Abstract
The content of intrinsically disordered protein (IDP) is related to organism complexity, evolution, and regulation. In the Plantae, despite their high complexity, experimental investigation of IDP content is lacking. We identified by mass spectrometry 682 heat-resistant proteins from the green alga, Chlamydomonas reinhardtii. Using a phosphoproteome database, we found that 331 of these proteins are targets of phosphorylation. We analyzed the flexibility propensity of the heat-resistant proteins and their specific features as well as those of predicted IDPs from the same organism. Their mean percentage of disorder was about 20%. Most of the IDPs (~70%) were addressed to other compartments than mitochondrion and chloroplast. Their amino acid composition was biased compared to other classic IDPs. Their molecular functions were diverse; the predominant ones were nucleic acid binding and unfolded protein binding and the less abundant one was catalytic activity. The most represented proteins were ribosomal proteins, proteins associated to flagella, chaperones and histones. We also found CP12, the only experimental IDP from C. reinhardtii that is referenced in disordered protein database. This is the first experimental investigation of IDPs in C. reinhardtii that also combines in silico analysis.
Introduction
Some biologically active proteins have no well-defined tertiary structure in their native state and are known as intrinsically disordered proteins (IDPs) while other proteins possess structural elements with some disordered (flexible) regions (IDRs)1–3. It is well-known that the lack of protein structure is determined by the amino acid sequence4 and indeed, IDPs or IDRs have a biased amino acid composition. Compared to other proteins, they are enriched in charged and structure-breaking residues (Pro and Gly) and in Ala residues while they are depleted in hydrophobic and aromatic residues and have low content of Cys and Asn residues5–10.
Although proteins may have different conformations and be folded or unfolded depending on different conditions11, in IDPs, order-disorder transitions can be triggered by pH, temperature, redox potential, mechanical force, light exposure and various types of interactions. IDPs or IDRs are often the target of phosphorylation, ubiquitination, methylation, breakage of disulfide bridges and disorder-order transitions can result from these post- translational modifications (PTMs)12,13. Recently 4588 phosphoproteins and 115 protein kinases in C. reinhardtii were detected using phosphorylation and kinome enrichment strategies coupled to mass spectrometry but without considering intrinsic flexibility of these proteins14.
Because of their dynamic properties and flexibility allowing them to bind a wide range of partners, IDPs are often central hubs and play multiple roles in biological processes2,13,15,16. According to previous proteome-wide studies, intrinsic flexibility is widespread in all kingdoms of Life17, with eukaryotes having a significantly larger fraction of intrinsic disorder in their proteomes than prokaryotes18. The average content of flexible proteins is 3.8% in archaea, 5.7% in bacteria, and 18.9% in eukaryotes suggesting that increasing protein flexibility is related to the complexity of an organism19. Transcription factors containing IDRs are likely key factors contributing to the evolution of organismic complexity as they have important roles in the regulation of the cell cycle, division, differentiation and proliferation and in cell size20,21. IDRs in proteins, as well as the alternative splicing of their precursor mRNA and their phosphorylation, constitute a driving force in the evolution of complex multicellularity22. Flexibility or plasticity allows functional diversification and environmental responsiveness23 and since photosynthetic organisms are complex and require a high level of regulation to cope with their changeable environment, a large number of flexible proteins are expected within their proteome. However; only 51 IDPs from photosynthetic species are referenced in the database for disordered proteins24,25. This number is significantly lower than the 157 bacterial IDPs, the 62 IDPs from fungi and the 400 IDPs from vertebrates. This relatively low proportion of identified IDPs from photosynthetic organisms among the 804 IDPs of the DisProt database25 illustrates the lack of study of structural disorder on these organisms, and does not reflect the true proportion of IDPs within the different Life kingdoms.
In Plantae, two specific families of proteins relying on disorder for their functioning have been well described: the dehydrins including protein chaperones such as ERD10 and ERD1426,27 and the GRAS family28,29. Dehydrins play major roles under specific conditions including responses to abiotic stress including drought28,30 and GRAS proteins are involved in hormone responses. They are therefore critical for plant adaptation and survival31. Nevertheless, as mentioned above, only a few analyses of the global IDP content in photosynthetic organisms are available, and are based on bioinformatic search32,33. Experimental methods to identify the flexible proteins have been proposed and applied to other organisms34 including the bacterium, Escherichia coli, the yeast, Saccharomyces cerevisiae35 and the mouse36. In the higher plant, Arabidopsis thaliana, a systematic analysis of the seed phosphoproteome was performed using heat-treatment followed by phosphoaffinity chromatography to identify phosphorylated IDPs. This study showed that several late-embryogenesis-abundant (LEA) proteins and storage-like proteins were major components of the seed phosphoproteome37. While the characterization of the flexible proteins is the focus of numerous studies, experimental identifications of IDPs are still lacking and are thus needed to bring an added value to the set of bioinformatic data already available.
The eukaryotic green alga, Chlamydomonas reinhardtii, is a well-known biological model, and has been extensively studied and referred to as the photosynthetic yeast38. There are only a few IDPs reported from this green alga, such as the Chloroplast Protein (CP12), which forms a supramolecular complex with two key Calvin-Benson-Bassham (CBB) cycle enzymes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and phosphoribulokinase (PRK)39–42. This protein regulates the association–dissociation of this complex, thereby allowing the CBB cycle to be inactive in the dark and active in the light, but has moonlighting activities43, for instance, chaperone function44 and metal ions binding45. Another IDP recently found in this alga is the Essential Pyrenoid Component 1 (EPYC1), a low complexity repeat protein that binds the ribulose-1, 5-bisphosphate carboxylase-oxygenase to form the pyrenoid matrix46.
While an entire proteome bioinformatic analysis has been performed for ten eukaryotes, including C. reinhardtii, providing a reliable collection of disorder annotations, statistics, and relevant disorder parameters from protein amino acid sequences33, these authors concluded that these results need to be confronted with experimental data.
To bring new information on amino acid compositions, cellular compartments and molecular functions of algal IDPs, we searched for flexible proteins from C. reinhardtii based on their heat-resistance property and we characterized them by mass spectrometry coupled to in silico approaches. We compared our experimental results to the whole proteome of C. reinhardtii using a bioinformatics analysis. This work will help to bring a conceptual breakthrough for an in-depth understanding of the molecular mechanisms of IDPs and their role in the cellular physiology of this alga.
Results
IDPs enrichment in C. reinhardtii extracts and their identification
IDPs or IDR-containing proteins are well-known to remain soluble under some critical conditions, such as extreme pH and temperature whereas globular proteins unfold, aggregate and precipitate. Therefore, to characterize proteins with flexibility in C. reinhardtii, proteins (about 2.4 mg) extracted from this alga were either acid- or heat-treated for at least 5 min. About 7% of the total proteins were heat-resistant while only 0.3 to 0.5% were acid-resistant. When heated for longer time, up to 1 h, the amount of proteins in the supernatant or soluble fraction did not change. To study as many experimental IDPs as possible, heat-treatment was chosen (Table 1). The heat-stable proteins (proteins remaining soluble after heat-treatment) expected to be IDPs or IDR-containing proteins were further analyzed by SDS-PAGE (Fig. 1 and Supplementary Fig. S1). The most intense bands were analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS) after trypsin digestion. We identified 791 heat-resistant proteins from NCBI database search (15313 proteins) and also searched against Phytozome v12.1 (19526 proteins). Among the 791 heat-resistant proteins, the sequence of 109 proteins was only partial and thus 682 proteins were analyzed further. These 682 proteins are listed in Supplementary Table S1. Their theoretical biophysical properties are: a broad range of isoelectric points (4 < pI < 12) and of molecular masses with most proteins ranging within 10 to 200 kDa as expected from SDS-PAGE (Fig. 2). The most represented proteins were ribosomal proteins (57), proteins associated to flagella (33), chaperones (20) and histones (9). We also found the proteins that are biochemically well-characterized in details as being IDPs such as CP12 and EPYC1 or IDR-containing protein such as adenylate kinase 3.
Table 1.
Heat and acid-treatments.
| Treatment | Protein (mg) | % of putative IDPs |
|---|---|---|
| Control | 2.38 ± 0.05 | NA |
| 5% TCA | 0.007 ± 0.003 | 0.3 |
| 10% PCA | 0.010 ± 0.005 | 0.5 |
| 98 °C, 1 h | 0.164 ± 0.021 | 6.9 |
| 98 °C, 30 min | 0.161 ± 0.023 | 6.8 |
| 98 °C, 5 min | 0.159 ± 0.018 | 6.7 |
Content (mg) and percent of heat- or acid-resistant proteins in C. reinhardtii. Control corresponds to the total protein concentration in the samples before treatment. Values are means with standard error, n = 5. TCA and PCA stands for trichloroacetic acid and perchloric acid, respectively.
Figure 1.
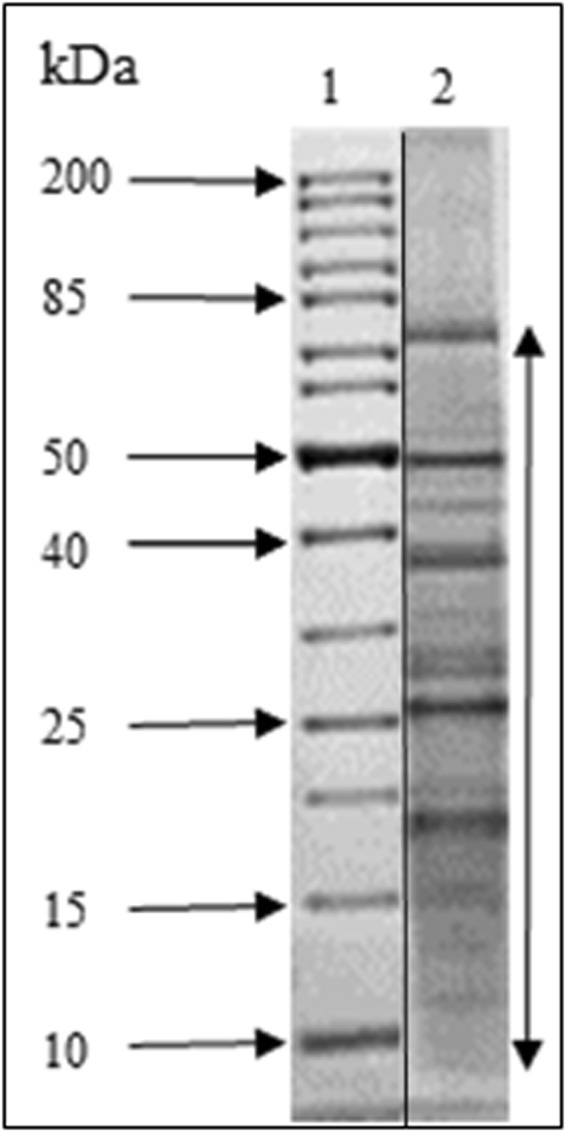
SDS-PAGE of heat-treated proteins from C. reinhardtii. 10 µg were loaded and separated on 12% polyacrylamide gels under denaturing conditions and stained with Coomassie Blue (Lane 2), molecular weight markers (Euromedex. unstained protein ladder, Lane 1). The two lanes came from different parts of the same gel (see Fig. S1). The most abundant bands in the range of molecular weight (Mw) shown by the arrow were sliced and identified by mass spectrometry.
Figure 2.
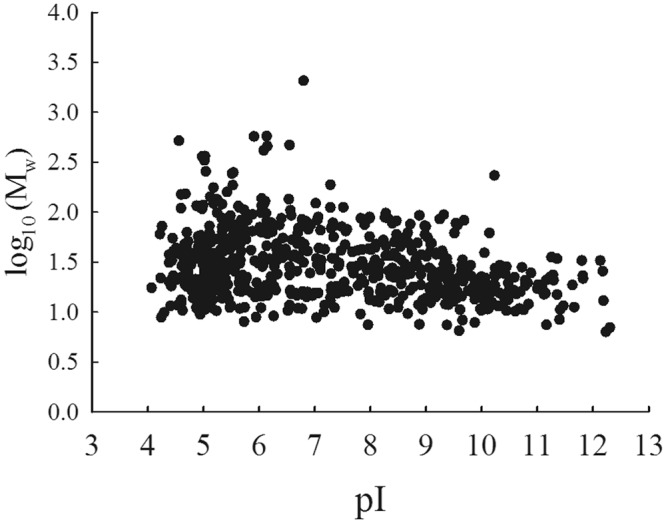
Distribution of isoelectric point (pI) value and molecular weight (Mw) of 682 experimentally identified IDPs from C. reinhardtii. The pI and the Mw were theoretical and given by the mass spectrometry software.
Even though proteases inhibitors were added, ten proteins (1.5% of the 682 heat-resistant proteins) were obviously degraded. These proteins were Type I polyketide synthase, Dicer-like protein, flagellar-associated protein, SNF2 superfamily protein, and hypothetical proteins (see Supplementary Table S1), and they have a high theoretical molecular mass, out of the range of the SDS-PAGE but their degraded fragments contain disordered residues. This is in agreement with the analysis by PONDR that showed that these large multi-domain proteins have a high proportion of disordered residues from 26 to 97%.
A side-observation concerns phosphorylation of 19 proteins. Indeed, since our approach was not aimed at enriching phosphorylated proteins, a few phosphorylated proteins were experimentally highlighted and are listed in Supplementary Table S2. We then searched how many proteins within the 682 proteins were phosphorylated using the phosphoproteome data from the literature14. We found 331 proteins corresponding to about 50% of the total proteins extracted (682) that were phosphorylated. The entire list of the 331 phosphorylated proteins can be found as Supplementary Table S3.
In silico analysis of “experimentally found” IDPs
After analyzing the properties of the heat-resistant proteins, we specifically investigated their content of flexibility using bioinformatic analysis. Searching for flexible region higher than 30 consecutive amino acid residues as previously reported in the literature10,47, 506 proteins (74.1% of the 682 identified proteins, Table 2) were validated as flexible using PONDR while IUPred, DisEMBL and FoldIndex, validated 244 (35.7%), 101 (14.8%) and 260 (38.1%) proteins, respectively. Only 43 proteins were consistently selected by all the four predictors as each predictor relies on different philosophies: some are a priori algorithms and others are trained on existing datasets. Agreement among them therefore, should not be expected for all the proteins. We then modified our criteria and included proteins with the longest disorder length (LDR comprised between 10 and 30 residues) but with a percent of flexibility higher than 10. In that case, PONDR considered 98% (669/682) of the proteins to be IDPs confirming our experimental procedure. FoldIndex, DisEMBL and IUPred considered 498 (73%), 448 (66%), and 348 (51%) proteins to be IDPs, respectively. 299 proteins (44%) were selected as IDPs by all four software (Table 2). These results highlight the importance to combine many predictors and approaches to increase reliability of disorder analysis47. We also searched for the IDPs found in our study and for all predicted IDPs from a recent in silico approach that used other criteria for disorder33. After removing partial sequences, among the 9418 proteins left, we found 2152 IDPs that had a disorder percent higher than 10. Among these 2152 IDPs, 205 proteins from our experimental dataset with disorder percent higher than 10 were listed. The mean percentage of flexibility of this subset of proteins was 23% compared to 17.4% for the initial set of 2152 proteins. The relationship between the protein length and the disordered residues was analyzed for these two sets of IDPs. A linear relationship was observed, meaning that the longer the protein, the more the disordered residues (Fig. 3).
Table 2.
Number of experimental proteins with the longest disordered region determined by different predictors.
| Predictor | Length range | ||||
|---|---|---|---|---|---|
| 0~9 | 10~29 | 30~49 | 50~99 | 100< | |
| PONDR | 16 (2.3%) | 160 (23.6%) | 198 (29%) | 181 (26.5%) | 127 (18.6%) |
| IUPred | 300 (44.1%) | 138 (20.2%) | 73 (10.7%) | 95 (13.9%) | 76 (11.1%) |
| DisEMBL | 175 (25.8%) | 406 (59.4%) | 62 (9.0%) | 33 (4.8%) | 6 (0.9%) |
| FoldIndex | 262 (38.3%) | 160 (23.4%) | 99 (14.6%) | 111(16.3%) | 50 (7.3%) |
Percentage is given in parentheses taking into account a total of 682 proteins.
Figure 3.
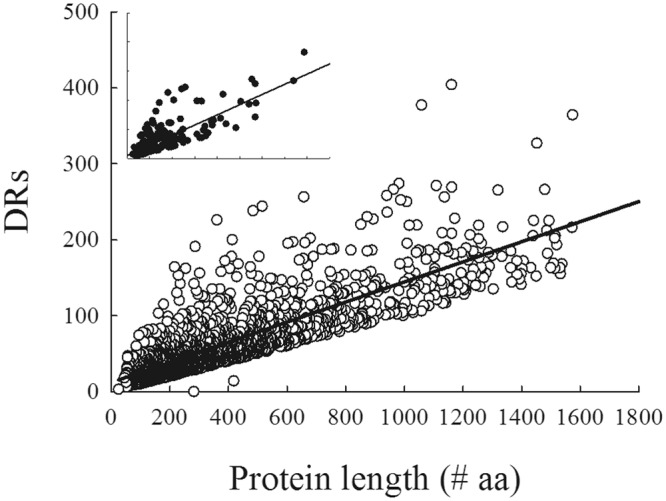
Relationship between disordered residues (DRs) and protein length. Open circles and full circles represent the 2152 IDPs analyzed from33 and the 299 experimental IDPs from this study (inset); in both case, only proteins with a percent disorder ≥10% were analyzed. A linear relationship between DR calculated from the disorder percent from Vincent and Schnell33 and protein length was found with a regression coefficient a = 0.17 and a correlation coefficient R2 = 0.6.
Putative subcellular localization
Using PredAlgo, we further analyzed the location of (i) the 299 experimental IDPs, (ii) the 2152 IDPs and (iii) the whole C. reinhardtii proteome (9418 proteins that are listed in Supplementary Table S4) mentioned above (Fig. 4). Among the 299 experimental IDPs, 8.4% proteins were predicted to be addressed to the mitochondrion (M) and 21.4% proteins to the chloroplast (C). The rest (70.2%) was located in other compartments ((O), e.g. the nucleus, the endoplasmic reticulum, etc.) but could not be analyzed any further because there was no predictor available for these compartments. The same trend, less IDPs in the mitochondrion, in the chloroplast and in the other compartments (M < C < O), was followed for all the other predicted IDPs. However, in the mitochondrion, less disordered proteins were found in our experimental dataset compared to the predicted IDPs (14.8%). As regard to the whole proteome the same percent of proteins were also found with 14.7% in the mitochondrion and around 20% in the chloroplast.
Figure 4.
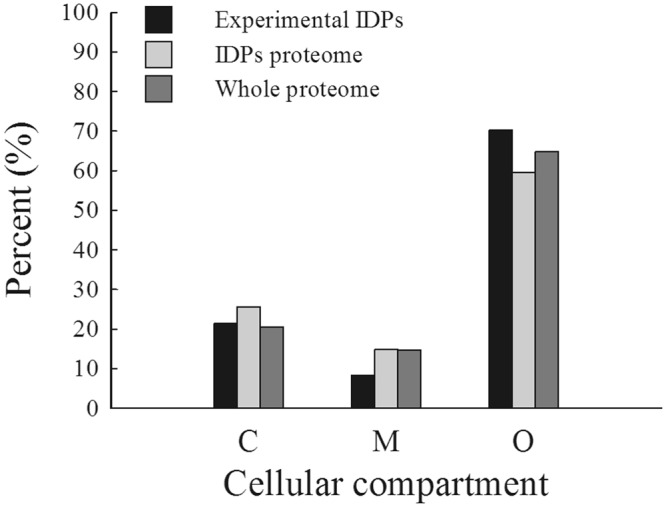
Subcellular localization of IDPs. The distributions of the 299 experimental IDPs (black bars), of the 2152 IDPs from C. reinhardtii proteome (gray bars) and from the whole algal proteome (9418 proteins since protein partial sequences were removed (see list in Supplementary Table S4, dark gray bars) in the chloroplast (C), in the mitochondrion (M) and in the other compartments (O) are shown. These localizations were predicted using PredAlgo (https://giavapgenomes.ibpc.fr/predalgo/).
Analysis of amino acid composition
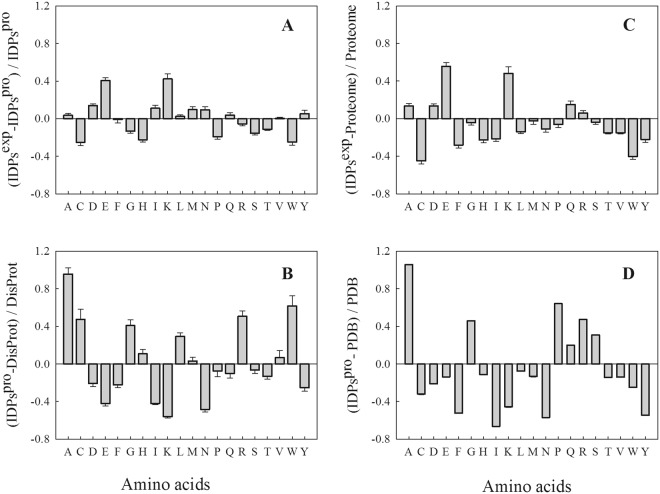
Using Composition profiler, we analyzed the amino acid compositions of the experimental IDPs and compared them to those of (i) the 2152 predicted IDPs, (ii) the IDPs from DisProt 3.4 database, (iii) the whole C. reinhardtii proteome and (iv) to those of the globular proteins from the Protein Data Bank (PDB Select 25). When experimental IDPs were compared to the 2152 predicted IDPs, a few differences were observed compared to all the other sets. However, some residues such as Glu, and Lys were higher and Cys, His, Pro and Trp were lower in the experimental IDPs (Fig. 5A). The 2152 predicted IDPs from C. reinhardtii vs classic IDPs24,25 had a biased amino acid composition (Fig. 5B). They had a higher content in Ala, Arg, Gly, Leu and unexpectedly, in Trp and Cys residues than classic IDPs, a lower content in Asn, Ile, Glu, and Lys and a similar content in other residues. When the experimental IDPs were compared to the whole proteome (Fig. 5C), as expected they had a higher content in charged residues such as Glu, Lys and lower content in Cys, and all aromatic residues (Phe, Trp and Tyr). The 2152 predicted IDPs compared to globular proteins from the PDB S25 (Fig. 5D), were depleted in Asp, Glu and Lys unexpectedly, but were enriched in structure-breaking residues (Pro and Gly) and in Ala, Arg and Ser residues; as expected, they were also depleted in hydrophobic and aromatic residues (Phe, Trp, Tyr), and had low content of order-promoting amino acid residues (Ile, Met, Leu, Val, Asn, Cys).
Figure 5.
Amino acid composition of IDPs. Amino acid comparison, using Composition profiler (http://www.cprofiler.org/)82, of IDPs experimentally (IDPsexp) found and the 2152 IDPs (IDPsPro) from C. reinhardtii (A), of the 2152 IDPs from C. reinhardtii (IDPsPro) and IDPs from DisProt 3.424,25 (B), of IDPs experimentally found (IDPsexp) and the whole proteome from C. reinhardtii (C), of the 2152 IDPs (IDPsPro) from C. reinhardtii and of the structured or globular proteins from the protein data bank PDB Select 2583 (D).
Molecular functions of IDPs
We analyzed the predicted molecular functions associated with the flexible proteins identified systematically with the four disorder algorithms and classified them using GO terms from the molecular function ontology. We searched for the proteins having the same molecular function in the whole proteome. For the two sets we calculated the frequency of proteins being in the same molecular function category (Fig. 6). The results showed that IDPs were most abundant in the GO terms, RNA binding, unfolded protein binding, translation and DNA binding; they were also more abundant in the GO terms antioxidant activity, transcription and transcription factor activity. Most proteins from the whole proteome could be clustered in the GO term catalytic activity but only few IDPs were present in this category. Proteins clustered in the GO terms, nucleotide binding, metal ion binding, protein binding and transporter activity were slightly more frequent in the whole proteome than in the experimental IDPs.
Figure 6.
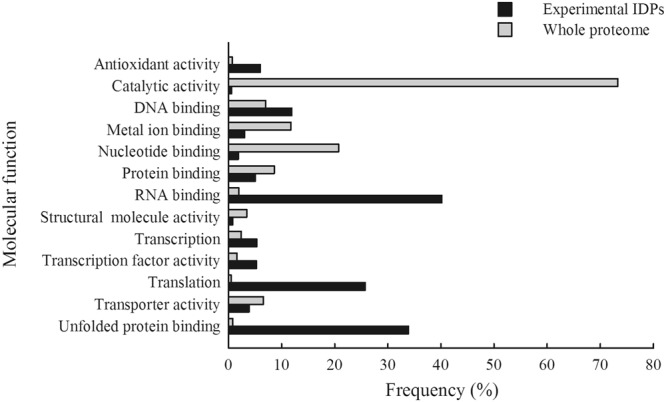
Classification of molecular functions of IDPs and of the proteome from C. reinhardtii. Classification of the 299 experimental IDPs (black bars) and of the 7058 proteins (gray bars) with known molecular functions (https://genome.jgi.doe.gov/cgi-bin/ToGo?accession = GO:0003674&species = Chlre4) is based on gene ontology (GO) terms.
Discussion
IDPs contain three times less aggregation prone regions than globular proteins, in particular, they lack a hydrophobic core that can be exposed under denaturing conditions, and remain soluble even at high temperature and under acid treatment34. Therefore as previously reported for other organisms34–36, we used these treatments to isolate IDPs. In C. reinhardtii, heat-treatment was a better method to isolate IDPs than acid-treatment and allowed 20 times more IDPs to be recovered in the soluble fraction. This fraction contained 7% of the total protein extract from which we identified 682 soluble proteins called heat-resistant proteins. This set of proteins is probably not an exhaustive list of heat-resistant proteins since some proteins may have precipitated due to aggregation of globular domain surrounded by IDR; others were out of the range of molecular mass investigated in this study and others may have not been found in the LC-MS/MS analysis.
As a result of amino acid bias, sequence complexity, hydrophobicity, charge and other sequence attributes, sites of PTM are frequently associated with IDPs. Phosphorylation was reported to be one of the most common PTMs and over-represented in the flexible regions of eukaryotic proteins, including plants12,48. Indeed, we experimentally identified Serine/arginine-rich splicing factors (SR proteins) consistent with what has been shown for other organisms49,50. Moreover, we found that 50% of the 682 proteins studied in this work were present in the recently published phosphoproteome of C. reinhardtii14,51 indicating that they can be phosphorylated. It has been shown in silico that there is a positive relationship between phosphorylation and content of flexibility in in algae proteome analysis52. Combining two non-targeted experimental approaches, the characterization of flexible proteins (this work) and the phosphoproteome by Wang et al. of C. reinhardtii14, we were able to list proteins that were both flexible and phosphorylated. Since PTM, especially phosphorylation, and flexibility are two key factors involved in protein-protein interaction and regulation, further research is likely to detect important regulators in C. reinhardtii. This study will help to develop driven approach to answer more specific biological questions.
The different percent of proteins in the different compartments of the cell reveals that the mitochondrion and the chloroplast contain a lower proportion of IDPs as previously shown53 and that other compartments (including the nucleus) contain a higher proportion of IDPs as described for other organisms10. The chloroplast and mitochondria are ancient organelles with a prokaryotic origin, which probably explains their low level of disordered elements54. In addition, evolutionary pressure might have forced nuclear proteins to acquire disordered regions.
Though the experimental IDPs vs all predicted IDPs from C. reinhardtii were enriched in Glu, Lys and contained less Trp residues, both have common features with classic IDPs5,8,9,47 and present some peculiarities. The content in Ala and Gly residues is even higher in IDPs from this green alga than in higher plants55. All together these results imply that IDPs have amino acid compositions that are distinct from globular proteins but are also species-specific within the same kingdom, Plantae.
Disorder is less frequent in enzymes and many proteins involved in catalytic activity are structured, and as expected, we found only few IDPs clustered in this category. However, benefitting from their biased amino acid composition and thereby highly conformational flexibility, IDPs can bind multiple partners to perform their particular functions10,30. Indeed many IDPs in our study are associated with nucleic acids binding. Moreover, as reported in the literature, IDPs found in plants are associated with many stress-response processes, acting as protein chaperones56 or protecting other cellular components2. We have identified 20 IDPs related to unfolded protein binding or chaperone-function, with some illustrative examples, Hsp3357–60, Hsp70 and Hsp9061, belonging to the family of heat-shock proteins. Hsp70 and Hsp90 were also found in the Chlamydomonas phosphoproteome14 indicating that they are phosphorylated. Though these chaperones play crucial roles in relation to their flexibility, they are understudied in C. reinhardtii compared to other organisms62,63.
The similarity between the chloroplast ribosome and the 70S bacterial ribosome64 is such that a lower content of disorder is expected for the chloroplast ribosomal proteins compared to the cytosolic ones. This is in agreement with our results. We experimentally identified 14 chloroplast, 42 cytosolic and one mitochondrial ribosomal proteins (L29) (Tables 3 and 4). 21 of these ribosomal proteins were confirmed as flexible by the four algorithms, among which were 17 cytosolic ribosomal proteins. Three large chloroplast ribosomal proteins were also confirmed by the four predictors, two (L15 and L34), were found in other plants53 while one (L32) was specific to C. reinhardtii and of interest, was present in the phosphoproteome. On the contrary, other chloroplast ribosomal proteins were not confirmed as IDPs by all the predictors but have been described as flexible in higher plants such as S5, S21, L11, L18 and L2453 (Table 3). This suggests that flexibility in ribosomal proteins is probably under estimated when the four predictors are taken into account. By homology with the E. coli 70S ribosome, it is expected that the L7/L12 stalk of the chloroplast ribosome remains flexible65 and it was experimentally confirmed. As expected, we also identified the core histones (H2A, H2B, H3, H4) and the linker histone (H1 family) as IDPs66–68.
Table 3.
Experimental disordered proteins within the chloroplast ribosome in C. reinhardtii. Accession numbers are from Phytozome v12.1 and from UniProt. NF stands for accession number not found. P, D, I and F corresponds to PONDR, DisEMBL, IUPred and FoldIndex, respectively.
| Accession | Accession | Name | P | D | I | F |
|---|---|---|---|---|---|---|
| Cre16.g659950.t1.1 | A8J8M5 | S5 | + | − | + | + |
| Cre12.g494450.t1.2 | A8JDN8 | S16 | + | − | − | + |
| NF | NP_958370 | S19 | + | + | + | + |
| Cre12.g494750.t1.2 | A8JDN4 | S20 | + | + | − | + |
| Cre01.g017300.t1.2 | A8HPN4 | S21 | + | − | − | + |
| Cre13.g581650.t1.2 | A8HTY0 | L7/L12 | + | + | − | − |
| Cre10.g423650.t1.2 | A8ICE4 | L11 | + | − | − | − |
| Cre14.g612450.t1.2 | A8JAL6 | L15 | + | + | + | + |
| Cre01.g052100.t1.2 | A8HNJ8 | L18 | + | − | − | + |
| Cre16.g652550.t1.2 | A8J9D9 | L24 | + | − | − | + |
| Cre07.g352850.t1.2 | A8IUC3 | L32 | + | + | + | + |
| Cre01.g030050.t1.2 | A8HQG3 | L34 | + | + | + | + |
| Cre02.g083950.t1.1 | A8I8A3 | P3 | + | + | − | + |
| Cre12.g519180.t4.1 | A8J641 | P7 | + | − | + | + |
Table 4.
Experimental disordered proteins within the cytosolic ribosome in C. reinhardtii. P, D, I and F corresponds to PONDR, DisEMBL, IUPred and FoldIndex, respectively. Accession numbers were from Phytozome v12.1 and from UniProt. NF stands for accession number not found.
| Accession | Accession | Name | P | D | I | F | Accession | Accession | Name | P | D | I | F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cre02.g102250.t1.2 | A8I4P5 | S3 | + | − | − | − | Cre12.g528750.t1.2 | A8J597 | L12 | + | − | − | + |
| Cre13.g568650.t1.2 | A8HS48 | S3a | + | + | − | + | Cre14.g630100.t1.2 | A8IUV7 | L13 | + | + | + | + |
| Cre09.g400650.t1.2 | A8J1G8 | S6 | + | + | + | + | Cre12.g532550.t1.1 | A8IVU0 | L13a | + | − | − | + |
| Cre11.g467578.t1.1 | A8JF05 | S8 | + | + | + | + | Cre17.g701200.t2.1 | A8IQE3 | L14 | + | + | − | + |
| Cre04.g214503.t1.1 | A8J9T0 | S12 | + | − | − | − | Cre12.g512600.t1.2 | A8IKZ2 | L18 | + | + | − | + |
| Cre07.g331900.t1.2 | A8IGY1 | S13 | + | + | − | + | Cre02.g075700.t1.2 | A8IA18 | L19 | + | + | + | + |
| Cre12.g498250.t1.2 | A8JGK1 | S17 | + | + | + | + | Cre06.g278135.t1.1 | A8J951 | L21 | + | + | + | + |
| Cre16.g660150.t1.2 | A8J8M9 | S20 | + | + | − | + | Cre07.g357850.t1.2 | A8JI94 | L22 | + | + | − | + |
| Cre03.g203450.t1.2 | A8IXG3 | S21 | + | − | − | − | Cre09.g391097.t2.1 | A8J1A3 | L24 | + | + | + | + |
| Cre10.g456200.t1.2 | A8I0I1 | S24 | + | + | + | + | Cre01.g040000.t1.2 | A8HMG7 | L26 | + | + | + | + |
| Cre08.g382500.t1.2 | A8IZ36 | S25 | + | + | + | + | Cre11.g467578.t1.1 | A8JF05 | L28 | + | + | + | + |
| Cre06.g273600.t1.2 | A8HVK4 | S27a | + | + | + | + | NF | A8ILG8 | L31 | + | + | − | + |
| Cre12.g510450.t1.2 | A8IKP1 | S28 | + | − | − | + | Cre14.g617900.t1.2 | A8HNX3 | L35 | + | − | − | + |
| Cre08.g358556.t1.1 | A8JIE5 | S29 | − | − | − | + | Cre12.g484050.t1.2 | A8JHU2 | L36 | + | − | + | + |
| Cre16.g666301.t1.2 | A8JF66 | S30 | + | + | + | + | Cre06.g310700.t1.2 | A8IM74 | L36a | + | − | − | + |
| Cre14.g621450.t1.2 | A8HP55 | L5 | + | + | − | + | Cre10.g430400.t1.2 | A8IBG1 | L37 | + | + | + | + |
| Cre01.g011000.t1.2 | A8HP90 | L6 | + | + | − | + | Cre06.g257150.t1.2 | A8HY08 | L37a | + | − | − | + |
| Cre12.g529651.t1.1 | A8J567 | L7a | + | + | − | + | Cre07.g325746.t1.1 | Q8GUQ9 | L38 | + | − | − | + |
| Cre12.g535851.t1.1 | A8IVK1 | L8 | + | + | + | + | NF | A8J1Q3 | L39 | + | + | + | + |
| NF | P50884 | L12 | + | − | − | + | Cre01.g007051.t1.2 | A8JCX9 | L40 | + | + | − | + |
| Cre12.g520500.t1.1 | A8J5Z0 | P0 | + | + | − | + | Cre02.g143050.t1.2 | A8J0R4 | P2 | + | + | + | − |
Thirteen flagellar associated proteins over the 29 predicted to be highly flexible, were found in the phosphoproteome, in agreement with previous reports showing that phosphorylation is a key modification involved in flagellar assembly/disassembly in C. reinhardtii69,70. We also found 27 disordered proteins that play a very critical role in cytoskeleton assembly.
IDPs in C. reinhardtii may provide a fast and efficient mechanism to respond to changing environmental conditions and therefore play very important roles as described in other organisms53. Indeed, we also found many IDPs involved in the regulation of translation and transcription.
To conclude, although disorder is emerging to have numerous important functions in a cell, in plants it has been largely understudied, and the work reported here is the first large-scale experimental investigation of the intrinsically disordered proteome in C. reinhardtii. Indeed, few IDPs have been biochemically characterized in C. reinhardtii, CP1243,71–73, EPYC146 and an IDR-containing protein, adenylate kinase 374. In this work, a central pipeline for the extraction, identification, characterization and analysis of IDPs was developed. Taken together, our experimental results and bioinformatic analysis lead to a greater knowledge of IDPs and show that structural flexibility is widespread, and likely important, in many biological processes in C. reinhardtii.
Methods
Protein extraction
C. reinhardtii was grown mixotrophically in Tris-acetate-phosphate (TAP) medium at 25 °C with vigorous shaking 90 rpm under 50 µmol photon m−2 s−1 photosynthetically active radiation75. Cultures (50 mL, 5 replicates) from the exponential phase were centrifuged at 4,000 g for 15 min at 4 °C (Beckman Coulter Allegra® X-15R Centrifuge (Pasadena, CA, USA); rotor: 4750 A), then stored at −80 °C. Cells were broken by sonication in lysis buffer (15 mM Tris, 0.1 mM EDTA at pH 7.5) supplemented by protease inhibitors 40 µg mL−1 (Sigma-Aldrich, P2714); the homogenate was centrifuged at 11,000 g, 4 °C for 30 min (2–16KC centrifuge using a 12132-H rotor, Sigma-Aldrich, Saint-Louis, MO, USA) to isolate the supernatant that mainly contained non-membrane proteins.
Acid and heat treatments
Acid-resistant proteins were extracted by treating with 10% perchloric acid (PCA) or 5% trichloroacetic acid (TCA), on ice for 15 min. The heat-resistant proteins were extracted by boiling the samples at 98 °C for 5 min, 30 min and 1 h. After cooling to room temperature, the insoluble fractions were removed by centrifugation at 11,000 g, 4 °C for 30 min (2–16KC centrifuge using a 12132-H rotor, Sigma-Aldrich, Saint-Louis, MO, USA) and thus, only the soluble extracts containing heat-resistant or acid-resistant proteins were kept and analyzed further35. Protein concentration was determined, using the Bio-Rad reagent protein assay (Bio-Rad Laboratories, Hercules, CA, USA) with bovine serum albumin as a standard.
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis (PAGE) of soluble proteins
Protein migration was performed on 12% polyacrylamide gel Mini-PROTEAN® Tetra Cell (Biorad, Hercules, USA). Extracts were incubated for 5 min at 94 °C with 10% SDS, 10 mM DTT, 20% glycerol, 0.2 M Tris-HCl pH 6.8 and 0.05% Bromophenol Blue and 10 µg of each heated-sample were loaded onto the gels. After running, the gels were stained with Coomassie Brilliant Blue R250.
Identification of proteins by mass spectrometry
The most intense bands separated by SDS-PAGE were cut and submitted to trypsin digestion as previously described76. LC-MS/MS analyses were performed on an ESI-Q-Exactive plus mass spectrometer (ThermoFisher) coupled to a nano liquid chromatography (Ultimate 3000, Dionex). Solubilized tryptic peptides in 0.05% (v/v) TFA/2% (v/v) acetonitrile were loaded onto a nano trap (Acclaim PepMap100, 100 µm × 2 cm, 5 µm, 100 Å, Dionex) before elution onto a C18 column (Acclaim PepMap RSLC, 75 µm × 150 mm, 2 µm, 100 Å, Dionex). A linear gradient from 6% to 40% of mobile phase B (0.1% (v/v) formic acid (FA)/80% (v/v) acetonitrile) in mobile phase A (0.1% (v/v) FA) was applied for 52 min. The peptides were detected into the mass spectrometer in a positive ion mode, using a Top 10 Data Dependent workflow with a 60 s dynamic exclusion. One scan event full MS in the Orbitrap at 70 000, in a 350–1900 m/z range, was followed by a fragmentation MS/MS step, at 17 500, of the 10 top ions, in the Higher Energy Collisional Dissociation cell set at 27.
For protein identification, spectra were processed by the Proteome Discoverer software (Thermo Fisher Scientific, versions: 1.4.0.288 and 2.1.0.81) using the Sequest HT algorithm including the Protein Center annotation aspects (biological process, cellular component, molecular function).
To identify the heat-resistant proteins, the search was performed using C. reinhardtii databank (Taxonomy ID 3055, 15313 sequence entries) downloaded from the non-redundant NCBI databank and/or from Phytozome v12.1 (19526 sequence entries). The following parameters were set: enzyme: trypsin; dynamic modifications: oxidation/+ 15.995 Da (M), phosphorylation/+ 79.966 Da (Y, S, T), static modification: carbamidomethyl/+ 57.021 Da (C); mass values: monoisotopic; precursor mass tolerance: ± 10 ppm; fragment mass tolerance: ± 0.02 Da; missed cleavages: 2. Proteins were considered as identified by 2 unique “rank 1” peptides, as shown by the two best Peptide Spectrum Matches (PSM), passing the high confidence filter, with validation on q-Value (Strict Target FDR: 0.01) and maximum Delta Cn: 0.05.
Computational evaluation of disorder
We selected proteins with PSM higher than two and removed proteins where only partial sequence was available. We used four different algorithms47: (i) PONDR VL-XT (http://www.pondr.com/cgi-bin/PONDR/pondr.cgi) that is based on artificial neural networks, using a variety of physiochemical properties of the input protein chain including amino acid compositions, aromaticity, flexibility, hydropathy, and hydrophobicity77; (ii) FoldIndex (http://bip.weizmann.ac.il/fldbin/findex) that is based on the average residue hydrophobicity and net charge of the sequence78; (iii) DisEMBL Remark-465 (http://dis.embl.de/) that is also a method based on artificial neural networks trained for predicting several definitions of disorder, in particular, it is trained on evolutionarily conserved sequence features of disordered regions that have missing residues in high-resolution X-ray structures; (iv) IUPred (http://iupred.enzim.hu/) that predicts intrinsic disorder based solely on propensities/properties of amino acids of the input protein sequences79. In our study we therefore used these different types of algorithms to increase reliability of our analysis.
Cellular compartment
To predict where the proteins were targeted (mitochondrion, chloroplast, and other compartments), PredAlgo (https://giavap-genomes.ibpc.fr), the most reliable software for Chlamydomonas and related green algae species (Chlorophyta), was used80.
Amino acid residues comparison
The comparison of the amino acid composition of the different sets of proteins from C. reinhardtii was analyzed by Composition profiler (http://www.cprofiler.org/)24,25.
Functional classification
The molecular function of each disordered protein was attributed according to the Gene Ontology (http://geneontology.org/) and JGI Genome Portal (http://genome.jgi.doe.gov/)54,81.
Electronic supplementary material
Supplemental Data Fig S1 Tables S2 and S3
List of the 682 heat-resistant proteins experimentally identified as potential IDPs, from Chlamydomonas reinhardtii.
List of the C. reinhardtii proteins (full length) and their cellular compartment predicted by PredAlgo.
Acknowledgements
YZ was supported by Chinese Scholarship Council (201404910544). HL was supported by French National Research Agency (ANR-13-BSV5-0013). AS was supported by the Ministère de l′Education Nationale, de la Recherche et de la Technologie (MRT). Financial support was provided by CNRS, Aix Marseille Université, the Region PACA, IBiSA (BG). We thank Vincent and Schnell for kindly sharing their database and Véronique Brechot for helpful discussion. The authors are grateful to Pr. Stephen Maberly, native English speaker who read our manuscript and made helpful comments.
Author Contributions
Y.Z., R.L. and B.G. planned and designed the research. Y.Z. and R.L. performed the experiments. Y.Z., H.L., A.S. and B.G. analyzed the data. Y.Z., H.L. and B.G. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/29/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24772-7.
References
- 1.Dunker AK, et al. What’s in a name? Why these proteins are intrinsically disordered: Why these proteins are intrinsically disordered. Intrinsically Disordered Proteins. 2013;1:e24157. doi: 10.4161/idp.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uversky VN. Unreported intrinsic disorder in proteins: Building connections to the literature on IDPs. Intrinsically Disordered Proteins. 2014;2:1–42. doi: 10.4161/21690693.2014.970499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;293:321–331. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- 4.Habchi J, Tompa P, Longhi S, Uversky VN. Introducing protein intrinsic disorder. Cheml Rev. 2014;114:6561–6588. doi: 10.1021/cr400514h. [DOI] [PubMed] [Google Scholar]
- 5.Campen A, et al. TOP-IDP-scale: a new amino acid scale measuring propensity for intrinsic disorder. Protein Pept Lett. 2008;15:956. doi: 10.2174/092986608785849164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iakoucheva LM, et al. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32:1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathura, V., et al, The protein non-folding problem: amino acid determinants of intrinsic order and disorder. Paper presented at Pacific Symposium on Biocomputing: Disorder and flexibility in protein structure and function, Mauna Lani, Hawaii, USA, World Scientific publisher, 2001, January. [DOI] [PubMed]
- 8.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–533. doi: 10.1016/S0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 9.Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269:2–12. doi: 10.1046/j.0014-2956.2001.02649.x. [DOI] [PubMed] [Google Scholar]
- 10.Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol. 2004;337:635–645. doi: 10.1016/j.jmb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Yegambaram K, Bulloch E, Kingston R. Protein domain definition should allow for conditional disorder. Protein Sci. 2013;22:1502–1518. doi: 10.1002/pro.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurotani A, et al. Correlations between predicted protein disorder and post-translational modifications in plants. Bioinformatics. 2014;30:1095–1103. doi: 10.1093/bioinformatics/btt762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakob U, Kriwacki R, Uversky VN. Conditionally and transiently disordered proteins: awakening cryptic disorder to regulate protein function. Chem Rev. 2014;114:6779–6805. doi: 10.1021/cr400459c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, et al. The global phosphoproteome of Chlamydomonas reinhardtii reveals complex organellar phosphorylation in the flagella and thylakoid membrane. Mol Cell Proteomics. 2014;13:2337–2353. doi: 10.1074/mcp.M114.038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 16.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 2008;37:215–246. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 17.Xue B, Dunker AK, Uversky VN. Orderly order in protein intrinsic disorder distribution: disorder in 3500 proteomes from viruses and the three domains of life. J Biomol Struct Dyn. 2012;30:137–149. doi: 10.1080/07391102.2012.675145. [DOI] [PubMed] [Google Scholar]
- 18.Schad E, Tompa P, Hegyi H. The relationship between proteome size, structural disorder and organism complexity. Genome Biol. 2011;12:1–13. doi: 10.1186/gb-2011-12-12-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Z, et al. Exceptionally abundant exceptions: comprehensive characterization of intrinsic disorder in all domains of life. Cell Mol Life Sci. 2015;72:137–151. doi: 10.1007/s00018-014-1661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yruela I. Plant development regulation: Overview and perspectives. J Plant Physiol. 2015;182:62–78. doi: 10.1016/j.jplph.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Yruela I, Oldfield CJ, Niklas KJ, Dunker AK. Evidence for a strong correlation between transcription factor protein disorder and organismic complexity. Genome Biol Evol. 2017;9:1248–1265. doi: 10.1093/gbe/evx073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niklas KJ, Bondos SE, Dunker AK, Newman SA. Rethinking gene regulatory networks in light of alternative splicing, intrinsically disordered protein domains, and post-translational modifications. Front Cell Dev Biol. 2015;3:1–13. doi: 10.3389/fcell.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunker AK, Bondos SE, Huang F, Oldfield CJ. Intrinsically disordered proteins and multicellular organisms. Semin Cell Dev Biol. 2015;37:44–55. doi: 10.1016/j.semcdb.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Piovesan D, et al. DisProt 7.0: a major update of the database of disordered proteins. Nucleic Acids Res. 2017;45:D219–D227. doi: 10.1093/nar/gkw1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sickmeier M, et al. DisProt: the Database of Disordered Proteins. Nucleic Acids Res. 2007;35:D786–D793. doi: 10.1093/nar/gkl893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacs D, Kalmar E, Torok Z, Tompa P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 2008;147:381–390. doi: 10.1104/pp.108.118208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tompa P, Kovacs D. Intrinsically disordered chaperones in plants and animals. Biochem Cell Biol. 2010;88:167–174. doi: 10.1139/O09-163. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Rikkerink EH, Jones WT, Uversky VN. Multifarious roles of intrinsic disorder in proteins illustrate its broad impact on plant biology. Plant Cell. 2013;25:38–55. doi: 10.1105/tpc.112.106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, et al. A functionally required unfoldome from the plant kingdom: intrinsically disordered N-terminal domains of GRAS proteins are involved in molecular recognition during plant development. Plant Mol Biol. 2011;77:205–223. doi: 10.1007/s11103-011-9803-z. [DOI] [PubMed] [Google Scholar]
- 30.Pazos F, Pietrosemoli N, García-Martín JA, Solano R. Protein intrinsic disorder in plants. Front Plant Sci. 2013;4:1–5. doi: 10.3389/fpls.2013.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Covarrubias AA, Cuevas-Velazquez CL, Romero-Pérez PS, Rendón-Luna DF, Chater CC. Structural disorder in plant proteins: where plasticity meets sessility. Cell Mol Life Sci. 2017;74:3119–3147. doi: 10.1007/s00018-017-2557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietrosemoli N, García-Martín JA, Solano R, Pazos F. Genome-wide analysis of protein disorder in Arabidopsis thaliana: implications for plant environmental adaptation. PloS one. 2013;8:e55524. doi: 10.1371/journal.pone.0055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent M, Schnell S. A collection of intrinsic disorder characterizations from eukaryotic proteomes. Sci Data. 2016;3:1–9. doi: 10.1038/sdata.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csizmók V, Dosztányi Z, Simon I, Tompa P. Towards proteomic approaches for the identification of structural disorder. Curr Protein Pept Sci. 2007;8:173–179. doi: 10.2174/138920307780363479. [DOI] [PubMed] [Google Scholar]
- 35.Cortese MS, Baird JP, Uversky VN, Dunker AK. Uncovering the unfoldome: enriching cell extracts for unstructured proteins by acid treatment. J Proteome Res. 2005;4:1610–1618. doi: 10.1021/pr050119c. [DOI] [PubMed] [Google Scholar]
- 36.Galea CA, et al. Proteomic studies of the intrinsically unstructured mammalian proteome. J Proteome Res. 2006;5:2839–2848. doi: 10.1021/pr060328c. [DOI] [PubMed] [Google Scholar]
- 37.Irar S, Oliveira E, Goday A. Towards the identification of late-embryogenic-abundant phosphoproteome in Arabidopsis by 2-DE and MS. Proteomics. 2006;6:175–185. doi: 10.1002/pmic.200500387. [DOI] [PubMed] [Google Scholar]
- 38.Rochaix J-D. Chlamydomonas reinhardtii as the photosynthetic yeast. Annual Review of Genetics. 1995;29:209–230. doi: 10.1146/annurev.ge.29.120195.001233. [DOI] [PubMed] [Google Scholar]
- 39.Avilan L, et al. Regulation of glyceraldehyde-3-phosphate dehydrogenase in the eustigmatophyte Pseudocharaciopsis ovalis is intermediate between a chlorophyte and a diatom. Eur J Phycol. 2012;47:207–215. doi: 10.1080/09670262.2012.687459. [DOI] [Google Scholar]
- 40.Graciet E, et al. The small protein CP12: a protein linker for supramolecular complex assembly. Biochem. 2003;42:8163–8170. doi: 10.1021/bi034474x. [DOI] [PubMed] [Google Scholar]
- 41.Launay H, et al. Absence of residual structure in the intrinsically disordered regulatory protein CP12 in its reduced state. Biochem Biophys Res Commun. 2016;477:20–26. doi: 10.1016/j.bbrc.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Moparthi SB, et al. FRET analysis of CP12 structural interplay by GAPDH and PRK. Biochem Biophys Res Commun. 2015;458:488–493. doi: 10.1016/j.bbrc.2015.01.135. [DOI] [PubMed] [Google Scholar]
- 43.Gontero B, Maberly SC. An intrinsically disordered protein, CP12: jack of all trades and master of the Calvin cycle. Biochem Soc Trans. 2012;40:995–999. doi: 10.1042/BST20120097. [DOI] [PubMed] [Google Scholar]
- 44.Erales J, Lignon S, Gontero B. CP12 from Chlamydomonas reinhardtii, a permanent specific “chaperone-like” protein of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 2009;284:12735–12744. doi: 10.1074/jbc.M808254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delobel A, et al. Mass spectrometric analysis of the interactions between CP12, a chloroplast protein, and metal ions: a possible regulatory role within a PRK/GAPDH/CP12 complex. Rapid Commun Mass Spectrom. 2005;19:3379–3388. doi: 10.1002/rcm.2192. [DOI] [PubMed] [Google Scholar]
- 46.Mackinder LC, et al. A repeat protein links Rubisco to form the eukaryotic carbon-concentrating organelle. Proc Natl Acad Sci USA. 2016;113:5958–5963. doi: 10.1073/pnas.1522866113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng F, Uversky V, Kurgan L. Comprehensive review of methods for prediction of intrinsic disorder and its molecular functions. Cell Mol Life Sci. 2017;74:3069–3090. doi: 10.1007/s00018-017-2555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bah A, Forman-Kay JD. Modulation of intrinsically disordered protein function by post-translational modifications. J Biol Chem. 2016;291:6696–6705. doi: 10.1074/jbc.R115.695056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haynes C, Iakoucheva LM. Serine/arginine-rich splicing factors belong to a class of intrinsically disordered proteins. Nucleic Acids Res. 2006;34:305–312. doi: 10.1093/nar/gkj424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanford JR, Bruzik JP. Developmental regulation of SR protein phosphorylation and activity. Genes Dev. 1999;13:1513–1518. doi: 10.1101/gad.13.12.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werth EG, et al. Probing the global kinome and phosphoproteome in Chlamydomonas reinhardtii via sequential enrichment and quantitative proteomics. Plant J. 2017;89:416–426. doi: 10.1111/tpj.13384. [DOI] [PubMed] [Google Scholar]
- 52.Kurotani A, Sakurai T. In silico analysis of correlations between protein disorder and post-translational modifications in algae. Int J Mol Sci. 2015;16:19812–19835. doi: 10.3390/ijms160819812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yruela I, Contreras-Moreira B. Protein disorder in plants: a view from the chloroplast. BMC Plant Biol. 2012;12:165–176. doi: 10.1186/1471-2229-12-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Botstein D, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yruela I, Contreras-Moreira B. Genetic recombination is associated with intrinsic disorder in plant proteomes. BMC genomics. 2013;14:772–781. doi: 10.1186/1471-2164-14-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bardwell JC, Jakob U. Conditional disorder in chaperone action. Trends Biochem Sci. 2012;37:517–525. doi: 10.1016/j.tibs.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cremers CM, Reichmann D, Hausmann J, Ilbert M, Jakob U. Unfolding of metastable linker region is at the core of Hsp33 activation as a redox-regulated chaperone. J Biol Chem. 2010;285:11243–11251. doi: 10.1074/jbc.M109.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ilbert M, et al. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat Struct Mol Biol. 2007;14:556–563. doi: 10.1038/nsmb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reichmann D, et al. Order out of disorder: working cycle of an intrinsically unfolded chaperone. Cell. 2012;148:947–957. doi: 10.1016/j.cell.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segal N. a. & Shapira, M. HSP33 in eukaryotes – an evolutionary tale of a chaperone adapted to photosynthetic organisms. Plant J. 2015;82:850–860. doi: 10.1111/tpj.12855. [DOI] [PubMed] [Google Scholar]
- 61.Borges JC, Seraphim TV, Dores-Silva PR, Barbosa LR. A review of multi-domain and flexible molecular chaperones studies by small-angle X-ray scattering. Biophys Rev. 2016;8:107–120. doi: 10.1007/s12551-016-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Genest O, Hoskins JR, Camberg JL, Doyle SM, Wickner S. Heat shock protein 90 from Escherichia coli collaborates with the DnaK chaperone system in client protein remodeling. Proc Natl Acad Sci USA. 2011;108:8206–8211. doi: 10.1073/pnas.1104703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genest O, Hoskins JR, Kravats AN, Doyle SM, Wickner S. Hsp70 and Hsp90 of E. coli directly interact for collaboration in protein remodeling. J Mol Biol. 2015;427:3877–3889. doi: 10.1016/j.jmb.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beligni MV, Yamaguchi K, Mayfield SP. The translational apparatus of Chlamydomonas reinhardtii chloroplast. Photosynth Res. 2004;82:315–325. doi: 10.1007/s11120-004-2440-5. [DOI] [PubMed] [Google Scholar]
- 65.Christodoulou J, et al. Heteronuclear NMR investigations of dynamic regions of intact Escherichia coli ribosomes. Proc Natl Acad Sci USA. 2004;101:10949–10954. doi: 10.1073/pnas.0400928101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen JC, Lu X, Ross ED, Woody RW. Intrinsic protein disorder, amino acid composition, and histone terminal domains. J Biol Chem. 2006;281:1853–1856. doi: 10.1074/jbc.R500022200. [DOI] [PubMed] [Google Scholar]
- 67.Lazar T, et al. Intrinsic protein disorder in histone lysine methylation. Biol Direct. 2016;11:30–39. doi: 10.1186/s13062-016-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng Z, Mizianty MJ, Xue B, Kurgan L, Uversky VN. More than just tails: intrinsic disorder in histone proteins. Mol BioSyst. 2012;8:1886–1901. doi: 10.1039/c2mb25102g. [DOI] [PubMed] [Google Scholar]
- 69.Liu G, Huang K. Phosphorylation regulates the disassembly of cilia. Sci China Life Sci. 2015;58:621–623. doi: 10.1007/s11427-015-4874-8. [DOI] [PubMed] [Google Scholar]
- 70.Pan J, et al. Protein phosphorylation is a key event of flagellar disassembly revealed by analysis of flagellar phosphoproteins during flagellar shortening in Chlamydomonas. J Proteome Res. 2011;10:3830–3839. doi: 10.1021/pr200428n. [DOI] [PubMed] [Google Scholar]
- 71.Del Giudice A, et al. Unravelling the shape and structural assembly of the photosynthetic GAPDH-CP12-PRK complex from Arabidopsis thaliana by small-angle X-ray scattering analysis. Acta Crystallogr D Biol Crystallogr. 2015;71:2372–2385. doi: 10.1107/S1399004715018520. [DOI] [PubMed] [Google Scholar]
- 72.Fermani S, et al. Conformational selection and folding-upon-binding of intrinsically disordered protein CP12 regulate photosynthetic enzymes assembly. J Biol Chem. 2012;287:21372–21383. doi: 10.1074/jbc.M112.350355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marri L, et al. In vitro characterization of Arabidopsis CP12 isoforms reveals common biochemical and molecular properties. J Plant Physiol. 2010;167:939–950. doi: 10.1016/j.jplph.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Thieulin-Pardo G, et al. The intriguing CP12-like tail of adenylate kinase 3 from Chlamydomonas reinhardtii. FEBS J. 2016;283:3389–3407. doi: 10.1111/febs.13814. [DOI] [PubMed] [Google Scholar]
- 75.Avilan L, Gontero B, Lebreton S, Ricard J. Memory and imprinting effects in multienzyme complexes. Eur J Biochem. 1997;246:78–84. doi: 10.1111/j.1432-1033.1997.00078.x. [DOI] [PubMed] [Google Scholar]
- 76.Abdelkafi S, et al. Identification and biochemical characterization of a GDSL-motif carboxylester hydrolase from Carica papaya latex. Biochim Biophys Acta Mol Cell Biol Lipids. 2009;1791:1048–1056. doi: 10.1016/j.bbalip.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Peng K, et al. Optimizing long intrinsic disorder predictors with protein evolutionary information. J Bioinform Comput Biol. 2005;03:35–60. doi: 10.1142/S0219720005000886. [DOI] [PubMed] [Google Scholar]
- 78.Prilusky J, et al. FoldIndex©: a simple tool to predict whether a given protein sequence is intrinsically unfolded. Bioinformatics. 2005;21:3435–3438. doi: 10.1093/bioinformatics/bti537. [DOI] [PubMed] [Google Scholar]
- 79.Radivojac P, et al. Intrinsic disorder and functional proteomics. Biophys J. 2007;92:1439–1456. doi: 10.1529/biophysj.106.094045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tardif M, et al. PredAlgo: a new subcellular localization prediction tool dedicated to green algae. Mol Biol Evol. 2012;29:3625–3639. doi: 10.1093/molbev/mss178. [DOI] [PubMed] [Google Scholar]
- 81.Merchant SS, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Sci. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vacic V, Uversky VN, Dunker AK, Lonardi S. Composition Profiler: a tool for discovery and visualization of amino acid composition differences. BMC Bioinformatics. 2007;8:1471–2105. doi: 10.1186/1471-2105-8-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berman HM, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data Fig S1 Tables S2 and S3
List of the 682 heat-resistant proteins experimentally identified as potential IDPs, from Chlamydomonas reinhardtii.
List of the C. reinhardtii proteins (full length) and their cellular compartment predicted by PredAlgo.