Abstract
Aims
In HIV-infected individuals, non-injection drug use (NIDU) compromises many health outcomes. In HIV primary care, the efficacy of brief motivational interviewing (MI) to reduce NIDU is unknown, and drug users may need greater intervention. We designed an enhancement to MI, HealthCall (HC), for daily patient self-monitoring calls to an interactive voice response (IVR) phone system, and provided participants with periodic personalized feedback. To reduce NIDU among HIV primary care patients, we compared the efficacy of MI+HealthCall to MI-only and an educational control condition.
Design
Participants age >18 with >4 days of NIDU during the prior 30 days were recruited from large urban HIV primary care clinics. Of the 240 participants, 83 were randomly assigned to control, 77 to MI-only, and 80 to MI + HC. Counselors provided educational control, MI-only or MI + HC at baseline. At 30 and 60 days (end-of-treatment), counselors briefly discussed drug use, moods and health behaviors, using HealthCall-generated graphs with MI + HC patients. Primary outcomes (last 30 days) were number of days used primary drug (NumDU), and total quantity of primary drug used (dollar amount spent; QuantU), derived from the Time-Line Follow-Back.
Findings
Across all groups, at end-of-treatment, frequency and quantity of NIDU decreased, with significantly greater reductions in the MI-Only group. A twelve-month post-treatment follow-up indicated sustained benefits of MI + HC and MI-only relative to control.
Conclusions
Brief interventions can be successfully used to reduce non-injection drug use in HIV primary care. IVR-based technology may not be sufficiently engaging to be effective. Future studies should investigate mobile technology to deliver a more engaging version of HealthCall to diverse substance abusing populations.
Keywords: HIV, Drug use, IVR intervention, Motivational interviewing
1. Introduction
HIV infection is highly prevalent among non-injection cocaine and heroin users in the US (Des Jarlais et al., 2014; Keen, Khan, Clifford, Harrell, & Latimer, 2014; Mitchell & Latimer, 2009). In HIV patients, non-injection drug use (NIDU) is associated with multiple adverse outcomes, including shortened survival (Carrico et al., 2007; Colfax & Guzman, 2006; Cook et al., 2008; Kapadia et al., 2005; Lucas et al., 2006), worse prognosis due to poor antiretroviral medication adherence (Baum et al., 2009; French et al., 2009; Kipp, Desruisseau, & Qian, 2011; Moore et al., 2012; Van & Koblin, 2009; Wynn, Cozza, Zapor, Wortmann, & Armstrong, 2005) and sexual transmission of HIV (Khan et al., 2013; Strathdee & Sherman, 2003).
Among HIV-infected individuals in primary medical care, 20–40% use drugs (Durvasula & Miller, 2014), often non-injected cocaine or crack (Pence, Miller, Whetten, Eron, & Gaynes, 2006; Pisu et al., 2010; Shacham, Onen, Donovan, Rosenburg, & Overton, 2014; Skalski, Sikkema, Heckman, & Meade, 2013). While HIV primary care has long been recommended as an entry point for addressing drug use in HIV-infected individuals (Aberg et al., 2004; Centers for Disease Control and Prevention, 2004; Del Rio, 2003; Wilson et al., 2006), barriers in primary care settings include lack of in-clinic resources offering extended substance abuse interventions, and lack of patient interest in such interventions when they are available.
Given these concerns, brief evidence-based interventions such as Motivational Interviewing (MI) would seem to offer advantages over more complex and lengthy interventions (Martins & McNeil, 2009; Miller & Rollnick, 2002; Miller et al., 2006; Neushotz & Fitzpatrick, 2008). However, in patients with complex problems, including HIV, brief MI almost certainly needs enhancement by some form of ongoing intervention or repeated contact to be effective (Emmons & Rollnick, 2001). Although brief interventions to reduce drug use among patients in primary care are currently being rolled out across the United States (Substance Abuse and Mental Health Services Administration (SAMHSA), 2016), recent findings of limited efficacy for such interventions (Roy-Byrne et al., 2014; Saitz, 2014; Saitz et al., 2014) have led to calls to rethink delivery of brief interventions for drug abuse in primary care (Hingson & Compton, 2014) and to find more effective methods of addressing drug abuse in such patients.
Technology offers innovative ways to extend health interventions (Kempf, Huang, Savage, & Safren, 2015; Lester et al., 2010; Marsch, Carroll, & Kiluk, 2014). Automated telephone interactive voice response (IVR) systems can be designed to provide daily questions for self-monitoring, with patients’ answers stored in a database. When combined with another intervention, self-monitoring techniques reduce addictive behaviors (Moore et al., 2013; Mullen et al., 1997; Rose, Skelly, Badger, Naylor, & Helzer, 2012). In recent years, IVR has been used to improve the medical management of various health-related behaviors (David et al., 2012; Naylor, Naud, Keefe, & Helzer, 2010; Oake, Jennings, van Walraven, & Forster, 2009; Swendeman et al., 2015; Wolin et al., 2015). IVR does not require literacy, technical knowledge or special equipment, suggesting that it could be useful in low-income, low-literacy populations (Schroder, Johnson, & Wiebe, 2007). Therefore, we designed ‘HealthCall’ to incorporate IVR in enhancing brief intervention. HealthCall combines two main process elements: (1) daily calls to an interactive voice response (IVR) system for daily self-monitoring of the behavior targeted for change; and (2) personalized feedback (Emmons & Rollnick, 2001; Miller & Rollnick, 1991) based on the daily call data, presented to the patient and discussed briefly at two 30-day intervals. HealthCall, delivered over 60 days, includes the following steps: (1) after an initial brief MI session, the participant is instructed in how to make a daily call of ~2.5 min to self-monitor the targeted behavior and is advised to make the calls as an aid to decreasing the behavior; (2) individual personalized feedback graphs reflecting participants’ HealthCall-reported target behavior are presented to patients by their MI counselors at 30-day intervals to facilitate brief (~10 min) discussions of patients’ reductions of targeted behaviors.
Previously, we conducted a trial showing that MI+HealthCall achieved significantly greater drinking reduction than MI-only or educational control in HIV primary care alcohol-dependent patients (Hasin et al., 2013). We adapted HealthCall for non-injection drug users, and then conducted a randomized pilot study (N = 40) that (a) suggested better results with MI+HealthCall than with MI-only, and (b) indicated that HealthCall was acceptable to non-injection drug users in HIV primary care (Aharonovich et al., 2012). We subsequently investigated the efficacy of HealthCall to reduce non-injection drug use (NIDU) in HIV primary care patients. We now report the results of a large randomized trial comparing MI+HealthCall to MI-only and to an education control condition among largely minority, low SES non-injection drug users participating in HIV primary care clinics.
2. Method
2.1. Setting and participants
The study was conducted at three large urban HIV primary care clinics. Inclusion criteria included being age ≥18 years, enrollment in one of two New York City hospital-affiliated clinics, NIDU on ≥4 days during the prior 30 days, and English- or Spanish-speaking. Exclusion criteria included active psychosis, suicidality, gross cognitive impairment, injection drug use during the prior 30 days, and alcohol or marijuana as the patient’s primary substance. Participants provided written informed consent. Institutional review boards at the New York State Psychiatric Institute, St Luke’s-Roosevelt Hospital and Mt. Sinai Medical Center in New York City approved all procedures.
2.2. Procedures
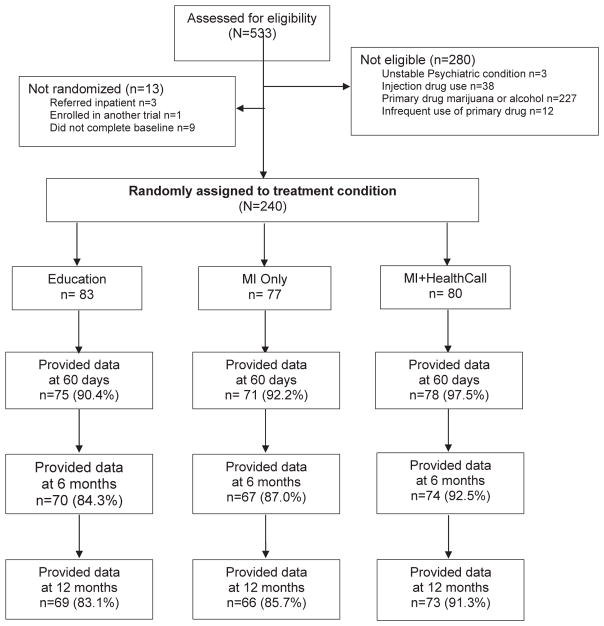
Substance-using patients attending a primary HIV clinic visit were informed about the study by their providers, who referred them, if interested, to meet with a study coordinator for written informed consent and assessment of eligibility. Potential participants were told that the purpose of the study was to investigate whether a brief meeting with a trained health care worker that was followed or not followed by brief daily phone calls about drug use would help patients reduce their drug use. Of 533 individuals assessed, 240 met eligibility, completed baseline assessments, and were randomized (see Study CONSORT Fig. 1). In a parallel three-arm randomized design (1:1:1 allocation ratio), participants were assigned to one of three conditions: MI+ HealthCall; MI-only, or educational control (viewing a DVD on HIV self-care) that did not include drug use content. Randomization was stratified on drug use severity, depression and unstable housing using urn randomization (Zhao, Weng, Wu, & Palesch, 2012). All baseline assessments were completed prior to random assignment to treatment condition. Counselors administering MI-only or MI+HealthCall were blind to participants’ assignment to these two treatment conditions until after the MI was administered, when they received the assignment via text message. Counselors and participants were not blinded to treatment condition after assignment. Study procedures were conducted in English or Spanish (participants’ preference). Participants were compensated with gift cards for their assessments at each study visit.
Fig. 1.
CONSORT diagram of participants flow and data availability1 in a study of HealthCall based IVR to reduce non-injection drug use. 1Participants who missed assessment provided data for that period by completing a retrospective TLFB at their subsequent assessment.
2.3. Interventions
Bi-lingual study counselors (BA and MA levels) delivered the interventions. Visit time required for the baseline intervention (30 min) was equally balanced across the three conditions. The total length of the treatment period was 60 days for all three conditions. Counselors were trained and certified in MI and attended weekly supervision meetings. MI sessions were audiotaped and reviewed for quality assurance and to prevent counselor drift. Participants returned at two 30-day intervals after the baseline intervention session (30-day and 60-day) for assessments and a brief booster session (10–15 min) with the study counselor. Follow-up assessment visits occurred at 3, 6 and 12 months after baseline.
2.4. Education arm (control)
Study counselors informed participants that their use of non-injection drugs was at levels potentially harmful to their health, and showed participants a 30-min educational HIV self-care DVD (English or Spanish versions) that did not include specific content about substance use. Counselors then provided a digital alarm wrist watch to patients, and suggested that they use it as a medication reminder. (This was given to be parallel with the other two arms.) At 30 and 60 days, participants returned for assessment and brief booster advice sessions, where substance use reduction was encouraged.
2.5. MI-only arm
The study counselor administered a 25–30 min individual MI session using standard MI techniques. These techniques included a dialogue with the patient about health consequences of drug use, exploring ambivalence, pros and cons of use, patient’s readiness to change, and perceived importance of change and when possible, working with the patient to set a drug reduction goal for the next 30 days. At the end of the session, counselors provided an alarm wrist watch, suggesting that participants use it daily as a medication reminder. Patients returned at 30 and 60 days for assessments and booster sessions in which counselors discussed patients’ drug use during the past 30 days, evaluated the drug goal and worked with the patient to set a new (lower) goal if desired.
2.6. MI+HealthCall arm
The study counselor conducted a MI session with the techniques and goals described for the MI-only arm. Counselors then briefly introduced and explained HealthCall, and guided the participant through a first practice use of HealthCall. Counselors and participants then determined the most convenient time to call HealthCall daily and set the watch alarm accordingly. Participants were asked to make a daily call for the next 30 days. Participants who did not use HealthCall for more than two consecutive days (>48 h) received a reminder call from their counselor to resume calling.
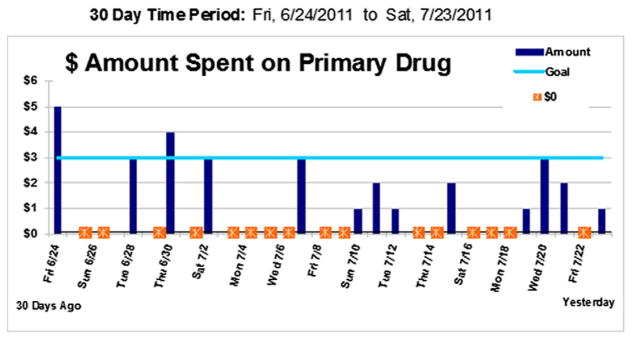
Participants accessed HealthCall via a toll-free number for daily, ~2.5 min calls, answering pre-recorded questions about ‘yesterday’ (morning, afternoon, evening) to ensure consistent reporting periods regardless of the hour called. A set of brief self-monitoring pre-recorded questions in English or Spanish (participant’s choice) about the previous day asked about use of participant’s primary drug, dollar amount of the drug used, use of other drugs, HIV medication adherence, and feelings of wellness, stress, and overall life quality. The IVR daily data were used to produce personalized feedback, including a graph showing days used primary drug, dollar amount spent on each day, and initial drug reduction goal set in the MI session (Fig. 2). Below the graph, summary statistics of other data were shown (e.g., daily average dollar amount spent; reasons for using or not using drugs). At the 30-day booster visit, the counselor showed and used the personalized graph, and summary statistics as the basis for a brief ~15-min discussion of participant’s drug use, mood, and overall health. The drug reduction goal was re-evaluated and re-set on the IVR system if participants wished. Participants were then asked to resume calling for another 30 days. At the following visit, 60 days after baseline (end of treatment), a booster session was conducted including discussion of the graph and the summary statistics that were generated based on the 31–60-day period.
Fig. 2.
Sample personalized HealthCall data based graph showing goal set at $3; $0 = no use. Graphs were part of the feedback given in the MI+HealthCall arm at 30 and 60-day visits.
2.7. MI training and fidelity
A PhD-level MI Network Trainer trained the counselors and made standardized periodic fidelity ratings (Moyers, Martin, Manuel, Hendrickson, & Miller, 2005) on 8% of randomly chosen MI sessions (13/160). These showed acceptable to excellent fidelity on the following domains: mean % complex reflections (52.05%), reflection/question ratio (7.85), MI spirit scores (3.84/5), MI empathy scores (3.92/5), and mean number of MI non-adherent statements (1.0).
2.8. Assessments
Assessments included an interviewer-administered Computer-Assisted Personal Interview (CAPI), administered at baseline prior to randomization and subsequently, including 60 days (end of treatment), and 6 and 12-month post-baseline follow-ups. Drug use was assessed at all time-points with a 30-day Timeline-follow back (TLFB), which uses a calendar and memory aids to reconstruct estimates of drug use levels (Sobell & Sobell, 1995). If participants missed an assessment but returned for subsequent assessments, a retrospective TLFB was administered to cover the missed time periods. The mean/median recall interval was 88/69 days (range, 21–319), which results in little or no loss of data reliability (Robinson, Sobell, Sobell, & Leo, 2014).
Drug use reports were confirmed with rapid urine screens (iScreens® panel for cocaine, amphetamine, methamphetamine, THC, opiates, PCP, barbiturates and benzodiazepines) on each of the assessments. Agreement between the TLFB and confirmatory drug screen was 97.23% for those reporting positive use in the past 3 days and 73.63% for those reporting abstinence in the past 3 days. This high concordance rate is consistent with the accuracy and use of TLFB data for alcohol and substance use in other studies (Dillon, Turner, Robbins, & Szapocznik, 2005; Fals-Stewart, O’Farrell, Freitas, McFarlin, & Rutigliano, 2000).
2.9. Outcomes
The primary study outcomes were frequency, the total number of days of primary drug used in the prior 30 days (NumDU), and quantity, represented by the total dollar amount of primary drug used (QuantU) in the prior 30 days, as derived from the TLFB.
2.10. Statistical analysis
Pre-study power computations indicated that n = 130 per group would provide 80% power at α = 0.05 to detect a moderate treatment effect (effect size = 0.36) on days of drug use, guided by our pilot results (Aharonovich et al., 2012; Hasin, Aharonovich, & Greenstein, 2014). We stopped enrollment before reaching our target due to a change in hospital ownership that precluded further enrollment. All tests were two-tailed; p < 0.05 indicated significance, with trends toward significance at p = 0.05–0.09. Participants were all analyzed in their originally assigned treatment group.
2.11. Primary analysis – treatment effect on frequency (NumDU) & Quantity (QuantU)
Descriptive information (e.g. mean, SD) is presented on levels of NumDU and QuantU within each treatment group over time. The primary analysis was designed to examine efficacy at the end of treatment and at 6 and 12 months after baseline. For this, we utilized a generalized linear model (PROC GENMOD, SAS 9.4) to test the main effect of treatment on changes in NumDU and QuantU between baseline and the three time points: 60 days (end of treatment), 6- and 12-month follow-up. Values for NumDU and QuantU were log-transformed and specified as having a negative binomial distribution due to skewness. QuantU was shifted by 1 to avoid infinite values for participants who achieved abstinence at one or more study time points. In models of NumDU and QuantU at different study time points, we controlled for participants’ baseline levels of NumDU and QuantU, respectively. Also presented are pre-planned contrasts of NumDU and QuantU between treatment groups.
2.12. Sensitivity analyses
First, as a sensitivity analysis, a different analytic strategy was used to determine the robustness of the findings if all assessment time points were included. This analysis therefore included data on NumDU and QuantU at baseline (30 days, 60 days, and 3, 6 and 12 months). These data were analyzed using a generalized linear mixed effects repeated measures model. This model is robust to the effects of missing data, uses a random intercept to account for within-subject correlation across time point and between-subject heterogeneity at baseline (PROC GLIMMIX, SAS 9.4). Treatment effects were tested with interactions between treatment group and time.
Because preliminary examination of outcome means over time suggested a marked difference in the trends in substance use over time during the first 30 days compared with the subsequent study period, we tested piece-wise linear models with a knot at 30 days. These tests yielded Bayesian Information Criterion (BIC) scores that were significantly reduced compared to models with time specified as a simple linear effect (Raftery, 1995), indicating better model fit in the models incorporating the knot at 30 days. Thus, a knot at 30 days was retained in the model.
Second, we substituted results from the urine drug tests for TLFB data, and examined the results for end of treatment (60 days). For this, we used logistic regression since the outcome was binary, and controlled for baseline urine test result.
2.13. Exploratory analysis
Based on our previous HealthCall trial for HIV-infected heavy drinkers showing that HealthCall primarily had its effects among participants with alcohol dependence (Hasin et al., 2013), we explored treatment differences among the subset of 39 participants with current DSM-IV drug dependence at baseline. Participants were diagnosed with current drug dependence if they met criteria for DSM-IV dependence for their primary drug during the 30 days prior to baseline. We used Kruskal-Wallis non-parametric tests of whether the treatment groups differed on changes in NumDU and QuantU between baseline and their values at 60 days (end of treatment), 6 and 12-month follow-up assessments.
3. Results
3.1. Sample characteristics
As per study CONSORT (Fig. 1), of 533 individuals assessed, 240 met eligibility criteria and were randomized to MI+HealthCall (n = 80), MI-only (n = 77) or education/control (n = 83). Six participants withdrew consent (3 MI-Only, 2 MI+HealthCall, 1 control) and five participants expired during the study period due to HIV medical complications, unrelated to the study protocol (3 education/control, 1 MI-Only, 1 MI+HealthCall) and were therefore dropped from the analysis. Of those randomized, 234 were confirmed as HIV positive; six participants were HIV-negative and receiving preventive HIV services at the clinic. Mean years since initial HIV diagnosis was 15.4 years (SD = 8.0).
Participants were primarily male (83.7%), black or Hispanic (80.8%), single (89.2%), unemployed (85.8%), and had at least a high-school education (68.8%) as presented in Table 1. Mean age was 46.5 (SD = 9.3) years. The majority (70.4%) used crack/cocaine as their primary drug, and on average used their primary drug on 8.7 days (SD = 6.8) and an amount equivalent to $15.78 worth per day (SD = 19.47) overall during the 30 days prior to baseline (See Table 1). Analysis of variance and chi-square analyses indicated no significant differences by treatment conditions on any of the variables presented in Table 1.
Table 1.
Baseline characteristics of study participants.
| Education (control) (n = 83) No. (%) |
MI-Only (n = 77) | MI + HealthCall (n = 80) | Full sample (N = 240) | p-value | |
|---|---|---|---|---|---|
| Female | 14 (16.87) | 10 (12.99) | 15 (18.75) | 39 (16.25) | 0.61 |
| Ethnicity | 0.64 | ||||
| African American | 48 (57.83) | 39 (50.65) | 45 (56.25) | 132 (55.00) | b |
| Hispanic | 17 (20.48) | 23 (29.87) | 22 (27.50) | 62 (25.83) | b |
| Other | 18 (21.69) | 15 (19.48) | 13 (16.25) | 46 (19.17) | b |
| Spanish-speaking | 1 (1.20) | 0 (0.00) | 3 (3.75) | 4 (1.67) | 0.17 |
| High school education | 62 (74.70) | 49 (63.64) | 54 (67.50) | 165 (68.75) | 0.31 |
| Married/stable relationship | 9 (10.84) | 6 (7.79) | 11 (13.75) | 26 (10.83) | 0.49 |
| Employed | 10 (12.20) | 12 (15.58) | 12 (15.00) | 34 (14.23) | 0.81 |
| Current DSM-IV drug dependencea | 14 (17.07) | 12 (15.58) | 13 (16.25) | 39 (16.32) | 0.97 |
| Primary drug type | 0.95 | ||||
| Crack | 42 (50.60) | 38 (49.35) | 39 (48.75) | 119 (49.58) | b |
| Cocaine | 14 (16.87) | 18 (23.38) | 18 (22.50) | 50 (20.83) | b |
| Meth | 18 (21.69) | 15 (19.48) | 15 (18.75) | 48 (20.00) | b |
| Heroin | 9 (10.84) | 6 (7.79) | 8 (10.00) | 23 (9.58) | b |
| Mean (SD) | |||||
| Age, years | 46.72 (10.32) | 47.38 (8.31) | 45.53 (9.23) | 46.54 (9.34) | 0.46 |
| Years since HIV diagnosisc | 14.96 (8.15) | 15.58 (6.88) | 15.75 (8.85) | 15.42 (7.99) | 0.81 |
| Number of days used (NumDU)d | 8.18 (6.46) | 9.29 (7.21) | 8.54 (6.86) | 8.65 (6.83) | 0.58 |
| Amount $ spent (QuantU)e | 16.12 (23.53) | 15.02 (17.61) | 16.15 (16.52) | 15.78 (19.47) | 0.92 |
MI = motivational interviewing; SD = standard deviation.
DSM-IV drug dependence on participant’s primary drug during the 30 days prior to baseline.
P-values not given for pair-wise contrasts between each ethnic group or each primary drug type because these-variables were tested as three- and four-level variables, respectively.
Mean years since HIV diagnosis computed among HIV+ participants (n = 234); six participants were provided HIV prevention services.
During 30 days prior to baseline.
Average dollar amount per day used over 30 days prior to baseline.
3.2. Retention, engagement and data availability by condition
At 60 days, retention in the MI+HealthCall, MI-only and education group was 88.8%, 81.8%, and 78.3%, respectively, a non-significant difference (p = 0.20). At 12-month follow-up, the retention rates were 91.3%; 84.4% and 83.1% respectively (p = 0.27). Among participants assigned to MI+HealthCall, the median of IVR calls made was 64.1%, after excluding incarcerated or hospitalized days when participants’ ability to call was not under their control. Of the 240 individuals randomized to treatment, Timeline-follow-back (TLFB) data were provided by 224 (96.5%) at the 60-day point (end of treatment), and 211 (87.9%) at the 6-month and 208 (86.7%) at the 12-month point (Fig. 1).
3.3. Descriptive drug use outcomes, by treatment group
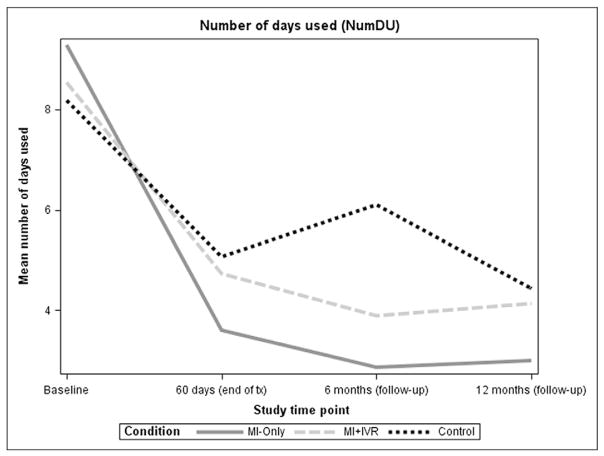
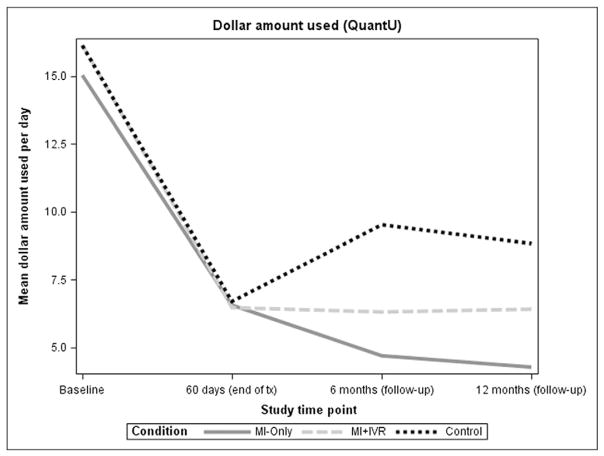
Figs. 3 and 4 present means of NumDU and QuantU, respectively, at baseline, 60 days, 6 and 12-month assessments, by treatment group. Compared to baseline, participants in all treatment groups decreased their NumDU and QuantU substantially by the end of treatment. Means (and SDs) of NumDU at 60 days were 5.07 (6.70) in control; 3.61 (5.79) in MI-only; and 4.73 (7.22) in MI+HealthCall group. Means (and SDs) of QuantU at 60 days were 6.56 (17.04) in control; 6.47 (8.65) in MI-only; and 6.70 (9.79) in MI+HealthCall group. Reduction in NumDU and QuantU was sustained for MI-only and MI+ HealthCall up to 12 months, while the control group did not show sustained reductions.
Fig. 3.
Means of number of drug used days by treatment group.
Fig. 4.
Means of quantity use (dollar amount) by treatment group.
3.4. Primary analysis: treatment effects on NumDU and QuantU
As presented in Table 2, NumDU and QuantU reduction between baseline and end of treatment did not differ significantly between participants in three arms at end of treatment, but did differ significantly at 6-month follow-up for NumDU and QuantU, and differed significantly at 12 months for QuantU with a trend toward significance for NumDU. Group contrasts indicated that the overall differences arose primarily from greater reduction in MI-only versus control, when, for example, reduction in NumDU was 51% greater in MI-Only than in control at six months, and 46% greater at 12 months. Similarly, reduction in QuantU was 50% greater in MI-only than control at 6 months, and 55% greater at 12 months.
Table 2.
Treatment effect on drug use reductions, at end of intervention, 6 and 12 month follow-up (generalized linear model).
| Overall treatment effectc
|
Contrasts
|
||||
|---|---|---|---|---|---|
| MI vs. Controld | MI+HealthCall vs. Controld | MI+HealthCall vs. MIe | |||
| χ2, df | p | Incidence risk ratio (95% confidence interval) | |||
| NumDUa | |||||
| End of intervention (at 60 days) (n = 224) | 2.78, 2 | 0. 25 | 0.68 (0.43–1.08) | 0.77 (0.49–1.21) | 1.13 (0.71–1.78) |
| 6 month follow-up (n = 211) | 7.27, 2 | 0.03 | 0.49 (0.29–0.85) | 0.59 (0.35–1.00) | 1.20 (0.70–2.06) |
| 12 month follow-up (n = 208) | 4.88, 2 | 0.09 | 0.54 (0.31–0.94) | 0.81 (0.47–1.38) | 1.51 (0.87–2.62) |
| QuantUb | |||||
| End of intervention (at 60 days) (n = 224) | 0.53, 2 | 0.77 | 0.83 (0.49–1.43) | 0.85 (0.51–1.44) | 1.02 (0.60–1.74) |
| 6 month follow-up (n = 211) | 15.92, 2 | <0.01 | 0.50 (0.28–0.90) | 0.75 (0.42–1.33) | 1.50 (0.84–2.67) |
| 12 month follow-up (n = 208) | 15.08, 2 | 0.04 | 0.45 (0.25–0.83) | 0.72 (0.40–1.31) | 1.60 (0.87–2.93) |
Incidence risk ratios of treatment group contrasts at study time points.
MI = motivational interviewing.
NumDU = mean number of days used primary drug during the 30 days prior to study visit.
QuantDU = mean dollar amount per day used over 30 days prior to study visit.
Corresponds to the omnibus test of treatment as a predictor of drug use outcome.
Control = Reference group.
MI = Reference group.
3.5. Sensitivity analyses: treatment effects on NumDU and QuantU
In the first sensitivity analysis, repeated measures analysis of NumDU and QuantU generally agreed with results of our primary analysis, indicating results that were robust across analysis types. The key differences were two between-group contrasts (Table 3): at the end of treatment, participants in MI-only reduced NumDU significantly more than those in control (p = 0.01); and at the end of 12 months, participants in MI-Only reduced NumDU significantly more than those in MI+HealthCall (p = 0.03).
Table 3.
Sensitivity analysis results: Treatment effect on drug use reduction at end of treatment and 6 and 12-month follow-up (generalized linear mixed effects repeated measures model).
| Overall model significance | Contrasts
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MI-only versus Controlc | MI+HealthCall versus Controlc | MI+HealthCall versus MI-onlyd | ||||||
| χ2, df | p | t, df | p | t, df | p | t, df | p | |
| NumDUa (N = 240) | ||||||||
| End of treatment (60 days) | 15.65, 4 | <0.01 | −2.60, 1082 | 0.01 | −1.34, 1082 | 0.18 | 1.27, 1082 | 0.2 |
| 6-month follow-up | −3.07, 1082 | <0.01 | −1.16, 1082 | 0.25 | 1.93, 1082 | 0.054 | ||
| 12-month follow-up | −2.73, 1082 | 0.01 | −0.63, 1082 | 0.53 | 2.13, 1082 | 0.03 | ||
| QuantUb (N = 240) | ||||||||
| End of treatment (60 days) | 15.83, 4 | <0.01 | −1.27, 1082 | 0.2 | −1.65, 1082 | 0.1 | −0.35, 1082 | 0.73 |
| 6-month follow-up | −1.88, 1082 | 0.06 | −1.35, 1082 | 0.18 | 0.55, 1082 | 0.58 | ||
| 12-month follow-up | −2.15, 1082 | 0.03 | −0.65, 1082 | 0.52 | 1.53, 1082 | 0.13 | ||
MI = motivational interviewing.
NumDU = mean number of days used primary drug during the 30 days prior to study visit.
QuantDU = mean dollar amount per day used over 30 days prior to study visit.
Control = Reference group.
MI = Reference group.
In the second sensitivity analysis examining urine test results at end-of-treatment (60 days), results were very similar to results based on the self-reported TLFB. The overall treatment effect on drug use missed significance (p = 0.10; Supplementary Table 1). When looking at individual contrasts between each treatment group, the MI-Only group had significantly lower odds of drug use than the control group (OR = 0.44, 95% CI = (0.21–0.94), p = 0.035). Neither of the other two between-group contrasts was significant.
3.6. Exploratory analysis: treatment effects within the subset of drug dependent participants
Median values of NumDU and QuantU are shown in Table 4 for the 39 participants with DSM-IV drug dependence at baseline. The Kruskal-Wallis test of differences in treatment effects between baseline and follow-up among these 39 participants did not show significant differences at end of treatment. At six months, MI-only appeared superior to MI+HealthCall for NumDU and QuantU. While treatment groups did not differ significantly at 12 months (p = 0.12 and 0.11 for NumDU and QuantU, respectively), drug use was lower at 12 months among participants in MI+HealthCall than in the other groups.
Table 4.
Median outcome values, participants with DSM-IV drug dependence at baseline (n = 39).
| Baseline | 60-day | p-valuea | 6-month | p-valuea | 12-month | p-valuea | |
|---|---|---|---|---|---|---|---|
| NumDU (frequency) | |||||||
| Control (n = 14) | 7.5 | 3.0 | 0.24 | 6.0 | 0.02 | 4.0 | 0.12 |
| MI-only (n = 12) | 12.0 | 0.0 | 0.0 | 3.0 | |||
| MI+HealthCall (n = 13) | 9.0 | 5.0 | 2.0 | 1.0 | |||
| QuantU (quantity) | |||||||
| Control (n = 14) | 10.7 | 3.3 | 0.77 | 7.8 | 0.04 | 4.3 | 0.11 |
| MI-only (n = 12) | 17.1 | 0.0 | 0.0 | 2.0 | |||
| MI+HealthCall (n = 13) | 18.2 | 8.5 | 5.3 | 0.8 | |||
p-Value from Kruskal-Wallis test of whether there are treatment group differences in reduction of drug use between baseline and 60-day, 6-month, or 12-month follow-up.
4. Discussion
In this randomized clinical trial, HealthCall, a technological enhancement of brief intervention was tested as a means of improving the efficacy of brief MI to reduce non-injection drug use among 240 HIV primary care participants (largely disadvantaged minorities). Overall retention in the study was excellent, with the highest retention rates among treatment arms in MI+HealthCall at the end of treatment (60 days; 89%) and 12 months (91%). Results indicated reduction of non-injection drug use (NIDU) in all three treatment groups, with participants significantly decreasing both frequency and quantity of drug use between baseline and end of treatment. Participants in MI-only showed the greatest reduction in the number of days they used their primary drug. Follow-up indicated sustained benefits of MI+HealthCall and MI-only relative to control.
We previously conducted two trials with HIV patients in primary care settings showing superior results for MI+HealthCall than for MI-only or control, one a pilot study showing reduction in non-injection drug use (Aharonovich et al., 2012) and the other reduction in heavy drinking (Hasin et al., 2013). In contrast, the present trial did not demonstrate a significant advantage of MI+HealthCall in reducing non-injection drug use among HIV primary care patients. One possible explanation of this difference is that the extra intervention provided by HealthCall primarily benefits participants with more severe substance problems. In the study of heavy drinking (Hasin et al., 2013), only alcohol dependent patients (about half the sample) did better with MI+HealthCall than MI-only, an effect not found among non-dependent heavy drinkers. In the MI+HealthCall NIDU pilot study, dependence was not diagnosed. However, the mean baseline frequency and quantity and of drug use (9.8 and $21.70) in the pilot study was greater than in the present study (8.7 and $15.80), suggesting that pilot study patients, who benefited more from MI+HealthCall than MI-only, had more severe drug problems initially. Our exploratory analyses of the small subset of drug dependent participants in the present study are also consistent with this explanation, since within this subset, both quantity and frequency of drug use were descriptively lower in the MI+HealthCall group at 12-month follow-up, although the difference in this small subset did not reach statistical significance.
Another possible explanation of the difference in results between this study and the other HealthCall studies may lie in changing attitudes that participants had toward an IVR-based intervention. Recruitment for the alcohol study was from 2007 to 2009, and for the pilot from 2008 to 2009. In the present HealthCall study, recruitment was from 2011 to 2014, when use of mobile technology and smartphones had become widespread and their technological features improved substantially. During this time, expectations of mobile technology may have increased as well, leading participants in the present study to find engagement in the IVR less interesting or compelling than in earlier studies. This speculation is supported by a more recent HealthCall pilot study we conducted in 2015 in which HealthCall was delivered via smartphone application (Aharonovich et al., 2016, under review). Among these HIV-infected individuals (all drug dependent), HealthCall engagement (indicated by mean percent of possible days the application could have been used) was substantially and significantly greater than in the IVR studies, and MI+HealthCall also appeared more beneficial than MI-only in reducing drug use. While further research is required to confirm this explanation, the combined implications of all four studies are that HealthCall is most effective in enhancing the effects of brief evidence-based intervention when delivered via smartphone, and when participants are individuals with substance use disorders rather than substance use that minimally meets common study inclusion frequency criteria. Additionally, future studies should examine patient characteristics predicting good HealthCall compliance, and whether good compliance predicts outcome, controlling for these characteristics.
We note that superior MI efficacy was shown not shown at the end of treatment, but only after the end of treatment, at 6- and 12-month follow-ups. An explanation for showing treatment effects only after the end of treatment may lie in the nature of the treatment received. During the 60-day final treatment session, the patient’s entire experience during study participation was reviewed, and taken into account in planning for further subsequent efforts to reduce substance use. The mechanism of post-treatment MI efficacy at 6 and 12 months may lie in the cumulative effects of the three sessions, culminating with the comprehensive look over the prior 60 days at the end of treatment, and the planning for future efforts that occurred in the final session.
Study limitations are noted. The current study consists of mostly minority disadvantaged adults in large urban HIV primary care clinics in the northeastern United States. The generalizability of these results to a broader range of individuals should be investigated, especially given the small proportions of White participants and somewhat low drug use severity of the sample. In a related point, the proportion of participants with drug dependence was low; future studies should either stratify randomization by drug dependence status, or require drug dependence or DSM-5 substance use disorder (at a moderate or severe level) as an additional inclusion criterion. In addition, data on participants’ route of HIV infection were not available in the current study, which would have provided useful descriptive information. Finally, drug use quantity was in the form of dollar amount spent, an imprecise proxy for quantity, since some individuals do not pay for drugs or exchange activities (e.g., sex) for drugs rather than paying, and market values fluctuate. The difficulties in quantifying actual amounts of drug used remain to be solved. Study strengths included the overall excellent retention rates, with the highest proportion retained in the HealthCall group, supporting feasibility, patient acceptability and generalizability. The bi-lingual counselors had backgrounds similar to personnel often found in such clinics, further enhancing generalizability.
Perhaps the most important finding of the present study is that the non-injection drug users reduced drug use through brief evidenced-based drug-specific motivational interviewing: a single session of 25–30 min at baseline, and two 10-min booster sessions at 30 and 60 days after baseline. These reductions were sustained even at the 12-month point. This is an important finding considering recent reports showing that brief drug-reduction interventions in primary care lack efficacy (Saitz, 2014; Saitz et al., 2014). One possible explanation of our findings relative to the other trials (Saitz, 2014; Saitz et al., 2014) is that the HIV context may be more generally supportive of reducing drug use (Korthuis et al., 2011) since the serious potential health consequences of continued drug abuse among HIV-infected individuals may make brief intervention to reduce such drug use more salient and important to them than to participants in general primary care who are not medically ill.
5. Conclusion
The current study expands our knowledge of the use of technology to enhance brief interventions aimed at reducing drug use (Aharonovich et al., 2012; Hasin et al., 2013). Specifically, results indicate that non-injection drug users can be treated successfully in HIV primary care clinics with brief behavioral interventions that do not require enhancement. However, the use of mobile technology to improve treatments to help individuals with drug use disorders achieve and maintain a meaningful and sustained recovery is an important NIDA priority (see Goal 3, Strategic Plan Workgroup, (National Institutes of Health (NIH), 2016). An important next step in this line of research is to investigate mobile technologies as the platform to deliver a more engaging version of HealthCall to diverse substance-abusing populations.
Acknowledgments
Role of funding source
This work was supported by the National Institutes of Health [grant numbers R01AA014323, T32DA031099]; and the New York State Psychiatric Institute (R01DA024606). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflicts of interest
None.
References
- Aberg JA, Gallant JE, Anderson J, Oleske JM, Libman H, Currier JS, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: Recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases. 2004;39(5):609–629. doi: 10.1086/423390. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Greenstein E, O’Leary A, Johnston B, Seol SG, Hasin DS. HealthCall: Technology-based extension of motivational interviewing to reduce non-injection drug use in HIV primary care patients - a pilot study. AIDS Care. 2012;24(12):1461–1469. doi: 10.1080/09540121.2012.663882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Stohl M, Cannizzaro D, Hasin D. Mobile HealthCall to reduce concurrent non-injection drug and alcohol use in a randomized pilot trial. 2016 doi: 10.1016/j.jsat.2017.09.013. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. Journal of Acquired Immune Deficiency Syndromes. 2009;50(1):93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, Charlebois ED, et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosomatic Medicine. 2007;69(8):785–792. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations for incorporating human immunodeficiency virus (HIV) prevention into the medical care of persons living with HIV. Clinical Infectious Diseases. 2004;38:104–121. doi: 10.1086/380131. [DOI] [PubMed] [Google Scholar]
- Colfax AW, Guzman R. Club drugs and HIV infection: A review. Clinical Infectious Diseases. 2006;15(42):1463–1469. doi: 10.1086/503259. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22(11):1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David P, Buckworth J, Pennell ML, Katz ML, DeGraffinreid CR, Paskett ED. A walking intervention for postmenopausal women using mobile phones and interactive voice response. Journal of Telemedicine and Telecare. 2012;18(1) doi: 10.1258/jtt.2011.110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio C. New challenges in HIV care: Prevention among HIV-infected patients. Topics in HIV Medicine. 2003;11(4):140–144. [PubMed] [Google Scholar]
- Des Jarlais DC, McKnight C, Arasteh K, Feelemyer J, Perlman DC, Hagan H, et al. A perfect storm: Crack cocaine, HSV-2, and HIV among non-injecting drug users in New York City. Substance Use & Misuse. 2014;49(7):783–792. doi: 10.3109/10826084.2014.880176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon FR, Turner CW, Robbins MS, Szapocznik J. Concordance among biological, interview, and self-report measures of drug use among African American and Hispanic adolescents referred for drug abuse treatment. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2005;19(4):404–413. doi: 10.1037/0893-164X.19.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula R, Miller TR. Substance abuse treatment in persons with HIV/AIDS: Challenges in managing triple diagnosis. Behavioral Medicine. 2014;40(2):43–52. doi: 10.1080/08964289.2013.866540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons KM, Rollnick S. Motivational interviewing in health care settings: Opportunities and limitations. American Journal of Preventive Medicine. 2001;20:68–74. doi: 10.1016/s0749-3797(00)00254-3. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The Timeline Followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the United States: A 10-year study. Journal of Acquired Immune Deficiency Syndromes. 2009;51(4):399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Aharonovich E, Greenstein E. HealthCall for the smartphone: Technology enhancement of brief intervention in HIV alcohol dependent patients. Addiction Science & Clinical Practice. 2014;9:5. doi: 10.1186/1940-0640-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Aharonovich E, Waxman R, Marcus SM, O’Leary A, Wainberg M, et al. Reducing heavy drinking in HIV primary care: A randomized trial of brief intervention, with and without technological enhancement. Addiction. 2013;108(7):1230–1240. doi: 10.1111/add.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Compton WM. Screening and brief intervention and referral to treatment for drug use in primary care: Back to the drawing board. Journal of the American Medical Association. 2014;312(5):488–489. doi: 10.1001/jama.2014.7863. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Cook JA, Cohen MH, Sohler N, Kovacs A, Greenblatt RM, et al. The relationship between non-injection drug use behaviors on progression to AIDS and death in a cohort of HIV seropositive women in the era of highly active antiretroviral therapy use. Addiction. 2005;100(7):990–1102. doi: 10.1111/j.1360-0443.2005.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen L, II, Khan M, Clifford L, Harrell PT, Latimer WW. Injection and non-injection drug use and infectious disease in Baltimore City: Differences by race. Addictive Behaviors. 2014;39(9):1325–1328. doi: 10.1016/j.addbeh.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf MC, Huang CH, Savage R, Safren SA. Technology-delivered mental health interventions for people living with HIV/AIDS (PLWHA): A review of recent advances. Current HIV/AIDS Reports. 2015;12(4):472–480. doi: 10.1007/s11904-015-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MR, Berger A, Hemberg J, O’Neill A, Dyer TP, Smyrk K. Non-injection and injection drug use and STI/HIV risk in the United States: The degree to which sexual risk behaviors versus sex with an STI-infected partner account for infection transmission among drug users. AIDS and Behavior. 2013;17(3):1185–1194. doi: 10.1007/s10461-012-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp AM, Desruisseau AJ, Qian HZ. Non-injection drug use and HIV disease progression in the era of combination antiretroviral therapy. Journal of Substance Abuse Treatment. 2011;40(4):386–396. doi: 10.1016/j.jsat.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, Saha S, Chander G, McCarty D, Moore RD, Cohn JA, et al. Substance use and the quality of patient-provider communication in HIV clinics. AIDS and Behavior. 2011;15(4):832–841. doi: 10.1007/s10461-010-9779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): A randomised trial. Lancet. 2010;376(9755):1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: A longitudinal study in the era of highly active antiretroviral therapy. American Journal of Epidemiology. 2006;163(5):412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Carroll KM, Kiluk BD. Technology-based interventions for the treatment and recovery management of substance use disorders: A JSAT special issue. Journal of Substance Abuse Treatment. 2014;46(1):1–4. doi: 10.1016/j.jsat.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clinical Psychology Review. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. xvii. New York: Guilford Press; 1991. p. 348. [Google Scholar]
- Miller W, Rollnick S. Motivational interviewing: Preparing people for change. 2. xx. New York: Guilford Press; 2002. p. 428. [Google Scholar]
- Miller W, Baca C, Compton WM, Ernst D, Manuel JK, Pringle B, et al. Addressing substance abuse in health care settings. Alcoholism, Clinical and Experimental Research. 2006;30(2):292–302. doi: 10.1111/j.1530-0277.2006.00027.x. [DOI] [PubMed] [Google Scholar]
- Mitchell MM, Latimer WW. Unprotected casual sex and perceived risk of contracting HIV among drug users in Baltimore, Maryland: Evaluating the influence of non-injection versus injection drug user status. AIDS Care. 2009;21(2):221–230. doi: 10.1080/09540120801982897. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, et al. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care. 2012;24(12):1504–1513. doi: 10.1080/09540121.2012.672718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fazzino T, Barry DT, Fiellin DA, Cutter CJ, Schottenfeld RS, et al. The recovery line: A pilot trial of automated, telephone-based treatment for continued drug use in methadone maintenance. Journal of Substance Abuse Treatment. 2013;45(1):63–69. doi: 10.1016/j.jsat.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. Journal of Substance Abuse Treatment. 2005;28(1):19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Mullen PD, Simons-Morton DG, Ramirez G, Frankowski RF, Green LW, Mains DA. A meta-analysis of trials evaluating patient education and counseling for three groups of preventive health behaviors. Patient Education and Counseling. 1997;32(3):157–173. doi: 10.1016/s0738-3991(97)00037-2. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (NIH) Strategic plan workgroup reports and past plans. 2016 Available from: https://www.drugabuse.gov/about-nida/strategic-plan/strategic-plan-workgroup-reports-past-plans.
- Naylor MR, Naud S, Keefe FJ, Helzer JE. Therapeutic interactive voice response (TIVR) to reduce analgesic medication use for chronic pain management. The Journal of Pain. 2010;11(12):1410–1419. doi: 10.1016/j.jpain.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neushotz LA, Fitzpatrick JJ. Improving substance abuse screening and intervention in a primary care clinic. Archives of Psychiatric Nursing. 2008;22(2):78–86. doi: 10.1016/j.apnu.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Oake N, Jennings A, van Walraven C, Forster AJ. Interactive voice response systems for improving delivery of ambulatory care. American Journal of Managed Care. 2009;15(6):383–391. [PubMed] [Google Scholar]
- Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the southeastern United States. Journal of Acquired Immune Deficiency Syndromes. 2006;42(3):298–306. doi: 10.1097/01.qai.0000219773.82055.aa. [DOI] [PubMed] [Google Scholar]
- Pisu M, Cloud G, Austin S, Raper JL, Stewart KE, Schumacher JE. Substance abuse treatment in an urban HIV clinic: Who enrolls and what are the benefits? AIDS Care. 2010;22(3):348–354. doi: 10.1080/09540120903193658. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–163. [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors. 2014;28(1):154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Rose GL, Skelly JM, Badger GJ, Naylor MR, Helzer JE. Interactive voice response for relapse prevention following cognitive-behavioral therapy for alcohol use disorders: A pilot study. Psychological Services. 2012;9(2):174–184. doi: 10.1037/a0027606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, et al. Brief intervention for problem drug use in safety-net primary care settings: A randomized clinical trial. Journal of the American Medical Association. 2014;312(5):492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R. Screening and brief intervention for unhealthy drug use: Little or no efficacy. Frontiers in Psychiatry. 2014;5:121. doi: 10.3389/fpsyt.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, et al. Screening and brief intervention for drug use in primary care: The ASPIRE randomized clinical trial. Journal of the American Medical Association. 2014;312(5):502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder KEE, Johnson CJ, Wiebe JS. Interactive voice response technology applied to sexual behavior self-reports: A comparison of three methods. AIDS and Behavior. 2007;11:313–323. doi: 10.1007/s10461-006-9145-z. [DOI] [PubMed] [Google Scholar]
- Shacham E, Onen NF, Donovan MF, Rosenburg N, Overton ET. Psychiatric diagnoses among an HIV-infected outpatient clinic population. Journal of the International Association of Providers of AIDS Care. 2014 doi: 10.1177/2325957414553846. [DOI] [PubMed] [Google Scholar]
- Skalski LM, Sikkema KJ, Heckman TG, Meade CS. Coping styles and illicit drug use in older adults with HIV/AIDS. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2013;27(4):1050–1058. doi: 10.1037/a0031044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback users’ manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- Strathdee SA, Sherman SG. The role of sexual transmission of HIV infection among injection and non-injection drug users. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 2003;80(4 Suppl 3):iii7–ii14. doi: 10.1093/jurban/jtg078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Screening, brief intervention, and referral to treatment (SBIRT) 2016 Available from: http://www.samhsa.gov/sbirt.
- Swendeman D, Jana S, Ray P, Mindry D, Das M, Bhakta B. Development and pilot testing of daily interactive voice response (IVR) calls to support antiretroviral adherence in India: A mixed-methods pilot study. AIDS and Behavior. 2015;19(Suppl 2):142–155. doi: 10.1007/s10461-014-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van TH, Koblin BA. HIV, alcohol, and noninjection drug use. Current Opinion in HIV and AIDS. 2009;4(4):314–318. doi: 10.1097/COH.0b013e32832aa902. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Vlahov D, Crystal S, Absalon J, Klein SJ, Remein RH, et al. Integrating HIV prevention activities into the HIV medical care setting: A report from the NYC HIV centers consortium. Journal of Urban Health. 2006;83(1):18–30. doi: 10.1007/s11524-005-9004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin KY, Steinberg DM, Lane IB, Askew S, Greaney ML, Colditz GA, et al. Engagement with eHealth self-monitoring in a primary care-based weight management intervention. PLoS One. 2015;10(10):e0140455. doi: 10.1371/journal.pone.0140455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn GH, Cozza KL, Zapor MJ, Wortmann GW, Armstrong SC. Med-psych drug-drug interactions update. Antiretrovirals, part III: Antiretrovirals and drugs of abuse. Psychosomatics. 2005;46(1):79–87. doi: 10.1176/appi.psy.46.1.79. [DOI] [PubMed] [Google Scholar]
- Zhao W, Weng Y, Wu Q, Palesch Y. Quantitative comparison of randomization designs in sequential clinical trials based on treatment balance and allocation randomness. Pharmaceutical Statistics. 2012;11(1):39–48. doi: 10.1002/pst.493. [DOI] [PMC free article] [PubMed] [Google Scholar]