimica et Cosmochimica Acta. 2008;72:1598–1610. doi: 10.1016/j.gca.2008.01.010. [DOI] [Google Scholar]
- 30.Hagemann N, et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nature Communications. 2017;8:1089. doi: 10.1038/s41467-017-01123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conte P, Laudicina A. V. Mechanisms of Organic Coating on the Surface of a Poplar Biochar. Current Organic Chemistry. 2017;21:559–565. doi: 10.2174/1385272821666161216122035. [DOI] [Google Scholar]
- 32.Mia, S., Dijkstra, F. A. & Singh, B. In Advances in Agronomy Vol. Volume 141 (ed. L. Sparks Donald) 1–51 (Academic Press, 2017).
- 33.Yao FX, et al. Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor. Chemosphere. 2010;80:724–732. doi: 10.1016/j.chemosphere.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Naisse C, et al. Effect of physical weathering on the carbon sequestration potential of biochars and hydrochars in soil. GBC Bioenergy. 2015;7:488–496. [Google Scholar]
- 35.IUSS Working Group. World reference base for soil resources 2014 - International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports 106 (2014).
- 36.Sanaullah M, et al. Effects of drought and elevated temperature on biochemical composition of forage plants and their impact on carbon storage in grassland soil. Plant and Soil. 2014;374:767–778. doi: 10.1007/s11104-013-1890-y. [DOI] [Google Scholar]
- 37.Paetsch, L. et al. A multi-technique approach to assess the fate of high-temperature biochar in soil and to quantify its effect on soil organic matter composition. Organic Geochemistry, 10.1016/j.orggeochem.2017.06.012 (2017).
- 38.Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry. 1987;19:703–707. doi: 10.1016/0038-0717(87)90052-6. [DOI] [Google Scholar]
- 39.Balesdent J, Balabane M. Maize root-derived soil organic carbon estimated by natural 13C abundance. Soil Biology and Biochemistry. 1992;24:97–101. doi: 10.1016/0038-0717(92)90264-X. [DOI] [Google Scholar]
- 40.Bimüller C, et al. Decoupled carbon and nitrogen mineralization in soil particle size fractions of a forest topsoil. Soil Biology and Biochemistry. 2014;78:263–273. doi: 10.1016/j.soilbio.2014.08.001. [DOI] [Google Scholar]
- 41.Durner, W. & Peters, A. SHYPFIT 2.0 – Software zur Anpassung hydraulischer Funktionen an Messdaten. Böden - eine endliche Ressource (2009).
- 42.Kosugi K. Lognormal distribution model for unsaturated soil hydraulic properties. Water Resources Research. 1996;32:2697–2703. doi: 10.1029/96WR01776. [DOI] [Google Scholar]
- 43.R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2016).
- 44.Koide RT, et al. Biochar amendment of soil improves resilience to climate change. GCB Bioenergy. 2015;7:1084–1091. doi: 10.1111/gcbb.12191. [DOI] [Google Scholar]
- 45.Liao N, et al. Effects of biochar on soil microbial community composition and activity in drip-irrigated desert soil. European Journal of Soil Biology. 2016;72:27–34. doi: 10.1016/j.ejsobi.2015.12.008. [DOI] [Google Scholar]
- 46.Ameloot N, et al. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biology and Biochemistry. 2013;57:401–410. doi: 10.1016/j.soilbio.2012.10.025. [DOI] [Google Scholar]
- 47.Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes PC. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biology and Biochemistry. 2011;43:2304–2314. doi: 10.1016/j.soilbio.2011.07.020. [DOI] [Google Scholar]
- 48.Maestrini B, Nannipieri P, Abiven S. A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy. 2015;7:577–590. doi: 10.1111/gcbb.12194. [DOI] [Google Scholar]
- 49.Jones D, et al. Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biology and Biochemistry. 2011;43:1723–1731. doi: 10.1016/j.soilbio.2011.04.018. [DOI] [Google Scholar]
- 50.Smith JL, Collins HP, Bailey VL. The effect of young biochar on soil respiration. Soil Biology and Biochemistry. 2010;42:2345–2347. doi: 10.1016/j.soilbio.2010.09.013. [DOI] [Google Scholar]
- 51.Jiang X, Denef K, Stewart CE, Cotrufo MF. Controls and dynamics of biochar decomposition and soil microbial abundance, composition, and carbon use efficiency during long-term biochar-amended soil incubations. Biol Fertil Soils. 2016;52:1–14. doi: 10.1007/s00374-015-1047-7. [DOI] [Google Scholar]
- 52.Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biology and Biochemistry. 2009;41:210–219. doi: 10.1016/j.soilbio.2008.10.016. [DOI] [Google Scholar]
- 53.Farrell M, et al. Microbial utilisation of biochar-derived carbon. Science of The Total Environment. 2013;465:288–297. doi: 10.1016/j.scitotenv.2013.03.090. [DOI] [PubMed] [Google Scholar]
- 54.Cross A, Sohi SP. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biology and Biochemistry. 2011;43:2127–2134. doi: 10.1016/j.soilbio.2011.06.016. [DOI] [Google Scholar]
- 55.Naisse C, Girardin C, Davasse B, Chabbi A, Rumpel C. Effect of biochar addition on C mineralisation and soil organic matter priming in two subsoil horizons. J Soils Sediments. 2015;15:825–832. doi: 10.1007/s11368-014-1002-5. [DOI] [Google Scholar]
- 56.Luo Y, et al. Microbial biomass growth, following incorporation of biochars produced at 350 °C or 700 °C, in a silty-clay loam soil of high and low pH. Soil Biology and Biochemistry. 2013;57:513–523. doi: 10.1016/j.soilbio.2012.10.033. [DOI] [Google Scholar]
- 57.Ventura M, et al. Biochar mineralization and priming effect on SOM decomposition in two European short rotation coppices. GBC Bioenergy. 2015;7:1150–1160. [Google Scholar]
- 58.Jan MT, Roberts P, Tonheim SK, Jones DL. Protein breakdown represents a major bottleneck in nitrogen cycling in grassland soils. Soil Biology and Biochemistry. 2009;41:2272–2282. doi: 10.1016/j.soilbio.2009.08.013. [DOI] [Google Scholar]
- 59.Badalucco L, Nannipieri P, Grego S, Ciardi C. Microbial biomass and anthrone-reactive carbon in soils with different organic matter contents. Soil Biology and Biochemistry. 1990;22:899–904. doi: 10.1016/0038-0717(90)90127-L. [DOI] [Google Scholar]
- 60.Lou Y, et al. Water extract from straw biochar used for plant growth promotion: an initial test. BioResources. 2015;11:249–266. [Google Scholar]
- 61.Hale SE, Hanley K, Lehmann J, Zimmerman AR, Cornelissen G. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environmental Science & Technology. 2011;45:10445–10453. doi: 10.1021/es202970x. [DOI] [PubMed] [Google Scholar]
- 62.Pietikäinen J, Kiikkilä O, Fritze H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos. 2000;89:231–242. doi: 10.1034/j.1600-0706.2000.890203.x. [DOI] [Google Scholar]
- 63.Ameloot N, et al. C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biology and Biochemistry. 2014;78:195–203. doi: 10.1016/j.soilbio.2014.08.004. [DOI] [Google Scholar]
- 64.Durenkamp M, Luo Y, Brookes PC. Impact of black carbon addition to soil on the determination of soil microbial biomass by fumigation extraction. Soil Biology and Biochemistry. 2010;42:2026–2029. doi: 10.1016/j.soilbio.2010.07.016. [DOI] [Google Scholar]
- 65.Ascough PL, Sturrock CJ, Bird MI. Investigation of growth responses in saprophytic fungi to charred biomass. Isotopes in Environmental and Health Studies. 2010;46:64–77. doi: 10.1080/10256010903388436. [DOI] [PubMed] [Google Scholar]
- 66.Smith P. Soil carbon sequestration and biochar as negative emission technologies. Global Change Biology. 2016;22:1315–1324. doi: 10.1111/gcb.13178. [DOI] [PubMed] [Google Scholar]
- 67.Wardle DA, Ghani A. A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance and ecosystem development. Soil Biology and Biochemistry. 1995;27:1601–1610. doi: 10.1016/0038-0717(95)00093-T. [DOI] [Google Scholar]
- 68.Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI. Environmental and stoichiometric controls on microbial carbon‐use efficiency in soils. New Phytologist. 2012;196:79–91. doi: 10.1111/j.1469-8137.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- 69.von Lützow M, et al. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biology and Biochemistry. 2007;39:2183–2207. doi: 10.1016/j.soilbio.2007.03.007. [DOI] [Google Scholar]
- 70.Waldrop, M. P. & Firestone, M. K. Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia138(2), 275–284 (2004). [DOI] [PubMed]
- 71.Chen J, et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Applied Soil Ecology. 2013;71:33–44. doi: 10.1016/j.apsoil.2013.05.003. [DOI] [Google Scholar]
- 72.Chen J, et al. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Science of The Total Environment. 2017;574:24–33. doi: 10.1016/j.scitotenv.2016.08.190. [DOI] [PubMed] [Google Scholar]
- 73.Gomez JD, Denef K, Stewart CE, Zheng J, Cotrufo MF. Biochar addition rate influences soil microbial abundance and activity in temperate soils. European Journal of Soil Science. 2014;65:28–39. doi: 10.1111/ejss.12097. [DOI] [Google Scholar]
- 74.Smith OL. An analytical model of the decomposition of soil organic matter. Soil Biology and Biochemistry. 1979;11:585–606. doi: 10.1016/0038-0717(79)90027-0. [DOI] [Google Scholar]
- 75.Craine JM, Morrow C, Fierer N. Microbial nitrogen limitation increases decomposition. Ecology. 2007;88:2105–2113. doi: 10.1890/06-1847.1. [DOI] [PubMed] [Google Scholar]
- 76.Harris, R. In Water potential relations in soil microbiology (eds J. F. Parr, W. R. Gardner, & L. F. Elliott) 23–95 (American Society of Agronomy, 1981).