Abstract
Acute lung injury (ALI) leads to progressive loss of breathing capacity and hypoxemia, as well as pulmonary surfactant dysfunction. ALI’s pathogenesis and management are complex, and it is a significant cause of morbidity and mortality worldwide. Exogenous surfactant therapy, even for research purposes, is impractical for adults because of the high cost of current surfactant preparations. Prior in vitro work has shown that poly-N-substituted glycines (peptoids), in a biomimetic lipid mixture, emulate key biophysical activities of lung surfactant proteins B and C at the air-water interface. Here we report good in vivo efficacy of a peptoid-based surfactant, compared with extracted animal surfactant and a synthetic lipid formulation, in a rat model of lavage-induced ALI. Adult rats were subjected to whole-lung lavage followed by administration of surfactant formulations and monitoring of outcomes. Treatment with a surfactant protein C mimic formulation improved blood oxygenation, blood pH, shunt fraction, and peak inspiratory pressure to a greater degree than surfactant protein B mimic or combined formulations. All peptoid-enhanced treatment groups showed improved outcomes compared to synthetic lipids alone, and some formulations improved outcomes to a similar extent as animal-derived surfactant. Robust biophysical mimics of natural surfactant proteins may enable new medical research in ALI treatment.
Introduction
Lung surfactant is a complex lipid-protein mixture that coats alveolar surfaces and reduces surface tension at the air/liquid interface, enabling normal respiratory function1. The absence or inactivation of lung surfactant results in lung collapse and respiratory distress syndrome (RDS)2,3. The lack of surfactant related to infant RDS (IRDS)4 is routinely treated with animal-derived exogenous surfactant, which improves outcomes1,4,5. Acute lung injury (ALI) is a more complex disease resulting from a diverse set of etiologies2,6,7. Lung inflammation and alterations to endogenous surfactant result in hypoxemia and decreasing pulmonary function2,8.
Progressive lung dysfunction results from alterations in the lipid profile and surfactant-associated protein amounts, or changes in the relative amounts of surfactant aggregate forms9,10. Animal and clinical pilot studies suggest a sound rationale for using surfactant replacement therapy to mitigate some of these changes in a subset of ALI patients2,7 but unfortunately, larger Phase III clinical trials have had variable outcomes11–13. Factors contributing to inconsistent clinical results include: 1) mode, dose, and timing of surfactant delivery, 2) post-administration ventilation strategy, and 3) surfactant characteristics2. Reduced alveolar delivery of the surfactant has also been suggested as a contributing factor based on recent computer modeling14.
The biophysical activity, biostability/bioavailability, metabolic fate, and availability and cost of exogenous surfactant preparations impact their suitability for treatment of ALI patients. “Biomimetic” synthetic surfactants, designed to emulate features of animal-derived surfactant, are an emerging class of treatments that could provide benefits for ALI patients, as well as address the resource limitations inherent in animal-derived medications. Lipid and protein components are essential to animal-derived surfactant function. Lung surfactant proteins B and C (SP-B and SP-C) are two hydrophobic proteins essential for the adsorption and spreading of the surfactant film at the air-liquid interface15. These proteins have proven challenging to synthesize in active form (either chemically or recombinantly), thus most research has focused on development of simpler analogs of these proteins that replicate key features for bioactivity16–18.
Biomimetic surfactants comprising lipids and mimics of SP-B or SP-C made with helical, poly-N-substituted glycines (peptoids) exhibit promising in vitro biophysical activity19–23. Peptoids are non-natural compounds with a polypeptide backbone, but with side chains appended to the backbone nitrogens rather than the α-carbons24. Peptoid structure is advantageous for biomedical applications; their highly stable structure is protease-resistant, thus improving biostability and bioavailability while reducing immunogenic response; and they can be designed to form stable amphipathic helices25.
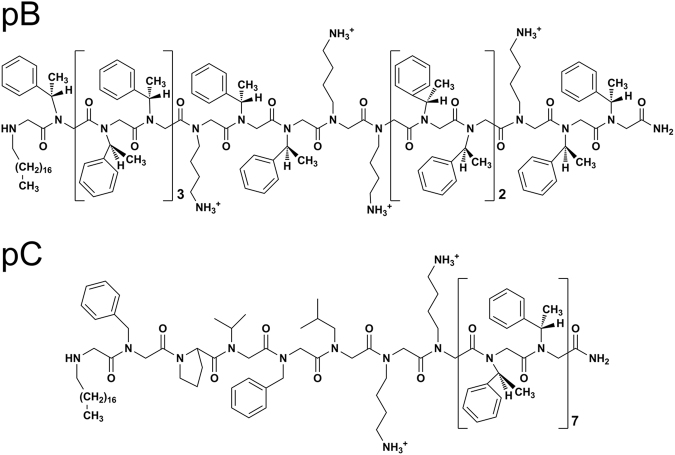
We examined the ability of peptoid-based biophysical mimics of SP-B and/or SP-C, termed pB and pC, to mitigate deleterious physiological and biochemical responses associated with ALI. pB and pC (Fig. 1) were designed with sequence attributes that mimic the overall hydrophobicity, amphipathicity, and helical structures of SP-B and SP-C, respectively26,27. Both molecules are N-terminally C18-alkylated to mimic the palmitoyl moieties of natural SP-C, a feature known to improve in vitro surface activity26,27. The design of the SP-B mimic is more loosely biomimetic of the natural protein, while the SP-C mimic is more similar to the natural protein. Our hypothesis was that peptoid-enhanced surfactants could demonstrate in vivo efficacy in an animal model of ALI that matches or exceeds that of animal-derived surfactant.
Figure 1.
Chemical structures of peptoid-based mimics of SP-B (pB)26 and SP-C (pC)27. The eight N-terminal residues of pC contain side chains that are analogous to SP-C5–12, and the remaining 14 aromatic hydrophobic residues form a helix that mimics the membrane spanning, hydrophobic helix of native SP-C. The N-terminal octadecyl amine of pC is a motif intended to mimic the post-translational modification of palmitoylated residues 5 and 6 in human SP-C. The mimic pB was designed to emulate the insertion region and helical amphipathic patterning of SP-B1-25, with the added feature of an N-terminal octadecylamine.
Methods
Peptoid Synthesis and Surfactant Preparation
Peptoids were synthesized according to previously published protocols19,26,28. Tanaka lipids (DPPC:POPG:PA 68:22:9 [wt]) were prepared and dried26,29, with 2 mol% peptoid (1 mol% each for pB/pC). On the day of the experiment, sterile saline was added to the dehydrated surfactant mixture to make a homogeneous, fluid lipid-peptoid surfactant suspension at a concentration of 25 mg/mL. Additional details can be found in the Supplementary Information.
Animal Experiments
Procedures were approved by the Animal Use Subcommittee (AUS) at University of Western Ontario, London, Ontario, Canada, according to the Canadian Council on Animal Care (CCAC). All experiments were performed in accordance with relevant guidelines and regulations. Sprague-Dawley rats (360–410 g) (Charles River, St. Constant, PQ, Canada) were weighed, anesthetized by intraperitoneal (i.p.) injection (75 mg/kg ketamine hydrochloride, 5 mg/kg xylazine, sterile 0.15 M saline), and given 0.05–0.1 mg/kg of buprenorphine subcutaneously. Right carotid artery access via isolation and cannulation permitted blood gas sampling, measuring vital signs, and instilling fluids (0.15 M saline, 0.5 mL/kg/hour), while similarly obtained jugular venous line access allowed administering of ~1.0 mg/kg/min propofol and fluids. Following tracheostomy and endotracheal tube placement, pancuronium bromide (1 mg/kg i.v.) was administered to inhibit spontaneous movement. Ventilator settings were: tidal volume, 8 mL/kg; positive end expiratory pressure (PEEP), 5 cm H2O; respiratory rate, 55–60 breaths/minute; and fraction of inspired oxygen (FiO2), 1.0 (volume-cycle mechanical rodent ventilator, Harvard Instruments, St. Laurent, PQ, Canada; airway pressure monitor, Caradyne Ltd, Indianapolis, IN). Initial inclusion criterion was baseline average blood oxygen level (PaO2) >400 mmHg.
Whole lung lavage was performed30–33. After ventilator removal, 0.15 M NaCl (10 mL, 37 °C) was instilled/withdrawn from the lungs, followed by mechanical ventilation. Lungs were lavaged four times, 5 minutes apart preceding a blood gas measurement. Study inclusion required PaO2 <120 mmHg. Non-inclusive animals were re-lavaged until the inclusion criterion was satisfied30.
Animals were randomized into five treatment groups: 1) bovine lipid extract surfactant (BLES, BLES Biochemicals, London, Ontario, CA, the more widely utilized of the two bovine extracted surfactants available in Canada34,35 and the market leader in India and several countries outside of the USA and Europe) (positive control, n = 7), 2) pB (n = 6), 3) pC (n = 7), 4) pB/pC (n = 7), 5) Tanaka lipids (negative control, n = 5). Note that as a commercially available surfactant BLES was used as is while additional preparative work, described above, was needed to make the other surfactant treatments prior to dosing. After ventilator removal, upright animals were instilled with a 50 mg/kg surfactant bolus endotracheally via syringe, and then a 3-mL air bolus that ensured distribution to distal regions. This surfactant dosage was the same for all treatment groups. Ventilation and monitoring occurred for 2 hours, with blood gas sampling at semi-regular timepoints. Recovery vital signs were monitored for adequate perfusion, as was anesthetic state. Measured physiological responses included PaO2, blood pH, shunt fraction, Alveolar-arterial (A-a) gradient, and peak inspiratory pressure (PIP). Post-experiment, animals were euthanized via sodium pentobarbital, exsanguinated, the chest wall opened, lung-lavaged five times, and total lavage volume was recorded36.
Surfactant Analysis
After broncheoalveolar lavage fluid (BAL) centrifugation (150 g, 10 minutes), 5 mL of supernatant “Total Surfactant (TS)” was aliquoted for further analysis, and the remainder centrifuged (40,000 g, 15 minutes) to separate supernatant Small Aggregates (SA) from pellet. Resuspended pellets (in 2 mL saline) produced Large Aggregates (LA). Aliquots were extracted (Bligh/Dyer method37), phospholipids quantified (Duck-Chong phosphorous assay38), and BAL total protein content determined (micro-BCA protein assay, Pierce Biotechnology, Rockford, IL).
Statistical Analysis
Presented data are means ± SEM and were analyzed via one-way ANOVA using the Tukey-Kramer method (p < 0.05).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Physiological Responses
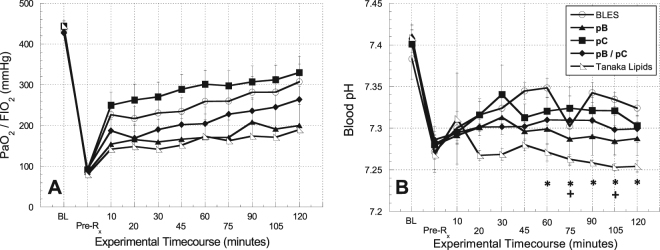
In general, the surgical procedure was well-tolerated by the animals. Three animals died during the procedure, and 13 animals did not meet the study inclusion criteria. For the 32 animals included in the study, the average baseline blood oxygen level (PaO2), normalized to the fraction of inspired oxygen (FiO2) (1.0 throughout all experiments), of 435.7 ± 4.9 mm Hg was reduced to 88.3 ± 2.5 mm Hg post-lavage, reflective of surfactant deficiency (Fig. 2A). Similarly, the average baseline peak inspiratory pressure (PIP) of 12.0 ± 0.3 cm H2O was increased to 20.8 ± 0.4 cm H2O post-lavage. The average blood pressure and heart rate for each treatment group over the time course of the experiments are shown in Supp. Figure S1.
Figure 2.
Physiological indicators of pulmonary gas exchange function over time. (A) PaO2/FIO2 and (B) Blood pH over the time course of the experiment. Error bars indicate the standard error of the mean (SEM). Statistical significance indicators: * indicates p < 0.05 between BLES treatment group and Tanaka Lipids; + indicates p < 0.05 between pC treatment group and Tanaka Lipids.
The average PaO2/FIO2 as a function of time is shown for each treatment group in Fig. 2A. The immediate response to surfactant treatment is reflected in changes from the pre-treatment (Pre-Rx) condition to the 10-minute data time point. The ability of surfactant treatments to sustain a response was gleaned by comparing the 10-minute time point data to conditions at the end of the two-hour observation period. Animals treated with the positive control BLES (p < 0.001), pC (p < 0.0005), and pB/pC (p < 0.007) showed a statistically significant, immediate improvement in oxygenation upon treatment. The immediate improvements demonstrated by the negative control Tanaka lipids group (p < 0.23) and pB group (p < 0.07) were not significant. Treatment with BLES (p < 0.16), pC (p < 0.15), and pB/pC (p < 0.11) also demonstrated better sustained oxygenation throughout the two-hour observation period compared with the pB (p < 0.47) and Tanaka lipid (p < 0.60) treatment groups. Animals in the pC treatment group were correlated with the highest arterial blood oxygenation levels throughout the study.
The blood pH as a function of time is shown for each treatment group in Fig. 2B. Comparing baseline to pre-treatment conditions, pulmonary lavage caused a significant (p < 0.005) and uniform lowering of the blood pH in all treatment groups. On average, the highest blood pH outcome was achieved by the BLES treatment group, which was found to be statistically different (p < 0.05) from the Tanaka lipids treatment group at t > 45 minutes. Among peptoid-enhanced surfactants, treatment with pC exhibited the most complete return to baseline conditions; the blood pH of this treatment group was statistically different (p < 0.05) from the Tanaka lipids treatment group at 75 and 105 minutes.
Figure 3 displays three additional indicators of pulmonary function, including shunt fraction, A-a gradient, and PIP. Ten minutes after treatment, the shunt fraction decreased significantly for the BLES (p < 0.0005), pC (0.001), and pB/pC (p < 0.01). The further decrease in shunt fraction observed from the 10-minute time point until the end of the two-hour observation period was statistically significant for the pC (p < 0.05) and pB/pC (p < 0.05) treatment groups. As shown in Fig. 3A, the pC treatment group was shown to be statistically different (p < 0.05) from the pB and Tanaka lipids treatment groups at selected timepoints. The A-a gradient data shown in Fig. 3B exhibits a statistically significant, immediate response for all treatment groups (p < 0.05) except Tanaka lipids (p < 0.20). The pC treatment group resulted in the most significant immediate decrease (p < 0.0003), but the pB/pC treatment group resulted in the best sustained response from the 10-minute time point throughout the observation period (p < 0.1). The PIP data shown in Fig. 3C demonstrated statistically significant immediate improvement at 10 minutes for BLES (p < 0.05) and pC (p < 0.01) (pB and pB/pC exhibited p < 0.1). The Tanaka lipid treatment group immediate improvement was not significant (p < 0.2). No treatment groups demonstrated sustained improvement of PIP throughout the 2-hour observation period.
Figure 3.
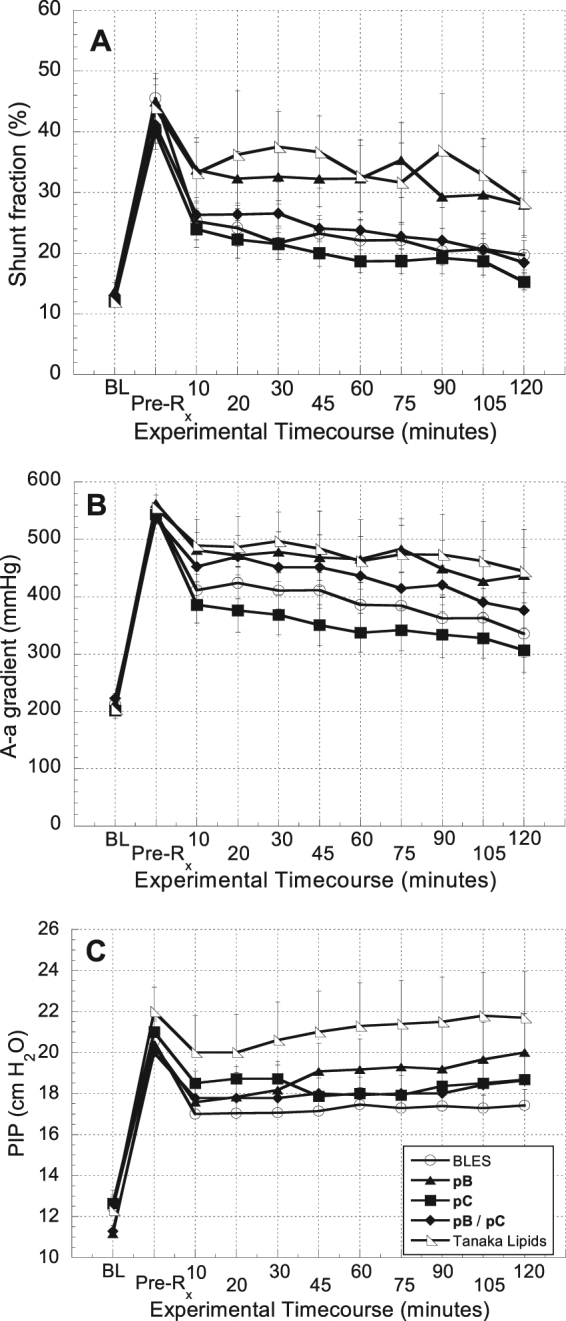
Physiological indicators of pulmonary function. (A) Shunt fraction (B) A-a gradient, and (C) Peak inspiratory pressure (PIP) over the time course of the experiments. Error bars indicate the standard error of the mean (SEM).
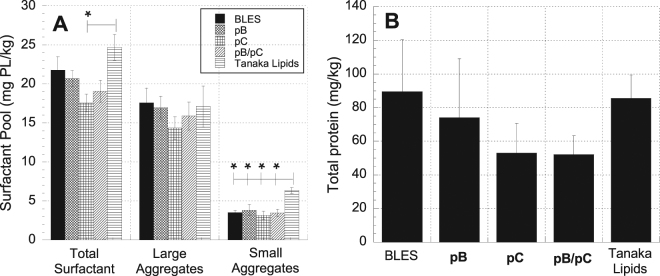
Surfactant Pool Evaluation
Since the efficacy of surfactant may be influenced by its metabolism within the airspace, phospholipid pools and total protein content in the broncheoalveolar lavage (BAL) fluid from each animal were evaluated at the end of the ventilation period. The average amount of total surfactant (TS), large aggregates (LA), and small aggregates (SA) obtained from the BAL of each treatment group is shown in Fig. 4A. While there was no statistically significant difference between the large aggregate contents of the treatment groups, the amount of small aggregates was higher in the Tanaka lipid treatment group than in any other (p < 0.05). The average amount of total surfactant was highest for the Tanaka lipids treatment group, and statistically higher (p < 0.05) than the pC treatment group. The average total protein content of the BAL for each treatment group is shown in Fig. 4B. The data show that there was no statistically significant difference in the total protein content among the various treatment groups.
Figure 4.
Surfactant pool characterization in broncheoalveolar lavage (BAL). (A) Average amounts of total surfactant, large aggregates, and small aggregates in BAL. (B) Average total protein content in the BAL of each treatment group. Error bars indicate the standard error of the mean. Statistical significance indicators: * indicates p < 0.05 for the difference between the designated group and TL alone group.
Discussion
While the extensive alveolar network and capillary vasculature of the pulmonary parenchymal tissue are critical to achieving efficient gas exchange, these delicate structures are highly susceptible to systemic pathogens and environmental toxins7. A broad spectrum of direct pulmonary insults and indirect systemic maladies results in lung surfactant deficiency and dysfunction, which leads to ALI2,7,39. There is currently no cure for ALI, and while exogenous surfactant treatment as part of a multimodal therapy has been shown to mitigate symptoms of the disease for a subset of patients2,7, outcomes of clinical trials have been varied2,7,13. We propose that peptoid-based, biomimetic surfactant replacements provide a novel technology platform with characteristics amenable to the treatment of ALI. In this inaugural study designed to investigate the in vivo efficacy of peptoid-based surfactants, we demonstrate, using the lung lavage model of ALI, that these lung surfactant replacements can improve physiological and biochemical outcomes to an equivalent extent as treatment with animal-derived surfactant. Peptoid-enhanced surfactant preparations demonstrated statistically significant immediate (within 10 minutes of treatment) and/or sustained (10 minutes–2 hours) improvements in PaO2/FIO2, shunt fraction, A-a gradient, and PIP. This is an encouraging result for biomimetic surfactants as it marks the first reporting of peptoid-enhanced surfactants demonstrating in vivo efficacy. In addition, for two measurements, PaO2 and A-a gradient (Figs 2A and 3B, respectively), the pC preparation appeared to perform slightly better than BLES based on the observed values, highlighting the potential of peptoid-based surfactants.
While peptoids have been shown previously to mimic the in vitro surface activity of SP-B20,22,23,26 and SP-C19,21,27, this study evaluated the in vivo efficacy of peptoid-based SP-B and SP-C mimics formulated separately and in combination. Tanaka lipids29 were selected as the lipid carrier for these synthetic formulations based on its similarity to the lipid/fatty acid component of natural surfactant29 and superior in vitro surface activity compared to other lipid formulations23. Though peptoid-enhanced surfactants contain a higher amount of protein mimic (~10 wt%) relative to the quantities of SP-B and SP-C found in extracted surfactant (~0.5–3 wt% each), this is reasonable because peptoids pB and pC (20 and 22 residues, respectively) represent only a portion of the natural proteins’ structures (79 and 35 residues for SP-B and SP-C, respectively). Moreover, the therapeutic dose of peptoid-enhanced surfactant has not been optimized in this study. The 50 mg/kg dose was chosen for this study based on previous experimental work in this animal model with BLES32. Additionally, the intent of this study is to test activity of the surfactants in vivo rather than clinical efficacy in an animal model. A reduced BLES dosage may highlight differences among the preparations, allowing the tested surfactants an opportunity to demonstrate superiority, inferiority, or equivalency whereas a higher BLES dose would only be a test for inferiority or equivalency. It is also notable that synthetic surfactants containing functional protein (peptide-based) mimics are not unprecedented. Lucinactant® (Surfaxin)40 from Discovery Labs contains KL4 (sinapultide), a 21mer peptide comprised of lysine and leucine, that was approved for use in the United States by the Food and Drug Administration in 2012 as a treatment for RDS in premature infants. Previous work has shown that pB and pC - formulated separately - showed improved in vitro surface activity compared to KL4 in a Tanaka lipid formulation26,27. Another synthetic surfactant formulation consisting of phosphatidylcholine, phosphatidylglycerol, and peptide analogs of SP-B and SP-C, designated CHF5633, is being developed by Chiesi Farmaceutici17 and compares favorably to animal-derived surfactant41,42. Most recently, this synthetic surfactant has been tested successfully in a Phase I clinical trial of preterm infants with RDS43. The success of Lucinactant® and the ongoing clinical development of CHF5633 is encouraging for the therapeutic potential of peptoid-enhanced surfactants.
We utilized a lung lavage model of surfactant deficiency in adult rats, a model that is well-characterized and has previously been shown to respond to animal-derived surfactant preparations36,44. The average heart rate and blood pressure of the various treatment groups showed no notable difference among any of the groups (Figure S1). The average post-lavage decrease in the PaO2 and increase in PIP showed that pulmonary gas exchange and lung compliance were significantly and uniformly damaged, a condition associated with ALI. While this animal model of surfactant deficiency does not capture all aspects of the pathophysiology associated with ALI (i.e. surfactant alterations) and is not intended to model IRDS, this simple, established protocol was deemed well-suited for direct comparison of surfactant preparations.
Previous work with this model has shown that animals which receive no treatment (air bolus only) have low PaO2/FIO2 values throughout the experiment (~100 mmHg)30, indicative of surfactant deficiency. Similar low values were observed in the animals in this study after lavage and prior to surfactant treatment as would be expected. PIP values for an air bolus only control group were consistently greater than 19 cmH2O30. Again, we see comparable values in our studies after lavage but prior to treatment where the PIP values were ~20 cmH2O. The five physiological responses measured in this study consistently showed that the negative control treatment group (Tanaka lipids) resulted in the least improvement in pulmonary function. Figures 2 and 3 show that the ‘Tanaka lipids alone’ formulation neither achieved the same initial degree of recovery, nor effectively maintained activity throughout the observation period. The notable and consistent improvement in physiological response to peptoid-enhanced Tanaka lipid formulations compared to the lipid carrier alone provide evidence for the bioactivity of peptoids to effect improved outcomes using a lung lavage model of ALI.
We have demonstrated the in vivo efficacy of peptoid-enhanced surfactant to mitigate the conditions associated with ALI, and this is a significant result because biomimetic exogenous lung surfactants afford several advantages over animal-derived surfactant replacements. The high cost of natural surfactant coupled with the large quantities required to treat adults for ALI can make treatment prohibitively expensive. Moreover, the use of a biomimetic surfactant avoids the risk of immune response that is inherent with animal-derived products. Biomimetic surfactants also offer the possibility of a “designer” treatment that could be customized to mitigate specific types of surfactant dysfunction or deactivation induced by the myriad of clinical maladies that result in ALI45. It is conceivable, for example, that additives could be included in a synthetic formulation, not only to improve surface activity, but also to prevent surfactant inhibition, regulate surfactant homeostasis, and control inflammatory response7,18,45. Finally, peptoids designed as biomimetics specifically exhibit secondary structure that makes them less prone to aggregation, which can result in enhanced shelf-life and facilitates synthesis and purification46–48.
Whereas all peptoid-containing surfactants improved physiological lung function, there were differences in the responses to the individual preparations. The formulation pC demonstrated a more significant initial improvement in physiological responses and exhibited sustained benefit throughout the recovery period, compared to the other preparations. The pB treatment group, however, consistently had a less significant impact on measured outcomes, and on average appeared only marginally better than Tanaka lipids.
A second observation regarding the responses of the individual preparations lies in comparing the performance of pB and pC formulated separately to that of the pB/pC combination formulation. By all physiological measures, the pC treatment group achieved a more favorable outcome than did the pB treatment group. Interestingly, however, the pB/pC group achieved the best sustained response in PaO2/FIO2, shunt fraction, and A-a gradient. The literature provides mixed evidence as to whether natural SP-B and SP-C or other SP-B/SP-C mimics interact synergistically, although most of the research suggests the presence of both SP-B and SP-C (or their mimics) is beneficial49–54. The degree of synergistic or additive surface-active behaviors of these mimics is ostensibly related to their underlying mechanisms of action. Moreover, it is likely that variability in the dynamic in vivo environment and lipid composition can influence the extent to which proteins and protein mimics interact. Because the synergistic interaction of protein mimics is dependent on both their chemical structures and the conditions in vivo, it is difficult to generalize observations relevant to a particular system. In this study, however, co-dosing pB and pC in the pB/pC formulation enabled a better sustained response in some physiological outcomes over the two-hour recovery period.
The way in which exogenously administered surfactant is metabolized is another factor that can influence its efficacy. Surfactant delivered to the airspace can subsequently be taken up by alveolar type II cells for recycling or by alveolar macrophages for degradation. In addition, within the airspace, exogenous surfactant can be converted from the active large aggregates to inactive small aggregates. These processes would all impact the efficacy of the exogenous material, and the surfactant pool characterization at the end of the ventilation period provided some insight into these effects. The data showed that the Tanaka lipids treatment group had a larger total surfactant pool than any other group and was statistically different from that of the pC treatment group (Fig. 4A). This difference could be due to disparate surfactant uptake rates for the various surfactant preparations. Because the Tanaka lipid formulation contains no proteins or protein mimics, it is possible that it may not be as readily taken up by type II cells. The rate of conversion from large to small aggregates within the surfactant pool has also been shown to increase under conditions pervasive in an injured lung: 1) increased protease activity55,56, 2) altered surfactant composition57,58, and 3) dynamic changes in surface area due to mechanical ventilation59,60. Injured lungs, therefore, often exhibit an increased amount of total surfactant and a concomitant increase in the less surface-active small aggregates2,9,10,61. Figure 4A shows that indeed the small aggregate component of the BAL from the Tanaka lipid treatment group was statistically greater than that of any other group. The increase in total surfactant of this group appears to be due to primarily an increase in the less active small aggregates.
The results of this in vivo study demonstrate that peptoid-enhanced lung surfactant replacements exhibit promising bioactivity and can improve physiological and biochemical outcomes using the lung lavage model of ALI. Further investigation is warranted to explore several areas building on the results of this foundational work. Evaluating the bioactivity of different peptoid-based protein mimics (separately and in combination) and lipid carriers, as well as optimization of the surfactant preparation protocol, are important for maximizing the bioactivity of this class of surfactants. It is also important to understand the efficacy of peptoid-enhanced surfactants in alternative animal models including surfactant dysfunction, acid aspiration, endotoxin inhalation, and sepsis-causing cecal ligation and perforation. Lastly, the metabolic fate of peptoids such as these, when delivered to the lung is currently unknown, and it will be important to understand their mechanism of clearance for in vivo applications. However, previous studies in which helical, amphipathic peptoids designed to mimic antimicrobial peptides have been used in vivo for the treatment of intraperitoneal bacterial infection, have shown them to be well-tolerated and show efficacy in treating infections62. A pharmacokinetic study conducted using 64Cu-labeled antimicrobial peptoids delivered per oral, intravenously, or intraperitoneally demonstrated that overall the peptoids had higher tissue accumulation, slower elimination, and higher in vivo stability compared to peptides63.
In conclusion, we have demonstrated for the first time, in vivo, that peptoid-enhanced lung surfactant replacements can improve physiological and biochemical outcomes to an extent equivalent to or better than animal-derived surfactant. While all peptoid-enhanced formulations tended to improve outcomes compared to treatment with the lipid carrier alone, pC exhibited the best and most sustained in vivo response. However, pB (in combination with pC) will likely still be essential for bioactivity in a successful biomimetic lung surfactant replacement. These promising results demonstrate the potential of peptoid-enhanced surfactants to be functional biomaterials for the treatment of ALI.
Electronic supplementary material
Acknowledgements
We acknowledge the following sources of support: US National Institutes of Health (Grant 2 R01 HL067984), the National Science Foundation (Grant BES-0101195 and Grant CHE-0404704), a 3 M Corporation fellowship (AMC), and a NIH Biotechnology Training Fellowship (NJB). BLES was kindly provided by BLES Biochemicals, London, Ontario, Canada.The Molecular Foundry has also provided support for this project; work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Author Contributions
A.M.C. wrote the manuscript. A.M.C. and L.A.B. synthesized and purified the peptoids used in these studies and prepared them for in vivo testing. M.T.D. invented and performed the in vitro experiments for the S.P.-B. peptoid mimic. N.J.B. invented and performed the in vitro experiments for the S.P.-C. mimic. L.M.M. conducted the animal studies. L.J.Y., J.F.L., and R.V. did the data analysis. J.S.L. helped write, revise, and refine the manuscript. M.K.D. helped with final revisions of the manuscript and provided useful comments. A.E.B. conceived the experiments and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25009-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruud Veldhuizen, Email: rveldhui@uwo.ca.
Annelise E. Barron, Email: aebarron@stanford.edu
References
- 1.Notter, R. H. Lung Surfactants: Basic Science and Clinical Applications. Vol. 149 (Marcel Dekker, Inc., 2000).
- 2.Lewis JF, Veldhuizen R. The role of exogenous surfactant in the treatment of acute lung injury. Annu Rev Physiol. 2003;65:613–642. doi: 10.1146/annurev.physiol.65.092101.142434. [DOI] [PubMed] [Google Scholar]
- 3.Possmayer F, Yu SH, Weber JM, Harding PG. Pulmonary surfactant. Can J Biochem Cell Biol. 1984;62:1121–1133. doi: 10.1139/o84-146. [DOI] [PubMed] [Google Scholar]
- 4.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 5.Suresh GK, Soll RF. Exogenous surfactant therapy in newborn infants. Ann Acad Med Singapore. 2003;32:335–345. [PubMed] [Google Scholar]
- 6.Gunther A, et al. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res. 2001;2:353–364. doi: 10.1186/rr86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghavendran K, Willson D, Notter RN. Surfactant Therapy for Acute Lung Injury and Acute Respiratory Distress Syndrome. Crit Care Clin. 2011;27:525−+. doi: 10.1016/j.ccc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard GR, et al. The American-European consesus conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Gunther A, et al. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med. 1996;153:176–184. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 10.Veldhuizen RA, McCaig LA, Akino T, Lewis JF. Pulmonary surfactant subfractions in patients with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:1867–1871. doi: 10.1164/ajrccm.152.6.8520748. [DOI] [PubMed] [Google Scholar]
- 11.Wiswell TE, et al. Bronchopulmonary segmental lavage with surfaxin (KL4-surfactant) for acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:1188–1195. doi: 10.1164/ajrccm.160.4.9808118. [DOI] [PubMed] [Google Scholar]
- 12.Willson DF, Truwit JD, Conaway MR, Traul CS, Egan EE. The Adult Calfactant in Acute Respiratory Distress Syndrome Trial. Chest. 2015;148:356–364. doi: 10.1378/chest.14-1139. [DOI] [PubMed] [Google Scholar]
- 13.Meng H, et al. Exogenous surfactant may improve oxygenation but not mortality in adult patients with acute lung injury/acute respiratory distress syndrome: a meta-analysis of 9 clinical trials. J Cardiothorac Vasc Anesth. 2012;26:849–856. doi: 10.1053/j.jvca.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grotberg JB, Filoche M, Willson DF, Raghavendran K, Notter RH. Did Reduced Alveolar Delivery of Surfactant Contribute to Negative Results in Adults with Acute Respiratory Distress Syndrome? Am J Respir Crit Care Med. 2017;195:538–540. doi: 10.1164/rccm.201607-1401LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curstedt T, Johansson J. New synthetic surfactants–basic science. Biol Neonate. 2005;87:332–337. doi: 10.1159/000084881. [DOI] [PubMed] [Google Scholar]
- 16.Choi Y-S, Chung S-H, Bae C-W. A Combination of Short and Simple Surfactant Protein B and C Analogues as a New Synthetic Surfactant: In Vitro and Animal Experiments. Yonsei Med J. 2017;58:823–828. doi: 10.3349/ymj.2017.58.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seehase, M. et al. New Surfactant with SP-B and C Analogs Gives Survival Benefit after Inactivation in Preterm Lambs. Plos One7, 10.1371/journal.pone.0047631 (2012). [DOI] [PMC free article] [PubMed]
- 18.Notter RH, et al. Synthetic lung surfactants containing SP-B and SP-C peptides plus novel phospholipase-resistant lipids or glycerophospholipids. PeerJ. 2016;4:e2635. doi: 10.7717/peerj.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown NJ, Wu CW, Seurynck-Servoss SL, Barron AE. Effects of hydrophobic helix length and side chain chemistry on biomimicry in peptoid analogues of SP-C. Biochemistry. 2008;47:1808–1818. doi: 10.1021/bi7021975. [DOI] [PubMed] [Google Scholar]
- 20.Seurynck-Servoss SL, Dohm MT, Barron AE. Effects of including an N-terminal insertion region and arginine-mimetic side chains in helical peptoid analogues of lung surfactant protein B. Biochemistry. 2006;45:11809–11818. doi: 10.1021/bi060617e. [DOI] [PubMed] [Google Scholar]
- 21.Wu CW, Seurynck SL, Lee KY, Barron AE. Helical peptoid mimics of lung surfactant protein C. Chem Biol. 2003;10:1057–1063. doi: 10.1016/j.chembiol.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Seurynck SL, Patch JA, Barron AE. Simple, helical peptoid analogs of lung surfactant protein B. Chem Biol. 2005;12:77–88. doi: 10.1016/j.chembiol.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Seurynck-Servoss SL, Brown NJ, Dohm MT, Wu CW, Barron AE. Lipid composition greatly affects the in vitro surface activity of lung surfactant protein mimics. Colloids Surf B Biointerfaces. 2007;57:37–55. doi: 10.1016/j.colsurfb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Simon RJ, et al. Peptoids: a modular approach to drug discovery. Proc Natl Acad Sci USA. 1992;89:9367–9371. doi: 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller SM, et al. Comparison of the proteolytic susceptibilites of homologous L-amino-acid, D-amino-acid, and N-substituted glycine peptide and peptoid oligomers. Drug Dev Res. 1995;35:20–32. doi: 10.1002/ddr.430350105. [DOI] [Google Scholar]
- 26.Dohm MT, Brown NJ, Seurynck-Servoss SL, Bernardino de la Serna J, Barron AE. Mimicking SP-C palmitoylation on a peptoid-based SP-B analogue markedly improves surface activity. Biochim Biophys Acta. 2010;1798:1663–1678. doi: 10.1016/j.bbamem.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Brown NJ, Dohm MT, Bernardino de la Serna J, Barron AE. Biomimetic N-terminal alkylation of peptoid analogues of surfactant protein C. Biophys J. 2011;101:1076–1085. doi: 10.1016/j.bpj.2011.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuckermann RN, Kerr JM, Kent SBH, Moos WH. Efficient Method for the Preparation of Peptoids [Oligo(N-Substituted Glycines)] by Submonomer Solid-Phase Synthesis. J Am Chem Soc. 1992;114:10646–10647. doi: 10.1021/ja00052a076. [DOI] [Google Scholar]
- 29.Tanaka Y, et al. Development of synthetic lung surfactants. J Lipid Res. 1986;27:475–485. [PubMed] [Google Scholar]
- 30.Brackenbury AM, et al. Evaluation of alveolar surfactant aggregates in vitro and in vivo. Eur Respir J. 2002;19:41–46. doi: 10.1183/09031936.02.00211202. [DOI] [PubMed] [Google Scholar]
- 31.Keating E, et al. Effect of cholesterol on the biophysical and physiological properties of a clinical pulmonary surfactant. Biophys J. 2007;93:1391–1401. doi: 10.1529/biophysj.106.099762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey TC, et al. Physiological and inflammatory response to instillation of an oxidized surfactant in a rat model of surfactant deficiency. J Appl Physiol (1985) 2004;96:1674–1680. doi: 10.1152/japplphysiol.01143.2003. [DOI] [PubMed] [Google Scholar]
- 33.Milos S, et al. The effect of diet-induced serum hypercholesterolemia on the surfactant system and the development of lung injury. Biochemistry and biophysics reports. 2016;7:180–187. doi: 10.1016/j.bbrep.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemyre B, et al. Poractant alfa versus bovine lipid extract surfactant for infants 24+0 to 31+6 weeks gestational age: A randomized controlled trial. PLOS ONE. 2017;12:e0175922. doi: 10.1371/journal.pone.0175922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuart S, McMillan D. Surfactant use outside the tertiary care centre. Paediatrics & Child Health. 2005;10:100–102. [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey TC, et al. Physiological effects of oxidized exogenous surfactant in vivo: effects of high tidal volume and surfactant protein A. Am J Physiol Lung Cell Mol Physiol. 2006;291:L703–709. doi: 10.1152/ajplung.00538.2005. [DOI] [PubMed] [Google Scholar]
- 37.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 38.Duck-Chong CG. Rapid sensitive method for determining phospholipid phosphorus involving digestion with magnesium nitrate. Lipids. 1979;14:492–497. doi: 10.1007/BF02533467. [DOI] [Google Scholar]
- 39.Lewis JF, et al. Evaluation of exogenous surfactant treatment strategies in an adult model of acute lung injury. J Appl Physiol (1985) 1996;80:1156–1164. doi: 10.1152/jappl.1996.80.4.1156. [DOI] [PubMed] [Google Scholar]
- 40.Braide-Moncoeur O, Tran NT, Long JR. Peptide-based synthetic pulmonary surfactant for the treatment of respiratory distress disorders. Curr Opin Chem Biol. 2016;32:22–28. doi: 10.1016/j.cbpa.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Ricci F, Murgia X, Razzetti R, Pelizzi N, Salomone F. In vitro and in vivo comparison between poractant alfa and the new generation synthetic surfactant CHF5633. Pediatr Res. 2017;81:369–375. doi: 10.1038/pr.2016.231. [DOI] [PubMed] [Google Scholar]
- 42.Rey-Santano C, et al. Cerebral and lung effects of a new generation synthetic surfactant with SP-B and SP-C analogs in preterm lambs. Pediatr Pulmonol. 2017;52:929–938. doi: 10.1002/ppul.23685. [DOI] [PubMed] [Google Scholar]
- 43.Sweet, D. G. et al. A first-in-human clinical study of a new SP-B and SP-C enriched synthetic surfactant (CHF5633) in preterm babies with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed, 10.1136/archdischild-2017-312722 (2017). [DOI] [PMC free article] [PubMed]
- 44.Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand. 1980;24:231–236. doi: 10.1111/j.1399-6576.1980.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 45.Holm BA, Waring AJ. Designer surfactants. The next generation in surfactant replacement. Clin Perinatol. 1993;20:813–829. [PubMed] [Google Scholar]
- 46.Sanborn TJ, Wu CW, Zuckermann RN, Barron AE. Extreme stability of helices formed by water-soluble poly-N-substituted glycines (polypeptoids) with alpha-chiral side chains. Biopolymers. 2002;63:12–20. doi: 10.1002/bip.1058. [DOI] [PubMed] [Google Scholar]
- 47.Wu CW, et al. Structural and spectroscopic studies of peptoid oligomers with alpha-chiral aliphatic side chains. J Am Chem Soc. 2003;125:13525–13530. doi: 10.1021/ja037540r. [DOI] [PubMed] [Google Scholar]
- 48.Wu CW, Sanborn TJ, Huang K, Zuckermann RN, Barron AE. Peptoid oligomers with alpha-chiral, aromatic side chains: sequence requirements for the formation of stable peptoid helices. J Am Chem Soc. 2001;123:6778–6784. doi: 10.1021/ja003154n. [DOI] [PubMed] [Google Scholar]
- 49.Walther FJ, Hernandez-Juviel J, Bruni R, Waring AJ. Spiking Survanta with synthetic surfactant peptides improves oxygenation in surfactant-deficient rats. Am J Respir Crit Care Med. 1997;156:855–861. doi: 10.1164/ajrccm.156.3.9611053. [DOI] [PubMed] [Google Scholar]
- 50.Curstedt T, Calkovska A, Johansson J. New Generation Synthetic Surfactants. Neonatology. 2013;103:327–330. doi: 10.1159/000349942. [DOI] [PubMed] [Google Scholar]
- 51.Walther FJ, Hernandez-Juviel J, Bruni R, Waring AJ. Protein composition of synthetic surfactant affects gas exchange in surfactant-deficient rats. Pediatr Res. 1998;43:666–673. doi: 10.1203/00006450-199805000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Gurel O, Baatz JE, Notter RH. Differential activity and lack of synergy of lung surfactant proteins SP-B and SP-C in interactions with phospholipids. J Lipid Res. 1996;37:1749–1760. [PubMed] [Google Scholar]
- 53.Almlen A, et al. Synthetic surfactant based on analogues of SP-B and SP-C is superior to single-peptide surfactants in ventilated premature rabbits. Neonatology. 2010;98:91–99. doi: 10.1159/000276980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walther, F. J., Hernandez-Juviel, J. M., Gordon, L. M. & Waring, A. J. Synthetic surfactant containing SP-B and SP-C mimics is superior to single-peptide formulations in rabbits with chemical acute lung injury. Peerj2, 10.7717/peerj.393 (2014). [DOI] [PMC free article] [PubMed]
- 55.Gross NJ, Schultz RM. Serine protease requirement for the extracellular metabolism of pulmonary surfactant. Biochim Biophys Acta. 1990;1044:222–230. doi: 10.1016/0005-2760(90)90306-I. [DOI] [PubMed] [Google Scholar]
- 56.Gross NJ, Schultz RM. Requirements for extracellular metabolism of pulmonary surfactant: tentative identificationof serine protease. Am J Physiol. 1992;262:L446–L453. doi: 10.1152/ajplung.1992.262.4.L446. [DOI] [PubMed] [Google Scholar]
- 57.Veldhuizen RAW, Inchley K, Hearn SA, Lewis JF, Possmayer F. Degradation of surfactant-associated protein B (SP-B) during in vitro converstion of large to small surfactant aggregates. Biochem J. 1993;295:141–147. doi: 10.1042/bj2950141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veldhuizen RAW, Yao LJ, Hearn SA, Possmayer F, Lewis JF. Surfactant-associated protein A is important for maintaining surfactant large-aggregate forms during surface-area cycling. Biochem J. 1996;313:835–840. doi: 10.1042/bj3130835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veldhuizen RA, et al. Effects of lung injury on pulmonary surfactant aggregate conversion in vivo and in vitro. Am J Physiol. 1997;272:L872–878. doi: 10.1152/ajplung.1997.272.5.L872. [DOI] [PubMed] [Google Scholar]
- 60.Veldhuizen RA, et al. Alveolar surfactant aggregate conversion in ventilated normal and injured rabbits. Am J Physiol. 1996;270:L152–158. doi: 10.1152/ajplung.1996.270.1.L152. [DOI] [PubMed] [Google Scholar]
- 61.Gregory TJ, et al. Surfactant chemical composition and biophysical activity in acutre respiratory distress syndrome. J Clin Investigations. 1991;88:1976–1981. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Czyzewski AM, et al. In Vivo, In Vitro, and In Silico Characterization of Peptoids as Antimicrobial Agents. PLoS One. 2016;11:e0135961. doi: 10.1371/journal.pone.0135961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seo J, et al. In vivo biodistribution and small animal PET of (64)Cu-labeled antimicrobial peptoids. Bioconjug Chem. 2012;23:1069–1079. doi: 10.1021/bc300091d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.