Abstract
The stability and unconventional reactivity of 1,13‐diamino‐4,7,10‐trioxatridecane in the presence of NH3, H2O2, and (NH4)2S2O8 are described. The ether‐diamine is an ingredient marketed to hair salons and consumers for so‐called “plex” services to compensate for hair damage during bleaching. The main reaction product identified is an unexpected azanyl ester derivative. This is considered relevant for the safety evaluation when used in cosmetic products. The mechanism of reaction was explored through DFT calculations. This study represents the first attempt to assess the stability of a plex active in an oxidative environment.
Keywords: azanyl ester, nucleophilic substitution, oxidation, peroxide, persulfate
Cosmetic hair bleaching is a popular procedure applied all over the world to lighten hair for aesthetic purposes. It is based on the oxidation of melanin pigments upon treatment with hydrogen peroxide and persulfate salts under alkaline conditions (ammonia or monoethanolamine).1 In addition to the desired lightening results, hair bleaching is known to cause damage to the hair shaft over repeated treatments,2 and this has led to a recent increase in popularity of “plex” technologies. The term plex refers to a number of products marketed under diverse brands, containing different active molecules, which claim, for example, to repair damage, prevent breakage, or give strengthening effects.3 Despite being used by millions of consumers, to the best of our knowledge, no experimental data are published to elucidate the stability or reactivity of these products in the strong oxidative environment of the bleach products.
Generally, persulfate, in combination with hydrogen peroxide or alone, is known for its ability to degrade organics, after activation with heat, transition metals, ultraviolet light, or other means that produce the sulfate radical, and it finds extensive use in groundwater remediation.4 Also, without activation, the persulfate anion is known to react with some organic chemicals, although with slow kinetics.5 Recently, persulfate salt has been shown to be a suitable reagent for the remote C(sp3)−H oxygenation of protonated aliphatic amines.6 In general, organic peracids have been described to oxidize aliphatic amines to hydroxylamines and further to oximes.7 Azanyl ester derivatives, which will be shown in this work to be a major reaction product of the ether‐diamine in bleach, have been successfully synthesized starting from the corresponding hydroxylamine and chlorosulfuric acid.8 To the best of our knowledge, the synthesis of azanyl esters starting from persulfate has not previously been described in the literature.
Herein, we propose, for the first time, an approach to characterize the stability and/or reactivity of the so‐called plex ingredients in cosmetic bleach, hence, in the presence of an excess of persulfate and hydrogen peroxide. We provide both experimental and computational proof of the reactivity of 1,13‐diamino‐4,7,10‐trioxatridecane (Figure 1), a molecule manufactured by the chemical industry for epoxy applications, which is, nowadays, prominently featured in the patent literature as a hair repair agent, both in combination with an organic acid or as a stand‐alone active ingredient.3e–3j
Figure 1.

1,13‐diamino‐4,7,10‐trioxatridecane.
The reactivity of the diamine was first investigated by 1H NMR spectroscopy in a model bleach, prepared by using deuterated water, ammonia solution in water, ammonium persulfate, and hydrogen peroxide (details in Table 1). Spectra were recorded at time 0, 12, 48, and 144 h and compared with a control.
Table 1.
1H NMR experiment setup. Aliquots of these solutions are directly measured in the NMR spectrometer.
| Chemical | Weight [g] |
Volume [mL] |
Concentration [mmol] |
|---|---|---|---|
| Solution A: Control | |||
| D2O | 49 | 44.3 | 2450 |
| 25 % ammonia | 6.8 | 7.51 | 100 |
| ether‐diamine | 3.6 | 3.62 | 16 |
| Solution B: Reaction in model bleach | |||
| D2O | 31 | 28.0 | 1550 |
| 25 % ammonia | 6.8 | 7.51 | 100 |
| (NH4)2S2O8 | 21 | 92 | |
| H2O2 30 % | 18 | 16.2 | 159 |
| ether‐diamine | 3.6 | 3.62 | 16 |
Continuous gas development over at least 12 h, deriving from (NH4)2S2O8 and H2O2 decomposition in alkaline environment,9 negatively impacted the resolution of the spectra recorded at 0 and 12 h (for full spectra see the Supporting Information). After 12 h, the signal of a new molecular entity is clearly visible; whereas, only after 48 h, full resolution is regained. Details of the 1H NMR spectra of Solutions A and B after 48 h are displayed in Figure 2, and chemical shift assignments are depicted in Figure 3. The spectrum recorded for Solution B after 48 h suggests the presence of two main components: 1) unreacted diamine with a characteristic quintet signal at 1.97 ppm (notice that the protons chemical shifts for the diamine in Solution B are different from the ones in Solution A, because of the different chemical composition of the solutions in which the spectrum is recorded; cf. Figure 3 a and 3 b); 2) a new molecule giving a quintet signal at 1.85 ppm, which is compatible with the azanyl ester derivative depicted in Figure 3 b.
Figure 2.
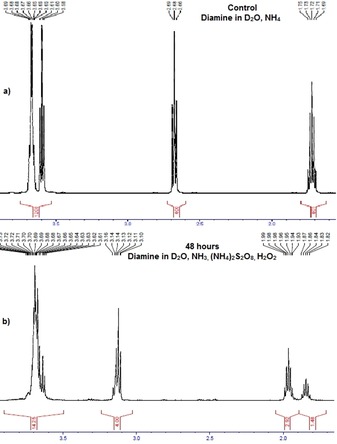
a) H NMR spectrum of Solution A. b) H NMR spectrum of Solution B, after 48 h.
Figure 3.
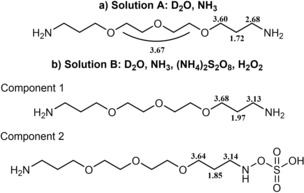
Chemical shift assignment for a) Solution A and b) proposed composition of Solution B after 48 hours.
Preliminary LC–MS analysis of Solution B after 48 h revealed the presence of a molecular mass of 317 Da, which is compatible with the [M+H]+ for the proposed azanyl ester derivative. The newly formed molecule was subsequently isolated through preparative HPLC and analyzed by high‐resolution mass spectrometry, which showed a m/z value of 317.1372 against a theoretical value of 317.1377 (Δ=−1.54 ppm), thus confirming, together with 1H NMR and 13C NMR spectra (see the Supporting Information), the proposed structure for the main reaction product.
To gain insight into the behavior of the ether‐diamine over the standard application time of 40 min, we analyzed the reaction by using a tandem mass spectrometer. Operating the instrument in the negative mode, we first confirmed the presence of m/z of 315 after 40 min, which corresponds to the azanyl ester derivative. The fragmentation pattern of the molecule was also recorded, which is displayed in Figure 4.
Figure 4.
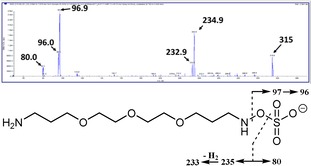
Azanyl ester derivative mass spectrum and proposed fragmentation pattern.
The ions with m/z 96, 97, and 233 (the latter derives from a rearrangement of the parent ion leading to the loss of a hydrogen molecule) are characteristic for aliphatic azanyl esters.10
By using a multiple reaction monitoring (MRM) method for the transitions m/z 315→97 and 315→80, we followed the formation of the reaction product through the time points 0, 10, 20, 30, and 40 min. The results are shown in Figure 5 as peak area of m/z 315→97 versus time. A linear increase over the analyzed timeframe is pointed out.
Figure 5.
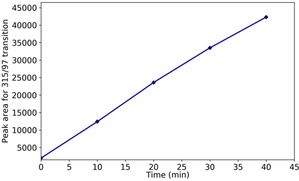
Semi‐quantitative analysis of the azanyl ester in a mixture of ether‐diamine and model bleach, at different time points.
The ether‐diamine is marketed worldwide under the brand name of Olaplex Bond Multiplier No. 1, in which the molecule is formulated in water with maleic acid and other minor components. We decided to investigate the stability of the diamine when the commercial product is mixed with the bleach. A mass spectrometric MRM method coupled to reverse‐phase chromatography was developed for the quantification of the diamine in Olaplex, resulting in 9.15 % w/w. The same analytical method was used to monitor the molecule content at time points 0, 10, 20, 30 and 40 min, when Olaplex is mixed with a model bleach (excess of persulfate and peroxide; see the Supporting Information). The results are shown in Figure 6, expressed as diamine concentration in the bleach mixture versus time. The time point 0 min, which also served as a quality control, displayed a recovery value of 97.6 % and the linear correlation coefficient for a seven‐point calibration curve was 0.998. The diamine degradation found over 40 min was 17.5 %.
Figure 6.
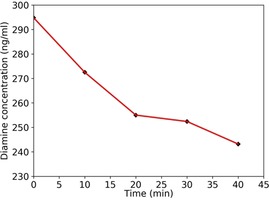
Quantitative analysis of the ether‐diamine in a mixture of Olaplex and model bleach, at different time points.
To assess the performance of the developed analytical method with a commercial bleach, we also attempted the analysis of the diamine content in a mixture of Olaplex, Blondor Multi‐Blond powder, an ammonium and sodium persulfate‐containing powder, and Welloxon Perfect 12 %, a hydrogen‐peroxide‐containing cream, prepared according to usage instructions for highlights, [11] which resulted in a 7.09±0.88 % depletion in diamine content between 0 and 40 min (the experiment was performed in duplicate). The highly complex matrix of a blend of Welloxon and Blondor imposes time‐consuming extraction and sample preparation steps prior to LC–MS analysis. Thus, time 0 min already contains partially reacted diamine, contributing to the low recovery value for this measurement (73.05±0.45 %).
Therefore, to avoid the challenges related to analyzing the real product, we recommend using a model bleach system for evaluating the stability of plex actives.
The formation of the azanyl ester could be explained by considering a radical mechanism, as suggested by the literature: it is acknowledged that persulfate in alkaline environment produces the sulfate radical anion,12 which can subsequently react with water or, at basic pH, with OH−, releasing hydroxyl radicals,13 which can in turn react with 1,13‐diamino‐4,7,10‐trioxatridecane, thus producing NH2−R−NH⋅.14 R indicates the 4,7,10‐trioxatridecane moiety. Finally, SO4 −. could react with NH2−R−NH⋅, thus forming the azanyl ester derivative.
Although consistent with the available literature, a radical mechanism as the main pathway of the formation of the azanyl ester would imply an additional high occurrence of more drastic cleavages, which are not observed in the H NMR spectra (after 12 to 144 h). We, therefore, explored an alternative mechanism through DFT calculations. More specifically, we hypothesized a nucleophilic substitution through an SN2 mechanism, as described in Scheme 1. Again, NH2−R−NH2 indicates the 1,13‐diamino‐4,7,10‐trioxatridecane.
Scheme 1.
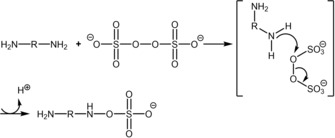
Proposed SN2 mechanism.
To assess the possibility that this reaction can occur under our experimental conditions, we calculated the activation Gibbs free energy ( ) and the reaction Gibbs free energy ( ) by means of DFT calculations (the interested reader is referred to the Supporting Information for methodological details).
Keeping in mind the reaction time (about 40 min), a non‐prohibitive activation barrier was determined ( =21.57 kcal mol−1), thus supporting the idea that the described SN2 mechanism might take place under the adopted experimental conditions. It is worth noting that a similar SN2 reaction mechanism has recently been hypothesized to occur between persulfate and a tertiary amine.15 The computed transition state geometry (TS) with an imaginary frequency equal to −245.43 cm−1 is depicted in Figure 7 along with the free energy profile of the reaction. The TS was further confirmed by the intrinsic reaction coordinate calculation (IRC) reported in Figure S1 of the Supporting Information.
Figure 7.
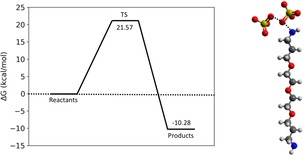
Free energy profile (left) of the SN2 reaction mechanism and optimized geometry of the corresponding transition state (TS) computed at the B3LYP/6–311++G(d,p) level of theory (right). Solvent (water) and dispersion effects were taken into account through the integral equation formalism of the polarizable continuum model (PCM) and the GD3BJ model, respectively.
In conclusion, we have performed a study to explore the reactivity of the 1,13‐diamino‐4,7,10‐trioxatridecane in a bleach containing ammonia, ammonium persulfate, and hydrogen peroxide. The main reaction product is an unexpected azanyl ester derivative, resulting from a so‐far‐undescribed reaction mechanism, as suggested by DFT calculations. We have shown that the azanyl ester is formed over a time period of 40 min and that the diamine contained in the commercialized product degrades by approximately 17 % over the same time, equivalent to 0.031 g, considering 2 g of Olaplex (1.875 mL recommended for full head service) containing 0.183 g of diamine.11
Correspondingly, the data indicate that the azanyl ester represents a substantial fraction, considered relevant for the safety evaluation specially when used in cosmetic products. We want to emphasize that the systematic approach to the stability assessment of ether‐diamine can be generally reapplied for the analysis of molecules reacting under bleach conditions, such as plex actives.
Conflict of interest
E.M.G., I.W., C.G., W.R., and S.W. are employees of Coty, selling cosmetic hair products including “plex” products. The ingredients studied in this paper are currently used in commercial products marketed by this company or other companies. The authors G.F.M., D.A., and O.N. (from the University of Bari) participated as experts in computational chemistry. The authors alone are responsible for the content and writing of the paper.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We are grateful to Armin Osan, Gerd Schlotzhauer, and Dr. Thomas Koepke for the purification of the 1,13‐diamino‐4,7,10‐trioxatridecane. We thank Spectral Service AG for the NMR spectra and ReseaChem GmbH for isolating the azanyl ester. We also thank Renzo Luisi and Leonardo Degennaro for useful discussions. We acknowledge CINECA award nos. HP10CJSIFE‐AQP4‐NT and HP10BGY23X‐OAP‐AQP4 under the ISCRA initiative for the availability of high‐performance computing resources.
E. M. Gargano, G. F. Mangiatordi, I. Weber, C. Goebel, D. Alberga, O. Nicolotti, W. Ruess, S. Wierlacher, ChemistryOpen 2018, 7, 319.
Contributor Information
Dr. Emanuele M. Gargano, Email: Emanuele_gargano@cotyinc.com.
Dr. Giuseppe F. Mangiatordi, Email: Giuseppe.mangiatordi@uniba.it
References
- 1.
- 1a. Smith R. A. W., Garrett B., Naqvi K. R., Fülöp A., Godfrey S. P., Marsh J. M., Chechik V., Free Radical Biol. Med. 2017, 108, 110–117. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Kaliyadan F., Gosai B. B., Al Melhim W. N., Feroze K., Qureshi H. A., Ibrahim S., Kuruvilla J., Int. J. Trichology 2016, 8, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a.K. C. Hamilton, C. R. F. Goget, D. Danielski, G. Provot, F. B. Boulineau, (L'OREAL), Pat. Coop. Treaty Int. Appl WO2016179017A1, 2016;
- 3b.A. Kleen, H. Höffkes, D. Hollenberg, O. Brabänder, F. Naumann, (Henkel AG & Co. KGaA), Eur. Pat. Off EP1326577B2, 2012;
- 3c. S. Nagase, K. Ando, E. Kariya, S. Shibuichi, N. Satoh, (Kao Corporation), Eur. Pat. Off EP0978272A1, 2000;
- 3d.A. Flohr, (Wella), Eur. Pat. Off EP3175837, 2017;
- 3e.E. D. Pressly, C. J. Hawker, (Liqwd, Inc.), Off. Gaz. U. S. Pat. Trademark Off US9144537B1, 2015;
- 3f.E. D. Pressly, C. J. Hawker, (Liqwd, Inc.), Off. Gaz. U. S. Pat. Trademark Off US2016081899A1, 2015;
- 3g.E. D. Pressly, C. J. Hawker, (Liqwd, Inc.), Off. Gaz. U. S. Pat. Trademark Off US2015328102A1, 2015;
- 3h.E. D. Pressly, C. J. Hawker, (Liqwd, Inc.), Off. Gaz. U. S. Pat. Trademark Off US2016263003A1, 2015;
- 3i.E. D. Pressly, C. J. Hawker, (Liqwd, Inc.), Off. Gaz. U. S. Pat. Trademark Off US2016193129A1, 2016;
- 3j.E. D. Pressly, C. J. Hawker, (Liqwd, Inc.), Off. Gaz. U. S. Pat. Trademark Off US2016206535A1, 2016.
- 4.
- 4a. Yan N., Li M., Liu Y., Liu F., Brusseau M. L., Water Air Soil Pollut. 2017, 228, 453; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. Liu Z., Guo W., Han X., Li X., Zhang K., Qiao Z., Environ. Sci. Pollut. Res. Int. 2016, 23, 19707–19712; [DOI] [PubMed] [Google Scholar]
- 4c. Dulova N., Kattel E., Trapido M., Chem. Eng. J. 2017, 318, 254–263. [Google Scholar]
- 5.
- 5a. Matzek L. W., Carter K. E., Chemosphere 2016, 151, 178–188; [DOI] [PubMed] [Google Scholar]
- 5b. Liu H., Bruton T. A., Doyle F. M., Sedlak D. L., Environ. Sci. Technol. 2014, 48, 10330–10336; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Herscu-Kluska R., Masarwa A., Saphier M., Cohen H., Meyerstein D., Chem. Eur. J. 2008, 14, 5880–5889. [DOI] [PubMed] [Google Scholar]
- 6. Lee M., Sanford M. S., Org. Lett. 2017, 19, 572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patil V. V., Gayakwad E. M., Shankarling G. S., New J. Chem. 2015, 39, 6677–6682. [Google Scholar]
- 8. Strom A. E., Hartwig J. F., J. Org. Chem. 2013, 78, 8909–8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.
- 9a. da Silva-Rackov C. K. O., Lawal W. A., Nfodzo P. A., Vianna M. M. G. R., do Nascimento C. A. O., Choi H., Appl. Catal. B 2016, 192, 253–259; [Google Scholar]
- 9b.S.-C. Fang, S.-L. Lo, Second International Conference on Mechanic Automation and Control Engineering (MACE), IEEE 2011, 2362–2366.
- 10. Yi L., Dratter J., Wang C., Tunge J. A., Desaire H., Anal. Bioanal. Chem. 2006, 386, 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. https://help.olaplex.com/detail/lightener-bleach-foils (accessed 09 January, 2018).
- 12.
- 12a. Furman O. S., Teel A. L., Watts R. J., Environ. Sci. Technol. 2010, 44, 6423–6428; [DOI] [PubMed] [Google Scholar]
- 12b. Luke T. L., Mohan H., Manoj V. M., Manoj P., Mittal J. P., Aravindakumar C. T., Res. Chem. Intermed. 2003, 29, 379–391. [Google Scholar]
- 13. Liang C., Su H. W., Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar]
- 14.
- 14a. Ball R. E., Chako A., Edwards J. O., Levey G., Inorg. Chim. Acta 1985, 99, 49–58; [Google Scholar]
- 14b. Huang L., Li L., Dong W., Liu Y., Hou H., Environ. Sci. Technol. 2008, 42, 8070–8075. [DOI] [PubMed] [Google Scholar]
- 15. Zhang S., Shi Z., Xu H., Ma X., Yin J., Tian M., Soft Matter 2016, 12, 2575–2582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


