Abstract
Chronic insufficient sleep is a major societal problem and is associated with increased risk of metabolic disease. Hypothalamic inflammation contributes to hyperphagia and weight gain in diet-induced obesity, but insufficient sleep-induced neuroinflammation has yet to be examined in relation to metabolic function. We therefore fragmented sleep of adult male C57BL/6 J mice for 18 h daily for 9 days to determine whether sleep disruption elicits inflammatory responses in brain regions that regulate energy balance and whether this relates to glycemic control. To additionally test the hypothesis that exposure to multiple inflammatory factors exacerbates metabolic outcomes, responses were compared in mice exposed to sleep fragmentation (SF), high-fat diet (HFD), both SF and HFD, or control conditions. Three or 9 days of high-fat feeding reduced glucose tolerance but SF alone did not. Transient loss of body mass in SF mice may have affected outcomes. Comparisons of pro-inflammatory cytokine concentrations among central and peripheral metabolic tissues indicate that patterns of liver interleukin-1β concentrations best reflects observed changes in glucose tolerance. However, we demonstrate that SF rapidly and potently increases Iba1 immunoreactivity (-ir), a marker of microglia. After 9 days of manipulations, Iba1-ir remains elevated only in mice exposed to both SF and HFD, indicating a novel interaction between sleep and diet on microglial activation that warrants further investigation.
Keywords: Astrocytes, Microglia, Cytokines, Glucose metabolism, Hypothalamus
Highlights
-
•
Sleep disruption and high-fat diet feeding independently induce inflammation and alter glycemic control.
-
•
In this protocol, high-fat diet feeding reduces glucose tolerance, but sleep fragmentation does not.
-
•
Inflammatory responses in hypothalamus persist only in mice exposed to both high-fat diet feeding and sleep fragmentation.
1. Introduction
The number of adults with diabetes worldwide has more than doubled in recent decades (Danaei et al., 2011) while the age of diagnosis has decreased (Carstensen et al., 2008, Holden et al., 2013, Koopman et al., 2005, Kitagawa et al., 1994, Dabelea et al., 2014). Concurrent declines in societal sleep health likely contribute to these trends (Liu et al., 2016, Basch et al., 2014, Matricciani et al., 2012; but see Youngstedt et al., 2016). Sleep disturbances such as fragmented, nonrestorative sleep are more frequently observed in individuals with type 2 diabetes than in those without (Sokwalla et al., 2017, Knutson et al., 2006, Trento et al., 2008), and glycemic control in these patients relates directly to their sleep quality (Knutson et al., 2006, Knutson et al., 2011, Tsai et al., 2012, Tang et al., 2014). Additionally, poor sleep may exacerbate metabolic outcomes when combined with other risk factors. For example, sleep fragmentation and obesity are common characteristics of obstructive sleep apnea. Patients with obstructive sleep apnea are at increased risk of type 2 diabetes (Tasali et al., 2008), whereas those that achieve weight loss through diet and exercise demonstrate improved apnea-hypopnea index and diabetes control (Foster et al., 2009).
Although associations between chronic insufficient sleep and type 2 diabetes are well established (Leng et al., 2016, Kowall et al., 2016, Anothaisintawee et al., 2015), the mechanisms underlying these relationships are less clear. Chronic, systemic inflammation is a well-recognized mechanism in diabetes and obesity pathophysiology (Donath and Shoelson, 2011, Gregor and Hotamisligil, 2011), and many of the same inflammatory pathways activated during metabolic stress are stimulated by sleep loss. For example, macrophage infiltration and release of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) contribute to impaired islet beta-cell function, insulin action, and glucose homeostasis in pathogenic conditions such as hyperglycemia and obesity (Maedler et al., 2002, Larsen et al., 2007, Stanley et al., 2011, Weisberg et al., 2003, Ehses et al., 2007). Sleep fragmentation-induced insulin resistance is likewise associated with increased numbers of M1 macrophages, circulating concentrations of IL-6 (Poroyko et al., 2016), and IL-6 and TNF-α mRNA expression in visceral adipose tissue of mice (Zhang et al., 2014). A recent study that found systemic inflammation partially mediates the significant relationship between insufficient sleep and insulin resistance in women (Kim et al., 2016).
Metabolic deficits in diet-induced obesity are also associated with increased inflammatory markers in the hypothalamus (De Souza et al., 2005, Thaler et al., 2012, Milanski et al., 2009, Kleinridders et al., 2009, Zhang et al., 2008, Berkseth et al., 2014, Posey et al., 2009, Andre et al., 2017), a brain region critical to energy homeostasis (Morton et al., 2006). Chronic consumption of saturated fatty acids leads to hypothalamic insulin and leptin resistance, impaired anorectic signaling, and subsequent weight gain in rodents and are associated with increased local IL-1β, IL-6, and TNF-α expression and endoplasmic reticulum stress (De Souza et al., 2005, Milanski et al., 2009). Acute (i.e., 1 day) sleep disruption in rodents also increases cytokine gene expression in the hypothalamus (Dumaine and Ashley, 2015) and basal forebrain in general (Zielinski et al., 2014), but how these findings translate to protein levels and metabolic function remains to be determined.
To address these questions, we used a mouse model of sleep fragmentation to determine whether insufficient sleep elicits neuroinflammation in metabolically relevant brain regions and how this relates to metabolic function. We compared inflammatory responses between central and peripheral tissues, and additionally tested the hypothesis that exposure to multiple inflammatory factors exacerbates metabolic outcomes. Sleep and diet of mice were therefore manipulated for 3 or 9 days to determine individual and combined effects of sleep fragmentation and high-fat feeding on inflammation, energy balance, and glucose metabolism.
2. Materials and methods
2.1. Animals and housing
Male C57BL/6 J mice (age 6–8 weeks upon arrival) were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were acclimated to handling and individual housing in standard shoebox cages in controlled conditions of a 12:12 h light:dark cycle at an ambient temperature of 28 ± 1 °C. Mice were then habituated to housing in sleep disruption devices for 3 days under the same light and temperature conditions. Ad libitum rodent chow (PicoLab Rodent Diet 20; 13.1% kcal from fat; Labdiet, St. Louis, MO) and water were provided throughout habituation. During the experimental period, mice were maintained on chow or a high-fat diet (HFD; 60% kcal from fat, D12492, Research Diets, New Brunswick, NJ) with ad libitum access to food and water except where noted. All procedures involving the use of animals were approved by the University of Washington Institutional Animal Care and Use Committee.
2.2. Sleep fragmentation
Sleep of mice was manipulated using a novel sleep disruption device as previously described (Ho et al., 2016, Ringgold et al., 2013, Sutton and Opp, 2014). Sleep disruption devices consisted of a Plexiglas® cylindrical chamber divided into two separate compartments with each compartment housing one mouse. The floor of the chamber is a disc that is programmed to rotate for 8 s during each 30-s interval. Parameters of disc rotation are such that 1) the direction of rotation is randomized and 2) placement of the 8-s rotation within each 30-s period is varied. Mice were subjected to sleep fragmentation (SF) for 18 h per every 24-h period. Under this protocol, mice were allowed 6 h of uninterrupted sleep opportunity at the start of the light cycle in which they could freely behave, including sleep, when the disc was not rotating.
2.3. Experimental design
Prior to habituation in sleep disruption devices, mice were assigned to one of four experimental groups: 1) undisturbed sleep with ad libitum chow diet (Rested Chow); 2) undisturbed sleep with ad libitum HFD (Rested HFD); 3) 18-h SF with ad libitum chow diet (SF Chow); 4) 18-h SF with ad libitum HFD (SF HFD). To control for non-specific effects of housing conditions, all mice were housed in sleep disruption devices throughout the study regardless of group assignment. Mice of the same experimental group were paired together in the same sleep disruption device. After 3 days of habituation in sleep disruption devices, mice were subjected to 3 or 9 days of sleep and diet manipulation. Daily measures of body mass, food intake, and water consumption were recorded throughout habituation and manipulation periods of the study. Food spillage was taken into account by subtracting the weight of food spilled from the difference in food hopper weight between days. Due to disc rotation and shared occupancy of sleep disruption devices, food spillage could not be accurately ascribed to individual mice during periods of SF. Food intakes of SF mice were therefore calculated as a mean value among pairs of mice housed within the same device during the period of manipulation. Although this method limits accuracy of individual food intakes, mice paired together in a given device were subjected to the same experimental manipulations. Our measures thus reflect overall response patterns to sleep and diet manipulations.
2.4. Glucose tolerance tests
Glucose tolerance tests (GTTs) were performed after 3 (n = 12/group) and 9 (n = 6/group) days of manipulation. To habituate mice to testing procedures, mice were handled daily prior to the start of manipulations and were given an intraperitoneal (i.p.) injection of 0.9% saline on 3 separate days prior to testing. Mice were fasted at the start of the light cycle for 6 h on test days. Fasting blood glucose was measured with a glucometer (AlphaTRAK2, Abbott Laboratories, North Chicago, IL) via tail vein blood sampling. Mice were then given a bolus i.p. injection of glucose (2 g/kg, Sigma-Aldrich, St. Louis, MO) and blood glucose was sampled at 15, 30, 60, 90, and 120 min post-injection. Food was returned at the end of testing.
2.5. Determination of cytokine and hormone concentrations
2.5.1. Blood and tissue collection
Plasma and fresh tissues were collected 2 h into the light cycle following days 3 (n = 8/group) or 9 (n = 7–8/group) of manipulation (i.e., 2 h after the last bout of SF). Mice were deeply anesthetized with isoflurane (Henry Schein, Dublin, OH) and a terminal blood sample was taken via cardiac puncture. Blood samples were collected in EDTA-coated tubes and kept on ice until centrifugation at 17910 g for 20 min at 4 °C to collect plasma. Immediately following blood sampling, brains were dissected on ice and brain and peripheral tissues samples were flash-frozen in liquid nitrogen. All samples were stored at -80°C until further processing.
2.5.2. Cytokine assays
Multiplex bead sets were produced in-house using Luminex (Austin, TX) MagPlex microspheres (regions 34, 38, and 65 for IL-1β, IL-6, and TNF-α, respectively). Beads were conjugated to capture antibodies from R&D Systems (Minneapolis, MN) DuoSets (DY401, DY406, and DY410 for IL-1β, IL-6, and TNF-α, respectively) as per manufacturer’s instructions with the exception of overnight incubations. These were performed using the kit’s wash buffer, followed by removal in wash buffer for long-term storage at 4 °C in Stabiliguard (product code SG01, SurModics, Eden Prairie, MN).
All reagents and samples were allowed to warm to room temperature prior to use. To generate a standard series, lyophilized recombinant protein standards from R&D Systems (401-ML, 406-ML, and 410-ML for IL-1β, IL-6, and TNF-α, respectively) were reconstituted as per manufacturer’s instructions. Diluents were generated with cell lysis buffer (BioRad, Hercules, CA, catalog #171–304012). Diluents and reconstituted standards were used to generate a series of 7 standards (for IL-1β: 34, 103, 309, 926, 2,778, 8,333, and 25,000 pg/ml; for IL-6: 3, 10, 31, 93, 278, 833, and 2500 pg/ml; for TNF-α: 7, 21, 62, 185, 556, 1667, and 5000 pg/ml). The diluents also served as the blanks for the appropriate sample type. Standards and samples were run in duplicate in a 96-well plate. Protein samples were standardized at 10 µg of total protein per well and brought to a total volume of 150 µl per well with the addition of PBS with 1% BSA.
Beads for specific analytes were added to incubation buffer (PBS + 0.1% BSA + 0.05% Tween 20) for a final concentration of 50 beads/µl. Upon addition of bead solution (50 µl), plates were covered using a foil plate sealer then agitated at 600 rpm overnight at 4 °C. The following day, plates and reagents were brought to room temperature. Plates were set upon a magnetic separator (Luminex, part number CN-0269-01) and beads were allowed to settle for 2 min. The supernatant was then discarded and beads were washed twice with wash buffer (PBS + 0.05% Tween 20) prior to the addition of 25 µl of detection antibodies (R&D Systems BAF401, BAF406, and BAF410 for IL-1β, IL-6, and TNF-α, respectively) in incubation buffer. The plate was covered again and incubated for 30 min at 600 rpm at room temperature. The plate was then placed on the magnetic separator and beads were washed twice as described above. A streptavidin-phycoerythrin solution (Invitrogen, Carlsbad, CA, catalog #S866) of 4 µg/ml in incubation buffer was added at a volume of 25 µl per well. The plate was sealed once more and incubated at room temperature for 30 min.
At the end of the incubation, the plate was placed on the magnetic separator and washed twice. After washing, 100 µl of wash buffer was added to each well and the plate was read on a Bio-Plex 200 system (Bio-Rad). Data were analyzed using Bio-Plex Manager 4.1 software with five-parameter logistic regression (5PL) curve fitting. Observed concentrations were obtained from the Bio-Plex software for all sample types. The cut-off for levels of detection was set at the lowest point on the standard curve with an observed concentration between 70–130% of the (observed/expected)*100 value, as suggested by the manufacturers of the Bio-Plex 200 system. Sensitivities were based on the lower limit of detection for each analyte and are as follows: IL-1β, 34 pg/ml; IL-6, 3 pg/ml; TNF-α, 7 pg/ml.
2.5.3. Corticosterone assays
Plasma corticosterone concentrations were measured by enzyme immunoassay using commercially available kits (Arbor Assays, Ann Arbor, MI) following manufacturer’s instructions. Samples were assayed in duplicate with an intra-assay covariance of 3.5% and lower limit of detection at 78 pg/ml.
2.6. Determination of glial cell activation
To determine effects of sleep and diet on glial cell activation, brains were collected from an additional set of mice subjected to the 3- (n = 6/group) or 9-day (n = 6/group) study protocol. These mice were transcardially perfused with 0.1 M PBS followed by 10% formalin (Fisher Scientific, Pittsburgh, PA). Brains were post-fixed overnight in 10% formalin, transferred to 30% sucrose solution for two days, then stored in Tissue Tek (Sakura Finetek, Torrance, CA) at -80°C. Brain tissue was sectioned coronally in a cryostat (Leica CM1950, Leica Biosystems, Buffalo Grove, IL) at a thickness of 14 µm and thaw-mounted onto electrostatically charged microscope slides (Superfrost Plus, Fisher). Slides were stored at -80°C until immunohistochemical processing.
2.6.1. Immunohistochemistry
Brain tissue was immunohistochemically processed in a Sequenza slide rack (Ted Pella Inc., Redding, CA) with all 4 experimental conditions represented in each batch. Slides were allowed to warm to room temperature for 20–30 min before rehydrating in 0.1 M PBS 3 × 5 min. Slides were then incubated in blocking solution (5% normal donkey serum in 0.1 M PBS + 1% bovine serum albumin [BSA]) for 30 min at room temperature. Primary antibodies (1:1000; goat anti-GFAP, Abcam ab53554, Abcam Inc., Cambridge, MA; rabbit anti-Iba1, Code No. 019-19741 lot# WEK6254, Wako Chemicals USA, Inc., Richmond, VA) were applied directly thereafter and incubated overnight at 4 °C. The following day, slides were washed 6 × 5 min in PBS then incubated with secondary antibodies (1:400; Alexa Fluor 488 donkey anti-rabbit, Code No. 711-545-152; Alexa Fluor 594 donkey anti-goat, Code No. 705-585-147; Jackson ImmunoResearch, West Grove, PA) at room temperature for 30 min. Slides were again washed 6 × 5 min before coverslipped with Vectastain Hardset Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA). Slides were stored in light-tight slide boxes at 4 °C.
2.6.2. Quantification of immunohistochemical staining
Iba1 and GFAP immunoreactivity (-ir; as markers of microglia and astrocytes, respectively) was visualized through a fluorescence microscope (Olympus BX51; Center Valley, PA) fitted with a digital camera (C11440, Hamamatsu Photonics, Japan) using StereoInvestigator software (MicroBrightField Inc., Williston, VT). Omission of primary antibodies in control studies confirmed the absence of nonspecific immunoreactivity (data not shown). The arcuate nucleus (ARC), lateral hypothalamus (LH), and ventromedial hypothalamus (VMH) were examined at approximately -1.58, -1.70, and -1.82 mm from bregma (with the additional coordinate of -1.46 mm for ARC and VMH) based on the Paxinos and Franklin mouse brain atlas (Paxinos and Franklin, 2001). Areas of the brainstem examined included the area postrema (AP) and the nucleus of the solitary tract (NTS) at approximately -7.32, -7.56, and -7.76 mm from bregma (as well as -7.08 mm for the NTS).
Iba1- and GFAP-ir in captured images was quantified in Adobe Photoshop (San Jose, CA) by an independent researcher blinded to treatment condition. Borders of nuclei were traced in similar size with respect to each nucleus and Iba1-positive cells were counted manually within outlined regions of interest. The brightness and contrast of images were adjusted to a firm setting for all sections to aid in visualization of immunostaining. GFAP-ir was quantified by subtracting the optical density from areas with minimal staining from that of outlined nuclei to account for variation in background staining. Iba1- and GFAP-ir values were averaged from both sides of bilateral nuclei (ARC, LH, VMH, and NTS).
2.7. Statistical analyses
Cumulative changes in body mass, food intake, and water consumption were analyzed using repeated-measures analysis of variance (ANOVA) followed by post-hoc independent t-tests. Mean Iba1-positive cell number and GFAP optical density were analyzed across 3–4 sections of each nucleus with repeated-measures ANOVAs followed by post-hoc comparisons with Bonferroni correction where significance was achieved. Protein concentrations were analyzed with 2-way ANOVAs with post-hoc Bonferroni correction. Data that did not meet requirements of homogeneity of variance were log10-transformed (protein concentrations) or re-analyzed using non-parametric tests (Mann-Whitney U for glucose tolerance and ingestive behavior). To account for group differences in body mass, glycemic responses to GTTs were quantified by dividing the incremental increase in area under the curve (AUC; determined by trapezoidal integration) by body mass. Corrected AUC values were analyzed within each time point with 2-way ANOVAs and post-hoc tests with Bonferroni correction.
3. Results
3.1. Effects of SF and/or HFD on body mass and ingestive behavior
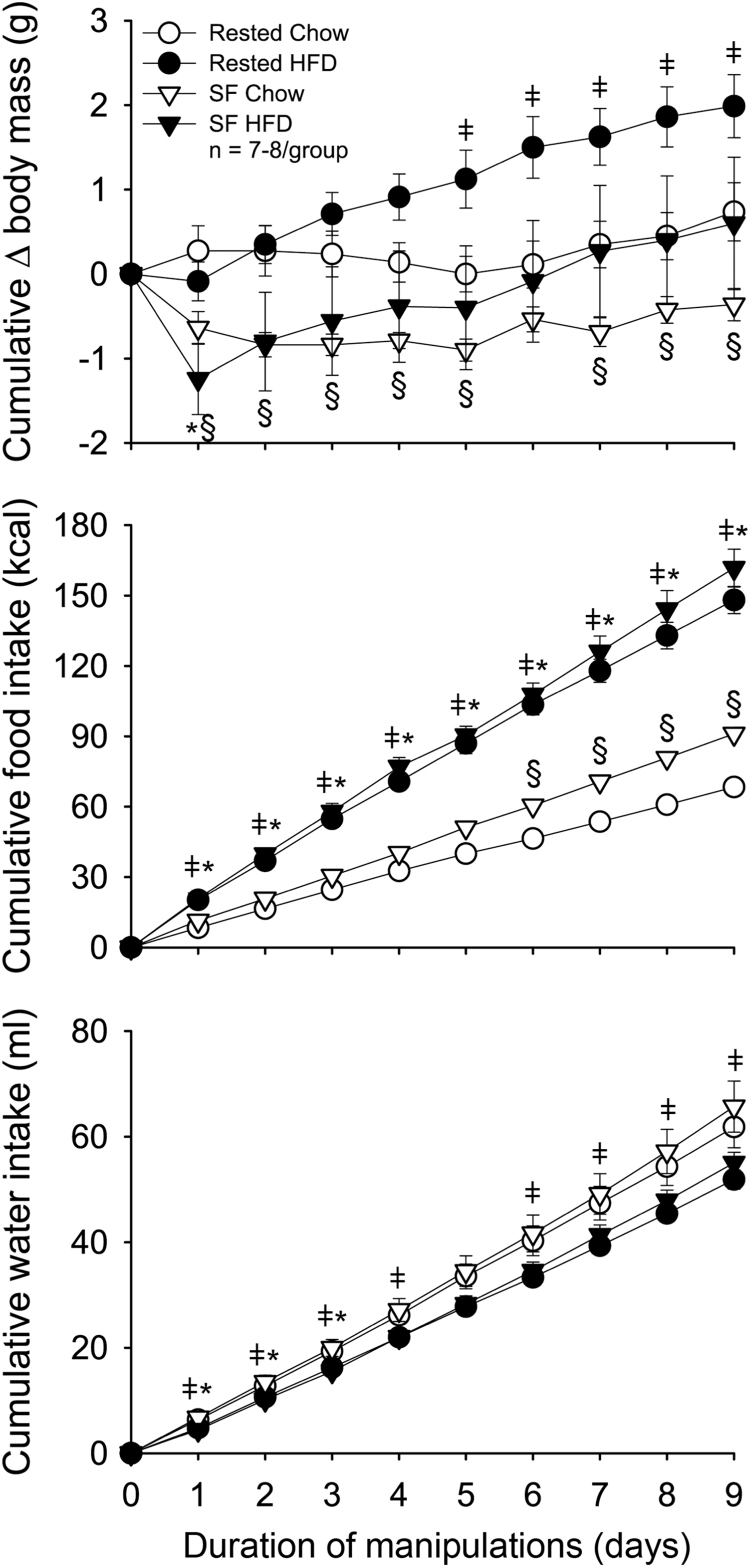
SF significantly reduced body mass throughout the 9-day protocol (F(1,27) = 10.097, p = 0.004, Fig. 1). Post-hoc tests revealed that mice subjected to SF weighed significantly less than undisturbed, chow-fed controls across all days except after day 6 (Rested Chow vs. SF Chow, p < 0.05). Exposure to HFD increased body mass and reduced weight loss in mice exposed to SF, but overall effects of HFD on body mass were not statistically significant (p = 0.097; Fig. 1). Mice exposed to SF and/or HFD demonstrated greater food intake (sleep: F(1,27) = 11.870, p = 0.002; diet: F(1,27) = 33.652, p < 0.001; Fig. 1)with less cumulative food intake in Rested Chow mice compared to all other groups (p < 0.05). Mice fed HFD consumed significantly less water than chow-fed mice across all experiment days (F(1,27) = 8.890, p = 0.006; Fig. 1).
Fig. 1.
Effects of sleep fragmentation (SF) and/or high-fat diet (HFD) on body mass and ingestive behavior. Cumulative changes in body mass (top panel), food intake (middle panel), and water intake (bottom panel) were recorded in response to 9 days of sleep and diet manipulation. Data are presented as means ± SEM and were analyzed with repeated-measures ANOVAs. Post-hoc comparisons were made with independent t-tests or Mann-Whitney U tests where appropriate. ǂ p < 0.05 Rested HFD vs Rested Chow; § p < 0.05 SF Chow vs Rested Chow; *p < 0.05 SF HFD vs Rested Chow.
3.2. Effects of SF and/or HFD on glucose tolerance
To determine the individual and combined effects of SF and HFD on glucose tolerance, blood glucose responses to a bolus i.p. injection of glucose were quantified as incremental increase in area under the curve divided by body mass. Three days of high fat intake significantly reduced glucose tolerance (F(1,43) = 20.773, p < 0.001; Fig. 2). After 9 days, significant main effects of sleep and diet were observed (sleep: F(1,17) = 10.275, p = 0.005; diet: F(1, 17) = 24.428, p < 0.001; Fig. 2) with the greatest impairments in mice exposed to both SF and HFD. However, post-hoc comparisons revealed no significant differences between SF mice and their rested controls within either diet (p > 0.05). At both time points, fasting blood glucose concentrations were significantly elevated with HFD and significantly reduced with SF (day 3: sleep, F(1,43) = 65.671, p < 0.001; diet, F(1,43) = 6.717, p = 0.013; interaction: F(1,43) = 14.153, p = 0.001; day 9: sleep: F(1,17) = 20.870, p < 0.001; diet: F(1,17) = 5.212, p = 0.036; interaction: p > 0.05; Fig. 2).
Fig. 2.
Effects of sleep fragmentation (SF) and/or high-fat diet (HFD) on glucose tolerance. Intraperitoneal glucose tolerance tests were performed after a 6-h fast at the start of the light phase after 3 (top panel) or 9 (bottom panel) days of sleep and diet manipulations. Glycemic responses were quantified by calculating the incremental increase in area under the curve (IncrAUC) from fasting (time 0) conditions and were corrected for body mass (BM; insets). Data are presented as means ± SEM and were analyzed with two-way ANOVAs and post-hoc comparisons with Bonferroni correction. *p < 0.05 vs Rested Chow.
3.3. Effects of SF and/or HFD on glial activation
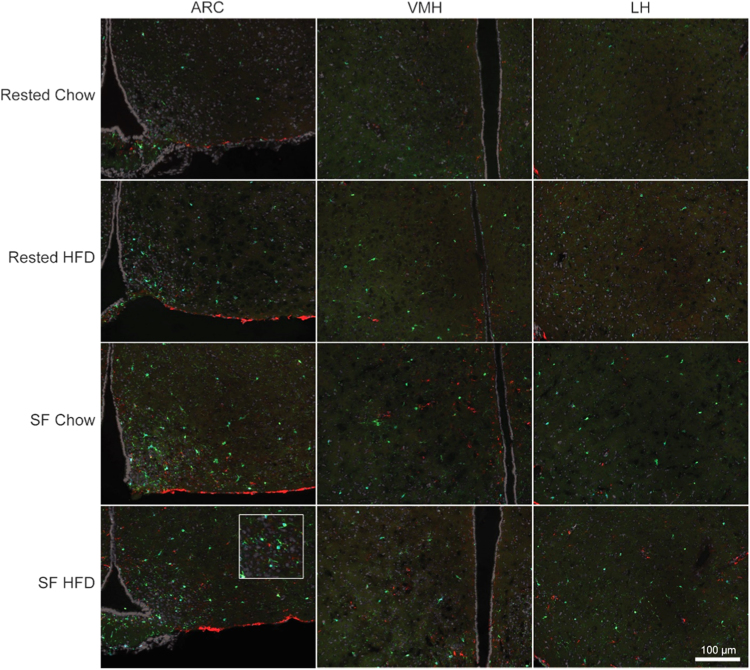
Exposure to SF and/or HFD increased glial activation in the hypothalamus (Fig. 3). Three days of exposure to SF or HFD significantly increased Iba1-ir in the ARC (sleep: F(1, 18) = 28.212, p < 0.001; diet: F(1, 18) = 5.541, p = 0.030) and VMH (sleep: F(1, 19) = 35.241, p < 0.001; diet: F(1, 19) = 11.289, p = 0.003). SF HFD mice exhibited significantly more Iba1-positive cells in these nuclei than either group of rested mice (p < 0.05; Fig. 4). The LH demonstrated significantly greater Iba1-ir only with SF (F(1, 19) = 22.367, p < 0.001). A significant interaction between sleep and diet was observed with respect to GFAP-ir in the VMH (F(1, 19) = 5.046, p = 0.037; Fig. 4), but group means did not differ significantly from one another in post-hoc comparisons (p > 0.05).
Fig. 3.
Representative photomicrographs of hypothalamic nuclei in mice exposed to 3 days of sleep fragmentation (SF) and/or high-fat diet (HFD), or control conditions. Iba1 (green)and GFAP (red) immunoreactivity were used as respective markers of microglia and astrocytes in the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), and lateral hypothalamus (LH). Inset depicts immunostaining at higher magnification.
Fig. 4.
Effects of sleep fragmentation (SF) and/or high-fat diet (HFD) on glial activation. Immunoreactivity (-ir) of Iba1 and GFAP was quantified in the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), lateral hypothalamus (LH), nucleus of the solitary tract (NTS), and area postrema (AP). Data are presented as means ± SEM and were analyzed with two-way ANOVAs and post-hoc comparisons with Bonferroni correction. *p < 0.05 vs Rested Chow.
Iba1-ir remained elevated in the ARC, VMH, and LH of SF HFD mice after 9 days of manipulations. Effects of diet on Iba1-ir cell number persisted in the ARC (F1,9 = 27.146, p = 0.001; Fig. 4) and a nearly significant interaction with sleep (p = 0.058) resulted in significant differences between SF HFD mice and chow-fed controls (p < 0.05). GFAP-ir in ARC was similarly affected by diet (F1,10 = 5.199, p = 0.046; Fig. 4) with a slight trend towards an interaction (p = 0.088) but no statistically significant differences among individual groups (p > 0.05). Although main effects of sleep or diet were not statistically significant in the VMH or LH, Iba1-ir was visibly greater in SF HFD mice and a significant interaction was observed in the LH (F1,10 = 5.905, p = 0.035; Fig. 4). Neither Iba1-ir nor GFAP-ir was significantly altered with sleep or diet manipulations in the NTS or AP at either time point (Fig. 4).
3.4. Effects of SF and/or HFD on cytokine and hormone concentrations
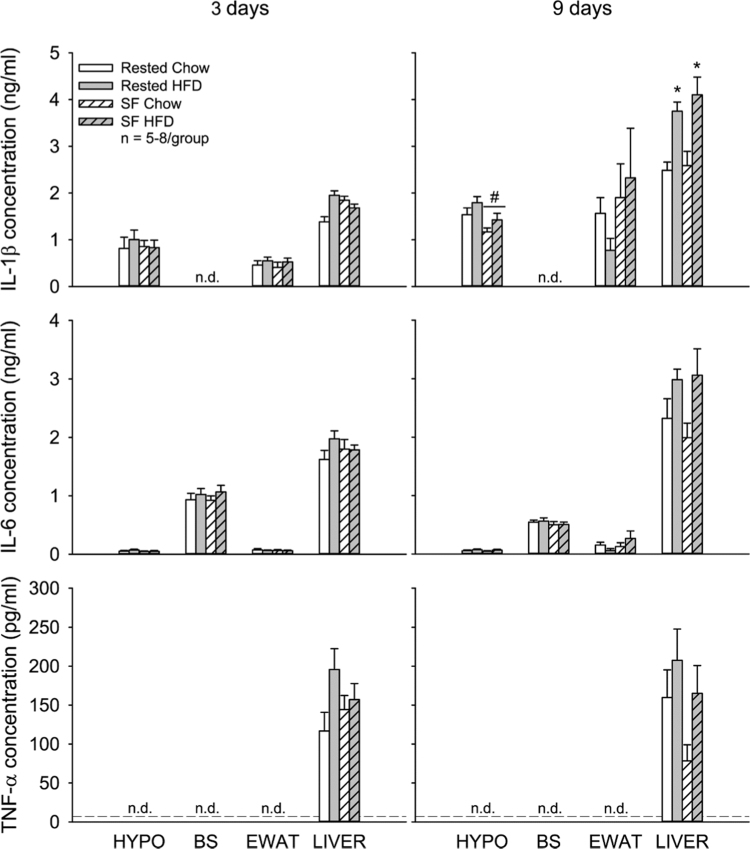
IL-1β, IL-6, and TNF-α concentrations in the hypothalamus or brainstem were not appreciably altered after 3 days of sleep and diet manipulations (p > 0.05). After 9 days, hypothalamic IL-1β concentrations were elevated with high-fat feeding (p = 0.054) and significantly reduced with SF (p = 0.007)(Fig. 5). However, the largest cytokine responses to sleep and diet manipulations were observed in the liver. High fat intake significantly increased liver IL-1β concentrations after 3 days and both IL-1β and IL-6 concentrations after 9 days (Fig. 5). No significant changes in cytokine concentrations were observed in epididymal white adipose tissue (EWAT; p > 0.05) at either time point. Plasma corticosterone concentrations were significantly elevated after 3 days of SF (F1,28 = 5.067, p = 0.032) and significantly reduced by 9 days of high-fat intake (F1,27 = 5.996, p = 0.021)(Fig. 6).
Fig. 5.
Effects of sleep fragmentation (SF) and/or high-fat diet (HFD) on cytokine concentrations. Concentrations of interleukin (IL-)1β, IL-6, and tumor necrosis factor-α (TNF-α) were determined in the hypothalamus (HYPO), brainstem (BS), epididymal white adipose tissue (EWAT) and liver. Data are presented as means ± SEM and were analyzed with two-way ANOVAs and post-hoc comparisons with Bonferroni correction. The dashed line represents the minimal level of detection (threshold set at lowest concentration of known standards). Values that fall below this level are designated as not detectable (n.d.). *p < 0.05 vs Rested Chow; #p < 0.05 SF vs Rested groups.
Fig. 6.
Effects of sleep fragmentation (SF) and/or high-fat diet (HFD) on corticosterone concentrations. Two-way ANOVAs demonstrate that 3 days of SF significantly elevates, and 9 days of high fat intake significantly reduces, plasma corticosterone concentrations (p < 0.05). Post-hoc comparisons with Bonferroni correction indicate no significant differences among individual treatment groups. Data are presented as means ± SEM. *p < 0.05 SF vs Rested groups; #p < 0.05 HFD vs Chow groups.
4. Discussion
Previous studies in humans and rodents indicate that poor sleep negatively affects metabolic function and that inflammation may play a role. Whereas systemic inflammatory responses have been associated with insufficient sleep and insulin resistance (Kim et al., 2016, Zhang et al., 2014), sleep-related neuroinflammation has yet to be examined within metabolic contexts. In the present study, we sought to determine whether sleep disruption elicits neuroinflammation in brain regions that regulate energy balance, and the extent to which this reflects changes in metabolic function. We further examined individual and combined effects of sleep disruption and saturated fat consumption to determine whether exposure to multiple inflammatory factors exacerbates metabolic outcomes.
In contrast to 3 or 9 days of high-fat feeding, SF alone did not impair glucose tolerance, as determined by integrated responses over a 2-h test period. However, after 3 days of manipulations blood glucose concentrations of rested and SF mice reached similar absolute values at the 15-min time point despite SF mice starting at significantly lower fasting blood glucose concentrations. This heightened glucose excursion suggests that early insulin responses may be impaired with SF, at least after this acute (3 day) period of manipulation, and should be further examined in future studies. Whereas the use of IPGTTs in our study allowed for gross metabolic phenotyping without the added stress of surgical catheterization and large-volume sampling, interpretation of our findings remains limited. The use of more sophisticated measures, such as those that determine insulin secretion (e.g., hyperglycemic clamps) and insulin action (e.g., hyperinsulinemic-euglycemic clamps), could be incorporated in future studies to more thoroughly investigate interactions between sleep and diet on glucose metabolism.
A notable effect of our SF protocol was the initial loss of body mass, which was more pronounced in mice exposed to both SF and HFD. Collectively, the loss of body mass and greater food intake suggest increased energy expenditure, and may explain why we did not observe the expected decrements in glucose tolerance with SF that have been previously observed (Zhang et al., 2014; Baud et al., 2013). In these previous studies, 8–12 h daily SF for 10–14 days reduces glucose tolerance in the absence of weight changes. Transient loss of body mass may have precluded detrimental effects of SF on glucose tolerance. However, one study in rats found that even with weight loss, 8 days of sleep disruption impairs glucose tolerance (Barf et al., 2010). One key difference among protocols may be that mice in our study were allowed 6 h of undisturbed sleep opportunity during the light phase and this recovery period may be sufficient to negate detrimental effects of SF on glucose tolerance. Although other studies allow longer durations of undisturbed sleep, these coincide with more active periods of the rodents’ 24-h day (i.e., dark phase) and may not be as restorative due to conflict with circadian rhythms. It is possible that thermoregulatory demands may have some influence on our outcomes as our mice were housed without bedding to facilitate measures of food intake. However, mice were maintained at ambient temperatures within their thermoneutral zone to minimize energetic demands associated with maintaining body temperature. Physiological responses to energy perturbations are also supported by the observed changes in plasma corticosterone concentrations. Patterns of change (i.e., reduced with SF after 3 days but elevated with HFD after 9 days) best reflect those in body mass and suggest the need to mobilize glucose in response to periods of energy deficit and excess, respectively. Future studies with more accurate and temporally acute measures of energy intake and expenditure would provide information as to how energy balance contributes to glucose metabolism in rodent models of sleep fragmentation.
Our results nonetheless demonstrate that SF potently induces hypothalamic inflammation. Three days of SF produced clear effects on Iba1-ir and the significant main effect of HFD at this time point appears largely driven by responses associated with sleep disruption. After 9 days, Iba1-ir remained elevated only in mice that had been exposed to both SF and HFD, revealing a novel interaction between sleep and diet on microglial activation. Transient neuroinflammatory responses to sleep restriction (Zielinski et al., 2014) or HFD (Thaler et al., 2012, Baufeld et al., 2016) have been previously reported in rodents, with acute responses subsiding after 1–3 days and returning only after prolonged (4–8 weeks) exposure (Baufeld et al., 2016, Thaler et al., 2012). However, our findings suggest that simultaneous exposure to multiple subthreshold inflammatory responses may exacerbate outcomes when combined. Inhibiting microglia expansion reduces food intake and weight gain and restores hypothalamic leptin sensitivity in HFD-fed mice (Andre et al., 2017), suggesting a key role for microglia in diet-induced obesity. Perhaps with longer exposure, microglial responses to SF and HFD may, too, exacerbate hypocretinergic behaviors that promote weight gain. Investigation of these interactions in brain regions such as the amygdala, ventral tegmental area, and striatum may also provide critical insight as to how sleep and diet affect glucose sensing, insulin action, and reward processes in the brain (Diepenbroek et al., 2013).
The additive (3-day) and synergistic (9-day) effects of SF and HFD in our study may be due to their independent abilities to disrupt sleep. Three or 9 days of exposure to our SF protocol reduces rapid eye movement (REM) and non-rapid eye movement (NREM) sleep (Ho et al., 2016), and high-energy diets increase SF (Perron et al., 2015) and NREM sleep in rodents (Danguir, 1987, Hansen et al., 1998, Jenkins et al., 2006). Mice fed a HFD demonstrate a blunted sleep-wake rhythm with increased NREM sleep and reduced wakefulness during the active night phase, as well as lower cortical activity and insulin sensitivity in the brain (Sartorius et al., 2012). Interestingly, these effects are mediated in part by increased immune responses as treatment with a neutralizing IL-6 antibody improves cortical activity and insulin action in the brain, and mice with deficient TLR2/4 signaling exhibit greater cortical activity, insulin action, and sleep-wake rhythms than HFD-fed control animals (Sartorius et al., 2012).
Despite a robust effect of SF and HFD on microglial activation in energy-regulating regions of the hypothalamus, we found that cytokine responses (particularly of IL-1) in the liver best reflect the observed changes in glucose tolerance. The liver is a major target of insulin signaling, glucose uptake, and is responsible for de novo glucose production and insulin clearance. Pharmacological agents that improve insulin sensitivity improve liver function, highlighting its essential role in glucose metabolism (Home and Pacini, 2008). Hepatic inflammation profoundly affects local function and glucose metabolism in rodents (Cai et al., 2005), and the pro-inflammatory state induced by poor sleep and diet may contribute to observed deficits in systemic glucose clearance. Although adipose tissue is an established site of inflammation-induced impairments in glucose metabolism (Donath and Shoelson, 2011), the exposure period to HFD in our study is likely of insufficient duration to develop WAT-related impairments (Williams et al., 2014).
4.1. Conclusions and limitations
In the present study, naïve mice were simultaneously exposed to SF and HFD to determine their relative impacts on neuroinflammatory responses and metabolic function. We report that acute pro-inflammatory responses in the periphery (i.e., liver) better corresponds to patterns of glucose tolerance than do those in the brain. To our knowledge, this is the first time central and peripheral inflammatory responses have been compared simultaneously with respect to their association with glucose metabolism. Furthermore, we report the novel finding that sleep disruption induces a rapid and more potent increase in microglial activation than does high-fat feeding. The functional implications for these neurophysiological changes remain to be determined and should be examined beyond the relatively short period of manipulation in the present study. Given the critical role of the hypothalamus in numerous homeostatic processes, more prolonged exposure to an inflammatory milieu is likely to contribute to the adverse consequences of chronic insufficient sleep. Investigation of inflammatory responses in cortico-limbic regions may also provide important insight to interactions between sleep and diet on glucose metabolism.
Conflicts of interest
The authors declare they have no conflicts of interest.
Acknowledgments
The authors thank Dr. Kristyn Ringgold and Dr. Paulien Barf for their contributions to this project. This research was supported by NIH training grants 2T32DK007247 and 1F32DK103491 and was funded, in part, by the NIH National Institute of Allergy and Infectious Diseases grant AI115706, and by the Department of Anesthesiology & Pain Medicine of the University of Washington Seattle.
References
- Andre C., Guzman-Quevedo O., Rey C., Remus-Borel J., Clark S., Castellanos-Jankiewicz A., Ladeveze E., Leste-Lasserre T., Nadjar A., Abrous D.N., Laye S., Cota D. Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes. 2017;66:908–919. doi: 10.2337/db16-0586. [DOI] [PubMed] [Google Scholar]
- Anothaisintawee T., Reutrakul S., Van Cauter E., Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep. Med. Rev. 2015;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Barf R.P., Meerlo P., Scheurink A.J. Chronic sleep disturbance impairs glucose homeostasis in rats. Int. J. Endocrinol. 2010;2010:819414. doi: 10.1155/2010/819414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch C.E., Basch C.H., Ruggles K.V., Rajan S. Prevalence of sleep duration on an average school night among 4 nationally representative successive samples of American high school students, 2007–2013. Prev. Chronic. Dis. 2014;11:E216. doi: 10.5888/pcd11.140383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud M.O., Magistretti P.J., Petit J.M. Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice. J. Sleep. Res. 2013;22:3–12. doi: 10.1111/j.1365-2869.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- Baufeld C., Osterloh A., Prokop S., Miller K.R., Heppner F.L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132:361–375. doi: 10.1007/s00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkseth K.E., Guyenet S.J., Melhorn S.J., Lee D., Thaler J.P., Schur E.A., Schwartz M.W. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology. 2014;155:2858–2867. doi: 10.1210/en.2014-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J., Shoelson S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen B., Kristensen J.K., Ottosen P., Borch-Johnsen K. The Danish National Diabetes Register: trends in incidence, prevalence and mortality. Diabetologia. 2008;51:2187–2196. doi: 10.1007/s00125-008-1156-z. [DOI] [PubMed] [Google Scholar]
- Dabelea D., Mayer-Davis E.J., Saydah S., Imperatore G., Linder B., Divers J., Bell R., Badaru A., Talton J.W., Crume T., Liese A.D., Merchant A.T., Lawrence J.M., Reynolds K., Dolan L., Liu L.L., Hamman R.F. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G., Finucane M.M., Lu Y., Singh G.M., Cowan M.J., Paciorek C.J., Lin J.K., Farzadfar F., Khang Y.H., Stevens G.A., Rao M., Ali M.K., Riley L.M., Robinson C.A., Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- Danguir J. Cafeteria diet promotes sleep in rats. Appetite. 1987;8:49–53. doi: 10.1016/s0195-6663(87)80026-0. [DOI] [PubMed] [Google Scholar]
- De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C., Saad M.J., Velloso L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Dumaine J.E., Ashley N.T. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R1062–R1069. doi: 10.1152/ajpregu.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehses J.A., Perren A., Eppler E., Ribaux P., Pospisilik J.A., Maor-Cahn R., Gueripel X., Ellingsgaard H., Schneider M.K., Biollaz G., Fontana A., Reinecke M., Homo-Delarche F., Donath M.Y. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- Foster G.D., Borradaile K.E., Sanders M.H., Millman R., Zammit G., Newman A.B., Wadden T.A., Kelley D., Wing R.R., Pi-Sunyer F.X., Reboussin D., Kuna S.T., Sleep AHEAD Research Group of Look AHEAD Research Group A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch. Intern. Med. 2009;169(17):1619–1626. doi: 10.1001/archinternmed.2009.266. Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Hansen M.K., Kapás L., Fang J., Krueger J.M. Cafeteria diet-induced sleep is blocked by subdiaphragmatic vagotomy in rats. Am. J. Physiol. 1998;274:R168–R174. doi: 10.1152/ajpregu.1998.274.1.R168. [DOI] [PubMed] [Google Scholar]
- Ho J.M., Barf R.P., Opp M.R. Effects of sleep disruption and high fat intake on glucose metabolism in mice. Psychoneuroendocrinology. 2016;68:47–56. doi: 10.1016/j.psyneuen.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden S.H., Barnett A.H., Peters J.R., Jenkins-Jones S., Poole C.D., Morgan C.L., Currie C.J. The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes Obes. Metab. 2013;15:844–852. doi: 10.1111/dom.12123. [DOI] [PubMed] [Google Scholar]
- Home P.D., Pacini G. Hepatic dysfunction and insulin insensitivity in type 2 diabetes mellitus: a critical target for insulin-sensitizing agents. Diabetes Obes. Metab. 2008;10:699–718. doi: 10.1111/j.1463-1326.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- Jenkins J.B., Omori T., Guan Z., Vgontzas A.N., Bixler E.O., Fang J. Sleep is increased in mice with obesity induced by high-fat food. Physiol. Behav. 2006;87:255–262. doi: 10.1016/j.physbeh.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Kim T.H., Carroll J.E., An S.K., Seeman T.E., Namkoong K., Lee E. Associations between actigraphy-assessed sleep, inflammatory markers, and insulin resistance in the midlife development in the United States (MIDUS) study. Sleep. Med. 2016;27-28:72–79. doi: 10.1016/j.sleep.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa T., Owada M., Urakami T., Tajima N. Epidemiology of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in Japanese children. Diabetes Res. Clin. Pract. 1994;24(Suppl):S7–S13. doi: 10.1016/0168-8227(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Kleinridders A., Schenten D., Konner A.C., Belgardt B.F., Mauer J., Okamura T., Wunderlich F.T., Medzhitov R., Bruning J.C. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson K.L., Ryden A.M., Mander B.A., Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- Knutson K.L., Van Cauter E., Zee P., Liu K., Lauderdale D.S. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman R.J., Mainous A.G., III, Diaz V.A., Geesey M.E. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann. Fam. Med. 2005;3:60–63. doi: 10.1370/afm.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall B., Lehnich A.T., Strucksberg K.H., Fuhrer D., Erbel R., Jankovic N., Moebus S., Jockel K.H., Stang A. Associations among sleep disturbances, nocturnal sleep duration, daytime napping, and incident prediabetes and type 2 diabetes: the Heinz Nixdorf Recall Study. Sleep. Med. 2016;21:35–41. doi: 10.1016/j.sleep.2015.12.017. [DOI] [PubMed] [Google Scholar]
- Larsen C.M., Faulenbach M., Vaag A., Volund A., Ehses J.A., Seifert B., Mandrup-Poulsen T., Donath M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Leng Y., Cappuccio F.P., Surtees P.G., Luben R., Brayne C., Khaw K.T. Daytime napping, sleep duration and increased 8-year risk of type 2 diabetes in a British population. Nutr.Metab Cardiovasc.Dis. 2016 doi: 10.1016/j.numecd.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wheaton A.G., Chapman D.P., Cunningham T.J., Lu H., Croft J.B. Prevalence of healthy sleep duration among adults--United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016;65:137–141. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H.I., Spinas G.A., Kaiser N., Halban P.A., Donath M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matricciani L., Olds T., Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep. Med. Rev. 2012;16:203–211. doi: 10.1016/j.smrv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Milanski M., Degasperi G., Coope A., Morari J., Denis R., Cintra D.E., Tsukumo D.M., Anhe G., Amaral M.E., Takahashi H.K., Curi R., Oliveira H.C., Carvalheira J.B., Bordin S., Saad M.J., Velloso L.A. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. The Mouse Brain in Stereotaxic Coordinates. Second ed. Academic Press; San Diego: 2001. [Google Scholar]
- Perron I.J., Pack A.I., Veasey S. Diet/energy balance affect sleep and wakefulness independent of body weight. Sleep. 2015;38:1893–1903. doi: 10.5665/sleep.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poroyko V.A., Carreras A., Khalyfa A., Khalyfa A.A., Leone V., Peris E., Almendros I., Gileles-Hillel A., Qiao Z., Hubert N., Farre R., Chang E.B., Gozal D. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016;6:35405. doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey K.A., Clegg D.J., Printz R.L., Byun J., Morton G.J., Vivekanandan-Giri A., Pennathur S., Baskin D.G., Heinecke J.W., Woods S.C., Schwartz M.W., Niswender K.D. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringgold K.M., Barf R.P., George A., Sutton B.C., Opp M.R. Prolonged sleep fragmentation of mice exacerbates febrile responses to lipopolysaccharide. J. Neurosci. Methods. 2013;219:104–112. doi: 10.1016/j.jneumeth.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius T., Lutz S.Z., Hoene M., Waak J., Peter A., Weigert C., Rammensee H.G., Kahle P.J., Haring H.U., Hennige A.M. Toll-like receptors 2 and 4 impair insulin-mediated brain activity by interleukin-6 and osteopontin and alter sleep architecture. FASEB J. 2012;26:1799–1809. doi: 10.1096/fj.11-191023. [DOI] [PubMed] [Google Scholar]
- Sokwalla S.M., Joshi M.D., Amayo E.O., Acharya K., Mecha J.O., Mutai K.K. Quality of sleep and risk for obstructive sleep apnoea in ambulant individuals with type 2 diabetes mellitus at a tertiary referral hospital in Kenya: a cross-sectional, comparative study. BMC. Endocr. Disord. 2017;17:7. doi: 10.1186/s12902-017-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley T.L., Zanni M.V., Johnsen S., Rasheed S., Makimura H., Lee H., Khor V.K., Ahima R.S., Grinspoon S.K. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2011;96:E146–E150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton B.C., Opp M.R. Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization. Sleep. 2014;37:515–524. doi: 10.5665/sleep.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Meng L., Li D., Yang M., Zhu Y., Li C., Jiang Z., Yu P., Li Z., Song H., Ni C. Interaction of sleep quality and sleep duration on glycemic control in patients with type 2 diabetes mellitus. Chin. Med. J. 2014;127:3543–3547. [PubMed] [Google Scholar]
- Tasali E., Leproult R., Ehrmann D.A., Van C.E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl. Acad. Sci. USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O., Zhao X., Sarruf D.A., Izgur V., Maravilla K.R., Nguyen H.T., Fischer J.D., Matsen M.E., Wisse B.E., Morton G.J., Horvath T.L., Baskin D.G., Tschop M.H., Schwartz M.W. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trento M., Broglio F., Riganti F., Basile M., Borgo E., Kucich C., Passera P., Tibaldi P., Tomelini M., Cavallo F., Ghigo E., Porta M. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45:225–229. doi: 10.1007/s00592-008-0047-6. [DOI] [PubMed] [Google Scholar]
- Tsai Y.W., Kann N.H., Tung T.H., Chao Y.J., Lin C.J., Chang K.C., Chang S.S., Chen J.Y. Impact of subjective sleep quality on glycemic control in type 2 diabetes mellitus. Fam. Pract. 2012;29:30–35. doi: 10.1093/fampra/cmr041. [DOI] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Campbell F.M., Drew J.E., Koch C., Hoggard N., Rees W.D., Kamolrat T., Thi N.H., Steffensen I.L., Gray S.R., Tups A. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PLoS. One. 2014;9:e106159. doi: 10.1371/journal.pone.0106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt S.D., Goff E.E., Reynolds A.M., Khan N., Jeong M., Jean-Louis G. Objective measures of sleep quality have not declined over the last 50 years. Sleep. Med Rev. 2016;30:108–109. doi: 10.1016/j.smrv.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Zhang S.X., Khalyfa A., Wang Y., Carreras A., Hakim F., Neel B.A., Brady M.J., Qiao Z., Hirotsu C., Gozal D. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int. J. Obes. 2014;38:619–624. doi: 10.1038/ijo.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski M.R., Kim Y., Karpova S.A., McCarley R.W., Strecker R.E., Gerashchenko D. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci. Lett. 2014;580:27–31. doi: 10.1016/j.neulet.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]