Abstract
Background
The neurotoxicity of elemental mercury (Hg0) is well-recognized, but it is uncertain whether and for how long neurotoxicity persists; among studies that evaluated previously exposed workers, only one examined workers during and also years after exposure ceased.
Objective
To document the type, frequency, and dose-relatedness of objective neurological effects in currently exposed mercury workers and thereby provide first approximations of the effects one would have expected in previously exposed workers evaluated during exposure.
Methods
We systematically reviewed studies of neurotoxicity in currently exposed mercury workers identified by searching MEDLINE (1950 –2015), government reports, textbook chapters, and references cited therein; dental cohorts were not included. Outcomes on physical examination (PE), neurobehavioral (NB) tests and electrophysiological studies were extracted and evaluated for consistency and dose-relatedness.
Results
Forty-five eligible studies were identified, comprising over 3000 workers chronically exposed to a range of Hg0 concentrations (0.002 to 1.7 mg/m3). Effects that demonstrated consistency across studies and increased frequency across urine mercury levels (<50; 50–100; 100–200; ≥ 200 µg/L) included tremor, impaired coordination, and abnormal reflexes on PE, and reduced performance on NB tests of tremor, manual dexterity and motor speed. The data suggest response thresholds of UHg ≈275 µg/L for PE findings and ≈20 µg/L for NB outcomes.
Conclusion
These results indicate that PE is of particular value for assessing workers with UHg > 200 µg/L, while NB testing is more appropriate for those with lower UHg levels. They also provide benchmarks to which findings in workers with historical exposure can be compared.
Keywords: elemental mercury, occupational disease, neurotoxicity, tremor, physical examination, neurobehavioral testing, motor function, electrophysiological studies
Introduction
Elemental mercury (Hg0) is among the most recognized of neurotoxicants; various neurological effects have been documented in numerous cohorts occupationally exposed to its vapor (World Health Organization (WHO) 1991). Epidemiological studies of workers with long-term, ongoing exposure have reported disturbances of the central and peripheral nervous system, including objective findings of tremor and incoordination (Smith et al. 1970; Langolf et al. 1978; Roels et al. 1982; Fawer et al. 1983; Roels et al. 1985; Ehrenberg et al. 1991) peripheral neuropathy with abnormal motor and/or sensory nerve conduction (Angotzi et al. 1981; Albers et al. 1982; Levine et al. 1982), and deficits on neurobehavioral tests of manual dexterity, tapping, and color vision (Langolf et al. 1978; Piikivi et al. 1984; Liang et al. 1993; Cavalleri et al. 1995; Gunther et al. 1996). While the neurotoxicity of Hg0 is widely appreciated, less well known is whether and for how long objective findings of toxicity persist following exposure cessation. Review of case studies indicates that elemental mercury-induced neurotoxicity may be transient, with objective findings normalizing with the passage of time after exposures cease (Bidstrup et al. 1951; Vroom and Greer 1972; Wood et al. 1973; Adams et al. 1983; Florentine and Sanfilippo, II 1991), even in workers with evidence of massive exposures (e.g. 24-hour urine mercury levels of 1495–7950 µg) (Bidstrup et al. 1951). Others, however, have published case reports describing the persistence of neurological abnormalities (White et al. 1993; Cordeiro, Jr. et al. 2003). The informational value of such reports is limited by their lack of systematic focus and by potential publication bias (i.e. the tendency to publish significant results rather than null findings).
In order to better understand the persistence of objective neurological findings resulting from Hg0 exposure, we began a systematic review of published occupational cohort studies that evaluated the neurological health of workers examined years after cessation of long-term, continuous exposure to elemental mercury. Of the handful of studies so identified, only one had evaluated neurological effects longitudinally in workers during active exposure and then again years after the cessation of exposure. That study found no significant differences between exposed and control subjects’ performance on any of the neurobehavioral measures of dexterity, speed, attention, and reaction time for either time frame (Ellingsen et al. 2001; Bast-Pettersen et al. 2005). The remaining studies described the workers’ historical levels of Hg0 exposure, but only the results of neurological examinations performed after the cessation of those historical (i.e. ‘previous’) exposures. They reported sometimes significant, but seemingly inconsistent findings and, as a group, they have not been the subject of critical review or comparison. Moreover, because none of these studies evaluated the neurological status of the study workers while they were being actively exposed, they could not directly answer questions about the persistence of Hg0 –induced neurological effects. In other words, it is uncertain whether reported findings represent a change from the workers’ neurological status during active exposure.
Accordingly, we adopted an indirect, two-step approach to address the question of persistence. In the first step, we estimated the neurological effects one would have found in the ‘previously-exposed’ workers had they been examined while they were being exposed. To do that, we performed a systematic review of the medical literature to identify studies that described neurological findings in groups of workers during on-going Hg0 exposure (i.e. ‘currently-exposed’ workers). Identified studies were stratified into four exposure categories according to group mean urine mercury (UHg) levels. We then documented the type and frequency of objective neurological effects reported among the groups of workers belonging to each exposure category. Those dose-related findings in ‘currently-exposed’ workers provide a first approximation of the neurological effects one would expect to find in the ‘previously-exposed’ workers had they been examined during active exposure to comparable levels of Hg0. In the second step, we compared the type and frequency of dose-related neurological findings reported in those studies of ‘currently-exposed’ workers with the corresponding findings reported in the ‘previous-exposure’ studies. Differences or similarities in the prevalence of specific neurological findings could thus serve as indirect measures of their persistence over time.
The present report describes the methods and findings of the ‘first step’, the systematic evaluation of the range, consistency, and dose-relatedness of motor and sensory effects in workers currently exposed to various levels of Hg0. Additional objectives included identification of the specific tests that best demonstrated sensitivity, specificity and reliability to detect neurological effects among workers with a wide range of Hg0 exposure. Because of differences in neurological testing (e.g. different tests or test protocols) and in the reporting of results (e.g. some studies reported results of individual tests, others reported results for functional domains), the studies are not amenable to formal meta-analysis. Instead, study data was distilled into tabular format and then organized in ways that would allow the types of neurological effects and related exposure levels to be identified. A companion paper presents the comparison of these findings with the corresponding results from studies of workers previously exposed to elemental mercury.
Methods
Identification and selection of studies
We performed a comprehensive literature search to identify studies describing neurological effects in workers exposed to elemental mercury. Studies were located by searching MEDLINE (1950 – July 15, 2015) using multiple search terms: neurotoxicity or toxicity or health effects; and elemental mercury or mercury vapor or occupational exposure to mercury vapor. We also examined international and government agency reports (Friberg and Vostal 1971; WHO 1991; Agency for Toxic Substances and Disease Registry (ATSDR) 1999; International Programme on Chemical Safety (IPCS) 2003; American Conference of Governmental Industrial Hygienists (ACGIH) 2013), relevant book chapters (Berlin 1986; Feldman 1999), and reviewed reference lists from identified studies to ensure that all relevant studies were included in this review. Included for analysis were peer-reviewed studies in English, French, German, Italian, Portuguese, and Japanese languages that described: 1) workers with ongoing occupational exposure to Hg0 vapor, but not other forms of mercury, generally for at least 3 months; 2) the level of Hg0 exposure documented by measurements of mercury in urine, blood, or workplace air; 3) neurological effects involving motor function and/or sensory function; 4) testing methods and objective neurological findings from evaluations performed during active exposure, with the exception of two studies that examined workers after a break in exposure (Chang et al. 1995; Pranjic et al. 2003). Information from non-peer-reviewed publications was considered if it pertained to an eligible study.
We did not include studies of dental cohorts. The low-level exposures from mercury-containing dental amalgam result in urine Hg levels that overlap those of the US general population (Wang et al. 2012). Moreover, many dentists and dental technicians have occupational exposures to other neurotoxic agents, including nitrous oxide (National Institute for Occupational Safety and Health (NIOSH) 2015), an anesthetic associated with impaired neurobehavioral performance (Lucchini et al. 1996) and increased risk of neurological disease (Cohen et al. 1980), and methyl methacrylate, a monomer widely used in dentistry that has been associated with sensorimotor neuropathy (Verkkala et al. 1983; Seppalainen and Rajaniemi 1984; Rajaniemi 1986).
Our analysis focused on objective motor and sensory effects that could be measured using validated methods; studies that reported only symptoms, not signs of neurotoxicity were excluded. ‘Symptoms’ are subjective complaints (e.g. anxiety, headache, pain) perceivable only to the individual experiencing them. By contrast, ‘signs’ are objective findings that provide evidence of disease (e.g. unsteady gait, reflex abnormalities, tremor) perceivable to the patient and outside observers, and are generally measurable qualitatively (e.g. Romberg) or quantitatively (e.g. nerve conduction velocity) using clinical, neurobehavioral, and/or electrophysiological examinations. In addition, some study authors qualified their findings as either ‘clinical’ or ‘subclinical’. Unless otherwise stated, we assumed these terms were used in accordance with the following definition: ‘Subclinical toxicity refers to exposure-induced adverse effects that are too small to produce signs and symptoms evident in a standard clinical examination’ (National Research Council 1992). We did not consider neurological effects related to memory and cognitive function, as these findings were the subject of a meta-analysis (Meyer-Baron et al. 2002) and systematic review (Meyer-Baron et al. 2004).
Data extracted from each study were entered into Microsoft Excel spreadsheets and included information on the descriptive characteristics of study population, study design, exposure characteristics, testing methods and outcomes of interest, statistical methods, and variables considered as potential confounders. Studies written in other languages were translated to English prior to data extraction.
Exposure categorization
Because urine samples are considered ‘the best determinant of body burden…from long-term exposure to elemental…mercury’ (IPCS 2003), we stratified studies into exposure categories by their group mean urine mercury (UHg) concentrations measured at or around the time of the neurological examinations and expressed as µg/L. One study reported only median values (Langworth et al. 1992).
Most studies (70%) reported urine mercury levels in units of µg/L, thus levels reported in other units were converted to µg/L. For UHg concentrations reported in relation to creatinine, conversions were performed assuming a urine creatinine of 1.4 g/L, the mid-point of the upper and lower bounds on the expected range of creatinine concentrations in the US population (Barr et al. 2005). If studies reported only 24-hour UHg, levels were converted by assuming that workers excreted 1.5 L of urine per 24 hours. Finally, if only air Hg0 levels were reported, corresponding UHg levels were estimated using the air-to-urine ratio of 1 µg/m3 = 2.3 µg/L; that ratio corresponds to the midpoint of the range reported by the WHO (1991) for occupational exposures measured using static workplace samplers. Study-specific conversion methods are detailed in Supplemental Table 1.
Some studies reported findings for more than one group of workers (e.g. two cohorts included in one study, or one cohort stratified into several groups based on exposure). In these instances, information for each group was tabulated separately. Thus, some studies provided more than one study group. Accordingly, our analyses focused on study groups, which were stratified into the following four categories of exposure selected for their comparability to the historical exposure levels reported in the previous exposure studies:
| High | UHg ≥ 200 µg/L; |
| Medium | 100 µg/L ≤ UHg < 200 µg/L; |
| Low | 50 µg/L ≤ UHg < 100 µg/L; |
| <BEI | UHg < 35 µg/g creatinine ≈ <50 µg/L. |
The final category (‘<BEI’) refers to the Biological Exposure Index, a health-based guideline recommended by the ACGIH that ‘generally indicates the concentration below which nearly all workers should not experience adverse health effects’(ACGIH 2012). Prior to 2014, the era that included even the most recent of the reviewed studies, the BEI for elemental mercury was 35 µg/g creatinine (ACGIH 2013; 2014).
Neurological evaluations
Objective motor and sensory findings described in studies were extracted and tabulated into three categories reflecting the general types of evaluations used to examine workers: Physical Examination (PE); Neurobehavioral tests (NB); and Electrophysiological Studies (EPS). The PE category included mainly qualitative findings from clinical neurological examination; the NB category included results from functional performance tests that yield quantitative measures of tremor, motor and sensory functions, color vision, and balance; and the EPS category included quantitative findings from nerve conduction studies, electromyography, evoked potentials and electroencephalography. To permit comparisons across studies, examination findings and test results were further organized by domain (e.g. motor vs. sensory), and then, when sufficient details were provided, according to specific test (e.g. nerve conduction velocity), outcome evaluated (e.g. distal latency) and anatomic localization (e.g. median sensory nerve).
Tremor, ‘the hallmark of chronic mercury intoxication’ (Greenberg et al. 2003), is of particular importance for evaluating the effects of Hg0 exposure. Tremor can be classified as ‘resting’ or ‘action’, and action tremors can be further distinguished as postural, intention, or kinetic (National Institute of Neurological Disorders and Stroke (NINDS) 2012). Tremor can also be characterized according to physiological parameters such as frequency and amplitude. In this review, studies assessed tremor using PE and/or NB methods that sometimes also included ‘physiological techniques’ (Wastensson et al. 2006). Tremor detected on PE was rarely classified by study authors, and fewer still included the criteria used to distinguish them. By contrast, as described below, studies used a variety of NB functional performance tests to document and classify tremor and describe its parameters:
Tests of hand-eye coordination: These tests, which assess motor steadiness, included computer-based tests of static steadiness, aiming (e.g. hole tremor-meter) and tracking (e.g. laser-based system with visual feedback), and non-computerized tests that involve drawing of visually presented materials (e.g. Bender visual-motor gestalt test (BGT), and Benton visual retention test (B-VRT). Outcomes on tests of static steadiness, aiming, and tracking/drawing have been used to classify tremor as postural, intention, or kinetic, respectively (Louis et al. 2000; Louis 2007; Buijink et al. 2012; NINDS 2012; Gonzalez-Usigli & Espay 2013; Sternberg et al. 2013).
Physiological techniques: These methods, which involve the attachment of sensors to directly measure displacement while subjects perform tests of hand-eye coordination similar to those listed above, included the use of accelerometers, force transducers, and laser-based systems. The two most commonly reported physiological parameters, frequency and amplitude, have been used to characterize the etiology and severity of tremor (Berme et al. 1999; Louis & Pullman 2001; Gonzalez-Usigli & Espay 2013).
The interpretation of results from hand-eye coordination tests sometimes varied across studies, even when apparently similar results were obtained using the same instruments and similar protocols. For example, five studies evaluated tremor using a ‘static steadiness test’ (e.g. ‘hole-tremor-meter’): two described abnormal results as evidence of ‘intention’ tremor (Verberk et al. 1986; Ellingsen et al. 2001), while three described them as ‘postural’ tremor (Roels et al. 1982, 1989; Pranjic et al. 2003). To address such apparent inconsistencies, as well as differences in test methods and/or protocols, we evaluated tremor-related results as follows:
We first determined the dose-relatedness of tremor without regard to its classification or characteristics;
We then analyzed the dose-relatedness of tremor as categorized according to the classification shown in Table 1. Alternatively, because some authors’ tremor classifications differed from Table 1, we also performed these analyses using the classifications of tremor as reported by those authors;
Finally, we analyzed the dose-relatedness of tremor according to reported parametric characteristics (e.g. frequency and amplitude). Tremor parameters reported in fewer than three studies were not tabulated.
Table 1.
Tremor Classification Scheme
| Tremor Type |
Subtype | Occurrence | Examples of diagnostic tests |
|---|---|---|---|
|
| |||
| Rest | Rest/resting tremor | When limb is at rest and supported against gravity | PE: Hands resting at sides while lying down, resting in lap while seated, or relaxed at sides while standing. |
| NB: Accelerometry | |||
| EPS: Electromyography | |||
|
| |||
| Action | Postural/Sustention/“Static” tremor | When voluntarily elevating the limb against gravity | PE: Sustained arm extension |
| NB: Accelerometry; “Nine-Hole Steadiness Test” | |||
| EPS: Electromyography | |||
|
| |||
| Intention tremor | During a visually guided, movement that approaches a target. | PE: Finger-to-nose maneuver | |
| NB: Hand-eye coordination tests involving aim: “strike central area of discs” | |||
|
| |||
| Kinetic tremor | During any voluntary movement | PE: Finger-to-nose maneuver | |
|
| |||
| “Simple” | During any involuntary movement | EPS: Electromyography | |
|
| |||
| “Task-specific” | During a specific task | PE: Writing | |
| NB: Tracking: Neurobehavioral Evaluation System (NES, NES2); Drawing: “Bender visual-motor gestalt adult test (BGT), “Benton visual reproduction test (B-VRT)” | |||
Abbreviations: PE = Physical Examination; NB = Neurobehavioral; EPS = Electrophysiological Studies.
Sources: NIH NINDS Tremor 2014 (National Institute of Neurological Disorders and Stroke (NINDS) 2012); Buijink 2012 (Buijink et al. 2012); Merck Manual Tremor 2013 (Gonzalez-Usigli and Espay 2013); Louis 2007 (Louis 2007), and Sternberg 2013 (Sternberg et al. 2013).
Studies used a variety of NB functional performance tests to evaluate motor skills other than steadiness (used to detect tremor). Tests included those that assess only motor skills (i.e. motor coordination, dexterity, and speed) and those that assess motor ability as well as other abilities such as correct perception/information processing (i.e. perceptual motor speed, attention and reaction time). Because the latter set of tests does not provide information exclusively about motor function, we analysed those results (referred to as ‘motor accuracy’) separately from the results of tests that assessed only motor skills (referred to as ‘motor function’) (Goldstein and Sanders, 2004).
A different analytical challenge was posed by PE assessments of motor coordination (MC) because the various studies described 11 different outcomes, including results of six specific tests (i.e. finger-to-nose; finger-to-finger; heel-to-shin; heel-to-toe; gait; ‘bimanual coordination’) and five clinical findings (i.e. ‘ataxia’; ‘cerebellar’; dysdiadochokinesia (DDK); dysarthria; and nystagmus). Cerebellar abnormalities not otherwise associated with sensory dysfunction (e.g. positive Romberg) were included in our analysis of MC because tests of coordination ‘are mainly directed toward assessing cerebellar function’ (Reeves and Swenson 2008b). Most studies reported the total number of workers with one or more positive MC findings. Two studies (Suwa and Takahata 1969; Bunn et al. 1986) reported results separately for each of two or more individual tests but did not indicate the total number of individuals with abnormal MC tests. For those two, we selected the MC abnormality most frequently reported in each study to be used in calculating the overall prevalence of impaired MC in our primary analysis. As a precaution, we also performed a secondary analysis which considered that each of the reported MC abnormalities was experienced by different workers, discounting the possibility that ‘some workers had more than one abnormality’ as noted in one study (Ehrenberg et al. 1991) and was observable in several others (Miller et al. 1975; Bunn et al. 1986). Thus, our secondary analysis was highly conservative, estimating the highest possible prevalence by summing the number of workers for all reported MC abnormalities. Similar to our analysis of tremor, MC abnormalities were analyzed as follows:
We first determined the dose-relatedness of MC findings without regard to the type of outcome reported.
We then analyzed the dose-relatedness of each MC abnormality described in three or more studies: gait; nystagmus; finger-to-nose; heel-to-shin; ataxia; and DDK (Supplemental Table 8b). These were the terms used in the studies to describe these abnormalities; we recognize that some of these items may not be mutually exclusive (Reeves and Swenson 2008a; Stern 2010a).
Analytical methods
Most studies described results of PE, NB, and EPS based on two broad types of comparisons: (1) the proportion of exposed workers with an ‘abnormal’ effect (e.g. tremor) or an aggregate of effects (e.g. impaired coordination) compared to non-exposed controls or literature-based normative values; and (2) the mean or median values of quantitative results of NB and EPS in groups of exposed workers compared to controls or literature-based norms. Exposure correlations resulting from both types of comparisons were also evaluated in some studies. We refer to such comparisons as ‘exposure effects’.
A smaller number of studies evaluated the dose-response gradients of effects and/or test results across worker subgroups characterized by differing levels or patterns of exposure. We refer to such analyses as ‘dose effects’.
We report the statistical significance of such comparisons as presented in the original studies; significance was defined as p<0.05 using two-tailed statistical tests in all but two studies, which set alpha at p<0.10 (Miller et al. 1975; Langolf et al. 1978). However, some studies did not report statistical significance. In particular, studies that evaluated workers using PE characterized findings on the basis of clinical gestalt, and most reported ‘abnormal’ findings on the basis of the ‘clinical significance’, not statistical significance. In those cases, we simply report their results without statistical interpretation. By contrast, NB and EPS yielded objective quantitative results amenable to statistical analyses.
We evaluated study results qualitatively; results categorized as ‘positive’ included those that were statistically significantly associated with exposure (e.g. increased prevalence in exposed workers vs. controls, or positively correlated with exposure) and those that were judged ‘abnormal’ on the basis of clinical gestalt. The ‘null’ category included results that were not statistically significant or were otherwise described as ‘normal’ on the basis of clinical gestalt. Results that suggested a significantly protective effect of exposure were specifically noted as ‘paradoxical’.
Dose-relatedness
We evaluated the dose-relatedness of neurological effects using three approaches, based on the availability of individual (preferred) or group-level data.
-
Dose-relatedness of ‘exposure effects’: For each type of evaluation (PE, NB and EPS), we assessed the proportion of study groups (among those so evaluated) with ‘positive’ findings overall and within each of the four categories of exposure (i.e. ‘High’, ‘Medium’, ‘Low’, and ‘<BEI’). A similar dose-related analysis was performed for each of the most frequently reported outcomes, i.e. described in 10 or more study groups (e.g. tremor, motor coordination and motor accuracy). For these analyses, we assumed that studies would have reported the results of neurological evaluations if they had been clinically abnormal and/or statistically significant. Thus, for studies that described performing neurological evaluations/testing specific outcomes but did not report results, we categorized those results as ‘null’.
For the analyses of NB findings, we evaluated the dose-relatedness for each of the three most frequently reported findings: NB tremor, motor function, and motor accuracy. In our primary analysis, the results of tests of motor steadiness (Johnson and Baker 1987) were considered ‘secondary’ evidence of tremor (i.e. tests of static steadiness, aiming, tracking, and drawing) and were thus analyzed separately from other tests of motor function. As a secondary analysis, the results from tests of motor steadiness were included with the other tests of motor function.
- Dose-relatedness of ‘dose effects’: We summarized the ‘dose effects’ reported in individual studies that evaluated the dose-response gradients of neurological findings based on one or both of two general types of analyses:
- Exposure Correlation: correlations between specific neurological outcomes and various exposure metrics (e.g. average UHg; cumulative UHg; peak UHg);
- Exposure Intensity: comparisons of the prevalence of abnormal findings across categorical levels of exposure (e.g. UHg <50 µg/L vs. > 50 µg/L), or comparisons of mean UHg levels of workers with and without specific abnormalities.
- Prevalence analyses: The above analyses provided information about dose-relatedness of abnormal findings among studies and study groups, but did not indicate the actual number of workers affected. To gain additional perspective on dose-relatedness, we planned to evaluate prevalence data for each of the most frequently reported outcomes on PE, NB, and EPS. Most studies reported PE data amenable to determining the prevalence of specific effects among workers. By contrast, few studies reported the number of workers with NB and EPS abnormalities, instead reporting group mean values of quantitative test results. Thus, our analysis of prevalence was limited to PE outcomes reported in 10 or more study groups: tremor, abnormal deep tendon reflexes (DTRs), and impaired MC.
- Because tremor is considered the ‘classical neurological sign’ (WHO 1991) and ‘hallmark’ (Greenberg et al. 2003) of mercury intoxication, it seemed very unlikely that studies would not have looked for it. Thus, for our primary analysis, we assumed that all studies that performed PE had looked for the presence of tremor. As a secondary analysis, we evaluated only those studies that specifically indicated they had looked for tremor.
- By contrast, abnormal DTRs and impaired MC have been less frequently described as ‘classical’, ‘hallmark’ or ‘principal’ effects of mercury intoxication (WHO 1991; ATSDR 1999; Wastensson 2010). Thus, we did not assume that all studies that performed PEs had evaluated them; calculations of prevalence were limited to data from those studies that specifically described testing of DTRs and/or MC.
For each of the three PE findings, we present the prevalence reported in individual studies stratified by group mean UHg, and calculate the prevalence in all studies combined and across the four categories of exposure.
Assessment of study quality
Because of our interest in characterizing neurological effects across the widest range of exposures, we necessarily included studies of variable quality. To determine whether study quality affected analytical results we used a qualitative approach: studies were stratified into 3 tiers based on their use and appropriateness of control groups, inclusion/exclusion criteria, and analytical methods to minimize confounding. Tier 1 (highest quality) studies employed concurrent, matched controls and excluded workers with underlying medical causes of neuropathy or neurological disease. Tier 2 included two groups of studies: a) those that employed concurrent, matched controls, but did not employ exclusion criteria; and b) those that employed only non-concurrent or unmatched controls. Tier 3 studies did not use controls and most did not employ exclusion criteria. For each study, tier rankings were assessed separately for each type of neurological evaluation performed (PE, NB, EPS).
In addition to evaluating the effects of study quality, we assessed the possibility that factors other than dose might explain (1) trends observed in the frequency of positive results stratified across the four categories of exposure, and (2) differences between studies that reported positive vs. null results vs. studies that did not report their results vs. studies that did not perform evaluations. We evaluated the influence of the weighted averages of group mean UHg levels, age, and study quality Tier ratings on outcomes for each type of neurological evaluation (PE, NB, EPS). The large database on PE findings for tremor allowed us to evaluate the impacts of study group size and on tremor prevalence.
Results
Included studies
A total of 434 articles were identified in our initial search and review. Screening of titles and abstracts revealed 140 papers of potential relevance; in-depth inspection of these articles identified 57 published studies from 16 countries that described neurological effects in workers with current or recent on-going exposure to Hg0 and met our criteria. Ten studies described cohorts that were the subject of multiple publications: Angotzi (Angotzi et al. 1980, 1981; Camerino et al. 1981); Bidstrup (Bidstrup et al. 1951; Locket & Nazroo 1952); Bunn (McGill et al. 1964; Bunn et al. 1986); Cavalleri (Cavalleri et al. 1995; Cavalleri & Gobba 1998); Langolf (Langolf et al. 1978, 1981); McCullough (McCullough and Dick 1999, McCullough et al. 2001); Miller (Chaffin et al. 1973; Miller et al. 1975); Piikivi (Piikivi & Hanninen 1989; Piikivi & Tolonen 1989) Urban (Urban et al. 1996; Urban et al. 1999; Nerudova et al. 2000); and Wastensson (Wastensson et al. 2006, 2008). For each of those studies, data from the multiple publications were combined and treated as a single study; thus yielding a total of 45 distinct studies. Among those 45 studies, nine described findings for more than one study group. There were four that each described findings from two distinct cohorts (Gambini 1978; Roels et al. 1985; Bunn et al. 1986; Urban et al. 1999); we evaluated each of those four additional cohorts separately. In addition, seven studies each reported results for two or more groups categorized by exposure level (Bidstrup et al. 1951; Smith et al. 1970; Gambini 1978; Roels et al. 1985; Bunn et al. 1986; Soleo et al. 1990; Gunther et al. 1996; Tang and Li 2006); yielding a total of 16 study groups which we evaluated separately. Finally, four studies (Miller et al. 1975; Langolf et al. 1978; Albers et al. 1982; Levine et al. 1982) described findings in workers from the same chloralkali facilities studied over a six-year period (Langolf et al. 1981). However, because the numbers of workers, examinations, and tests performed differed across those studies such that they could not be combined into a single cohort, we evaluated them as four separate cohorts.
Thus, our review includes 45 published studies that evaluated the motor and sensory neurological effects in 48 distinct cohorts and one case-control study, with findings described in 58 specific study groups. Table 2 presents the descriptive characteristics for those studies, cohorts, and study groups. The majority of the studies were cross-sectional, but seven observed cohorts longitudinally. Studies were performed in a variety of industrial settings, including chloralkali facilities (n=25), factories manufacturing Hg-lamps (n=5) and thermometers (n=6), and mercury mines, mills and distillers (n=7), and described findings in a total of 3165 ‘currently-exposed’ mostly male workers and 2114 ‘non-exposed’ controls.
Table 2.
Studies included in review: Descriptive characteristics, summary of exposure metrics, neurological testing and tier ratings
| Studies* | Type of Work | Number of Workers† | Age of exposed ‡ | Study Design¶ |
Exposure Category§ |
Exposure Metrics‖ | Testing & Tiers# | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author Year | Country | Exposed/Controls study groups |
Mean | (range) or SD | UHg | BHg | AirHg | DUR | PE | NB | EPS | |||
| Albers 1982 | US | Chloralkali | 138/0 | NR | (NR) | XS | High | √ | 2 | 2 | 2 | |||
|
| ||||||||||||||
| Angotzi 1981 | Italy | Hg distillation | 55/29 | 39‡ | (NR) | XS | Low | √ | √ | √ | √ | 2 | 2 | 2 |
|
| ||||||||||||||
| Bidstrup 1951 | England | a) DC meter | 161/0 (a)=103; (b)=58 | NR | (19–65) | XS/D | (a)=High; (b)=Medium | 24-hrs | √x | √ | 3 | – | – | |
| b) AC meter | ||||||||||||||
|
| ||||||||||||||
| Bunn 1986* | US | Chloralkali | (1): 115/0 | NR | (NR) | Longitudinal: 1: (1976–79) | Cohort 1: High | √ | √ | √ | 2 | – | – | |
| (2): 101/0 (a)=51; (b)=50 | NR | (NR) | 2: (1957–78) | Cohort 2: (a)=Medium; (b)=Low | ||||||||||
|
| ||||||||||||||
| Camerino 2002 | Italy | Chloralkali | 38/34 | 39 | (23–57) | XS | ≤BEI | √s | √ | – | 1 | – | ||
|
| ||||||||||||||
| Cavalleri 1995 | Italy | Thermometer | 33/33 | 27 | ± 9 | XS; F/U: 1-yr | Medium | √s | √ | 2 | 2 | – | ||
|
| ||||||||||||||
| Chang 1995 | China | Chloralkali | 26/52 | 43 | (33–54) | XS | Low | 24-hrs | √ | √ | 3 | – | 2 | |
|
| ||||||||||||||
| Chapman 1990 | US | Hg-Zn battery | 18/27 | 33 | (20–57) | XS | ≤BEI | 24-hrs | √ | 3 | 1 | – | ||
|
| ||||||||||||||
| Ehrenberg 1991 | US | Thermometer | 83/79 | 35 | (18–68) | XS | Medium | √s | √ | √ | 2 | – | – | |
|
| ||||||||||||||
| Ellingsen 2001 | Norway | Chloralkali | 47/47 | 42 | (24–67) | XS | ≤BEI | √ | √ | √ | – | 1 | – | |
|
| ||||||||||||||
| El Sadik 1970* | Egypt | Chloralkali | 39/10 | NR | (20–49) | XS | High | √s | √x | √ | 2 | – | – | |
|
| ||||||||||||||
| Fawer 1983 | Switzerland | Chloralkali/Hg-lamps/Acetaldehyde | 26/25 | 44 | ± 2 | XS | ≤BEI | √s | √ | √ | √ | – | 2 | – |
|
| ||||||||||||||
| Gambini 1978 | Italy | Chloralkali | (1): 131/130 | 34 | (NR) | 1: XS | Cohort 1: Low | √ | √ | √ | 2 | – | – | |
| (2): 129/0 (a)=61; (b)=68 | NR | (NR) | 2: 2-yr P | 2: (a)=Low; (b)= ≤BEI | ||||||||||
|
| ||||||||||||||
| Gilioli 1976 | Italy | Chloralkali | 86/0 | 43 | ± 10 | XS/D | High | √ | √x | 3 | – | 3 | ||
|
| ||||||||||||||
| Gonzalez-Fernandez 1984 | Spain | Hg-glass relays | 5/0 | NR | (NR) | XS; F/U: 2-month, post-chelation | High | √ | √ | √ | √ | 3 | – | – |
|
| ||||||||||||||
| 71/43 | ||||||||||||||
| Gunther 1996* | Germany | Chloralkali | (a)=14(14–21); (b)=33(34–50) | 44 | (NR) | Longitudinal: (1979–87) | (a)=Medium; (b)= ≤BEI | √ | √ | √ | 2NR | 2 | – | |
| 43 | (NR) | |||||||||||||
|
| ||||||||||||||
| Iwata 2007 | China | Hg mine/smelter | 27/52 | 41 | ± 10 | XS | High | √s | √ | – | 1 | – | ||
|
| ||||||||||||||
| Langolf 1978 | US | Chloralkali | 79/51 | 37 | ± 11 | XS ‡ F/U: 1-yr | High | √ | √ | √ | 1 | 1 | 1 | |
|
| ||||||||||||||
| Langworth 1992 | Sweden | Chloralkali | 89/75 | 42 | (22–64) | XS | ≤BEI | √s | √ | √ | 1 | 1 | – | |
|
| ||||||||||||||
| Levine 1982 | US | Chloralkali | 18/138 | 31 | (19–56) | XS ‡ | High | √ | 3 | – | 2 | |||
|
| ||||||||||||||
| Li 2007 | China | Hg smelter | 22/40 | 42 | (30–63) | XS | High | √s | √ | 2 | – | – | ||
|
| ||||||||||||||
| Liang 1993 | China | Hg lamps | 88/70 | 34 | ± 7 | XS | ≤BEI | 24-hrs | √ | √ | – | 2 | – | |
|
| ||||||||||||||
| McCullough 2001 | US | Hg recycling | 16/0 | 33 | (18–47) | XS/D | High | √ | √ | 3 | 2 | – | ||
|
| ||||||||||||||
| Miller 1975 | US | Chloralkali | 77/65 | 36 | ± 10 | XS F/U: 4–6 mo. | High | √s | √ | √ | 3 | 2 | 2 | |
|
| ||||||||||||||
| Piikivi 1984 | Finland | Chloralkali | 36/36 | 40 | ± 10 | XS | Low | √ | √ | √ | 2NR | 1 | – | |
|
| ||||||||||||||
| Piikivi 1989 | Finland | Chloralkali | 60/60 | 38 | (26–56) | XS | ≤BEI | √s | √ | √ | 1NR | 2 | 1 | |
|
| ||||||||||||||
| Pranjic 2003 | Bosnia | Chloralkali | 45/32 | 35 | (23–58) | XS | High | √ | √x | √ | 1NR | 1 | – | |
|
| ||||||||||||||
| Rentos 1968 | US | Hg mill/mine | 13/9 | NR | (NR) | XS | High | √s | √ | 2 | – | – | ||
|
| ||||||||||||||
| Roels 1982 | Belgium | Chloralkali/Hg-Zn battery | 43/47 | 38 | (23–57) | XS | Medium | √s | √ | √ | 2 | 2 | – | |
|
| ||||||||||||||
| Roels 1985 | Belgium | Chloralkali/Hg-Zn battery | Male Cohort:131/114 | 31 | (19–57) | XS | (♂, ♀) = Low | √s | √ | √ | – | 1 | – | |
| Female Cohort: 54/48 | 29 | (19–51) | ||||||||||||
|
| ||||||||||||||
| Roels 1989 | Belgium | Chloralkali/Hg-Zn battery | 54/48 | 35 | (20–57) | XS | Medium | √ | √ | √ | 1NR | 1 | – | |
|
| ||||||||||||||
| Schuckmann 1979 | Germany | Chloralkali | 39/39 | NR | (NR) | XS | Medium | √ | √ | √ | √ | – | 2 | – |
|
| ||||||||||||||
| 567/382 | ||||||||||||||
| Smith 1970 | US | Chloralkali | (a)=27; (b)=61; (c)=145; (d)=334 | NR | (19–69) | XS | (a)=High; (b)=High; c)=Medium; (d)=Low | √ | √ | √x | √ | 2 | – | – |
|
| ||||||||||||||
| Soleo 1990 | Italy | Hg Lamps | 28/22 (a)=8; (b)=20 | 40 | (NR) | XS | (a,b) = ≤BEI | 24-hr | √ | √ | – | 2 | – | |
|
| ||||||||||||||
| Suwa 1969 | Japan | Hg mine | 77/0 | NR | (NR) | XS/D | High | √s | 3 | – | – | |||
|
| ||||||||||||||
| Tang 2006 | China | Thermometer | 34/109 (a)=9; (b)=25 | 29 | (18–55) | XS | (a)=Low; (b)= ≤BEI | √s | √ | √ | 2 | – | – | |
|
| ||||||||||||||
| Triebig 1982 | Germany | Thermometer | 18/18 | 20‡ | (18–66) | XS | Medium | √ | √ | √ | √ | 3 | – | 2 |
|
| ||||||||||||||
| Urban 1996 | Slovakia | Hg smelter: | Cohort 1: 77/(0 PE; 46 EPS) | 39 | (21–58) | XS | Cohort 1: High | 24-hrs | √ | √ | 3 | – | 2 | |
| Chloralkali: | Cohort 2: 36/(0 PE; 46 EPS) | 39 | (21–61) | Cohort 2: Medium | 24-hr | |||||||||
|
| ||||||||||||||
| Urban 2003 | Slovakia | Chloralkali | 24/24 | 42 | (26–59) | XS | ≤BEI | 24-hrs | √ | √ | – | 2 | – | |
|
| ||||||||||||||
| Verberk 1986 | Netherlands | Hg Lamps | 21/0 | 51 | (28–61) | XS/D | Low | √ | √ | 3NR | 3 | – | ||
|
| ||||||||||||||
| Vroom 1972 | US | Thermometer | 9/(0 PE; 9 EMG; 40 NCV) | 51 | (33–71) | XS | High | 24-hrs | 3 | 3 | 2 | |||
|
| ||||||||||||||
| Wastensson 2006 | Sweden | Chloralkali | 43/22 | 41 | (25–65) | XS | ≤BEI | √ | √ | 1 | 1 | – | ||
|
| ||||||||||||||
| West 1968* | US | Hg Mill | 14/9 | 38 | (21–63) | XS | High | √s | √ | √ | 2 | – | – | |
|
| ||||||||||||||
| Zampollo 1987 | Italy | Thermometer | 17/0 | 42 | (21–63) | XS/D | High | √s | √ | √ | 3 | – | 2 | |
|
| ||||||||||||||
| Zedda 1980 | Italy | Hg Lamps | 7/0 | 31 | (22–49) | XS/D | Low | √ | √ | √ | 3 | – | 3 | |
= Study has an explanatory note; explanatory notes are available in Supplemental notes for Table 2.
= For studies that described neurological results separately for more than one study group: identifies the number of exposed workers in individual cohorts (enumerated as (1) or (2)), and/or the number of exposed workers within the same cohort, but stratified by exposure into two or more groups (indicated by lowercase letters (a)(b)(c)(d) and listed right to left in the order of highest to lowest exposure).
= Angotzi et al: mean age was calculated from the weighted average of the mean ages reported for two or more study groups; Triebig: median age.
= Study design identified as Cross-sectional (XS), Descriptive (D), Prospective (P), or Longitudinal; also notes if workers were followed-up (F/U).
= Identifies the exposure category into which each study, cohort, or study group was stratified (High, Medium, Low, or ≤BEI).
= Exposure metrics used by each study: urine (UHg), blood (BHg), air (AirHg), or duration of exposure (DUR) measurements. UHg measurements are spot samples unless otherwise indicated; determinations based on single sample are noted as √s, exposure metric used to determine Exposure Category, if other than Current UHg is noted as √x.
= Type of evaluation (i.e. Physical Exam (PE), Neurobehavioral (NB), Electrophysiological Studies (EPS)) used in each study to evaluate neurological effects, and methodological adequacy of the evaluation (Tier 1=highest, Tier 3=lowest; see Method section in text for details).
Dash symbol "–" indicates study did not perform testing, and subscript NR indicates study performed testing but did not report results.
Five of the studies deserve special note because they are not directly comparable to the others. Gunther et al. (1996) assessed workers prospectively, reporting results from four different testing periods; we tabulated findings as positive if they were statistically significant for at least two of the testing periods. Chang et al. (1995) evaluated workers 40–70 days after their last exposure, while Pranjic et al. (2003) performed evaluations 90–180 days after their last exposure. Vroom and Greer (1972) examined only the ‘most severely affected’ workers from an untold number of symptomatic employees of a thermometer factory. This study was included for its descriptive value: it was the only study of highly exposed workers that detailed individual-level results for all three types of testing (PE, NB, and EPS). However, because of obvious selection bias, results were not included in analyses of prevalence. Albers et al. (1982) was the only study that used a nested case-control design to evaluate the dose-relatedness of results for PE, NB and EPS in an unspecified number of exposed and unexposed workers. It contrasted findings among those with clinical evidence of peripheral neuropathy (PN) vs. those without. However, they reported only the UHg levels for exposed and unexposed workers combined. Workers with PN had UHg levels >250 µg/L in the previous year, and thus most certainly represented a High Exposure group. Therefore we discuss the study to document qualitative effects of dose, but do not include it in our analyses of dose or prevalence.
Study subjects
The basis for the selection of exposed workers and controls differed across cohorts. In 41% (20/49) of cohorts, workers were unselected, i.e. the cohorts included either the entire workforce or its random sample. In another 29% (14/49), workers were selected on the basis of duration and/or level of exposure. In one cohort, workers with the most severe signs and symptoms were selected (Vroom & Greer 1972). In the remaining cohorts, studies did not describe inclusion criteria and it was therefore unclear whether the workers studied were representative of the entire worker populations.
For most cohorts (41 of 49), a ‘non-exposed’ control group was included for comparison in at least one type of testing (PE, NB, and EPS). However, in six cohorts, the controls had possible historical (Miller et al. 1975; Soleo et al. 1990) and/or current Hg0 exposure (Rentos and Seligman 1968; El-Sadik and Abdel-Aziz 1970; Smith et al. 1970; Langolf et al. 1978), as evidenced by individual or group mean UHg levels that were greater than generally accepted upper background limits in non-exposed adult populations (e.g. 20 µg/L) (US Environmental Protection Agency (EPA) 1984; ATSDR 1999; US EPA 2012). Nevertheless, comparative UHg levels in the exposed groups were nearly 3-to 23-fold higher than their respective ‘non-exposed’ control groups.
In sixteen cohorts (33%), workers were excluded who had underlying medical disorders commonly associated with neurological abnormalities (e.g. diabetes; renal failure; head trauma; alcohol abuse; specific medications) and of these, most also excluded workers with a history of occupational exposure to other neurotoxicants (e.g. lead, solvents). In five other cohorts, only limited exclusion criteria were applied (Roels et al. 1982; Gonzalez-Fernandez et al. 1984; Piikivi et al. 1984; Verberk et al. 1986; Cavalleri et al. 1995). In the remaining 27 cohorts such a priori exclusions were not utilized, although eight acknowledged the possible adverse effects of conditions such as diabetes, alcohol abuse, and family or personal history of neurological disorders (Vroom & Greer 1972; Gilioli et al. 1976; Zedda et al. 1980; Angotzi et al. 1981; Roels et al. 1982; Bunn et al. 1986; Soleo et al. 1990; Urban et al. 1996). Among 35 cohorts evaluated using NB and/or EPS evaluations, 24 (69%) used either matching or statistical analyses to control for the possible effects of height, weight, education, smoking, consumption of alcohol and caffeine, and use of tremorigenic medications (e.g. adrenergic asthma medications, thyroid hormone) on specific outcomes. The quality rankings (i.e. Tiers) of studies are shown in Table 2; the individual components of those rankings (use and appropriateness of control groups, inclusion/exclusion criteria, and analytical methods of minimize confounding) are detailed in Supplemental Table 2.
Exposure assessment
Studies used a variety of approaches to characterize worker exposure levels and determine exposure characteristics associated with neurological abnormalities. Most studies reported measurements of UHg; a smaller number considered blood mercury (BHg) and/or air Hg0 (Table 2). In 25 cohorts (51%), exposure was characterized on the basis of a single urine sample. In the other 24 cohorts, multiple UHg samples were obtained over time, and exposure was assessed in terms of group or individual mean, peak (i.e. number of times UHg levels exceeded a given threshold), or cumulative (e.g. sum of all monthly UHg levels) values determined for a specific period of time (e.g. previous 3-, 6-, 12-, or 24-months). Exposure levels reported in individual studies are summarized by highest to lowest UHg in Supplemental Table 1; Supplemental Tables 3 and 4 detail the study-specific methods used to evaluate exposure.
Correlations among various exposure metrics were assessed in 15 of 49 cohorts (Supplemental Table 5). Current mean UHg levels were significantly and consistently correlated with current BHg levels (Miller et al. 1975; Roels et al. 1982; Triebig and Schaller 1982; Fawer et al. 1983; Piikivi et al. 1984; Roels et al. 1985, 1989) and current air Hg0 levels (Smith et al. 1970; Gambini 1978; Gonzalez-Fernandez et al. 1984; Ehrenberg et al. 1991). Significant associations were also found with measures of peak UHg levels (Piikivi et al. 1984) and with UHg averaged over various time periods (Piikivi et al. 1984; Wastensson et al. 2006). By contrast, current UHg was not significantly correlated with cumulative urine levels (Wastensson et al. 2006). Exposure duration was also not significantly correlated with any of ten biological exposure metrics including current UHg and cumulative UHg (Miller et al. 1975; Piikivi et al. 1984; Langworth et al. 1992; Wastensson et al. 2006).
The exposure characteristics and distribution of study groups, cohorts, studies and exposed workers across the four exposure categories are shown in Table 3.
Table 3.
Summary results of study groups, cohorts, and studies stratified by exposure category
| Exposure category* (definitions) |
Study groups |
Cohorts | Studies | Exposed workers |
UHg levels (µg/L) mean (range)† |
Duration (yrs) mean (range)† |
|---|---|---|---|---|---|---|
| HIGH (≥200 µg/L) | n=21 | n= 20‡ | n= 20 | n = 1065 | 447 (3–7100) | 7.1 (0.25–40) |
| MEDIUM (100 – 199 µg/L) | n=11 | n= 9 | n = 7 | n = 581 | 145 (2–819) | 7.8 (0.08–30) |
| LOW (50 – 99 µg/L) | n=12 | n= 10 | n = 8 | n = 915 | 66 (6–1200) | 6.9 (0.03–37) |
| BEI (<50 µg/L) | n=14 | n= 10 | n = 10 | n = 604 | 23 (0.3–121) | 11.2 (0.03–45) |
|
| ||||||
| Summary Totals: | n =58 | n =49 | n =45 | 3165 | 189 (0.3–7100) | 8.4 (0.08 – 45) |
Key:
= Exposure categories used to stratify workers according to their group mean UHg levels (see text for details).
= Presents weighted average of group mean UHg levels (or exposure durations), and overall range of individual UHg levels (or exposure durations) among study groups within each exposure category.
= Includes Albers cohort that was evaluated using a nested case-control design.
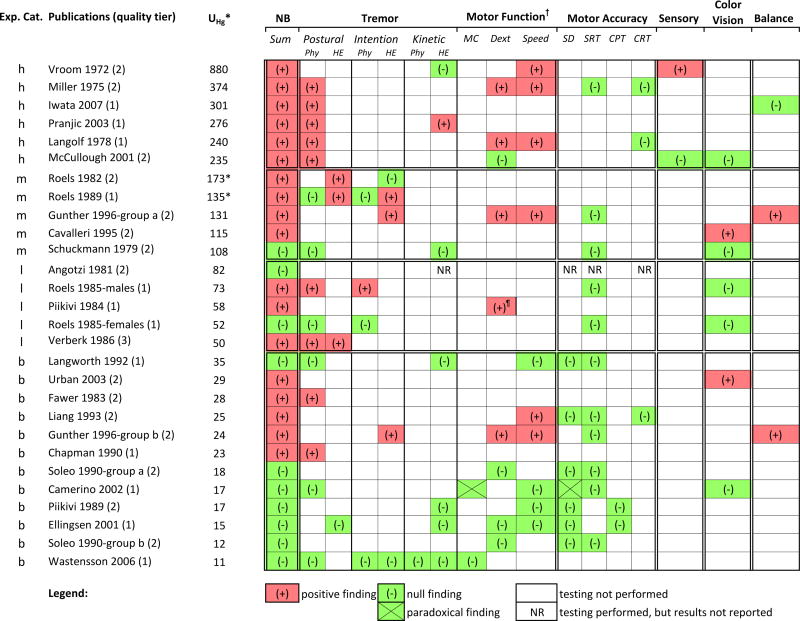
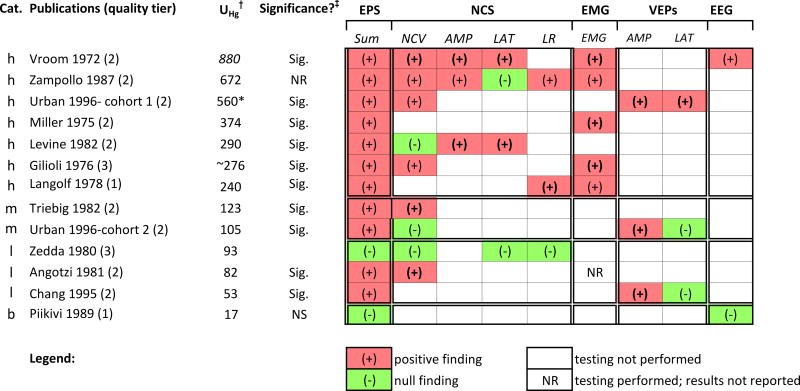
Neurological evaluations
The neurological findings described in the studies are presented below, grouped according to type of evaluation (PE, NB, and EPS). For each type of evaluation, the reported findings are summarized according to exposure categories.
Physical examination
PE was performed in 35 of 44 cohort studies (46 of 57 study groups) to evaluate neurological effects in 3724 workers (2480 exposed, 1244 controls). Exposed workers ranged in age from 18 to 71 years (weighted average mean: 38 years). PE was also performed in one nested case-control study of 138 workers (Albers et al. 1982). Most studies included comprehensive clinical neurological exams with mainly qualitative results reported as ‘normal’, ‘abnormal’ or ‘equivocal’. Semi-quantitative scales were used to judge strength (e.g. 0–5) and deep tendon reflexes (e.g. ‘absent’, ‘diminished’, ‘normal’, or ‘hyperactive’). Only 20 of the 36 studies (56%) evaluated non-exposed ‘controls’ and only nine (25%) performed statistical analyses to determine whether abnormalities in exposed workers were significantly increased compared to controls (Smith et al. 1970; Gambini 1978; Ehrenberg et al. 1991; Langworth et al. 1992; Tang & Li 2006; Wastensson et al. 2006) and/or significantly associated with exposure levels (Miller et al. 1975; Gilioli et al. 1976; Gambini 1978; Albers et al. 1982; Ehrenberg et al. 1991; Tang & Li 2006).
Studies provided varying levels of detail. Some reported results for each test (e.g. finger-to-nose), others reported results grouped by functional domains (e.g. motor coordination or sensory function), and a few described only aggregated findings (e.g. ‘conventional medical examinations failed to detect any neurotoxic effects’ (Langolf et al. 1981). Detailed PE results from each of the individual studies and study groups are available in Supplemental Tables 6a–d.
Exposure effects
Twenty-one of the 36 studies (35 cohorts and one case-control) described positive findings on PE in 22 of 47 study groups (Figure 1). As discussed below the proportion of cohort study groups with at least one positive finding on PE was respectively 0%, 40%, 20% and 79% of the <BEI, Low, Medium and High Exposure groups; similar dose-related trends were seen for each of the most frequently reported PE abnormalities (Table 4).
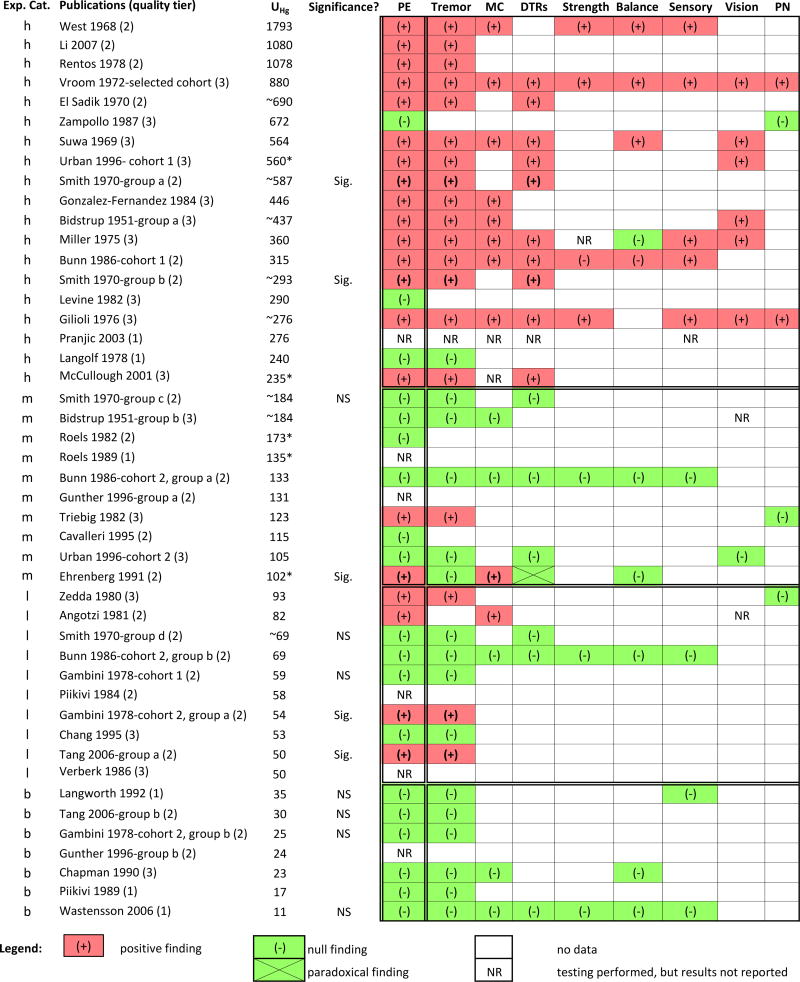
Figure 1.
Overview of occupational cohort studies that evaluated the association between mercury exposure and PE outcomes. Studies are listed top to bottom by decreasing group mean UHg values (µg/L) in exposed workers, and category of exposure is denoted (h=high, m=medium, l=low, b=<BEI).
Abbreviations: DTR = deep tendon reflexes; Exp. Cat. = exposure category; MC = motor coordination; NR = not reported; NS = not statistically significant; PE = physical examinations; PN=peripheral neuropathy; Sig. = statistically significant
* = UHg value was converted to µg/L from units originally reported in study, ~ = UHg estimated from average air Hg concentrations, as described in the Methods Section
Table 4.
Summary results of PE outcomes: exposure-effects and dose-effects reported in individual cohort studies
| ‘Exposure-effects’* | ‘Dose-effects’† | |||||
|---|---|---|---|---|---|---|
| PE outcomes | Overall | BEI | Low | Med | High | Individual study results |
| Abnormal PE: (# positive groups/# tested): | 46% (21/46) | 0% (0/7) | 40% (4/10) | 20% (2/10) | 79% (15/19) | 92% (12 of 13) |
|
| ||||||
| Tremor: | 41% (19/46) | 0% | 30% | 10% | 79% | 83% (10 of 12) |
| Motor Coordination: | 22% (10/46) | 0% | 10% | 10% | 42% | 80% (4 of 5) |
| Deep Tendon Reflexes: | 22% (10/46) | 0% | 0% | 0% | 53% | 60% (3 of 5) |
| Sensory Function: | 11% (05/46) | 0% | 0% | 0% | 26% | 75% (3 of 4) |
Key: Table presents summary results of all cohort studies that performed PE; data from the nested case-control study by Albers et al (1982) is not included.
= The proportion of cohort study groups with positive findings (i.e. # with ≥ 1 positive findings divided by # of study groups evaluated).
= The proportion of cohort studies that reported positive dose-relatedness among those cohort studies that evaluated dose-relatedness.
High Exposure
PEs were performed in 19 of the 20 High Exposure cohort study groups. Positive findings were described in 15 of those 19 (79%), of which all reported tremor. As shown in Figure 1, other abnormalities were found, but less frequently. Of four study groups that did not report positive findings, three described workers as ‘asymptomatic’ or ‘normal’ (Langolf et al. 1978; Levine et al. 1982; Zampollo et al. 1987) and the fourth, which had examined workers three to six months after cessation of exposure, did not report exam findings (Pranjic et al. 2003). The one study that did not perform PE described exposed workers (mean UHg: 301 µg/L) and controls as healthy: ‘neither….seemed to be apparently unhealthy’ (Iwata et al. 2007).
In the only nested case-control study, which compared workers with peripheral neuropathy (PN) vs. workers without PN, Albers et al. (1982) reported significantly increased prevalence of tremor, reduced DTRs, decreased sensation (distal vibratory and pin sensation), and decreased motor strength and tone in those with PN.
Medium Exposure
PEs were performed in 10 of 11 Medium Exposure study groups. Positive findings were described in two of those 10 groups (20%). Ehrenberg et al. (1991) found a significantly higher prevalence of impaired MC in exposed workers, a non-significant increase in tremor (19%), and a significant paradoxical finding for DTRs: exposed workers had fewer findings of hyporeflexia compared to controls (24% vs. 43%). Triebig and Schaller (1982) reported tremor in two of 18 workers (11%), but did not evaluate the significance of the finding. Of the remaining eight study groups, six described only null findings and two did not report findings. The one study (Schuckmann 1979) that did not perform physical exams (because study workers had been ‘under routine medical surveillance’) noted that ‘clinical intoxications … are not to be expected’ at the reported exposure levels (mean UHg: 108 µg/L).
Low Exposure
PEs were performed in 10 of 12 Low Exposure study groups. Four of the 10 (40%) reported positive findings, of which three reported tremor (Gambini 1978; Zedda et al. 1980; Tang & Li 2006); the fourth reported ‘cerebellar type’ abnormalities in 5% of exposed workers (Angotzi et al. 1981). Only null exam findings were reported in four study groups, and exam results were not reported in two other groups. PEs were not performed in one study (Roels et al. 1985) which evaluated two study groups.
<BEI Exposure
PEs were performed in 7 of the 14 <BEI Exposure study groups. Only null exam findings were reported for six groups; results were not reported for the seventh (Gunther et al. 1996). Physical exams were not performed in six studies which evaluated seven study groups.
Dose-effects
Only 13 of the 21 studies (62%) that described positive findings on PE also considered the dose-relatedness of their findings (i.e. ‘dose effects’) in 13 cohorts and one case-control study. All of these studies evaluated the effects of Exposure Intensity, while only three studies analyzed Exposure Correlations (i.e. the correlation between specific findings and dose metrics). We did not include data from Miller (1975), a study which performed PE on only a subset (32 of 142) of workers and found no dose-relatedness for tremor, DTRs or vibration in comparisons between exposed workers and controls. That subset included 18 ‘exposed’ workers (mean UHg: 500 µg/L) and 14 ‘controls’ not exposed during the prior 6 months (mean UHg: 152 µg/L). However, the study authors noted: ‘The Controls…were later found to have excessively high blood and urine mercury levels and cannot be classified as ‘normal’ controls in general’ (as reported in Chaffin et al. 1973). Thus, we regarded all 32 of those workers as heavily exposed and, therefore, the study’s PE findings not amenable to dose-related analyses. The dose-related findings reported within individual studies are discussed below; summary findings for specific outcomes are presented in the far-right column of Table 4. For additional perspective on dose-relatedness, we present results of our analyses of prevalence for the three most frequently reported PE abnormalities (tremor, MC, and DTRs).
Exposure intensity
The relationship between specific PE abnormalities and Exposure Intensity was evaluated in eight High Exposure studies (Bidstrup et al. 1951; Rentos & Seligman 1968; West & Lim 1968; El-Sadik & Abdel-Aziz 1970; Smith et al. 1970; Gilioli et al. 1976; Bunn et al. 1986; Urban et al. 1999), one Medium Exposure study (Ehrenberg et al. 1991), two Low Exposure studies which reported findings in three cohorts of workers (Gambini 1978; Tang & Li 2006), and one case-control study (Albers et al. 1982). Exposure Intensity was not evaluated in <BEI studies.
Tremor
Ten studies evaluated the dose-relatedness of tremor in eleven cohorts; their findings are summarized below.
Seven High Exposure studies evaluated the dose-relatedness of tremor; six reported a positive dose-response. In five of those studies, tremor was largely confined to subsets of the most highly exposed workers, in whom current individual (Bidstrup et al. 1951; Rentos & Seligman 1968; West & Lim 1968) or group mean UHg levels (Smith et al. 1970; Urban et al. 1999) approached or exceeded 300 µg/L. The sixth study reported that prevalence increased with exposure duration (< 3 vs. ≥ 3 years), but was unrelated to current UHg levels (El-Sadik & Abdel-Aziz 1970). The seventh study (Bunn et al. 1986) found no evidence that tremor prevalence was dose-related based on comparisons across three exposure subgroups.
One Medium Exposure study found tremor prevalence to be dose-related. Ehrenberg et al. (1991) reported that workers with ‘static’ tremor (i.e. postural tremor) had higher current UHg levels, and a significantly higher ‘chronic exposure index’ (i.e. an arbitrary unitless ‘index’ that combined duration and relative intensity of exposure) than did workers without such tremor, but no dose-relatedness was found for ‘resting’ tremor or ‘intention’ tremor.
Two Low Exposure studies described dose-relatedness in two of three cohorts. Tang & Li (2006) found that prevalence of tremor was significantly increased in workers with UHg ≥50 µg/L compared to those with UHg of 20–40 µg/L; the latter group did not differ from unexposed controls. Gambini (1978) evaluated two cohorts of chloralkali workers. In the first cohort, comprised of workers who were routinely rotated between high- and low-exposure jobs, tremor prevalence did not differ significantly across three subgroups with current mean UHg levels of 92, 59, or 25 µg/L (p>0.05). In the second cohort, new workers without prior exposure and not rotated between high- and low-exposure jobs were studied prospectively for two years. Tremor prevalence was significantly increased in workers with at least one UHg >50 µg/L compared to workers with UHg <50 µg/L.
Prevalence analysis
For additional perspective on the dose-relatedness of tremor, we examined tremor prevalence in individual studies and across exposure categories from 33 studies (44 study groups) that performed PEs in a total of 2414 exposed workers. We did not consider data from three studies. Two of those studies, Albers et al. (1982), a nested case-control study, and Vroom and Greer (1972), which selected workers based on the severity of their observed effects, were not included because of design particularities. A third study (Angotzi et al. 1981) which did not describe tremor, but did report ‘cerebellar’ abnormalities without further details in three workers, was excluded because we could not determine whether the authors considered tremor to be a cerebellar abnormality. The prevalence of tremor in exposed workers averaged 8%, 8%, 6% and 23% across <BEI, Low, Medium and High Exposure categories (Supplemental Table 7a). In seven control groups described in those studies, the prevalence of tremor averaged 8% (Supplemental Table 7b). Because the averaged proportions of exposed workers with tremor did not increase above that in controls until UHg >200 µg/L, the High Exposure groups were further divided into three subcategories (mean UHg 200–299, 300–499, and >500 µg/L) to identify a possible threshold of effect. In those subcategories, tremor prevalence was respectively 6%, 21% and 42%. These results indicated that on average, exposed workers did not experience tremor in excess of background until UHg ≥300 µg/L.
In the 18 study groups positive for tremor, the weighted average of group mean UHg levels was 4-fold higher than the average UHg level in the 26 groups null for tremor (434 vs 106 µg/L). Most study groups positive for tremor had group mean UHg levels ≥300 µg/L (67%; 12 of 18 groups) and maximum UHg levels >500 µg/L (86%; 12 of 14 groups; four groups lacked relevant data). By contrast, most null findings for tremor were associated with group mean UHg levels <200 µg/L (88%; 23 of 26 study groups) and maximum UHg levels <500 µg/L (68%; 15 of 22 study groups, 4 lacked relevant data) (detailed in Supplemental Table 7a). Results from a second analysis, limited to studies that had specifically indicated they had looked for tremor, were not substantially different from the first (data not shown).
Motor Coordination
Five studies evaluated the dose-relatedness of MC abnormalities; their findings are summarized below.
Three of four High Exposure studies that evaluated the dose-relatedness of MC abnormalities (e.g. ataxia, gait abnormalities) reported positive results. Bidstrup et al. (1951) diagnosed chronic Hg poisoning in 27 of 103 DC-meter repairmen based on findings that included ‘ataxia’; 21 of those 27 workers (78%) had 24-hr UHg levels >300 µg. By contrast, only 16% of workers without ‘clinical evidence of poisoning’ had such levels. In West & Lim (1968), workers with the most ‘severe’ cases of Hg poisoning, defined as ‘more intense nervous system findings such as tremors, muscle weakness, difficulty walking and balancing, numbness and tingling’, had higher UHg levels (1980 to 7100 µg/L) than workers without such findings (950–1880 µg/L). Gilioli et al. (1976) reported a dose-related increase in the prevalence of ‘ataxic signs’ (17%, 20%, 33%) across a 3-tiered ‘risk index’ of exposure (a combination of AirHg level, UHg and exposure duration not otherwise described). In the fourth study, Bunn et al. (1986) found no evidence of dose-related MC abnormalities based on comparisons across three exposure subgroups.
One Medium Exposure study evaluated the dose-relatedness of impaired MC. Ehrenberg et al. (1991) found that the ‘chronic exposure index’, but not current mean UHg levels, was significantly higher in workers with DDK and abnormal heel-to-toe walk compared to workers without such abnormalities.
Prevalence analyses
For additional perspective on the dose-relatedness of MC, we examined MC prevalence in individual studies and across exposure categories using data from 12 studies (15 study groups) that specifically described testing for MC abnormalities in 835 exposed workers. Data were not considered from Vroom and Greer (1972) due to its unique design, and from another study (McCullough et al. 1999, 2001) that reported workers had ‘at least one abnormal neurological finding’ on PE, ‘such as brisk reflexes’ and ‘tremor’, but did not specifically indicate whether or not MC was impaired. The prevalence of workers with one or more abnormal MC findings averaged 8%, 3%, 6%, and 17% across <BEI, Low, Medium, and High Exposure categories (Supplemental Table 8a). Of the two control groups described in those studies, abnormal MC was found in 2.5% (Ehrenberg et al. 1991) to 9% (Wastensson et al. 2006) of unexposed workers.
Positive MC findings on physical exam were associated with a weighted group mean UHg level that was 2.7-fold higher than the UHg level associated with null findings (351 vs. 129 µg/L). Of the nine study groups that described workers with one or more positive MC findings, seven had group mean UHg levels >275 µg/L, and eight had maximum UHg levels that approached or exceeded >500 µg/L (one group lacked relevant data). By contrast, five of the six study groups with null findings had group mean UHg levels ≤185 µg/L and four had maximum UHg levels <500 µg/L (one group lacked relevant data) (Supplemental Table 8a). Such positive dose-relatedness was also seen for each of six specific MC abnormalities that were described in three or more study groups (Supplemental Tables 8b and 8c).
Deep Tendon Reflexes
Five studies evaluated the dose-relatedness of DTRs; their findings are summarized below.
Three of four High Exposure studies that evaluated the dose-relatedness of abnormal DTRs reported positive findings. Urban et al. (1999) found abnormal DTRs in workers with current mean UHg levels of 840 µg/24 hrs, but not in a comparison group with mean UHg levels of 129 µg/24 hrs. Smith et al. (1970) reported that compared to controls, the prevalence of abnormal DTRs was significantly increased in two groups of workers exposed to TWA ambient Hg0 levels >100 µg/m3 (estimated mean UHg >230 µg/L), but not in those exposed to <100 µg/m3. In El-Sadik & Abdel-Aziz (1970), the prevalence of abnormal DTRs was increased in workers with longer duration of exposure (≥3 vs. <3 years) but unrelated to UHg levels. Bunn et al. (1986) found no evidence of dose-relatedness based on comparisons across three exposure subgroups.
One Medium Exposure study evaluated the dose-relatedness of abnormal DTRs. Ehrenberg et al. (1991) found that mean UHg levels and the ‘chronic exposure index’ were non-significantly increased in workers with hyperactive DTRs compared to workers with normal DTRs.
Prevalence analysis
For additional perspective on the dose-relatedness of DTRs, we examined data from 11 studies (17 study groups) that evaluated DTRs in 1265 exposed workers. Data from Albers et al. (1982) and Vroom and Greer (1972) were not considered due to their unique designs. Positive findings of abnormal DTRs were associated with a weighted group mean UHg level that was 4.0-fold higher than the comparative UHg level in groups with null DTR findings (435 vs. 108 µg/L, respectively). Eight of the nine cohort study groups that reported positive findings had group mean UHg levels >275 µg/L and all had maximum UHg levels >500 µg/L. By contrast, seven of the eight study groups with null findings had group mean UHg levels ≤184 µg/L, and all but two had maximum UHg levels <500 µg/L (details in Supplemental Table 9).
Stratified analysis of DTR prevalence across exposure categories included data from 698 workers described in 10 of those 11 studies; one study (Smith et al. 1970) did not provide sufficient information, indicating only that prevalence in exposed workers was ‘the same among controls’ until ‘exposure was greater than 0.10 mg/m3’. The prevalence of abnormal DTRs in exposed workers was not significantly greater than background prevalence in controls until UHg >275 µg/L (Supplemental Table 9). Of the four control groups described in those studies, two reported abnormal DTRs in 10% (El Sadik et al. 1970) and 0% (hyperactive) to 43% (hypoactive) (Ehrenberg et al. 1991) of unexposed workers, one did not indicate the number of affected controls (Smith et al. 1970), and the fourth found no evidence of abnormal DTRs (Wastensson et al. 2006).
Distal Strength
Two High Exposure studies evaluated the dose-relatedness of reduced distal strength; both reported positive findings. In West and Lim (1968), ‘muscle weakness’ was found among workers with current individual UHg levels that ranged from 1980–7100 µg/L, but not in those with UHg levels of 950–1880 µg/L. Gilioli et al. (1976) compared workers using a 3-tiered ‘risk index’ of exposure; the prevalence of reduced distal strength was significantly increased in workers within the highest-risk, but no differences were seen between workers with medium- and lowest-risk.
Balance
Two studies evaluated the dose-relatedness of balance. West and Lim (1968), a High Exposure study, reported ‘difficulty walking and balancing’ in workers with current individual UHg levels that ranged from 1980–7100 µg/L, but not in workers with UHg levels of 950–1880 µg/L. Ehrenberg et al. (1991), a Medium Exposure study, reported that exposed workers with abnormal Romberg tests had a non-significant increase in the ‘chronic exposure index’, but not current mean UHg, as compared to exposed workers with normal Romberg.
Sensory Function
Four studies evaluated the dose-relatedness of abnormal sensory function. Two of three High Exposure studies that evaluated dose-relatedness reported positive relationships. West and Lim (1968) reported ‘numbness and tingling’ in workers with current individual UHg levels that ranged from 1980–7100 µg/L, but not in workers with UHg levels of 950–1880 µg/L. Bunn et al. (1986) observed a monotonic dose-related increase in abnormal vibration sensation (7%, 14%, 17%), but not pinprick sensation (0%, 10%, 8%) across three subgroups of workers with increasing levels of exposure. Neither of those two studies reported statistical significance. In the third study, Gilioli et al. (1976) reported no statistically significant difference in the prevalence of ‘sensory signs’ (39%, 19%, 33%) across a 3-tiered ‘risk index’ of exposure (a combination of air level, UHg and exposure duration that was not otherwise described).
In the fourth study, a nested case-control study, Albers et al. (1982) found that in comparison to workers with normal clinical exam, workers with ‘mild sensory polyneuropathy’ on clinical exam had twice the number of UHg measurements >250 µg/L in the previous 6, 12, 24 and 36 months, and more than twice as many UHg >500 µg/L in the previous 24 and 36 months. All of those comparisons were statistically significant.
Exposure correlations
Only three studies evaluated correlations between PE abnormalities and various measures of exposure. Smith et al. (1970) evaluated 567 exposed workers divided into two High, one Medium and one Low Exposure study groups. Tremor was the only PE finding correlated with exposure; its prevalence was significantly correlated with one-year TWA levels of Hg0 in air, blood and urine. McCullough et al. (2001), a High Exposure study that evaluated 16 exposed workers, found ‘no association between having a tremor on examination and urinary mercury concentration’. Albers et al. (1982), a nested case-control study, used simple and multiple linear regressions to evaluate correlations between ‘selected’ clinical outcomes (not otherwise defined) and 14 exposure metrics in 138 workers. Only statistically significant correlations were reported. Significant correlations were found between ‘distal sensory loss’ and ‘weakness’ and the ‘majority of urine mercury indexes’, including current and average historical UHg levels from the prior 3, 6, 12, 24, and 36 months and also the number of UHg peaks >250 or >500 µg/L during the prior 6, 12, 24, and 36 months. No results were reported for tremor or DTRs.
Clinical significance
Only six studies explicitly commented on the clinical significance of the PE abnormalities they documented. Among four High Exposure studies that addressed such considerations, only one described clinically significant impairments. Vroom and Greer (1972) described nine workers with mean UHg of 1320 µg/24 hrs who had been ‘selected’ for the severity of their symptoms; in six of the nine, ‘eating, drinking, and dressing were performed with great difficulty and two … had virtually stopped walking because of unsteadiness’. By contrast, the other three High Exposure studies indicated that abnormalities were ‘clinically insignificant’. Miller and Chaffin et al. (1975) described clinical findings in 32 chloralkali workers with mean UHg of 360 µg/L, as ‘minor neurologic abnormalities’ with ‘no functional impairment with regard to work responsibilities, or habits of daily living … none of the workers reported being clinically ill’ (Chaffin et al. 1973). Langolf et al. (1978), in an expanded follow-up of the Miller study that included 79 exposed chloralkali workers with lower exposure (mean UHg of 240 µg/L), reported that ‘exposed employees revealed no evidence of signs or symptoms of excessive mercury exposure’ and ‘no functionally significant mercury related tremor effect’. Finally, Zampollo et al. (1987) concluded that among 17 thermometer factory workers with a group mean UHg of 672 µg/L, ‘none… presented overt clinical signs or symptoms of peripheral neuropathy or of central nervous system involvement’.
In the nested case-control study, Albers et al. (1982) diagnosed ‘mild’ polyneuropathy on clinical exam in 18 workers with current mean UHg of 130 µg/L and historical UHg levels >250 µg/L; ‘none [of the workers] were aware of the mild impairment.’
In a Low Exposure study that evaluated seven lamp manufacturing workers with two-year average UHg of 93 µg/L, Zedda et al. (1980) detected changes on EPS compatible with the ‘initial signs of neuropathy’, but noted that none of the workers showed signs of clinical neuropathy on neurological exam.
PE section summary
Positive findings on PE were associated with weighted mean UHg of 403 µg/L (range of means: 50 – 1793 µg/L), nearly 4-fold greater than the weighted mean associated with null findings (104 µg/L, range of means: 11 – 672 µg/L). Weighted average UHg levels in studies that did not report their PE results (117 µg/L) and those that did not perform PE (60 µg/L) were similar to or lower than those that reported null results (104 µg/L) (Table 5a). This lends support that studies that performed PE would have reported the results of neurological evaluation if they had been clinically abnormal and/or statistically significant.
Table 5.
| a. Sensitivity analysis: comparisons between studies that did and did not perform PE, and by type of outcome reported | |||||
|---|---|---|---|---|---|
| Type of PE result | UHg (µg/L) | Age of exposed | Number of workers | Quality tier | |
| % (number) | Mean (range) | Mean (range)* | Exposed/Controls† | Mean‡ | |
| Performed PE: | 81% (46 of 57 groups) | 212 (11 –1793) | 38 (20–51) | 2480/1244 | 2.0 |
|
| |||||
| Positive results: | 46% (21 of 46 groups) | 403 (50 –1793) | 38 (20–51) | 929/667 | 2.2 |
|
| |||||
| Null results: | 41% (19 of 46 groups) | 104 (11 –672) | 37 (27–43) | 1324/909 | 1.9 |
|
| |||||
| Not reported results: | 13% (6 of 46 groups) | 117 (24 –273) | 41 (35–51) | 227/159 | 1.6 |
|
| |||||
| PE not performed: | 19% (11 of 57 groups) | 60 (12 –301) | 36 (29–44) | 502/475 | NA |
| b. Sensitivity analysis: summary of PE results, UHg, descriptive data, and Tier ratings, stratified by category of exposure | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
|
Exposure Category |
PE Results | UHg (µg/L) | Age of exposed | Number of workers | Quality tier¶ | |
| % with positive results* | Mean (range)† | Mean (range)† | Exposed/Controls‡ | All | (+) | |
| Overall: | 46% (21 of 46 groups) | 205 (11 –1793) | 38 (20 – 51) | 2480/1244 | 2.0 | 2.2 |
|
| ||||||
| HIGH | 79% (15 of 19 groups) | 454 (235 – 1793) | 39 (31 – 51) | 855/533 | 2.2 | 2.4 |
|
| ||||||
| MED | 20% (2 of 10 groups) | 147 (102 – 184) | 35 (20 – 47) | 542/632 | 2.0 | 2.1 |
|
| ||||||
| LOW | 40% (4 of 10 groups) | 65 (50 – 93) | 38 (29 – 51) | 730/686 | 2.0 | 2.0 |
|
| ||||||
| < BEI | 0% (0 of 7 groups) | 24 (11 – 35) | 40 (29 – 47) | 353/309 | 1.5 | NA |
Table summarizes data from 44 studies (57 study groups); data from Albers' case-control study not included.
= Weighted average of group means and range of group means; calculated from data reported for individual study groups.
= When a study reported findings for multiple study groups and results on PE were positive in one or more groups and null in the other(s), the total number of controls in that study was assigned to each relevant Type of PE outcome, as occurred with 2 studies (Smith, and Tang); thus the # of controls listed for "Performed PE" does not agree with the sum of controls listed for Positive, Null, and Not Reported PE results.
= Average of Tier ratings, weighted by study group size (i.e. total number of exposed and controls)
Table summarizes data from 46 groups described in 35 cohort studies; data from Albers' case-control study not included.
= The proportion of study groups with positive findings (i.e. # with ≥1 positive finding on PE divided by # of study groups evaluated using PE).
= Weighted average of group means and (the range of means); calculated from data reported for individual study groups.
= When a study reported findings for multiple study groups, the total number of controls in that study was assigned to each study group that was stratified into a separate exposure category (i.e. Smith 1970, Gunther 1996, and Tang 2006); thus the sum of controls listed across exposure categories does not agree with the Overall sum of controls.
= Average of Quality Tier value weighted by study group size (i.e. total number of exposed and controls) for all study groups evaluated using PE, and those study groups with positive (+) results.
NA = not applicable.
As shown in Table 5b, the proportion of study groups reporting one or more positive finding on PE increased across exposure categories and with increasing UHg levels; the dose-related trend did not appear to be age-related.
Neurobehavioral testing
NB testing was performed in 25 of the 44 cohort studies (28 of the 57 study groups) to evaluate neurological function in 2343 workers (1251 exposed, 1092 controls) aged 19 to 71 years (weighted average mean: 38 years). NB testing was also performed in one nested case-control study of 138 workers (Albers et al. 1982). Studies used a variety of quantitative tests to evaluate a range of neurological functions. All but three studies (Vroom & Greer 1972; Verberk et al. 1986; McCullough et al. 2001) evaluated non-exposed controls, and all but one (Vroom & Greer 1972) performed statistical analyses to determine whether abnormalities in exposed workers were significantly increased compared to controls and/or literature-based normative values, or significantly associated with exposure levels. Figure 2 presents summary results from the cohort studies across six functional domains: tremor, motor function, motor accuracy, sensory function, color vision, and balance.
Figure 2.
Overview of occupational cohort studies that evaluated the association between mercury exposure and neurobehavioral outcomes across six functional domains. Studies are listed top to bottom by decreasing group mean UHg values (µg/L), and category of exposure is denoted (h=high, m=medium, l=low, b=<BEI).
Abbreviations: CPT=Continuous Performance Test; CRT=Choice Reaction Time; Dext=dexterity; HE=hand-eye coordination tests of motor steadiness used to measure NB tremor; MC=motor coordination; NB=neurobehavioral testing; Phy=physiological tests of NB tremor; SD=Symbol Digit/Digit Symbol; SRT=Simple Reaction Time
* indicates UHg was converted to µg/L from units originally reported in study as described in the Methods Section
† Results of motor steadiness tests were tabulated as Tremor (see HE columns for Postural, Intention and Kinetic tremor)
¶ Mixed results: study reported performance in exposed workers was significantly poorer in comparison to controls, but analysis of dose-effects were paradoxical (i.e. higher exposure was associated with better performance).
Studies varied in the amount of detail provided regarding their results and specific methods. Some studies reported results for each specific test; others reported results by functional domain (e.g. sensory function). Some provided details of the instruments used, testing protocols and scoring criteria; others described the name of the test or function measured and reported test results without further detail. Detailed results from each of the individual studies and study groups are available in Supplemental Tables 10a–d.
Exposure effects
Eighteen of the 26 studies (25 cohorts and one case-control) described positive NB findings in 19 of 29 study groups tested. As discussed below and summarized in Table 6, the proportion of cohort study groups with at least one positive NB finding was respectively 42%, 60%, 80% and 100% of the <BEI, Low, Medium, and High Exposure groups. Of the three most frequently reported NB abnormalities, dose-related trends were seen for tremor and motor function, but not motor accuracy.
Table 6.
Summary results of NB outcomes: exposure-effects and dose-effects reported in individual studies
| ‘Exposure-effects’* | ‘Dose-effects’† | |||||
|---|---|---|---|---|---|---|
| NB outcomes | Overall | BEI | Low | Med | High | Individual study results |
| % positive on NB: (# groups pos./# tested): | 64% (18/28) | 42% (5/12) | 60% (3/5) | 80% (4/5) | 100% (6/6) | 59% (13 of 22) |
|
| ||||||
| NB Tremor: | 69% (13/22) | 38% (3/8) | 50% (2/4) | 75% (3/4) | 83% (5/6) | 52% (9 of 17) |
| Motor Function‡: | 47% (7/15) | 22% (2/9) | 100% (1/1) | 100% (1/1) | 75% (3/4) | 36% (4 of 11) |
| Motor Accuracy¶: | 0% (0/15) | 0% (0/8) | 0% (0/3) | 0% (0/2) | 0% (0/2) | 25% (3 of 12) |
Table presents summary results for overall NB and for specific NB outcomes evaluated in ≥10 cohort study groups; findings from the case-control study (Albers et al 1982) are not included.
= The proportion of cohort study groups with positive findings (i.e. # with ≥ 1 positive finding divided by # of study groups evaluated).
= The proportion of cohort studies that reported positive dose-relatedness among those cohort studies that evaluated dose-relatedness.
= Summary results from tests of motor coordination, manual dexterity and motor speed. Analysis does not include results from tests of motor steadiness that provided secondary evidence of tremor, those test results are included above in the analysis of "NB tremor"; the findings from these analyses of motor function were similar to the results from an analysis that included tests of motor steadiness with the other tests of motor function (data not shown).
= Summary results from tests of attention, response speed and perceptual motor speed.
NB tremor
Twenty of the 25 cohort studies performed NB testing of tremor in 22 study groups; the case-control study did not evaluate NB tremor. Twelve studies employed hand-eye coordination tests of motor steadiness: nine used tests of static steadiness (Roels et al. 1982; Verberk et al. 1986; Roels et al. 1989; Ellingsen et al. 2001), aiming (Roels et al. 1982, 1989; Gunther et al. 1996; Wastensson et al. 2008), and tracking (Schuckmann 1979; Piikivi & Hanninen 1989; Langworth et al. 1992; Wastensson et al. 2008), and four used tests that involved drawing of geometrical designs (Vroom & Greer 1972; Angotzi et al. 1981; Ellingsen et al. 2001; Pranjic et al. 2003). Fourteen studies employed physiological techniques that measure specific tremor parameters (e.g. amplitude, frequency): ten used accelerometers (Schuckmann 1979; Fawer et al. 1983; Roels et al. 1985, 1989; Langworth et al. 1992; McCullough et al. 2001; Camerino et al. 2002; Pranjic et al. 2003; Wastensson et al. 2006; Iwata et al. 2007), including one that also used a laser-based system (Wastensson et al. 2006), and four used force transducers (Miller et al. 1975; Langolf et al. 1978; Verberk et al. 1986; Chapman et al. 1990).
High Exposure
NB tests of tremor were administered in six of the 20 High Exposure cohort study groups; five of those groups reported positive findings on one or more tests (Figure 2). Postural tremor was assessed in five groups using physiological techniques; all reported statistically significant results. Evidence for kinetic tremor was mixed in two groups evaluated using the BGT drawing test; positive results were reported in one (Pranjic et al. 2003), but not the other (Vroom & Greer 1972).
Medium Exposure
NB tests of tremor were administered in four of the 11 Medium Exposure study groups; positive findings were noted in three. Postural tremor was assessed in three groups: two reported positive findings on tests of static steadiness (Roels et al. 1982; 1989), while two reported null results using accelerometers (Schuckmann 1979; Roels et al. 1989). Intention tremor was assessed in three groups: positive results were found in two of the three groups using tests of aiming; null results were reported for the only group tested with an accelerometer (Roels et al. 1989). No evidence of kinetic tremor was observed in one group evaluated using a tracking test (Schuckmann 1979).
Low Exposure
NB tests of tremor were administered to 4 of 12 Low Exposure study groups; positive findings were found in two. One study (Roels et al. 1985) using accelerometry reported positive findings for postural and intention tremor in a cohort of men, but not in a cohort of women. The second study (Verberk et al. 1986) reported positive findings for postural tremor on tests of accelerometry and static steadiness. In the third study, Angotzi et al. (1981) evaluated drawing on B-VRT (kinetic tremor) but only reported results aggregated with other NB tests (results reported in Camerino et al. 1981).
<BEI Exposure
NB tests of tremor were administered to eight of 14 <BEI Exposure study groups; three of those groups reported one or more positive findings. Tests of postural tremor were positive in two of six study groups. Null findings reported in four groups included one (Wastensson et al. 2006) that tested workers using two different methods (i.e. accelerometry and a laser-based system). Intention tremor was assessed in two groups using tests of aiming; mixed results were reported. No evidence of kinetic tremor was found in four groups assessed using tests of tracking (Piikivi & Hanninen 1989; Langworth et al. 1992; Wastensson et al. 2006) and the B-VRT drawing test (Ellingsen et al. 2001).
Motor function
Motor function (other than steadiness) was assessed in 13 studies that evaluated 15 study groups using a variety of tests that assess motor coordination, manual dexterity, and motor speed (Johnson & Baker 1987).
High Exposure
Motor function was abnormal in three of the four High Exposure study groups evaluated. Manual dexterity was positive in two groups tested using the Michigan Maze (Miller et al. 1975; Langolf et al. 1978), but not in a third group tested using the Grooved Pegboard (McCullough et al. 2001). Motor speed was significantly reduced in three groups evaluated using tapping tests (Vroom & Greer 1972; Miller et al. 1975; Langolf et al. 1978).
Medium Exposure
Motor function was evaluated in one Medium Exposure study group, which reported abnormal results on tests of ‘finger dexterity’ and tapping (Gunther et al. 1996).
Low Exposure
Motor function was evaluated in one Low Exposure study group, which had positive results for manual dexterity on the Santa Anna Dexterity Test (Piikivi et al. 1984).
<BEI Exposure
Motor function was abnormal in only two of the nine <BEI Exposure study groups evaluated. Motor coordination was normal in two groups evaluated for rapid alternative movements using Diadochokinesimetry in one (Wastensson et al. 2008), and Branches Alternate Movement Task and the Luria-Nebraska Neuropsychological Battery in the other (Camerino et al. 2002). One of those groups yielded paradoxical results; exposed workers performed significantly better on the Luria Battery than controls (Camerino et al. 2002).
Manual dexterity was assessed in four groups. Results were positive in one group using a ‘finger dexterity’ test (Gunther et al. 1996). Null results were reported in one study that evaluated two groups using the Santa Anna Dexterity Test (Soleo et al. 1990), and in one group using Grooved Pegboard (Ellingsen et al. 2001).
Motor speed, evaluated using tests of tapping, was significantly reduced in two of six groups tested.
Motor accuracy
Motor accuracy was assessed in 12 studies that evaluated 15 study groups using tests of attention and response speed (Simple Reaction Time (SRT), Choice Reaction Time (CRT), and Continuous Performance Tests (CPT)); and perceptual motor speed (Symbol Digit/Digit Symbol (SD)).
High Exposure
Motor accuracy was normal in two study groups evaluated for SRT (Miller et al. 1975) and/or CRT (Miller et al. 1975; Langolf et al. 1978).
Medium Exposure
Motor accuracy was normal in two study groups assessed on visual (Schuckmann 1979; Gunther et al. 1996) and auditory SRT (Gunther et al. 1996).
Low Exposure
Motor accuracy was evaluated in three study groups. Angotzi et al. (1981) found an increase in the percentage of exposed workers with abnormal findings compared to controls based on the combined results of three different tests: SRT, CRT, and SD, but did not report the statistical significance of the comparison (results reported in Camerino et al. 1981). Roels et al. (1985) found no significant differences in SRT in two study groups (one male, one female) when compared to controls.
<BEI Exposure
Motor accuracy was normal in all eight study groups evaluated. All eight groups were evaluated for attention/response time using tests of SRT, CRT, and/or CPT, and seven were tested using SD. Of the seven groups tested on SD, one reported paradoxical results (Camerino et al. 2002).
Balance
Balance was evaluated in three study groups assessed for postural sway (with eyes open). One study (Gunther et al. 1996) reported significant deficits of balance in two, one Medium Exposure group and one <BEI Exposure group. A second study reported null results in a group of High Exposure workers (Iwata et al. 2007).
Sensory function
Sensory function (other than color vision) was quantitatively evaluated in three study groups. Positive results were reported in one High Exposure study group of ‘severely affected’ workers on two tests of tactile function (Vroom & Greer 1972), and a nested case-control study that showed significant differences on tests of two-point discrimination, vibration, pin-pain, and touch-pressure in higher-exposed workers with clinical PN vs. lower-exposed workers without PN (Albers et al. 1982). Null results were reported for the third study group, which tested a small number of High Exposure workers (n=16) using a Neurometer (McCullough et al. 2001).
In two other High Exposure study groups evaluated using sensory tests (e.g. two-point discrimination) amenable to quantitative analysis, only qualitative results (e.g. positive or null) were reported (Miller et al. 1975, as reported in Chaffin et al. 1973; Pranjic et al. 2003). As such, findings could not be tabulated with other quantitative NB results, and were thus included in the discussion of PE results.
Color vision
Color vision was evaluated in seven study groups; statistically significant differences in the Color Confusion Index (CCI) were found in one Medium Exposure group (Cavalleri et al. 1995) and one <BEI Exposure group (Urban et al. 2003).
Dose-effects
Twenty-three of the 26 studies that performed NB evaluations also considered the dose-relatedness of their findings. The exceptions were Vroom and Greer (1972), Schuckmann (1979), and Pranjic et al. (2003). The dose-relatedness of NB outcomes reported within individual studies is discussed below; summary findings for all NB outcomes and for the three most frequently reported outcomes are presented in the far-right column of Table 6.
NB tremor
The dose-relatedness of NB tremor was evaluated in 17 of the 20 studies (including 18 study groups) using hand-eye coordination tests of motor steadiness (n=9 study groups) and/or physiological techniques (n=10 study groups).
Physiological tests of tremor
Ten of 14 studies that used physiological techniques also evaluated the dose-relatedness of their findings. Five of those 10 described statistically significant associations between exposure levels and at least one tremor parameter (e.g. frequency, amplitude, Tremor Index, or power spectrum).
High Exposure
Dose-relatedness of postural tremor was evaluated in four studies; positive results were reported in three. Miller et al. (1975) reported significant positive correlations between tremor frequency and current UHg, current BHg, and duration of exposure in ‘exposed’ workers, but no such correlations for tremor amplitude (alpha set at p<0.10). In an expanded follow-up of that cohort, Langolf et al. (1978) used a step-wise regression analysis to determine correlations between postural tremor, duration of exposure, and ten metrics of historical average or peak UHg levels. The number of UHg peaks >500 µg/L in the previous year was the strongest predictor of increased tremor power (p<0.005). Tremor amplitude and frequency were also noted to increase among workers with the ‘the highest urine mercury’, but statistical significance was not reported. McCullough et al. (2001) reported that mean Tremor Index was significantly greater in 13 workers with group mean UHg of 200 µg/g creatinine compared to three <BEI workers (mean UHg: 27 µg/g creatinine), but frequency and amplitude were not significantly increased. In an additional analysis, the prevalence of abnormal tremor parameters (e.g. amplitude and frequency) was not significantly increased across three categorical levels of five-month average UHg (<81, 149–235 or >235 µg/g creatinine). The fourth study (Iwata et al. 2007) found no correlations between current UHg and tremor amplitude or frequency.
Medium Exposure
Dose-relatedness of physiological tremor was not evaluated in the Medium Exposure studies.
Low Exposure
Dose-relatedness of postural tremor was evaluated in two studies; positive findings were reported in one. Verberk et al. (1986) reported a significant correlation between tremor amplitude and current UHg, but not UHg averaged over the prior year; tremor frequency was not dose-related. Roels et al. (1985) found no correlations between current UHg or BHg levels in male workers and tremor parameters that integrated measures of frequency and amplitude. Exposure duration was positively correlated with one of four tremor parameters, a finding that the authors discounted as ‘fortuitous’. Further analyses that considered the prevalence of abnormal tremor parameters across categorical exposure metrics revealed no significant correlations with exposure duration (1–4, 5–9, ≥10 years) or current UHg levels (<50, 50–74.9, >75 µg/g creatinine). Roels et al. (1985) also found no evidence of dose-relatedness of intention tremor in male workers.
<BEI Exposure
A positive result was reported in one of four <BEI Exposure studies that evaluated dose-relatedness of postural tremor. In Fawer et al. (1983), frequency and amplitude were both significantly correlated with duration of exposure; amplitude, but not frequency was correlated significantly with current UHg. In the remaining three studies, tremor frequency and amplitude showed no significant correlations with current UHg (Chapman et al. 1990; Camerino et al. 2002; Wastensson et al. 2006), historical UHg (5-yr average and lifetime cumulative) (Wastensson et al. 2006), or duration of exposure (Chapman et al. 1990; Camerino et al. 2002). One of those studies, Wastensson et al. (2006), which also used a laser-based system, found no dose-relatedness between postural or kinetic tremor and current UHg or historical UHg (5-yr average and lifetime cumulative).
Hand-eye coordination tests of tremor
Nine of 13 studies that assessed NB tremor using hand-eye coordination tests of motor steadiness also evaluated the dose-relatedness of their findings. Of those, five reported statistically significant associations between various exposure metrics and mean scores and/or the prevalence of abnormal scores.
High Exposure
Dose-relatedness was not evaluated in the High Exposure studies.
Medium Exposure
Three studies evaluated the dose-relatedness between Exposure Intensity and postural, or intention tremor (Roels et al. 1982, 1989; Gunther et al. 1996). The only positive finding was reported in Roels et al. (1982), which found dose-relatedness for postural tremor.
In two studies, Roels et al. (1982, 1989) used a static steadiness test to assess postural tremor and an aiming test (orthokinesiometer) to assess intention tremor. In the first study (Roels et al. 1982), the prevalence of abnormal scores for postural tremor was significantly increased among workers with UHg >50 µg/g creatinine or BHg >1 µg/dL. By contrast, no ‘clear-cut dose response relationships’ were found for categorical levels of current exposure (UHg or BHg) (Roels et al. 1982, 1989), historical exposure (lifetime average or cumulative UHg) (Roels et al. 1989), or duration of exposure (Roels et al. 1982, 1989) and the prevalence of abnormal scores for intention tremor in both studies (Roels et al. 1982, 1989) or postural tremor in the second study (Roels et al. 1989). In the third study, Gunther et al. (1996) used analysis of covariance (ANCOVA) that controlled for effects of age and verbal IQ to compare performance on repeated tests of aiming (intention tremor) in one Medium Exposure group (UHg : 111–152 µg/L), one <BEI Exposure group (UHg: 21–25 µg/L), and a control group. Over a seven-year time period, ‘no clear tendency could be demonstrated between the exposed groups’ on tests of aiming.
All three studies also evaluated Exposure Correlations; results were mostly null. No significant associations were found between test scores for postural tremor (Roels et al. 1989) or intention tremor (Roels et al. 1982, 1989; Gunther et al. 1996) and current exposure (UHg or BHg) (Roels et al. 1982, 1989; Gunther et al. 1996), historical exposure (lifetime average or cumulative UHg) (Roels et al. 1989), or duration of exposure (Roels et al. 1982, 1989). One study (Roels et al. 1982) found that current UHg correlated ‘marginally’ with only one of seven parameters of postural tremor.
Low Exposure
Two studies reported significant dose-relatedness for postural and kinetic tremor; intention tremor was not assessed. Verberk et al. (1986) reported significant correlations between the individuals’ summary scores on tests of static steadiness (postural tremor) and current UHg, but not one-year average UHg levels. Angotzi et al. (1981) found evidence of dose-related kinetic tremor: significantly worse performance on the B-VRT was observed in workers with three or more UHg peaks >50 µg/L during the prior year, compared to controls (results reported in Camerino et al. 1981).
<BEI Exposure
Five studies evaluated the dose-relatedness of NB tremor using hand-eye coordination tests of motor steadiness. Of these, two of three studies found significant dose-relatedness for kinetic tremor, one found no evidence of dose-relatedness for postural tremor, and two found no evidence of dose-related intention tremor.
Langworth et al. (1992) found only limited evidence of dose-related kinetic tremor; scores on tests of tracking were significantly correlated with the number of peak BHg >3 µg/dL during the prior five years, but not to current UHg, current BHg, one- or five-year average BHg, or exposure duration. In addition, ‘No notable dose-response relations’ were found between the prevalence of abnormal scores and current UHg (<17.5, 17.5–43.75, >43.75 µg/g creatinine). In Ellingsen et al. (2001), performance on B-VRT (kinetic tremor) was inversely and significantly correlated with current BHg, but not with current UHg, lifetime average UHg or lifetime cumulative UHg. However, no significant correlations were found between exposure metrics and test scores for static steadiness (postural tremor). By contrast, Piikivi and Hanninen (1989) found no evidence that kinetic tremor was dose-related: no significant correlations were found between scores on tracking and current UHg, or current or lifetime average BHg, and analysis of Exposure Intensity revealed no significant differences in scores on tracking between workers categorized as ‘High’ or ‘Low’ exposure based on median levels of current UHg, current BHg, or lifetime average BHg. Wastensson et al. (2008) and Gunther et al. (1996) found no significant correlations between intention tremor, assessed using tasks of aiming, and current UHg (Gunther et al. 1996; Wastensson et al. 2008), five-year average UHg (Wastensson et al. 2008) or lifetime cumulative UHg (Wastensson et al. 2008).
NB tremor analyses
For additional perspective on dose-relatedness of NB tremor, we considered data from all 22 study groups that evaluated NB tremor in a total of 1949 workers (1042 exposed). Because most studies only reported differences between group mean scores in exposed and controls (rather than the number of workers with abnormal scores) we were unable to calculate the prevalence of workers with abnormal findings. Accordingly, we calculated the proportion of study groups that reported positive results for NB tremor and tremor subtypes overall and across exposure categories, and examined the influence of age, UHg, and type of testing (physiological techniques vs. test of hand-eye coordination) on positive vs. null results.
As shown in Table 7, positive tremor on NB testing in 13 study groups was associated with a weighted group mean UHg that was nearly 3-fold higher than the weighted mean UHg level in the nine study groups with null findings (169 vs 59 µg/L, respectively). Positive dose-relatedness was also seen for postural and kinetic tremor, but not for intention tremor. However, results for kinetic tremor were based on only one positive study. Age and type of testing used to evaluate tremor did not appear to influence results (Supplemental Table 11).
Table 7.
Summary results of NB tremor and tremor subtypes overall and across exposure categories
| Exposure Categories | Group Mean UHg* | Mean Age† | # Examined | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exam Outcome | Overall | BEI | Low | Med | High | All groups | (+) vs null results | (+) vs null results | Exposed / Controls |
| NB Tremor (# pos. groups/# tested) | 59% (13/22) | 38% (3/8) | 50% (2/4) | 75% (3/4) | 83% (5/6) | 123 µg/L | 169 vs. 59 | 37 vs. 39 yrs | 1042/907 |
|
| |||||||||
| POSTURAL Tremor: | 65% (11/17) | 33% (2/6) | 67% (2/3) | 67% (2/3) | 100% (5/5) | 130 µg/L | 184 vs. 39 | 36 vs. 39 yrs | 847/726 |
| INTENTION Tremor: | 57% (4/7) | 50% (1/2) | 50% (1/2) | 67% (2/3) | NT | 80 µg/L | 81 vs. 77 | 36 vs. 35 yrs | 396/322 |
| KINETIC Tremor: | 12.5% (1/8) | 0% (0/4) | 0% (0/1) | 0% (0/1) | 50% (1/2) | 89 µg/L | 276 vs. 64 | 35 vs. 41 yrs | 387/353 |
= Comparison of weighted average of group mean UHg levels in study groups that were tested for tremor, and among study groups with positive vs. null tremor results.
NT=not tested.
= Weighted average of group mean age in study grouprs tested for tremor.
Motor function
The dose-relatedness of motor function (other than steadiness) was evaluated in 11 of the 13 studies that performed testing of motor coordination, manual dexterity, and motor speed; significant results in the expected direction were reported in four studies.
High Exposure
Two studies found evidence of a positive dose-response for tests of motor speed (tapping) and manual dexterity (Michigan Maze). Miller et al. (1975) found that performance on tapping was significantly and inversely correlated with current UHg and BHg, but not with duration of exposure. Langolf et al. (1978), the expanded follow-up of the Miller study, assessed correlations between tapping and ten metrics of historical average or peak UHg levels, or duration of exposure: performance on tapping ‘showed statistically significant changes related to urine mercury’. In both studies, manual dexterity was significantly and inversely correlated with exposure; increases in ‘erratic’ performance (i.e. increased variability in hole-to-hole times) correlated significantly (alpha set at p <0.10) with increasing levels of current UHg and current BHg (Miller et al. 1975), and historical average UHg and peak UHg metrics (Langolf et al. 1978). For both outcomes (i.e. tapping and Michigan Maze), Langolf et al. (1978) found that the number of UHg peaks >500 µg/L in the previous year was the ‘best predictor of psychomotor performance’.
Medium Exposure
One study evaluated the dose-relatedness of manual dexterity. Gunther et al. (1996) found no significant correlations between current UHg and performance on tests of ‘finger dexterity’ or tapping. However, analyses of Exposure Intensity revealed significantly decreased performance on ‘finger dexterity’ tests in the Medium Exposure group vs. <BEI Exposure group (current UHg levels: 111–152 µg/L vs. 21–25 µg/L), a finding confirmed by ANCOVA that controlled for the effects of age and verbal IQ. During the seven-year investigation, only one of four testing periods revealed significant differences between exposure groups on tests of tapping.
Low Exposure
Paradoxical results were reported in the only Low Exposure study that evaluated the relationship between manual dexterity and Exposure Intensity. Piikivi et al. (1984) compared group mean performances on the Santa Ana Dexterity Test in subgroups of workers stratified above vs. below group median levels for three exposure metrics (current UHg, median = 56 µg/L; current BHg, median = 1.5 µg/dL; lifetime average UHg, median = 110 µg/L) and those with peak UHg >300 vs. <300 µg/L. In all four analyses, statistically significant decrements in manual dexterity were observed only among the subgroups with lower exposures.
<BEI Exposure
Eight studies evaluated the dose-relatedness of motor function in nine study groups; null results were reported in all but one study (Langworth et al. 1992).
Motor coordination, tested in two studies, showed no evidence of dose-relatedness. Camerino et al. (2002), who found that exposed workers performed significantly better than controls on a test of rapid alternative movement (Luria Battery), performed correlation analyses to explain this paradoxical finding. The observed difference was mainly related to age (younger) and education (higher), not current UHg or duration of exposure. Wastensson et al. (2008) reported no significant correlations between six parameters of a rapid alternative movement test (Diadochokinesimetry) and either five-year average UHg or lifetime cumulative UHg. No significant correlations were found for five of six parameters and current UHg. In analyses of Exposure Intensity, no significant differences in Diadochokinesimetry were found between subgroups of workers stratified above and below median levels of current UHg (median = 5.9 µg/g creatinine), or 5-year average UHg (median = 6.75 µg/g creatinine).
Manual dexterity, tested in three studies (Soleo et al. 1990; Gunther et al. 1996; Ellingsen et al. 2001), also showed no evidence of dose-relatedness. Ellingsen et al. (2001) found performance on Grooved Pegboard was not correlated with current UHg, current BHg, lifetime average UHg, or lifetime cumulative UHg. By contrast, significant decrements in performance were seen in smokers compared to nonsmokers. Soleo et al. (1990) reported no significant differences in Santa Ana Dexterity Test results between controls and two study groups, stratified by job titles that had different mean current UHg (12 vs. 18 µg/L), weighted 9-year average group mean UHg levels (14 vs. 28 µg/L), and 9-year ranges of UHg (2–66 µg/L vs. 9–138 µg/L). Likewise, Gunther et al. (1996) found no significant correlations between performance on ‘finger dexterity’ tests and current UHg in one <BEI Exposure group.
Motor speed, evaluated by tests of tapping in six studies (Piikivi & Hanninen 1989; Soleo et al. 1990; Langworth et al. 1992; Liang et al. 1993; Gunther et al. 1996; Ellingsen et al. 2001), showed little evidence of dose-relatedness. In Langworth et al. (1992), scores on finger tapping were significantly correlated with the number of peak BHg >3 µg/dL during the prior five years, but not to current UHg, current BHg, one- or five-year average BHg, or exposure duration, and ‘No notable dose-response relations’ were found between the prevalence of abnormal scores and current UHg (<17.5, 17.5–43.75, >43.75 µg/g creatinine). The remaining five studies found no evidence that performance on tapping was dose-related: scores on tapping were not significantly correlated with current UHg (Piikivi & Hanninen 1989; Gunther et al. 1996; Camerino et al. 2002), current BHg (Piikivi & Hanninen 1989), lifetime average of BHg (Piikivi & Hanninen 1989), lifetime average UHg (Ellingsen et al. 2001), lifetime cumulative UHg (Ellingsen et al. 2001), or duration of exposure (Liang et al. 1993; Camerino et al. 2002). One study (Piikivi & Hanninen 1989) also reported that scores on tapping did not differ between subgroups of workers stratified above vs. below median levels of current UHg, current BHg, or lifetime average BHg.
Motor accuracy
The dose-relatedness of motor accuracy was evaluated in 10 of the 12 studies that tested motor accuracy; significant results in the expected direction were reported in three studies.
High Exposure
Two High Exposure studies using tests of reaction time (SRT, CRT) found no evidence of dose-related performance. Miller et al. (1975) described a tendency for slower responses on SRT and two tests of CRT among workers with elevated BHg and UHg levels, but the authors were ‘reluctant to draw any conclusions’ because of a ‘lack of consistency’ in their findings. Langolf et al. (1978) reported no significant correlations (alpha set at p <0.10) between performance on CRT and ten metrics of historical average or peak UHg levels.
Medium Exposure
Paradoxical results were reported in the only study that evaluated the relationship between Exposure Intensity and SRT. In Gunther et al. (1996), auditory reaction time was significantly faster in a Medium Exposure group of workers (UHg: 111–152 µg/L) compared to a <BEI Exposure group (UHg: 21–25 µg/L), a finding confirmed by ANCOVA that controlled for the effects of age and verbal IQ. No evidence of dose-relatedness was observed for visual reaction times.
Low Exposure
One Low Exposure study that evaluated motor accuracy using tests of reaction time (SRT, CRT) and symbol digit (SD) reported dose-related changes. Angotzi et al. (1981) found that performance on tests of SRT, CRT or SD was significantly worse in a subgroup of workers with 3 or more peak UHg levels >50 µg/L during the prior year in comparison to controls (results reported in Camerino et al. 1981).
<BEI Exposure
Two of seven <BEI Exposure studies reported inconsistent evidence of dose-related performance on tests of motor accuracy. Ellingsen et al. (2001), using multiple linear regression analyses that considered intellect and other potential confounders, reported ‘a weak statistical association’ between SD scores and current BHg and lifetime average UHg, but not with current UHg or lifetime cumulative UHg. Visual reaction time, measured by Continuous Performance Tests (CPT), was not significantly correlated with any dose metric (Ellingsen et al. 2001). By contrast, Liang et al. (1993) reported that visual reaction time (CRT) was significantly and positively correlated with exposure duration (<10, 10–19, ≥20 years) using analyses of covariance to control for age, but results on SD and visual SRT were not significantly correlated with exposure. The remaining five studies found no positive dose-related findings. Piikivi and Hanninen (1989) used two analytical approaches and found no evidence of dose-relatedness for CPT or SD and current UHg, current BHg, or lifetime average BHg. In a similar analysis, Soleo et al. (1990) found no differences on SRT and SD scores when compared across controls and two study groups of workers stratified by job titles with significantly different mean levels of current and historical UHg. Langworth et al. (1992) found no significant correlations between performance on SRT or SD tests and current UHg, current BHg, one- or five-year average BHg, exposure duration, or the number of peak BHg >3 µg/dL during the prior five year. As described above, Gunther et al. (1996) reported paradoxical results for auditory SRT; response time was significantly slower in a <BEI Exposure group compared to a Medium Exposure group. No evidence of dose-relatedness was observed for visual SRTs. Finally, in a study that reported paradoxical results (i.e. exposed workers performed significantly better than controls on SD and SRT testing), Camerino et al. (2002) found those differences were mainly influenced by age and level of schooling, but better performance on SD was also significantly correlated with increasing current UHg and duration of exposure.
Balance
Dose-relatedness of balance was evaluated in two studies that performed quantitative tests of postural sway. Iwata et al. (2007), a High Exposure study that used multiple regression analysis to control for the effects of age, height, alcohol, and smoking, found a significant correlation between transversal sway (eyes open) and current UHg, but no correlations were found for transversal sway (eyes closed) or sagittal sway (eyes open or closed). By contrast, Gunther et al. (1996), using ANCOVA to compare controls and two groups of workers, one Medium Exposure group (UHg : 111–152 µg/L) and one <BEI Exposure group (UHg: 21–25 µg/L), concluded that correlations between current UHg and postural sway (eyes open) ‘did not support the assumption of a dose related effect.’
Sensory function
The only study that evaluated the dose-relatedness of sensory function reported positive results. Albers et al. (1982), a nested case-control study, evaluated correlations between quantitative results of three tests of sensory function (Touch Pressure, Vibratory Sensation, Pin-pain) and 14 dose-metrics using a multiple linear regression analysis that controlled for the effects of age, height, weight, and alcohol use. Reduced sensation in all three tests was significantly correlated with the number of peak UHg >500 µg/L, but apparently not correlated with current UHg, or average UHg during the prior 3, 6, 12, 24, or 36 months.
Color vision
Both studies that reported positive findings for abnormal color vision also evaluated its dose-relatedness using the Color Confusion Index (CCI). Cavalleri et al. (1995), a Medium Exposure study, compared mean CCI values between controls and two subgroups of workers stratified according to current UHg (>50 or <50 µg/L). Compared to controls, mean CCI values were significantly increased (i.e. greater color vision deficits) in the workers with UHg >50 µg/L, but not in workers with lower exposures. Urban et al. (2003), a <BEI Exposure study, using a multiple regression analysis that included age, alcohol, and smoking, found no significant correlations between CCI values and the following exposure metrics: current UHg; exposure duration; cumulative UHg (defined as the product of UHg and exposure duration); and UHg following a DMPS chelation challenge.
NB section summary
On average, positive NB findings were associated with a weighted mean UHg of 149 µg/L (range of means: 23 – 880 µg/L) nearly 4-fold greater than the mean level associated with null findings (39 µg/L, range of means: 11 – 108 µg/L). As summarized in Table 8, the proportion of study groups reporting one or more positive NB finding increased monotonically across exposure categories and with increasing UHg levels; the dose-related trend did not appear to be influenced by age.
TABLE 8.
Sensitivity analysis: summary of NB results, UHg, descriptive data, and Tier ratings across category of exposure
|
Exposure Category |
NB Results | UHg (µg/L) in exposed | Age (yrs) of exposed | Number of workers | Quality Tier¶ | |
|---|---|---|---|---|---|---|
| % with positive results* | Mean (range)† | Mean (range)† | Exposed/Controls‡ | All | (+) | |
| Overall: | 64% (18 of 28 groups) | 110 (11 – 880) | 38 (27–51) | 1257/1092 | 1.5 | 1.6 |
|
| ||||||
| HIGH | 100% (6 of 6 groups) | 316 (235 – 880) | 37 (35 – 51) | 253/249 | 1.4 | 1.4 |
|
| ||||||
| MED | 80% (4 of 5 groups) | 134 (108 – 173) | 36 (27– 47) | 190/210 | 1.7 | 1.8 |
|
| ||||||
| LOW | 60% (3 of 5 groups) | 52 (50 –82) | 35 (29 –51) | 297/227 | 1.2 | 1.1 |
|
| ||||||
| < BEI | 42% (5 of 12 groups) | 23 (11 – 35) | 40 (33–47) | 511/471 | 1.6 | 1.9 |
Table summarizes data from 28 cohort study groups described in 25 cohort studies; data from Albers case-control study not included.
= The proportion of study groups with positive findings (i.e. # with ≥ 1 positive finding on NB testing, divided by # of study groups evaluated using NB tests.
= Weighted average of group means and (the range of means); calculated from data reported for individual study groups.
= When a study reported findings for multiple study groups, the total number of controls in that study was assigned to each study group if stratified into a separate exposure category (i.e. Gunther 1996, Soleo 1990, Tang 2006); thus the sum of controls listed across exposure categories does not agree with the Overall sum of controls.
= Average of Quality Tier value weighted by study group size (i.e. total number of exposed and controls)) for all study groups evaluated using NB testing, and those study groups with positive (+) results.
Electrophysiological testing
Twelve of the 44 cohort studies (13 of the 57 study groups) described results of EPS in 1039 workers (546 exposed, 493 controls). Exposed workers ranged in age from 18 to 71 years (weighted average mean: 38 years). EPS findings were also reported in one nested case-control study of 138 workers (Albers et al. 1982). Studies used a variety of EPS to evaluate a range of neurologic and/or neuromuscular functions (Figure 3). Most used nerve conduction studies (NCS) and/or electromyography (EMG) to evaluate responses to electrical stimulation in nerves (NCS) or neuromuscular tissues (EMG) of the peripheral nervous system. A few used evoked potentials studies (EPs) or electroencephalograms (EEGs) to evaluate the electrical activity of the central nervous system.
Figure 3.
Overview of occupational cohort studies that evaluated the association between mercury exposure and four types of electrophysiological outcomes. Studies are listed top to bottom by decreasing group mean UHg values (µg/L), and category of exposure is denoted (h=high, m=medium, l=low, b=<BEI).
Abbreviations: AMP=amplitude; EEG=electroencephalogram; EMG=electromyography; EPS=electrophysiological studies; L=lower limbs; LAT=latency; LR=late responses (H-reflex, F-wave latencies, and myotatic stretch reflex); NCS=nerve conduction studies; NCV=nerve conduction velocity; Sig. = statistically significant; U=upper limbs; VEPs=visual evoked potentials
† : UHg values with an asterisk * indicate UHg was converted from units originally reported in study, ~ indicates UHg was estimated from average air Hg concentrations, as described in the Methods Section
‡ Studies that evaluated the statistical analyses of their findings did not report the results for all outcomes tested; positive findings reported as statistically significant have been emboldened.
All studies used statistical analyses to determine the significance of results in exposed workers on at least one type of EPS, with one exception (Zedda et al. 1980). Comparisons were made to currently examined controls (Vroom & Greer 1972; Angotzi et al. 1981; Triebig & Schaller 1982; Piikivi & Hanninen 1989; Chang et al. 1995), normal values determined in their laboratories (Albers et al. 1982; Levine et al. 1982; Zampollo et al. 1987; Urban et al. 1999), and/or the published literature (Vroom & Greer 1972; Zampollo et al. 1987; Urban et al. 1999). Other studies did not indicate the source of their reference values. Eight studies evaluated correlations between effects and exposure levels (Miller et al. 1975; Gilioli et al. 1976; Langolf et al. 1978; Albers et al. 1982; Levine et al. 1982; Triebig & Schaller 1982; Zampollo et al. 1987; Piikivi & Hanninen 1989).
Most studies analyzed differences between group mean values of test results (Vroom & Greer 1972; Miller et al. 1975; Langolf et al. 1978; Zedda et al. 1980; Angotzi et al. 1981; Albers et al. 1982; Levine et al. 1982; Triebig & Schaller 1982; Piikivi & Hanninen 1989; Chang et al. 1995; Urban et al. 1999), while some also considered individual values (Vroom & Greer 1972; Miller et al. 1975; Langolf et al. 1978; Zedda et al. 1980; Levine et al. 1982; Urban et al. 1999).
Head-to-head comparisons of EPS outcomes across studies were complicated due to the complex set of testing-related variables. Such variables included the specific testing instruments, techniques and protocols used, the large number of nerves and muscles amenable to testing, and the potential to measure a variety of parameters for each nerve or muscle selected. In addition, test results can be affected by study subjects’ characteristics such as body temperature, height, and smoking. Details of the testing performed and their results are provided in Supplemental Tables 12 and 13, respectively. Discussed below are the results from four types of EPS testing that were reported in two or more studies: NCS (n=9 studies); EMG (n=7); EPs (n=2); and EEG (n=2). To address the difficulty of comparisons across studies, the results section includes a consideration of the dose-relatedness across exposure categories for all EPS findings and the dose-relatedness and patterns of reported abnormalities for each of the four types of EPS.
Exposure effects
As discussed below, positive findings on EPS were reported in 0%, 67%, 100% and 100% of the <BEI, Low, Medium, and High BEI Exposure study groups, respectively, in which testing was performed.
High Exposure
EPS were performed in seven High Exposure study groups. Five of the seven were evaluated using NCS; positive results were reported in all (Figure 3). Likewise, all five evaluated by EMG had positive findings. In the only study group evaluated using visual evoked potentials (VEP), findings were positive (Urban et al. 1999). Only one study group was evaluated by EEG; in that study, which selected workers on the basis of the severity of their effects, Vroom and Greer (1972) reported ‘diffuse slowing’ in 56% (5 of 9).
Albers et al. (1982), a nested case-control study, reported significant group mean differences on NCS and EMG in higher-exposed workers with clinical PN vs. lower-exposed workers without PN.
Medium Exposure
EPS were performed in two of 11 Medium Exposure study groups. Both were evaluated using NCS; significant group mean differences were reported in one group (Triebig & Schaller 1982), but not the other (Urban et al. 1999). In the one study group also assessed by VEP, findings were positive (Urban et al. 1999).
Low Exposure
EPS were performed in three of 12 Low Exposure study groups. Results were mostly null in two studies that performed NCS testing: Angotzi et al. (1981) reported a significant difference between group means on only one of three sensory NCS parameters, while Zedda et al. (1980) found no significant differences between group means of seven NCS parameters. Angotzi et al. (1981) also performed EMG testing, but did not report results. In the only study group assessed by VEP, Chang et al. (1995) reported significant group mean differences in only one of five parameters tested.
<BEI Exposure
EPS were performed in only one of the 14 <BEI Exposure study groups. Piikivi & Tolonen (1989) found no significant difference between the prevalence of abnormal findings on visually interpreted EEGs in 41 exposed workers compared with 41 matched controls (24% vs. 15%, respectively).
Nerve conduction studies
NCS were performed in nine studies (10 study groups) that each assessed a variety of motor and/or sensory functions in up to five different nerves and up to five different parameters, yielding 24 unique combinations (e.g. ‘ulnar motor latency’). Nerve conduction velocity was the most frequently evaluated parameter (36 group outcomes), followed by latency (17 group outcomes) and amplitude (14 group outcomes), while late responses were recorded less often: F-wave (2 group outcomes) and H-reflex (3 group outcomes). The term ‘group outcome’ refers to results of a specific nerve/specific function/specific parameter reported in a specific study group.
Table 9 presents summary results for all NCS outcomes combined and for each of the three most frequently reported parameters. To examine patterns of effects, results are stratified by motor vs. sensory outcomes and by upper vs. lower limbs.
Table 9.
Nerve conduction study results: analysis of patterns of effect
| Patterns of Effect | All NCS Outcomes* | Velocity (NCV) | Latency | Amplitude |
|---|---|---|---|---|
| ALL NCS: | 29% | 22% | 41% | 36% |
| (21/72)‡ | (8/36) | (7/17) | (5/14) | |
|
| ||||
| Sensory: | 44% | 38% | 67% | 40% |
|
| ||||
| vs. | (12/27) | (6/16) | (4/6) | (2/5) |
|
| ||||
| Motor: | 20% | 10% | 27% | 33% |
|
| ||||
| (9/45) | (2/20) | (3/11) | (3/9) | |
|
| ||||
| Upper Limbs: | 29% | 13% | 55% | 44% |
|
| ||||
| vs. | (13/45) | (3/24) | (6/11) | (4/9) |
|
| ||||
| Lower Limbs: | 30% | 42% | 17% | 20% |
|
| ||||
| (8/27) | (5/12) | (1/6) | (1/5) | |
Table presents the percentage of positive outcomes divided by the total number of outcomes that were evaluated. Two studies described performing specific NCS tests but did not report each outcome (Urban 1999- cohort 1; Angotzi 1981); the results that were not reported were categoried as "null" findings and included in the denominator.
= The analysis of patterns for All NCS outcomes includes NCV, latency, amplitude, and late responses (i.e. F-wave and H-reflexes). The limited number of late responses (n=5) precluded a separate analysis of their patterns of effect.
Sensory abnormalities were more common than motor abnormalities across all three parameters (i.e. velocity, latency and amplitude), and for all NCS group outcomes combined (44% vs. 20%, respectively). A less consistent pattern was observed when upper vs. lower extremity abnormalities were compared. Only one parameter, conduction velocity, had abnormalities that were more prevalent in the lower extremities (45% vs. 16%, respectively). By contrast, abnormalities of latency and amplitude were each more prevalent in the upper extremities.
Late responses (i.e. F-wave and H-Reflex) showed significant abnormalities in only one of five group outcomes. Group mean H-reflex latency was decreased significantly in one High Exposure group (Zampollo et al. 1987), increased (non-significantly) in a second High Exposure group (Vroom & Greer 1972), and ‘within the norm’ in a Low Exposure group (Zedda et al. 1980). In a separate High Exposure group, Langolf et al. (1978) reported significantly decreased latency on myotatic (stretch) reflex, a measure closely related to H-reflex (Ball 2005). The only study that evaluated F-wave latency found ‘no detectable change…either upward or downward’ (Zampollo et al. 1987).
Electromyography
EMG was performed in seven studies; one did not report results (Angotzi et al. 1981). Three studies performed needle EMGs (Vroom & Greer 1972; Albers et al. 1982; Zampollo et al. 1987), three performed surface EMGs (Miller et al. 1975; Gilioli et al. 1976; Langolf et al. 1978), and one did not describe its method (Angotzi et al. 1981). EMGs were generally characterized as abnormal on the basis of one or more of the following: irregular, polyphasic muscle unit action potential (MUP); altered MUP amplitude; increased MUP duration; fibrillation; positive spike waves.
Most frequently reported was an increase in polyphasic MUPs in four studies (Vroom & Greer 1972; Miller et al. 1975, as described in Chaffin et al. 1973; Langolf et al. 1978; Zampollo et al. 1987). Three studies reported increased MUP amplitude (Vroom & Greer 1972; Miller et al. 1975, as described in Chaffin et al. 1973; Albers et al. 1982), two reported the presence of muscle fibrillations (Albers et al. 1982; Zampollo et al. 1987), and one each described increased MUP duration (Vroom & Greer 1972) and the presence of positive spike waves (Albers et al. 1982). One study reported a significant inverse association between the number of motor units and ‘dispersion values between fast and slow conducting motor fibres’ (Gilioli et al. 1976).
Visual evoked potentials
VEPs were evaluated in only two studies of three study groups, which described findings for two parameters of amplitude and three parameters of latency. Urban et al. (1999) evaluated High and Medium Exposure groups, while Chang et al. (1995) assessed a Low Exposure group. The findings of these studies were inconsistent. Group mean amplitude was significantly decreased (both parameters) in the two groups of workers described by Urban et al. (1999), but increased in those same parameters tested by Chang et al. (1995), of which one was significantly increased. Group mean latency was decreased in all nine group outcomes, but differences were statistically significant for only one outcome which was seen in the High Exposure group (Urban et al. 1999).
Electroencephalograms
EEGs were performed in only two studies. Vroom and Greer (1972) assessed workers in a High Exposure group, while Piikivi and Tolonen (1989) evaluated a <BEI Exposure group. Vroom and Greer (1972) described ‘diffuse slowing’ that was ‘marked temporally’ on EEG in 56% (5 of 9) of exposed workers, but did not evaluate a control group for comparison. Piikivi and Tolonen (1989) described ‘mild’ EEG abnormalities in 24% (10 of 41) of exposed workers on visually interpreted EEG, but the prevalence of such findings was not significantly different from controls (15%; 6 of 41). The studies differed in the types of abnormalities observed. Vroom and Greer (1972) described generalized (i.e. ‘diffuse slowing’) EEG abnormalities. By contrast, Piikivi and Tolonen (1989) found a greater prevalence of focal abnormalities in exposed compared to controls (15% vs. 5%) while the prevalence of generalized abnormalities was lower (10% vs. 12%). Similar to the Vroom study, most focal abnormalities in the Piikivi study were located in the temporal lobe, however they were unilateral, ‘situated in the left hemisphere’ (Piikivi & Tolonen 1989), whereas Vroom described abnormalities that were mainly ‘bilateral’. Piikivi and Tolonen (1989) also evaluated quantitative EEG (qEEG); differences between exposed and controls largely reflected the influence of shiftwork rather than mercury exposure: ‘shiftwork was an obvious confounding factor’. These qEEG data were not tabulated or analyzed in our review because in addition to confounding, qEEG is regarded as having little clinical utility (American Clinical Neurophysiology Society 1997; Nuwer 1997).
Dose-effects
The dose-relatedness of EPS was evaluated in 11 of the 13 studies that performed EPS. Of those eleven studies, eight reported significant dose-related associations.
Nerve conduction studies
Seven of nine studies that performed NCS also evaluated the dose-relatedness of their findings; four described statistically significant associations between exposure and/or dose and at least one NCS parameter.
Three of four High Exposure studies reported significant dose-related NCS findings. Levine et al. (1982) evaluated correlations between five ulnar nerve parameters (3 motor and 2 sensory) and 14 metrics of current and historical UHg. Prolonged sensory and motor latencies were significantly correlated with most historical metrics of Average UHg and Peak UHg (>250 or >500 µg/L) from the previous 3-, 6-, 12-, 24- and 36-months, but were not correlated with current UHg. By contrast, motor conduction velocity was significantly correlated with current UHg, and only the most recent historical metrics. No dose-relatedness was found for sensory or motor amplitudes. Urban et al. (1999) compared conduction velocity (in 3 motor and 3 sensory nerves) across two groups of exposed workers, one High (24-hr UHg: 840 µg) and one Medium Exposure group (24-hr UHg: 129 µg). After controlling for diabetes and ‘alcohol abuse’, abnormal results were seen only in workers in the highest exposure group. Gilioli et al. (1976) reported a significant negative correlation between conduction velocity (peroneal motor) and a 3-tiered ‘risk index’ of exposure. Langolf et al. (1978) reported positive dose-related findings for myotatic (stretch) reflex. Zampollo et al. (1987) found no significant correlations between 14 NCS parameters (10 motor and 4 sensory) and current UHg, current BHg, or exposure duration.
Albers et al. (1982), a nested case-control study, evaluated correlations between 13 NCS parameters and 14 metrics of current and historical UHg. Four sensory parameters (3 latencies and 1 conduction velocity) and one motor parameter (amplitude) showed significant correlations with ‘most’ of the UHg dose-metrics.
One Medium Exposure study found no significant correlations between median and ulnar conduction velocity (in 3 sensory and 1 motor nerve) and current UHg (Triebig & Schaller 1982).
One Low Exposure study found no evidence of dose-related nerve conduction velocity. In Angotzi et al. (1980), group mean sensory NCVs (2 nerves, 3 parameters) showed no significant correlation with cumulative UHg, and no difference between groups of workers stratified by current UHg above vs. below 50 µg/L, or stratified by job titles into three groups that had mean current UHg levels of 39, 84, 108 µg/L respectively.
Nerve conduction studies were not performed in the <BEI Exposure studies.
Electromyography
Four of the six studies that reported results of EMG also evaluated the dose-relatedness of their findings; two described statistically significant associations between exposure and/or dose levels and abnormal EMG findings.
Two of four High Exposure studies reported significant dose-related EMG findings. Miller et al. (1975) evaluated correlations between 6 EMG parameters (5 frequency, 1 amplitude) and current UHg, current BHg, and duration of exposure in exposed workers using a step-wise regression analysis that included the effects of age, height, weight, and smoking (alpha = p≤0.10). Several of the frequency parameters were significantly correlated with current UHg and BHg, but not with exposure duration, while increased amplitude was significantly correlated with exposure duration, but not current UHg or BHg. Langolf et al. (1978) evaluated correlations between EMG parameters, duration of exposure and 10 historical indices of average and peak UHg mercury using a step-wise regression analysis that included the effects of age, height, weight, smoking, blood pressure and education; a shift in EMG power towards lower frequencies was significantly associated with increasing levels of historical UHg, but details were not provided. Gilioli et al. (1976) found no significant correlation between ‘the number of motor units’ and a 3-tiered ‘risk index’ of exposure. Zampollo et al. (1987) found no significant correlations between changes in EMG (polyphasic motor unit potentials and/or fibrillation activity) and current UHg, BHg, or duration of exposure.
The only case-control study that evaluated EMG did not report the dose-relatedness of EMG findings separately. In that study, Albers et al. (1982) found that workers with clinical evidence of PN had a higher prevalence of EMG abnormalities; a significantly larger than expected proportion of those workers had UHg >50 µg/L vs. <50 µg/L.
Angotzi et al. (1980), the only Low Exposure study to evaluate EMG, did not report their results, but used them in combination with clinical exam and motor NCV results to diagnose PN. There was no evidence that PN was dose-related.
Electromyography was not performed in the <BEI Exposure studies.
Visual evoked potentials
Both of the studies that evaluated VEPs also assessed the dose-relatedness of their findings; each reported significant results.
Urban et al. (1999) used ANOVA to compare differences between group mean values of five VEP parameters in controls and two groups of exposed workers, one High (24-hr UHg: 840 µg) and one Medium Exposure group (24-hr UHg: 129 µg). The findings were inconsistent. An apparent dose-related trend was seen for one of two parameters of amplitude (N1P1), but the second (P1N2) showed an apparently paradoxical dose-response. A non-monotonic dose-response was found for the only latency parameter that yielded a significant result.
In a separate report, Urban et al. (1996) also performed correlation analyses. In the High Exposure group, multiple regression analyses including age, gender, and alcohol, found a significant correlation between current UHg and only one of three latency parameters and neither of two amplitude parameters. No correlations were found between duration of exposure and any of the VEP parameters. Such correlations were not evaluated in the Medium Exposure group.
In the second study, Chang et al. (1995) also used ANOVA to evaluate the dose-relatedness of the same five VEP parameters in a group of Low Exposure workers stratified by work history into three exposure subgroups (UHg: 17, 18, and 47 µg/L). Group mean VEP values were compared to those in matched controls from their laboratory database; a significant increase was found for only one parameter, increased amplitude in the highest exposure subgroup.
Testing of evoked potentials was not performed in the <BEI Exposure studies.
Electroencephalograms
Neither of the two studies that evaluated conventional EEGs assessed the dose-relatedness of their findings (Vroom & Greer 1972; Piikivi & Tolonen 1989). However, Piikivi and Tolonen (1989), using two-way ANOVA to evaluate correlations between qEEG parameters and current and historical exposures (UHg, inorganic BHg, organic BHg, total BHg, and TWA of total BHg), as well as shiftwork, found ‘no suggestion of a dose effect relation’ on quantitative qEEG. Piikivi and Tolonen (1989) also noted that the level of organic BHg was associated with the ‘generous consumption of fish’ in controls and exposed workers: ‘the possible influence of additional exposure to methyl Hg on the [qEEG] could not be controlled in the study.’
EPS section summary
The limited number of studies performing EPS precluded evaluation of the influence of age, study quality, and UHg on EPS summary results.
Influence of study quality
The cohort studies included in our review showed marked heterogeneity with respect to levels of exposure, eras of study publication, and methodological adequacy. Assessment of study quality indicated that risk of bias was lowest for NB studies and highest for PE studies; 43% of NB study groups were categorized as Tier 1 (highest quality) and only one group was considered Tier 3, whereas 37% of PE study groups were considered Tier 3 (lowest quality) and only six groups were Tier 1. The limited number of studies performing EPS precluded meaningful assessment of study quality.
Analyses of the impact of study quality on study results suggested that lower quality studies were more likely to report abnormal findings. Because of the relatively large number of studies that considered PE findings, it was possible to identify an apparent interaction between study era, study quality, and level of exposure. As seen in Supplemental Table 14, the highest exposure studies tended to be the oldest. Not surprisingly, they favored PE over NB and EPS testing, diagnostic methods that were more recently adopted. Likewise, those older studies were performed in an era when higher exposure levels were permissible and when research methods and statistical standards were less sophisticated and less widely implemented. The increasing use of NB and EPS testing methods, and a growing appreciation of methodological standards, coincided with often substantially decreased workplace exposure levels. Accordingly, we cannot determine whether the much higher rate of positive findings in those earlier studies was solely due to their much higher exposure levels; the marked dose-relatedness and possible threshold suggests that was so. However, effects of study quality, era of study, and level of exposure confound one another making such conclusions less certain.
Summary
The principal goal of this review was to provide a first approximation of the neurological effects one would have expected in previously-exposed mercury workers had they been evaluated years before, during active exposure. To that end, studies of currently-exposed workers were stratified into exposure categories (i.e. <BEI, Low, Medium, and High) selected a priori to reflect the range of group mean UHg levels reported in those previous exposure studies. Overall, neurological effects were reported in 41 of 58 (71%) study groups of workers with long-term exposure to elemental mercury vapor. The proportion of groups with positive findings increased across increasing exposure strata in each of three types of testing: PE, NB and EPS and in accordance with the sensitivity of the neurological evaluation (EPS>NB>PE) (Supplemental Table 15).
Dose-relatedness was also seen for those specific tests and outcomes observed in sufficient numbers of groups to provide the data necessary for dose-response assessments. Among PE tests, there were sufficient data to document dose-relatedness for the three most frequently reported findings: tremor, impaired MC, and abnormal DTRs. Dose-relatedness was also seen for NB tests that incorporated motor function such as tremor, manual dexterity, and motor speed. There is a suggestion of dose-relatedness in the EPS data, but the number of EPS studies was too small and focused on highest exposure strata, so no definite conclusion could be made.
Although limited, the existing data suggest the possibility of response thresholds that characterize individual tests and functions. With few exceptions, PE findings of tremor, impaired MC, and abnormal DTRs were more prevalent in exposed workers with group mean UHg levels ≥275 µg/L than in corresponding controls.
Among studies that reported positive PE findings, six did not fit the dose-response patterns described above. Those studies, which reported positive PE findings at mean UHg levels <200 µg/L, suffered from a variety of methodological limitations (e.g. small sample size, confounding, and lack of statistical testing) that are discussed below.
NB findings of tremor and motor function were seen at much lower levels of exposure, but not below UHg levels of 20 µg/L. There was considerable agreement among studies that NB tests of motor accuracy (i.e. tests which requires motor ability as well as other abilities such as correct perception/information processing) do not yield abnormal results in mercury-exposed workers regardless of exposure levels. No significant differences were seen between exposed workers and controls in the 15 groups evaluated on attention/response speed and the eight groups evaluated on perceptual motor speed.
Discussion
Our systematic review, which considered objective neurological effects associated with occupational exposure to elemental mercury is the largest of its kind, spanning nearly six decades (1951–2007) of published findings for over 3,000 workers exposed for up to 45 years across a broad range of Hg0 concentrations (0.002 to 1.7 mg Hg/m3) in a variety of industries.
The purpose of this review was three-fold: (1) to identify the types and patterns of neurological effects most frequently observed in workers currently exposed over a range of Hg0 exposures; (2) to evaluate the dose-relatedness of those effects; and, (3) to determine the effects most likely to occur following specific levels of exposure.
We encountered a heterogeneous collection of studies that varied with respect to exposure levels, study size, tests performed, presentation of results, use of statistical testing, and methodological adequacy. Because some studies reported only aggregated results (e.g. impaired motor coordination on PE or NB testing, without detailed results for individual test components), we could not always identify the specific effects observed, or not observed. The latter situation occurred when studies (primarily focused on NB testing and/or EPS) reported only that PE was ‘normal’, but did not specify which PE tests had been performed.
Other studies, particularly the PE studies, failed to consider the prevalence of abnormalities in controls and fewer performed statistical tests. In those, it was often not possible to determine whether the reported effects had occurred significantly more often than expected. In addition, some of the NB and EPS studies compared exposed workers vs. controls in terms of the group means of quantitative test results, but did not describe the actual distributions of those results or the numbers of workers with abnormal results. When such studies reported significant effects, it was generally not possible to determine whether exposure had caused relatively small effects in a large proportion of the workers, or relatively large effects in only a few.
Such limitations had little impact on our ability to identify the most frequently observed neurological effects or to demonstrate their dose-relatedness. But, as described below, they provided a challenge to identifying the specific effects that might be expected at particular exposure levels. They also made it more difficult to determine which specific tests would most reliably detect adverse effects in individual workers at various levels of exposure.
We have probably overstated the number of study groups positive for effects on PE because we regarded studies that reported any abnormal findings in groups of exposed workers as ‘positive’ even when they did not consider background prevalence and determine statistical significance. For example, in seven PE studies that included controls, point prevalence of tremor in control groups averaged 8.2% and ranged up to 20%. In light of such background prevalence rates, it is likely that some of the studies listed as positive for tremor (Supplemental Table 7a) described effects that actually were not significantly increased. Lack of control data and statistical testing also limited our ability to conclude whether other effects reported in PE studies had occurred significantly more often than expected, as demonstrated in Supplemental Tables 8a and 9 for MC and DTRs, respectively.
Patterns of effects
Overall, motor abnormalities were much more frequently reported than sensory abnormalities, which might suggest a predominant effect of elemental mercury on the motor system. However, because abnormal sensation is not considered a ‘classical neurological sign’ of mercury intoxication (WHO 1991), it is possible that sensory function was not evaluated in some of the study groups. Among those studies that reported results of sensory testing, six of 11 reported abnormalities on PE, two of three reported sensory deficits on NB testing, and EPS testing of exposed workers documented a greater frequency of NCS deficits in sensory rather than motor nerves. Accordingly, we find no evidence that mercury toxicity tends to target the motor system or spare the sensory system.
Among studies with positive findings on PE, tremor was reported at least twice as often as other motor abnormalities (i.e. MC, DTRs, and reduced strength). NB testing of motor function suggests that exposure status is associated with postural and intention tremor, but not kinetic tremor, and with abnormalities in tests of dexterity and motor speed, but not in tests of perceptual motor speed, attention or reaction time. It is not possible to compare NB tremor subtypes to corresponding findings on PE, because PE studies rarely characterized the tremors they observed.
As noted above, sensory abnormalities on NCS were more common than motor abnormalities. The NCS parameters most frequently documented as abnormal were prolonged latencies and reduced amplitudes. However, those abnormalities were found more often in upper extremities, not lower extremities, a finding that differs from expectations (Spencer et al. 2000). Conduction velocity was the only parameter that had abnormal findings in the expected direction: (lower > upper) was observed across both sensory nerves (75% vs. 25%) and motor nerves (33% vs. 0%). EMG was less frequently performed, but reported results were always abnormal; the most commonly described effect was polyphasic MUPs. The EMG findings were indicative of active denervation (fibrillations, positive waves) and reinnervation (prolonged MUP duration, polyphasic MUPs) (Feldman 1999b).
The pattern of the most commonly reported effects on EPS (reduced amplitudes, prolonged distal latencies and normal to slightly reduced conduction velocity on NCS, along with the EMG abnormalities noted above) is compatible with sensorimotor polyneuropathy due to axonal degeneration (Albers et al. 1982; Spencer et al. 2000; Franssen and van den Bergh 2006).
Dose-relatedness of effects
We next considered dose. We presumed that effects reported with consistency and in a dose-related pattern across studies stratified by increasing UHg levels were those most likely to be the consequence of mercury exposure. On PE, the three most frequently reported positive findings were all in the High Exposure category, with the exception of a handful of outlier studies that are discussed below. Evidence of dose-relatedness for tremor, and impaired MC and DTRs was also reported in most of the individual studies that examined dose-response.
On NB testing, tremor and motor function outcomes both showed a dose-related increase in the frequency of positive studies across increasing exposure categories. However, in our analysis of tremor subtypes, evidence of dose-relatedness was strongest for postural tremor, suggestive for kinetic tremor, and lacking for intention tremor. The small number of studies evaluating intention tremor or kinetic tremor may have limited our ability to evaluate dose-relatedness. Results from dose-response analyses performed in individual studies found dose-relatedness for postural (6/13 studies) and kinetic tremor (3/5 studies), but not intention tremor (0/6 studies). Individual studies that evaluated the dose-response of motor function found dose-relatedness for tests of dexterity and motor speed.
On EPS testing, results of NCS and EMG each showed dose-related increases in the frequency of positive study groups across increasing categories of exposure, but the trends were based on small numbers of observations. Dose-response analyses performed in individual studies provided additional support for those findings: results were positive only among High Exposure studies; significant associations were observed for the ‘highest urine indexes’ (Langolf et al. 1978; Albers et al. 1982), mean 24-hr UHg of 840 µg (Urban et al. 1999), and the number of UHg peaks >250 or >500 µg/L (Albers et al. 1982; Levine et al. 1982).
Effects most likely to occur at specific exposure levels
The reported study results provide perspective on those mercury-induced effects that can be expected at various levels of exposure and, therefore, the sorts of testing most likely to be diagnostically appropriate. As a general rule, abnormalities on PE should not be expected in studies of currently-exposed workers with group mean UHg <200 µg/L. In studies with higher urine levels (i.e. group mean UHg >275 µg/L), tremor, impaired MC, and abnormal DTRs are likely. By contrast, those PE findings should not be so readily ascribed to mercury toxicity in currently-exposed workers with lower UHg levels, and other causes of neurological dysfunction should be pursued.
Because few studies described the type of tremor observed on PE, we were unable to identify which specific tests of tremor would be most useful. Similarly, abnormal findings on MC testing were more often reported for ‘DDK’ and ‘ataxia’ than for ‘gait’, ‘nystagmus’ or ‘heel-to-shin’ testing, but variations in testing details and possible overlap of outcomes limited our ability to identify which specific test(s) were most sensitive or most useful.
Among workers with UHg <200 µg/L, PE offers little diagnostic value. Instead, these individuals should be evaluated by means of NB or EPS testing. Abnormalities on EPS testing of NCS and EMG can be seen at levels >80 µg/L. As for NB, it was the only type of evaluation that identified neurological effects, particularly those inclusive of a motor component (e.g. tremor and motor function), in groups with mean UHg levels <50 µg/L. On NB testing of tremor, the frequency of abnormal findings was similar between studies that used physiological techniques vs. hand-eye coordination tests of motor steadiness. Tests of manual dexterity (e.g. Grooved Pegboard, Santa Anna Dexterity) and motor speed (e.g. Finger Tapping) showed consistency across studies and were also dose-related.
This finding for NB testing is consistent with the conclusions of a recent meta-analysis that described mainly motor-related NB deficits in groups of workers with mean UHg <50 µg/g creatinine and estimated individual UHg mainly <100 µg/g creatinine (Meyer-Baron et al. 2002). That analysis also reported evidence that the magnitudes of NB effects were dose-related.
On EPS, testing of NCS and EMG revealed abnormalities in all but a few studies. The pattern of findings in the reviewed studies is consistent with axonal sensorimotor polyneuropathy. However, the same findings have been associated with a variety of metabolic disorders (e.g. diabetes, hypothyroidism, nutritional deficiencies) and chronic alcohol abuse, thus findings of subclinical PN in individual workers should be interpreted with caution. Testing of VEPs and EEGs was rarely performed, thus our analyses cannot conclude their usefulness for the evaluation of Hg0-exposed workers.
Outlier studies
As noted above, six ‘outlier’ PE studies with group mean UHg levels <200 µg/L reported abnormalities on PE (ie. Tremor, impaired MC) that were inconsistent with the dose-response patterns seen in the other 47 studies. These studies suffered from a variety of methodological limitations.
Four studies did not determine statistical significance, a deficiency of particular importance for Triebig and Schaller (1982), who described tremor in two of 18 (11%) exposed workers, and Angotzi et al. (1981), which found ‘cerebellar’ abnormalities in three of 55 (5%) exposed workers. Because both reported prevalence of abnormalities in the range generally seen in unexposed controls, and because neither considered control populations, it is possible that these two studies actually did not document Hg0-related adverse PE effects. Moreover, UHg levels ranged up to 380 µg/L in one study and 1200 µg/L in the other, thus some of the positive findings may have resulted from High exposures.
Two other studies suffered from small sample size, which can result in unrepresentative findings by chance (Coggon et al. 2003), and confounding. In the first, Tang and Li (2006) reported tremor in seven of nine workers with ‘mercury poisoning’; mean UHg levels were ≥50 µg/L, but the range was not indicated. Because the workplace was small and poorly ventilated, and mercury drops were seen on the ground and tables, we suspect that some had substantially higher levels. Other factors noted as possibly contributing to tremor ‘physical conditions and age’ and ‘malnutrition’.
In the second small sample study, Zedda et al. (1980) reported ‘intentional tremors’ in six of seven workers (and subclinical PN in five of seven) with UHg concentrations <200 µg/L during the previous two years. The study authors noted the ‘absence of other neurotoxic causes (diabetes, alcoholism, etc.)’, but they also reported that four workers drank 0.5 to 1 liter of wine per day and a fifth drank more than 1.5 liters per day, equivalent to ≈3 to >10 standard drinks per day (CDC 2014). Such alcohol consumption, commensurate with ‘Alcohol Use Disorder’ and ‘Heavy Drinking’ (National Institute on Alcohol Abuse and Alcoholism 2016), has been associated with intention tremor (National Institute of Neurological Disorders and Stroke 2016) and EPS findings of peripheral neuropathy (Monforte et al. 1995).
A fifth study (Ehrenberg et al. 1991) reported results that were generally inconsistent with the other studies in our review. On PE, there was a statistically increased prevalence of abnormal MC, but not tremor or hyperactive DTRs. The finding of significantly increased MC abnormalities for ‘heel-to-toe walk’, but not for three other tests of MC (i.e. finger-to-nose, DDK, and ‘gait disturbance’) was also inconsistent with patterns of MC effects reported in other studies. Finally, the most common DTR abnormality, hyporeflexia, was significantly and paradoxically more common in the controls. Thus, the pattern of Ehrenberg findings does not align with one of the ‘cardinal tenets of neurotoxic disease’: ‘most chemicals … produce a consistent pattern of disease, commensurate with the dose and duration of exposure’ (Spencer et al. 2000).
The authors concluded that their finding of such a high prevalence of hyporeflexia in exposed and control workers was ‘not readily explained’ (Ehrenberg et al. 1991). However, a possible explanation not discussed by Ehrenberg et al. was alcohol abuse, perhaps a response to the substantial psychological stresses reported among the workers and their community, including closure of the facility and loss of jobs following documentation of ‘off-site contamination’ (Anon 1988; Ehrenberg et al. 1991; Hudson et al. 1987; Zirschky & Witherell 1987). Abnormal heel-to-toe walking is regarded as a ‘reliable and rapid screening test’ for alcoholic cerebellar dysfunction (Walker 1990) and hyporeflexia is considered one of the ‘minor criteria for the diagnosis of alcoholism’ (Kissin 1977).
The sixth study (Gambini 1978) described two cohorts of workers at the same chloralkali facility. The first comprised 131 workers with mean UHg of 59 µg/L who routinely rotated through various jobs with differing exposure levels to reduce individual Hg0 exposures. Compared to controls, those workers had no significant increase in tremor. The second cohort was composed of 129 workers not previously exposed who spent two years renovating the facility. These workers were evaluated prospectively with quarterly PE exams and monthly UHg measurements; they were not rotated between high- and low-exposure jobs. Compared to controls, tremor was significantly more prevalent (13 of 61) in workers with at least one UHg >50 µg/L (group mean: 54 µg/L), but not in 68 workers who never had UHg >50 µg/L. The range of UHg levels in the second cohort was not described, but urine levels in the first cohort ranged up to 520 µg/L. It seems likely that some of the affected workers may have been exposed to very high exposure levels.
Accordingly, we are skeptical about the ability of these six studies to inform our thinking about the dose-relatedness and possible thresholds associated with the toxic effects of elemental mercury.
Variability of PE outcomes across studies
Although the results of prevalence analyses showed consistent positive dose-related trends across exposure categories, within each exposure strata the results of individual studies varied substantially. Possible reasons include small sample size, misclassification of exposure, selection bias, and individual differences in susceptibility related to job characteristics, lifestyle factors (i.e. smoking, alcohol intake, and nutrition) and genetics. Several authors have suggested that only resistant workers remain in mercury-exposed jobs, while those more susceptible self-select out of the workforce (Piikivi & Hanninen 1989; Roels et al. 1989).
Small Sample Size
To better understand the possibility that small samples may yield unrepresentative results, we reanalyzed the large database of PE tremor findings, comparing results from all studies vs. studies restricted to only those with at least 20 exposed workers. Overall, the average point prevalence rates across exposure categories in the restricted analysis were similar to those in the original analysis, but within each category, tremor prevalence differed substantially among individual studies. In our original ‘unrestricted’ analysis, tremor prevalence rates in the three High Exposure subcategories (i.e. UHg of 200–299; 300–499; and, >500 µg/L) ranged widely: respectively, 0–86%, 10–100%, and 0–100%. By contrast, much less variability was seen among studies in the restricted analysis: 0–21%, 10–31%, and 18–74%, respectively. There is also evidence that at least some of the smaller studies were subject to selection bias (Vroom & Greer 1972; Tang & Li 2006). Accordingly, we urge caution about relying on such smaller studies.
Exposure Misclassification
Another explanation for the variability of results across studies involves potential exposure misclassification. One example stems from the practice of rotating workers between high- and low/no exposure tasks. Temporary ‘medical removal protection’ of over-exposed workers has long had a place in workplace safety programs (e.g. OSHA 1978, 1992), and rotation of workers to reduce cumulative exposures and in response to acute Hg0 toxicity was specifically described in a number of the reviewed studies (Miller et al. 1975; Gambini 1978; Langolf et al. 1978; Langolf et al. 1981; Piikivi et al. 1984; Bunn et al. 1986). Because the urinary half-life of inorganic mercury is relatively short (approximately 60 days (Clarkson et al. 2003)), spot urines obtained in cross-sectional assessments of rotating workers would tend to understate the exposures experienced by those workers rotated away from high-exposure tasks. Thus following removal from exposure, workers with tremor after High Exposures might be wrongly categorized as Medium or Low Exposure based on current UHg despite high body burdens. On the other hand, workers more recently rotated into high exposure tasks might have high current UHg levels, but relatively low body burdens and no objective findings of toxicity (Lauwerys & Hoet 2001).
Another example of exposure misclassification is found in a study of five workers exposed for 5 to 7 years (Gonzalez-Fernandez et al. 1984). During their last seven months of work, plant production decreased, TWA air levels fell from 800 to 80 µg/m3, and mean UHg levels declined from 979 µg/L (range: 875–1100) to 84 µg/L (range: 63–97). PE at the end of that seven month period documented tremor and impaired MC in all five (100%). Had these workers been categorized based on only urines obtained when they were examined, they would have been described as a Low Exposure (50–99 µg/L) group with 100% abnormal findings, and strikingly inconsistent with other Low Exposure groups. By contrast, their history of markedly elevated UHg levels indicates that they were High Exposure workers and their PE findings were consistent with other High Exposure groups.
Exposure misclassification is also a likely explanation for the paucity of dose-related findings in two High Exposure PE studies. Bunn et al. (1986) found no evidence of dose-relatedness of tremor, abnormal MC or DTRs in comparisons across workers stratified by work history into three groups associated with increasing UHg levels. Notably, workers with symptoms of toxicity or UHg >250 µg/L were routinely removed from exposure. Moreover, UHg levels in the high- and intermediate-subgroups overlapped: 64% of the high subgroup and 29% of the intermediate subgroup had UHg levels in the 200–500 µg/L range. El-Sadik & Abdel-Aziz (1970) reported that point prevalence of tremor and abnormal DTRs that increased with duration of exposure (≥3 vs. <3 years), but not with current UHg levels. The study authors attributed this lack of association to the development of mercury-induced renal disease, noting that UHg had increased with duration of exposure during the first years of exposure, but then decreased as exposure continued.
Confounders and Effect Modifiers
In contrast to small sample size and exposure misclassification seen across all ranges of exposures, the effects of confounding are more likely to impact lower dose studies: ‘As we approach the lower end of the dose response curve, the health endpoints become more and more nonspecific. Confounders play an increasing role and become more difficult to control’ (Clarkson 1998). This can be seen by considering the impacts of smoking on tremor, an acute effect of nicotine exposure (Louis 2007). In the general population, studies have reported significant associations between smoking and postural tremor detected using NB tests (Lippold et al. 1980) and kinetic tremor detected on PE (Louis 2007). However, the importance of smoking as a potential confounder or effect modifier has received little attention in studies of occupational neurotoxicants (Ellingsen et al. 2001).
In five studies with UHg above 50 µg/L that specifically considered smoking, three reported its effects on tremor were significant, but secondary to the significant effects of mercury exposure (as measured by BHg or Uhg) (Chaffin et al. 1973; Verberk et al. 1986; Iwata et al. 2007), while two others found no significant correlation between smoking and tremor (Langolf et al. 1978; Langworth et al. 1992). In three <BEI studies that found no difference between exposed workers and controls on NB tests of tremor, smoking was associated with postural tremor (Ellingsen et al. 2001; Camerino et al. 2002; Wastensson et al. 2006) and poorer performance on tests of motor function (Ellingsen et al. 2001; Camerino et al. 2002). In Ellingsen et al. (2001), current smokers had significantly worse scores on Static Steadiness and borderline worse scores on Grooved Pegboard than non-smokers. Similar results were found when the workers were re-examined several years after cessation of exposure (Bast-Pettersen et al. 2005; Ellingsen et al. 2006). The authors cautioned that smoking ‘may act as an important confounder in epidemiological studies of mercury vapor’ (Bast-Pettersen et al. 2005) and that these effects might be modulated by age (Ellingsen et al. 2006).
A different confounding concern was raised by (Roels et al. 1982) in a study of 43 exposed workers: smoking during the work shift ‘significantly increases the exposure to mercury vapor’. Among 25 smokers, median UHg was 159 µg/L and median BHg was 25.5 µg/L, compared to 18 non-smokers with UHg of 64 µg/l and BHg 15.7 µg/L. We are not aware that this finding, which might be due to smoking-induced volatilization of Hg0 from the hands of contaminated smokers (Colquitt 2003), has been corroborated.
Implications of these results
In addition to their significance for the clinical assessment of mercury intoxication, the findings of our analysis have potentially important implications for future studies of mercury-exposed workers and, perhaps, for neurotoxicants more generally. They also lend independent support for the recently adopted ACGIH BEI of 20 µg/g creatinine (ACGIH 2013).
Exposure levels and dose metrics should be considered in the context of each subject’s exposure history. The longer half-life of Hg in urine than blood makes UHg a preferred dose metric for chronic exposure studies, but the informational value of spot urines is limited because they principally reflect recent exposures. In workers who rotate jobs and in others whose exposures are inconstant, spot urines can misclassify actual exposures. Differing results have been reported when effects were related to current UHg, average UHg, peak UHg, or cumulative UHg (i.e. average UHg × duration). The accumulated evidence suggests that peak exposures are the most important determinants of mercury toxicity; regardless of current UHg levels, future studies should seek evidence of such peaks in each worker’s history.
The choice of tests to perform and functions to assess should reflect the levels of exposure under consideration. PE can usefully discriminate among high exposure workers, but its value is severely limited in the assessment of groups exposed to lower Hg0 levels, for whom NB testing is more appropriate. Testing of motor function that includes assessment of other abilities in addition to motor skills (i.e. tests of reaction time and perceptual motor speed) is apparently not useful for assessment of mercury toxicity. Further, the selection of tests should include at least a minimum battery of standardized tests that ‘detected positive effects in published studies’ (Rohlman et al. 2003; Stern 2010b; Anger 2014), the results for each component should be presented along with appropriate statistical comparisons to well-matched controls and/or historical baselines. The goal should be not only to document the effects of exposure, but to also determine the best and most economical ways to detect those effects.
Concerns about potential confounders and effect modifiers have been too often ignored in these and other occupational studies. The use of controls matched on the basis of general demographics (with or without exclusion criteria) may not be sufficient to control for the confounding effects of smoking, alcohol, and medications. In the near future, it will also be necessary to control for genetic differences (Schulte et al. 2015). Much as specific susceptibility to trichloroethylene has been linked to polymorphisms in genes affecting its reductive metabolism (Moore et al. 2010), we expect that differential susceptibility to Hg0 is linked to variations in genes that affect its oxidative metabolism by catalase, and possibly its reduction by tissue thiols (Hursh et al. 1980; Khayat and Dencker 1984; Custodio et al. 2005; Gundacker et al. 2010; Goodrich et al. 2011; Ogata et al. 2016).
Limitations of this systematic review
The main limitations of the present review are the design and methodology of older occupational studies, particularly those conducted at a time when Hg0 exposures were substantially higher than occurs today in the modern workplace. Their failure to describe detailed findings including relevant negative results and their lack of statistical evaluations using matched controls or historical baselines limited our ability to evaluate the utility and value of specific individual tests. Likewise, even among more recent studies, few used and described the results of the same tests, although most tested the same neurological domains. Thus, we could document dose-relatedness and estimate response thresholds for a small number of PE, NB and EPS effects in general, but dose-relatedness and response thresholds could not be determined for most of the specific tests used in the various studies.
Another limitation was that various studies reported their urine measures in differing ways (e.g. µg Hg/L, µg Hg/g creatinine, or µg Hg per 24 hours), requiring values to be converted to a common metric to enable comparisons across studies. As discussed in the methods section, conversions to µg/L were made by using the midpoint of the range reported for creatinine concentration, or urinary volume. However, we also performed conversions using alternatives conversion factors (i.e. the lower and upper bounds of creatinine concentration, 1.0 to 1.8 g/L, and urinary excretion rates, 1.0 to 2.0 L/24 hrs); it did not alter the conclusions of our review.
We also considered the possibility that our inclusion criterion of exposure to Hg ‘generally for at least 3 months’ might have biased our results. Based on our literature review, the choice of the three month criterion seemed reasonable. For example, ACGIH has repeatedly summarized evidence that following onset of exposure to Hg0 there is a latency period in urinary excretion that reflects renal accumulation of Hg. Until some threshold is reached, urine Hg understates body burden. ACGIH estimates that such latency ‘can take 10 days for high exposure and six months for low exposure’ (ACGIH 1996, 2013). Because most of the studies we considered involved either high-dose exposures (and thus would have shorter latency periods) or lower-dose exposures of longer than three months, the three month criterion seemed appropriate. Most of the studies that we reviewed described exposures of 6 months or more; of eight studies that did not specifically report duration of exposure, seven were High Exposure studies that presumably would have achieved the renal threshold after relatively short latencies. Accordingly, we think that this specific inclusion criterion did not affect our study findings.
Conclusion
According to a 1991 NIOSH Health Hazard Evaluation of Hg-exposed workers: ‘…studies of the health effects of mercury exposure have shown difficulties in defining which health effect may be expected at specific exposure levels’ (Reh et al. 1991). We were able to address these limitations by expanding the focus beyond individual study findings and examining the consistency, patterns, and dose-relatedness of objective motor and sensory neurological effects described in all eligible studies stratified across categories of exposure. This type of systematic review allowed us to identify the types of neurological effects most commonly associated with ongoing exposure to elemental mercury vapor and the exposure/dose levels at which they occurred. These findings suggest that for diagnosing mercury intoxication in currently exposed workers, PE is of particular value in those with UHg >200 µg/L but not in those with lesser exposures. By contrast, NB testing is of particular diagnostic value in those with lower UHg levels. The results of these analyses in currently exposed workers can now be compared to the type and frequency of neurological findings reported in workers with historical Hg exposure to shed light on the questions about the persistence of Hg-induced neurological effects.
Supplementary Material
Acknowledgments
We wish to thank Ms. J. Rivera for her bibliographic excellence, and Dr. M. Russi, Mr. G. Munemitsu, and Mr. E. Fiel for help and advice regarding translations. We are very grateful to the five anonymous reviewers selected by the Editor for the value of their comments in improving the manuscript.
Declaration of interest
Jonathan Borak and Cheryl Fields are employees of Jonathan Borak & Company, which provides consulting services on environmental and occupational health issues (including concerns related to mercury exposure) to government and private clients. Elan Louis is a full-time employee of Yale University. The authors have not appeared in the last five years in any legal or regulatory proceedings related to the contents of this paper and have no plans to participate in such proceedings in the future. Dr. Borak and Ms. Fields contributed to the research reported in this paper during their normal course of employment without supplementary funding or external support. Dr. Louis was supported by grant R01 NS094607 from NINDS.
Contributor Information
Cheryl A. Fields, Department of Environmental Health Sciences, Yale School of Public Health, New Haven, Connecticut, USA.
Jonathan Borak, Departments of Medicine and Epidemiology & Public Health, Yale School of Medicine, New Haven, Connecticut, USA.
Elan D. Louis, Department of Neurology, Yale School of Medicine, and Department of Chronic Disease Epidemiology, Yale School of Public Health, New Haven, Connecticut, USA
References
- State of Vermont, Village of Poultney v. STACO, Inc., Chase Instruments Corporation, Chase instruments Sales Corporation, Keeper Corporation, Roberts Sirkus, I. Walter Munzer, Robert Munzer (CivA. No 86–190) United States District Court; District of Vermont: 1988. [Google Scholar]
- Adams CR, Ziegler DK, Lin JT. Mercury intoxication simulating amyotrophic lateral sclerosis. J Am Med Assoc. 1983;250:642–643. [PubMed] [Google Scholar]
- [ATSDR] Agency for Toxic Substances and Disease Registry. Toxicological Profile For Mercury (update) Washington, DC: U.S. Department of Health and Human Services; 1999. [Google Scholar]
- Albers JW, Cavender GD, Levine SP, Langolf GD. Asymptomatic sensorimotor polyneuropathy in workers exposed to elemental mercury. Neurology. 1982;32:1168–1174. doi: 10.1212/wnl.32.10.1168. [DOI] [PubMed] [Google Scholar]
- American Clinical Neurophysiology Society. [Accessed: 04/17/2015];Assessment of Digital EEG, Quantitative EEG, and EEG Brain Mapping. 1997 doi: 10.1212/wnl.49.1.277. (at: https://www.acns.org/pdf/guidelines/QEEG-Statement.pdf) [DOI] [PubMed]
- [ACGIH] American Conference of Governmental Industrial Hygienists. Mercury, Elemental and Inorganic Forms; Cincinnati. American Conference of Governmental Industrial Hygienists.1996. [Google Scholar]
- [ACGIH] American Conference of Governmental Industrial Hygienists. 2012 Threshold Limit Values (TLVs) for Chemical Substances and Physical Agents and Biological Exposure Indices (BEIs); Cincinnati. American Conference of Governmental Industrial Hygienists.2012. [Google Scholar]
- [ACGIH] American Conference of Governmental Industrial Hygienists. Mercury, Elemental: BEI® 7th Edition Documentation; Cincinnati. American Conference of Governmental Industrial Hygienists.2013. [Google Scholar]
- [ACGIH] American Conference of Governmental Industrial Hygienists. 2014 Threshold Limit Values (TLVs) for Chemical Substances and Physical Agents and Biological Exposure Indices (BEIs); Cincinnati. American Conference of Governmental Industrial Hygienists.2014. [Google Scholar]
- Anger WK. Reconsideration of the WHO NCTB strategy and test selection. Neurotoxicology. 2014;45:224–231. doi: 10.1016/j.neuro.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angotzi G, Cassitto MG, Camerino D, Cioni R, Desideri E, Franzinelli A, Gori R, Loi F, Sartorelli E. Relation between mercury exposure and health conditions in a group of workers employed in a mercury distillation factory in Siena Province. Med Lav. 1980;71:463–480. [PubMed] [Google Scholar]
- Angotzi G, Battistini N, Carboncini F, Cioni R, Desideri E, Paradiso C, Nuti D, Sartorelli E. Impairment of nervous system in workers exposed to inorganic mercury. Toxicol Eur Res. 1981;3:275–278. [PubMed] [Google Scholar]
- Ball RD. Electrodiagnostic Evaluation of the Peripheral Nervous System. In: DeLisa JL, Gans BM, Walsh NE, Bockenek WL, et al., editors. Physical Medicine & Rehabilitation: Principles and Practice. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast-Pettersen R, Ellingsen DG, Efskind J, Jordskogen R, Thomassen Y. A neurobehavioral study of chloralkali workers after the cessation of exposure to mercury vapor. Neurotoxicology. 2005;26:427–437. doi: 10.1016/j.neuro.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Berlin M. Mercury. In: Friberg L, Nordberg GF, Vouk VB, editors. Handbook on the Toxicology of Metals. 2. II. Amsterdam: Elseiver; 1986. pp. 387–445. [Google Scholar]
- Berme N, Oggero E, Pagnacco G. Power spectrum characteristics of physiologic and pathologic tremor. Acta Bioeng Biomech. 1999;1:71–88. [Google Scholar]
- Bidstrup PL, Bonnell JA, Harvey DG, Locket S. Chronic mercury poisoning in men repairing direct - current meters. Lancet. 1951;2:856–861. doi: 10.1016/s0140-6736(51)91825-9. [DOI] [PubMed] [Google Scholar]
- Buijink AW, Contarino MF, Koelman JH, Speelman JD, van Rootselaar AF. How to tackle tremor - systematic review of the literature and diagnostic work-up. Front Neurol. 2012;3:146. doi: 10.3389/fneur.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn WB, McGill CM, Barber TE, Cromer JW, Jr, Goldwater LJ. Mercury exposure in chloralkali plants. Am Ind Hyg Assoc J. 1986;47:249–254. doi: 10.1080/15298668691389720. [DOI] [PubMed] [Google Scholar]
- Camerino D, Cassitto MG, Desideri E, Angotzi G. Behavior of some psychological parameters in a population of a Hg extraction plant. Clin Toxicol. 1981;18:1299–1309. doi: 10.3109/00099308109035070. [DOI] [PubMed] [Google Scholar]
- Camerino D, Buratti M, Rubino FM, Somaruga C, Belluigi V, Bordiga A, Bordini L, Maraschi R, Molinari M, Colosio C, Soleo L, Colombi A. Evaluation of the neurotoxic and nephrotoxic effects following long-term exposure to metallic mercury in employed at a chlorine/sodium-hydroxide plant. Med Lav. 2002;93:238–250. [PubMed] [Google Scholar]
- Cavalleri A, Belotti L, Gobba F, Luzzana G, Rosa P, Seghizzi P. Colour vision loss in workers exposed to elemental mercury vapour. Toxicol Lett. 1995;77:351–356. doi: 10.1016/0378-4274(95)03317-3. [DOI] [PubMed] [Google Scholar]
- Cavalleri A, Gobba F. Reversible color vision loss in occupational exposure to metallic mercury. Environ Res. 1998;77:173–177. doi: 10.1006/enrs.1997.3814. [DOI] [PubMed] [Google Scholar]
- CDC. Alcohol and Public Health. Atlanta: Centers for Disease Control and Prevention; 2014. (at: http://www.cdc.gov/alcohol/faqs.htm) [Google Scholar]
- Chaffin DB, Dinman BD, Miller JM, Smith RG, Zontine DH. An Evaluation of the Effects of Chronic Mercury Exposures on EMG and Psychomotor Functions (HSM-099-71-62 Final Report) National Institutes of Health; 1973. [Google Scholar]
- Chang Y-C, Yeh C-Y, Wang J-D. Subclinical neurotoxicity of mercury vapor revealed by a multimodality evoked potential study of chloralkali workers. Am J Ind Med. 1995;27:271–279. doi: 10.1002/ajim.4700270211. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Sauter SL, Henning RA, Dodson VN, Reddan WG, Matthews CG. Differences in frequency of finger tremor in otherwise asymptomatic mercury workers. Br J Ind Med. 1990;47:838–843. doi: 10.1136/oem.47.12.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW. Human toxicology of mercury. J Trace Elem Exp Med. 1998;11:303–317. [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury--current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Coggon D, Rose G, Barker DJP. Epidemiology for the uninitiated. London: British Medical Journal; 2003. (at: http://www.bmj.com/about-bmj/resources-readers/publications/epidemiology-uninitiated) [Google Scholar]
- Cohen EN, Brown BW, Wu ML, Whitcher CE, Brodsky JB, Gift HC, Greenfield W, Jones TW, Driscoll EJ. Occupational disease in dentistry and chronic exposure to trace anesthetic gases. J Am Dent Assoc. 1980;101:21–31. doi: 10.14219/jada.archive.1980.0345. [DOI] [PubMed] [Google Scholar]
- Colquitt PJ. Smoking increases mercury exposure in hospital workers-13 July, 2003. British Medical Journal. 2003;327:1–4. [Google Scholar]
- Cordeiro Q, Jr, de Araujo Medrado FM, Fraguas R., Jr Depression, insomnia, and memory loss in a patient with chronic intoxication by inorganic mercury. J Neuropsychiatry Clin Neurosci. 2003;15:457–458. doi: 10.1176/jnp.15.4.457. [DOI] [PubMed] [Google Scholar]
- Custodio HM, Harari R, Gerhardsson L, Skerfving S, Broberg K. Genetic influences on the retention of inorganic mercury. Arch Environ Occup Health. 2005;60:17–23. doi: 10.3200/AEOH.60.1.17-23. [DOI] [PubMed] [Google Scholar]
- Ehrenberg RL, Vogt RL, Smith AB, Brondum J, Brightwell WS, Hudson PJ, McManus KP, Hannon WH, Phipps FC. Effects of elemental mercury exposure at a thermometer plant. Am J Ind Med. 1991;19:495–507. doi: 10.1002/ajim.4700190407. [DOI] [PubMed] [Google Scholar]
- El-Sadik YM, Abdel-Aziz E-D. Effects of exposure of workers to mercury at a sodium hydroxide producing plant. Am Ind Hyg Assoc J. 1970;31:705–710. doi: 10.1080/0002889708506317. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Bast-Pettersen R, Efskind J, Thomassen Y. Neuropsychological effects of low mercury vapor exposure in chloralkali workers. Neurotoxicology. 2001;22:249–258. doi: 10.1016/s0161-813x(01)00012-2. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Bast-Pettersen R, Efskind J, Gjolstad M, Olsen R, Thomassen Y, Molander P. Hand tremor related to smoking habits and the consumption of caffeine in male industrial workers. Neurotoxicology. 2006;27:525–533. doi: 10.1016/j.neuro.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [EPA] U.S.Environmental Protection Agency. Mercury Health Effects Update (Health issue assessment) (EPA 600/8-84-019F) North Carolina: Research Triangle Park; 1984. [Google Scholar]
- [EPA] U.S.Environmental Protection Agency. Integrated Risk Information System: Mercury elemental (CASRN 7439-97-6) Washington DC: US Environmental Protection Agency; 2012. [Google Scholar]
- Fawer RF, DeRibaupierre Y, Gullemin MP, Berode M, Lob M. Measurement of hand tremor induced by industrial exposure to metallic mercury. Br J Ind Med. 1983;40:204–208. doi: 10.1136/oem.40.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RG. Mercury. In: Feldman RG, editor. Occupational & Environmental Neurotoxicology. Philadelphia: Lippincott-Raven; 1999a. pp. 92–114. [Google Scholar]
- Feldman RG. Occupational & Environmental Neurotoxicology. Philadelphia: Lippincott-Raven; 1999b. [Google Scholar]
- Florentine MJ, Sanfilippo DJ. Elemental mercury poisoning. Clin Pharm. 1991;10:213–221. [PubMed] [Google Scholar]
- Franssen H, van den Bergh PY. Nerve conduction studies in polyneuropathy: practical physiology and patterns of abnormality. Acta Neurol Belg. 2006;106:73–81. [PubMed] [Google Scholar]
- Friberg L, Vostal J. Mercury in the Environment: An Epidemiological and Toxicological Appraisal. Research Triangle Park, U.S. Environmental Protection Agency; 1971. [Google Scholar]
- Gambini G. Correlation between the concentrations of inorganic mercury vapors in the air hydrargyriuria and symptoms of chronic mercurialism in a Cloro-Soda plant, using electrolytic cells with mercury cathodes (Italian) Med Lav. 1978;69(suppl 3):379–392. [PubMed] [Google Scholar]
- Gilioli R, Bulgheroni C, Caimi L, Foa V, Filippini C, Boiardi A, Bussone G, Quarti M, Boeri R. Correlations between subjective complaints and objective neurophysiological findings in workers of a chlor-alkali plant. In: Horvath M, Frantik E, editors. Adverse Effects of Environmental Chemicals and Psychotropic Drugs. Neurophysiological and behavioural tests. Vol. 2. Amsterdam: Elsevier Scientific Publishing Company; 1976. pp. 157–164. [Google Scholar]
- Goldstein G, Sanders RD. Sensory-Perceptual and Motor Function. In: Goldstein G, Beers SR, Hersen M, editors. Comprehensive Handbook of Psychological Assessment: Intellectual and Neuropsychological Assessment. Vol. 1. Hoboken: John Wiley & Sons; 2004. pp. 309–319. [Google Scholar]
- Gonzalez-Fernandez E, Mena J, Diaz-Gonzalez M, Martinez-Gil de Arana JM. Long-term survey of environmental, blood and urine mercury levels and clinical findings in workers manufacturing mercury relays. Ind Health. 1984;22:97–106. doi: 10.2486/indhealth.22.97. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Usigli HA, Espay A. Tremor: A Merck Manual of Patient Symptoms podcast. Whitehouse Station: Merk & Company, Inc.; 2013. ( http://www.merckmanuals.com/professional/neurologic_disorders/movement_and_cerebellar_disorders/tremor.html) [Google Scholar]
- Goodrich JM, Wang Y, Gillespie B, Werner R, Franzblau A, Basu N. Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan dental professionals. Toxicol Appl Pharmacol. 2011;257:301–308. doi: 10.1016/j.taap.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg MI, Hamilton RJ, Phillips SD, McCluskey GJ. Clinical Toxicology: Mercury Pathophysiology. In: Greenberg MI, Hamilton RJ, Phillips SD, McCluskey GJ, editors. Occupational, Industrial, and Environmental Toxicology. 2. St. Louis: Mosby; 2003. pp. 91–95. [Google Scholar]
- Gundacker C, Gencik M, Hengstschlager M. The relevance of the individual genetic background for the toxicokinetics of two significant neurodevelopmental toxicants: mercury and lead. Mutat Res. 2010;705:130–140. doi: 10.1016/j.mrrev.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Gunther W, Sietman B, Seeber A. Repeated neurobehavioral investigations in workers exposed to mercury in a chloralkali plant. Neurotoxicology. 1996;17:605–614. [PubMed] [Google Scholar]
- Hudson PJ, Vogt RL, Brondum J, Witherell L, Myers G, Paschal DC. Elemental mercury exposure among children of thermometer plant workers. Pediatrics. 1987;79:935–938. [PubMed] [Google Scholar]
- Hursh JB, Greenwood MR, Clarkson TW, Allen J, Demuth S. The effect of ethanol on the fate of mercury vapor inhaled by man. J Pharmacol Exp Ther. 1980;214:520–527. [PubMed] [Google Scholar]
- [IPCS] International Programme on Chemical Safety. Concise International Chemical Assessment Document 50: Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. Geneva: World Health Organization; 2003. [Google Scholar]
- Iwata T, Sakamoto M, Feng X, Yoshida M, Liu XJ, Dakeishi M, Li P, Qiu G, Jiang H, Nakamura M, Murata K. Effects of mercury vapor exposure on neuromotor function in Chinese miners and smelters. Int Arch Occup Environ Health. 2007;80:381–387. doi: 10.1007/s00420-006-0144-1. [DOI] [PubMed] [Google Scholar]
- Johnson BL, Baker EL. Prevention of Neurotoxic Illness in Working Populations. New York: Wiley; 1987. [Google Scholar]
- Khayat A, Dencker L. Organ and cellular distribution of inhaled metallic mercury in the rat and Marmoset monkey (Callithrix jacchus): influence of ethyl alcohol pretreatment. Acta Pharmacol Toxicol (Copenh) 1984;55:145–152. doi: 10.1111/j.1600-0773.1984.tb01977.x. [DOI] [PubMed] [Google Scholar]
- Kissin B. Medical Management of the Alcoholic Patient. In: Kissin B, Begleiter H, editors. The Biology of Alcoholism: Treatment and Rehabiliation of the Chronic Alcholic. Vol. 5. Boston: Springer; 1977. pp. 53–103. [Google Scholar]
- Langolf GD, Chaffin DB, Henderson R, Whittle HP. Evaluation of workers exposed to elemental mercury using quantitative tests of tremor and neuromuscular functions. Am Ind Hyg Assoc J. 1978;39:976–984. doi: 10.1080/0002889778507898. [DOI] [PubMed] [Google Scholar]
- Langolf GD, Smith PJ, Henderson R, Whittle H. Measurements of neurological functions in the evaluations of exposure to neurotoxic agents. Ann Occup Hyg. 1981;24:293–296. doi: 10.1093/annhyg/24.3.293. [DOI] [PubMed] [Google Scholar]
- Langworth S, Almqvist O, Soderman E, Wikstrom BO. Effects of occupational exposure to mercury vapour on the central nervous system. Br J Ind Med. 1992;49:545–555. doi: 10.1136/oem.49.8.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwerys RR, Hoet P. Industrial Chemical Exposure: Guidelines for Biological Monitoring. 3. Boca Raton: CRC Press; 2001. [Google Scholar]
- Levine SP, Cavender GD, Langolf GD, Albers JW. Elemental mercury exposure: Peripheral neurotoxicity. Br J Ind Med. 1982;39:136–139. doi: 10.1136/oem.39.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y-X, Sun R-K, Sun Y, Chen Z-Q, Li L-H. Psychological effects of low exposure to mercury vapor: Application of a computer-administered neurobehavioral evaluation system. Environ Res. 1993;60:320–327. doi: 10.1006/enrs.1993.1040. [DOI] [PubMed] [Google Scholar]
- Lippold OCJ, Williams EJ, Wilson CG. Finger tremor and cigarette smoking. Br J Clin Pharmacol. 1980;10:83–86. doi: 10.1111/j.1365-2125.1980.tb00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locket S, Nazroo IA. Eye changes following exposure to metallic mercury. Lancet. 1952;259:528–532. [Google Scholar]
- Louis ED, Yousefzadeh E, Barnes LF, Yu Q, Pullman SL, Wendt KJ. Validation of a portable instrument for assessing tremor severity in epidemiologic field studies. Movement Disorders. 2000;15:95–102. doi: 10.1002/1531-8257(200001)15:1<95::aid-mds1015>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Louis ED, Pullman SL. Comparison of clinical vs. electrophysiological methods of diagnosing of essential tremor. Movement Disorders. 2001;16:668–673. doi: 10.1002/mds.1144. [DOI] [PubMed] [Google Scholar]
- Louis ED. Kinetic tremor: differences between smokers and non-smokers. Neurotoxicology. 2007;28:569–575. doi: 10.1016/j.neuro.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R, Placidi D, Toffoletto F, Alessio L. Neurotoxicity in operating room personnel working with gaseous and nongaseous anesthesia. Int Arch Occup Environ Health. 1996;68:188–192. doi: 10.1007/BF00381630. [DOI] [PubMed] [Google Scholar]
- McCullough J, Dick R. Mercury Waste Solutions, Inc., Union Grove, Wisconsin (HETA 98-0320-2751) Cincinnati: National Institute for Occupational Safety and Health, Centers for Disease Control; 1999. [Google Scholar]
- McCullough JE, Dick R, Rutchik J. Chronic mercury exposure examined with a computer-based tremor system. J Occup Environ Med. 2001;43:295–300. doi: 10.1097/00043764-200103000-00022. [DOI] [PubMed] [Google Scholar]
- McGill CM, Ladd AC, Jacobs MB, Goldwater LJ. Mercury exposure in a chlorine plant. J Occup Med. 1964;6:335–337. doi: 10.1097/00043764-196408000-00003. [DOI] [PubMed] [Google Scholar]
- Meyer-Baron M, Schaeper M, Seeber A. A meta-analysis for neurobehavioral results due to occupational mercury exposure. Arch Toxicol. 2002;76:127–136. doi: 10.1007/s00204-002-0327-9. [DOI] [PubMed] [Google Scholar]
- Meyer-Baron M, Schaeper M, van Thriel C, Seeber A. Neurobehavioural test results and exposure to inorganic mercury: in search of dose-response relations. Arch Toxicol. 2004;78:207–211. doi: 10.1007/s00204-003-0531-2. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chaffin DB, Smith RG. Subclinical psychomotor and neuromuscular changes in workers exposed to inorganic mercury. Am Ind Hyg Assoc J. 1975;36:725–733. doi: 10.1080/0002889758507331. [DOI] [PubMed] [Google Scholar]
- Monforte R, Estruch R, Valls-Sole J, Nicolas J, Villalta J, Urbano-Marquez A. Autonomic and peripheral neuropathies in patients with chronic alcoholism. A dose-related toxic effect of alcohol. Arch Neurol. 1995;52:45–51. doi: 10.1001/archneur.1995.00540250049012. [DOI] [PubMed] [Google Scholar]
- Moore LE, Boffetta P, Karami S, Brennan P, Stewart PS, Hung R, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, et al. Occupational trichloroethylene exposure and renal carcinoma risk: Evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res. 2010;70:6527–6536. doi: 10.1158/0008-5472.CAN-09-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health. NIOSH Alerts: Controlling Exposures to Nitrous Oxide during Anesthetic Administration. Atlanta: Centers for Disease Control and Prevention; 2015. (DHHS (NIOSH) Publication No. 94-100)( http://www.cdc.gov/niosh/docs/94-100/) [Google Scholar]
- [NINDS] National Institute of Neurological Disorders and Stroke. Tremor. Bethesda: National Institute of Neurological Disorders and Stroke, National Institute of Health; 2012. (NIH Publication No. 12-4734) [Google Scholar]
- [NINDS] National Institute of Neurological Disorders and Stroke. Tremor Fact Sheet. Bethesda: NIH Neurological Institute; 2016. ( http://www.ninds.nih.gov/disorders/tremor/detail_tremor.htm) [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. Bethesda: National Institutes of Health; 2016. ( http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking) [Google Scholar]
- National Research Council. Environmental Neurotoxicology. Washington, DC: The National Academies Press; 1992. [PubMed] [Google Scholar]
- Nerudova J, Cabelkova Z, Frantik E, Lukas E, Urban P, Blaha K, Pelclova D, Lebedova J, Cikrt M. Mobilization of mercury by DMPS in occupationally exposed workers and in model experiments on rats: evaluation of body burden. Int J Occup Med Environ Health. 2000;13:131–146. [PubMed] [Google Scholar]
- Nuwer M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 1997;49:277–292. doi: 10.1212/wnl.49.1.277. [DOI] [PubMed] [Google Scholar]
- Ogata M, Wang DH, Ogino K. Mammalian acatalasemia: the perspectives of bioinformatics and genetic toxicology. Acta Med Okayama. 2016;62:345–361. doi: 10.18926/AMO/30951. [DOI] [PubMed] [Google Scholar]
- OSHA. Occupational Exposure to Lead. Fed Reg. 1978;43:52952–53007. [Google Scholar]
- OSHA. Occupational Exposure to Cadmium. Final Rule. Fed Reg. 1992;57:42102–42463. [Google Scholar]
- Piikivi L, Hanninen H, Martelin T, Mantere P. Psychological performance and long-term exposure to mercury vapors. Scand J Work Environ Health. 1984;10:35–41. doi: 10.5271/sjweh.2365. [DOI] [PubMed] [Google Scholar]
- Piikivi L, Hanninen H. Subjective symptoms and psychological performance of chlorin-alkali workers. Scand J Work Environ Health. 1989;15:69–74. doi: 10.5271/sjweh.1880. [DOI] [PubMed] [Google Scholar]
- Piikivi L, Tolonen U. EEG findings in chlor-alkali workers subjected to low long term exposure to mercury vapour. Br J Ind Med. 1989;46:370–375. doi: 10.1136/oem.46.6.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranjic N, Sinanovic O, Jakubovic R. Chronic psychological effects of exposure to mercury vapour among chlorine-alkali plant workers. Med Lav. 2003;94:531–541. [PubMed] [Google Scholar]
- Rajaniemi R. Clinical evaluation of occupational toxicity of methylmethacrylate monomer to dental technicians. J Soc Occup Med. 1986;36:56–59. doi: 10.1093/occmed/36.2.56. [DOI] [PubMed] [Google Scholar]
- Reeves AG, Swenson RS. Evaluation of the Ataxic Patient. In: Reeves AG, Swenson RS, editors. Disorders of the Nervous System. A Primer. Hanover: Dartmouth Medical School; 2008a. [Google Scholar]
- Reeves AG, Swenson RS. Motor System Examination. In: Reeves AG, Swenson RS, editors. Disorders of the Nervous System. A Primer. Hanover: Dartmouth Medical School; 2008b. [Google Scholar]
- Reh CM, Deitchman SD, Moss CE. NIOSH Health Hazard Evaluation Report: HETA: 87-402-2145, LCP Chemicals and Plastics, Inc., Brunswick, Georgia. Cincinnati: National Institute for Occupational Safety and Health, Centers for Disease Control; 1991. [Google Scholar]
- Rentos PG, Seligman EJ. Relationship between environmental exposure to mercury and clinical observation. Arch Environ Health. 1968;16:794–800. doi: 10.1080/00039896.1968.10665154. [DOI] [PubMed] [Google Scholar]
- Roels H, Lauwerys R, Buchet JP, Bernard A, Barthels A, Oversteyns M, Gaussin J. Comparison of renal function and psychomotor performance in workers exposed to elemental mercury. Int Arch Occup Environ Health. 1982;50:77–93. doi: 10.1007/BF00432495. [DOI] [PubMed] [Google Scholar]
- Roels H, Gennart JP, Lauwerys R, Buchet JP, Malchaire J, Bernard A. Surveillance of workers exposed to mercury vapour: Validation of a previously proposed biological threshold limit value for mercury concentration in urine. Am J Ind Med. 1985;7:45–71. doi: 10.1002/ajim.4700070106. [DOI] [PubMed] [Google Scholar]
- Roels H, Abdeladim S, Braun M, Malchaire J, Lauwerys R. Detection of hand tremor in workers exposed to mercury vapour: a comparative study of three methods. Environ Res. 1989;49:152–165. doi: 10.1016/s0013-9351(89)80060-x. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Gimenes LS, Eckerman DA, Kang SK, Farahat FM, Anger WK. Development of the Behavioral Assessment and Research System (BARS) to detect and characterize neurotoxicity in humans. Neurotoxicology. 2003;24:523–531. doi: 10.1016/s0161-813x(03)00023-8. [DOI] [PubMed] [Google Scholar]
- Schuckmann F. Study of preclinical changes in workers exposed to inorganic mercury in chloralkali plants. Int Arch Occup Environ Health. 1979;44:193–200. doi: 10.1007/BF00381134. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Whittaker C, Curran CP. Considerations for using genetic and epigenetic information in occupational health risk assessment and standard setting. J Occup Environ Hyg. 2015;12(suppl 1):S69–S81. doi: 10.1080/15459624.2015.1060323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppalainen AM, Rajaniemi R. Local neurotoxicity of methyl methacrylate among dental technicians. Am J Ind Med. 1984;5:471–477. doi: 10.1002/ajim.4700050606. [DOI] [PubMed] [Google Scholar]
- Smith RG, Vorwald AJ, Patil LS, Mooney TF. Effects of exposure to mercury in the manufacture of chlorine. Am Ind Hyg Assoc J. 1970;31:687–700. doi: 10.1080/0002889708506315. [DOI] [PubMed] [Google Scholar]
- Soleo L, Urbano ML, Petrera V, Ambrosi L. Effects of low exposure to inorganic mercury on psychological performance. Br J Ind Med. 1990;47:105–109. doi: 10.1136/oem.47.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH, Ludolph AC. Experimental and Clinical Neurotoxicology. New York: Oxford University Press; 2000. [Google Scholar]
- Stern Y. Gait Disorders. In: Rowland LP, Pedley TA, editors. Merritt's Neurology. 12. Philadelphia: Lippincott Williams & Wilkins; 2010a. [Google Scholar]
- Stern Y. Neuropsychological Evaluation. In: Rowland LP, Pedley TA, editors. Merritt's Neurology. 12. Philadelphia: Lippincott Williams & Wilkins; 2010b. [Google Scholar]
- Sternberg EJ, Alcalay RN, Levy OA, Louis ED. Postural and intention tremors: a detailed clinical study of essential tremor vs. Parkinson's disease. Front Neurol. 2013;4(Article 51):1–8. doi: 10.3389/fneur.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa N, Takahata N. Clinical studies of chronic inorganic mercury poisoning [in japanese] Rec Adv Res Nerv System. 1969;13:89–92. [PubMed] [Google Scholar]
- Tang N, Li YM. Neurotoxic effects in workers of the clinical thermometer manufacture plant. Int J Occup Med Environ Health. 2006;19:198–202. doi: 10.2478/v10001-006-0023-8. [DOI] [PubMed] [Google Scholar]
- Triebig G, Schaller KH. Neurotoxic effects in mercury--exposed workers. Neurobehav Toxicol Teratol. 1982;4:717–720. [PubMed] [Google Scholar]
- Urban P, Lukas E, Benicky L, Moscovicova E. Neurological and electrophysiological examination on workers exposed to mercury vapors. Neurotoxicology. 1996;17:191–196. [PubMed] [Google Scholar]
- Urban P, Lukas E, Nerudova J, Cabelkova Z, Cikrt M. Neurological and electrophysiological examinations on three groups of workers with different levels of exposure to mercury vapors. Eur J Neurol. 1999;6:571–577. doi: 10.1046/j.1468-1331.1999.650571.x. [DOI] [PubMed] [Google Scholar]
- Urban P, Gobba F, Nerudova J, Lukas E, Cabelkova Z, Cikrt M. Color discrimination impairment in workers exposed to mercury vapor. Neurotoxicology. 2003;24:711–716. doi: 10.1016/S0161-813X(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Verberk MM, Salle HJA, Kemper CH. Tremor in workers with low exposure to metallic mercury. Am Ind Hyg Assoc J. 1986;47:559–562. doi: 10.1080/15298668691390232. [DOI] [PubMed] [Google Scholar]
- Verkkala E, Rajaniemi R, Savolainen H. Local neurotoxicity of methylmethacrylate monomer. Toxicol Lett. 1983;18:111–114. doi: 10.1016/0378-4274(83)90079-6. [DOI] [PubMed] [Google Scholar]
- Vroom FQ, Greer M. Mercury vapour intoxication. Brain. 1972;95:305–318. doi: 10.1093/brain/95.2.305. [DOI] [PubMed] [Google Scholar]
- Walker HK. The Cerebellum. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3. Boston: Butterworths; 1990. pp. 356–359. [PubMed] [Google Scholar]
- Wang Y, Goodrich JM, Gillespie B, Werner R, Basu N, Franzblau A. An investigation of modifying effects of metallothionein single-nucleotide polymorphisms on the association between mercury exposure and biomarker levels. Environ Health Perspect. 2012;120:530–534. doi: 10.1289/ehp.1104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastensson G, Lamoureux D, Sallsten G, Beuter A, Barregard L. Quantitative tremor assessment in workers with currrent low exposure to mercury vapor. Neurotoxicol Teratol. 2006;28:681–693. doi: 10.1016/j.ntt.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Wastensson G, Lamoureux D, Sallsten G, Beuter A, Barregard L. Quantitative assessment of neuromotor function in workers with current low exposure to mercury vapor. Neurotoxicology. 2008;29:596–604. doi: 10.1016/j.neuro.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Wastensson G. Quantitative Methods for Evaluation of Tremor and Neuromotor Function. Goteborg, Sweden: University of Gothenburg; 2010. (at: https://gupea.ub.gu.se/handle/2077/23133) [Google Scholar]
- West I, Lim J. Mercury poisoning among workers in California's mercury mills. J Occup Med. 1968;10:697–701. doi: 10.1097/00043764-196812000-00004. [DOI] [PubMed] [Google Scholar]
- White RF, Feldman RG, Moss MB, Proctor SP. Magnetic resonance imaging (MRI), neurobehavioral testing, and toxic encephalopathy: two cases. Environ Res. 1993;61:117–123. doi: 10.1006/enrs.1993.1055. [DOI] [PubMed] [Google Scholar]
- Wood RW, Weiss AB, Weiss B. Hand tremor induced by industrial exposure to inorganic mercury. Arch Environ Health. 1973;26:249–252. doi: 10.1080/00039896.1973.10666268. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Environmental Health Criteria 118: Inorganic Mercury. Geneva: World Health Organization; 1991. [Google Scholar]
- Zampollo A, Baruffini A, Cirla AM, Pisati G, Zedda S. Subclinical inorganic mercury neuropathy: neurophysiological investigation 17 occupationally exposed subjects. Ital J Neurol Sci. 1987;8:249–254. doi: 10.1007/BF02337482. [DOI] [PubMed] [Google Scholar]
- Zedda S, Cirla AM, Ratti R, Sala C, Zampollo A. Rischio da mercurio nella fabbricazione di lampade fluorescenti studio clinico e ambientale. G Ital Med Lav. 1980;2:187–192. [Google Scholar]
- Zirschky J, Witherell L. Cleanup of mercury contamination of thermometer worker's homes. Am Ind Hyg Assoc J. 1987;48:81–84. doi: 10.1080/15298668791384418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.