Abstract
Although flea-borne rickettsiosis is endemic in Los Angeles County, outbreaks are rare. In the spring of 2015 three human cases of flea-borne rickettsiosis among residents of a mobile home community (MHC) prompted an investigation. Fleas were ubiquitous in common areas due to presence of flea-infested opossums and overabundant outdoor cats and dogs. The MHC was summarily abated in June 2015, and within five months, flea control and removal of animals significantly reduced the flea population. Two additional epidemiologically-linked human cases of flea-borne rickettsiosis detected at the MHC were suspected to have occurred before control efforts began. Molecular testing of 106 individual and 85 pooled cat fleas, blood and ear tissue samples from three opossums and thirteen feral cats using PCR amplification and DNA sequencing detected rickettsial DNA in 18.8% of the fleas. Seventeen percent of these cat fleas tested positive for R. felis-specific DNA compared to under two (<2) percent for Candidatus R. senegalensis-specific DNA. In addition, serological testing of 13 cats using a group-specific IgG-ELISA detected antibodies against typhus group rickettsiae and spotted fever group rickettsiae in six (46.2%) and one (7.7%) cat, respectively. These results indicate that cats and their fleas may have played an active role in the epidemiology of the typhus group and/or spotted fever group rickettsial disease(s) in this outbreak.
Author summary
Outbreaks of flea-borne rickettsiosis are rare despite the endemic status in Los Angeles County. In the spring of 2015 three human cases of flea-borne rickettsiosis among residents of a mobile home community (MHC) prompted an investigation. Fleas were found in all common areas at the MHC due to presence of flea-infested opossums and overabundant outdoor cats and dogs. The MHC was summarily abated in June 2015, and within five months, flea control and removal of animals significantly reduced the flea population. Two additional epidemiologically-linked human cases detected at the MHC were considered to have occurred before control efforts began. Molecular testing of cat fleas, immunological testing of opossums and feral cats collected at the site indicated active transmission of flea-borne rickettsiosis. This study represents the first flea-borne rickettsial outbreak that summary abatement approach was used to reduce its intensity.
Introduction
Acute febrile flea-borne rickettsial diseases are caused by intracellular gram-negative bacteria Rickettsia typhi and Rickettsia felis, which are known to be transmitted to humans by the bite of Xenopsylla cheopis (oriental rat flea) and Ctenocephalides felis (cat flea); both fleas are found on many domestic and peri-domestic vertebrate hosts [1]. In recent years, additional flea transmitted Rickettsia has been identified in fleas and their hosts, both living in close proximity to people, warranting such rickettsial infections in humans referred to as flea-borne rickettsiosis. Unfortunately, when rickettsial infections are detected in humans the clinical diagnostic tests almost never distinguish between rickettsial species–R. typhi (typhus group—TG) or R. felis (spotted fever group- SFG) [2]. The commonly reported flea-borne rickettsiosis in humans is murine typhus whose known causative agent is R. typhi. Commercially available clinical serological diagnostic tests commonly show cross-reactivity among the pathogens in the TG and SFG. Confirmation of these infections requires positive serology in paired acute and convalescent serum samples of a four-fold or greater change in immunoglobulin G (IgG) and M (IgM)-specific antibody titer reactive to R. typhi or other Rickettsia species antigen by indirect immunofluorescence assay (IFA). In most clinical settings, rarely are paired acute and convalescent serum samples collected, and California Department of Public Health resolved this shortcoming through special guidelines that achieved confirmation of murine typhus serologically or by nucleic amplification in a single serum specimen of elevated IgG and IgM antibody reactive to R. typhi or other Rickettsia species by IFA or DNA amplification, respectively (CDPH 2011) [3]. Consequently, laboratory confirmation of murine typhus may not represent true cases, but cases of flea-borne rickettsiosis. Although these cases are reported as murine typhus without identifying the actual rickettsial pathogen but for purposes of consistent reporting they are reported as murine typhus. Here we identify such cases as flea-borne rickettsiosis and attempt to determine the responsible rickettsial agent for the current outbreak.
On average, there are more than 200 human cases of murine typhus reported in the United States each year. This disease is not nationally reportable, so the true number of cases is unknown [4]. In areas where this disease is endemic (California, Hawai’i, and Texas), providers and clinical laboratories are mandated to report cases to their local public health departments [2,5]. In California, most of the flea-borne rickettsial disease cases occur in Los Angeles and Orange counties, but concentrated outbreaks of the disease are rare [2]. Prior to 2015, the last documented cluster of human flea-borne rickettsial disease in Los Angeles County occurred in 2009 [6,7]. Over the past decade, the incidence of flea-borne rickettsial disease in Los Angeles County (LAC) has increased. In 2014, 51 cases were reported statewide [44 (86%) in LAC], 88 cases [69 (78%) in LAC] in 2015, and 90 cases [70 (78%) in LAC] in 2016. These cases in LAC occur in suburban communities through interactions between wildlife, domestic animals, and humans.
Since the initial discovery of R. felis in C. felis in 1990 [8,9,10], the association of R. felis with C. felis has been well-documented and the connection between zoonotic diseases and free-roaming animals has become an issue [8,9,10,11,7]. The last two decades have witnessed an increase in the recognition of new R. felis-like organisms (RFLOs), particularly Rickettsia asembonensis and Candidatus Rickettsia senegalensis, whose distributions and host ranges appear to mimic those of R. felis [12]. Although there are no studies published concerning its ability to cause clinical illness, R. asembonensis was recently detected in the blood of monkeys (Macaca fascicularis) in Malaysia [13], and in dogs’ blood in South Africa [14]. Another agent with close genetic composition to Ca. R. senegalensis was detected in the blood of febrile patients from Senegal [15].
Free-roaming animals have increasingly become a source of flea-borne infectious diseases that are controlled in domestic cat populations through routine veterinary care and flea control. When this care is lacking, the consequence is increased potential health risks for other domestic animals and humans [11]. Incidental infection transfer also occurs when free-roaming animals are fed outdoors. Wildlife, free-roaming cats, and domestic animals are brought in proximity when food is left outdoors, increasing the potential for exchanging fleas. When ectoparasites of wildlife become too numerous, they infest new hosts, increasing the risk that they may transmit disease to domestic animals and humans [16,17].
Three human case reports of flea-borne rickettsiosis among residents of a single 95-unit mobile home community (MHC), with disease onsets from April 23rd to June 9th, 2015 prompted an initial investigation, and subsequent abatement order by the San Gabriel Valley Mosquito and Vector Control District (District) [18]. The Los Angeles County Department of Public Health (LACDPH) initiated active case finding to look for additional outbreak-associated cases during this time period and helped to coordinate a multi-agency notification, and abatement in the MHC [19,4]. The District conducted the abatement order in the MHC and coordinated overall surveillance, control, and mitigation efforts [20]. Here we discuss the outbreak, samples collected to identify the etiological agent(s), and efforts made to mitigate the risk factors to public health.
Materials and methods
The San Gabriel Valley Mosquito and Vector Control District (District) is located in Los Angeles County, California. It includes more than 54,000 hectares of Los Angeles County and is bordered to the north by the San Gabriel Mountains where a plethora of wild animals exist. From April to June 2015, the District received reports from LACDPH of three active cases of flea-borne typhus in its jurisdiction from a 95-unit MHC. The cases were investigated to determine the environmental conditions supporting this outbreak and necessary efforts to prevent its recurrence.
Human cases
In April 2015, case A from the MHC was hospitalized for four nights and case B was hospitalized for six nights (Table 1). In June, case C from the same MHC was hospitalized for five nights [4]. A hospital infection preventionist reported the cases to LACDPH and they were forwarded to LACDPH Environmental Health Department and the District. From April to June 2015, LACDPH conducted enhanced case finding for Rickettsia typhi-positive (Quest Diagnostic, Inc.; IFA test) cases at all acute care facilities and local clinics within the catchment area of the MHC. Outbreak-associated cases were defined as MHC residents with symptom onset of fever with headache or rash from March 1st, 2015 through August 31st 2015, and/or positive R. typhi or R. rickettsii laboratory test (immunoglobulin M (IgM) >1:128 and/or immunoglobulin G (IgG) >1:128. Additional criteria include elevated liver function tests (ALT or AST), decreased platelet counts, and proximity in time and space for epidemiological-linkage to the outbreak. Cases A, B, and C were initially tested by Quest Diagnostics, Inc (San Juan Capistrano, CA) and two additional cases were tested and confirmed via indirect fluorescent antibody assays (IFA) by LAC Public Health Laboratory (part of LACDPH). Paired acute and convalescent specimens of the cases were not available. Unlike LACDPH Laboratory, it’s not common practice for clinical commercial tests to include a full rickettsial panel of R. typhi and R. rickettsii. The State of California Department of Public Health guidelines interprets the detection of elevated IgG and IgM antibody reactive to R. typhi or other Rickettsia species antigen by IFA titer of ≥ 128 in a single serum specimen in addition to having specified clinical symptoms as confirmed cases [3]. These cases were part of anonymized LA County public health surveillance data.
Table 1. Case characteristics of five flea-borne rickettsiosis cases residing in the investigated mobile home community in Los Angeles County, California during the outbreak period of March 1st through August 31st, 2015.
| Case | Age | Sex | Cat Owner | Dog Owner | Onset Date | Fever | Headache | Rash | Hospitalized | Hosp Nights | ALT (U/L) | AST (U/L) | Platelets (K/mm3) | R. typhi | R. rickettsii | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgG | IgM | ||||||||||||||
| A | 42 | F | No | Yes | 4/9/15 | Yes | Yes | No | Yes | 4 | 92 | 84 | 72 | 1:128 | ≥1:256 | ND | ND |
| B | 51 | M | Yes | Yes | 4/20/15 | Yes | Yes | No | Yes | 6 | 57 | 120 | 56 | <64 | 1:64 | ND | ND |
| C | 67 | F | Yes | Yes | 6/5/15 | Yes | Yes | No | Yes | 5 | 189 | 140 | 119 | 1:128 | 1:128 | ND | ND |
| D | 48 | F | No | Yes | Unsp* | No | Yes | Yes | No | 0 | ND | ND | ND | 1:128 | <64 | 1:64 | <64 |
| E | 47 | F | No | Yes | 8/20/15 | Yes | Yes | Yes | No | 0 | ND | ND | ND | 1:64 | <64 | 1:128 | <64 |
* Denotes unspecified indicating that other ongoing health conditions made it difficult to assess a true onset date for flea-borne rickettsial infection.
Environmental investigation
The initial investigation began in June 2015 with a survey of the property which included counting and photographing the number of pets and animals outdoors, the number of outdoor feeding sources (water bowls and food), the presence and locations of free roaming animals, available harborage, and the presence of uncovered garbage bins. A door to door inspection and discussion with the residents of this MHC was conducted to inform them of the outbreak and symptoms of rickettsial disease, and the association of fleas and illnesses in the community. Discussions regarding appropriate cause of action were conducted between LACDPH, Environmental Health, and District staff. Opossums in the neighborhood were trapped using two live traps (Tomahawk Live Trap, Tomahawk, WI) set on selected properties in the MHC. Fleas were combed off the opossums trapped, identified to species level [21], and tested by species-specific molecular methods for presence of rickettsial pathogens.
A summary abatement order was issued by the District to the MHC on June 24, 2015 to clean up the property. Specific requests included removal of animal feces, enforcement of MHC property rules limiting the pets to one animal per property, and regular flea control to reduce the population of fleas. The order also mandated that MHC residents cease outdoor feeding of pets and contract a pest control company to reduce the number of feral animals on the property. In addition, the property manager notified residents to provide flea control for all animals under their care, those tenants with more than one pet against the MHC homeowner rules and regulations to give them up and register their “single animal pet” with the property manager. This directive was necessary because most tenants had more pet animals some up to 32, contrary to their property lease contracts.
On August 24, 2015 a multi-agency community event was held at a shopping center adjacent to the MHC by LACDPH, the District, the state Senator’s office District 20, the city, and other regulatory agencies, to discuss public health risk posed by flea-borne rickettsiosis and encourage residents of the MHC to participate in reducing this risk. Residents of the MHC with symptoms of flea-borne rickettsial disease per case definition within the past three months were encouraged to provide blood samples for testing at no cost to them. Five individuals participated in the blood draw event and their blood tested by commercially available IFA tests, two of them were positive and was considered epidemiologically-linked human cases from this MHC. The tests were performed by Quest Diagnostics and/or confirmed by LAC Public Health Laboratory using commercially available FDA-approved IFA clinical tests.
The property owner contracted with two pest control companies, one to control fleas and the other to remove feral animals on the property. The numbers of outdoor feeding sources were recorded monthly to determine the effectiveness of the property owners’ efforts. Flea control was conducted every 14 days, and the population of fleas was monitored bi-weekly by placing six 16 cm x 11 cm glue boards (PIC Corporation, Linden, NJ) throughout the property. Fleas collected on glue boards were counted and averaged by month to assess flea control efforts. Free-roaming domestic animals were counted monthly during morning walks of the property to monitor the impact of vertebrate/animal trapping efforts. A regression analysis (JMP v 10/0: http://www.jmp.com) was used to calculate the coefficient of determination (static) which correlated control measures conducted at the MHC against the number of outdoor wildlife, feeding sources, and flea activity. Fleas, blood, and ear tissue samples were retrieved from all cats removed by the vertebrate trapper and the opossums by the District from the MHC (13 cats, and 3 opossums) for epidemiologic studies. One rat retrieved from the trap was dead and no flea, blood, or ear tissue samples were collected for testing. The Naval Medical Research Center tested all samples collected from the MHC epidemiologic study to determine the prevalence of rickettsial agents responsible for the outbreak.
Molecular and serological testing
Fleas combed off animals trapped at the MHC were washed in molecular grade water and mechanically disrupted with disposable pellet pestles (Fisher Scientific, Pittsburgh, PA). Genomic DNA was extracted with Prepman Ultra sample preparation kits (Applied Biosystems, Foster City, CA). Genomic DNA from cat and opossum ear tissues and blood clots were extracted using the DNeasy blood and tissue kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions with a final elution volume of 50 μl.
All fleas from cats (n = 46) and 20 fleas from each opossum (n = 60) were processed individually. Remaining fleas from the three opossums were processed in pools of 18–20 fleas (n = 1,553). Flea DNA, and DNA from the cat and opossum blood and tissues were initially screened for rickettsial DNA using a genus-specific quantitative real-time PCR (qPCR) assay (Rick17b) targeting the 17-kDa antigen gene [22]. DNA from the individual flea, cat, and opossum blood, and tissue samples that tested positive by the Rick17b assay were subsequently tested using a group-specific qPCR assay (RfelB) [23] and three species-specific assays, namely, (1) R. felis specific assay (Rfel_phosp_MB) that targets the membrane phosphatase gene from R. felis [24], (2) the R. typhi species specific qPCR assay (Rtyph) which targets a fragment of the R. typhi ompB gene [23], and (3) the Rickettsia asembonensis-specific qPCR assay (Rasem), which targets a fragment of R. asembonensis ompB gene [25,26]. Similarly, the DNA from the pooled fleas was screened using the Rick17b, Rtyph, and the Rasem qPCR assays.
PCR amplification and sequencing of gltA was attempted for a subset of 7 individual flea DNA including one DNA sample that was positive for rickettsial DNA using the Rick17b qPCR assay but negative for R. felis DNA based on the Rfel_phosp_MB qPCR assay, 2 samples that had discordant cycle threshold (Ct) values between Rick17b qPCR and Rfel phosp_MB qPCR assays and 4 flea DNA samples positive with both the Rick17b and the Rfel_phosp_MB qPCR assays to confirm the specificity of Rfel_phosp_MB. PCR amplification of gltA gene was attempted for all ear tissue DNA that tested positive for rickettsia DNA by qPCR, as previously described [20]. Sequencing reactions were performed in the forward and reverse directions utilizing the Big Dye Terminator v3.1 Reaction Cycle sequencing kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions using an ABI 3500 genetic analyzer (Applied Biosystems). Sequence assembly was performed using CodonCode Aligner version 5.0.1 (CodonCode Corporation, Centerville, MA). Blast searches were performed in NCBI websites.
Evidence of previous infection of animals with spotted fever group (SFGR) and typhus group (TGR) rickettsiae was assessed using group-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assays (ELISAs) as previously described [25,27,28], using R. typhi str. Wilmington and R. conorii str. Morocco as the TGR and SFGR ELISA antigens, respectively. Serum samples were diluted 1:100 and screened for antibodies against rickettsiae using both the SFGR and TGR ELISAs. Screen positive samples (those with net absorbance of ≥ 0.5) were titered by 4-fold serial dilution (100–6,400). Commercial anti-cat IgG (KPL, Gaithersburg, MD) and anti-opossum IgG (Alpha Diagnostics Intl. Woodlake Center, San Antonio, TX) antibodies labeled with horseradish peroxidase (HRP) were used in both ELISAs.
Ethics Statement: The San Gabriel Valley Mosquito and Vector Control District (SGVMVCD) do not have a formal Institutional Animal Care and Use Committee (IACUC) since it is not considered a research institution. However, it does follow the protocols for animal handling for disease surveillance purposes as outlined by the California Department of Public Health, Vector Borne Disease Section, and adhered to American Veterinary Medical Association (2013) guidelines for animal euthanasia. SGVMVCD as a cooperative member of Mosquito and Vector Control Association of California is exempt from requirement of holding a scientific permit under FG code 1002, 4005, and 4011 of the California Department of Fish and Wildlife (CDFW) to collect and sample small mammals for disease surveillance purposes.
Results
Human cases
Initially, three human (A, B, and C) cases were identified from the MHC with illness onset ranging from April to June 2015. The LACDPH active case finding reported two additional epidemiologically-linked (D and E) cases of the outbreak for the period from March 1st through August 31st 2015 (Table 1). They were predominantly female (4/5) their ages ranged from 42 to 67 years; all were dog owners and two also owned cats. All experienced headaches, three had fever (A, B, and C), and two (D and E) had a rash. The first three (A, B and C) cases were hospitalized for a total of 15 days (mean 5); the remaining two cases were discovered from on-site blood draw at the community event (D and E). The same three cases (A, B, and C) showed lower platelet count and elevated liver function enzymes (ALT and AST). All cases recovered without complication. All five cases had antibodies reactive to R. typhi or other Rickettsia antigens with titers ≥ 1:128 via IFA testing except case B which together with cases C and D are considered epidemiologically-linked and part of the outbreak (Table 1).
Environmental investigation
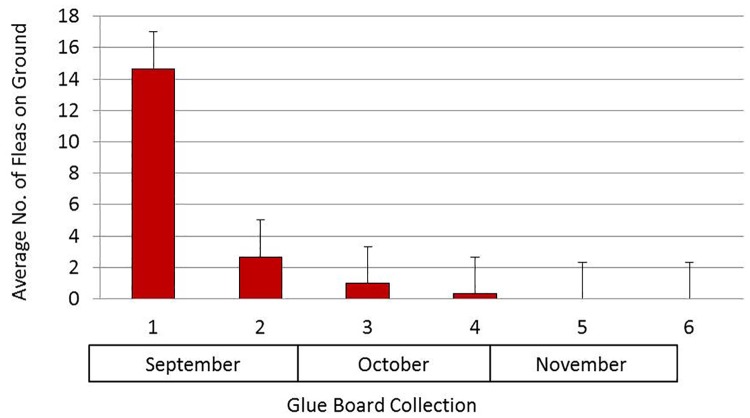
Two live traps set in June 2015 on the selected plots at the MHC yielded two opossums infested with 615, and 1,087 cat fleas, respectively. Another opossum was trapped in September and was infested with 487 cat fleas. Flea control was conducted at the MHC every 14 days beginning in September 2015 by a professional pest control company. To assess its effectiveness, glue boards strategically distributed in the MHC at six locations were retrieved every two weeks from September to November. The glue boards placed on location prior to treatment contained as many as 39 fleas. The number of fleas on the glue boards declined significantly after the first treatment (n = 36, r² = 0.5813, p≤ 0.05), and the trend continued over time (number of months), until no fleas were collected for two consecutive collection sessions (Fig 1).
Fig 1. Average number of fleas (plus standard error) collected on glue boards (n = 36) from Sep to Nov 2015 at the mobile home community in San Gabriel Valley, California.
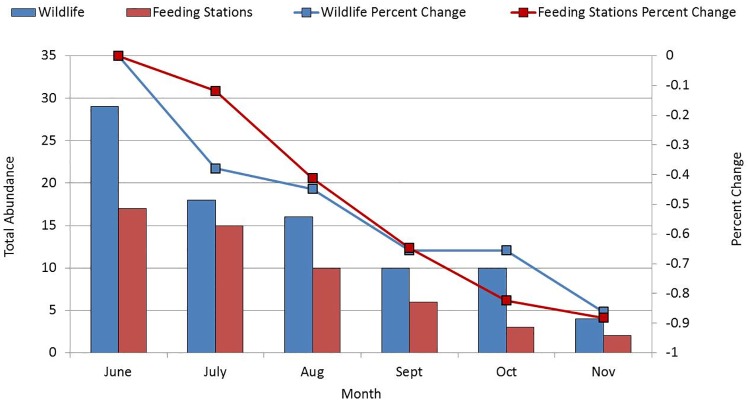
The MHC hired a wildlife trapper to remove outdoor vertebrate pests from the MHC. Thirteen cats were removed by the trapper from September to November. The number of fleas on the cats removed from the MHC declined over time (n = 13, r² = 0.7512, p < 0.05) with the first cat collected in September having 13 fleas on it, and the one collected in November having no fleas. Subsequently, the number of cats outdoors from June through November also significantly declined from 29 to 4 (n = 87, r² = 0.914, p < 0.05) over time (Fig 2).
Fig 2. Abundance of outdoor wildlife and outdoor feeding sources from Jun to Nov 2015 at the mobile home community in San Gabriel Valley, California.
The number of outdoor feeding stations at the MHC also decreased significantly (n = 90, r² = 0.9697, p < 0.05; Fig 2). The decline in the population of fleas on the ground and on cats, free-roaming animals, and wildlife, and the decreased prevalence of outdoor feeding ultimately decreased the risk at the MHC of acquiring flea-borne typhus.
Molecular and serological testing
A total of 13 cats and three opossums were trapped around the MHC over the study period. Pooled fleas (n = 1,553) combed from these animals were screened for rickettsiae with the genus specific qPCR assay (Rick17b), R. typhi specific qPCR assay, and R. asembonensis- specific qPCR assay. Eighty-five pools were tested, with 74 (85.33%) testing positive for rickettsiae (Table 2). Further screening was qPCR assay negative for both R. typhi and R. asembonensis.
Table 2. Summary of Rickettsia genus-specific qPCR assay (Rick17b) assessment of Ctenocephalides felis pools (n ≤ 20 individual fleas/pool) from 3 opossums at the mobile home community in Los Angeles County, California.
| Opossum (n = 3) Identification Number# | Total # of Fleas | Total # of Pools Tested | Total # of Pools Rick17b qPCR Positive | % Rick17bpositive | MIR |
|---|---|---|---|---|---|
| 1 | 119 | 10 | 10 | 100 | 8.4 |
| 2 | 967 | 52 | 51 | 97.87 | 5.3 |
| 3 | 467 | 23 | 13 | 56.52 | 2.8 |
| Total | 1553 | 85 | 74 | 85.33 | 4.8 |
One hundred and six individual fleas from cats and opossums were tested. From 9 of 13 cats, 46 fleas, and from three opossums, 60 fleas making a total of 106 fleas were tested for rickettsial DNA (Table 3). Twenty of these fleas (18.8%) were positive for Rickettsia DNA using the genus-specific assay Rick17b. Of these, 18 were positive for R. felis using the Rfel_phosp_MB assay. None of the fleas were positive for R. typhi or R. asembonensis-specific qPCR assays.
Table 3. Summary of qPCR assay results for individual fleas from 3 opossums and 13 cats at the mobile home community in Los Angeles County, California.
| Total # of animals | # of fleas | # of fleas tested | #Rickettsia genus-specific Rick71b positive (%) | R. felis-specific Rfel_phosp_MB positive (%) | R. typhi-specific Rtyph positive (%) | Ca. R. senegalensis-sequence positive (%) | # of Positive animals (%) | MIR | |
|---|---|---|---|---|---|---|---|---|---|
| Opossums | 3 | 2189 | 60 | 9 (15) | 8 (13.33) | 0 | 1 (1.66) | 3 (100) | 4.1 |
| Cats | 13 | 46 | 46 | 11 (23.91) | 10 (21.73) | 0 | 1 (2.17) | 9 (69.23) | 23.9 |
| Total | 16 | 2235 | 106 | 20 (18.8) | 19 (16.9) | 0 | 2 (1.88) | 12 (75) | 8.9 |
To confirm the identity of rickettsiae identified by the qPCR assays, PCR amplification and sequencing of the gltA gene was done in a subset of seven individual flea DNA preparations. Seven 1186-bp gltA sequences were generated, of which five were 100% identical to each other and to R. felis URRWXCal2 (Accession no. CP000053). The five samples included four that had tested positive for both Rick17b and Rfel_phosp_MB, and one of the two samples that had discordant Ct. The remaining two sequences were 100% identical to each other and to Candidatus Rickettsia senegalensis (Accession number KF666472), and included one that was positive for Rick17b but negative for Rfel_phosp_MB, assay, and one that had discordant Ct values.
A total of 13 cat sera and three opossum sera were assessed for antibodies against TGR and SFGR by ELISA and the presence of Rickettsia DNA by qPCR assays. Of the sera tested for the presence of SFGR- and TGR-specific IgG, 6/13 (46.15%) had IgG antibodies reactive against TGR antigens with endpoint titers ranging from 1600 to 6400 (Table 4). One of the 13 cat sera (7.69%) was positive for antibodies against SFGR antigens with an endpoint titer of 100. None of the opossum sera were positive for antibodies against SFGR- or TGR-specific IgG.
Table 4. Summary of percent (%) prevalence of IgG antibodies in 3 opossums and 13 cat sera from animals collected at the mobile home community in Los Angeles County, California.
| Total # | SFG Screen | SFG Titer | TG Screen | TG Titer | Overall Prevalence | |
|---|---|---|---|---|---|---|
| Opossums | 3 | 0 | 0 | 0 | 0 | 0 |
| Cats | 13 | 1 (7.69)* | 100 | 6 (46.15) | ≥ 1600 | 6 (46.15) |
* One cat serum sample was positive for SFG and TG IgG ELISA at titers of 6400 and 100, respectively.
The blood clots and tissues from 13 cats and 3 opossums were also assessed for the presence of Rickettsia DNA. All blood clot DNA preparations from cats and opossums were negative for Rickettsia DNA whereas three of thirteen cat tissues tested positive for Rickettsia DNA (Rick 17b with Ct values > 35). One of three cat tissue DNA preparations tested positive for R. felis by group-specific qPCR RfelB assay, but all three cat tissue preparations were negative for other PCR assays and none produced amplicons for sequencing.
Discussion
An outbreak of five cases of flea-borne rickettsial disease occurred at the MHC within the District. It is highly likely that additional infections occurred due to the abundance of fleas on the property, but went undetected due to a mild presentation and/or lack of testing.
The etiologic agent of flea-borne rickettsial diseases has been debated for years. R. typhi has been known historically as the etiologic agent of murine typhus [29,1,30,2]. There were three hospitalized and two non-hospitalized epidemiologically-linked cases at the MHC that had R. typhi or other Rickettsia antibody titers ≥ 1:128 except one of hospitalized cases with titers ≥ 1:64 via IFA test, all in private clinical laboratory or/and LAC Public Health Laboratory. Although human testing met state guidelines the only limitation of the clinical diagnostic testing of all five cases was the use of a single serological sample instead of the paired acute and convalescent samples. Such samples were not available because it is uncommon for clinicians to collect paired samples from patients in clinical settings. Physicians often overlook rickettsial infections and by the time such tests are deemed necessary for patient care a dose of antibiotics/chemotherapy would have been administered rendering collection of paired serological sample impossible. The clinical IFA test conducted on single patient sera does not confirm murine typhus but confirms rickettsial infection either of TG or SFG. The epidemiology of rickettsial diseases in southern California, and especially San Gabriel Valley in Los Angeles County show that R. typhi is the predominant human Rickettsia pathogen compared to SFG-transmitted R. rickettsii, thus it has not been important from a public health standpoint to differentiate between TG and SGF infections [2,4]. Current assays examining IgG/IgM titers with indirect fluorescent antibody assays do not always differentiate between antibodies against R. typhi and other Rickettsia species, and the former is assumed to be the etiologic agent for murine typhus, therefore of flea-borne rickettsiosis [31]. A more accurate differentiation between R. typhi and other Rickettsia species could be made through PCR based assays if a blood specimen could have been available from acute symptomatic individuals. Unfortunately, at the time of investigation, acute blood specimens from the three hospitalized cases were unavailable.
Samples from the animals and fleas removed from the MHC were tested for the presence of the different etiologic agents. Rickettsia felis was detected in fleas obtained from animals at the MHC, which supports prior research that elevated the potential role of R. felis previously referred to as ELB as an etiological agent of flea-borne rickettsial disease in relation to R. typhi, and implicated opossums and rodents as the main hosts [2,32,33]. However, results showed that both R. felis and Ca. R. senegalensis were present in cat fleas, although the association of R. felis with human disease seems poor [34]. The focus was on R. typhi where 46.15% of cat sera removed from the MHC were positive, and none of the opossum blood had any rickettsial DNA. Low level IgG-positive opossum and lack of rickettsial DNA in opossums aligns with findings from previous studies [12,34]. Alternatively, the presence of antibodies against R. typhi in cats suggests that it could still be active within the peri-domestic and domestic animal community and this may suggest that cats provide another mechanism for maintaining typhus in southern California. Furthermore, R. typhi was not detected in the cat fleas, which corroborates past findings [35] that R. typhi may still be present in the environment at a very low but infectious level and/or possibly carried by another flea species and hosted by other mammals beside opossum and cats.
Beside R. felis, the present study detected Ca. R. senegalensis in cat fleas. This corroborates the findings of two previous studies that reported existence of the “RFLO” in southern California [12,35]. Although the previous studies reported R. asembonensis at a relatively low rate (0.3% of 597 fleas tested) in the same region [12], the present study did not confirm that finding. Although two RFLOs have been detected in the blood of dogs, monkeys, and humans [15,13,14], their ability to cause disease in mammalian species has not yet been proven. The relationship (symbiosis, mutualism, or parasitism) between the host and the rickettsiae has not been elucidated for any of the RFLOs. It has been suggested that all rickettsiae can potentially be pathogenic to vertebrate hosts [36]. This is evidenced by the findings that R. slovaca, R. helvetica and R. parkeri tick endosymbionts were associated with human disease years after they were discovered [37,38,39].
Within five months of focused abatement implementing mitigation measures, the potential risk of rickettsial and other flea-borne diseases infection was reduced based on several observations within the neighborhood under investigation. The achievement of mitigation measures involved flea control, trapping and removal of feral cats, and opossums, providing flea-collars to residents for cats and dogs, and removal of outdoor feeding sources by the property owner and tenants. The role of public health agencies was education and coordination, and that of vector control was identifying the responsible parties–property owner and tenants–and ensuring they contracted with professional pest control whose work was certified at completion. More importantly no additional cases of flea-borne rickettsioses were detected. Ultimately, the success of this approach as spearheaded by public health agencies was measured by the absence of new cases of flea-borne typhus, but the MHC must continue to adhere to its policies to ensure that public health risk previously present do not re-occur.
Acknowledgments
We thank the Los Angeles County Department of Public Health for coordinating the community engagement, surveillance, and control of this flea-borne rickettsiosis outbreak; especially Jeffrey Gunzenhauser, the Public Health Officer, Laurene Mascola and Benjamin Schwartz of the Acute Communicable Disease for their leadership in identifying the public health threat and coordinating the response; Cristin Mundy, Area Health Officer of Service Area 3 of Los Angeles County, for coordinating the investigation and ensuring that the public health threat was mitigated; Terri Williams, LAC Environmental Health Director, and the team of Brenda Lopez, Graceline Shin, Yvette Boston-Darton, Kelsey Onaga, and Maria Dalusong for outbreak responses and field inspections. Sincere thanks go to Senator Connie Leyva’s office, especially to her District Director Manuel Saucedo and District Representative Benny Ayala for their involvement in ensuring all local and state agencies responsible for mobile homes were engaged, especially for involving the state mobile home regulator, Sal Poidomani and his local representative Sonia Semlow of California Housing and Community Development to enforce mobile home park regulations, accordingly. Furthermore, we thank Marco Metzger and Renjie Hu of the State Department of Public Health, Vector Borne Disease Section, Ontario office for their oversight; Mark Gluba and the City of Pomona for their cooperation in this investigation. Finally, great thanks go to Carrie Fogarty and Robert Cummings of the Orange County Mosquito and Vector Control District for their generosity in providing preliminary testing of fleas initially collected from the subject property. Preliminary results from this study were presented at the 84th Annual Conference of Mosquito and Vector Control Association of California; February 28- March 2, 2016, Sacramento, California, USA.
Disclaimers
The views expressed in this article are those of the authors and do not necessarily represent the official policy or position of the Department of the Navy, Department of Defense, Centers for Disease Control and Prevention, or the U.S. Government.
Data Availability
All relevant data are within the paper and its supporting files. Additional data is also available from the GenBanks accession numbers CP000053, KF666472.
Funding Statement
This study was in part funded by the United States Department of Defense Global Emerging Infections Surveillance and Response System (GEIS) to ALR and ANM, work unit number 847705.82000.25GB.A0074. In addition, this study was supported in part by an appointment of CF to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists (CSTE) and funded by the Centers for Disease Control and Prevention (CDC) Cooperative Agreement Number 1U38OT000143-04. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Azad AF, Radulovic S, Higgins JA, Noden BH, Troyer JM. Flea-borne rickettsioses: Ecologic considerations. Emerg Infect Dis. 1997; 3:319–327. doi: 10.3201/eid0303.970308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Civen R, Ngo V. Murine typhus: An unrecognized suburban vector-borne disease. Clin Infect Dis. 2008; 46:913–918. doi: 10.1086/527443 [DOI] [PubMed] [Google Scholar]
- 3.(CDPH) California Department of Public Health. Typhus and other non-spotted fever rickettsioses case report (CDPH working case definition, 2011). http://publichealth.lacounty.gov/acd/Diseases/EpiForms/TyphusOtherNonSpottedRickettsiosesCaseReport-CDPH8580.pdf (Accessed February 2, 2018).
- 4.Foo C, Croker C, Nelson K, Wekesa JW, Fujioka K, Civen R. An outbreak of flea-borne typhus associated with a mobile home community- Los Angeles County, California, 2015. Acute Communicable Disease Control Annual Report. http://publichealth.lacounty.gov/acd/Publications.htm
- 5.(CDPH) California Department of Public Health. Flea-Borne Typhus. 2014 http://www.cdph.ca.gov/HealthInfo/discond/Documents/Flea-borneTyphus.pdf
- 6.Acute Communicable Disease Control 2005 Special Reports. A suburban neighborhood outbreak of murine typhus, South Pasadena, May 2005. (http://www.publichealth.lacounty.gov/acd/reports/spclrpts/spcrpt05/Murine_SS05.pdf) (Accessed September 1, 2016)
- 7.Wekesa JW, Nelson K, Brisco A, Cook M, Fujioka K. History of flea-borne typhus in Los Angeles County, California. Proc Mosq Vector Control Assoc Calif. 2016a; 84:1–4. [Google Scholar]
- 8.Adams JR, Schmidtmann ET, Azad AF. Infection of colonized cat fleas, Ctenocephalides felis (Bouche), with a rickettsia-like microorganism. Am J Trop Med Hyg. 1990; 43:400–409. [DOI] [PubMed] [Google Scholar]
- 9.Reif KE, Macaluso KR. Ecology of Rickettsia felis: a review. J Med Ent. 2009; 46:723–736. [DOI] [PubMed] [Google Scholar]
- 10.Cummings R, Krueger L, Nguyen K, Fogarty C, Bennett S, Velten R, et al. The conflicting roles of vector control and animal control agencies in mitigating the rise of human cases of flea-borne typhus in Orange County, California. In: Proc 26th Vert Pest Conf, (R.M. Timm and J.M. O’Brien, Editors). Published at UC Davis; pp. 316–322.
- 11.Gerhold RW, Jessup DA. Zoonotic diseases associated with free-roaming cats. Zoonoses and Public Health. 2013; 60:189–195. doi: 10.1111/j.1863-2378.2012.01522.x [DOI] [PubMed] [Google Scholar]
- 12.Maina AN, Fogarty C, Krueger L, Macaluso KR, Odhiambo A, Nguyen K et al. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County, California. PLoS ONE. 2016a; 11:e0160604 doi: 10.1371/journal.pone.0160604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tay ST, Koh FX, Kho KL, Sitam FT. Rickettsial infections in monkeys, Malaysia. Emerg Infect Dis. 2015; 21: 545–547. doi: 10.3201/eid2103.141457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolo AO, Sibeko-Matjila KP, Maina AN, Richards AL, Knobel DL, Matjila PT. Molecular detection of zoonotic rickettsiae and Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector-Borne and Zoonotic Diseases. 2016; 16:245–52. doi: 10.1089/vbz.2015.1849 [DOI] [PubMed] [Google Scholar]
- 15.Socolovschi C, Pages F, Ndiath MO, Ratmanov P, Raoult D. Rickettsia species in African Anopheles mosquitoes. 2012; PLoS One 7: e48254 doi: 10.1371/journal.pone.0048254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomel B, Kasten R, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, et Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996; 34:1952–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longcore T, Rich C, Sullivan LM. Critical assessment of claims regarding management of feral cats by trap–neuter–return. Conservation Biology. 2009; 23:887–894. doi: 10.1111/j.1523-1739.2009.01174.x [DOI] [PubMed] [Google Scholar]
- 18.Wekesa, JW, Nelson K, Brisco A, Fujioka K. 2016b. Vector control has a role to play in mitigating the high incidence of flea-borne typhus in Los Angeles County, California. In: Proc 27th Vertebrate Pest Conf (RM Timm and RA Baldwin, Editors). Published at UC Davis 2016; pp. 288–292.
- 19.Croker C, Foo C, Tormey M, Nelson KJ, Wekesa JW, Fujioka K, et al. Opossums, fleas and human disease: A collaborative approach for mitigating an outbreak of murine typhus in a mobile home community, Los Angeles County, California. 2015. Ann Conf Council of State and Territorial Epidemiologists, 19–23 June 2016, Anchorage, Alaska.
- 20.Nelson K, Brisco A, Holquin G, Foo C, Croker C, Cook M, Civen R, Fujioka K, and Wekesa W. Use of abatement to reduce intensity of a flea-borne typhus outbreak in the San Gabriel Valley, Los Angeles County, California. Proc Mosq Vector Control Assoc Calif. 2016; 84:104–106. [Google Scholar]
- 21.Pratt HD, Stojanovich CJ. Fleas: Illustrated key to species found on domestic rats in southern United States. In: Pictorial keys to arthropods, reptiles, birds and mammals of public health significance. US Department of Health, Education, and Welfare, Communicable Disease Center, Atlanta, Georgia 1966; 167–174.
- 22.Jiang J, Stromdahl EY, Richards AL. 2012. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector Borne Zoonotic Dis. 2012; 12:175–182. doi: 10.1089/vbz.2011.0614 [DOI] [PubMed] [Google Scholar]
- 23.Odhiambo AM, Maina AN, Taylor ML, Jiang J, Richards AL. Development and validation of a quantitative real-time polymerase chain reaction assay specific for the detection of Rickettsia felis and not Rickettsia felis-like organisms. Vector Borne Zoonotic Dis. 2014; 14: 476–481. doi: 10.1089/vbz.2013.1518 [DOI] [PubMed] [Google Scholar]
- 24.Leulmi H, Socolovschi C, Laudisoit A, Houemenou G, Davoust B, Bitam I, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014; 8:e3152 doi: 10.1371/journal.pntd.0003152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry KM, Jiang J, Rozmajzl PJ, Azad AF, Macaluso KR, Richards AL. Development of quantitative real-time PCR assays to detect Rickettsia typhi and Rickettsia felis, the causative agents of murine typhus and flea-borne spotted fever. Mol Cell Probes. 2007; 21:17–23. doi: 10.1016/j.mcp.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 26.Jiang J, Maina AN, Knobel DL, Cleaveland S, Laudisoit A, Wamburu K, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013; 13:550–558. doi: 10.1089/vbz.2012.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graf PC, Chretien JP, Ung L, Gaydos JC, Richards AL. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse US sample. Clin Infect Dis. 2008; 46:70–77. doi: 10.1086/524018 [DOI] [PubMed] [Google Scholar]
- 28.Maina AN, Luce-Fedrow A, Omulo S, Hang J, Chan TC, Ade F, Jima DD, Ogola E, Ge H, Breiman RF, Njenga MK. Isolation and characterization of a novel Rickettsia species (Rickettsia asembonensis sp. nov.) obtained from cat fleas (Ctenocephalides felis). Int J Syst Evol Microbiol. 2016b; 66:4512–7. [DOI] [PubMed] [Google Scholar]
- 29.Adams WH, Emmons RW, Brooks JE. The changing ecology of murine (endemic) typhus in Southern California. Am J Trop Med Hyg. 1970; 19:311–318. [DOI] [PubMed] [Google Scholar]
- 30.Adjemian J, Parks S, McElroy K, Campbell J, et al. Murine typhus in Austin, Texas, USA, 2008. Emerg Infect Dis. 2010; 16:412–417. doi: 10.3201/eid1603.091028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eremeeva ME, Karpathy SE, Krueger L, Hayes EK, Williams AM, Zaldivar Y, Bennett S, Cummings R, Tilzer A, Velten RK, Dasch GA. Two pathogens and one disease: detection and identification of flea-borne Rickettsia in areas endemic for murine typhus in California. J Med Entomol 2012; 49: 1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boostrom A, Beier MS, Macaluso JA, Macaluso KR, Sprenger D, Hayes J, et al. Geographic association of Rickettsia felis-infected opossums with human murine typhus, Texas. Emerg Infect Dis. 2002; 8:549–554. doi: 10.3201/eid0806.010350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schriefer ME, Sacci JB Jr, Dumler JS, Bullen MG, Azad AF. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol. 1994; 32:949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eremeeva ME, Warashina WR, Sturgeon MM, Buchholz AE, Olmsted GK, et al. (2008) Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawai’i. Emerg Infect Dis. 2008; 14:1613–1615. doi: 10.3201/eid1410.080571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billeter SA, Diniz PP, Jett LA, Wournell AL, Kjemtrup AM, Padgett KA, Yoshimizu MH, Metzger ME, Barr MC. Detection of Rickettsia species in fleas collected from cats in regions endemic and nonendemic for flea-borne rickettsioses in California. Vector Borne Zoonotic Dis. 2016; 16:151–156. doi: 10.1089/vbz.2015.1869 [DOI] [PubMed] [Google Scholar]
- 36.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997; 35: 2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raoult D, Berbis P, Roux V, Xu W, Maurin M. A new tick-transmitted disease due to Rickettsia slovaca. Lancet. 1997; 350: 112–113. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson K, Lindquist O, Pahlson C. Association of Rickettsia helvetica with chronic perimyocarditis in sudden cardiac death. Lancet. 1999; 354: 1169–1173. doi: 10.1016/S0140-6736(99)04093-3 [DOI] [PubMed] [Google Scholar]
- 39.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004; 38: 805–811. doi: 10.1086/381894 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its supporting files. Additional data is also available from the GenBanks accession numbers CP000053, KF666472.